Abstract
The bitter taste receptor T2R38 has been shown to regulate mucosal innate immune responses in the upper airway epithelium. Furthermore, SNPs in T2R38 influence the sensitivity to 6-n-propylthiouracil (PROP) and are associated with caries risk/protection. However, no study has been reported on the role of T2R38 in the innate immune responses to oral bacteria. We hypothesize that T2R38 regulates oral innate immunity and that this regulation is genotype-specific. Primary gingival epithelial cells carrying three common genotypes, PAV/PAV (PROP super-taster), AVI/PAV (intermediate) and AVI/AVI (non-taster) were stimulated with cariogenic bacteria Streptococcus mutans, periodontal pathogen Porphyromonas gingivalis or non-pathogen Fusobacterium nucleatum. QRT-PCR analyzed T2R38 mRNA, and T2R38-specific siRNA and ELISA were utilized to evaluate induction of hBD-2 (antimicrobial peptide), IL-1α and IL-8 in various donor-lines. Experiments were set up in duplicate and repeated three times. T2R38 mRNA induction in response to S. mutans was highest in PAV/PAV (4.3-fold above the unstimulated controls; p<0.05), while lowest in AVI/AVI (1.2-fold). In PAV/PAV, hBD-2 secretion in response to S. mutans was decreased by 77% when T2R38 was silenced. IL-1α secretion was higher in PAV/PAV compared to AVI/PAV or AVI/AVI with S. mutans stimulation, but it was reduced by half when T2R38 was silenced (p<0.05). In response to P. gingivalis, AVI/AVI showed 4.4-fold increase (p<0.05) in T2R38 expression, whereas the levels in PAV/PAV and AVI/PAV remained close to that of the controls. Secretion levels of IL-1α and IL-8 decreased in AVI/AVI in response to P. gingivalis when T2R38 was silenced (p<0.05), while the changes were not significant in PAV/PAV. Our data suggest that the regulation of gingival innate immunity by T2R38 is genotype-dependent and that the ability to induce a high level of hBD-2 by PAV/PAV carriers may be a reason for protection against caries in this group.
Keywords: Innate Immunity, T2R38 signaling, Caries, Taste receptors
1. Introduction
As early as 1932, it was recognized that large, genetically-determined, individual differences exist in taste sensitivity for the bitter compounds phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP) (Blakeslee and Fox 1932; Griffin and Fischer 1960). The ability to taste these compounds varies dramatically in the population, ranging from those virtually unable to detect them at any concentration (non-tasters) to those who find even trace amounts extremely bitter. More recently, a family of G-protein-coupled receptors expressed in taste receptor cells of the tongue and palate were identified as the receptor proteins responsible for transducing bitter taste in mammal (Bufe, Breslin et al. 2005). Later, T2R38 was identified as the primary taste receptor that binds to PTC and PROP, imparting their bitter taste (Bufe, Breslin et al. 2005). Studies have identified five polymorphisms in the T2R38 receptor gene (TAS2R38), three of which cause amino acid substitutions in the T2R38 receptor protein at positions 49, 262, and 296 (Kim, Jorgenson et al. 2003). Two haplotypes of the T2R38 receptor gene are common outside of sub-Saharan Africa – these are the dominant PAV haplotype, which confers taster status, and the recessive AVI haplotype (Kim, Jorgenson et al. 2003). Individuals with AVI/AVI genotypes are almost invariably non-tasters.
Since the original discovery of T2R receptors in taste buds, evidence of these receptors has been detected in other tissues, with extensive expression observed in the upper airway and lungs (Kinnamon 2012). T2R receptors are expressed in solitary chemosensory cells of the nasal cavity (Finger, Bottger et al. 2003), in ciliated epithelial cells of the human airway (Shah, Ben-Shahar et al. 2009), and in smooth muscle cells lining the airways of the lungs (Deshpande, Wang et al. 2010). In the upper airway, these receptors seem to trigger innate host defense systems. Most recently, human sinonasal cells were demonstrated to express the T2R38 receptor, and the receptor was demonstrated to respond to Pseudomonas quorum-sensing molecules by regulating mucociliary clearance and antibacterial effects through calcium-dependent NO production (Lee, Xiong et al. 2012). Importantly, genetic variation in the T2R38 receptor gene was found to modulate these innate host defense responses of the sinonasal cells, with cells from homozygous dominant individuals (PAV/PAV) eliciting a greater innate defense response than cells from heterozygous (PAV/AVI) or homozygous recessive (AVI/AVI, non-taster) individuals. These findings seem to have clinical importance for chronic rhinosinusitis. In a study of 28 patients with chronic rhinosinusitis, only 1 PAV/PAV individual was identified, versus the expected five to six individuals in a population this size (Adappa, Howland et al. 2013), suggesting a protective effect of the PAV/PAV genotype.
