Abstract
The present study was undertaken to explore the possible mechanisms of the behavioral alterations that develop in response to cancer and to cancer therapy. For this purpose we used a syngeneic heterotopic mouse model of human papilloma virus (HPV)-related head and neck cancer in which cancer therapy is curative. Mice implanted or not with HPV+ tumor cells were exposed to sham treatment or a regimen of cisplatin and radiotherapy (chemoradiation). Sickness was measured by body weight loss and reduced food intake. Motivation was measured by burrowing, a highly prevalent species specific behavior. Tumor-bearing mice showed a gradual decrease in burrowing over time and increased brain and liver inflammatory cytokine mRNA expression by 28 days post tumor implantation. Chemoradiation administered to healthy mice resulted in a mild decrease in burrowing, body weight, and food intake. Chemoradiation in tumor-bearing mice decreased tumor growth and abrogated liver and brain inflammation, but failed to attenuate burrowing deficits. PCR array analysis of selected hypoxia and mitochondrial genes revealed that both the tumor and chemoradiation altered the expression of genes involved in mitochondrial energy metabolism within the liver and brain and increased expression of genes related to HIF-1α signaling within the brain. The most prominent changes in brain mitochondrial genes were noted in tumor-bearing mice treated with chemoradiation. These findings indicate that targeting mitochondrial dysfunction following cancer and cancer therapy may be a strategy for prevention of cancer-related symptoms.
Keywords: Cancer, Human papilloma virus, Sickness behavior, Chemoradiotherapy, Hypoxia, Mitochondria
1. Introduction
Cancer and its therapy are associated with symptoms including fatigue and depressed mood. These symptoms are often present at diagnosis, peak during therapy, and can persist long after completion of therapy. As these symptoms have a striking parallel to inflammation-induced sickness behavior [1, 2], the inflammation hypothesis has been the primary mechanism under investigation. Preclinical models of cancer confirm that tumors induce inflammation in the periphery and brain [3-5] and a few reports indicate that chemotherapy agents can increase brain expression of proinflammatory cytokines [6-8]. Moreover, various clinical studies report associations between cancer-related symptoms and various biomarkers of inflammation [9-13]. However, there are also numerous reports where no association between symptoms and cytokines could be identified [14-18]. This is not surprising as chemotherapy is often immunosuppressive [19, 20]. Therefore, other mechanisms, including mitochondrial dysfunction, have been proposed as potential mechanisms of chemotherapy-induced symptoms [21]. The objective of the present study was to investigate the mechanisms of the behavioral alterations that develop in response to cancer and cancer therapy in an animal model of cancer. We hypothesized tumor-induced behavioral changes would be linked to inflammation, while chemoradiation-induced behavioral changes would be more closely associated with alterations in mitochondrial gene expression. For this purpose we selected an inflammatory and metabolically active murine oropharynx squamous cell carcinoma model in which mice undergo heterotopic implantation of tonsil epithelial cells transfected with H-Ras and human papilloma virus (HPV) oncogenes E6 and E7 [22]. The rates of HPV-related head and neck cancer are on the rise, particularlly among middle aged white males [23, 24]. Although HPV-related head and neck cancer responds relatively well to a regimen of chemotherapy and radiotherapy (chemoradiation) [25], this treatment is associated with the development of important local and systemic symptoms including mucositis, pain, fatigue, and distress [26-28].
The tumor developed by mice injeced into their hind leg with HPV-related tumor cells responds to a combined regimen of cisplatin chemotherapy and radiotherapy similar to the one used in HPV-related head and neck cancer [22]. Mice implanted or not with tumor cells were exposed or not to chemoradiation according to a 2 × 2 factorial design. Sickness was assessed by decreases in body weight and food intake and reduced burrowing, a species-specific motivated behavior that is very sensitive to variations in well being [29, 30]. Using this model we confirmed that the signs of sickness that developed in tumor bearing mice were associated with inflammation propagating from the tumor to the liver and brain. However, the signs of sickness that developed in tumor bearing mice treated with chemoradiation were no longer associated with inflammation. In view of the highly metabolic nature of the tumor [31, 32] and the well known damaging effects of cisplatin on mitochondria [33-39], we investigated the relationship between behavioral alterations and expression of genes involved in mitochondrial energy metabolism and hypoxia in the liver and brain using PCR arrays. We observed additive effects of tumor and chemoradiation on burrowing and alterations in expression of genes involved in mitochondrial energy metabolism in the brain, pointing to mitochondrial dysfunction as a possible cause of cancer-related symptoms.
2. Materials and Methods
2.1 Mice
All procedures described in this study were approved by the Institutional Animal Care and Use Committees of the University of Texas MD Anderson Cancer Center. Experiments were conducted on adult male C57BL/6 mice individually housed in temperature and humidity controlled environments on 12 hour light-dark cycles. Food and water were available ad libitum.
2.2 Tumor Model
A heterotopic syngeneic murine tumor model of HPV-related head and neck cancer was used. This model has been described in detail previously [22, 40, 41]. The tumor cells were derived from normal C57BL/6 mouse oropharyngeal epithelial cells that were transfected with HPV E6 and E7 oncogenes and hRAS. Mice were inoculated with 1 × 106 tumor cells into the right hind leg. The advantage of this heterotopic location is that tumor growth does not interfere with eating and drink, as would be observed if implanted in the oral cavity, and it allows for irradiation to be presented to the tumor without direct effects on the brain or abdominal cavity. Tumor volume was determined from three mutually orthogonal tumor diameters (d1, d2, d3) measured using Vernier calipers [Volume = (π/6)(d1*d2*d3)] as previously described [42, 43].
2.3 Cancer therapy
Mice were treated with a regimen of cisplatin chemotherapy and radiotherapy that reliably suppresses tumor growth [41]. This regimen included 3 rounds of once weekly cisplatin (Calbiochem, EMD Millipore, Billerica, MA) plus 8 Gy local tumor irradiation of the leg beginning 12 days after tumor implantation. Cisplatin was dissolved in sterile saline and administered by intraperitoneal injection at a dose of 5.28 mg/kg (equivalent to approximately 20 mg/m2). Radiotherapy was administered via a small animal cesium137 irradiator that collimates the parallel opposed radiation beams to a 3 cm diameter circular field and thereby avoids irradiation of surrounding tissues, including the abdomen.
2.4 Assessing Sickness
Sickness was assessed by evaluating body weight, food consumption (by measuring food disappearance), and burrowing. For the burrowing task, mice were provided access to a slightly elevated tube filled with 200 +/− 1.0 g of standard rodent chow. The amount of chow removed from the tube after 30 min was recorded [29, 30]. Healthy mice exposed to the burrowing task usually remove most of the food pellets from the burrowing tube during the 30 min time period they are allocated and generally do not eat them. Mice were trained for 3-5 sessions prior to baseline assessment. Mice burrowing less than 30 grams following training were excluded from the experiment as non-burrowers (< 5% of mice).
