Abstract
An unfolded protein response (UPR) in addition to oxidative stress and the inflammatory response is known to be activated in age-related ocular disorders, such as macular degeneration, diabetic retinopathy, glaucoma, and cataracts. Therefore, we aimed to investigate whether healthy aged retinas display UPR hallmarks, in order to establish a baseline for the activated UPR markers for age-related ocular diseases. Using western blotting, we determined that the hallmarks of the UPR PERK arm, phosphorylated (p) eIF2a, ATF4, and GADD34, were significantly altered in aged vs. young rat retinas. The cleaved pATF6 (50) and CHOP proteins were dramatically upregulated in the aged rodent retinas, indicating the activation of the ATF6 UPR arm. The UPR activation was associated with a drop in rhodopsin expression and in the NRF2 and HO1 levels, suggesting a decline in the anti-oxidant defense in aged retinas. Moreover, we observed down-regulation of anti-inflammatory IL-10 and IL-13 and upregulation of pro-inflammatory RANTES in the healthy aged retinas, as measured using the Bio-plex assay. Our results suggest that cellular homeostasis in normal aged retinas is compromised, resulting in the concomitant activation of the UPR, oxidative stress, and inflammatory signaling. This knowledge brings us closer to understanding the cellular mechanisms of the age-related retinopathies and ocular disorders characterized by an ongoing UPR, and highlight the UPR signaling molecules that should be validated as potential therapeutic targets.
Introduction
The cellular mechanisms of age-related ocular disorders, such as age-related macular degeneration (AMD) [3, 4, 14, 25], diabetic retinopathy [19, 23, 41], glaucoma [1, 9, 11], and cataracts [5, 37], are tightly associated with oxidative stress, inflammation, and the unfolded protein response (UPR), suggesting cross talk between these cellular signals. Oxidative stress has been found to play a deteriorating role in aged retinas by producing reactive oxygen species (ROS) that are known to trigger different retinopathies, causing damage to the mitochondrial DNA. The generation of ROS is part of the normal metabolism in a biological system; however, free radicals (extremely reactive species), once formed, can begin a series of reactions that are harmful to the cell. Normally present, different cellular defense mechanisms have been launched to combat the consequences of acute oxidative stress. However, if the stress becomes chronic, and the cell cannot overcome the consequences, the pathological events in the retinas could be detrimental. ROS are also capable of lipid peroxidation, protein misfolding, and nucleic acid damage [18].
Aging is characterized by increased low-grade chronic pro-inflammation markers that circulate in the body, such as cytokines, C-reactive proteins (CRPs), and mannose-binding lectin (MBL) [13]. Among the factors that chronically trigger the immune system in the elderly, the epigenetic factor, viral CMV and HHV5 infections, and post-translational macromolecule modifiers are present in aged tissue. All of these antigens can provoke the innate immune system to secrete pro-inflammatory mediators and launch the inflammatory response [13].
Another cellular signal involved in the pathology of many age-related ocular disorders is the UPR. The UPR is comprised of a set of signaling pathways that collectively adjust the cell's ER protein folding capacity according to need. Three independently activated UPR signals, RNA-activated protein kinase (PKR)-like endoplasmic reticulum kinase (PERK), activating transcriptional factor 6 (ATF6), and inositol requiring kinase 1 (IRE1), are known to be activated upon stress. The main function of the UPR is to recognize misfolded proteins that occur, with the help of the immunoglobulin protein (BiP), and to lower the protein load via the phosphorylation of eukaryotic initiation factor 2α (peIF2α). The transcription factors, x-box binding protein 1 (Xbp1) and activating transcription factors 4 and 6, act to reestablish the equilibrium of the endoplasmic reticulum (ER) [15]. Oxidative stress and inflammation [35] have been highlighted for their associations with normal aging; however, the UPR and its role in the aged cell receiving internal or external insult has not yet been established. Moreover, the proteomics of normal aged retinas have not been investigated in detail, suggesting that ocular geriatrics must move forward to advance our knowledge and the treatment of age-related ocular disorders.
