Abstract
Recently, a family of innate cells has been identified that respond to IL-25 and IL-33 in murine intestinal helminths. Termed Type 2 innate lymphoid cells (ILC2s) they facilitate the development of Th2 responses responsible for helminth clearance. We evaluated these cells in a tissue-invasive helminth model. Using Litomosides sigmodontis (a strong Th2 polarizing filarial infection) we observed a robust Th2 response in the pleural cavity, where adult worms reside, marked by increased levels of IL-5 and IL-13 in infected mice. In parallel, ILC2s were expanded in the pleural cavity early in the infection, peaking during the pre-patent period. L. sigmodontis also elicits a strong systemic Th2 response, which includes significantly increased levels of IgG1, IgE and IL-5 in the plasma of infected mice. Although ILC2s were expanded locally, they were not expanded in the spleen, blood, or mediastinal lymph nodes in response to L. sigmodontis infection, suggesting that ILC2s function primarily at the site of infection. The increase in ILC2s in the pleural cavity and the expansion in Th2 responses indicates a probable role for these cells in initiating and maintaining the Th2 response and highlights the importance of these cells in helminth infections and their role in Th2 immunity.
Keywords: lymphocytes, filaria, pleural cavity, innate lymphoid cells, ILC2s
1. Introduction
Recently, a family of innate cells was identified that responded to IL-25 and IL-33 in the context of murine intestinal helminth infection and facilitated the development of the Th2 immune response necessary to expel the worms. Originally termed nuocytes, innate helper type 2 cells, multipotent progenitor cells and natural helper cells, 3 of the 4 cell types are now classified into a two families of cells, the multipotent progenitor cells and the rest of the cells falling into the family of innate lymphoid cells (ILCs) [1] based on analogous functions and shared surface molecule expression patterns.
ILCs are comprised of subsets termed ILC1, ILC2 and ILC3 that have specific cytokine profiles driven by discrete transcription factors [1]. ILC1s have been shown to produce IL-12 primarily and rely on the transcription factor Tbet; ILC2s produce IL-13, IL-5 and some IL-4 and their differentiation is driven by GATA3; and ILC3s express Rorγt and produce IL-22 and IL-17. These ILC subsets parallel those seen among CD4+ T cells and are thought to influence Th subset differentiation [1]. Based on these definitions, the ILCs identified initially in mice [2–4] were from the ILC2 subset.
While ILC2s [2–4] have been shown to be important for the initiation of the Th2 response to intestinal helminths, there have been no reports to date of these cells types in more systemic helminth infection nor of their presence in sites other than the skin, gastrointestinal tract or the lung proper. ILC2s have been shown to be expanded in the lung during infection with the tissue transiting helminth Strongyloides venezuelensis, and have been shown to produce IL-13 and IL5 in response to epithelial-cell derived IL-33 [5].
Here, using a model of the tissue-invasive filarial parasite L. sigmodontis, we investigated the presence of ILC2s within the pleural cavity, the location where adult L. sigmodontis worms reside.
2. Materials and methods
2.1 Parasites and mouse infection
Litomosoides sigmodontis infective stage (L3) parasites were isolated by lavage from the pleural cavity of 4 day infected Meriones unguiculatus jirds obtained from TRS Laboratory, Athens, GA, as previously described [6]. Wild type female BALB/c mice 6 weeks of age were infected by subcutaneous injection of 40 L3. The mice were maintained at the Uniformed Services University of the Health Sciences animal facility and all experiments were performed under a protocol approved by the Uniformed Services University Institutional Animal Care and Use Committee (Bethesda, MD). Animals were anesthetized with pentabarbitol at the end points of the experiments.
Three separate experiments were conducted. In the first experiment, 3 mice were infected and 4 were used as control mice for each time point. Spleens, whole blood, and cells from a pleural lavage were obtained at days 1, 5, 14, 42 and 60 post-infection. Whole blood and pleural lavage samples were pooled for each experimental condition at each time point, and cells were isolated from these samples as described below. For the second experiment, 5 mice were infected as above and 5 mice were used as uninfected controls for each time point; samples were collected day 5, 14, 42, and 60 post-infection. Again, spleens, whole blood, and pleural lavage fluid were collected as were mediastinal lymph nodes (MLN). Cells collected from the pleural lavage fluid and MLN were pooled for each experimental condition at each time point. Plasma was isolated from the whole blood and used for antibody and cytokine analysis (see below). The third experiment again used 5 infected and 5 uninfected mice per time point. Spleens, pleural lavage (both cells and fluid) and plasma were collected at days 36 and 42 post-infection. Pleural lavage fluid and pleural lavage cells were pooled at each time point for each experimental condition. The course of infection in the mouse and the time points taken are depicted in Fig. 1.
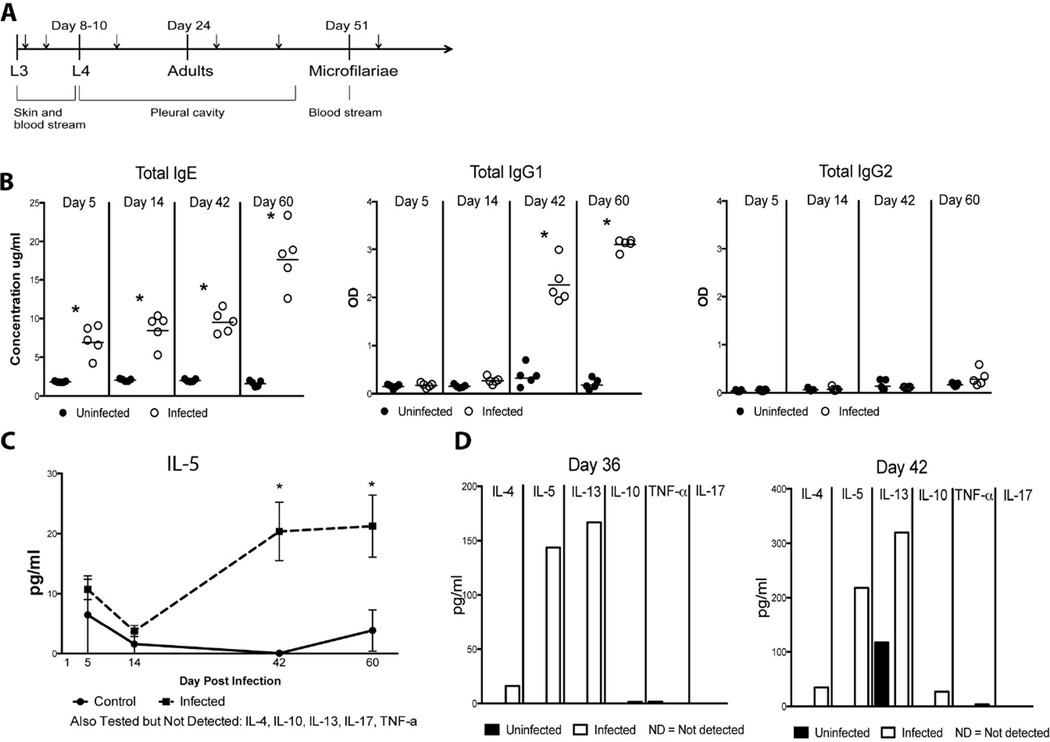
Fig. 1.
