Abstract
Background
A substantial proportion of patients with clinical stage I NSCLC have more advanced disease on final pathologic review. We studied potentially modifiable factors that may predict pathologic upstaging.
Methods
Data of patients with clinical stage I NSCLC undergoing resection were obtained from the National Cancer Database (NCDB). Univariate and multivariate analyses were performed to identify variables that predict upstaging.
Results
From 1998–2010, 55,653 patients with clinical stage I NSCLC underwent resection; of these 9,530 (17%) had more advanced disease on final pathologic review. Of the 9,530 upstaged patients, 27% had T3 or T4 tumors, 74% had positive lymph nodes (N>0), and 4% were found to have metastatic disease (M1). Patients with larger tumors (38mm vs. 29mm, p<0.001) and a delay >8 weeks from diagnosis to resection were more likely to be upstaged. Upstaged patients also had more lymph nodes examined (10.9 vs. 8.2, p<0.001) and were more likely to have positive resection margins (10% vs. 2%, p<0.001). Median survival was lower in upstaged patients (39 months vs. 73 months). Predictors of upstaging in multivariate regression analysis included larger tumor size, delay in resection >8 weeks, positive resection margins, and number of lymph nodes examined. There was a linear relationship between the number of lymph nodes examined and the odds of upstaging (1–3 nodes, OR 2.01; >18 nodes OR 6.14).
Conclusions
Pathologic upstaging is a common finding with implications for treatment and outcomes in clinical stage I NSCLC. A thorough analysis of regional lymph nodes is critical to identify patients with more advanced disease.
INTRODUCTION
Surgical resection is the optimal treatment for early stage non-small cell lung cancer (NSCLC). Current data suggest that patients with stage I disease who undergo complete resection can experience long-term survival in the majority of cases.1 Accurate clinical staging not only provides valuable prognostic information, but is also important for identifying patients with more advanced disease who would benefit from multi-modality therapy.2
Despite advances in the diagnosis and pre-operative staging of lung malignancies using computed tomography (CT), positron emission tomography (PET), and endobronchial ultrasound (EBUS), pathologic upstaging of early stage disease remains a common finding.3,4 Studies indicate that current staging protocols may underestimate the extent of disease in up to 28% of patients with clinical stage I NSCLC.5,6
The National Cancer Database (NCDB) is a program developed in 1989 by the Commission on Cancer, the American College of Surgeons, and the American Cancer Society.7 Data is submitted by more than 1,500 accredited cancers centers across the United States and Puerto Rico, and it captures approximately 70% of all new cancer cases diagnosed in the U.S. annually. We queried the NCDB to further quantify the incidence of pathologic upstaging in clinical stage I NSCLC on a national level. We hypothesized that there are potential predictors of pathologic upstaging in early stage NSCLC patients undergoing resection, and some of these variables may be modifiable. If identified, such predictors could have important implications regarding treatment of patients with early stage NSCLC.
Material and Methods
For patients treated from 1998–2010, information was abstracted from the NCDB participant user file for those with clinical stage I NSCLC (T1 or T2a, N0 according to the 7th edition AJCC staging manual) who underwent surgical resection.8 All information was de-identified so IRB approval for the study was waived at Washington University. Patients with T2b tumors were specifically excluded.
Patients recorded as clinical stage I NSCLC with T2 status but lacking T2a/T2b differentiation in the database were presumed T2a and therefore included. Patient- and tumor-related variables, treatment details, and outcomes were extracted. Using information on race, income, and population size of the area, we created dichotomized groups in which a patient was either Caucasian or not Caucasian, had an annual income less than or greater than $35,000, and presented from a rural location (regional population less than 250,000) or urban location, respectively. The Charlson/Deyo score was used as a measure of comorbidity (categorized as 0, 1, or ≥ 2). The NCDB combines those with scores of 2 or greater into a single group, as very few patients have scores greater than two. Treatment facilities were classified as community cancer programs, comprehensive community cancer programs, and academic/research centers. For the analysis, community cancer programs and comprehensive community cancer programs were categorized as nonacademic centers.