A great deal of published literature suggests that there is a critical need to establish a baseline for activated UPR markers in aged retinas, which would bring us closer to understanding the cellular mechanisms of age-related retinopathies and ocular disorders characterized by ongoing UPR. This has motivated us to initiate a study to analyze the UPR markers in aged retinas, creating a parallel between the UPR activation and the expression of the cellular markers of oxidative stress and inflammation. This knowledge would allow not only the design of common therapeutic treatments for age-related ocular disorders, but also the creation of a “fountain of youth” to delay aging in humans.
Materials and Methods
Animals
The samples consisting of 4-month and 24-month-old enucleated F344 rat eyes were obtained from the National Institute of Aging tissue bank. The one-year C57BL6 mouse retinas were a generous gift from Dr. Daniel Smith Jr. (University of Alabama at Birmingham).
Western Blot Analysis
For the protein extraction, whole retinas were isolated from the enucleated eyes using surgical excision. The total protein was extracted via sonication in a protein extraction buffer containing 970 uL of RIPA, 10 uL of 100 mM PMSF, 10 uL of 100 mM EGTA, and a mixture of protease inhibitors (PMSF, TLCK, aprotinin, leupeptin, and pepstatin). The protein concentrations were determined using BioRad Protein Assays and based on the Bradford method of protein quantitation. Next, the proteins (30–120 ug) were separated in 4–20% Criterion Precast gels and 5% polyacrylamide gels (BioRad), transferred to a polyvinylidene difluoride (PVDF) membrane using the Trans-Blot Turbo Transfer System (BioRad), and incubated with primary antibodies overnight at 4°C under agitation. Goat anti-rabbit (1:10,000, #926-68021), donkey anti-goat (1:10000, #926-32214), and donkey anti-mouse (1:10000, #926-32210) secondary antibodies were used (LI-COR Odyssey). In addition, β-actin, GAPDH, or Tubulin was used as the gel loading control, and was detected using an anti-β-actin antibody (1:5000, Sigma-Aldrich, #A1978) or anti-GAPDH antibody (1:1000, Abcam, #ab9485). Finally, the developed membrane was imaged using the LI-COR Odyssey Quantitative Fluorescence Imaging System.
Fixation of the Retinal Sections
The F344 enucleated rat eyes were fixed overnight in 4% paraformaldehyde, freshly made in phosphate-buffered saline (PBS). Then, the retinal cryosections were rinsed in PBS and blocked in 2% normal goat serum and 0.3% Triton X-100 in 0.01% BSA in PBS for 1 hour at room temperature. The sections were stained with primary anti-GFAP antibodies (Sigma Aldrich, C9205) which were diluted in PBS with 0.1% Triton X-100 and 1% BSA, and incubated overnight at 40°C. The Cy2-labeled anti-IgG secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:500 in PBS was applied at room temperature for 1 hour; then, the sections were mounted using Vectashield Mounting Medium (Vector Lab) and cover slipped. Images were taken using a confocal microscope (Leica SP1 UV Confocal Laser Scanning Microscope).
Cytokine Detection
The retina lysates (900 mg in 1 mL) were prepared using the Bio-Plex Cell Lysis Kit (BioRad 171-304011). The IL-10, IL-13, and RANTES cytokine levels were determined in the lysate according to the manufacturer's instructions, using the Bio-Plex set, Bio-Plex reader, and Bio-Rad L600013F25 machine.
Results
A review of the current literature has suggested that the UPR is involved in age-related ocular pathologies [15]. Recently, we have demonstrated that the UPR, once persistently activated, can also promote retinal degeneration [30]. These data together indicate that the UPR could affect the initiation and the progression of ocular disease in the elderly; therefore, we began our experiment with a determination of the UPR markers in 24 month old F344 retinas. The choice of 24 months was based on published data correlating rat age with human age [33] that would equate 24 months of rat age to 60 years of human age.