2.5 Tissue Collection
Mice were euthanized by CO2 inhalation. For assessment of gene expression, mice were saline perfused and tissue (i.e., brain, liver, and tumor tissue) was collected, snap-frozen in liquid nitrogen, and stored at −80°C until RNA extraction. Brain tissue was crushed with a mortar and pestle on liquid nitrogen and divided for assessment of inflammatory cytokines and for assessment of mitochondrial energy metabolism and hypoxia signaling by RT2 Profiler PCR arrays. To verify the lack of metastatic disease in the liver, liver tissue was fixed with 4% formalin and stained with hematoxylin and eosin (H&E).
2.6 Real-time PCR for inflammatory cytokines
For assessment of inflammatory cytokines, total RNA was isolated using TRIzol reagent (Ambion by Life Technologies, Grand Island, NY) and E.Z.N.A. RNA Isolation Columns (Omega Bio-Tek, Norcross, GA). Reverse transcription was conducted using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems by Life Technologies, Grand Island, NY) according to manufacturer's instructions.
Real time RT-PCR was carried out with TaqMan gene expression assays. Targets include IL-6 (cat number: Mm.PT.51.12387735), IL-1β (cat number: Mm.PT.51.17212823), TNF-α (cat number: Mm.PT.51.16622079) and GAPDH (cat number: Mm.PT.39a.1) from Integrated DNA Technologies (Coralville, IA). GAPDH served as the endogenous housekeeping control. Reactions were performed in duplicate, and relative fold difference for each target gene was calculated using the 2−ΔΔCT method, where CT is the threshold concentration.
2.7 RT2 Profiler PCR arrays of Hypoxia Signaling Pathways and Mitochondrial Energy Metabolism
Total RNA was isolated from brain and liver murine samples using TRIzol reagent (Ambion by Life Technologies, Grand Island, NY) and E.Z.N.A. RNA Isolation Columns (Omega Bio-Tek, Norcross, GA). Approximately 120 ng of extracted RNA were converted to first strand cDNA using the RT2 First Strand Kit (SA Biosciences, Qiagen, Valencia, CA) according to the manufacturer's instructions. The mouse Mitochondrial Energy Metabolism RT2 Profiler PCR Array (PAMM_008ZE-4, SA Biosciences, Qiagen, Valencia, CA) and the mouse Hypoxia Signaling Pathway RT2 Profiler PCR Array (PAMM-032Ze-4, SA Biosciences, Qiagen, Valencia, CA) were run according to manufacturer's instructions. The Mitochondrial Energy Metabolism array assesses the expression of 84 genes involved in modulating ATP synthesis and oxidative phosphorylation. The Hypoxia Signaling Pathway array evaluates 84 genes that are responsive to low oxygen levels. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin (ACTB) were used as reference genes. All samples were tested in duplicate. Reactions were carried out on ABI PRISM 7900HT sequence detection system.
The data were normalized and analyzed using fold change from control with positive values indicating an upregulation and negative values indicative of a downregulation compared to control. Canonical pathway analyses were conducted using Ingenuity Pathway Analysis (Qiagen, Silicon Valley, Redwood City, CA). In this analysis, all groups were independently compared to the control mice (no tumor, no chemoradiation). Heat maps were generated from the log transformed fold change using CIMminer (http://discover.nci.nih.gov/cimminer).
2.8 Hematoxylin and Eosin (H&E) staining for liver pathology
Fixed liver sections were evaluated by a pathologist from the Department of Veterinary Medicine and Surgery from MD Anderson Cancer Center. The pathologist also scored hypertrophy of Kupffer cells on a 4 point scale (Grade 1= modest, rare < 10%, Grade 2 = mild, infrequent 10-20%, Grade 3 = moderate, frequent 20-50%, and Grade 4 = severe, extensive > 50%).
2.9 Experimental Design
To evaluate the effects of chemoradiation in tumor-bearing animals, we employed a 2 (control vs tumor) × 2 (placebo vs chemoradiation) factorial design (n=7 mice/group). Mice were injected or not with tumor cells on day 0 and treated or not with chemoradiation on days 12, 19, and 26. Brain, liver, and tumor were collected one day after the final session of chemoradiation for analysis. Signs of sickness were measured prior to tumor implantation (baseline), prior to the start of chemotherapy (day 6), and the day after each session of chemoradiation (day 13, 20, and 27). In a separate group of animals (n=4 mice/group), liver tissue was collected to evaluate for possible tumor metastasis. On day 28 post-tumor implantation tissue was collected from healthy and tumor-bearing mice and was stained for H&E.
2.10 Statistical Analysis
Data were analyzed using SPSS (version 19, Chicago, IL). Data are expressed as mean +/− standard error of the mean. Depending on the experimental design, one- or two-way analyses of variances (ANOVAs) with tumor and chemoradiation as between subject factors were run to evaluate differences in cytokine expression levels. Two-way repeated measures ANOVAs were conducted to evaluate behavioral changes over time. Post hoc analyses were conducted with the least significant test to clarify group differences, as needed. P-values of less than 0.05 were considered significant.
3. Results
3.1. Chemoradiation and HPV-related tumors induce sickness
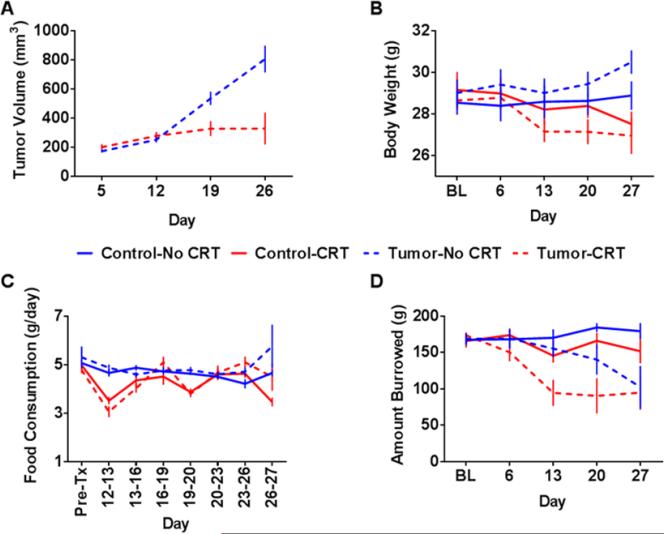
In line with previously reported data using this murine model of HPV-related head and neck cancer (Spanos et al., 2009), tumor growth was inhibited by chemoradiation (F(3,36) = 13.76, p<0.001; Figure 1A). Chemoradiation significantly reduced body weight over time in control and tumor-bearing mice, F(4,96)=16.10, p<0.001 (Figure 1B), and reduced food consumption in the 24 h following each treatment, F(7,168)=5.35, p<0.001 (Figure 1C). There was no evidence of cachexia or anorexia in tumor-bearing mice. Instead, a subtle increase in food consumption was observed in tumor-bearing mice toward the end of the experiment, F(7,168)=2.13, p<0.05.