The Th2 response to L. sigmodontis is initiated in the pre-patent period Systemic and local immune responses were examined to determine the timing and extent of the Th2 response to L. sigmodontis. (A) Infection of L. sigmodontis in BALB/c mice is illustrated with approximate times of molting and patency as well the anatomical location of the various life cycle stages, adapted from [19]. Time points for the experiments (Days 1, 5, 14, 36, 42, and 60 post-infection) are denoted with small arrows. The pre-patent period is defined as the period after the adults are established in the pleural cavity prior to microfilariae appearing in the blood. (B) Levels of total IgE, total IgG1 and total IgG2 were determined by ELISA in the plasma of infected (n=5) or control (n=5) mice. Total IgE values are expressed in ug/ml and total IgG1 and total IgG2 values are expressed as ODs. (C) The mean levels of IL-5 (expressed as pg/ml) in the plasma from 5 control (solid line) and 5 infected mice (dotted line). All statistically significant differences (p<0.05) are indicated with a star. (D) Cytokine levels (IL-4, Il-5, IL-10, IL-13, IL-17 and TNF-α) were determined in concentrated and pooled pleural lavage fluid by Luminex™ for control mice (black bars) and infected mice (gray bars). All values are expressed as pg/ml.
2.2 Cell processing
Mouse splenocytes were isolated from female wild type BALB/c mice. Briefly, spleens were taken from anesthetized mice and placed in 5 ml of RPMI (Cellgro, Manassas, VA) supplemented with 3% FBS (Valley Biomedical, Winchester, VA), 1% penicillin/streptomycin (Cellgro), 1% L-glutamine (Cellgro) and 20 mM HEPES (Cellgro). The spleens were then pushed through a 70 um filter (BD Biosciences) in a 100mm petri dish using a syringe plunger from a 3ml syringe (BD Biosciences) and washed with an additional 5 ml of 3% RPMI. Cells were spun at 400 rpm for 5 min at RT, resuspended in 2 ml of ACK Lysing buffer (Quality Biological, Gaithersburg, MD) and lysed for 5 min. After lysing, 5 ml of 3% RPMI was added, the cells centrifuged as before and resuspended in 10 ml of 3% RPMI for counting. Then the cells were centrifuged and stained with Violet Live/Dead (Invitrogen) stain at 1:4000, fixed for 5 minutes in 4% paraformaldehyde (Sigma Aldrich) and cryopreserved in 1X PBS (Quality Biological) with 10% DMSO (Sigma Aldrich). Sections of harvested mouse lungs were processed in the same manner, as were lymph nodes but without the lysing step. Additional sections of mouse lungs were fixed whole in paraformaldehyde or perfused with OTC (Sakura Finetek, Torrance, CA), frozen and stored in liquid nitrogen.
Pleural lavage cells were obtained by cutting a small window in the diaphragm and using a transfer pipette to instill 3 ml of RPMI into the cavity to collect the cells in that space. After harvesting, the pleural lavage cells were centrifuged and resuspended in 3% RPMI for counting, Live/Dead stained and cryopreserved as detailed above.
The pooled pleural lavage from uninfected or infected mice was concentrated from 15 ml to 1.5 ml each using Amicon Ultra Ultracel 3K centrifugal filters (Millipore) and transferred to tubes for storage at −80 degrees Celsius.
Whole blood was taken from each mouse by puncture of the inferior vena cava. The blood was lysed at a ratio of 1 part blood to 9 parts Immuno-lyse (1:25 dilution for 1X solution) (Beckman Coulter, Brea, CA) and vortexed for 1 min or until the solution was no longer cloudy. 2–3 volumes of 1X PBS was added and samples were centrifuged at 400 rpm for 5 min. Supernatants were aspirated, and the cells resuspended in 3% RPMI for counting, staining with Live/Dead (Invitrogen) stain and cryopreservation (First experiment). For analysis of plasma cytokines, blood was collected in Microtainer plasma separator tubes with lithium heparin (BD), centrifuged and the plasma placed in cryovials (Sarstedt) and stored at −20°C
2.3 Cytokine analysis
IL-4, IL-5, IL-13, IL-10, IL-17, IFN-γ and TNF-α concentrations in plasma and concentrated pleural lavage fluid samples were measured using a multiplex bead array assay per the manufacturer’s instructions. The minimum detection limits for these assays were as follows: 1.1 pg/ml for IFN-γ, 3.1 pg/ml for TNF-α, 0.3 pg/ml for IL-4, 0.4 pg/ml for IL-5, 2.6 pg/ml for IL-10, 12.4 pg/ml for IL-13 and 0.7 pg/ml for IL-17.
2.4 Flow cytometry
Cryopreserved spleen, pleural lavage, MLN and whole blood cells were thawed and washed once in 1X PBS supplemented with 1% BSA. The cells were then incubated in 1% BSA for 20 minutes. The cells were stained with anti-mouse lineage cocktail (anti-CD3, anti-CD45R, anti-CD11b, anti-TER-119 and anti-Ly-G6) in Pacific Blue (eBioscience), cKit in AlexaFluor 700 (eBioscience), Sca1 in FITC (eBioscience), CD127 in PE-Cy7 (eBioscience), T1/ST2 in PE (R&D Systems), CD90.2 in APC-eFluor 780 (eBioscience), and CD44 in PerCPCy5.5 (eBioscience) for 30 minutes in 1% BSA. An additional subset of pleural lavage cells were stained with IL-5 in PE (omitting the ST2) to check for intracellular cytokine levels. The cells were washed once with 1% BSA and resuspended for analysis on a LSRII flow cytometer (BD Biosciences). ILC2s are defined as lineage−/CD90.2+/ST2+.