UPR Markers are Elevated in Aged Retinas
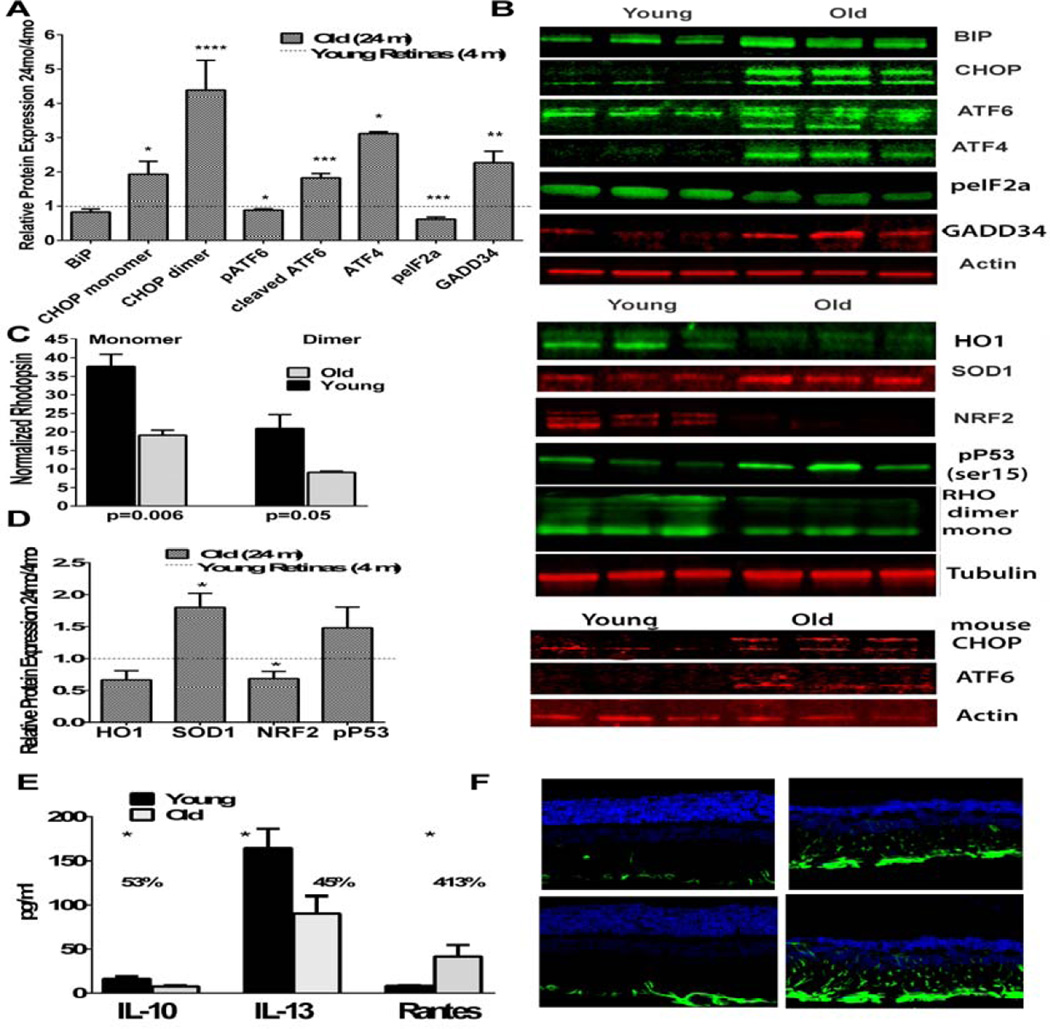
The activation of the UPR was measured using a western blot analysis of the retinal protein lysates from 4-month or 24-month-old F344 rats, quantifying the BiP, cleaving pATF6, peIF2α, ATF4, CHOP, and GADD34 (Figs. 1A and B, Table S1). The binding of the immunoglobulin protein GRP78 showed a slight decrease by 1.2 fold (p = 0.19) in the 24-month-old retinas, compared to the young retinas, confirming our previous findings in the nigrostriatal system in which the Grp78 was lower in the aged rat brains vs. the young (2 months) animals [32]. However, the markers of the ATF6 and PERK pathways were significantly modified in the aged retinas. Thus, we found that the phosphorylated (p) ATF6 (90 kD) was slightly decreased in the aged retinas (p < 0.05), while its active form, cleaved pATF6 (50 kD), was significantly increased by 1.8 fold (p < 0.001), suggesting the activation of the ATF6 UPR arm in the aged retinas.