Figure 1. Sickness is induced by both chemoradiation and the tumor.
Mice were implanted or not with tumor cells on day 0 and were exposed to chemoradiation (CRT) (5.28 mg/kg cisplatin + 8 Gy leg irradiation) or sham treatment on days 12, 19, and 26. (A) CRT suppressed tumor growth as indicated by a significant main effect of time (p<0.001), CRT (p<0.05), and the time by CRT interaction (p<0.001). (B) CRT also resulted in a reduction in body weight as indicated by a significant main effect of time (p<0.05) and a time by CRT interaction (p<0.001). (C) There was a significant main effect of time, CRT, and a time by CRT interaction (all p<0.001) for food consumption, indicating at transient reduction in consumption following CRT. (D) A main effect of time (p<0.001), CRT (p<0.01), tumor (p<0.001), a time by tumor interaction (p<0.001), and a time by CRT interaction (p<0.05) was observed for burrowing behavior. Data represented as mean +/− SEM. n=7 mice/group.
Burrowing levels declined significantly over time in tumor bearing mice, F(4,96)=8.07, p<0.001, with a greater than 40% decline in performance by day 27. Further, chemoradiation significantly suppressed burrowing in control mice and further suppressed burrowing in tumor-bearing mice, F(4,96)=2.95, p<0.05 (Figure 1D). Tumor-bearing mice treated with chemoradiation showed reduced burrowing following the first session of chemoradiation, while sham-treated tumor-bearing mice showed a more gradual decline in performance.
3.2 Chemoradiation abrogates tumor-induced liver and brain proinflammatory cytokine expression
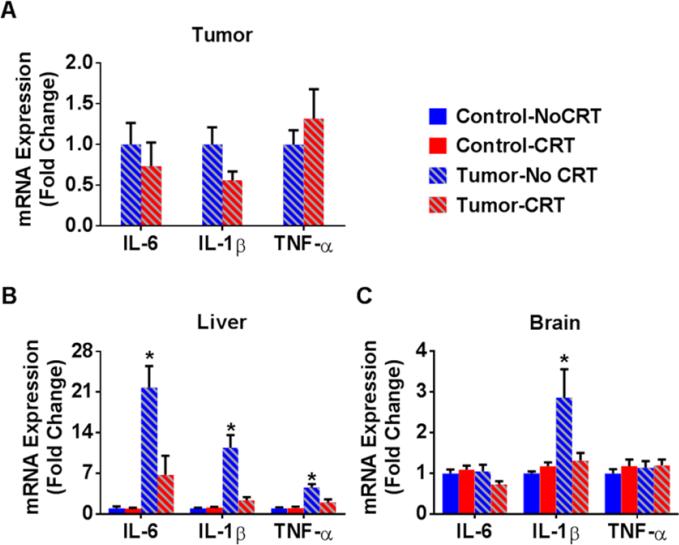
Analysis of the tumor showed no significant change in proinflammatory cytokine expression following 3 rounds of cisplatin and tumor irradiation (Figure 2A). There was a trend toward a decrease in tumor IL-1β mRNA expression within the tumor following chemoradiation (p<0.1). However, in view of the robust decrease in tumor volume following chemoradiation, the total cytokines produced by the tumor would likely be much lower.
Figure 2. Tumor-induced cytokine expression is abrogated by chemoradiation.
Mice were implanted or not with tumors on day 0 and were exposed to chemoradiation (5.28 mg/kg cisplatin + 8 Gy leg irradiation) or sham treatment on day 12, 19, and 26. Tissue was collected on day 27 and was analyzed by RT-PCR for proinflammatory cytokine expression. (A) There were no statistically significant effects of chemoradiation on tumor cytokine expression. (B) There were significant main effects of tumor and chemoradiation on liver cytokines (all ps<0.05) as well a significant tumor by chemoradiation interactions for IL-6, F(1,24)=9.02, p<0.01, IL-1β, F(1,24)=16.89, p<0.001, and TNF-α, F(1,24)=9.89, p<0.005. (C) Within the brain, there was a main effect of tumor, F(1,24)=6.97, p<0.05, and a tumor by chemoradiation interaction, F(1,24)=4.48, p<0.05, on IL-1β mRNA expression. Post hoc analyses show that untreated tumor-bearing mice had significantly higher cytokine mRNA expression. Data are represented as mean fold differences compared to control mice +/− SEM. * p<0.05, n= 7/group
Tumor-bearing mice had increased proinflammatory cytokine expression in the liver (Figure 2B) and brain (Figure 2C). Within the liver IL-6, IL-1β, and TNF-α mRNA expression (all p<0.01) were elevated and within the brain IL-1β was elevated (p<0.05). Chemoradiation significantly abrogated these effects (all p<0.05). Despite the fact that tumor-bearing mice treated with chemoradiation had cytokine expression levels that were not different from healthy controls, they showed the most severe behavioral effect.
3.3 Inflammation is associated with tumor volume in tumor bearing mice
In view of the natural variability in tumor volume in tumor bearing mice we searched for possible associations between tumor size and cytokine expression within the liver and brain. As anticipated, there was a significant positive correlation between tumor volume and liver IL-6, IL-1β, and TNF-α as well as brain IL-1β mRNA expression (Table 1). This provides confirmation that the suppression in cytokine expression in liver and brain observed following chemoradiation is related to reducing the tumor volume.
Table 1.
Pearson correlations between tumor volume on day 26 and cytokine mRNA expression in tumor-bearing mice (n=14). An adjusted p-value of 0.006 was set to adjust for multiple comparisons.