Last known vital status and the time between diagnosis and the follow-up date were used to determine survival. According to the NCDB, diagnosis date refers to the date of histologic confirmation of NSCLC when available. In cases where the diagnosis was made based on imaging and patients proceeded directly to resection without biopsy, diagnosis date refers to the date of radiologic imaging identifying the lesion. Patients found to be pathologic stage II or higher were categorized as pathologically upstaged.
All analyses were performed using SPSS 21.0 (SPSS Inc, Chicago, IL). Descriptive statistics were expressed as means +/− standard deviation unless otherwise specified. Independent samples t-tests and one-way ANOVA were used to compare continuous variables. Chi-square tests were used to compare categorical data. Overall survival was estimated by the Kaplan-Meier method. Multivariate logistic regression models were fitted to evaluate variables influencing pathologic upstaging. Factors accounted for in the multivariate analysis include: age, gender, race, facility type (academic vs. non-academic), income, urban location, Charlson score, tumor size, distance travelled for treatment, T status (T1 vs. T2), treatment delay >8 weeks, pathologic margin status, tumor histology, and number of lymph nodes examined. In regards to the latter, patients with zero lymph nodes sampled serve as the reference for the odds ratios generated by logistic regression. Finally, survival analysis was performed using the Cox proportional hazards model. The variables considered were age, gender, race, facility type, income, urban location, Charlson score, tumor size, distance travelled for treatment, T status, pathologic margin status, presence or absence of upstaging, and number of lymph nodes examined. We also performed a subgroup analysis in the non-upstaged patients and studied the same variables on overall survival in a Cox proportional hazards model. For all analyses, p-values < 0.05 were considered statistically significant.
Results
From 1998 to 2010, 55,653 patients with surgically resected clinical stage I NSCLC were identified in the database (Table 1). Of these, 9,530 (17%) were found to have more advanced disease on final pathology. Pathologic upstaging was due to histologically positive lymph nodes (N>0) in 74% of upstaged cases (N1 = 50%, N2 = 24%, N3 < 1%). Final pathologic review demonstrated T3 or T4 status in 16% and 11% of upstaged patients, respectively. Pathologic evidence of metastatic disease was found in 4%. Of upstaged patients, 18% were upstaged based on more than 1 TNM characteristic. On univariate analysis, upstaged patients were slightly younger, more likely to be male, and more likely to receive treatment at an academic center. In addition, upstaged patients had larger tumors (38mm vs. 29mm, p<0.001) and were more likely to have a delay of greater than 8 weeks from diagnosis to treatment (p=0.003).
Table 1.
Demographics and clinical information for patients with clinical stage I NSCLC that were or were not upstaged following surgical resection – Continuous variables are displayed as mean +/− standard deviation. Categorical variables are displayed as number (% total).