For characterization of the pleural infiltrate, cryopreserved pleural lavage cells were thawed, washed, blocked as above and stained with anti-mouse F4/80 in Pacific Blue (BD Pharmingen), CD11c in Alexa Fluor 700 (BD Pharmingen), IgE in FITC (BD Pharmingen), CD11b in PE-Cy5 (BD Pharmingen), and cKit in APC (BD Pharmingen). In a separate tube, cells were stained with anti-mouse CD45 in FITC (eBioscience), SiglecF in PE (BD Pharmingen), CD4 in PE-Cy7 (BD Pharmingen), CD8 in APC-Cy7 (BD Pharmingen), Grl/Ly6 in APC (eBioscience), and CD19 and PE-Cy5 (eBioscience). Cells from tube 1 were washed once, incubated for 10 minutes in 100ul 4% paraformaldehyde and 100ul 1X permeabilizaton buffer (eBioscience), washed in 1X permeabilization buffer and stained with anti-mouse IL-33 in PE (R&D Systems), washed again in 1X permeabilization buffer then resuspended in FACS Buffer. Cells in tube 2 were washed once and then resuspended in FACS Buffer. Analysis was performed on a Fortessa flow cytometer (BD Biosciences) using Diva software v 7.0 and the cell types were defined as follows; macrophages were F4/80+, dendritic cells (DCs) were CD11c+, eosinophils were SiglecF+ and CD45+, neutrophils were Gr1+, mast cells were IgE+ and cKit+, B cells were CD19+, and T cells were either CD4+ or CD8+. Cutoffs for surface marker positivity was determined using the fluorescence minus one approach.
2.5 Antibody determination
Immulon 4 plates (Thermo Scientific, Waltham, MA) were coated with either purified mouse anti-IgE capture antibody (BD Biosciences), purified mouse anti-IgG1 (BD Pharmingen, Franklin Lakes, NJ) capture antibody or purified mouse anti-IgG2 (BD Pharmingen) capture antibody in 1X PBS and incubated overnight at 4°C. Then plates were washed with 1X PBS supplemented with 0.025% Tween 20 and blocked for 2 hours at 37 °C in 1X PBS supplemented with 5% BSA (Sigma Aldrich) and 0.05% Tween. Purified mouse IgE (BD Biosciences), purified mouse IgG1 (BD Biosciences) and purified mouse IgG2 (BD Biosciences) were used as standards. Blocked plates were washed with wash buffer and samples incubated for 2 hours at 37 °C. Plates were then washed and biotinylated anti-mouse IgE (BD Biosciences), anti-mouse IgG1 (BD Biosciences) or anti-mouse IgG2 (BD Biosciences) was added and incubated for 2 hours at 37 °C. Following a washing step, 1:1000 dilution of streptavidin conjugated alkaline phosphatase (Jackson Immunoresearch, West Grove, PA) was added to the plates and incubated for 1 hour at 37 °C. 4-Nitrophenyl phosphate disodium salt hexahydrate (PNPP) (Sigma Aldrich) was used as the substrate. Plates were read on an ELISA reader at 405/650.
2.6 Immunohistochemistry
Immunohistochemistry was performed by Vitrovivo Biotechnology (Rockville, MD) per standard protocol. Briefly, formalin fixed lungs were paraffin-embedded and sectioned. Antigen retrieval was done using citrate buffer and the slides were blocked with PBS with normal serum. Rabbit anti-Il-33 (Enzo Life Sciences, Farmingdale, NY) was added to the slides at a 1:1000 dilution and incubated overnight at 4 °C. Biotin labelled goat-anti-rabbit IgG was used as a secondary antibody and incubated with the slides for 40 min at room temperature. HRP-strepavadin was then added for 40 min at room temperature and the slides were visualized with DAB plus H2O2 for 2–5 min.
3. Results
3.1 The Th2 response to L. sigmodontis is initiated in the pre-patent period
The kinetics of the immune response to L. sigmodontis are linked to the developmental cycle of the parasite and have been described previously [7] with increases in polyclonal IgE levels occurring early (4 weeks) and parasite specific IgE appearing by 6 weeks (pre-patent period). By 10 weeks, when the adult worms are fully developed, there is a peak of parasitespecific IgE as well as IL-4 and IL-5 production from splenocytes [7]. To characterize further the nature of the immune response to L. sigmodontis systemically and at the site of infection, plasma and pleural lavage fluid were examined for antibody and cytokine levels at different time points during the course of infection (Fig. 1a). For the purposes of these experiments, the prepatent period was considered to be at both days 36 and day 42 post-infection. When IgE, IgG1 (Th2-surrogates), and IgG2 (a Th1-surrogate) antibodies were measured in the plasma of uninfected and infected mice it was noted that total IgE levels were significantly increased in infected mice early at day 5 of infection (p=0.008), and that this increase continued throughout the course of infection (Fig. 1b) with significant increases compared to uninfected mice throughout the infection (day 14 p=0.012, day 42 p=0.008, day 60 p=0.008) (Fig. 1b). IgG1 increased from baseline by day 14 (p=0.016) and remained significantly elevated at both day 42 (p=0.008) and day 60 (p=0.008) (Fig. 1b). In contrast, no differences in IgG2 levels were observed between infected and uninfected mice at any time point (Fig. 1b). Cytokine levels in the plasma were also used to measure the systemic immune response to L. sigmodontis. Th2-associated (IL-5, IL-13, IL-4 and IL-10), Th1-associated (TNF-α), and Th17-associated (IL-17) cytokines were assessed in the plasma of control (n=5) and infected (n=5) mice (Fig. 1c). The only cytokine that was detectable in plasma was IL-5, which showed significantly increased levels compared to uninfected controls at day 42 (p=0.016) and day 60 (p=0.008) of infection (Fig. 1c). These data illustrate that the systemic Th2 immune response elicited by L. sigmodontis peaks between weeks 5 and 6, just prior to the onset of microfilaremia that typically occurs between 7 and 8 weeks post-infection.
Given that the site of infection, the pleural cavity, is a relatively constrained immunological space and that the Th2 response systemically is heightened right before patency when microfilariae are present in the bloodstream, we sought to gain a more complete picture of the immune response in the pleural cavity in the pre-patent period. Thus, pooled pleural lavage fluid taken from infected (n=5) and uninfected (n=5) mice at day 36 and day 42 post-infection was concentrated and cytokine levels determined. Although little to no IL17 or TNF-α was detectable at either time point in both groups of mice, pleural lavage IL-4, IL-5, IL-10 and IL-13 levels, though unmeasurable in control mice, were substantially elevated at day 36 in infected mice (16.05 pg/ml IL-4, 143.71 pg/ml IL-5, 1.51 pg/ml IL-10 and 166.94 pg/ml IL-13) (Fig. 1d). By day 42, the concentration of cytokines in the pleural lavage of infected mice had increased from the previous week by 2.2-fold for IL-4 (34.7 pg/ml), 1.9-fold for IL-13 (319.75 pg/ml), and 18-fold for IL-10 (27.12 pg/ml) with IL-5 (218.35 pg/ml) being unchanged. Inexplicably, control mice had some IL-13 production at day 42 (117.2 pg/ml) (Fig. 1d).
