Abstract
Alcohol use disorder represents a significant human health problem that leads to substantial loss of human life and financial cost to society. Currently available treatment options do not adequately address this human health problem, and thus, additional therapies are desperately needed. The endocannabinoid system has been shown, using animal models, to modulate ethanol-motivated behavior, and it has also been demonstrated that chronic ethanol exposure can have potentially long-lasting effects on the endocannabinoid system. For example, chronic exposure to ethanol, in either cell culture or preclinical rodent models, causes an increase in endocannabinoid levels that results in down-regulation of the cannabinoid receptor 1 (CB1) and uncoupling of this receptor from downstream G protein signaling pathways. Using positron emission tomography (PET), similar down-regulation of CB1 has been noted in multiple regions of the brain in human alcoholic patients. In rodents, treatment with the CB1 inverse agonist SR141716A (Rimonabant), or genetic deletion of CB1 leads to a reduction in voluntary ethanol drinking, ethanol-stimulated dopamine release in the nucleus accumbens, operant self-administration of ethanol, sensitization to the locomotor effects of ethanol, and reinstatement/relapse of ethanol-motivated behavior. Although the clinical utility of Rimonabant or other antagonists/inverse agonists for CB1 is limited due to negative neuropsychiatric side effects, negative allosteric modulators of CB1 and inhibitors of endocannabinoid catabolism represent therapeutic targets worthy of additional examination.
Keywords: ethanol, cannabinoid, alcohol, endocannabinoid, CB1
1. Introduction
Alcohol dependence is a highly prevalent disorder, which affects an estimated eight million Americans and inflicts a tremendous cost (in excess of $223.5 billion annually) to society (Grant et al., 2004; Bouchery et al., 2011). Pharmaceutical treatments for alcohol dependence include the use of the opioid receptor antagonist naltrexone, and the broadly-acting drug, acamprosate. While naltrexone has been shown clinically to reduce alcohol intake and relapse (Latt et al., 2002; Kranzler et al., 2004; Foa et al., 2013), acamprosate appears to be more effective, compared to placebo, at increasing the percent of abstinent days (Mason et al., 2006). A recent meta-analysis of the efficacy of both naltrexone and acamprosate for alcohol treatment demonstrated that these effects, though significant, are modest. Only 12-19% of individuals treated with naltrexone and 7-13% treated with acamprosate had better outcomes compared to those treated with placebo (Kranzler and Van Kirk, 2001; Grant et al., 2004; Bouchery et al., 2011). These studies illustrate the limitations of currently available pharmacotherapies and emphasize the need to expand therapeutic options for treating alcohol dependence.
Here we review the most current evidence demonstrating that ethanol can modulate endocannabinoid system (ECS) signaling in preclinical rodent models. Our review complements and builds on other excellent recent reviews on the interatctions between the ECS and alcohol (Pava and Woodward, 2012). Preclinical rodent models have been used to show that genetic and pharmacological inhibition of ECS signaling can profoundly reduce voluntary ethanol consumption, reward for ethanol, as well as reinstatement and relapse of ethanol-motivated behaviors. Understanding the precise mechanisms through which alcohol influences the ECS and vice versa has the potential to positively impact treatment of alcohol use disorder. Recent preclinical work has investigated the therapeutic potential of drugs that manipulate endocannaboid levels directly (Ramesh et al., 2013), or act as allosteric modulators of the cannabinoid receptor 1 (CB1) (Gamage et al., 2014; Jing et al., 2014). Both approaches are capable of providing therapeutic benefits through modulation of the ECS while avoiding possible side effects associated with direct CB1 inverse agonism.
2. Endocannabinoid Signaling System
The ECS consists of three main components: the endogenous ligands (endocannabinoids), the cannabinoid receptors, and the enzymes that are responsible for synthesis and catabolism of endocannabinoids. Two cannabinoid receptors have been cloned and characterized (Matsuda et al., 1990; Munro et al., 1993). CB1 is predominately presynaptic (Katona et al., 1999) and is expressed widely throughout the nervous system (Devane et al., 1988; Herkenham et al., 1990; Matsuda et al., 1990; Herkenham et al., 1991; Mailleux and Vanderhaeghen, 1992; Tsou et al., 1998). CB1 is responsible for mediating the psychoactive effects of delta-9-tetrahydrocannabinol (△9-THC), the principal psychoactive cannabinoid in the cannabis plant (Ledent et al., 1999; Monory et al., 2007). Within the nervous system, CB1 is involved in modulation of a diverse range of physiological functions including pain (Lichtman and Martin, 1997; Ledent et al., 1999), synaptic plasticity related to learning and memory (Kreitzer and Regehr, 2001b; Wilson et al., 2001; Marsicano et al., 2002), reward signaling (Hungund et al., 2003; Riegel and Lupica, 2004), and mood (Martin et al., 2002; Hill and Gorzalka, 2005). CB1 is also present in peripheral “non-neuronal” tissues including liver, adipose tissue, and pancreas, where it has a prominent role in metabolism (Cota et al., 2003; Ravinet Trillou et al., 2004; Osei-Hyiaman et al., 2005). The other cannabinoid receptor, CB2, was first cloned from a human promelocytic leukemia cell line (HL60) and is most abundant in immune cells (Munro et al., 1993), although low neuronal CB2 expression has been reported in the brain (Van Sickle et al., 2005). CB1 is a G protein-coupled receptor that is typically coupled to Gαi/o proteins. Agonist activation has been shown to lead to stimulation of MAPK (Bouaboula et al., 1995) and G protein-coupled inward rectifying potassium channels (GIRKs) (Mackie et al., 1995) as well as inhibition of adenylyl cyclase (Howlett and Fleming, 1984; Howlett, 1985; Howlett et al., 1986) and voltage-gated calcium channels (VGCCs) (Mackie and Hille, 1992; Mackie et al., 1993).
Endocannabinoid production is post-synaptic and occurs in response to increased levels of intracellular calcium and/or excitatory post-synaptic potential (EPSP)-induced depolarization of the plasma membrane (Maejima et al., 2001; Maejima et al., 2005). Endocannabinoids diffuse in a retrograde manner across the synapse where they act at pre-synaptic CB1 receptors to suppress neurotransmission (Kreitzer and Regehr, 2001a; Maejima et al., 2001; Wilson et al., 2001). Two main endocannabinoids have been identified, N-arachidonoylethanolamine (AEA; anandamide) (Devane et al., 1992) and 2-arachidonoyl-glycerol (2-AG) (Sugiura et al., 1995; Stella et al., 1997). However, three other putative endocannabinoid ligands, N-arachidonoyl dopamine, (NADA) (Huang et al., 2002), O-arachidonoyl ethanolamine (virodhamine) (Porter et al., 2002), and 2-arachidonoyl glyceryl ether (noladin ether), (Hanus et al., 2001) have also been identified.
Endocannabinoids are synthesized on demand from plasma membrane phospholipids (Piomelli, 2003). The production of AEA from phosphatidylethanolamine (PE) can occur via multiple synthetic pathways. However, one synthetic pathway that is particularly relevant to the effect of ethanol on the ECS involves phospholipase A2 (PLA2) conversion of N-arachidonoyl phosphatidylethanolamine (NAPE) to Lyso-NAPE, which is then cleaved, by lyso-phospholipase D, to AEA (Sun et al., 2004). The majority of 2-AG is produced via a two-step process involving sequential action of phospholipase C (PLC) to produce diacylglycerol (DAG) from phosphatidylinositol 4,5-bisphosphate (PIP2), followed by the action of sn-1-diaglyerol lipase alpha and beta (DAGL α/β) to convert DAG to 2-AG (Bisogno et al., 2003). Signaling by these modulatory lipids is terminated by their breakdown. Hydrolysis of AEA is catalyzed by fatty-acid amide hydrolase (FAAH) (Cravatt et al., 1996) while 2-AG is hydrolyzed by either monoacylglycerol lipase (MAGL; 85% of 2-AG breakdown) (Dinh et al., 2002) or the alpha/beta hydrolase domain (ABHD)-containing proteins ABHD6 and ABHD12 (Blankman et al., 2007; Marrs et al., 2010).
3. Voluntary ethanol drinking
Using the two bottle choice assay, CB1 knock-out (KO) mice have been shown to exhibit decreased voluntary drinking of ethanol. Consumption of 10% ethanol was decreased in CB1 KO mice given six or eight hours of limited access to ethanol per day (Poncelet et al., 2003; Thanos et al., 2005). Under continuous access conditions, ethanol consumption was decreased for multiple ethanol concentrations in both male and female CB1 KO mice compared to wild-type controls (Hungund et al., 2003; Naassila et al., 2004; Vinod et al., 2008b). However, the inhibitory effect of CB1 deletion on ethanol intake was more profound in female mice, which generally show higher overall basal ethanol consumption than their male counterparts (Hungund et al., 2003). Additionally, age-related decreases in ethanol drinking were shown to be absent in CB1 KO mice, suggesting that the decrease in ethanol consumption that occurs during aging is mediated by endocannabinoid signaling (Wang et al., 2003).