Figure 1.
The unfolded protein response is activated in aged rat retinas concomitantly with oxidative stress and inflammatory signaling (N=5). A. The hallmarks of an activated UPR are significantly modified in aged retinas. B. Images of representative western blots probed with antibodies against UPR, oxidative stress markers, and the rhodopsin protein. Red or green coloration indicates use of a 700 or 800 channel imaged secondary, respectively. C. The expression of the rhodopsin protein was significantly downregulated (N=5). D. The expression of the oxidative stress markers was significantly modified (N=5). E. The activation of the UPR and impaired oxidative stress defense in aged retinas were associated with a decrease in the anti-inflammatory cytokines and increase in the pro-inflammatory RANTES cytokine (N=4). F. Images of young and old retinas treated with GFAP demonstrating immunoreactivity of the glia with aging.
Another UPR marker, the PERK kinase substrate eukaryotic initiation factor 2α (peIF2α), was dramatically decreased in the old retinas, and a 1.8 fold reduction in the peIF2a level was found in the old retinas when compared to the young rats (p < 0.001). Interestingly, this decrease was in agreement with the level of the growth arrest and DNA damage inducible protein 34 (GADD34), which is known to be a subunit for a peIF2α phosphatase. A 2.3-fold increase between 4 months and 24 months was found in the retinal extracts (p < 0.01), suggesting that this increase could be responsible for reducing the level of peIF2a in aged rat retinas. As shown in Figure 1A, the UPR downstream transcriptional factors, activating transcription factor 4 (ATF4), and C/EBP homologous protein (CHOP) dimer were increased by 1.6 and 5.6-fold (p < 0.001 and p < 0.0001, respectively). The CHOP monomer, known to act as a pro-apoptotic transcription factor [15], was found to be elevated by 1.9 fold in the aged rat retinas (p < 0.05), proposing an increase in the level of apoptosis in aged retinas.
To verify whether or not the UPR activation in aged animals is associated with the inability of the retinas to produce melanin in albino retinas, making them vulnerable to light damage [29], we performed an analysis of one-year-old C57BL6 pigmented mouse retinas and found up-regulation of the CHOP and cleaved pATF6 UPR markers, suggesting that UPR activation is a common feature in aged retinas.
Rhodopsin Expression is Reduced in Aged Retinas
We have previously demonstrated that the rhodopsin protein and mRNA levels are significantly down regulated in the mouse model of inherited retinal degeneration with UPR activation [20, 27]. Therefore, we became interested in analyzing the RHO in aged retinas with ongoing UPR, and found that the protein level in the aged retinas was significantly two-fold down regulated (Figs. 1B and C, Table S1). This suggests either an impairment in the RHO expression machinery or upregulation of the E3 ubiquitin ligases responsible for rhodopsin degradation in aging photoreceptors.
Oxidative Stress and Inflammation Increases with Age
Numerous reports suggest that oxidative stress ubiquitously increases with age. We analyzed the antioxidant proteins nuclear factor erythroid-derived factor 2 (NRF2), superoxide dismutase 1 (SOD1), heme oxygenase 1 (HO1), and serine 15 phosphorylated protein 53 (p-p53 ser15) via western blot analysis (Figs. 1B and D, Tables S1). The NRF2 shows a 1.9 fold downregulation with age (p = 0.002), confirming the previously found decline in the NRF2 transcriptional binding activity with age [36]. Both the HO1 and SOD1 are antioxidant-responsive element (ARE) genes [10, 16] found to be modified in aged retinas. Surprisingly, the HO1, as shown in Figures 1B and C, was decreased by 1.3-fold (p = 0.05) while the SOD1 was increased by 1.8 fold (p < 0.01). The other key coordinator of oxidative stress and aging, protein 53, was increased in its active phosphorylated form at serine 15 [22] by 2.1 fold (p = 0.05). The phosphorylation of Ser at 15 is known to occur during ultraviolet light-induced DNA damage and/or hydrogen peroxide damage [2, 7, 34]; therefore, this data points out the fact that light and ROS can damage normally aged retinas.