3.2 ILC2s are increased in the pleural lavage of Ls-infected BALB/c mice
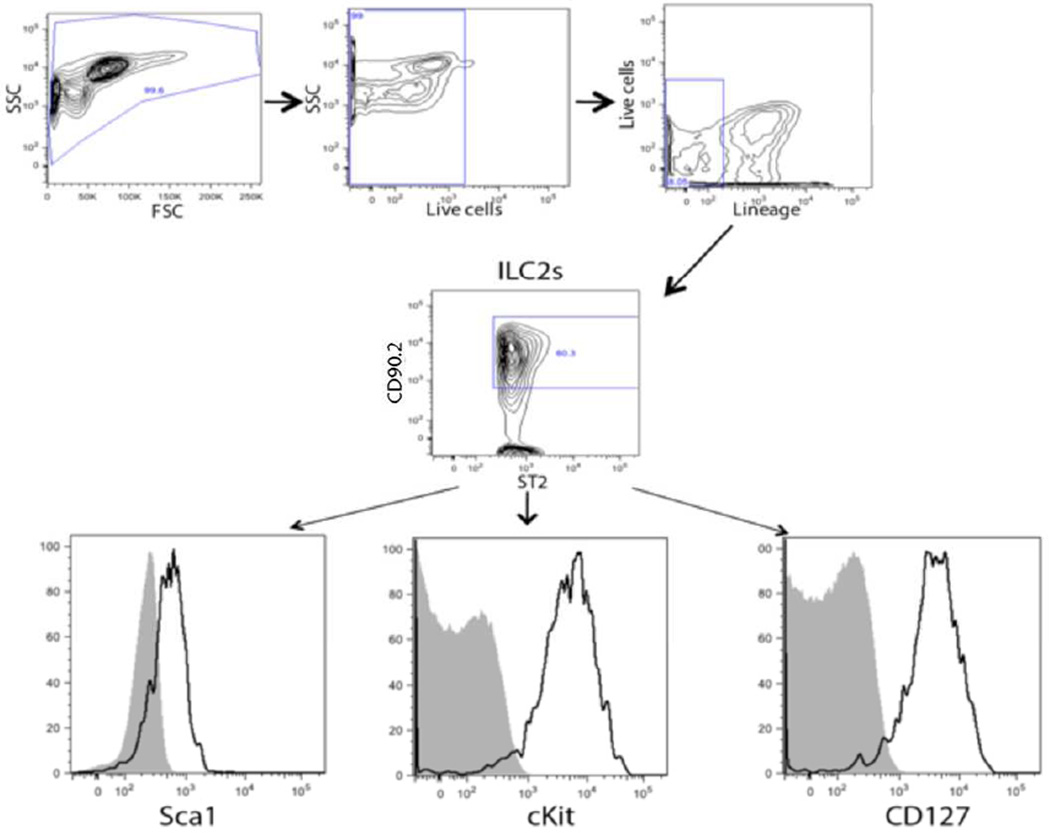
Given that L. sigmodontis infection induces a Th2 response both systemically and locally and the recent evidence indicating that ILC2s may play a significant role in initiating this response, we investigated the presence of ILC2s in the pleural cavity during L. sigmodontis infection. The presence of ILC2s in the cellular infiltrate obtained from the pleural space of infected mice was assessed using multi-color flow cytometry. ILC2s were defined as lineage−/ST2+/CD90.2+ cells. The ILC2s also expressed cKit, Sca1 and CD127 (Fig. 2).
Fig. 2.
ILC2s are present in the pleural cavity of mice at homeostasis and during L. sigmodontis infection. Representative flow cytometric plots from cells in a pleural lavage sample from a L. sigmodontis-infected mice showing the ILC2s (lineage−/ST2+/CD90/2+) that are also positive for cKit+, Sca1+ and CD127.
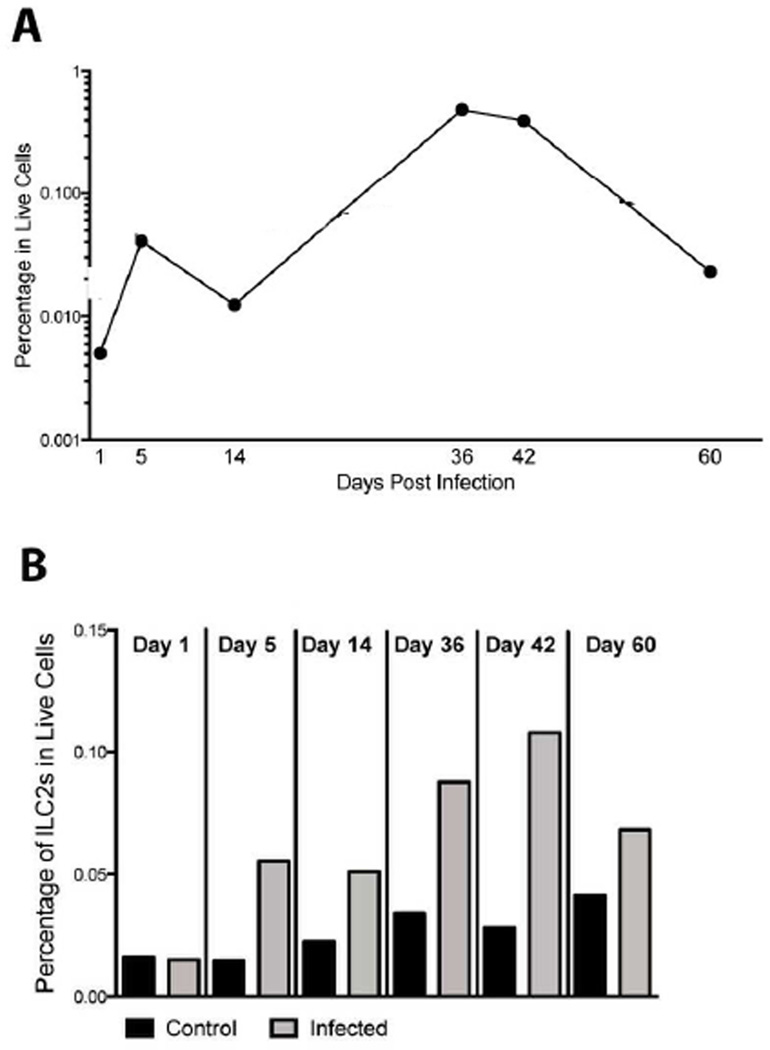
ILC2s were identified in the pleural cavity of control and infected mice. Figure 3a shows an overview of the frequencies of ILC2s in the pleural cavity of infected mice as a function of time post-infection. By day 5 post-infection ILC2s were increased by 3.7-fold compared to day 1 frequencies. This elevation compared to baseline was sustained throughout the course of infection; with peak frequencies at days 36 and 42 (Fig. 3a).
Fig. 3.
ILC2s are increased in the pleural lavage just prior to patency in L. sigmodontis infection. (A) ILC2s (lineage−/ST2+/CD90/2+) over time in L. sigmodontis-infected mice. (B) The frequency of ILC2s in control (black bar) and sigmodontis-infected (gray bar) mice by day postinfection.
