Abstract
Background
Prescription opioid misuse is a significant public health problem as well as a patient safety concern. Primary care providers (PCPs) are the leading prescribers of opioids for chronic pain, yet few PCPs follow standard practice guidelines regarding assessment and monitoring. This cluster randomized controlled trial will determine whether four implementation strategies; nurse care management, use of a patient registry, academic detailing, and electronic tools, will increase PCP adherence to chronic opioid therapy guidelines and reduce opioid misuse among patients, relative to electronic tools alone. The implementation strategies and intervention content are based on the Chronic Care Model.
Methods
We include 53 PCPs from three Boston-area community health centers and one urban safety-net hospital-based primary care practice who have at least four patients meeting the following inclusion criteria: 1) age ≥ 18; 2) one or more completed visits to the primary care practice in the past year; 3) long-term opioid treatment defined as three or more opioid prescriptions written at least 21 days apart within six months and 4) an inpatient or outpatient ICD-9-CM diagnosis for musculoskeletal or neuropathic pain. We consider PCPs to be study subjects, and obtained a waiver of informed consent for patients because the study is promoting an established standard of care. We enrolled participants (PCPs) from December 2012 through March 2015. PCPs were randomized to receive the intervention, which includes four components: 1) nurse care management, 2) use of a patient registry, 3) academic detailing, and 4) electronic tools, or a control condition, which includes only the use of the electronic tools. The intervention PCPs receive the services of a nurse-managed registry for planning individual patient care and conducting population-based care for patients receiving opioids for chronic pain. In academic detailing visits, trained co-investigators provide intervention PCPs with individualized education to change prescribing practice. Electronic tools, located on a website external to the EMR, www.mytopcare.org, include validated instruments to assess patient status, and management resources to facilitate PCP adherence to suggested monitoring. Electronic tools are available to PCPs in both study arms. The primary outcomes are PCP adherence to chronic opioid therapy guidelines and patient opioid misuse. Secondary outcomes include measures of substance abuse, possible opioid diversion, and level of opioid risk among patients. We will follow PCPs and their estimated 1200 chronic pain patients for one year after study enrollment. To determine whether the intervention condition achieves greater adherence to guidelines and reduced opioid misuse after one year compared to the control condition, we will compare the baseline and follow-up measures of the individual patients, stratifying by intervention status and noting differences that are statistically significant at the p=0.05 level. Analyses will be based on intent-to-treat.
Results
Randomization resulted in groups with similar baseline characteristics. The ages of PCPs are evenly distributed, with inclusion of both PCPs who have recently completed training and those who have been in practice for more than twenty years. Two-thirds of enrolled PCPs are women and one-third are non-white.
Discussion
The study will determine the impact of this multicomponent intervention on improving PCP adherence to guidelines and reducing opioid misuse among patients.
Keywords: Prescription opioid misuse, primary care, cluster randomized trial, nurse care management, patient registry, academic detailing
Introduction
Prescription opioid misuse is a significant public health problem and a patient safety concern. Studies have shown alarming rises in opioid abuse, addiction, diversion and unintentional overdoses over the last 15 years (Substance Abuse and Mental Health Services Administration, 2007). Primary care providers (PCPs) are the leading prescribers of opioids for chronic pain, yet few follow practice guidelines regarding assessment and monitoring (Ballantyne & Mao, 2003; Morasco, Duckart, & Dobscha, 2011; Starrels et al., 2011). Based on the best available evidence, clinical guidelines endorse universal assessment for opioid misuse risk and monitoring for subsequent harm (Chou R, 2009; Gourlay, Heit, & Almahrezi, 2005). The guidelines suggest that monitoring strategies for all chronic pain patients on long-term opioid therapy should be implemented according to patient risk level for opioid misuse. Patient risk level should be identified through individual risk factors, such as substance use disorders or psychiatric diagnoses. Recommended monitoring strategies include a controlled substance agreement, urine drug testing, frequent PCP visits, pill counts (to ensure that a patient is not diverting or misusing medications), use of state prescription monitoring programs (PMPs) that provide data on individual pharmacy fills of controlled substances, and addressing aberrant opioid taking behaviors (Passik & Kirsh, 2004). Given that evidence supports these individual components (Carter & Hall, 2008; Chou R, 2009; Katz et al., 2010; Manchikanti et al., 2006; Wang & Christo, 2009), the goal of this study is to implement and test an enhancement to usual care to improve uptake of the monitoring strategies, thus addressing the pressing need for an effective clinical approach to the mounting problems of opioid misuse.