Retinal age-related diseases such as diabetic retinopathy have been characterized by an increase in inflammation [17, 38]. Therefore, we analyzed inflammatory markers during the normal aging process by measuring the interleukin 10 (IL-10), interleukin 13 (IL-13), and chemokine ligand 5 (CCL5/RANTES) cytokines using magnetic bead flow cytometry (Fig. 1E, Table S2). We found that the IL-10, an anti-inflammatory cytokine, was decreased by 2.1 fold with age (p = 0.027), while another anti-inflammatory marker, the IL-13 cytokine, was found to be decreased by 1.8 fold (p = 0.036). Given that the human recombinant IL-13 significantly inhibits ocular inflammation in the LPS-induced rat model of endotoxin-induced uveitis, results in diminished TNFa, pro-inflammatory IL-6, and IL-1b [21], this suggests that the anti-inflammatory defense in aged retinas is weakened. In support of this hypothesis, we found a distinct increase in the pro-inflammatory marker RANTES, which was elevated 5 fold in the aged retinas, compared to the young retinas (p = 0.024).
Next, we confirmed glial activation in the aged retinas via immunohistochemistry (Fig. 1F). The microglial activation marker glial fibrillary acidic protein (GFAP) was found to be dramatically increased in aged vs. young retinas, supporting the findings of the distinct rises in the pro-inflammatory signals between 4 and 24 months of age.
Discussion
It has been demonstrated that the UPR is implicated not only in inherited retinal degenerative disorders, but also in age-associated ocular diseases [15]. Our findings suggest that the normal aging process is marked by activation of the UPR, dysregulation of antioxidant response proteins, and disruption of the anti and pro-inflammatory markers towards a pro-inflammatory state. They also indicate that, when launched in aged retinas, these cellular pathways must be taken into consideration while investigating the cellular mechanisms of age-associated eye disorders.
PERK signaling is activated in aged retinas, and both the ATF4 and CHOP proteins are significantly elevated, pointing out pro-apoptotic events overcoming anti-apoptotic. For example, it is known that the CHOP significantly affects the ratio of the BCL2 anti-apoptotic vs. pro-apoptotic events, shifting the balance towards apoptosis. In addition, the CHOP could promote cell death by the induction of miR-708, which is known to regulate the expression of many genes, including the rhodopsin. Perhaps, the miR-708 is elevated in aged retinas, and might be responsible for the reduction in the RHO found in aged retinas in addition to age-related photoreceptor cell loss [31] and potential epigenetic changes [28, 40].
In our experiments, we found that during persistently activated UPR the peIF2α level becomes reduced, suggesting that deregulated protein and chaperone synthesis are age-associated events. The phosphorylation of eIF2a is known to inhibit the GTP-GDP exchange, affecting protein translation in general. Thus, we speculate that aged retinas undergo translational dysregulation that plays a deleterious role in aging retinas. However, to answer whether lowered peIF2a affects, for example, photoreceptor specific proteins in aged retinas, additional experiments need to be conducted in the future.
Our data also indicate an increase in protein phosphatase 1 activity in aged retinas; the GADD34 expression was elevated, in agreement with a study by Naidoo et al. [26]. These authors have demonstrated that aged mice do not display an increase in BiP expression, while a decline in eIF2α phosphorylation and higher levels of the GADD34 and pro-apoptotic CHOP protein were found in the aged mouse cerebral cortex. This study also proposed that young animals possess an efficient ER adaptive response that declines with aging [26].
An interesting finding was the decrease in the NRF2 expression in aged retinas. One logical explanation for this discovery could be the negative regulation of NRF2 by either KEAP1 or HRD1 E3 ligases that are known to promote Nrf2 degradation [6, 8, 39, 42]. Perhaps, the expression or activities of these ligases are altered with aging in the retina, leading to more pronounced NRF2 degradation. In support of this hypothesis, a study with NRF2 knockout mice has demonstrated that these mice undergo accelerated aging by developing age-related RPE and choroidal degeneration resembling the cardinal features of human AMD, exhibiting extensive hair loss and a decreasing body weight [42]. Altogether, these facts point to NRF2 as a promising therapeutic target to slow down aging.