| Tumor Volume | |||
|---|---|---|---|
| Correlation coefficient | p-value | ||
| Tumor | IL-6 | 0.577 | 0.031 |
| IL-1β | 0.548 | 0.042 | |
| TNF-α | 0.037 | 0.901 | |
| Liver | IL-6 | 0.911 | <0.001 |
| IL-1β | 0.842 | <0.001 | |
| TNF-α | 0.951 | <0.001 | |
| Brain | IL-6 | 0.594 | 0.025 |
| IL-1β | 0.711 | 0.004 | |
| TNF-α | 0.217 | 0.456 | |
3.4 Both the tumor and chemoradiation are associated with altered expression of genes related to mitochondrial energy metabolism and hypoxia
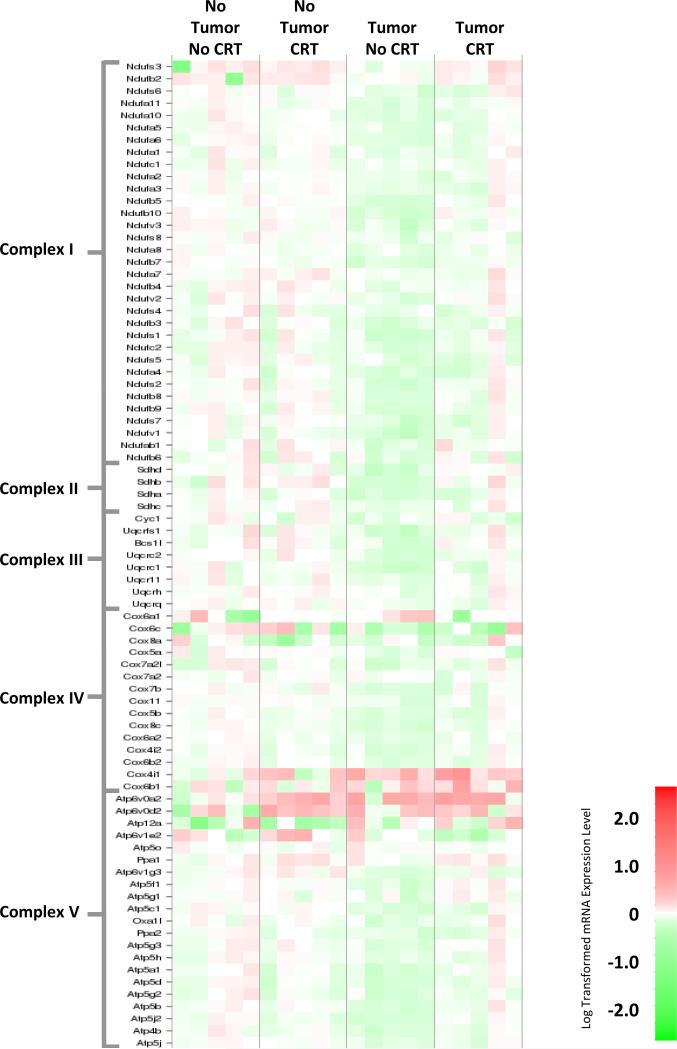
To explore the possible role of mitochondial energy metabolims and hypoxia in cancer-related symptoms we conducted real time RT-PCR array analyses in the liver and brain collected 28 days after the start of the experiment. Within the liver, the presence of the tumor was associated with significant downregulation of mitochondrial respiratory chain complex genes (Figure 3, Table S1A). Chemoradiation attenuated tumor-induced changes in mitochondrial respiratory chain complex gene expression (see Table 2). Pathway analyses also indicated that the HIF-1α signaling pathway was upregulated within the liver in response to the tumor alone, while chemoradiation in the presence of the tumor reduced this upregulation (refer to Figure S1, Table S2A).
Figure 3. Heat map of mitochondria complex gene expression in the liver.
Gene expression changes in mitochondrial complex genes in liver samples. Red corresponds to increased gene expression, while green corresponds to decreased expression.
Table 2.
Summary of altered mitochondrial gene expression in the liver and brain by complex. The percent of significantly altered genes (p<0.05) in each complex is represented by arrows. ↑ indicate an upregulation, while ↓ indicates a downregulation. Increasing number of arrows indicate increased number of genes significantly dysregulated: 3 arrows indicate 50-100%, 2 arrows indicate 25-49.9%, 1 arrow indicate 12.5-24.9%, and no arrows indicate <12.5% of genes significantly altered.
| Complex | ||||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Liver | No Tumor -CRT | ↓ | ||||
| Tumor-No CRT | ↓ ↓ ↓ | ↓ ↓ ↓ | ↓ ↓ ↓ | ↓ ↓ ↓ | ↓ ↓ ↓ | |
| Tumor -CRT | ||||||
| Brain | No Tumor -CRT | ↑ | ↑ | ↑ | ||
| Tumor-No CRT | ↑ ↑ | ↑ ↑ ↓ | ↑ | ↑ | ||
| Tumor-CRT | ↑ ↑ | ↑ ↑ | ↑ ↑ ↑ ↓ ↓ | ↑ ↑ | ||
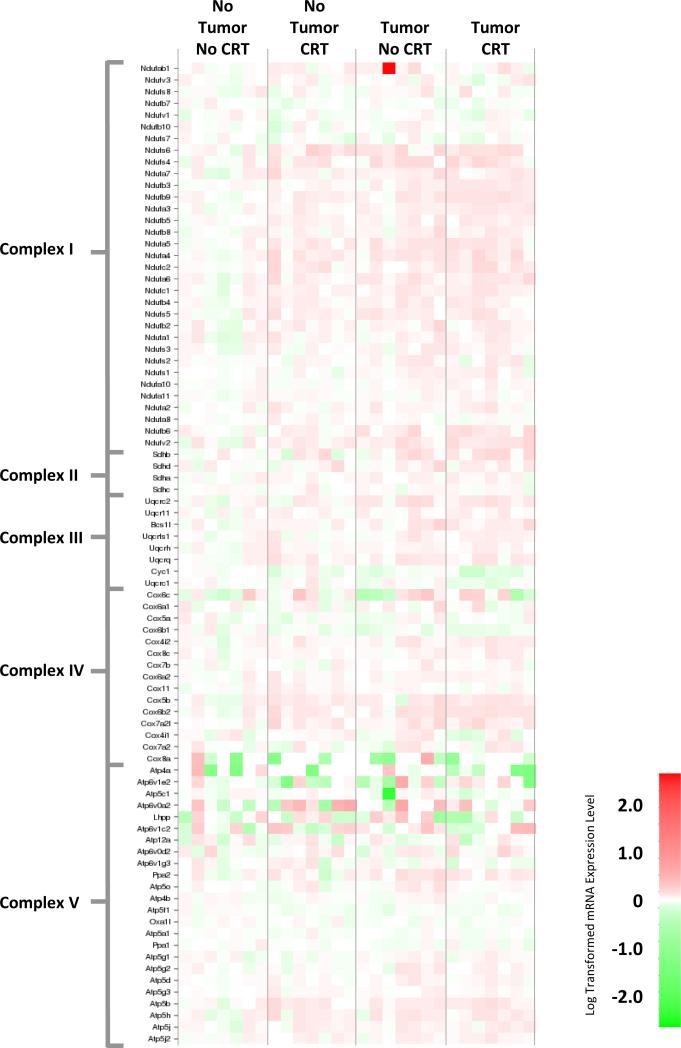
Within the brain a different pattern was observed. While both chemoradiation and the tumor resulted in changes in gene expression within the mitochondrial respiratory chain (Figure 4, Table S1B), the highest degree of change was observed in tumor-bearing mice treated with chemoradiation, particularly within complex II, III, and IV (Table 2). Further, pathway analyses indicates that both the tumor and chemoradiation resulted in the upregulation of genes associated with HIF-1α activation within the brain (Figure 2S, Table S2B).