Voluntary ethanol intake is attenuated in rodents following treatment with CB1 inverse agonists, including SR141716A (Rimonabant). Treatment with SR141716A decreased ethanol drinking and sensitization to locomotor effects of ethanol (Marinho et al., 2015) in ethanol-preferring C57Bl6 mice as well as in non-preferring DBA/2J mice (Arnone et al., 1997; Vinod et al., 2008b). In Sardinian ethanol-preferring rats selected for high ethanol consumption (sP), systemic SR141716A treatment decreased voluntary consumption of 10% ethanol under limited and continuous access conditions (Colombo et al., 1998; Serra et al., 2001). Likewise, treatment with the CB1 inverse agonists AM251 and SR147778 reduced ethanol consumption in both Fawn Hooded (Femenia et al., 2010) and Wistar (Lallemand and De Witte, 2006) rats chronically exposed to ethanol vapor. These results demonstrate that disruption of CB1 signaling in mice and rats causes a robust and highly reproducible reduction in voluntary ethanol consumption.
The effect of CB1 agonists on voluntary drinking of ethanol has been examined but is generally less understood than the effect of CB1 deletion or pharmacological blockade on consumption of ethanol. Systemic administration of a CB1 full agonist (WIN 55,212-2), enhanced ethanol consumption in C57Bl6 mice subjected to the drinking in the dark (DID) model of binge-like ethanol drinking (Linsenbardt and Boehm, 2009). Systemic injection of WIN 55,212-2 or CP 55,940, another full agonist of CB1, has been shown to elicit a dose-dependent increase in voluntary ethanol drinking in sP rats given continuous access to 10% ethanol (Colombo et al., 2002). The stimulatory effects of systemic WIN 55,212-2 and CP 55,940 on voluntary ethanol drinking were blocked by pretreatment with SR141716A, providing evidence that these effects are CB1-mediated (Colombo et al., 2002).
The effect of endocannabinoid levels on voluntary ethanol drinking has been examined using both FAAH KO mice and mice treated with the FAAH inhibitor, URB597. Mice lacking FAAH, which modulates catabolism of the endocannabinoid AEA, exhibit increased voluntary consumption and preference for ethanol across a wide range of concentrations (Basavarajappa et al., 2006; Blednov et al., 2007; Vinod et al., 2008a). Systemic injection of URB597 in wild-type mice found a male-specific increase in ethanol consumption and preference (Blednov et al., 2007). It has also been reported that systemic administration of URB597 in wild-type male C57Bl6 mice increased preference for 12% ethanol (Vinod et al., 2008a). The possibility that sex-specific differences in ethanol drinking behavior might be driven through the ECS is an interesting question in need of further examination. A summary of the effects of pharmacological and genetic manipulation of CB1 and endocannabinoid levels is shown in Table 1.
Table 1.
Voluntary Ethanol Drinking
| Species | Strain or Phenotype |
Gender | Pharmacological compound |
Alcohol drinking paradigm |
Alcohol Consumption |
References |
|---|---|---|---|---|---|---|
| Mice | CB1 −/− | Male | - | Limited Access |
⬇ | Poncelet et al., 2003 |
| Mice | CB1 −/− | Male | - | Limited Access |
⬇ | Thanos et al., 2005 |
| Mice | CB1 −/− | Male and Female |
- | Continuous Access |
⬇ | Hungund et al., 2003 |
| Mice | CB1 −/− | Male and female |
- | Continuous Access |
⬇ | Naassila et al., 2004 |
| Mice | CB1 −/− | Male | - | Continuous Access |
⬇ | Wang et al., 2003 |
| Mice | C57Bl6 | Male | SR141716A | Limited Access |
⬇ | Arnone et al., 1997 |
| Mice | C57Bl6 | Male | WIN 55,212-2 | Drinking in the Dark |
⬇ | Linsenbardt & Boehm, 2009 |
| Mice | FAAH −/− | Female | - | Continuous Access |
⬇ | Basavarajappa et al., 2006 |
| Mice | FAAH −/− | Male | - | Continuous Access |
⬇ | Vinod et al., 2008a |
| Mice | FAAH −/− | Male | - | Continuous Access |
⬇ | Blednov et al., 2007 |
| Mice | FAAH −/− | Female | - | Continuous Access |
⬇ | Blednov et al., 2007 |
| Mice | C57Bl6 | Male | URB597 | Continuous Access |
⬇ | Blednov et al., 2007 |
| Mice | C57Bl6 | Male | URB597 | Continuous Access |
⬇ | Vinod et al., 2008a |
| Rat | Sardinian ethanol- preferring |
Male | SR141716A | Limited Access |
⬇ | Colombo et al., 1998 |
| Rat | Sardinian ethanol- preferring |
Male | SR141716A | Continuous Access |
⬇ | Serra et al., 2001 |
| Rat | Fawn Hooded | Male | AM251 | Continuous Access |
⬇ | Femenia et al., 2010 |
| Rat | Wistar | Male | SR147778 | Chronic Ethanol Vapor |
⬇ | Lallemand & De Witte, 2006 |
| Rat | Sardinian ethanol- preferring |
Male | WIN 55,212-2 or CP 55,940 |
Continuous Access |
⬇ | Colombo et al., 2002 |
| Rat | Sardinian ethanol- preferring |
Male | Pre-Treatment with SR141716A blocked WIN 55,212-2 or CP 55,940 stimulatory effect |
Continuous Access |
No ▲ | Colombo et al., 2002 |
4. CB1 Involvement in Self-Administration
In addition to modulating voluntary ethanol consumption, CB1 is involved in the “motivation” of rodents to work for oral ethanol access using operant self-administration (SA) procedures. Ethanol SA is frequently assessed using a fixed-ratio (FR) schedule of reinforcement in which a specific number of operant responses (~1-4) allows access to a small volume (~0.1 mL) of ethanol solution. Systemic pretreatment with, or microinjection directly into the nucleus accumbens (Caille et al., 2007) of the CB1 inverse agonist SR141716A (Cippitelli et al., 2005; Economidou et al., 2006; Cippitelli et al., 2007; Cippitelli et al., 2008) attenuated ethanol SA using a FR schedule in Wistar rats with no inherent alcohol preference. In this study, however, it should be noted that the SR141716A doses used are non-selective for ethanol-motivated behavior and also decreased SA of sucrose and saccharin-containing solutions (Cippitelli et al., 2005; Economidou et al., 2006). SR141716A treatment did not attenuate SA of sodium chloride or standard rat chow in sodium-depleted (Economidou et al., 2006) and food-restricted (Rodriguez de Fonseca et al., 1999) rats. This suggests that CB1 mediates goal-directed behaviors with high hedonic impact (i.e., ethanol or sucrose) versus need-based behaviors that are required for essential homeostatic physiology.
Progressive ratio (PR) schedules of reinforcement, as opposed to FR schedules, are used to determine the “break point” or upper limit to the amount of “work” a subject is willing to do to obtain reward/reinforcement. SR141716A pretreatment decreased the response break points for both ethanol and sucrose in Wistar rats (Economidou et al., 2006). The selective CB1 neutral antagonist, SLV330, decreased the nose-poke operant response for 12% ethanol in Wistar rats (de Bruin et al., 2011). One pitfall of FR and PR schedules is that it is difficult to dissociate an operant response for drug from the consumption component of SA. To avoid this confounder, Freedland and colleagues (Freedland et al., 2001) used a sipper-tube model (Samson et al., 1999) with a single response requirement (i.e., 16 lever presses) prior to gaining 20 minutes of unrestricted access to ethanol. Under these conditions, SR141716A reduced both consumption and operant responding for 10% ethanol (Freedland et al., 2001).
The willingness of animals to ‘work’ for calorically rich rewards, such as sucrose, highlights the need, in SA paradigms, for controls that account for the both the taste and caloric content of ethanol. One interesting approach, developed by Gallate and McGregor to address this issue, involved SA of beer containing 4% ethanol or isocaloric ‘near’-beer containing <0.5% ethanol. Using this paradigm, they found that low dose SR141716A (0.3 mg/kg) selectively decreased the response break point for beer under a PR schedule of reinforcement while higher doses of SR141716A (0.6 to 3.0 mg/kg) decreased motivation for near-beer as well (Gallate and McGregor, 1999; Gallate et al., 2004).
FR, PR, and sipper-tube models of reinforcement have been used to show that pharmacological inhibition of CB1 can decrease ethanol SA in rats that are not ethanol-dependent (Gallate and McGregor, 1999; Rodriguez de Fonseca et al., 1999; Freedland et al., 2001; Gallate et al., 2004; Cippitelli et al., 2005; Economidou et al., 2006; Cippitelli et al., 2007; Cippitelli et al., 2008; de Bruin et al., 2011). Likewise, systemic treatment with the CB1 inverse agonist SR141716A selectively decreased ethanol SA in the following rat models: 1) Alko Alcohol (AA) rats (Malinen and Hyytia, 2008) bred selectively for high ethanol intake and preference; 2), P rats selected for high ethanol intake (Getachew et al., 2011), and 3) sP rats selected for high ethanol intake (Colombo et al., 2004)). Microinjection of SR141716A into the nucleus accumbens or ventral tegmental area (VTA) also decreased ethanol SA in AA rats, suggesting that cannabinoid signaling in these brain regions can modulate ethanol consumption (Malinen and Hyytia, 2008).