| Patient Characteristics | Pathologic Stage I Patients n= 46,123 | Upstaged patients n=9,530 | p-Value | |
|---|---|---|---|---|
| Age at diagnosis (years) | 68.1 +/− 10.0 | 67.3 +/− 10.1 | <0.001 | |
| Male Gender | 21,794 (47%) | 4,902 (51%) | <0.001 | |
| Caucasian | 41,163 (89%) | 8,440 (89%) | 0.05 | |
| Academic Center | 16,492 (36%) | 3,597 (38%) | 0.001 | |
| Annual Income >$35,000 | 29,233 (67%) | 6,028 (67%) | 0.61 | |
| Urban Area | 29,824 (65%) | 6,232 (65%) | 0.18 | |
| Charlson/Deyo Score (CCI) | 0 | 23,270 (50%) | 4,943 (52%) | 0.03 |
| 1 | 16,507 (36%) | 3,341 (35%) | ||
| 2 | 6,346 (14%) | 1,246 (13%) | ||
| Tumor Size (mm) | 28.6 +/− 21.1 | 37.7 +/− 23.7 | <0.001 | |
| Latency >8 weeks | 12,857 (28%) | 2,801 (29%) | 0.003 | |
| Clinical T Stage | 1 | 30,404 (66%) | 4,572 (48%) | <0.001 |
| 2 | 15,719 (34%) | 4,958 (52%) | ||
| Pathologic T Stage | 1 | 26,804 (58%) | 2,390 (25%) | <0.001 |
| 2 | 19,319 (42%) | 4,563 (48%) | ||
| 3 | n/a | 1,491 (16%) | ||
| 4 | n/a | 1,086 (11%) | ||
| Pathologic N Stage | 0 | 46,123 (100%) | 2,503 (26%) | <0.001 |
| 1 | n/a | 4,736 (50%) | ||
| 2 | n/a | 2,272 (24%) | ||
| 3 | n/a | 19 (0.2%) | ||
| Pathologic M Stage | 1 | n/a | 340 (4%) | <0.001 |
| Complete Resection | 45,018 (98%) | 8,605 (90%) | <0.001 | |
| Mean Number of Lymph Nodes Examined | 8.19 +/− 7.0 | 10.9 +/− 8.2 | <0.001 | |
| Adjuvant Radiation | 1,300 (3%) | 1,582 (17%) | <0.001 | |
| Adjuvant Chemotherapy | 4,440 (10%) | 4,649 (51%) | <0.001 | |
| Surgery | Lobectomy | 37,940 (82%) | 7,801 (82%) | <0.001 |
| Pneumonectomy | 1,072 (2%) | 929 (10%) | ||
| Wedge/segment | 7,111 (15%) | 800 (8%) | ||
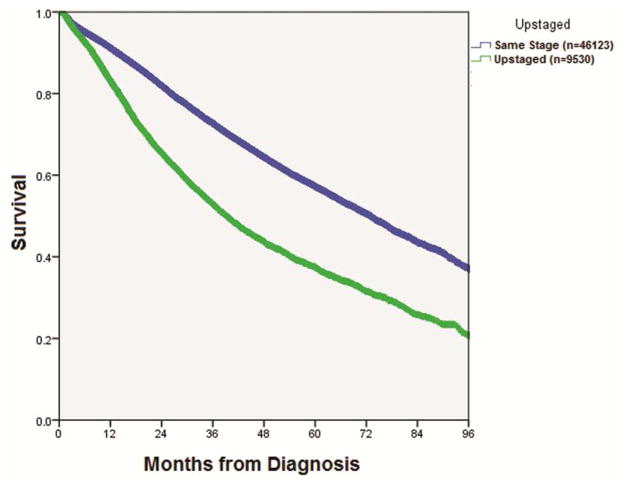
On pathologic review, upstaged patients were more likely to have positive resection margins (10% vs. 2%, p<0.001) and had a greater number of lymph nodes examined (mean= 10.9 vs. 8.2, p<0.001). Upstaged patients were more likely to undergo adjuvant radiation and chemotherapy, and were more likely to require pneumonectomy (10% vs. 2%, p<0.001). Despite a higher likelihood of receiving additional treatment, median overall survival was lower in the upstaged cohort (39.2 vs. 73.0 months, p<0.001) (Figure 1).
Figure 1.
Kaplan-Meier curve for overall survival in patients with clinical stage I NSCLC who were later found to be pathologic stage 1 versus those that were pathologically upstaged (p<0.001). The table below indicates the number at risk and percent survival for both groups at years 1 through 8.
| Same Stage | N at Risk | Upstaged | N at Risk | |
|---|---|---|---|---|
| 1 Year | 91.2% | 35458 | 82.1% | 6697 |
| 2 Year | 82.0% | 24161 | 65,5% | 4001 |
| 3 Year | 72.7% | 15498 | 52.9% | 2375 |
| 4 Year | 64.6% | 9916 | 43.7% | 1426 |
| 5 Year | 57.2% | 6435 | 37.5% | 911 |
| 6 Year | 50.6% | 3664 | 31.6% | 523 |
| 7 Year | 43.3% | 1633 | 25.8% | 237 |
| 8 Year | 37.0% | 432 | 20.8% | 47 |
Multivariate logistic regression analysis identified several predictors of upstaging including larger tumor size, delay in resection > 8 weeks, positive resection margins, adenocarcinoma histology, and number of lymph nodes examined (Table 2). When analyzed as a categorical variable, there was a linear relationship between the number of lymph nodes examined and the odds of pathologic upstaging (1–3 nodes, OR 2.01; >18 nodes, OR 6.14).