Figure 4. Heat map of mitochondria complex gene expression in the brain.
Gene expression changes in mitochondrial complex genes in brain samples. Red corresponds to increased gene expression, while green corresponds to decreased expression.
3.5 There is no evidence of metastatic disease within the liver of tumor-bearing mice
Given that the inflammatory and mitochondrial gene expression changes observed within the liver showed an expression pattern that would be expected within a tumor microenvironment, we sought verify that these changes were not related to the development of metastatic disease. A pathologist evaluated H&E stained liver sections from a healthy and tumor bearing mice (n=4/group). No evidence of metastatic disease or hepatocyte hypertrophy was noted; however, the pathologist did note the presence of grade 2 (n=2) and 3 (n=2) hypertrophy of Kupffer cells in tumor-bearing mice. These activated cells are likely a significant source of the increased inflammation observed within the liver of tumor-bearing mice. All non-tumor bearing mice were assigned a score of 1 (see Figure S3 for representative images). A Pearson chi square analysis shows this difference to be statistically significant (χ2 (2) = 8, p<0.05).
4. Discussion
The results from this study demonstrate that a syngeneic HPV-related tumor model induced sickness behavior and inflammation. In contrast, chemoradiation in tumor-bearing mice reduced tumor volume and inflammation within the liver and brain while exacerbating tumor-induced sickness behavior. Chemoradiation by itself induced sickness behavior but had no effect on cytokine gene expression in the liver and brain. Both the tumor and chemoradiation induced alterations in the expression of genes associated with mitochondrial energy metabolism within the liver and brain as well as activation of genes associated with HIF-α signaling within the brain.
4.1 Effects of the Tumor
Tumor-bearing mice developed deficits in burrowing without any evidence of anorexia and cachexia. Deficits in burrowing in these mice were associated with evidence of inflammation in the periphery and brain, as evidenced by increased IL-6, IL-1β, and TNF-α mRNA expression within the liver and increased IL-1β mRNA expression within the brain. As there is a strong role for inflammation in the pathogenesis of HPV-related head and neck cancers [44-46], it is not surprising to observe a robust inflammatory profile in tumor-bearing mice. Further, the finding of a sickness response in mice implanted with tumor cells is consistent with other studies that show behavioral and neuroinflammatory changes in animal models of cancer [3, 5].
The livers of tumor-bearing mice presented reduced expression of mitochondrial respiratory chain genes, which likely indicates a shift from mitochondrial energy metabolism toward glycolysis. Further support for this shift toward glycolysis can be observed within a cluster of metabolism genes of the hypoxia array; specifically genes involved in glycolysis showed a general increase in expression within the liver of tumor-bearing mice (e.g., HK2, LDHA, and PFKP). Gene expression changes indicative of hypoxia signaling and alterations in mitochondrial respiratory chain complex genes were observed within the brain. In general, mild increases in genes encoding components of mitochondrial complex were observed (see Table 2). The functional implications of such an upregulation remain to be determined by e.g., assessing mitochondrial respiratory capacity. Similar changes have been described in the brain of aged mice [47] and the postmortem brains of unmedicated patients with psychiatric diseases [48]. These changes have been interpreted as evidence of mitochondrial dysfunction and oxidative damage; however, it is yet unknown whether they represents a compensatory increase or dysfunctional state.
Further, it is yet unclear whether brain expression of IL-1β or mitochondrial dysfunction is ultimately responsible for decreased burrowing in tumor bearing mice. Future studies will evaluate this through administration of an IL-1 receptor antagonist and/or mitochondrial protectant agents. It will also be necessary to examine whether the gene expression changes observed within the whole brain represent subtle widespread alterations or the diluted expression of strong localized changes within specific brain regions.
More research is needed to understand the mechanism by which the tumor alters the expression of mitochondrial genes in distant organs. It is possible that the effects observed in the liver are a downstream consequence of chronic inflammation. The gene expression pattern observed in the liver is in accordance with the metabolic signature of inflammation, characterized by a switch from oxidative phosphorylation to glycolysis [49]. The information concerning the impact of inflammation on brain mitochondrial activity is still quite limited. However, an altered pattern of mitochondrial respiratory chain complex genes has been reported in animal models of septic shock, with decreased mitochondrial complex I and possibly complex IV predominating [50, 51]. A possible explanation for the distant metabolic effects of tumors is that tumor-induced exosomes or microvesicles, which contain double-stranded DNA, RNA, and proteins (including HPV oncogene proteins E6 and E7) enter circulation and interact with liver and brain cells (e.g., innate immune cells) to induce distant metabolic changes [52, 53].
4.2 Effects of Chemoradiation
The chemoradiation protocol we used induced body weight loss, temporarily decreased food intake, and reduced burrowing. These findings are in line with the literature. Both cisplatin and radiation have been shown to reduce body weight [54-57]. Further, chemotherapy treatment can suppress voluntary activity, including decreased running wheel [17, 58-61] and open field activity [56, 57].
Although the behavioral alterations induced by cancer therapy are usually assumed to be mediated by inflammation [1, 6-8] there was no evidence of liver or brain proinflammatory cytokine gene expression in response to chemoradiation in mice implanted or not with tumor cells. The lack of an inflammatory response in mice exposed to chemoradiation in the present study also contrasts with the pronounced behavioral and neuroinflammatory effects that develops in response to partial body irradiation [62, 63]. However, these last effects are likely secondary to gut leakage following irradiation of the abdominal cavity [64, 65], which did not occur in the hind leg irradiation model employed in the current study.
In contrast to the lack of effect of chemoradiation on inflammation there was clear evidence of altered mitochondrial energy metabolism and activation of hypoxia signaling pathway in response to chemoradiation. Cisplatin is already known to induce mitochondrial dysfunction through the formation of mitochondrial DNA adducts [37, 38]. Further, cisplatin-induced fragmentation of mitochondria has been described in kidneys of cisplatin-treated mice and can be prevented by pharmacological inhibition of dynamin-related protein-1 (Drp-1), a critical mitochondrial fission protein [33]. Radiation can enhance the effects of chemotherapy on mitochondrial dysfunction through generation of radical oxygen species [35].
As expected the curative regimen of chemoradiation used in this study decreased tumor volume in tumor-bearing mice [22]. It also abrogated the expression of peripheral and central proinflammatory cytokines, likely through suppression of tumor growth. Despite this inhibition of inflammation, chemoradiation did not reverse or attenuate sickness in tumor bearing mice. Rather tumor-bearing mice treated with chemoradiation showed severe sickness behavior. This indicates that while inflammation may mediate sickness associated with growth of this tumor, this is clearly not the case for signs of sickness associated with chemoradiation. While chemoradiation attenuated tumor-induced suppression of mitochondrial gene suppression in the liver, the highest level of mitochondria gene dysregulation was noted in the brains of tumor-bearing mice treated with chemoradiation. While some genes within complex I and III were down regulated within the brain, the majority were upregulated compared to healthy control mice. As previously mentioned the functional implication of this gene expression pattern is yet to be determined.