Extinction testing provides another measure to assess the motivation of rodents to obtain reward/reinforcement (Gallate and McGregor, 1999; Hansson et al., 2007; Millan et al., 2011). During an extinction session, continued operant responding for a reinforcer does not result in the delivery of reward (i.e., ethanol or sucrose). The continued responding (termed extinction responding) by the animal is thought to be an indication of the motivation (or craving) to seek that reinforcer. Pretreatment of sP rats with the inverse agonist SR141716A (Colombo et al., 2004) or SR147778 (Gessa et al., 2005) resulted in decreased extinction responding for an ethanol reward. However, the effect of SR141716A was nonselective for ethanol as this drug also attenuated extinction responding for sucrose, saccharin, and ‘near’-beer. One study that was able to dissociate operant ethanol-seeking behavior from ethanol consumption suggested that lower doses of SR141716A might selectively attenuate the motivation to seek ethanol (Freedland et al., 2001).
The effect of CB1 agonists on ethanol SA is less well understood since CB1 agonists often depress locomotor activity, which can make the experiments difficult to perform and can be a confounder for data interpretation. Systemic administration of the CB1 full agonist CP 55,940 to Wistar rats with no inherent preference for alcohol increased the response break point for beer, ‘near’-beer, sucrose (8.4%), and lite beer (2.7% ethanol) (Gallate et al., 1999). Increased SA of ethanol was also found in male alcohol-preferring AA rats following systemic administration of a different cannabinoid receptor full agonist, WIN 55,212-2 (Malinen and Hyytia, 2008). However, in both of those studies, the highest dose of agonist failed to increase ethanol SA, possibly as a consequence of the confounding condition of decreased locomotor activity in animals treated with these agonists.
In Wistar rats, manipulation of endocannabinoids through inhibition of the catabolic enzyme FAAH, using URB597, did not alter the response break point for ethanol delivery (Cippitelli et al., 2008). However, microinjection of URB597 directly into the prefrontal cortex (PFC) increased ethanol SA under a FR schedule (Hansson et al., 2007). Since AEA is only a partial CB1 agonist, it is possible that elevation of AEA in the brain does not fully mimic treatment with full agonists. An alternate approach to investigate the effects of manipulation of endocannabinoid levels on the response break point would be to inhibit MAGL, the catabolic enzyme that hydrolyzes the endocannabinoid 2-AG, using low doses of the MAGL inhibitor JZL-184 that do not adversely affect motor activity. It will be interesting to see whether enhancement of 2-AG levels in this manner can potentiate ethanol SA. Table 2 illustrates the impact of the ECS on operant ethanol self-administration behaviors.
Table 2.
Ethanol Self-Administration
| Species | Strain or Phenotype |
Gender | Pharmacological compound |
Self- Administration Schedules |
Alcohol Self- Administration |
References |
|---|---|---|---|---|---|---|
| Rat | Wistar | Male | SR141716A | Fixed-ratio | ⬇ | Cippitelli et al., 2005;2007;2008 |
| Rat | Wistar | Male | SR141716A | Fixed-ratio | ⬇ | Economidou et al., 2006 |
| Rat | Wistar | Male | SR141716A | Fixed-ratio | ⬇ | Caille et al., 2007 |
| Rat | Wistar | Male | SR141716A | Fixed and progressive-ratio |
⬇ | Economidou et al., 2006 |
| Rat | Wistar | Male | SLV330 | Fixed-ratio | ⬇ | de Bruin et al., 2011 |
| Rat | Long-Evans | Male | SR141716A | Fixed-ratio second order schedule |
⬇ | Freedland et al., 2001 |
| Rat | Wistar | Male | SR141716A | Progressive-ratio | ⬇ | Gallate & McGregor, 1999 |
| Rat | Wistar | Male | SR141716A | Progressive-ratio | ⬇ | Gallate et al., 2004 |
| Rat | Alko Alcohol (AA) |
Male | SR141716A | Fixed-ratio | ⬇ | Malinen & Hyytia, 2008 |
| Rat | P | Male | SR141716A | Fixed-ratio reinstatement |
⬇ | Getachew et al., 2011 |
| Rat | sP | Male | SR141716A | Fixed-ratio extinction responding |
⬇ | Colombo et al., 2004 |
| Rat | Wistar | Male | CP 55,940 | Progressive-ratio | ⬇ | Gallate et al., 1999 |
| Rat | Alko Alcohol (AA) |
Male | WIN 55,212-2 | Fixed-ratio | ⬇ | Malinen & Hyytia, 2008 |
| Rat | Wistar | Male | URB597 | Progressive-ratio | No▲ | Cippitelli et al., 2008 |
| Rat | Wistar | Male | URB597 into PFC (prefrontal cortex) |
Progressive-ratio | ⬇ | Hansson et al., 2007 |
5. Cannabinoid Involvement in Ethanol Withdrawal
For alcohol-dependent individuals, sudden termination of excessive alcohol intake can elicit the onset of alcohol withdrawal, characterized by anxiety, insomnia, irritability, and the less common, but potentially fatal conditions of delirium tremens (DTs) and tonic-clonic seizures. Rodents generally do not readily consume sufficient amounts of ethanol to consistently produce measurable withdrawal symptoms upon forced abstinence. Therefore, rodent models of ethanol dependence typically involve involuntary ethanol consumption via an ethanol liquid diet, ethanol vapor inhalation, and/or intragastric ethanol administration. When such models are used, the severity of ethanol withdrawal is assessed through quantification of handling-induced convulsions (HICs) (Becker, 2000).
For two strains of wild-type mice (C57Bl6 and DBA/2J), daily administration of a CB1 inverse agonist (SR141716A) decreased the severity of ethanol withdrawal in mice chronically exposed to ethanol vapor inhalation (Vinod et al., 2008b). It has been reported that CB1 deletion increases the severity of ethanol withdrawal (Naassila et al., 2004), however, there are also reports that CB1 deletion decreases or eliminates ethanol withdrawal as well (Racz et al., 2003; Vinod et al., 2008b). Factors including genetic background, paradigm employed, and time point when withdrawal testing was conducted can influence the results of this type of ethanol withdrawal study and likely contribute to the discrepancies between the reported findings. Reduction of endocannabinoid catabolism in FAAH KO mice resulted in decreased severity of ethanol withdrawal (Vinod et al., 2008a). To our knowledge, the effect of CB1 agonists on the severity of ethanol withdrawal has not been reported. However, this question is important given the recent legalization of recreational marijuana and the high rate of comorbidity of marijuana usage among alcoholics.
6. CB1 Involvement in Ethanol Relapse Drinking and Reinstatement
One of the biggest challenges facing alcoholics is the risk of relapse, characterized by a return to heavy drinking following a period of abstinence. Relapse is a significant problem in treating alcohol abuse and can be triggered by several factors, including stress, alcohol-associated cues and contextual environments. Reinstatement of operant ethanol-seeking behavior caused by exposure to stress, ethanol-priming injection, or ethanol-associated cues is used to assess relapse in rodents. A return to heavy drinking is often modeled in rodents by measuring the magnitude of the alcohol deprivation effect (ADE), a transient increase in ethanol intake or ethanol SA following a period of forced abstinence in animals previously given long-term access to ethanol (Sinclair and Senter, 1968).
There are few reports investigating the effect of CB1 deletion on reinstatement of operant ethanol-seeking behavior in mice given access to ethanol after a period of abstinence. Exposure to mild, intermittent foot-shock stress was reported to increase preference for ethanol and also increased alcohol intake in wild-type mice but not CB1 KO mice (Racz et al., 2003), suggesting that deletion of CB1 may inhibit stress-induced relapse of ethanol drinking. Pretreatment with a CB1 inverse agonist (SR141716A), or a neutral CB1 antagonist (SLV330), attenuated cue-induced reinstatement of ethanol-seeking behavior in Wistar rats (Cippitelli et al., 2005; Economidou et al., 2006). Interestingly, SR141716A pretreatment did not suppress stress-induced ethanol reinstatement (Economidou et al., 2006).
In rats genetically selected for high ethanol consumption, pretreatment with CB1 inverse agonists blocked relapse. Both SR141716A (Serra et al., 2002; Vinod et al., 2012) and SR147778 (Gessa et al., 2005) were able to suppress the ADE following reinstatement of access to ethanol. Similarly, pretreatment with SR141716A blunted cue-induced reinstatement of ethanol-seeking behavior in ethanol-preferring msP rats (Cippitelli et al., 2005). In female alcohol-preferring (P) rats, pretreatment with SR141716A attenuated Pavlovian Spontaneous Recovery, which is, after a delay, the re-emergence of a conditioned response that had previously become extinct (Getachew et al., 2011). Although CB1 antagonists attenuate relapse and reinstatement of ethanol-seeking behavior, the effects are non-selective for ethanol at higher doses.