The T2R38 receptor gene has also been associated with caries in the primary dentition (Wendell, Wang et al. 2010). Specifically, the PAV (taster) haplotype has been shown to have a protective effect against caries (Wendell, Wang et al. 2010). Previous explanations for this protective effect have pointed to the role of the T2R38 receptor gene in influencing dietary choice (Wendell, Wang et al. 2010; Oter, Ulukapi et al. 2011). Now that T2R38 receptor is known to play a role in triggering innate host defense responses in sinonasal cells, it was of interest to investigate whether similar innate host defense responses may be triggered by the same receptor in gingival epithelial cells (GECs). Such involvement would provide potential points of intervention for boosting innate host defense systems against oral bacteria. We hypothesize that T2R38 receptor regulates innate immune responses in the oral cavity by inducing the release of antimicrobial peptides along with inflammatory markers in response to different oral bacteria. The goal of this study is to examine the extent of T2R38’s regulation of gingival innate immunity.
2. Materials and Methods
2.1. Growth of GECs
Primary GECs were grown in the Cell Culture Core at the University of Washington School of Dentistry as described in our previous publications (Chung and Dale 2008; Chung, An et al. 2010). The GECs used in our laboratory are grown under calcium levels that permit them to begin to express differentiation markers as they would in tissues. Donor lines carrying each of three common genotypes were chosen: AVI/AVI (non-taster), AVI/PAV (intermediate), and PAV/PAV (super-taster). At least two different donors carrying the same genotype were tested to look for consistency.
2.2. Comparing various donor lines from different SNP carriers
We extracted genomic DNA from the cells using the Wizard® Genomic DNA Isolation Kit (Promega Corporation, Madison, WI) and measured DNA concentration and purity using small volume spectrophotometry (NanoDrop, Wilmington, DE). Samples were diluted to 5 ng/μl in distilled water and this template was used in Taqman assays (Applied Biosystems, Foster City, CA). Genotypes were assayed in duplicate using previously established methods (Mennella, Pepino et al. 2011). For the three variant sites within the TAS2R38 gene, we report genotyping results as amino acids codes, rs713598, A49P; rs1726866, V262A, and I296V, rs10246939. We constructed haplotypes (e.g., AVI or PAV) and diplotypes (e.g., AVI/AVI or PAV/PAV) using previously established population frequencies (Kim, Jorgenson et al. 2003; Bufe, Breslin et al. 2005; Kim, Wooding et al. 2005), with the AVI and PAV haplotypes being the most frequently observed in populations of European ancestry.
2.3. Synthesis of specific siRNA and transfection of GECs
siRNA transfection was carried out as previously reported in our earlier studies (Dommisch, Chung et al. 2007; Chung, An et al. 2010; Rohani, Beyer et al. 2010). Unstimulated GECs and GECs transfected with non-silencing (NS) control siRNA served as controls. Following bacterial stimulation, total RNA was extracted for QRT-PCR. Specific and effective knock-down of T2R38 was verified using QRT-PCR with primers published in an earlier study (Jeon, Seo et al. 2011).
2.4. Conditions tested
Since T2R38 has been associated with caries risk, we investigated the role of T2R38 first by utilizing the cariogenic bacteria Streptococcus mutans. Because S. mutans is found in saliva, GECs will come in contact with the bacteria. Furthermore, our previous study showed GECs responded to S. mutans with increased antimicrobial peptide and chemokine expressions (Ock, DiJulio et al. 2011). In addition, it was informative to add Porphyromonas gingivalis, a Gram-negative periodontal pathogen and an excellent inducer of markers of innate immunity, as well as Gram-negative Fusobacterium nucleatum which plays a role as a bridging organism between the early colonizers and pathogens of the oral cavity. The initial time-course experiments on the induction of T2R38 mRNA by different bacteria were conducted by stimulating GECs for 4, 10 and 18 h. For investigating the effects of bacterial dose on the induction of T2R38 mRNA expression, GECs were stimulated with each bacterial species at the multiplicity of infection (MOI) of 10:1, 50:1 and 100:1. After the optimal MOI and time for bacterial stimulation were determined (see Results), the following conditions were set up in GECs: Unstimulated GECs without transfection; GECs transfected with siRNA for T2R38 or with NS control; GECs transfected with siRNA (T2R38 or NS) and subsequently stimulated with P. gingivalis, F. nucleatum or S. mutans at an MOI of 100:1 for 18 h; Control GECs exposed to each bacterial species as described. We have found that MOI higher than 100:1 puts stress on GECs, while MOI 100:1 for 18 h was sufficient to induce cellular stimulation as tested by the mRNA expression of various markers of innate immunity (Chung and Dale 2008; Chung, An et al. 2010).