A decrease in the NRF2 can explain the trend in the reduction of the HO1 protein encoded by the HO1 ARE-containing protein. However, the other ARE-dependent SOD1 gene encoding the SOD1 protein was increased in the aged retinas. Perhaps increased glial proliferation in aged retinas detected via GFAP contributes to SOD1 over-expression, indicating that in aged retinas there are mechanisms responsible for natural cytoprotection. In addition, altered miRNA expression or deficiency in Gp78 ER-associated E3 ligase (known to promote SOD1 degradation) might occur in aged retinas as well.
The role of P53 in aging remains controversial; for example, it has recently been proposed that P53 hyperactivity can accelerate aging through the insulin/IGF-1 pathway [24]. Therefore, it is not surprising that we found an increase in p-P53 in the aged retinas. Other studies have suggested that P53 plays a role as a longevity regulator through its tumor-suppressive function, giving an example of wild-type mice having a maximal life span of 3 years versus P53-null mice that succumb to tumors within several months [12]. Nevertheless, our results indicated that because retinal cells are highly metabolically active and aged retinas have a greater tendency for ROS production, P53 phosphorylation at Ser15 is increased. It would not be surprising to find that the insulin is also elevated in aged retinas, and additional studies on insulin level in healthy aged retinas are necessary.
Activated UPR and oxidative stress in aged retinas occur concomitantly with inflammation. The dramatic increases in the RANTES, chemotactic cytokines, and macroglial marker GFAP, commonly seen as hallmarks of glial activation after damage to retinal cells, are evidence that aged retinas experience stress. We have recently demonstrated that sustained UPR in wild-type mouse retinas can promote retinal degeneration [30]; therefore, it would be interesting to determine whether inflammation arises in healthy aged retinas due to oxidative stress, compromised redox potential, and activated UPR.
Finally, we showed an increase in the basal levels of the UPR, dysregulation of oxidative stress response, and elevation of inflammatory markers during the normal aging process of the retina, which might be helpful in the assessment of mechanisms responsible for age-related ocular dysfunction. Our study also pointed to UPR markers, which should be validated in order to postpone aging in humans.
Supplementary Material
Highlights.
We demonstrated activation of the Unfolded Protein Response in aged retinas
We found that the NRF2 level is dramatically reduced in aged retinas
Oxidative stress and inflammation are concomitantly activated in aging retina with ongoing UPR.
Acknowledgments
This work was supported by the National Institutes of Health Grant R01EY020905 and the VSRC Core Grant P30 EY003039.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anholt RR, Carbone MA. A molecular mechanism for glaucoma: endoplasmic reticulum stress and the unfolded protein response. Trends in molecular medicine. 2013;19:586–593. doi: 10.1016/j.molmed.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azizi B, Ziaei A, Fuchsluger T, Schmedt T, Chen Y, Jurkunas UV. p53-regulated increase in oxidative-stress--induced apoptosis in Fuchs endothelial corneal dystrophy: a native tissue model. Investigative ophthalmology & visual science. 2011;52:9291–9297. doi: 10.1167/iovs.11-8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cano M, Wang L, Wan J, Barnett BP, Ebrahimi K, Qian J, Handa JT. Oxidative stress induces mitochondrial dysfunction and a protective unfolded protein response in RPE cells. Free radical biology & medicine. 2014;69:1–14. doi: 10.1016/j.freeradbiomed.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cascella R, Ragazzo M, Strafella C, Missiroli F, Borgiani P, Angelucci F, Marsella LT, Cusumano A, Novelli G, Ricci F, Giardina E. Age-related macular degeneration: insights into inflammatory genes. Journal of ophthalmology. 2014;2014:582842. doi: 10.1155/2014/582842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celik SK, Aras N, Yildirim O, Turan F, Gorur A, Yildirim H, Tamer L. Glutathione S-transferase GSTM 1, null genotype may be associated with susceptibility to age-related cataract. Advances in clinical and experimental medicine : official organ Wroclaw Medical University. 