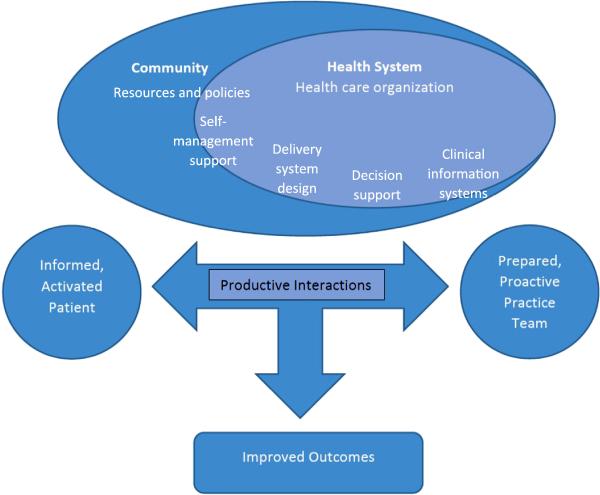
We based our intervention on the Chronic Care Model (Figure 1) (Bodenheimer, Wagner, & Grumbach, 2002). This model is designed to help practices improve patient outcomes by changing routine delivery of ambulatory care through six interrelated system changes (health care organization, clinical information systems, delivery system design, decision support, self-management support, and community resources) meant to make patient-centered, evidence-based care easier to accomplish. The aim of the model is to transform daily care for patients with chronic illnesses from acute and reactive to proactive, planned, and population-based. It is designed to accomplish these goals through effective team care and planned interactions; self-management support bolstered by use of community resources; integrated decision support; and patient registries and other supportive information technology (IT). These elements work together to strengthen the PCP-patient relationship and improve health outcomes.
Figure 1.
Chronic Care Model
Our intervention approach maps to the domains of the Chronic Care Model (Table 1) and is fashioned to take advantage of the documented efficacy of the individual intervention elements (nurse care management, patient registry, academic detailing, and electronic tools) to change PCP practices and improve patient outcomes. Quality improvement interventions in community health centers which have included disease registries in the setting of the Chronic Care Model, most notably in the Health Disparities Collaboratives of the Health Resources and Services Administration (HRSA), have shown improvement in processes of care for asthma and diabetes (Landon et al., 2007). Electronic tools (which map to the decision support domain of the Chronic Care Model) such as on-screen, point-of-care computer reminders have been shown to achieve improvements in PCP behavior, medication prescribing, and test ordering (Shojania et al., 2009). Academic detailing (which maps to the clinical information systems domain of the Chronic Care Model and includes feedback on PCP performance) has been shown to improve how health care professionals prescribe medications, which may affect hundreds of patients (O'Brien et al., 2007; Solomon et al., 2001). Nurse care management, which maps to the self-management support, delivery system design, and decision support domains of the Chronic Care Model), has been shown to improve patient pain outcomes for patients with chronic non-cancer pain (Kroenke et al., 2009; Kroenke et al., 2014). In this study, both intervention and control PCPs receive electronic tools on an external website (www.mytopcare.org) to facilitate guideline adherence. These tools include validated instruments to assess patient status and facilitate PCP adherence to suggested monitoring.
Table 1.