2.5. QRT-PCR with SYBR Green
After appropriate stimulation, total RNA was isolated from GECs by standard techniques using Qiagen RNeasy kit (Qiagen, Valencia, CA) and the mRNA expression levels of T2R38, IL-8, IL-1α and hBD-2 were determined utilizing real-time QRT-PCR with SYBR Green. The latter three innate immune markers were selected because our previous studies showed they play a significant role in the gingival innate immunity in response to various oral bacteria (Chung and Dale 2004; Dommisch, Chung et al. 2007; Chung, An et al. 2010). Each sample was set up in duplicate per each marker, along with housekeeping gene control GAPDH. QRT-PCR condition for the mRNA expression of another epithelial receptor protease-activated receptors (PARs) has been previously reported (Chung, Hansen et al. 2004; Dommisch, Chung et al. 2007). For all experiments, at least two different donors carrying the same genotype were tested, with each donor line tested three times.
2.6. IL-8, IL-1α and hBD-2 ELISA
GECs were cultured and treated with appropriate siRNA and bacteria as described above. Resultant cell culture supernatants were collected and corresponding ELISA kits were used to measure the secretion levels of IL-8, IL-1α (R&D Systems, Minneapolis, MN) and hBD-2 (PeproTech, Rocky Hill, NJ). Various donor lines were tested as described above, and experiments were performed in duplicates with each sample set up in duplicates. A seven-point standard curve was created using reagent diluents in a series of 2-fold serial dilutions ranging from 1000 - 15.625 pg/ml. The optical density (OD) measurements of the samples were normalized to the blank reagent diluent controls, and final concentrations of each sample were computed by calculating the average of the ODs according to the established sample dilution in the standard regression line.
2.7. Statistical analyses
Statistical significance was determined using t-test for multiple comparisons with ANOVA for Figure 1 and two-way ANOVA for Figures 2-5.
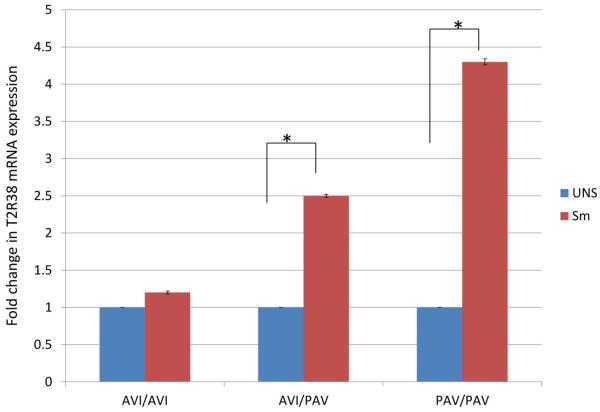
Figure 1.
T2R38 mRNA measured by QRT-PCR shows that T2R38 expression is differentially induced by oral bacteria S. mutans (Sm). In addition, the induction of T2R38 varied across donor cell lines with three different genotypes. Unstimulated GECs (UNS) served as controls. Each experiment was performed three times, in duplicate. The data (mean+SD) were normalized to the housekeeping gene GAPDH. Each asterisk indicates significance (p<0.05).
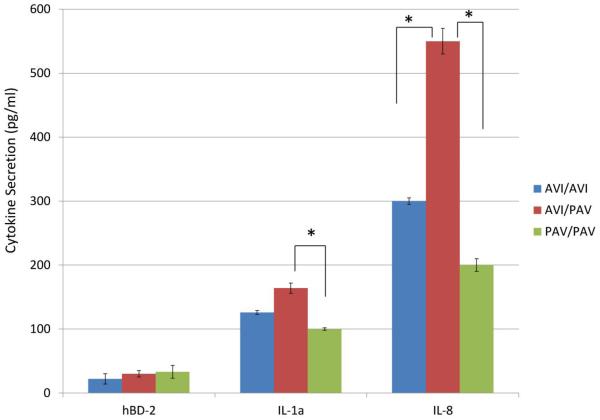
Figure 2.
Secreted levels of hBD-2, IL-1α and IL-8 in the unstimulated GECs of various genotypes are determined by ELISA. Each experiment was performed three times, in duplicate. The data are presented as mean+SD. *p<0.05.
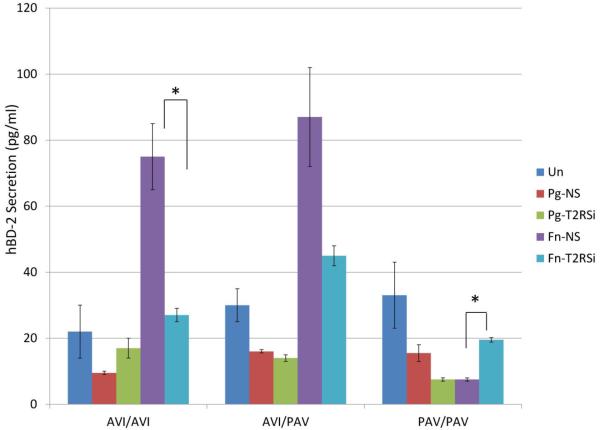
Figure 5.