2015;24:113–119. doi: 10.17219/acem/38143. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Wang L, Chen Y, Sternberg P, Cai J. Phosphatidylinositol 3 kinase pathway and 4-hydroxy-2-nonenal-induced oxidative injury in the RPE. Investigative ophthalmology & visual science. 2009;50:936–942. doi: 10.1167/iovs.08-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen K, Albano A, Ho A, Keaney JF., Jr Activation of p53 by oxidative stress involves platelet-derived growth factor-beta receptor-mediated ataxia telangiectasia mutated (ATM) kinase activation. The Journal of biological chemistry. 2003;278:39527–39533. doi: 10.1074/jbc.M304423200. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Wang J, Cai J, Sternberg P. Altered mTOR signaling in senescent retinal pigment epithelium. Investigative ophthalmology & visual science. 2010;51:5314–5319. doi: 10.1167/iovs.10-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YJ, Huang YS, Chen JT, Chen YH, Tai MC, Chen CL, Liang CM. Protective effects of glucosamine on oxidative-stress and ischemia/reperfusion-induced retinal injury. Investigative ophthalmology & visual science. 2015;56:1506–1516. doi: 10.1167/iovs.14-15726. [DOI] [PubMed] [Google Scholar]
- 10.Dreger H, Westphal K, Weller A, Baumann G, Stangl V, Meiners S, Stangl K. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovascular research. 2009;83:354–361. doi: 10.1093/cvr/cvp107. [DOI] [PubMed] [Google Scholar]
- 11.Ehlken C, Grundel B, Michels D, Junker B, Stahl A, Schlunck G, Hansen LL, Feltgen N, Martin G, Agostini HT, Pielen A. Increased expression of angiogenic and inflammatory proteins in the vitreous of patients with ischemic central retinal vein occlusion. PloS one. 2015;10:e0126859. doi: 10.1371/journal.pone.0126859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Z, Lin M, Wu R. The Regulation of Aging and Longevity: A New and Complex Role of p53. Genes & cancer. 2011;2:443–452. doi: 10.1177/1947601911410223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2015 doi: 10.1007/s10522-015-9578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao J, Liu RT, Cao S, Cui JZ, Wang A, To E, Matsubara JA. NLRP3 inflammasome: activation and regulation in age-related macular degeneration. Mediators of inflammation. 2015;2015:690243. doi: 10.1155/2015/690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorbatyuk M, Gorbatyuk O. Review: retinal degeneration: focus on the unfolded protein response. Molecular vision. 2013;19:1985–1998. [PMC free article] [PubMed] [Google Scholar]
- 16.He M, Pan H, Chang RC, So KF, Brecha NC, Pu M. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PloS one. 2014;9:e84800. doi: 10.1371/journal.pone.0084800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howcroft TK, Campisi J, Louis GB, Smith MT, Wise B, Wyss-Coray T, Augustine AD, McElhaney JE, Kohanski R, Sierra F. The role of inflammation in age-related disease. Aging. 2013;5:84–93. doi: 10.18632/aging.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Molecular aspects of medicine. 2012;33:399–417. doi: 10.1016/j.mam.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kowluru RA, Kowluru A, Mishra M, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Progress in retinal and eye research. 2015 doi: 10.1016/j.preteyeres.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunte MM, Choudhury S, Manheim JF, Shinde VM, Miura M, Chiodo VA, Hauswirth WW, Gorbatyuk OS, Gorbatyuk MS. ER stress is involved in T17M rhodopsin-induced retinal degeneration. Investigative ophthalmology & visual science. 2012;53:3792–3800. doi: 10.1167/iovs.11-9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemaitre C, Thillaye-Goldenberg B, Naud MC, de Kozak Y. The effects of intraocular injection of interleukin-13 on endotoxin-induced uveitis in rats. Investigative ophthalmology & visual science. 2001;42:2022–2030. [PubMed] [Google Scholar]