The ability of cannabinoid agonists to potentiate relapse and reinstatement of ethanol-seeking behavior has also been examined. Treatment of Wistar rats with a CB1 full agonist (WIN 55,212-2) (Alen et al., 2008) during ethanol deprivation resulted in long-lasting potentiation of the operant response to obtain ethanol reward when ethanol access was restored. Relapse of ethanol SA using both a FR and PR schedule was potentiated by WIN 55,212-2 across multiple deprivation periods. This potentiation of ethanol SA using a FR schedule was blocked by the D2 receptor antagonist raclopride, indicating the involvement of dopaminergic signaling in this process (Alen et al., 2008). Pretreatment of Wistar rats with △9-THC potentiated reinstatement of operant responding for both beer and near-beer, indicating that the effect of △9-THC on reinstatement of ethanol-seeking behavior was not completely selective for ethanol. Administration of △9-THC also resulted in reinstatement of operant responding for 10.4% sucrose, providing additional evidence that these effects of △9-THC are not selective for ethanol (McGregor et al., 2005).
7. Ethanol-stimulated dopamine release
Mice lacking CB1 do not show ethanol-stimulated dopamine release in the nucleus accumbens (NAc) (Hungund et al., 2003). CB1 KO mice exhibit increased expression of dopamine D2 receptors in the striatum, suggesting that dopaminergic reward signaling is impacted by alterations in endocannabinoid signaling (Houchi et al., 2005). Pharmacological blockade of CB1 using SR141716A prevents ethanol-stimulated dopamine release in the NAc of C57Bl6 wild-type but not CB1 KO mice (Hungund et al., 2003). Treatment with AM251, another CB1 inverse agonist, blocked ethanol-stimulated dopamine release in the NAc of ethanol-preferring Fawn Hooded rats (Femenia et al., 2010). Systemic treatment with SR141716A blocked sub-second dopamine increases in the NAc of Wistar rats subjected to in vivo voltammetry (Cheer et al., 2007). Interestingly, 2-AG-mediated synaptic plasticity (depolarization-induced suppression of inhibition) on GABAergic neurons projecting from the rostromedial tegmental nucleus to the ventral tegmental area (VTA) is increased for sP rats that consume more ethanol. This alteration in synaptic plasticity drives increased dopamine neuron firing providing a possible mechanism for the increased ethanol consumption in sP rats (Melis et al., 2014). Thus, the current data show that pharmacological and genetic inactivation of CB1 blocks ethanol-stimulated mesolimbic dopamine release in preclinical rodent models.
8. Conditioned place preference for ethanol
Mice lacking CB1 exhibit decreased conditioned place preference (CPP) for ethanol (Basavarajappa et al., 2003; Houchi et al., 2005; Thanos et al., 2005). However, CB1 KO mice display normal CPP for cocaine and quinpirole, indicating that CB1 KO mice possess the ability to acquire drug-associated contextual cues (Houchi et al., 2005). The effect of cannabinoid agonists on CPP for ethanol is difficult to assess as they often cause conditioned place aversion (Lepore et al., 1995; Cheer et al., 2000). One approach to avoid this complication is to examine CPP for ethanol in mice lacking the enzymes responsible for endocannabinoid hydrolysis or in animals treated with inhibitors for these enzymes. Surprisingly, there were no differences in CPP for 2 g/kg ethanol in either FAAH KO mice (Blednov et al., 2007) or in astrocyte glutamate transporter (EAAT1) KO mice that display decreased endocannabinoid signaling (Karlsson et al., 2012). Thus, the current literature demonstrates that genetic blockade of CB1 eliminates CPP for ethanol, a result that closely parallels the effect of CB1 disruption on ethanol-stimulated mesolimbic dopamine release (Hungund et al., 2003). However, testing the effect of enhanced ECS signaling on ethanol CPP warrants further investigation.
9. Sensitivity and tolerance for ethanol
Mice lacking CB1 exhibit marked differences in ethanol sensitivity. Mice lacking CB1 on either a C57Bl6 or DBA/2J genetic background display a longer duration of loss of righting reflex (LORR) following systemic injection of either 2 (Vinod et al., 2008b) or 4 g/kg ethanol (Naassila et al., 2004). Sensitivity to the anxiolytic effect of ethanol was unchanged in CB1 KO mice (Racz et al., 2003; Houchi et al., 2005). Reports of sensitivity to the hypothermic effects are mixed with some studies indicating that CB1 KO mice are less sensitive to ethanol (Racz et al., 2003; Vinod et al., 2008b) while others suggest that these mice are more sensitive to ethanol (Naassila et al., 2004).
FAAH KO mice exhibit decreased sensitivity to the hypothermic, sedative/hypnotic, and ataxic effects of ethanol (Basavarajappa et al., 2006; Blednov et al., 2007; Vinod et al., 2008a). Treatment with 0.5 mg/kg URB597 decreased sensitivity to the LORR effect caused by 3.2 g/kg ethanol while causing faster recovery from the ataxic effects of ethanol using the rotarod test. These results suggest that increased endocannabinoid signaling leads to a generalized decrease in sensitivity to multiple physiological and behavioral responses for ethanol. The converse is true for CB1 KO mice that generally display increased sensitivity to the sedative/hypnotic effects of ethanol. However, studies examining sensitivity to the hypothermic effect of ethanol in CB1 KO are mixed and additional work on this specific aspect of ethanol sensitivity would be valuable.
10. Effects of acute ethanol on endocannabinoids
Acute treatment of SK-N-SH neuroblastoma cells with 100 mM ethanol decreased production of AEA and NAPE, a precursor for AEA. However, in cultured hippocampal neurons, acute treatment with 50 mM ethanol increased 2-AG and AEA levels (Basavarajappa et al., 2008) raising the possibility that acute ethanol might produce cell-type specific effects on endocannabinoid levels. In Wistar rats, acute treatment with 4 g/kg ethanol caused elevated AEA in the NAc (Ceccarini et al., 2013). Likewise, acute ethanol exposure caused an increase in AEA and NAPE-PLD, an enzyme involved in AEA biosynthesis in the hippocampus and cortex of developing post-natal (PN) day 7 mouse pups (Subbanna et al., 2013). In contrast, acute ethanol failed to alter 2-AG in PN7 mice (Subbanna et al., 2013). Acute ethanol treatment in PN7 mice causes CB1-dependent caspase-3 activation, neurodegeneration, inhibition of ERK 1/2 signaling, and DNA methylation leading to long-term defects in novel object recognition memory (Subbanna et al., 2013; Nagre et al., 2015). These studies suggest that acute ethanol treatment likely increases endocannabinoid levels in native neuronal populations. Other studies have demonstrated that acute ethanol administration decreased the level of AEA in plasma, nucleus accumbens, cerebellum, and hippocampus of Wistar rats without having an effect on the activities of NAT, FAAH, and NAPE-PLD, enzymes involved in AEA biosynthesis and hydrolysis (Ferrer et al., 2007). This result is consistent with studies in neuroblastoma cells showing that acute ethanol exposure decreases AEA levels. Conflicting reports on the effect of acute ethanol on endocannabinoid levels make this a murky and unresolved issue. Additional work investigating the effect of acute ethanol on AEA and 2-AG in additional primary neuronal populations, and via other administration routes in vivo would help clarify this issue.
11. Effects of chronic ethanol on endocannabinoids
Much evidence demonstrates that chronic ethanol exposure stimulates endocannabinoid release in cultured cells as well as in the brain. Chronic treatment of SK-N-SH neuroblastoma cells with 100 mM ethanol for 72 hours activates phospholipase A2 (PLA2), an enzyme involved in the synthesis of AEA (Basavarajappa et al., 1997). PLA2 activity was also increased in the brains of Swiss-Webster mice subjected to four days of continuous ethanol vapor inhalation. Interestingly, shorter one or two day ethanol vapor exposures decreased PLA2 activity in the brain suggesting that acute and chronic ethanol exposure may have opposing effects on activation of PLA2 (Basavarajappa et al., 1998). Chronic treatment of neuroblastoma cells with ethanol for 72 hours caused an increase in AEA and NAPE synthesis (Basavarajappa et al., 2008). Similarly, chronic treatment of cultured cerebellar granule neurons with 100 or 150 mM ethanol stimulated extracellular AEA while inhibiting FAAH activity (Basavarajappa et al., 2003).
Involuntary 72-hour exposure to ethanol vapor inhalation in mice increased AEA while decreasing FAAH activity in the cortex (Vinod et al., 2006). Interestingly, the increase in cortical AEA returned to basal levels after 24 hours of withdrawal, suggesting that ethanol-stimulated endocannabinoid levels rapidly return to normal once chronic ethanol exposure is stopped. Chronic intermittent ethanol (CIE) exposure in C57Bl6 mice increased the amount of 2-AG but not AEA in the dorsolateral striatum (DePoy et al., 2013). Chronic exposure of non-selected Wistar rats to an ethanol-containing liquid diet increased AEA and 2-AG in the limbic forebrain (Gonzalez et al., 2004). Consistent with other studies showing a decrease in endocannabinoid levels during ethanol deprivation, the levels of AEA and 2-AG rapidly decreased in the limbic forebrain following cessation of ethanol treatment (Gonzalez et al., 2004).