Secreted levels of hBD-2 by ELISA show that the role of T2R38 in cytokine secretion is bacteria-specific and genotype-dependent. GECs transfected with control non-silencing (NS) siRNA and subsequently stimulated with different bacteria and unstimulated GECs served as controls. Each experiment was performed three times, in duplicate. The data are presented as mean+SD. Each asterisk denotes significant difference compared with NS siRNA plus bacteria controls (p<0.05). (T2RSi: T2R38-specific siRNA; Pg: P. gingivalis; Fn: F. nucleatum.)
3. Results
3.1. Dose-dependent T2R38 expression is observed in all SNP carriers
First to assess whether T2R38 is expressed and functioning in GECs, we used quantitative real-time RT-PCR (QRT-PCR) to measure T2R38 mRNA expression in both unstimulated GECs and GECs stimulated with different oral bacteria. The donor lines tested carry the following three genotypes: AVI/AVI (non-taster), AVI/PAV (intermediate), and PAV/PAV (super-taster). Across different SNP carriers, the level of T2R38 mRNA induction at MOI of 10:1 by different bacterial species was similar to the unstimulated control. The increase in T2R38 mRNA in response to S. mutans between MOIs 50:1 and 100:1 was two-fold in PAV/PAV and AVI/PAV carriers. The fold increase in the induction of T2R38 mRNA in response to P. gingivalis from MOIs of 50:1 to 100:1 was 1.7 and 1.4 among PAV/PAV and AVI/PAV carriers, respectively. For AVI/AVI carriers, the fold increase from 10:1 to 50:1 was 2.6, while to 100:1 was 4.4. For all SNP carriers, gradual but only slight (<1.4-fold) increase per step was observed in response to F. nucleatum MOIs 10:1, 50:1 and 100:1 (data not shown). For time-course studies, the level of T2R38 mRNA induction was negligible during the early hours, with significant increase shown at 18 h (data not shown). Therefore, the MOI of 100:1 and the stimulation time of 18 h were selected as optimal conditions for the remainder of this project.
3.2. T2R38 expression level is significantly up-regulated by cariogenic bacteria in PAV/PAV SNP carriers
Since T2R38 SNPs were shown to be associated with caries protection (PAV carriers) and risk (AVI carriers) (Wendell, Wang et al. 2010), it was of interest to investigate whether GEC donor lines with different genotypes would respond differently to the presence of bacteria. The difference in response by genotype was most prominent between the two extreme diplotypes, AVI/AVI and PAV/PAV. In particular, T2R38 expression in response to S. mutans was 4.3-fold above the unstimulated control in the PAV/PAV cell line, whereas little change was observed in the AVI/AVI cell line (Figure 1). All experiments for each donor line were performed three times, each in duplicate, and the data were normalized to the housekeeping control gene GAPDH. Additional donor lines of the same genotype were also tested, and the trends were consistent (data not shown). Our data suggest that the regulation of T2R38 by cariogenic bacteria S. mutans is genotype-dependent, with PAV/PAV donors showing the strongest ability to induce T2R38 mRNA in response to the bacteria.
3.3. Secreted levels of innate immune markers differ between various SNP carriers
Prior to bacterial stimulation, we investigated to determine if the base secretion levels of hBD-2, IL-1α and IL-8 differ between different SNP carriers. The difference in the amount of hBD-2 secretion between three different SNP carriers was not significant, although PAV/PAV donor line showed the most level of secretion (Figure 2). The carriers with AVI/PAV genotype secreted the highest amount of IL-1α and IL-8 among the three SNPs, while PAV/PAV carriers secreted the lowest amount of base level IL-1α and IL-8 (Figure 2).
3.4. Secretion of antimicrobial peptide hBD-2 in response to S. mutans occurs via T2R38
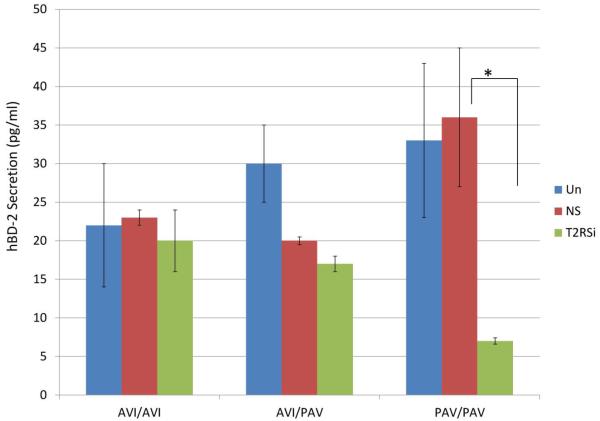
Earlier studies reported that the small cationic peptide hBD-2 showed an antimicrobial activity against several oral bacteria, including S. mutans (Joly, Maze et al. 2004; Ouhara, Komatsuzawa et al. 2005). In order to investigate if T2R38 plays a role in the secretion of hBD-2 in response to S. mutans, we post-transcriptionally knocked-down T2R38 and subsequently stimulated GECs with the bacteria. In response to S. mutans, PAV/PAV showed the highest level of both the mRNA expression and secretion of hBD-2 among the donor lines tested (Figures 2 and 3 for secretion; mRNA data not shown). In the absence of T2R38, the level of hBD-2 secretion in response to S. mutans is decreased by 77% in PAV/PAV carrier, while the decrease was by 13% and 15% in AVI/AVI and AVI/PAV donor lines, respectively (Figure 3). Each experiment per donor line was repeated three times, with each sample set up in duplicate. When additional donor lines carrying the same SNP were tested, consistent trends were observed (data not shown). Taken together, our data imply that T2R38 regulates secretion of the antimicrobial peptide hBD-2 and that the ability to induce a high level of hBD-2 by PAV/PAV carriers may be a defense mechanism behind this SNP carrier’s protective effect against caries
Figure 3.