- 22.Liu D, Xu Y. p53, oxidative stress, and aging. Antioxidants & redox signaling. 2011;15:1669–1678. doi: 10.1089/ars.2010.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma JH, Wang JJ, Zhang SX. The unfolded protein response and diabetic retinopathy. Journal of diabetes research. 2014;2014:160140. doi: 10.1155/2014/160140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, Sutherland A, Thorner M, Scrable H. Modulation of mammalian life span by the short isoform of p53. Genes & development. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitter SK, Song C, Qi X, Mao H, Rao H, Akin D, Lewin A, Grant M, Dunn W, Jr, Ding J, Bowes Rickman C, Boulton M. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy. 2014;10:1989–2005. doi: 10.4161/auto.36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:6539–6548. doi: 10.1523/JNEUROSCI.5685-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nashine S, Bhootada Y, Lewin AS, Gorbatyuk M. Ablation of C/EBP homologous protein does not protect T17M RHO mice from retinal degeneration. PloS one. 2013;8:e63205. doi: 10.1371/journal.pone.0063205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliver VF, Jaffe AE, Song J, Wang G, Zhang P, Branham KE, Swaroop A, Eberhart CG, Zack DJ, Qian J, Merbs SL. Differential DNA methylation identified in the blood and retina of AMD patients. Epigenetics. 2015;10:698–707. doi: 10.1080/15592294.2015.1060388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Organisciak DT, Vaughan DK. Retinal light damage: mechanisms and protection. Progress in retinal and eye research. 2010;29:113–134. doi: 10.1016/j.preteyeres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rana T, Shinde VM, Starr CR, Kruglov AA, Boitet ER, Kotla P, Zolotukhin S, Gross AK, Gorbatyuk MS. An activated unfolded protein response promotes retinal degeneration and triggers an inflammatory response in the mouse retina. Cell death & disease. 2014;5:e1578. doi: 10.1038/cddis.2014.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogala J, Zangerl B, Assaad N, Fletcher EL, Kalloniatis M, Nivison-Smith L. In vivo quantification of retinal changes associated with drusen in age-related macular degeneration. Investigative ophthalmology & visual science. 2015;56:1689–1700. doi: 10.1167/iovs.14-16221. [DOI] [PubMed] [Google Scholar]
- 32.Salganik M, Sergeyev VG, Shinde V, Meyers CA, Gorbatyuk MS, Lin JH, Zolotukhin S, Gorbatyuk OS. The loss of glucose-regulated protein 78 (GRP78) during normal aging or from siRNA knockdown augments human alpha-synuclein (alpha-syn) toxicity to rat nigral neurons. Neurobiology of aging. 2015;36:2213–2223. doi: 10.1016/j.neurobiolaging.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sengupta P. The Laboratory Rat: Relating Its Age With Human's. International journal of preventive medicine. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- 34.She QB, Chen N, Dong Z. ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation. The Journal of biological chemistry. 2000;275:20444–20449. doi: 10.1074/jbc.M001020200. [DOI] [PubMed] [Google Scholar]
- 35.Steinle JJ, Sharma S, Smith CP, McFayden-Ketchum LS. Normal aging involves modulation of specific inflammatory markers in the rat retina and choroid, The journals of gerontology. Series A. Biological sciences and medical sciences. 2009;64:325–331. doi: 10.1093/gerona/gln052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang HZ, Yang LM. Activation of the unfolded protein response in aged human lenses. Molecular medicine reports. 2015;12:389–393. doi: 10.3892/mmr.2015.3417. [DOI] [PubMed] [Google Scholar]
- 38.Tang J, Kern TS. Inflammation in diabetic retinopathy. Progress in retinal and eye research. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Chen Y, Sternberg P, Cai J. Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Investigative ophthalmology & visual science. 2008;49:1671–1678. doi: 10.1167/iovs.07-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan B, Yao J, Tao ZF, Jiang Q. Epigenetics and ocular diseases: from basic biology to clinical study. Journal of cellular physiology. 2014;229:825–833. doi: 10.1002/jcp.24522. [DOI] [PubMed] [Google Scholar]
- 41.Yu Z, Lu B, Sheng Y, Zhou L, Ji L, Wang Z. Andrographolide ameliorates diabetic retinopathy by inhibiting retinal angiogenesis and inflammation. Biochimica et biophysica acta. 2015;1850:824–831. doi: 10.1016/j.bbagen.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Z, Chen Y, Wang J, Sternberg P, Freeman ML, Grossniklaus HE, Cai J. Age-related retinopathy in NRF2-deficient mice. PloS one. 2011;6:e19456. doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.