4.3 Conclusion
Our data on the relationship between inflammation and sickness in tumor-bearing mice indicates that inhibition of inflammation could provide a strategy for reducing disease-driven symptoms, as would be observed in advanced cancer patients. This strategy is likely to be therapeutically safe as inhibition of tumor-induced inflammatory cytokines can attenuate tumor growth [31, 66-68]. However, our data on the effects of chemoradiation in tumor bearing mice would indicate that inflammation is unlikely to be the cause of symptoms that develop in response to cancer, meaning the use of anti-inflammatory treatments is not justified in this case. Agents aimed at mitochondrial protection and biogenesis should be preferred if it is demonstrated that such agents reverse the behavioral alterations associated with cancer therapy without adversely impacting the response of the tumor to cancer therapy.
Supplementary Material
Highlights.
A murine model of cancer induces sickness behavior and inflammation.
Chemoradiation attenuates tumor-induced inflammation.
Chemoradiation does not attenuate tumor-induced sickness.
Combined tumor and chemoradiation alters brain mitochondrial gene expression.
Acknowledgement
The work reported in this publication was supported the National Cancer Institute of the National Institutes of Health (R01CA193522). Additional support came from the University of Texas MD Anderson Cancer Center and the National Institutes of Health MD Anderson Cancer Center Support Grant (CA016672). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources. In addition, we would like to thank Devdeep Chandra and Myrna Garcia for their assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–25. doi: 10.1002/cncr.11382. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 2.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:971–82. doi: 10.1200/JCO.2007.10.7805. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norden DM, Bicer S, Clark Y, Jing R, Henry CJ, Wold LE, et al. Tumor Growth Increases Neuroinflammation, Fatigue and Depressive-like Behavior Prior to Alterations in Muscle Function. Brain, behavior, and immunity. 2014 doi: 10.1016/j.bbi.2014.07.013. doi: 10.1016/j.bbi.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pyter LM, El Mouatassim Bih S, Sattar H, Prendergast BJ. Peripheral tumors alter neuroinflammatory responses to lipopolysaccharide in female rats. Brain research. 2014;1552:55–63. doi: 10.1016/j.brainres.2014.01.012. doi: 10.1016/j.brainres.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pyter LM, Pineros V, Galang JA, McClintock MK, Prendergast BJ. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9069–74. doi: 10.1073/pnas.0811949106. doi: 10.1073/pnas.0811949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weymann KB, Wood LJ, Zhu X, Marks DL. A role for orexin in cytotoxic chemotherapy-induced fatigue. Brain, behavior, and immunity. 2014;37:84–94. doi: 10.1016/j.bbi.2013.11.003. doi: 10.1016/j.bbi.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borniger JC, Gaudier-Diaz MM, Zhang N, Nelson RJ, DeVries AC. Cytotoxic chemotherapy increases sleep and sleep fragmentation in non-tumor-bearing mice. Brain, behavior, and immunity. 2014 doi: 10.1016/j.bbi.2014.11.001. doi: 10.1016/j.bbi.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Smith LB, Leo MC, Anderson C, Wright TJ, Weymann KB, Wood LJ. The role of IL-1beta and TNF-alpha signaling in the genesis of cancer treatment related symptoms (CTRS): a study using cytokine receptor-deficient mice. Brain, behavior, and immunity. 2014;38:66–76. doi: 10.1016/j.bbi.2013.12.022. doi: 10.1016/j.bbi.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3517–22. doi: 10.1200/JCO.2011.36.1154. doi: 10.1200/jco.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5534–40. doi: 10.1158/1078-0432.CCR-08-2584. doi: 10.1158/1078-0432.ccr-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills PJ, Parker B, Dimsdale JE, Sadler GR, Ancoli-Israel S. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biological Psychology. 2005;69:85–96. doi: 10.1016/j.biopsycho.2004.11.007. doi: 10.1016/j.biopsycho.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain, behavior, and immunity. 2010;24:968–74. doi: 10.1016/j.bbi.2010.03.009. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang XS, Williams LA, Krishnan S, Liao Z, Liu P, Mao L, et al. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain, behavior, and immunity. 2012;26:699–705. doi: 10.1016/j.bbi.2011.12.007. doi: 10.1016/j.bbi.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahlberg K, Ekman T, Gaston-Johansson F. Levels of fatigue compared to levels of cytokines and hemoglobin during pelvic radiotherapy: a pilot study. Biological research for nursing. 2004;5:203–10. doi: 10.1177/1099800403259500. doi: 10.1177/1099800403259500. [DOI] [PubMed] [Google Scholar]
- 15.Geinitz H, Zimmermann FB, Stoll P, Thamm R, Kaffenberger W, Ansorg K, et al. Fatigue, serum cytokine levels, and blood cell counts during radiotherapy of patients with breast cancer. International journal of radiation oncology, biology, physics. 2001;51:691–8. doi: 10.1016/s0360-3016(01)01657-1. doi: 10.1016/S0360-3016(01)01657-1. [DOI] [PubMed] [Google Scholar]
- 16.Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. doi: 10.1016/j.cyto.2003.10.004. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Ray MA, Trammell RA, Verhulst S, Ran S, Toth LA. Development of a mouse model for assessing fatigue during chemotherapy. Comparative medicine. 2011;61:119–30. [PMC free article] [PubMed] [Google Scholar]