Table 2.
Multivariable logistic regression analysis identifying variables associated with upstaging in patients with clinical stage I NSCLC.
| Patient and Treatment Variables | Odds ratio (OR) with 95% Confidence Interval (CI) | p-Value |
|---|---|---|
| Age | 0.99 (0.99–0.99) | <0.001 |
| Male gender | 1.11 (1.05–1.17) | <0.001 |
| Tumor Size (mm) | 1.11 (1.10–1.13) | <0.001 |
| Clinical T2 Status | 1.52 (1.42–1.62) | <0.001 |
| Latency > 8 weeks | 1.10 (1.03–1.16) | 0.002 |
| Positive Tumor Margin | 4.14 (3.70–4.63) | <0.001 |
| Squamous Histology | 0.82 (0.78–0.87) | <0.001 |
| Number of Lymph Nodes Examined (n) | ||
| n=0 (2,565) | 1.00 | n/a |
| n=1–3 (9,523) | 2.01 (1.62–2.50) | <0.001 |
| n=4–6 (11,848) | 2.99 (2.42–3.69) | <0.001 |
| n=7–9 (9,406) | 3.81 (3.09–4.71) | <0.001 |
| n=10–12 (6,562) | 4.19 (3.38–5.19) | <0.001 |
| n=13–15 (4,126) | 4.51 (3.62–5.62) | <0.001 |
| n=16–18 (2,514) | 5.90 (4.70–7.40) | <0.001 |
| n>18 (4,528) | 6.14 (4.94–7.62) | <0.001 |
In a Cox regression model age, male gender, Caucasian race, increased Charlson score, increased tumor size, T2 status, positive margin, and presence of upstaging were associated with a greater risk of long-term mortality, while higher income, urban location, and treatment at an academic center were protective (Table 3). Sampling of at least one lymph node was associated with an improvement in survival (HR=0.80, 95% CI=0.74–0.86) and sampling a greater number of lymph nodes was associated with greater benefit (4–6 nodes, HR=0.69, 95% CI=0.65–0.75) though the relationship was non-linear.
Table 3.
Cox proportional hazards model identifying factors associated with long-term mortality in patients with clinical stage I NSCLC
| Patient and Treatment Variables | Hazard ratio (HR) with 95% Confidence Interval (CI) | p-Value |
|---|---|---|
| Age | 1.03 (1.03–1.03) | <0.001 |
| Male gender | 1.37 (1.33–1.42) | <0.001 |
| Caucasian Race | 1.08 (1.02–1.15) | 0.007 |
| Academic Facility | 0.96 (0.93–0.99) | 0.03 |
| Urban Location | 0.96 (0.92–0.99) | 0.02 |
| Income >$35,000 | 0.90 (0.87–0.93) | <0.001 |
| Charlson Score =1 | 1.14 (1.10–1.18) | <0.001 |
| Charlson Score =2 | 1.49 (1.42–1.56) | <0.001 |
| Tumor Size (mm) | 1.03 (1.02–1.03) | <0.001 |
| Clinical T2 Status | 1.24 (1.19–1.28) | <0.001 |
| Positive Tumor Margin | 1.49 (1.39–1.60) | <0.001 |
| Upstaged Tumor | 1.85 (1.78–1.92) | <0.001 |
| Number of Lymph Nodes Examined (n) – all patients | ||
| n=0 | 1.00 | n/a |
| n=1–3 | 0.80 (0.74–0.86) | <0.001 |
| n=4–6 | 0.69 (0.65–0.75) | <0.001 |
| n=7–9 | 0.67 (0.62–0.72) | <0.001 |
| n=10–12 | 0.64 (0.59–0.69) | <0.001 |
| n=13–15 | 0.65 (0.60–0.71) | <0.001 |
| n=16–18 | 0.62 (0.56–0.69) | <0.001 |
| n>18 | 0.66 (0.61–0.72) | <0.001 |
| Number of Lymph Nodes Examined – Non-upstaged only | ||
| n=0 | 1.00 | n/a |
| n=1–3 | 0.78 (0.72–0.84) | <0.001 |
| n=4–6 | 0.69 (0.64–0.74) | <0.001 |
| n=7–9 | 0.65 (0.60–0.71) | <0.001 |
| n=10–12 | 0.64 (0.58–0.69) | <0.001 |
| n=13–15 | 0.66 (0.60–0.73) | <0.001 |
| n=16–18 | 0.64 (0.57–0.72) | <0.001 |
| n>18 | 0.67 (0.61–0.74) | <0.001 |
In the subgroup analysis of non-upstaged patients, sampling of at least one lymph node was also associated with an improvement in survival (HR=0.78, 95% CI=0.72–0.84) (Table 3). We also noted that the HR for mortality in non-upstaged patients decreased to 0.69 (95% CI=0.64–0.74) if 4–6 lymph nodes were sampled and even further to 0.64 (95% CI=0.58–0.69) if 10–12 nodes were removed. The relationship however was non-linear.