Sixty days of voluntary 10% (v/v) ethanol drinking in alcohol-preferring sP rats increased 2-AG and AEA levels and decreased the amount of FAAH protein and activity in the striatum (Vinod et al., 2010). In this study, subsequent alcohol deprivation decreased striatal levels of AEA and 2-AG in a manner similar to the effect of withdrawal from involuntary ethanol administration. This study also found higher basal levels of AEA in the hippocampus and striatum and higher 2-AG in the cortex and hippocampus of sP rats compared to ethanol non-preferring sNP rats (Vinod et al., 2010). This finding raises the possibility that high basal levels of endocannabinoids might be a predisposing factor for elevated ethanol consumption and abuse. Additional studies have shown that fixed ratio self-administration of ethanol dose-dependently increased 2-AG but not AEA in the NAc shell of non-selected Wistar rats (Caille et al., 2007). Acute systemic injection of ethanol also increased 2-AG but decreased AEA in the NAc of ethanol-naïve Wistar rats (Alvarez-Jaimes et al., 2009). These studies demonstrate that contingent and non-contingent chronic ethanol exposure robustly stimulate endocannabinoid levels in the brain.
Acute or repeated withdrawal from chronic ethanol-containing liquid diet in Wistar rats decreased FAAH, MAGL, CB1, CB2, and GPR55 gene expression in the amygdala, and these decreases were more pronounced following repeated cycles of withdrawal (Serrano et al., 2012). Additional work demonstrates that endocannbinoid levels in the hippocampus of Sprague-Dawley rats are increased by acute or protracted withdrawal from CIE (Mitrirattanakul et al., 2007). Taken together, the rodent studies described in this section demonstrate that chronic ethanol causes long lasting increases in endocannabinoid levels across a wide range of different brain regions that can persist during protracted abstinence.
12. Effects of acute ethanol on CB1 coupling
Acute treatment of cultured hippocampal neurons with ethanol did not change CB1 protein levels (Basavarajappa et al., 2008). Acute treatment of Wistar rats with 4 g/kg ethanol had no effect on NAT activity or the expression of NAPE-PLD, FAAH, or CB1 transcripts in the hippocampus or cerebellum (Ferrer et al., 2007). However, other recent studies demonstrate that acute ethanol treatment increases CB1 transcript in the hippocampus and cortex of developing PN7 mice (Subbanna et al., 2013). Small animal position emission tomography (PET) imaging finds that acute ethanol treatment increases CB1 availability in the NAc (Ceccarini et al., 2013).
13. Effects of chronic ethanol on CB1 coupling
Most studies indicate that chronic ethanol treatment in rodents, regardless of whether ethanol administration is voluntary or not, causes decreased CB1 protein expression and G protein coupling. CB1 binding in Swiss-Webster mice subjected to 72 hours of involuntary ethanol vapor exposure is decreased in the striatum indicating down-regulation of CB1 in this brain region (Vinod et al., 2006). This study also found that CB1 G protein coupling, assessed by agonist-stimulated [35S]-GTPγS binding, was decreased in the striatum following chronic ethanol. The effects of involuntary chronic ethanol exposure were also examined using PET imaging. In this study, chronic exposure to an ethanol-containing liquid diet decreases CB1 availability in the striatum and hippocampus (Ceccarini et al., 2013). Likewise, inhibition of evoked GABAergic IPSCs by WIN 55,212-2 was absent in Layer II/III of mouse organotypic cortical slice cultures exposed to chronic ethanol (Pava and Woodward, 2014).
Chronic voluntary consumption of ethanol in mice decreases CB1 binding and agonist-stimulated [35S]-GTPγS binding in the mouse limbic forebrain (Basavarajappa et al., 2006). A similar effect of chronic voluntary access to ethanol on decreased CB1 coupling was observed in sP rats selected for high ethanol consumption. Interestingly, this decrease in CB1 coupling in sP rats was reversed by ethanol withdrawal (Basavarajappa et al., 2006), closely paralleling the ability of withdrawal to reverse ethanol-induced up-regulation of endocannabinoids in the brain. Decreased CB1 protein and coupling was detected in naïve ethanol-preferring AA rats suggesting the possibility that altered basal endocannabinoid signaling might underlie the increased ethanol consumption in this rat model. One possible explanation for this observation is that elevated basal endocannabinoid levels might be responsible for desensitizing and uncoupling CB1 in ethanol-preferring rats (Hansson et al., 2007; Vinod et al., 2012). Additional recent work has shown that CIE exposure causes uncoupling of CB1 and ablates CB1-mediated synaptic plasticity in organotypic cultures from the dorsal striatum (Adermark et al., 2011; DePoy et al., 2013). This work also finds enhancement of learning tasks mediated by the dorsal striatum that may underlie habit formation associated with drug taking behavior (DePoy et al., 2013).
Some studies suggest that the effect of withdrawal on CB1 coupling, binding, and transcript levels is similar to what is observed for brain endocannabinoids with ethanol-induced effects on CB1 returning to normal following 24 hours of ethanol deprivation (Vinod et al., 2006; Serrano et al., 2012). Another study found that withdrawal from CIE increased CB1 transcript in the rat prefrontal cortex (Rimondini et al., 2002). Other work suggests that withdrawal from CIE causes increased CB1 protein in the hippocampus (Mitrirattanakul et al., 2007; Ceccarini et al., 2013; Coelhoso et al., 2013), striatum (Ceccarini et al., 2013; Coelhoso et al., 2013), and NAc (Coelhoso et al., 2013) that persist for up to 40 days. These results suggest that persistent upregulation of CB1 occurs during acute withdrawal and protracted abstinence.
The collective evidence indicates that chronic ethanol exposure potentiates levels of 2-AG and AEA in the brain. However, work from Parsons and colleagues (Caille et al., 2007) shows that contingent operant SA of ethanol may selectively up-regulate 2-AG but not AEA. An opposite change occurs at the receptor level with chronic ethanol inducing a decrease in CB1 binding and coupling, raising the possibility that ethanol-induced increases in endocannabinoids drive CB1 uncoupling in the brain.
12. Human studies on the role of endocannabinoid signaling in alcoholism
Recent work has investigated the effects of alcohol dependence on ECS signaling in the human brain. Post-mortem analysis of individuals diagnosed with alcohol dependence found decreased CB1 and FAAH protein, decreased CB1 coupling to G proteins, and decreased FAAH activity (Vinod et al., 2010). A second post-mortem analysis of ECS components found a decrease in MAGL but not FAAH enzymatic activity without any differences in MAGL protein levels (Erdozain et al., 2014). Human studies using the PET CB1 ligand [18F] FMPEP-d2 found decreased CB1 binding in alcohol-dependent patients that correlated with the length of abuse and was not reversed after 2-4 weeks of abstinence (Hirvonen et al., 2013). A different PET imaging study found that acute alcohol in humans increased CB1 availability while chronic alcohol abuse caused a decrease in CB1 that persists during abstinence for at least one month (Ceccarini et al., 2014). A third PET imaging study found decreased CB1 during early abstinence in alcohol dependent patients (Neumeister et al., 2012). Although SR141716A (Rimonabant) decreases ethanol consumption in preclinical animal models, treatment with 20 mg/day Rimonabant had no effect on alcohol consumption in non-treatment seeking heavy alcohol drinkers (George et al., 2010) or relapse rate in recently detoxified alcohol-dependent patients (Soyka et al., 2008).
Several studies have demonstrated a possible genetic link between ECS components and risk of alcohol and poly-substance abuse. Studies examining a possible role of the silent 1359G/A Cnr1 (CB1) polymorphism in alcohol abuse found mixed results. Some studies showed this polymorphism was not associated with alcohol dependence (Zuo et al., 2007; Benyamina et al., 2010), while another suggested that homozygosity at this allele conferred increased risk of alcohol withdrawal delirium (Schmidt et al., 2002). Meta-analysis revealed that a polymorphism in the AAT Cnr1 repeat sequence is associated with substance dependence (Benyamina et al., 2010). Another Cnr1 polymorphism (rs2023239; also termed TAG or the C allele) has been associated with increased risk of poly-substance abuse in European, African American, and Japanese populations (Zhang et al., 2004). This polymorphism was linked to increased CB1 binding in the brain (Hutchison et al., 2008; Hirvonen et al., 2013), increased activation of ventromedial prefrontal cortex and midbrain by alcohol cues, and increased subjective reward for alcohol (Hutchison et al., 2008). A loss of function mutation in FAAH (P129T) that confers decreased FAAH protein and catalytic activity has been associated with problem alcohol and drug use (Sipe et al., 2002; Chiang et al., 2004). In summary, human studies suggest a complex interplay between endocannabinoid signaling and alcohol dependence by revealing that: 1) genetic variation in endocannabinoid signaling might be linked to alcohol dependence; 2) blockade of CB1 with Rimonabant does not attenuate alcohol consumption or relapse of problem drinking; 3) alcohol dependence decreases CB1 binding and coupling as well as MAGL activity and FAAH protein and activity.