- 18.Reinertsen KV, Grenaker Alnaes GI, Landmark-Hoyvik H, Loge JH, Wist E, Kristensen VN, et al. Fatigued breast cancer survivors and gene polymorphisms in the inflammatory pathway. Brain, behavior, and immunity. 2011;25:1376–83. doi: 10.1016/j.bbi.2011.04.001. doi: 10.1016/j.bbi.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Harris J, Sengar D, Stewart T, Hyslop D. The effect of immunosuppressive chemotherapy on immune function in patients with malignant disease. Cancer. 1976;37:1058–69. doi: 10.1002/1097-0142(197602)37:2+<1058::aid-cncr2820370813>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen L, Arvin A. Chemotherapy-induced immunosuppression. Environmental health perspectives. 1982;43:21–5. doi: 10.1289/ehp.824321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vichaya EG, Chiu GS, Krukowski K, Lacourt TE, Kavelaars A, Dantzer R, et al. Mechanisms of chemotherapy-induced behavioral toxicities. Frontiers in Neuroscience. 2015;9:131. doi: 10.3389/fnins.2015.00131. doi: 10.3389/fnins.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Archives of otolaryngology--head & neck surgery. 2009;135:1137–46. doi: 10.1001/archoto.2009.159. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 23.Mroz EA, Forastiere AA, Rocco JW. Implications of the oropharyngeal cancer epidemic. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4222–3. doi: 10.1200/JCO.2011.37.8893. doi: 10.1200/jco.2011.37.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols AC, Palma DA, Dhaliwal SS, Tan S, Theuer J, Chow W, et al. The epidemic of human papillomavirus and oropharyngeal cancer in a Canadian population. Current oncology (Toronto, Ont) 2013;20:212–9. doi: 10.3747/co.20.1375. doi: 10.3747/co.20.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. Journal of the National Cancer Institute. 2008;100:261–9. doi: 10.1093/jnci/djn011. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 26.de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Pretreatment factors predicting quality of life after treatment for head and neck cancer. Head & neck. 2000;22:398–407. doi: 10.1002/1097-0347(200007)22:4<398::aid-hed14>3.0.co;2-v. doi: 10.1002/1097-0347(200007)22:4<398::AID-HED14>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 27.Hickok JT, Roscoe JA, Morrow GR, Mustian K, Okunieff P, Bole CW. Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer. 2005;104:1772–8. doi: 10.1002/cncr.21364. doi: 10.1002/cncr.21364. [DOI] [PubMed] [Google Scholar]
- 28.Shi Q, Mendoza TR, Gunn GB, Wang XS, Rosenthal DI, Cleeland CS. Using group-based trajectory modeling to examine heterogeneity of symptom burden in patients with head and neck cancer undergoing aggressive non-surgical therapy. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2013;22:2331–9. doi: 10.1007/s11136-013-0380-2. doi: 10.1007/s11136-013-0380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cunningham C, Deacon R, Wells H, Boche D, Waters S, Diniz CP, et al. Synaptic changes characterize early behavioural signs in the ME7 model of murine prion disease. The European journal of neuroscience. 2003;17:2147–55. doi: 10.1046/j.1460-9568.2003.02662.x. doi: 10.1046/j.1460-9568.2003.02662.x. [DOI] [PubMed] [Google Scholar]
- 30.Deacon RM. Burrowing: a sensitive behavioural assay, tested in five species of laboratory rodents. Behavioural brain research. 2009;200:128–33. doi: 10.1016/j.bbr.2009.01.007. doi: 10.1016/j.bbr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Pavlides S, Vera I, Gandara R, Sneddon S, Pestell RG, Mercier I, et al. Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxidants & redox signaling. 2012;16:1264–84. doi: 10.1089/ars.2011.4243. doi: 10.1089/ars.2011.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. The Journal of clinical investigation. 2009;119:1275–85. doi: 10.1172/JCI37829. doi: 10.1172/jci37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devarajan P, Savoca M, Castaneda MP, Park MS, Esteban-Cruciani N, Kalinec G, et al. Cisplatin-induced apoptosis in auditory cells: role of death receptor and mitochondrial pathways. Hearing research. 2002;174:45–54. doi: 10.1016/s0378-5955(02)00634-2. doi: 10.1016/S0378-5955(02)00634-2. [DOI] [PubMed] [Google Scholar]
- 35.Gorman S, Tosetto M, Lyng F, Howe O, Sheahan K, O'Donoghue D, et al. Radiation and chemotherapy bystander effects induce early genomic instability events: telomere shortening and bridge formation coupled with mitochondrial dysfunction. Mutation research. 2009;669:131–8. doi: 10.1016/j.mrfmmm.2009.06.003. doi: 10.1016/j.mrfmmm.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Peters U, Preisler-Adams S, Lanvers-Kaminsky C, Jürgens H, Lamprecht-Dinnesen A. Sequence variations of mitochondrial DNA and individual sensitivity to the ototoxic effect of cisplatin. Anticancer research. 2002;23:1249–55. [PubMed] [Google Scholar]
- 37.Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K, et al. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiology of disease. 2011;41:661–8. doi: 10.1016/j.nbd.2010.11.017. doi: 10.1016/j.nbd.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Schumaker LM, Egorin MJ, Zuhowski EG, Guo Z, Cullen KJ. Cisplatin preferentially binds mitochondrial DNA and voltage-dependent anion channel protein in the mitochondrial membrane of head and neck squamous cell carcinoma: possible role in apoptosis. Clinical cancer research. 2006;12:5817–25. doi: 10.1158/1078-0432.CCR-06-1037. doi: 10.1158/1078-0432.CCR-06-1037. [DOI] [PubMed] [Google Scholar]
- 39.Yoon MS, Katsarava Z, Obermann M, Schafers M, Liedert B, Dzagnidze A, et al. Erythropoietin overrides the triggering effect of DNA platination products in a mouse model of cisplatin-induced neuropathy. BMC neuroscience. 2009;10:77. doi: 10.1186/1471-2202-10-77. doi: 10.1186/1471-2202-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoover AC, Spanos WC, Harris GF, Anderson ME, Klingelhutz AJ, Lee JH. The role of human papillomavirus 16 E6 in anchorage-independent and invasive growth of mouse tonsil epithelium. Archives of otolaryngology--head & neck surgery. 2007;133:495–502. doi: 10.1001/archotol.133.5.495. doi: 10.1001/archotol.133.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spanos WC, Hoover A, Harris GF, Wu S, Strand GL, Anderson ME, et al. The PDZ binding motif of human papillomavirus type 16 E6 induces PTPN13 loss, which allows anchorage-independent growth and synergizes with ras for invasive growth. Journal of virology. 2008;82:2493–500. doi: 10.1128/JVI.02188-07. doi: 10.1128/JVI.02188-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mason KA, Ariga H, Neal R, Valdecanas D, Hunter N, Krieg AM, et al. Targeting toll-like receptor 9 with CpG oligodeoxynucleotides enhances tumor response to fractionated radiotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:361–9. [PubMed] [Google Scholar]
- 43.Suit HD, Sedlacek R, Wagner M, Orsi L, Silobrcic V, Rothman KJ. Effect of Corynebacterium parvum on the response to irradiation of a C3H fibrosarcoma. Cancer research. 1976;36:1305–14. [PubMed] [Google Scholar]
- 44.Boccardo E, Lepique AP, Villa LL. The role of inflammation in HPV carcinogenesis. Carcinogenesis. 2010;31:1905–12. doi: 10.1093/carcin/bgq176. doi: 10.1093/carcin/bgq176. [DOI] [PubMed] [Google Scholar]