Comment
Our findings suggest that pathologic upstaging after surgery for clinical stage I NSCLC is common and is associated with larger tumors, delay of surgery, incomplete resection, and a greater number of lymph nodes sampled. Previous prospective studies have shown a discrepancy between clinical and pathologic staging in a significant proportion of patients with stage I NSCLC. A subgroup analysis of patients enrolled in Cancer and Leukemia Group B (CALGB) 9761 was performed to clarify the incidence of upstaging in resected patients with clinical stage I NSCLC.4,9 As part of the entry criteria, patients with N2 or N3 lymph nodes larger than 1cm were required to undergo mediastinoscopy to verify the absence of nodal metastasis. Despite the stringent entry criteria, 28.5% of patients had more advanced disease on final pathologic review (14% stage II, 13.5% stage III, 0.9% stage IV). One potential criticism is the infrequent use of PET scan as part of the staging workup for enrolled patients (PET data were reported for 12% of trial participants), which could falsely underestimate the sensitivity of current staging protocols. However, among 55 participants without evidence of additional disease on PET, 38.3% were later upstaged on final pathology suggesting that the use of PET did not significantly increase accuracy for detecting true stage I disease.
With the growing prominence of video-assisted thoracic surgery (VATS) for the treatment of early stage lung cancer, several studies have evaluated the incidence of upstaging as a surrogate for the oncologic quality of resection in VATS versus thoracotomy. While a discussion of the incidence of upstaging in VATS versus open resection is beyond the scope of this manuscript, these studies do provide some insight into the incidence of upstaging using more current staging paradigms. In a 2012 study using the Society of Thoracis Surgeons (STS) database to evaluate 11,500 patients with stage I NSCLC, Boffa et al described a 13.3% incidence of nodal upstaging alone.6 In 2014, Wilson et al evaluated the incidence of nodal upstaging in 302 patients with stage I NSCLC undergoing robotic-assisted pulmonary resections.3 Although 98% of patients underwent pre-operative PET imaging, over 10% of stage I patients had nodal metastases on final pathology and an additional 30 patients (10%) were pathologic T2b or higher. Therefore, despite the increased use of PET imaging, the accuracy of clinical staging of stage I NSCLC appears to have improved only slightly in the past decade. Other retrospective institutional studies have shown similar findings.10–11
According to current guidelines, the optimal treatment of stage I NSCLC is surgical resection while multi-modality therapy is recommended for patients with more advanced disease.2 Therefore, aside from being prognostically important, accurate identification of patients with locally advanced disease is critical for determining the appropriate course of treatment. Similarly, in patients undergoing ablative therapies such as stereotactic body radiotherapy (SBRT) and radiofrequency ablation, lack of a resected specimen precludes pathologic staging. Therefore accurate clinical staging becomes paramount for patient selection, as those with locally advanced disease would not be candidates for strictly local therapy.