13. Conclusions
Given the recent trend towards legalization of recreational marijuana, understanding how △9-THC and ECS signaling affect alcohol motivated behavior and addiction is of great importance. We reviewed results from a large number of laboratories showing that CB1 blockade or deletion disrupts voluntary consumption and self-administration of ethanol, reward for ethanol, and relapse and reinstatement of ethanol-motivated behavior in preclinical rodent models. Chronic exposure to ethanol stimulates endocannabinoid levels in the rodent brain leading to decreased CB1 binding and uncoupling of CB1 from G protein signaling pathways that persist into acute withdrawal and protracted abstinence (Figure 1). A similar effect is observed in humans where binding of a CB1 PET ligand is reduced in alcohol dependent individuals. Recent work has examined potential effects of negative allosteric modulators of CB1 in drug addiction models. It will be interesting to see whether negative allosteric modulators of CB1 might suppress ethanol-motivated behavior in preclinical models. Nonetheless, an improved and evolving understanding of the interplay between the ECS and alcohol consumption helps shed light on the mechanisms of alcohol use disorders and also furthers the possible development of ECS-directed drugs for treating this disease.
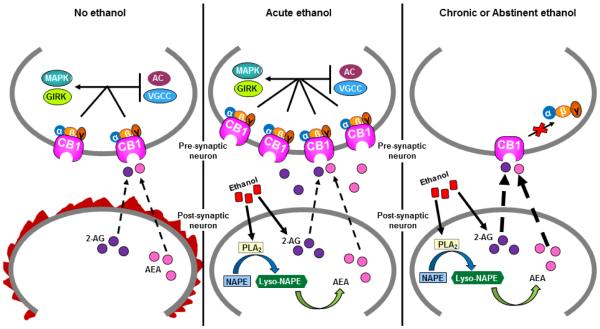
Figure 1. Chronic ethanol down-regulates and uncouples CB1 from G protein signaling pathways.
Under basal conditions in the absence of ethanol, the ECS is activated (designated as flames surrounding the post-synaptic neuron) when the plasma membrane of the post-synaptic neuron becomes depolarized by excitatory neurotransmission. Chronic treatment of cells and animals with ethanol increases endocannabinoid levels through activation of endocannabinoid-synthesizing enzymes such as PLA2 and inhibition of endocannabinoid hydrolytic enzymes such as FAAH. Consequently, elevated endocannabinoid levels cause down-regulation of CB1 and also uncouple this receptor from downstream G protein signaling pathways including modulation of ion channels (GIRKs and VGCCs), adenylyl cyclase (AC), and MAPKs. The effect of chronic ethanol treatment differs from acute ethanol exposure, which does not cause marked down-regulation or desensitization of CB1 that persists during acute withdrawal and abstinence.
Highlights.
An integrative analysis of literature related to the effects of endocannabinoid system activation and inactivation on ethanol-motivated behavior,
A comprehensive review of previous work related to the impact of acute versus chronic ethanol exposure on CB1-mediated endocannabinoid signaling,
Specific areas and questions on the topic of alcohol and endocannabinoid system interactions where additional work is needed have been identified.
Acknowledgements
The authors would like to thank Michael Zee, Dr. Diane McCloskey, Dr. Traci Czyzyk, and Dr. Andras Hajnal for critical review of this manuscript. This work has been supported by NIH grants DA036385 (DJM) and DA037355 (DJM) and is funded, in part, under a grant from the Pennsylvania Department of Health using Tobacco CURE Funds (DJM).
Abbreviations
- 2-AG
2-arachidonoyl-glycerol
- △9-THC
delta-9-tetrahydrocannabinol
- AA
Alko Alcohol Preferring
- ABHD
alpha/beta hydrolase domain-containing protein
- ADE
alcohol deprivation effect
- AEA
N-arachidonoylethanolamine
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- CPP
conditioned place preference
- DAG
diacylglycerol
- DID
drinking in the dark
- DTs
delerium tremens
- ECS
endocannabinoid system
- EPSP
excitatory post-synaptic potential
- FAAH
fatty-acid amide hydrolase
- FR
fixed-ratio
- GIRK
G Protein-coupled inward rectifying potassium channel
- GLAST
glutamate aspartate transporter
- GPR55
G protein-coupled receptor 55
- HIC
handling-induced convulsions
- KO
knock-out
- LORR
loss of righting reflex
- MAGL
monoacylglycerol lipase
- MAPK
Mitogen-activated protein kinase
- msP
Marchigian Sardinian alcohol-preferring rat
- NAc
nucleus accumbens
- NADA
N-arachidonoyl dopamine
- NAPE
N-acylphosphatidylethanolamine
- NAT
N-acetyltransferase
- nolandin ether
2-arachidonoyl glyceryl ether
- NP
non alcohol preferring
- P
alcohol preferring
- PE
phosphatidylethanolamine
- PET
positron emission tomography
- PFC
pre-frontal cortex
- PLA2
phospholipase-A2
- PLC
phospholipase C
- PLD
phospholipase-D
- PR
progressive ratio
- PSR
Pavlovian spontaneous recovery
- SA
self-administration
- sNP
Sardinian alcohol non-preferring
- sP
Sardinian alcohol preferring
- SR1
SR141716A
- O-arachidonoyl ethanolamine
virodamine
- VGCC
voltage-gated calcium channel
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adermark L, Jonsson S, Ericson M, Soderpalm B. Intermittent ethanol consumption depresses endocannabinoid-signaling in the dorsolateral striatum of rat. Neuropharmacology. 2011;61:1160–1165. doi: 10.1016/j.neuropharm.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Alen F, Moreno-Sanz G, Isabel de Tena A, Brooks RD, Lopez-Jimenez A, Navarro M, Lopez-Moreno JA. Pharmacological activation of CB1 and D2 receptors in rats: predominant role of CB1 in the increase of alcohol relapse. Eur J Neurosci. 2008;27:3292–3298. doi: 10.1111/j.1460-9568.2008.06302.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Jaimes L, Stouffer DG, Parsons LH. Chronic ethanol treatment potentiates ethanol-induced increases in interstitial nucleus accumbens endocannabinoid levels in rats. J Neurochem. 2009;111:37–48. doi: 10.1111/j.1471-4159.2009.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Cooper TB, Hungund BL. Effect of chronic ethanol exposure on mouse brain arachidonic acid specific phospholipase A2. Biochem Pharmacol. 1998;55:515–521. doi: 10.1016/s0006-2952(97)00501-7. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Ninan I, Arancio O. Acute ethanol suppresses glutamatergic neurotransmission through endocannabinoids in hippocampal neurons. J Neurochem. 2008;107:1001–1013. doi: 10.1111/j.1471-4159.2008.05685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Activation of arachidonic acid-specific phospholipase A2 in human neuroblastoma cells after chronic alcohol exposure: prevention by GM1 ganglioside. Alcohol Clin Exp Res. 1997;21:1199–1203. [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur J Pharmacol. 2003;466:73–83. doi: 10.1016/s0014-2999(03)01557-7. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology. 2006;50:834–844. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Becker HC. Animal models of alcohol withdrawal. Alcohol Res Health. 2000;24:105–113. [PMC free article] [PubMed] [Google Scholar]
- Benyamina A, Blecha L, Lebeau B, Reynaud M. Prevention of HIV transmission among intravenous drug users. Lancet. 2010;375:1782. doi: 10.1016/S0140-6736(10)60808-2. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL, 2nd, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570–1582. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312:637–641. doi: 10.1042/bj3120637. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarini J, Casteels C, Koole M, Bormans G, Van Laere K. Transient changes in the endocannabinoid system after acute and chronic ethanol exposure and abstinence in the rat: a combined PET and microdialysis study. Eur J Nucl Med Mol Imaging. 2013;40:1582–1594. doi: 10.1007/s00259-013-2456-1. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Hompes T, Verhaeghen A, Casteels C, Peuskens H, Bormans G, Claes S, Van Laere K. Changes in cerebral CB1 receptor availability after acute and chronic alcohol abuse and monitored abstinence. J Neurosci. 2014;34:2822–2831. doi: 10.1523/JNEUROSCI.0849-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Kendall DA, Marsden CA. Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl) 2000;151:25–30. doi: 10.1007/s002130000481. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet. 2004;13:2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Gorriti MA, Navarro M, Massi M, Piomelli D, Ciccocioppo R, Rodriguez de Fonseca F. The anandamide transport inhibitor AM404 reduces ethanol self-administration. Eur J Neurosci. 2007;26:476–486. doi: 10.1111/j.1460-9568.2007.05665.x. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology (Berl) 2008;198:449–460. doi: 10.1007/s00213-008-1104-0. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Hansson AC, del Arco I, Sommer W, Heilig M, Massi M, Bermudez-Silva FJ, Navarro M, Ciccocioppo R, de Fonseca FR. Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in rats. Eur J Neurosci. 2005;21:2243–2251. doi: 10.1111/j.1460-9568.2005.04056.x. [DOI] [PubMed] [Google Scholar]
- Coelhoso CC, Engelke DS, Filev R, Silveira DX, Mello LE, Santos-Junior JG. Temporal and behavioral variability in cannabinoid receptor expression in outbred mice submitted to ethanol-induced locomotor sensitization paradigm. Alcohol Clin Exp Res. 2013;37:1516–1526. doi: 10.1111/acer.12130. [DOI] [PubMed] [Google Scholar]