Application of the Chronic Care Model to Intervention Design and Measurement
| Chronic Care Model Domain | Intervention Component | Intervention Content | Measures |
|---|---|---|---|
| Health Care Organization | Nurse care management Patient registry Academic detailing Electronic tools | Create a standard of care across the organization | Baseline Survey: - Learning Organization Survey (Garvin, Edmondson, & Gino, 2008) |
| Clinical information systems | Nurse care management Patient registry Academic detailing | Identify relevant sub populations (patients at highest risk for opioid misuse) for proactive care | Structured Observations: -Interactions between nurse care manager (NCM) and patients -Interactions between academic detailer and primary care physician (PCP) |
| Nurse care management Patient registry Academic detailing | Plan individual patient care | Baseline Survey topics: -Prescription Monitoring Program (PMP) registration -PMP Utilization -Making decisions for patient care using PMP data |
|
| Nurse care management | Coordinate care by sharing information with patients and providers | Structured Observations: -Interactions between NCM and patients -Interactions between NCM and PCPs |
|
| Academic detailing | Monitor performance of the practice team and the care system using audit and feedback | Baseline Survey: - Learning Organization Survey (Garvin, et al., 2008) |
|
| Delivery system design | Nurse care management Patient registry | Define roles and distribute tasks among team members Support evidence-based care through use of planned interactions Provide clinical case management services for complex patients Provide regular follow-up by the care team Provide patient with culturally sensitive care |
Structured Observations: -Interactions between NCM and patients -Interactions between NCM and PCPs Qualitative interviews: -Interviews with PCPs regarding their experience of the intervention Baseline Survey: -Learning Organization Survey (Garvin, et al., 2008) |
| Decision support | Nurse care management | Share evidence-based guidelines and information with patients to encourage participation | Structured Observations: -Interactions between NCM and patients -Interactions between NCM and PCPs |
| Academic detailing Electronic tools | Use of proven provider education methods | Google Analytics: - Statistics on utilization and traffic for the website |
|
| Follow-Up Survey topics: -Usefulness of the website and its content -Suggestions for improvement |
|||
| Academic detailing | Integrate specialist expertise and primary care | Structured Observations: - Interactions between academic detailer and (PCP) |
|
| Self-management support | Nurse care management | Emphasize the patient's central role in managing their own health Use effective self-management support strategies, such as assessment, goal setting, action planning, problem solving, and follow-up Organize internal and community resources to provide ongoing self-management support to patients, such as referral to addiction treatment, food pantries, physical therapy, and behavior health resources |
Structured Observations: - Interactions between NCM and patients |
| The community | Nurse care management | Encourage patient participation in and provide referral to effective community programs, such as Alcoholics Anonymous, support groups, exercise groups, and the YMCA | Structured Observations: - Interactions between NCM and patients |
| Nurse care management Academic detailing | Advocate for policies to improve patient care by contacting the Department of Health for delegation capability for PMP lookups and by working with local pharmacies to distribute naloxone rescue kits | Field Notes: - Field notes on advocacy activities |
The intervention PCPs receive the services of a nurse-managed registry for planning individual patient care and conducting population-based care for patients receiving opioids for chronic pain. Finally, in academic detailing visits, trained co-investigators meet with intervention PCPs to provide them with individualized education (including audit and feedback) to change prescribing practice. We are unaware of studies that have implemented the combination of these approaches to improve management of patients with chronic pain on opioid therapy. We believe that this is a highly innovative approach to improving PCP adherence to guidelines and, potentially, to reducing patient risk for developing substance use disorders.
In this paper, we describe the protocol for a cluster randomized controlled trial of 53 PCPs at one urban safety-net hospital based practice and three community health centers, and their estimated 1200 patients on chronic opioid therapy. Intervention PCPs have access to a nurse-managed patient registry, and receive academic detailing visits. Intervention and control PCPs receive electronic decision support tools to improve adherence to guidelines. Primary outcomes are PCP adherence to chronic opioid therapy guidelines and patient opioid misuse. We hypothesize that PCP adherence to chronic opioid therapy guidelines will increase more in intervention patients relative to control patients and that the proportion of patients with opioid misuse will decrease more among intervention patients relative to control patients.