Values for all figures are expressed as the geometric means either 2 experiments (d 5, 14, 60) or 3 experiments (d 42) for day 5, day 14, day 42 and day 60. Day 1 and day 36 values are the frequency of ILC2s in live cells from one experiment.
When the frequencies of ILC2s in infected mice were examined in parallel with the uninfected controls (Fig. 3b) the increase in ILC2s at the time points preceding patency (day 36 and 42) is readily apparent. The ILC2s are not expanded at day 1 post-infection in infected mice compared to control mice (Freq=0.015 infected, Freq=0.016 controls) but are increased in frequency by 3.7-fold on day 5 post-infection in the infected mice (GM=0.055) compared to control mice (GM=0.015) (Fig. 3b). As indicated in Fig. 3a, the increased frequency of ILC2s in the infected mice remains consistent through the first 6 weeks of infection with a 2.2-fold increase at day 14 (GM=0.051 infected, GM=0.023 control), a 2.6-fold increase at day 36 (Freq=0.088 infected, Freq=0.034 control), and a 3.9-fold increase at day 42 (GM=0.11 infected, GM=0.028 control). By day 60, the ILC2s are not significantly increased in the infected mice (1.7-fold, GM=0.068 infected and GM=0.041 control), suggesting a return to homeostasis as the infection begins to be cleared. ILC2s exhibit the greatest expansion of the population at day 42 in the infected mice. In addition, when the day 42 pleural lavage cells were stained intracellulary with IL-5, over 80% of the ILC2s were producing IL-5 (Fig. S1). Only 4.7% of the Lin+ (non-ILC) cells taken from the same sample of day 42 pleural lavage cells stained positive for IL-5, highlighting the importance of the ILC2 population in IL-5 production in L. sigmodontis infection.
3.3 ILC2s are not significantly increased systemically during L. sigmodontis infection
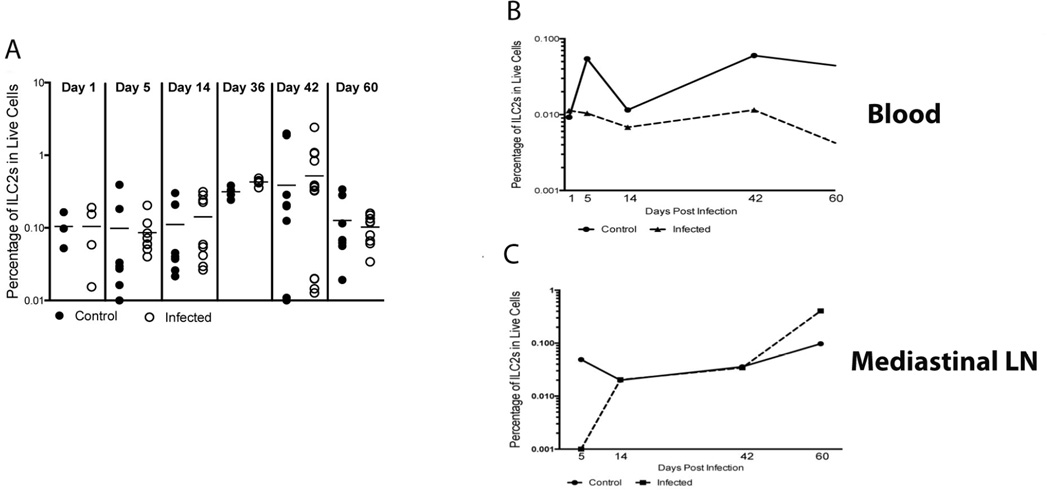
The type 2 immune response in L. sigmodontis infection is present both in the pleural cavity and systemically. Because ILC2s were present and increased during the course of infection in the pleural cavity, we assessed the effect of the parasite on ILC2s in secondary lymphoid organs. Little change was seen in the frequency of ILC2s in the Ls-infected compared to Ls-uninfected control spleens for day 1 (1.3-fold, GM=0.0717 infected, GM=0.0943 control), day 5 (1.9-fold, GM=0.0754 infected, GM=0.04 control), day 14 (1.4-fold, GM=0.0927 infected, GM=0.0688 control), day 36 (1.4-fold, GM=0.427 infected, GM=0.311 control), and day 60 (1.1-fold, GM=0.093 infected, GM=0.088 control) (Fig. 4a). However on day 42 there was a small increase in the ILC2 frequency in the Ls-infected spleens compared to the Ls-uninfected control (2.8-fold, GM=0.159 infected, GM=0.057 control) (Fig. 4a).
Fig. 4.
ILC2s are largely not increased systemically in L. sigmodontis infection. Cryopreserved spleen cells, non-red blood cells from pooled whole blood and pooled cells from the mediastinal lymph node were stained for ILC2s (lineage−/ST2+/CD90.2+). (A) The frequency in the live cell population of ILC2s in spleens of control (filled circles) and infected (open circles) mice. Horizontal lines indicate the geometric mean of the geometric means of the individual measurements. (B) The frequency in the live cell population of ILC2s in pooled samples of whole blood of control (solid line) and infected (dotted line) mice. Data are represented as the frequency values from one experiment for day 1 and day 36, and the geometric mean for either 2 experiments (d 5, 14, 60) or 3 experiments (d 42) for days 5, 14, 42 and 60. (C) The frequency in the live cell population of ILC2s in pooled samples of MLN for control (solid line) and infected (dotted line) mice. Data are represented as the frequency values for one experiment for day 1 and day 36, and the geometric mean of two experiments for either 2 experiments (d 5, 14, 60) or 3 experiments (d 42) for days 5, 14, 42 and 60. For all graphs, the x-axis indicates the day post-infection.
In addition to examining the spleen to determine the role of ILC2s in the systemic immune response, pooled cells from whole blood and the mediastinal lymph nodes were each assessed for the frequency of ILC2s (Fig. 4b–b). As seen in Fig. 4b, the frequency of ILC2s in Ls-infected mice was lower than the frequency in Ls-uninfected control mice beginning at day 5 (5.5-fold, GM=0.01 infected, GM=0.055 control) and continuing for the course of infection (day 14: 1.7-fold, GM=0.007 infected, GM=0.0112 control; day 42: 5.4-fold, GM=0.0112 infected, GM=0.06 control; day 60: 11-fold, GM=0.004 infected, GM=0.044 control) (Fig. 4b). This consistent decrease in the frequency of the ILC2s in the peripheral blood of infected animals compared to controls tracks with the increase of these cells seen in the pleural cavity on the same days, potentially signifying a migration of ILC2s from the peripheral blood to the pleural cavity.