- Colombo G, Vacca G, Serra S, Carai MA, Gessa GL. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR 141716, on alcohol's motivational properties in alcohol-preferring rats. Eur J Pharmacol. 2004;498:119–123. doi: 10.1016/j.ejphar.2004.07.069. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Fa M, Guano L, Lobina C, Loche A, Reali R, Gessa GL. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998;33:126–130. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, Carai MM, Gessa L. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology (Berl) 2002;159:181–187. doi: 10.1007/s002130100887. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thone-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- de Bruin NM, Lange JH, Kruse CG, Herremans AH, Schoffelmeer AN, van Drimmelen M, De Vries TJ. SLV330, a cannabinoid CB(1) receptor antagonist, attenuates ethanol and nicotine seeking and improves inhibitory response control in rats. Behav Brain Res. 2011;217:408–415. doi: 10.1016/j.bbr.2010.11.013. [DOI] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci U S A. 2013;110:14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, Trabace L, Ciccocioppo R. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology (Berl) 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- Erdozain AM, Rubio M, Valdizan EM, Pazos A, Meana JJ, Fernandez-Ruiz J, Alexander SP, Callado LF. The endocannabinoid system is altered in the post-mortem prefrontal cortex of alcoholic subjects. Addict Biol. 2014 doi: 10.1111/adb.12160. [DOI] [PubMed] [Google Scholar]
- Femenia T, Garcia-Gutierrez MS, Manzanares J. CB1 receptor blockade decreases ethanol intake and associated neurochemical changes in fawn-hooded rats. Alcohol Clin Exp Res. 2010;34:131–141. doi: 10.1111/j.1530-0277.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- Ferrer B, Bermudez-Silva FJ, Bilbao A, Alvarez-Jaimes L, Sanchez-Vera I, Giuffrida A, Serrano A, Baixeras E, Khaturia S, Navarro M, Parsons LH, Piomelli D, Rodriguez de Fonseca F. Regulation of brain anandamide by acute administration of ethanol. Biochem J. 2007;404:97–104. doi: 10.1042/BJ20061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Jr., Oslin D, O'Brien CP, Imms P, Riggs DS, Volpicelli J. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: a randomized clinical trial. JAMA. 2013;310:488–495. doi: 10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 2001;25:277–282. [PubMed] [Google Scholar]
- Gallate JE, McGregor IS. The motivation for beer in rats: effects of ritanserin, naloxone and SR 141716. Psychopharmacology (Berl) 1999;142:302–308. doi: 10.1007/s002130050893. [DOI] [PubMed] [Google Scholar]
- Gallate JE, Mallet PE, McGregor IS. Combined low dose treatment with opioid and cannabinoid receptor antagonists synergistically reduces the motivation to consume alcohol in rats. Psychopharmacology (Berl) 2004;173:210–216. doi: 10.1007/s00213-003-1694-5. [DOI] [PubMed] [Google Scholar]
- Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur J Pharmacol. 1999;370:233–240. doi: 10.1016/s0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- Gamage TF, Ignatowska-Jankowska BM, Wiley JL, Abdelrahman M, Trembleau L, Greig IR, Thakur GA, Tichkule R, Poklis J, Ross RA, Pertwee RG, Lichtman AH. In-vivo pharmacological evaluation of the CB1-receptor allosteric modulator Org-27569. Behav Pharmacol. 2014;25:182–185. doi: 10.1097/FBP.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Herion DW, Jones CL, Phillips MJ, Hersh J, Hill D, Heilig M, Ramchandani VA, Geyer C, Spero DE, Singley ED, O'Malley SS, Bishai R, Rawlings RR, Kunos G. Rimonabant (SR141716) has no effect on alcohol self-administration or endocrine measures in nontreatment-seeking heavy alcohol drinkers. Psychopharmacology (Berl) 2010;208:37–44. doi: 10.1007/s00213-009-1704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Serra S, Vacca G, Carai MA, Colombo G. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR147778, on alcohol intake and motivational properties of alcohol in alcohol-preferring sP rats. Alcohol Alcohol. 2005;40:46–53. doi: 10.1093/alcalc/agh114. [DOI] [PubMed] [Google Scholar]
- Getachew B, Hauser SR, Dhaher R, Katner SN, Bell RL, Oster SM, McBride WJ, Rodd ZA. CB1 receptors regulate alcohol-seeking behavior and alcohol self-administration of alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2011;97:669–675. doi: 10.1016/j.pbb.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Valenti M, de Miguel R, Fezza F, Fernandez-Ruiz J, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in reward-related brain regions of alcohol-exposed rats, and their possible relevance to alcohol relapse. Br J Pharmacol. 2004;143:455–464. doi: 10.1038/sj.bjp.0705963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Bermudez-Silva FJ, Malinen H, Hyytia P, Sanchez-Vera I, Rimondini R, Rodriguez de Fonseca F, Kunos G, Sommer WH, Heilig M. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2007;32:117–126. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci U S A. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol. 2005;15:593–599. doi: 10.1016/j.euroneuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Zanotti-Fregonara P, Umhau JC, George DT, Rallis-Frutos D, Lyoo CH, Li CT, Hines CS, Sun H, Terry GE, Morse C, Zoghbi SS, Pike VW, Innis RB, Heilig M. Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Mol Psychiatry. 2013;18:916–921. doi: 10.1038/mp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M, Naassila M. CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology. 2005;30:339–349. doi: 10.1038/sj.npp.1300568. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid inhibition of adenylate cyclase. Biochemistry of the response in neuroblastoma cell membranes. Mol Pharmacol. 1985;27:429–436. [PubMed] [Google Scholar]
- Howlett AC, Fleming RM. Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response in neuroblastoma cell membranes. Mol Pharmacol. 1984;26:532–538. [PubMed] [Google Scholar]
- Howlett AC, Qualy JM, Khachatrian LL. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol. 1986;29:307–313. [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci U S A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Haughey H, Niculescu M, Schacht J, Kaiser A, Stitzel J, Horton WJ, Filbey F. The incentive salience of alcohol: translating the effects of genetic variant in CNR1. Arch Gen Psychiatry. 2008;65:841–850. doi: 10.1001/archpsyc.65.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, Qiu Y, Zhang Y, Li JX. Effects of the cannabinoid CB(1) receptor allosteric modulator ORG 27569 on reinstatement of cocaine- and methamphetamine-seeking behavior in rats. Drug Alcohol Depend. 2014;143:251–256. doi: 10.1016/j.drugalcdep.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Adermark L, Molander A, Perreau-Lenz S, Singley E, Solomon M, Holmes A, Tanaka K, Lovinger DM, Spanagel R, Heilig M. Reduced alcohol intake and reward associated with impaired endocannabinoid signaling in mice with a deletion of the glutamate transporter GLAST. Neuropharmacology. 2012;63:181–189. doi: 10.1016/j.neuropharm.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1335–1341. [PubMed] [Google Scholar]
- Kranzler HR, Wesson DR, Billot L. Naltrexone depot for treatment of alcohol dependence: a multicenter, randomized, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2004;28:1051–1059. doi: 10.1097/01.alc.0000130804.08397.29. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001a;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001b;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand F, De Witte P. SR147778, a CB1 cannabinoid receptor antagonist, suppresses ethanol preference in chronically alcoholized Wistar rats. Alcohol. 2006;39:125–134. doi: 10.1016/j.alcohol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Latt NC, Jurd S, Houseman J, Wutzke SE. Naltrexone in alcohol dependence: a randomised controlled trial of effectiveness in a standard clinical setting. Med J Aust. 2002;176:530–534. doi: 10.5694/j.1326-5377.2002.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Lepore M, Vorel SR, Lowinson J, Gardner EL. Conditioned place preference induced by delta 9-tetrahydrocannabinol: comparison with cocaine, morphine, and food reward. Life Sci. 1995;56:2073–2080. doi: 10.1016/0024-3205(95)00191-8. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. The selective cannabinoid antagonist SR 141716A blocks cannabinoid-induced antinociception in rats. Pharmacol Biochem Behav. 1997;57:7–12. doi: 10.1016/s0091-3057(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL., 2nd Agonism of the endocannabinoid system modulates binge-like alcohol intake in male C57BL/6J mice: involvement of the posterior ventral tegmental area. Neuroscience. 2009;164:424–434. doi: 10.1016/j.neuroscience.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci U S A. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Devane WA, Hille B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol Pharmacol. 1993;44:498–503. [PubMed] [Google Scholar]
- Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Malinen H, Hyytia P. Ethanol self-administration is regulated by CB1 receptors in the nucleus accumbens and ventral tegmental area in alcohol-preferring AA rats. Alcohol Clin Exp Res. 2008;32:1976–1983. doi: 10.1111/j.1530-0277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- Marinho EA, Oliveira-Lima AJ, Santos R, Hollais AW, Baldaia MA, Wuo-Silva R, Yokoyama TS, Takatsu-Coleman AL, Patti CL, Longo BM, Berro LF, Frussa-Filho R. Effects of rimonabant on the development of single dose-induced behavioral sensitization to ethanol, morphine and cocaine in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:22–31. doi: 10.1016/j.pnpbp.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Marrs WR, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Goodman AM, Chabac S, Lehert P. Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: the role of patient motivation. J Psychiatr Res. 2006;40:383–393. doi: 10.1016/j.jpsychires.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Dam KD, Mallet PE, Gallate JE. Delta9-THC reinstates beer- and sucrose-seeking behaviour in abstinent rats: comparison with midazolam, food deprivation and predator odour. Alcohol Alcohol. 2005;40:35–45. doi: 10.1093/alcalc/agh113. [DOI] [PubMed] [Google Scholar]
- Melis M, Sagheddu C, De Felice M, Casti A, Madeddu C, Spiga S, Muntoni AL, Mackie K, Marsicano G, Colombo G, Castelli MP, Pistis M. Enhanced endocannabinoid-mediated modulation of rostromedial tegmental nucleus drive onto dopamine neurons in Sardinian alcohol-preferring rats. J Neurosci. 2014;34:12716–12724. doi: 10.1523/JNEUROSCI.1844-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking. Behav Brain Res. 2011;217:454–462. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Mitrirattanakul S, Lopez-Valdes HE, Liang J, Matsuka Y, Mackie K, Faull KF, Spigelman I. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clin Exp Res. 2007;31:855–867. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schutz G, Wotjak CT, Lutz B, Marsicano G. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5:e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46:243–253. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Nagre NN, Subbanna S, Shivakumar M, Psychoyos D, Basavarajappa BS. CB1-receptor knockout neonatal mice are protected against ethanol-induced impairments of DNMT1, DNMT3A, and DNA methylation. J Neurochem. 2015;132:429–442. doi: 10.1111/jnc.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Normandin MD, Murrough JW, Henry S, Bailey CR, Luckenbaugh DA, Tuit K, Zheng MQ, Galatzer-Levy IR, Sinha R, Carson RE, Potenza MN, Huang Y. Positron emission tomography shows elevated cannabinoid CB1 receptor binding in men with alcohol dependence. Alcohol Clin Exp Res. 2012;36:2104–2109. doi: 10.1111/j.1530-0277.2012.01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava MJ, Woodward JJ. A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol. 2012;46:185–204. doi: 10.1016/j.alcohol.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava MJ, Woodward JJ. Chronic ethanol alters network activity and endocannabinoid signaling in the prefrontal cortex. Front Integr Neurosci. 2014;8:58. doi: 10.3389/fnint.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Poncelet M, Maruani J, Calassi R, Soubrie P. Overeating, alcohol and sucrose consumption decrease in CB1 receptor deleted mice. Neurosci Lett. 2003;343:216–218. doi: 10.1016/s0304-3940(03)00397-5. [DOI] [PubMed] [Google Scholar]
- Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, Nomikos GG, Carter P, Bymaster FP, Leese AB, Felder CC. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M, Zimmer A. A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J Neurosci. 2003;23:2453–2458. doi: 10.1523/JNEUROSCI.23-06-02453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh D, Gamage TF, Vanuytsel T, Owens RA, Abdullah RA, Niphakis MJ, Shea-Donohue T, Cravatt BF, Lichtman AH. Dual inhibition of endocannabinoid catabolic enzymes produces enhanced antiwithdrawal effects in morphine-dependent mice. Neuropsychopharmacology. 2013;38:1039–1049. doi: 10.1038/npp.2012.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–11078. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Roberts AJ, Bilbao A, Koob GF, Navarro M. Cannabinoid receptor antagonist SR141716A decreases operant ethanol self administration in rats exposed to ethanol-vapor chambers. Zhongguo Yao Li Xue Bao. 1999;20:1109–1114. [PubMed] [Google Scholar]
- Samson HH, Sharpe AL, Denning C. Initiation of ethanol self-administration in the rat using sucrose substitution in a sipper-tube procedure. Psychopharmacology (Berl) 1999;147:274–279. doi: 10.1007/s002130051167. [DOI] [PubMed] [Google Scholar]
- Schmidt LG, Samochowiec J, Finckh U, Fiszer-Piosik E, Horodnicki J, Wendel B, Rommelspacher H, Hoehe MR. Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Depend. 2002;65:221–224. doi: 10.1016/s0376-8716(01)00164-8. [DOI] [PubMed] [Google Scholar]
- Serra S, Brunetti G, Pani M, Vacca G, Carai MA, Gessa GL, Colombo G. Blockade by the cannabinoid CB(1) receptor antagonist, SR 141716, of alcohol deprivation effect in alcohol-preferring rats. Eur J Pharmacol. 2002;443:95–97. doi: 10.1016/s0014-2999(02)01594-7. [DOI] [PubMed] [Google Scholar]
- Serra S, Carai MA, Brunetti G, Gomez R, Melis S, Vacca G, Colombo G, Gessa GL. The cannabinoid receptor antagonist SR 141716 prevents acquisition of drinking behavior in alcohol-preferring rats. Eur J Pharmacol. 2001;430:369–371. doi: 10.1016/s0014-2999(01)01379-6. [DOI] [PubMed] [Google Scholar]
- Serrano A, Rivera P, Pavon FJ, Decara J, Suarez J, Rodriguez de Fonseca F, Parsons LH. Differential effects of single versus repeated alcohol withdrawal on the expression of endocannabinoid system-related genes in the rat amygdala. Alcohol Clin Exp Res. 2012;36:984–994. doi: 10.1111/j.1530-0277.2011.01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci U S A. 2002;99:8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Koller G, Schmidt P, Lesch OM, Leweke M, Fehr C, Gann H, Mann KF. Cannabinoid receptor 1 blocker rimonabant (SR 141716) for treatment of alcohol dependence: results from a placebo-controlled, double-blind trial. J Clin Psychopharmacol. 2008;28:317–324. doi: 10.1097/JCP.0b013e318172b8bc. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Subbanna S, Shivakumar M, Psychoyos D, Xie S, Basavarajappa BS. Anandamide-CB1 receptor signaling contributes to postnatal ethanol-induced neonatal neurodegeneration, adult synaptic, and memory deficits. J Neurosci. 2013;33:6350–6366. doi: 10.1523/JNEUROSCI.3786-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Sun YX, Tsuboi K, Okamoto Y, Tonai T, Murakami M, Kudo I, Ueda N. Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem J. 2004;380:749–756. doi: 10.1042/BJ20040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res. 2005;164:206–213. doi: 10.1016/j.bbr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Xie S, Cooper TB, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619–625. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Sanguino E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem. 2008a;104:233–243. doi: 10.1111/j.1471-4159.2007.04956.x. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Kassir SA, Hungund BL, Cooper TB, Mann JJ, Arango V. Selective alterations of the CB1 receptors and the fatty acid amide hydrolase in the ventral striatum of alcoholics and suicides. J Psychiatr Res. 2010;44:591–597. doi: 10.1016/j.jpsychires.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Thanos PK, Vadasz C, Cooper TB, Volkow ND, Hungund BL. Genetic and pharmacological manipulations of the CB(1) receptor alter ethanol preference and dependence in ethanol preferring and nonpreferring mice. Synapse. 2008b;62:574–581. doi: 10.1002/syn.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Maccioni P, Garcia-Gutierrez MS, Femenia T, Xie S, Carai MA, Manzanares J, Cooper TB, Hungund BL, Colombo G. Innate difference in the endocannabinoid signaling and its modulation by alcohol consumption in alcohol-preferring sP rats. Addict Biol. 2012;17:62–75. doi: 10.1111/j.1369-1600.2010.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Zhang PW, Ishiguro H, Ohtsuki T, Hess J, Carillo F, Walther D, Onaivi ES, Arinami T, Uhl GR. Human cannabinoid receptor 1: 5' exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol Psychiatry. 2004;9:916–931. doi: 10.1038/sj.mp.4001560. [DOI] [PubMed] [Google Scholar]
- Zuo L, Kranzler HR, Luo X, Covault J, Gelernter J. CNR1 variation modulates risk for drug and alcohol dependence. Biol Psychiatry. 2007;62:616–626. doi: 10.1016/j.biopsych.2006.12.004. [DOI] [PubMed] [Google Scholar]