Our analysis of the NCDB reveals that pathologic upstaging continues to be a common finding at a national level. Similar to prior studies, we found that 17% of stage I NSCLC patients had more advanced disease on final pathology, with the majority (75%) involving nodal upstaging and an additional percentage (27%) demonstrating increased T-stage (T3/T4). Occult metastatic disease (M1) was much less frequent (4% of upstaged cases) suggesting that current staging methods are more effective at identifying distant metastatic disease than locoregional involvement.
Given the relatively high rate of upstaging, we sought to identify potential predictors that might be apparent prior to resection. We hypothesized that some of these might be modifiable, presenting a unique opportunity to alter the disease course and affect the need for additional treatment. Our univariate and multivariate analyses revealed several variables associated with pathologic upstaging. For some demographic factors such as age and gender, the absolute differences were fairly small between pathologic stage I and upstaged patients. Therefore the true clinical significance of these factors is unclear. However other significant factors including larger tumor size and a delay of more than 8 weeks from diagnosis to resection are more clinically meaningful. While the impact of tumor size on pathologic stage is not surprising, its utility as a modifiable factor is limited.
The impact of treatment delay is perhaps more actionable. The consequence of treatment delay on outcomes in lung cancer is controversial. Several retrospective series have suggested no significant impact on overall survival.12–14 Others have demonstrated a shorter wait time was associated with decreased survival.15–17 However in these studies, patients who presented with symptoms reflective of advanced disease such as hemoptysis or pneumonia tended to be treated in expedited fashion likely creating bias. A previous U.S. study of 84 patients undergoing resection for stage I or II NSCLC showed no difference in overall survival when patients were delayed more than 90 days from diagnosis to treatment.18 In our study, the delay in surgery as an independent predictor in multivariate analysis is likely clinically meaningful. If additional studies confirm its importance, renewed emphasis on an expedited staging evaluation and timely resection may generate meaningful improvement in oncologic outcomes.
A positive resection margin was also found to be significantly associated with pathologic upstaging. While incomplete resection certainly influences outcomes, it is only identified by pathologic analysis and thus cannot be used in pre-operative patient stratification. Rather, we suspect that the presence of a positive resection margin is likely a consequence of more advanced disease (higher incidence of T3/T4 lesions, higher rate of pneumonectomy) in the upstaged population.
Lastly, our data further stress the importance of a thorough lymph node evaluation for the accurate staging and prognostication of NSCLC. The impact of a complete nodal evaluation has been highlighted in previous population-based studies. Ludwig and colleagues performed a SEER database review of over 16,000 patients with resected stage I NSCLC between 1990 and 2000.19 Survival was significantly related to the number of lymph nodes sampled with a peak at 13–16 nodes. A SEER database analysis by Varlotto and colleagues examined over 24,000 patients with stage I NSCLC, and found a survival plateau around 11 nodes.20 In perhaps the largest and most recent SEER analysis by Osarogiagbon and colleagues, 24,650 patients with pathologically node-negative NSCLC (pT1-3) were evaluated.21 They show an incremental improvement in overall and cancer-specific mortality with an increasing number of lymph nodes examined, beginning at 6 and peaking at 18–21 lymph nodes. Interestingly, the mean number of nodes examined per patient was only 6, suggesting that in true practice routine lymph node evaluation “falls far short of optimal.”21
Our data further reinforce the importance of lymph node removal and its impact on stage migration in NSCLC. Upstaged patients had a greater number of lymph nodes examined (mean= 10.9 vs. 8.2, p<0.001) and multivariate logistic regression confirmed a distinct linear relationship between the number of lymph nodes examined and the incidence of pathologic upstaging. Since our odds ratio continued to increase to our maximum threshold of 18 nodes, we cannot formally comment on the optimal number of nodes to be sampled. However, our data appear to agree with Osarogiagbon and colleagues in that examination of up to 18 or more nodes shows continued benefit.21
In the survival analysis, we noted that a higher number of lymph nodes sampled was associated with lower long-term mortality in both upstaged and non-upstaged patients. Especially in the latter group, this information likely shows that greater nodal sampling is primarily a surrogate for accuracy in determining true pathologic stage I disease.