Secreted levels of hBD-2 in response to S. mutans by ELISA show that the role of T2R38 in hBD-2 secretion is genotype-dependent. GECs transfected with control non-silencing (NS) siRNA and subsequently stimulated with S. mutans and unstimulated GECs served as controls. Each experiment was performed three times, in duplicate. The data are presented as mean+SD. Each asterisk denotes significant difference compared with NS siRNA plus bacteria controls (p<0.05). T2RSi: T2R38-specific siRNA + S. mutans; NS: non-silencing siRNA + S. mutans.
3.5. T2R38 plays a role in the secretion of cytokines IL-8 and IL-1α
T2R38 is known to play a role in triggering innate host defense responses in sinonasal cells (Lee, Xiong et al. 2012), but its role in the oral cavity is still unknown. In order to examine whether T2R38 plays a role in the induction of cytokines in the oral mucosa, we utilized specific siRNA to knock down T2R38 and evaluated the change in the induction of IL-1α and IL-8 in GECs. Both of these markers play a significant role in oral mucosal innate immunity. GECs transfected with control NS siRNA and stimulated with each bacterial species as well as unstimulated GECs were used as controls. When T2R38 was post-transcriptionally silenced by transfecting GECs with specific siRNA and the cells were subsequently stimulated with S. mutans, secretion of IL-1α was significantly decreased compared with controls in PAV/PAV cell line but increased in the AVI/AVI cell line (Figure 4). Differences in the pattern of IL-8 secretion in response to S. mutans after T2R38 knockdown were observed between different donor lines, but the magnitude of the changes was not significant (Figure 4). The mRNA expression patterns of IL-1α and IL-8 were similar to that of the protein secretion (data not shown). Our data strongly suggest that T2R38 plays a role in the induction of innate immune responses in gingival innate immunity and that these responses are also genotype-dependent.
Figure 4.
ELISA data show the changes in secreted levels of IL-1α and IL-8 in response to S. mutans when T2R38 is silenced with siRNA (T2RSi). The figure suggests that the role of T2R38 in cytokine secretion is genotype-dependent. GECs transfected with control non-silencing (NS) siRNA and subsequently stimulated with S. mutans and unstimulated GECs served as controls. Each experiment was performed three times, in duplicate. The data are presented as mean+SD. Each asterisk denotes significant difference compared with NS siRNA plus bacteria controls (p<0.05). T2RSi: T2R38-specific siRNA + S. mutans; NS: non-silencing siRNA + S. mutans.
3.6. T2R38 regulates gingival innate immune responses to additional oral bacteria
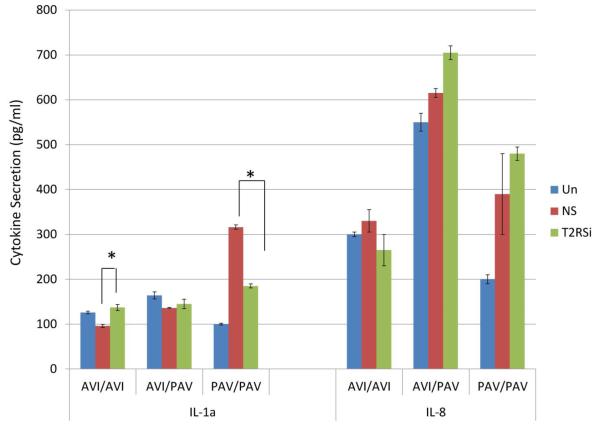
Since T2R38 plays a substantial role in the regulation of oral innate immunity in response to S. mutans, it was of interest to further investigate if T2R38 is involved in the regulation of gingival innate immune responses to periodontal bacteria. Similar to what we observed in response to S. mutans, genotype-dependent T2R38 mRNA expression was observed when different donor lines were stimulated with P. gingivalis or F. nucleatum. The difference was that the AVI/AVI cell line showed 4.4-fold increase in T2R38 expression compared to unstimulated controls, whereas the change was negligible in the PAV/PAV and AVI/PAV cell lines (data not shown). Thus our data suggest that T2R38 is differentially regulated by different types of bacteria present in the oral cavity and that this differential regulation is T2R38 genotype-dependent. Figure 5 shows the opposite trend between AVI/AVI vs. PAV/PAV carriers in the secretion of hBD-2 in response to P. gingivalis and F. nucleatum when T2R38 is silenced. Secretion of IL-1α was decreased in response to P. gingivalis compared with controls in PAV/PAV cell line but increased in the AVI/AVI cell line, similar to the pattern observed with S. mutans (Figure 6). On the contrary, the secretion pattern of IL-1α increased in response to F. nucleatum when T2R38 was silenced in all genotypes (Figure 6). The changes in IL-8 secretion in response to P. gingivalis showed opposite patterns between AVI/AVI and AVI/PAV donor lines, as is the case in response to F. nucleatum between AVI/AVI and AVI/PAV (Figure 6). mRNA expression patterns of IL-1α and IL-8 were similar to that of the protein secretion (data not shown).