Methods
Study design
We are performing a cluster randomized controlled trial of nurse care management, use of a patient registry, academic detailing, and electronic tools, relative to electronic tools alone, to promote PCP adherence to chronic opioid therapy guidelines and reduce opioid misuse among patients. Randomization to the study conditions is at the individual PCP level. Study recruitment is complete (we have recruited 53 PCPs); the intervention is complete at the first study site and in progress at three study sites. We included PCPs with a minimum of four patients receiving chronic opioid therapy for non-cancer pain. We will collect outcome data on all patients meeting the inclusion criteria whose PCP enrolls in the study. The Boston University Medical Center IRB approved the study protocol. Because the study is promoting an established standard of care, patients are not considered study subjects. Thus, we obtained a waiver of informed consent for patients.
Setting and Participants
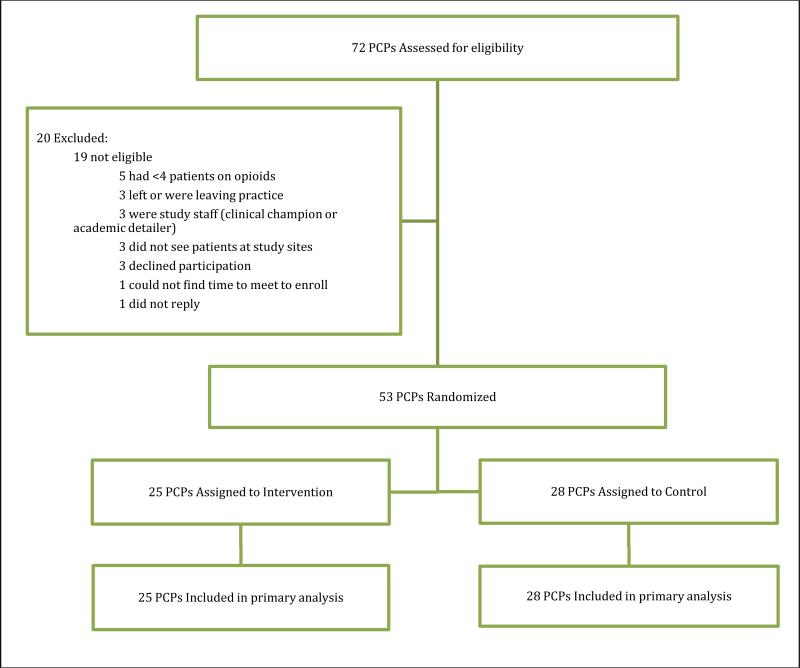
We are conducting the study at the adult primary care practice at Boston Medical Center, the largest safety-net hospital in New England, and at three affiliated community health centers. We pilot tested our intervention with two PCPs at Boston Medical Center, and one PCP at each of two community health centers. Through Boston Medical Center's clinical data warehouse, data were abstracted from the EMR (Centricity) used at all sites. We identified PCPs with at least four patients meeting the following patient inclusion criteria: 1) age ≥ 18; 2) one or more completed visits to the primary care practice in the past year; 3) long-term opioid treatment as defined by three or more opioid prescriptions written at least 21 days apart within six months and 4) an inpatient or outpatient ICD-9-CM diagnosis for musculoskeletal or neuropathic pain. We excluded resident PCPs from the study, since they are only present in the practice one week each month. Figure 2 shows the number of PCPs accrued and randomized to date. Of the 72 potentially eligible PCPs, 53 (74%) met study entry criteria and agreed to participate in the study.
Figure.
Consolidated Standards of Reporting Trials study flow diagram. PCP, primary care provider (ie, a physician, nurse practitioner, or physician assistant).