Given that ILC2s are present in increased numbers in the draining lymph nodes of the site of infection in intestinal helminth infections [3, 8] we investigated the presence of ILC2s in mediastinal lymph nodes (MLN), which are thought to drain the pleural cavity (Fig. 4c). There was no difference seen in the frequency of ILC2s between the infected and control mice through the initial stages of infection (day 1, 5, and 14) and at the time of patency (day 42). By the time the mice began to clear the infection (day 60) the frequency of ILC2s was increased in the infected mice compared to the controls in the MLN (4.2-fold, GM=0.406 infected, GM=0.097 control). Taken together, the relative lack of expansion of ILC2s in MLNs and spleen suggests that although these cell populations likely play a role at the site of infection, their contribution to the systemic immune response in L. sigmodontis infection is limited.
3.4 Multiple cell types are also increased in the pleural cavity in the pre-patent period in L. sigmodontis infection
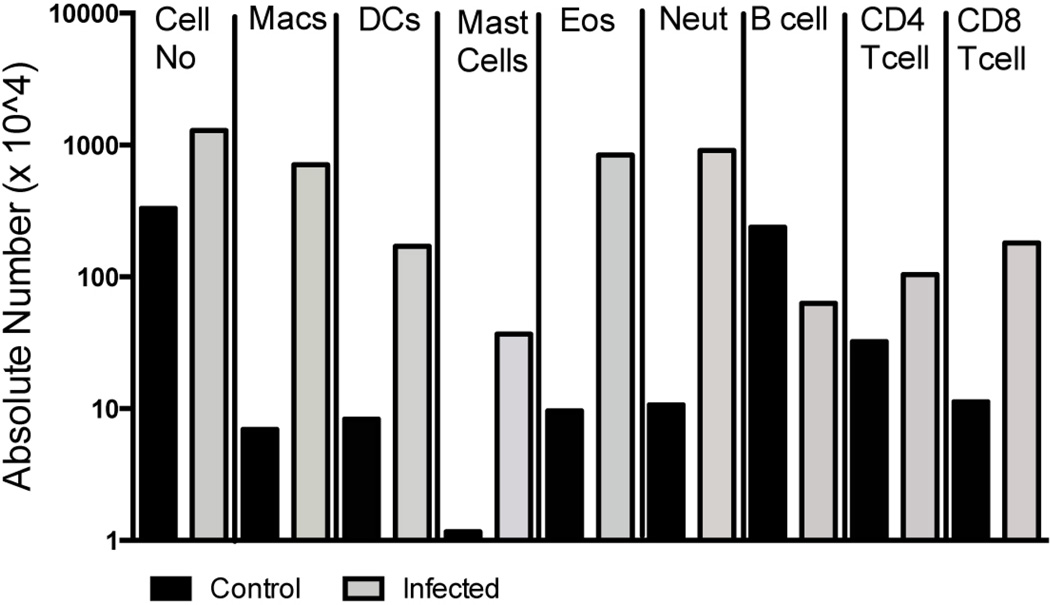
Since ILCs were expanded in the pleural cavity at the time of patency, we investigated what additional cell types were expanded in the pleural cavity during this time. Fixed and cryopreserved cells from pooled pleural lavage samples from the cellular infiltrates from day 36 and day 42 were characterized using multi-color flow cytometry (Fig. 5). By day 36 of the infection an overall increase (4-fold) in the total cell number in the pleural space was observed in the infected mice compared to the control mice (Fig. 5). This increase in cell number reflected an infection-related increase in the frequency of both myeloid-cells -- macrophages (102-fold), dendritic cells (20.4-fold), mast cells (31.6-fold), eosinophils (87.2-fold), neutrophils (85-fold) – and certain lymphoid cell lineages -- CD4+ T cells (3.3-fold) and CD8+ T cells (16-fold). Interestingly, in comparison to the other cells identified, the B cell population decreased by 3.8-fold (Fig. 5). At day 42, a similar pattern of myeloid and lymphoid cell expansion was seen though the magnitude of the differences between the Ls-infected and Ls-uninfected control mice was less pronounced (data not shown).
Fig. 5.
Multiple cell types are increased in the pleural cavity just prior to patency during L. sigmodontis infection. The absolute number of pooled cells from the pleural cavity of macrophages (F4/80+ and CD11b+), dendritic cells (CD11c+), mast cells (IgE+ and cKit+), eosinophils (SiglecF+ and CD45+), neutrophils (Gr1+), B cells (CD19+), and T cells (CD4+ or CD8+) at day 36 of L. simodontis-infected (gray bars) and -uninfected mice (black bars). Day 42 data was similar (data not shown).
3.5 IL-33 levels in the lungs are unaltered by L. sigmodontis infection
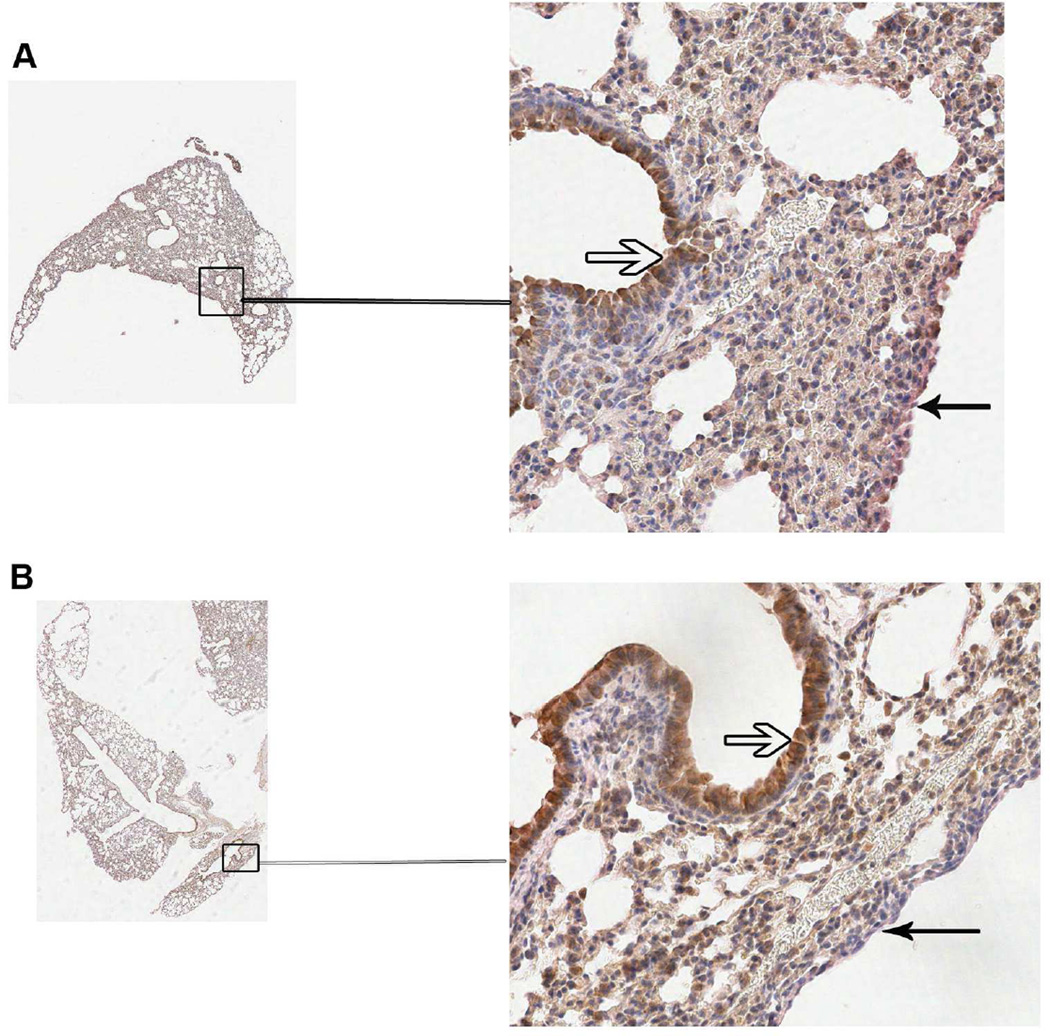
Because ILC2s are activated by IL-33 and IL-25 [9], and because IL-33 has recently been implicated in microfilarial clearance during L. sigmodontis infection [10], we examined the IL-33 expression in lung sections of L. sigmodontis -infected and -uninfected mice in the prepatent period (day 36 and day 42). Samples from 3 infected and 3 control mice from each time point were examined and Figure 6 shows IL-33 staining in a representative lung section of one control (Fig.6a) and one infected (Fig. 6b) mouse at from day 42. There was not an appreciable difference in the IL-33 staining between the infected and control mice either at day 42 (Fig. 6) or at day 36 (data not shown) for any of the lung sections examined. IL-33 staining was primarily found in the alveolar epithelium. IL-33 staining in the pleural epithelium was observed but was less prominent than that seen in the alveolar epithelium. These data suggest that IL-33 expression (at least in the lungs or pleural space) is not appreciably different between the infected mice and the control animals in the pre-patent period.