Figure 6.
IL-1α and IL-8 ELISA show that the role of T2R38 in cytokine secretion is bacteria-specific and genotype-dependent. GECs transfected with control non-silencing (NS) siRNA and subsequently stimulated with different bacteria and unstimulated GECs served as controls. Each experiment was performed three times, in duplicate. The data are presented as mean+SD. Each asterisk denotes significant difference compared with NS siRNA plus bacteria controls (p<0.05). (T2RSi: T2R38-specific siRNA; Pg: P. gingivalis; Fn: F. nucleatum.)
3.7. T2R38 may interact with other epithelial cell receptors in gingival innate immunity
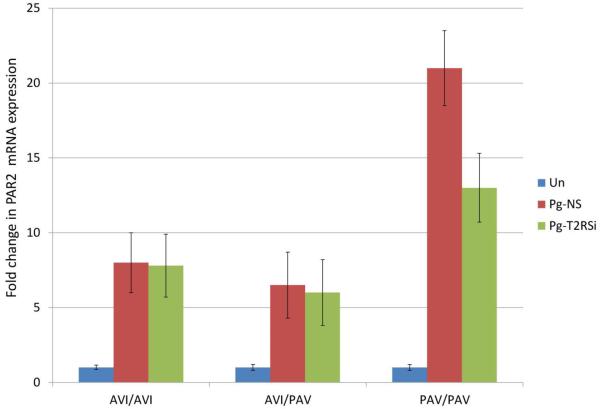
GECs are the first cells exposed to bacteria and inevitably utilize different receptors to induce necessary innate immune markers in response to bacteria. Our previous study showed that protease-activated receptors (PARs) and nucleotide-binding oligomerization domain receptors (NODs) can compensate for one another in epithelial responses to bacteria (Chung, An et al. 2010). In investigating whether T2R38 can also compensate for other epithelial receptors, we observed that when PAR1 or PAR2 is knocked down in GECs, T2R38 mRNA expression significantly increased (data not shown). In addition, our data show when T2R38 was knocked down in GECs, the PAV/PAV cell line decreased mRNA expression of PAR2 when stimulated with P. gingivalis (Figure 7). No significant changes were observed in other cell lines and also with other bacterial stimulation. The changes observed in PAR1 and NOD receptors were statistically not significant.
Figure 7.
QRT-PCR analyses show that PAR2 mRNA levels in PAV/PAV cell line were down-regulated when T2R38 was knocked down and GECs were subsequently stimulated with P. gingivalis (Pg). Each donor line was tested twice, in duplicate. T2RSi: GECs transfected with T2R38 siRNA.
4. Discussion
There has been limited account on the contribution of T2R38 in mucosal innate immune responses of airway epithelia via responses to bacterial quorum-sensing molecules. Although carriage of specific SNPs of this receptor has been implicated in caries protection, there has been no information on whether this receptor plays a role in the oral mucosa. To our knowledge, this is the first study to investigate if gingival innate immune responses are differentially regulated via T2R38 in responses to different oral bacteria, providing a new insight into the role this receptor plays in oral mucosal immunity. Furthermore, this is the first study to show how this innate immune response is differentially regulated by carriage of various SNPs. Regulation of mucosal innate immune responses in upper airway epithelium by T2R38 has been shown to work in part via up-regulating innate antimicrobial responses and also by responding to bacterial quorum-sensing molecules (Lee, Xiong et al. 2012; Adappa, Howland et al. 2013). Our current data show that primary GECs not only express T2R38, but differentially regulate the expression in response to different oral bacteria and that this expression is genotype-dependent. Because GECs were stimulated with whole bacteria grown to log-phase in this study, our data further suggest that T2R38 genotype differences in donor lines in response to bacteria itself are responsible for the differential regulations of T2R38 expression and not a response of the cells to differences in bacterial quorum-sensing molecules.