Study conditions
We randomly assigned PCPs to receive electronic tools or an intervention package consisting of nurse care management, use of a patient registry, academic detailing, and electronic tools. The intervention lasts for one year. We randomized individual PCPs to condition using random number generators in SAS. To ensure equivalence across treatment conditions, we stratified random assignment at each study site on the basis of PCP type defined by three levels: 1) physicians who prescribe suboxone; 2) physicians who do not prescribe suboxone; and 3) nurse practitioner or physician assistant. We stratified random assignment by prescription of suboxone because providers who prescribe suboxone may be systematically different than providers who do not prescribe suboxone. The former may be more familiar with the risks associated with prescription opioid use, and hence may be more likely to monitor patients with urine testing and to utilize treatment agreements (our study outcomes). We enrolled participants from December 2012 through March 2015 and are following them for one year after enrollment. The PCPs and patients are not blinded to intervention condition.
Control Condition (electronic tools)
Control PCPs receive web-based electronic tools at www.mytopcare.org. This content is common to both intervention and control conditions. By providing a tool that allows access to the current standard of care, we ensure that control condition providers at the very least have access to evidence-based tools that support adherence to clinical practice guidelines. The study PIs (KL and JL, both practicing PCPs), a PCP with expertise in informatics (CS), and the nurse care manager (NCM) developed the tools for the study. They consulted with a nationally recognized expert in opioid prescribing as well as a pharmacy expert. Nurses at one of the community health center sites provided patient education materials.
Web-based tools include patient pain assessments such as the Pain/Enjoyment/General Function (PEG) Screening Tool (Krebs et al., 2009), substance abuse screening tools such as the 10-item Alcohol Use Disorders Identification Test (AUDIT)(Figlie, Pillon, Dunn, & Laranjeira, 2000; Johnson, Lee, Vinson, & Seale, 2013; Lundin, Hallgren, Balliu, & Forsell, 2015; Meneses-Gaya et al., 2010) and the 11-item Drug Use Disorders Identification Test (DUDIT) (Durbeej et al., 2010; Figlie, et al., 2000; Lundin, et al., 2015; Mdege & Lang, 2011; Meneses-Gaya, et al., 2010), the Patient Health Questionnaire for depression (PHQ9)(Milette, Hudson, Baron, & Thombs, 2010; Zuithoff et al., 2010), and the Opioid Risk Tool (ORT) to assess risk for prescription drug misuse (Zgierska, Miller, & Rabago, 2012). The tools calculate scores with recommendations for specific action for each tool, when appropriate. For example, a low-risk score on the ORT prompts the PCP to perform urine drug testing every six months, while a high-risk score prompts PCPs to do more frequent urine testing as well as frequent PMP checks.
We used two different approaches to demonstrate the web-based tools to PCPs: one-on-one meetings with one of the study PIs, or demonstration of the tools during a provider meeting. In both forums, PCPs had the opportunity to ask questions about the tools.
Patients of control PCPs do not have interactions with the NCM and are not included in the registry. Intervention Condition (nurse care management, patient registry, academic detailing, and electronic tools)
Nurse Care Management
Table 1 summarizes how the intervention content maps to the different domains of the Chronic Care Model and how we are measuring the implementation of each area. The term “nurse care management intervention” refers only to the nurse care management intervention component, and not to the three other intervention components (patient registry, academic detailing, and electronic tools). We modeled the nurse care management intervention component on Boston Medical Center's successful collaborative care office-based opioid treatment (OBOT) program (Alford et al., 2011). The OBOT program uses a similar approach where nurses manage a population of patients and ensure that they receive assessments for risk and regular monitoring with urine tests.
In the present study, two NCMs work with PCPs assigned to the intervention condition. The NCMs are based centrally, in the Section of General Internal Medicine, and divide their time between the four study sites (three CHCs and one hospital-based clinic). The NCMs are dedicated to the project full-time. They attend the weekly research meeting for the project and have received training on the concept of contamination and the importance of avoiding interaction with control PCPs and their patients.