Fig. 6.
IL-33 expression in the lung is not altered by L. sigmodontis infection. Immunohistochemical staining for IL-33 in formalin-fixed lung tissue from 3 control and 3 L. sigmodontis-infected mice from day 36 and day 42 post-infection was performed. Pictured are representative sections from a control (A) and infected (B) lung at day 42. The open arrows denote bronchial epithelial cells and closed arrows indicate the lung pleural epithelium.
4. Discussion
The exact sequence of events that is responsible for the induction of a Th2 response in filarial infections is largely unknown. Early production of IL-4 from basophils was once hypothesized to potentially initiate Th2 immunity [11], but Th2 responses have subsequently been shown to occur despite basophil depletion in L. sigmodontis infection [7]. Alternatively activated macrophages (AAMs) have also been implicated as early influencers of a Th2 response in filarial infection [12]. However, AAMs are dependent on IL-4 for their differentiation and the initial source of IL-4, and other Th2-driving cytokines, has yet to be identified [13]. A new group of innate cells that produce large amounts of cytokines early in infection have been identified and taken on the name of innate lymphoid cells (ILCs)[2–4, 8]. Among these ILCs, those capable of producing Th2-type cytokines have recently been classified as ILC2s and are the focus of this study. [1]
Previous studies have shown a role for ILC2s in initiating the Th2 response to intestinal parasites [2–4, 8]. In this study, we evaluated the frequency and location of ILC2 cells during infection with a tissue-invasive nematode. We confirm that the filarial nematode L. sigmodontis causes a strong Th2 response in BALB/c mice, both locally in the pleural cavity and systemically (Fig 1). In parallel with the rise of the local Th2 response, we found that ILC2s are present in the pleural cavity and increase during the course of infection. Higher frequencies of ILC2s were detected in the pleural cavity as early as 5 days post-infection, and peaked in the pre-patent period (days 36 and 42, Figure 3). Subsequently, intracellular staining of pleural lavage cells indicates that the vast majority of ILC2s are making IL-5, a key cytokine for the initiation of the Th2 response. (Fig. S1) While the current state of flow cytometric antibodies prohibited the costaining of ST2 and IL-5 on these cells, the ILCs were positive for CD90.2 and CD127. ILC2s have been reported in secondary lymphoid organs such as the mesenteric lymph nodes and spleen [3, 8]. Although further investigation has confirmed the presence of multiple subsets of ILCs within the small intestine of mice [14], murine intestinal helminths have been shown to elicit robust responses from ILC2s systemically as evidenced by their expansion in lymph nodes and spleens of infected animals. In contrast to these findings, the involvement of ILC2s in L. sigmodontis infection seems confined to the site of infection, the pleural cavity. While an expansion of ILC2s was observed in the pleural space, there is little evidence of a systemic increase in frequency of ILC2s (Fig 4). ILC2s are not significantly increased in the spleens of infected mice and they decrease in whole blood over time in infected mice, leading to the conclusion that ILC2s act primarily at the site of infection in L. sigmodontis. This distinction between ILC2s in intestinal helminth infection and L. sigmodontis infection may be due to the unique nature of the site of infection. The role of ILC2s in the context of the pleural cavity may reflect a chemokine-mediated chemotaxis of innate cells and their activation as opposed to migration to a secondary lymphoid organ to activate cells there.
Eosinophils were also increased in the pleural cavity at day 36 and day 42 of infection and represented one of the primary cell types in the pleural lavage. Thus far, the exact mechanism of eosinophil recruitment in L. sigmodontis infection is unknown. Earlier studies reported ILC-like cells in the lungs of mice which responded to IL-25 and IL-33 by producing IL-5, which in turn regulated eosinophil recruitment [15]. Recently, ILC2s were implicated in maintaining tissue eosinophils through their production of IL-5 and the recruitment of eosinophils in the lung during type 2 inflammation via IL-13 production [16]. The sustained eosinophilia seen with L. sigmodontis infection could be driven by the IL-13 and IL-5 production by ILC2s since pleural lavage from control mice contained few eosinophils. Indeed, when the pleural lavage fluid was tested for cytokine levels, IL-5 production was seen only in infected mice and IL-13 was detected at increased levels in the infected mice (Fig. 1).