The PAV haplotype of the T2R38 gene has been associated with a protective effect against caries (Wendell et al., 2010). Heretofore, this observation has been thought to be due to the influence of T2R38 on food preferences and dietary intake. However, the relationship between T2R38 genotype and sweet preference is complex. In young children, the PAV/PAV genotype has been linked to increased liking for sweet (Mennella et al., 2005), which is opposite to the direction predicted to have a caries protective effect. Although the association between PAV haplotype and caries protection has been known for a few years, no molecular mechanism behind this protective effect has been reported. Based on our data, we propose that gingival epithelia increase the expression of T2R38 in the presence of oral bacteria, and that PAV/PAV carriers respond more strongly to cariogenic bacteria, whereas AVI/AVI carriers respond more towards periodontal pathogens. We also propose that the subsequent increase in the secretion of innate immune protein hBD-2 in PAV/PAV carriers after exposure to S. mutans exerts an antimicrobial effect against the cariogenic bacteria, potentially resulting in decreased susceptibility to caries. In a similar way, AVI haplotypes likely utilize secretion of hBD-2 as an innate host defense mechanism against F. nucleatum, which is susceptible to hBD-2 as reported previously (Joly, Maze et al. 2004).
GECs are one of the first host cell types that encounter colonizing bacteria. As a consequence, GECs utilize different receptors and elaborate signaling pathways in the induction of cytokines and antimicrobial proteins, leading to host innate immune responses to various bacteria. Different bacteria may induce different signals from the host (Ganz 2004; Chung, Dommisch et al. 2007; Kinane, Demuth et al. 2007). Nevertheless, it is not clear how these receptors and signaling cascades work in epithelial responses to different types of bacteria, i.e., pathogenic vs. non-pathogenic bacteria. Since bacteria have multiple Microbial-Associated Molecular Patterns and epithelial cells express multiple Pattern-Recognition Receptors (PRRs), it is reasonable to expect that there is cross-communication between these PRRs in epithelial responses to various bacteria. Innate immune response of GECs is the sum of activation by different PRRs, and information about how various epithelial cell PRRs alter their expression in response to different bacteria will help us better understand how oral epithelia produce appropriate innate immune responses to pathogenic and non-pathogenic bacteria. Utilizing siRNA specific for T2R38, we investigated the role of T2R38 in the induction of various innate immune markers. We also observed how the absence of T2R38 affects the expression of another epithelial receptor (PAR2) in response to P. gingivalis stimulation, but not to other bacteria. We have previously reported P. gingivalis induces hBD-2 in part via PAR 2 (Chung, Hansen et al. 2004). We also reported that appropriate innate immune responses to the different types of bacteria present within the oral cavity may require altered expression of different receptors (Chung, An et al. 2010). On the whole, our data imply that T2R38 may be part of a complex interplay of various epithelial receptors regulating the induction of proper innate immune responses in the oral cavity, where various receptors compensate for one another. Further studies on how different receptors present in the oral mucosa work together to activate appropriate innate immune responses will help us better understand how the complex innate immunity is modulated.
5. Conclusions
Our study addresses an area where very little information is currently available by demonstrating how gingival innate immune responses are triggered via utilization of T2R38, a receptor originally identified in the lingual epithelium, but now newly identified in the gingival epithelia. Our data strongly suggest the regulation of gingival innate immunity via T2R38 is genotype-specific. Both the T2R38 gene, which codes for the PROP/PTC receptor, and the taste intensity associated with this bitter compound have been shown to be associated with prevalence of caries in primary dentition. Several groups have proposed using sensitivity to PROP taste as a simple clinical assessment tool in order to establish caries risk. However, the mechanism by which the different forms of the T2R38 receptor protein confer caries protection and risk are not well understood. Our study showed a possible molecular mechanism behind the caries protective effect of PAV/PAV carrier. We envision that the results of our study will have direct implications for new understanding of oral innate immune responses and provide basis for developing powerful new translational application for various oral diseases.
Highlights.
The mRNA expression of the bitter taste receptor T2R38 is differentially regulated by SNPs.
Genotype- and bacteria-specific reduction of antimicrobial peptide hBD-2 secretion is observed in the absence of T2R38.
IL-1a and IL-8 secretion is also genotype-specific for T2R38.
T2R38 modulates innate oral immunity in a genotype-specific manner.
Modulation of hBD-2 secretion via T2R38 may be the mechanism by which the PAV haplotype confers caries protection.
Acknowledgements
The authors thank Dr. Danielle Reed at Monell Research Center (Philadelphia, PA) for genotyping the donor cell lines and for helpful discussions. The authors also thank Mr. Tanner Hudson for technical assistance. Funded by NIH/NIDCR R01DE19632 (to WOC), Washington Dental Service Endowed Professorship (to SEC) and University of Washington Morell Endowment (to WOC and SEC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors confirm there are no conflicts.