- 45.Ondrey FG, Dong G, Sunwoo J, Chen Z, Wolf JS, Crowl-Bancroft CV, et al. Constitutive activation of transcription factors NF-(kappa)B, AP-1, and NF-IL6 in human head and neck squamous cell carcinoma cell lines that express pro-inflammatory and pro-angiogenic cytokines. Molecular carcinogenesis. 1999;26:119–29. doi: 10.1002/(sici)1098-2744(199910)26:2<119::aid-mc6>3.0.co;2-n. doi: 10.1002/(SICI)1098-2744(199910)26:2<119::AID-MC6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 46.Tezal M, Scannapieco FA, Wactawski-Wende J, Hyland A, Marshall JR, Rigual NR, et al. Local inflammation and human papillomavirus status of head and neck cancers. Archives of otolaryngology--head & neck surgery. 2012;138:669–75. doi: 10.1001/archoto.2012.873. doi: 10.1001/archoto.2012.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manczak M, Jung Y, Park BS, Partovi D, Reddy PH. Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. Journal of neurochemistry. 2005;92:494–504. doi: 10.1111/j.1471-4159.2004.02884.x. doi: 10.1111/j.1471-4159.2004.02884.x. [DOI] [PubMed] [Google Scholar]
- 48.Iwamoto K, Bundo M, Kato T. Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum Mol Genet. 2005;14:241–53. doi: 10.1093/hmg/ddi022. doi: 10.1093/hmg/ddi022. [DOI] [PubMed] [Google Scholar]
- 49.Galvan-Pena S, O'Neill LA. Metabolic reprograming in macrophage polarization. Frontiers in immunology. 2014;5:420. doi: 10.3389/fimmu.2014.00420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Comim CM, Rezin GT, Scaini G, Di-Pietro PB, Cardoso MR, Petronilho FC, et al. Mitochondrial respiratory chain and creatine kinase activities in rat brain after sepsis induced by cecal ligation and perforation. Mitochondrion. 2008;8:313–8. doi: 10.1016/j.mito.2008.07.002. doi: 10.1016/j.mito.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 51.d'Avila JC, Santiago AP, Amancio RT, Galina A, Oliveira MF, Bozza FA. Sepsis induces brain mitochondrial dysfunction. Critical care medicine. 2008;36:1925–32. doi: 10.1097/CCM.0b013e3181760c4b. doi: 10.1097/CCM.0b013e3181760c4b. [DOI] [PubMed] [Google Scholar]
- 52.Honegger A, Leitz J, Bulkescher J, Hoppe-Seyler K, Hoppe-Seyler F. Silencing of human papillomavirus (HPV) E6/E7 oncogene expression affects both the contents and the amounts of extracellular microvesicles released from HPV-positive cancer cells. International journal of cancer Journal international du cancer. 2013;133:1631–42. doi: 10.1002/ijc.28164. doi: 10.1002/ijc.28164. [DOI] [PubMed] [Google Scholar]
- 53.Lee TH, Chennakrishnaiah S, Audemard E, Montermini L, Meehan B, Rak J. Oncogenic ras-driven cancer cell vesiculation leads to emission of double-stranded DNA capable of interacting with target cells. Biochemical and biophysical research communications. 2014;451:295–301. doi: 10.1016/j.bbrc.2014.07.109. doi: 10.1016/j.bbrc.2014.07.109. [DOI] [PubMed] [Google Scholar]
- 54.Ganesh S, Gonzalez-Edick M, Gibbons D, Ge Y, VanRoey M, Robinson M, et al. Combination therapy with radiation or cisplatin enhances the potency of Ad5/35 chimeric oncolytic adenovirus in a preclinical model of head and neck cancer. Cancer gene therapy. 2009;16:383–92. doi: 10.1038/cgt.2008.90. doi: 10.1038/cgt.2008.90. [DOI] [PubMed] [Google Scholar]
- 55.Mao-Ying QL, Kavelaars A, Krukowski K, Huo XJ, Zhou W, Price TJ, et al. The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. PloS one. 2014;9:e100701. doi: 10.1371/journal.pone.0100701. doi: 10.1371/journal.pone.0100701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient Receptor Potential Vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Molecular pain. 2010;6:15. doi: 10.1186/1744-8069-6-15. doi: 10.1186/1744-8069-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ta LE, Low PA, Windebank AJ. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Molecular pain. 2009;5:9. doi: 10.1186/1744-8069-5-9. doi: 10.1186/1744-8069-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahoney SE, Davis JM, Murphy EA, McClellan JL, Gordon B, Pena MM. Effects of 5-fluorouracil chemotherapy on fatigue: role of MCP-1. Brain, behavior, and immunity. 2013;27:155–61. doi: 10.1016/j.bbi.2012.10.012. doi: 10.1016/j.bbi.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahoney SE, Davis JM, Murphy EA, McClellan JL, Pena MM. Dietary Quercetin Reduces Chemotherapy-Induced Fatigue in Mice. Integrative cancer therapies. 2014 doi: 10.1177/1534735414523315. doi: 10.1177/1534735414523315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wood LJ, Nail LM, Perrin NA, Elsea CR, Fischer A, Druker BJ. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biological research for nursing. 2006;8:157–69. doi: 10.1177/1099800406290932. doi: 10.1177/1099800406290932. [DOI] [PubMed] [Google Scholar]
- 61.Zombeck JA, Fey EG, Lyng GD, Sonis ST. A clinically translatable mouse model for chemotherapy-related fatigue. Comparative medicine. 2013;63:491–7. [PMC free article] [PubMed] [Google Scholar]
- 62.Marquette C, Linard C, Galonnier M, Van Uye A, Mathieu J, Gourmelon P, et al. IL-1beta, TNFalpha and IL-6 induction in the rat brain after partial-body irradiation: role of vagal afferents. International journal of radiation biology. 2003;79:777–85. doi: 10.1080/09553000310001610998. doi: 10.1080/09553000310001610998. [DOI] [PubMed] [Google Scholar]
- 63.Rabin BM, Hunt WA, Lee J. Effects of dose and of partial body ionizing radiation on taste aversion learning in rats with lesions of the area postrema. Physiology & behavior. 1984;32:119–22. doi: 10.1016/0031-9384(84)90081-7. doi: 10.1016/0031-9384(84)90081-7. [DOI] [PubMed] [Google Scholar]
- 64.Kingham JG, Loehry CA. Permeability of the small intestine after intra-arterial injection of histamine-type mediators and irradiation. Gut. 1976;17:517–26. doi: 10.1136/gut.17.7.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maxwell A, Gaffin SL, Wells MT. Radiotherapy, endotoxaemia, and nausea. Lancet. 1986;1:1148–9. doi: 10.1016/s0140-6736(86)91857-x. [DOI] [PubMed] [Google Scholar]
- 66.Bollrath J, Greten FR. IKK/NF-kappaB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO reports. 2009;10:1314–9. doi: 10.1038/embor.2009.243. doi: 10.1038/embor.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Current opinion in genetics & development. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.