IL-33 is one of the cytokines known to activate ILC2s. The source of IL-33 during infection is pathogen dependent. The intestinal helminth Strongyloides venezuelensis migrates through the mouse lung on the way to the intestine. During that lung migration, Il-33 is produced by alveolar epithelial type II cells, which induces ILC2s to produce IL-5 and IL-13 [5]. Another study of ILC2s in the lung in response to influenza found that NKT cells, macrophages and dendritic cells were the primary sources of IL-33 in response to epithelial cell damage [17]. Macrophages, mast cells and dendritic cells isolated from the pleural lavage of L. sigmodontis infected mice all made IL-33 (data not shown) though no one cell type appeared to be the primary producer of IL-33 in response to the parasite. Additionally, when IL-33 production was assessed in lung tissues of control and infected mice at day 36 and day 42, there was no difference in the expression patterns (or staining intensity) of IL-33 between infected and control lungs. The lack of differential expression of IL-33 in the lungs during L. sigmodontis infection is not surprising given a recent study that illustrates an intact Th2 immune response in L. sigmodontis-infected mice lacking the IL-33R (ST2) [10]. Thus, although IL-33 plays a key role in Th2 initiation in other infections, the cells responsible for driving a localized (pleural space) Th2-dominated response in L. sigmodontis infection is unclear. Whether epithelial damage by the worm or secreted worm products, or both, are responsible for IL-33 induction that would lead to the expansion of ILC2s remains to be demonstrated. Additionally, it is possible that increased IL-33 production occurs earlier than the 36 day and 42 day time points evaluated in this study or that another innate cytokine such as TSLP or IL-25 is responsible for the induction of ILC2s. Further studies are required to determine the mechanisms responsible for induction of ILC2s in L. sigmodontis infection.
In addition to the amplification of the Th2 response seen around the time of patency, the beginnings of a regulatory response can also be observed. Total plasma IgG1 in infected mice is increased significantly compared to control mice at day 42. IgG1 is comparable to human IgG4, which while being a hallmark Th2 antibody, may also signify a regulatory response given that regulatory T cells have been implicated in the induction of IgG4 in filarial infection [18]. In addition to the production of murine IgG1, at day 42 there is also an increase in the IL-10 concentration in the pleural lavage of infected mice compared to control mice (Fig. 2). This regulatory response may limit the damage that would otherwise be inflicted by the robust Th2 response to L. sigmodontis infection.
In conclusion, L. sigmodontis infection induces a potent Th2 response both systemically and locally, which is amplified around the time of patency. While the ILC2 population does not appear to influence that response systemically, it is possible that it initiates and maintains the Th2 response at the site of infection in the pleural cavity. Further studies are needed to determine these cells’ roles in the eventual clearing of the infection and their interaction with the other immune cell lineages present in the pleural cavity. The primary source of IL-33 is yet to be identified, as are the molecules responsible for the worm’s ability to induce ILC2s. However, this report highlights the continued importance of ILCs in helminth infections and the processes leading to Th2-associated immune response.
Supplementary Material
ILC2s in the pleural lavage of L. sigmodontis infected mice produce IL-5. Pleural lavage cells collected day 42 post-infection were stained with live-dead stain, lineage cocktail, CD90.2, CD127 and IL-5. Shown is a representative flow diagram of the gating strategy (gates drawn in black around appropriate populations) for IL-5+ ILC2s. Pleural lavage cells were first gated on single cells and then gated on lymphocytes. ILC2s were defined as being CD90.2+, CD127+ and lin-. ILC2s were then gated based on positive intracellular IL-5 staining. Also shown is the IL-5 production for Lineage positive cells producing IL-5.
Highlights.
ILC2 expansion seen in the pleural space in Litomosoides sigmodontis infection
Little to no systemic expansion of ILC2s in response to infection
The site-specific ILC2 expansion paralleled the localized Th2 response
Acknowledgements
Alexis Boyd was a predoctoral student in the Microbiology and Immunology program of the Institute for Biomedical Sciences at the George Washington University. This work is from a dissertation that was presented in partial fulfillment of the requirements for the Ph.D. degree. The work was supported, in part, by the Division of Intramural Research (DIR), NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 2.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 3.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasuda K, Muto T, Kawagoe T, Matsumoto M, Sasaki Y, Matsushita K, et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci U S A. 2012;109:3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubner MP, Torrero MN, McCall JW, Mitre E. Litomosoides sigmodontis: a simple method to infect mice with L3 larvae obtained from the pleural space of recently infected jirds (Meriones unguiculatus) Exp Parasitol. 2009;123:95–98. doi: 10.1016/j.exppara.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torrero MN, Hubner MP, Larson D, Karasuyama H, Mitre E. Basophils amplify type 2 immune responses, but do not serve a protective role, during chronic infection of mice with the filarial nematode Litomosoides sigmodontis. J Immunol. 2010;185:7426–7434. doi: 10.4049/jimmunol.0903864. [DOI] [PubMed] [Google Scholar]
- 8.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li BW, Hendriks RW. Group 2 innate lymphoid cells in lung inflammation. Immunology. 2013;140:281–287. doi: 10.1111/imm.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ajendra J, Specht S, Neumann AL, Gondorf F, Schmidt D, Gentil K, et al. ST2 Deficiency Does Not Impair Type 2 Immune Responses during Chronic Filarial Infection but Leads to an Increased Microfilaremia Due to an Impaired Splenic Microfilarial Clearance. PLoS One. 2014;9:e93072. doi: 10.1371/journal.pone.0093072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitre E, Nutman TB. Basophils, basophilia and helminth infections. Chem Immunol Allergy. 2006;90:141–156. doi: 10.1159/000088886. [DOI] [PubMed] [Google Scholar]
- 12.Loke P, MacDonald AS, Allen JE. Antigen-presenting cells recruited by Brugia malayi induce Th2 differentiation of naive CD4(+) T cells. Eur J Immunol. 2000;30:1127–1135. doi: 10.1002/(SICI)1521-4141(200004)30:4<1127::AID-IMMU1127>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tait Wojno ED, Artis D. Innate lymphoid cells: balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe. 2012;12:445–457. doi: 10.1016/j.chom.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol. 2012;188:703–713. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- 16.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013 doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorski SA, Hahn YS, Braciale TJ. Group 2 Innate Lymphoid Cell Production of IL-5 Is Regulated by NKT Cells during Influenza Virus Infection. PLoS Pathog. 2013;9:e1003615. doi: 10.1371/journal.ppat.1003615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adjobimey T, Hoerauf A. Induction of immunoglobulin G4 in human filariasis: an indicator of immunoregulation. Ann Trop Med Parasitol. 2010;104:455–464. doi: 10.1179/136485910X12786389891407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris CP, Evans H, Larsen SE, Mitre E. A comprehensive, model-based review of vaccine and repeat infection trials for filariasis. Clin Microbiol Rev. 2013;26:381–421. doi: 10.1128/CMR.00002-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ILC2s in the pleural lavage of L. sigmodontis infected mice produce IL-5. Pleural lavage cells collected day 42 post-infection were stained with live-dead stain, lineage cocktail, CD90.2, CD127 and IL-5. Shown is a representative flow diagram of the gating strategy (gates drawn in black around appropriate populations) for IL-5+ ILC2s. Pleural lavage cells were first gated on single cells and then gated on lymphocytes. ILC2s were defined as being CD90.2+, CD127+ and lin-. ILC2s were then gated based on positive intracellular IL-5 staining. Also shown is the IL-5 production for Lineage positive cells producing IL-5.