References
- Adappa ND, Howland TJ, et al. Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int Forum Allergy Rhinol. 2013;3(3):184–7. doi: 10.1002/alr.21140. [DOI] [PubMed] [Google Scholar]
- Blakeslee A, Fox AL. Our different taste world: P.T.C. as a demonstration of genetic differences in taste. J Hered. 1932;23:97–107. [Google Scholar]
- Bufe B, Breslin PA, et al. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15(4):322–7. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WO, An JY, et al. Interplay of protease-activated receptors and NOD pattern recognition receptors in epithelial innate immune responses to bacteria. Immunol Lett. 2010;131(2):113–9. doi: 10.1016/j.imlet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WO, Dale BA. Innate immune response of oral and foreskin keratinocytes: utilization of different signaling pathways by various bacterial species. Infect Immun. 2004;72(1):352–8. doi: 10.1128/IAI.72.1.352-358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WO, Dale BA. Differential utilization of NFκB signaling pathways for gingival epithelial cell responses to oral commensal and pathogenic bacteria. Oral Microbiol Immunol. 2008;23:119–126. doi: 10.1111/j.1399-302X.2007.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WO, Dommisch H, et al. Expression of defensins in gingiva and their role in periodontal health and disease. Current Pharmaceutical Design. 2007;13:3073–3083. doi: 10.2174/138161207782110435. [DOI] [PubMed] [Google Scholar]
- Chung WO, Hansen SR, et al. Protease-activated receptor signaling increases epithelial antimicrobial peptide expression. J Immunol. 2004;173(8):5165–70. doi: 10.4049/jimmunol.173.8.5165. [DOI] [PubMed] [Google Scholar]
- Deshpande DA, Wang WC, et al. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16(11):1299–304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommisch H, Chung WO, et al. Protease-activated receptor 2 mediates human beta-defensin 2 and CC chemokine ligand 20 mRNA expression in response to proteases secreted by Porphyromonas gingivalis. Infect Immun. 2007;75(9):4326–33. doi: 10.1128/IAI.00455-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Bottger B, et al. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci U S A. 2003;100(15):8981–6. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of vertebrates. C R Biol. 2004;327(6):539–49. doi: 10.1016/j.crvi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Griffin F, Fischer R. Differential reactivity of saliva from 'tasters' and 'non-tasters' of 6-n-propylthiouracil. Nature. 1960;187:417–9. doi: 10.1038/187417b0. [DOI] [PubMed] [Google Scholar]
- Jeon TI, Seo YK, et al. Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem J. 2011;438(1):33–7. doi: 10.1042/BJ20110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly S, Maze C, et al. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42(3):1024–9. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, Wooding S, et al. Worldwide haplotype diversity and coding sequence variation at human bitter taste receptor loci. Hum Mutat. 2005;26(3):199–204. doi: 10.1002/humu.20203. [DOI] [PubMed] [Google Scholar]
- Kim UK, Jorgenson E, et al. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299(5610):1221–5. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- Kinane DF, Demuth DR, et al. Human variability in innate immunity. Periodontol 2000. 2007;45:14–34. doi: 10.1111/j.1600-0757.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- Kinnamon SC. Taste receptor signalling - from tongues to lungs. Acta Physiol (Oxf) 2012;204(2):158–68. doi: 10.1111/j.1748-1716.2011.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RJ, Xiong G, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122(11):4145–59. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Pepino MY, et al. Psychophysical dissection of genotype effects on human bitter perception. Chem Senses. 2011;36(2):161–7. doi: 10.1093/chemse/bjq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ock MS, DiJulio DH, et al. Role of Protease-Activated Receptors in host responses to Streptococcus mutans. International Association for Dental Research; San Diego, CA: 2011. [Google Scholar]
- Oter B, Ulukapi I, et al. The relation between 6-n-propylthiouracil sensitivity and caries activity in schoolchildren. Caries Res. 2011;45(6):556–60. doi: 10.1159/000332432. [DOI] [PubMed] [Google Scholar]
- Ouhara K, Komatsuzawa H, et al. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, {beta}-defensins and LL37, produced by human epithelial cells. J Antimicrob Chemother. 2005;55(6):888–96. doi: 10.1093/jac/dki103. [DOI] [PubMed] [Google Scholar]
- Rohani MG, Beyer RP, et al. Modulation of expression of innate immunity markers CXCL5/ENA-78 and CCL20/MIP3alpha by protease-activated receptors (PARs) in human gingival epithelial cells. Innate Immun. 2010;16(2):104–14. doi: 10.1177/1753425909339233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AS, Ben-Shahar Y, et al. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325(5944):1131–4. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendell S, Wang X, et al. Taste genes associated with dental caries. J Dent Res. 2010;89(11):1198–202. doi: 10.1177/0022034510381502. [DOI] [PMC free article] [PubMed] [Google Scholar]