Adequacy of nodal sampling is critically important in the current environment when nonoperative therapies such as SBRT are seen as potential alternatives even in normal risk patients with stage I NSCLC. The ability to sample locoregional lymph nodes has been proposed as a major advantage of surgical resection. However, lack of adequate nodal dissection could potentially ameliorate the benefit of surgery compared with other less invasive treatment modalities.
There are some limitations to the current study. Although the NCDB is a robust source of clinical information, the data are still retrospectively reviewed. In addition, certain factors such as the use of PET imaging, EBUS-FNA, or mediastinoscopy cannot be analyzed in our study since they are not prospectively collected data in the NCDB. Similarly, although we excluded patients specifically designated as T2b, we chose to include patients entered as T2 without further description if they were classified as stage I according to the NCDB. However, it is possible that imprecision in this staging information could result in some T2b patients being included in the current analysis. Lastly, although the American College of Surgeons Commission on Cancer requires at least a 90% patient follow-up rate as part of its accreditation program, our de-identified patient information cannot be used to further validate the accuracy of survival information submitted to the NCDB.
Although there have been considerable advances in the clinical evaluation of early stage lung cancer, pathologic upstaging remains a common occurrence. The detection of locoregional spread has important implications for prognosis and treatment. Data from the NCDB suggest that potentially modifiable predictors of pathologic upstaging include a delay from diagnosis to definitive therapy and the number of lymph nodes sampled during the operation. If confirmed in additional studies, this could provide a strong argument for the expedited evaluation and treatment of patients with early stage NSCLC. In addition, careful evaluation of regional lymph nodes is critical for the detection of advanced disease and meticulous evaluation of the nodes should be routine in the surgical management of these patients.
Acknowledgments
Grant Support
Varun Puri - NIH K07CA178120, K12CA167540-02 (Paul Calabresi Award)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P. The International Association for the Study of Lung Cancer Staging Project: Prognostic Factors and Pathologic TNM Stage in Surgically Managed Non-small Cell Lung Cancer. J Thorac Oncol. 2009;4:792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN Guidelines Version 5.2015 - Non-Small Cell Lung Cancer. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 3.Wilson JL, Louie BE, Cerfolio RJ, Park BJ, Vallieres E, Aye RW, Abdel-Razek A, Bryant A, Fariver AS. The Prevalence of Nodal Upstaging During Robotic Lung Resection in Early Stage Non-Small Cell Lung Cancer. Ann Thorac Surg. 2014;97:1901–1907. doi: 10.1016/j.athoracsur.2014.01.064. [DOI] [PubMed] [Google Scholar]
- 4.D’Cunha J, Herndon JE, Herzan DL, Patterson GA, Kohman LJ, Harpole DH, Kernstine KH, Kern JA, Green MR, Maddaus MA, Kratzke RA. Poor correspondence between clinical and pathologic staging in stage 1 non-small cell lung cancer: results from CALGB 9761, a prospective trial. Lung Cancer. 2005;48:241–246. doi: 10.1016/j.lungcan.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Encuentra A, Garcia-Lujan R, Rivas JJ, Rodriguez-Rodriguez J, Torres-Lanza J, Varela-Simo G. Comparison Between Clinical and Pathologic Staging in 2,994 Cases of Lung Cancer. Ann Thorac Surg. 2005;79:974–979. doi: 10.1016/j.athoracsur.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Boffa DJ, Kosinski AS, Paul S, Mitchell JD, Onaitis M. Lymph Node Evaluation by Open or Video- Assisted Approaches in 11,500 Anatomic Lung Cancer Resections. Ann Thorac Surg. 2012;94:347–353. doi: 10.1016/j.athoracsur.2012.04.059. [DOI] [PubMed] [Google Scholar]
- 7.Bilimoria KY, Stewart AK, Winchester D, Ko CY. The National Cancer Data Base: A Powerful Initiative to Improve Cancer Care in the United States. Ann Surg Onc. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- 9.D’Cunha J, Corfits AL, Herndon JE, Kern JA, Kohman LJ, Patterson GA, Kratzke RA, Maddaus MA. Molecular staging of lung cancer: real-time polymerase chain reaction estimation of lymph node micrometastatic tumor cell burden in stage I non-small cell lung cancer – preliminary results of Cancer and Leukemia Group B Trial 9761. J Thorac Cardiovasc Surg. 2002;123(3):484–491. doi: 10.1067/mtc.2002.119883. [DOI] [PubMed] [Google Scholar]
- 10.Meyers BF, Haddad F, Siegel BA, Zoole JB, Battafarano RJ, Veeramachaneni N, Cooper JD, Patterson GA. Cost-effectiveness of routine mediastinoscopy in computed tomography- and positron emission tomography- screened patients with stage I lung cancer. J Thorac Cardiovasc Surg. 2006;131:822–9. doi: 10.1016/j.jtcvs.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 11.Crabtree TD, Denlinger CE, Meyers BF, El Naqa I, Zoole J, Krupnick SA, Kreisel D, Patterson GA, Bradley JD. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010;140:377–86. doi: 10.1016/j.jtcvs.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 12.Bozcuk H, Martin C. Does treatment delay affect survival in non-small cell lung cancer? A retrospective analysis from a single UK centre. Lung Cancer. 2001;34:243–52. doi: 10.1016/s0169-5002(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 13.Aragoneses FG, Moreno N, Leon P, Fontan EG, Folque E. Influence of delays on survival in the surgical treatment of bronchogenic carcinoma. Lung Cancer. 2002;36:59–63. doi: 10.1016/s0169-5002(01)00458-5. [DOI] [PubMed] [Google Scholar]
- 14.Salomaa ER, Sallinen S, Hiekkanen H, Liippo K. Delays in the diagnosis and treatment of lung cancer. Chest. 2005;128:2282–2288. doi: 10.1378/chest.128.4.2282. [DOI] [PubMed] [Google Scholar]
- 15.Myrdal G, Lambe M, Hillerdal G, Lamberg K, Agustsson T, Stahle E. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax. 2004;59:45–49. [PMC free article] [PubMed] [Google Scholar]
- 16.Annakkaya AN, Arbak P, Balbay O, Bilgin C, Erbas M, Bulut I. Effect of symptom-to-treatment interval on prognosis in lung cancer. Tumori. 2007;93:61–67. doi: 10.1177/030089160709300111. [DOI] [PubMed] [Google Scholar]
- 17.Diaconescu R, Lafond C, Whittom R. Treatment delays in non-small cell lung cancer and their prognostic implications. J Thorac Oncol. 2011;6:1254–1259. doi: 10.1097/JTO.0b013e318217b623. [DOI] [PubMed] [Google Scholar]
- 18.Quarterman RL, McMillan A, Ratcliffe MB, Block MI. Effect of preoperative delay on prognosis for patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;125:108–113. doi: 10.1067/mtc.2003.93. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig MS, Goodman M, Miller DL, Johnstone PA. Postoperative Survival and the Number of Lymph Nodes Sampled During Resection of Node-Negative Non-Small Cell Lung Cancer. Chest. 2005;128:1545– 1550. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 20.Varlotto JM, Recht A, Nikolov M, Flickinger JC, DeCamp MM. Extent of Lymphadenectomy and Outcome for Patients With Stage I Nonsmall Cell Lung Cancer. Cancer. 2009;115:851–858. doi: 10.1002/cncr.23985. [DOI] [PubMed] [Google Scholar]
- 21.Osarogiagbon RU, Ogbata O, Yu X. Number of Lymph Nodes Associated with Maximal Reduction of Long-Term Mortality Risk in Pathologic Node-Negative Non-Small Cell Lung Cancer. Ann Thorac Surg. 2014;97:385–393. doi: 10.1016/j.athoracsur.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]