The main focus of the NCMs is to ensure that patients are receiving guideline-adherent care, which involves appropriate clinical assessments, controlled substance agreements, refill management, administration of monitoring tools according to risk level (urine toxicology screen, pill counts, PMP) and timely PCP visits to assess pain. To aid in carrying out these tasks, they utilize the electronic tools as well as the registry. When starting the intervention, the NCMs initially review the list of patients with each PCP to identify patients with risk factors for opioid misuse or dependence. Then the NCMs assess the status of high-risk intervention patients with regard to fulfillment of each aspect of guideline adherent care. Each week they assess patients scheduled for upcoming clinical visits with the PCP or for medication refills to see what care elements are lacking. The NCMs communicate to PCPs which of their patients require agreements and arrange for urine testing and pill counts according to standard registry reports. The NCMs counsel all high-risk intervention patients on safe medication storage and symptoms of addiction. Since the initial assessments require the largest time investment, the intervention is being rolled out to the four sites in sequence. If the NCMs are alerted to any (predetermined) high-risk results (e.g. urine toxicology screen with unexpected illicit substance), they contact the PCP to formulate an action plan to be put in place before the patient's next refill is due. The NCMs facilitate referrals to addiction treatment and community resources, as indicated. The way in which NCMs make these referrals depends on the severity of the patient's addiction, the risk level, and the patient's interest. For example, the NCM may call the OBOT nurse about a patient, or may hand a patient a list of methadone clinics.
The NCMs do not have a pre-determined number of visits and assessments for each patient; rather, they tailor an individualized care plan for each patient according to risk level. The NCMs and study PIs meet on a weekly basis to discuss challenging cases. Supervision is considered a necessary part of the NCM component. We define challenging cases as those 1) involving a high-risk patient where the PCP is not responding to or disagrees with the NCMs suggestions (e.g. to reduce dose of opioid); 2) where the PCP and NCM are unable to come up with a treatment plan and 3) where the NCM is having difficulty communicating with the PCP (e.g. the PCP does not respond to electronic messages). The team uses registry data in these discussions, specifically, reports by provider with percentage of patients having treatment agreements and urine tests.
Registry
We developed a freestanding centralized disease management application and web-based registry built using free open-source case-management software using SQL database technology (Sugar CRM Community Edition; http://www.sugarcrm.com/). Urine drug screening data from the EMR feeds into the registry on a daily basis via the hospital clinical data warehouse. The NCMs use a custom registry interface to monitor activities (e.g. whether the prescription monitoring program was checked, whether pill counts were done, and the frequency of aberrant behaviors such as failure to complete random urine tests) across the entire practice at each site and use population management tools to provide aggregate measures for quality monitoring. The measures include whether patients have a signed controlled substance agreement, and at least one completed urine drug test for controlled substances or illicit substances during the past year. All quality metrics can be downloaded in aggregate form for further analysis.
Academic detailing
PCPs in the intervention group receive one 45-60-minute individual visit from an expert in addiction and pain medication management two to three months after project implementation in the PCP's practice site. Prior to the visit, the NCMs provide specific data on the target PCP, including number of patients on chronic opioid therapy, percent of patients with guideline concordant care (at least one urine drug screen in 12 months, presence of controlled substance agreement in the EMR), percent of patients with multiple long-acting or multiple short-acting opioids, percent with morphine equivalent daily dose above 100 mg/day, percent with comorbid or high risk conditions (obstructive sleep apnea, mental health disorder, substance use disorder). Visit content combines elements of audit and feedback (e.g. the expert reviews registry reports of individual PCP compared with that of peers and goals) with traditional educational outreach (Grimshaw et al., 2004; O'Brien, et al., 2007; Soumerai et al., 1993). Specifically, experts review each aspect of guideline concordant care (assessment of risk and appropriateness for opioid medication, medication dosing, monitoring for harm/adherence, and pain outcomes) to solicit barriers to implementation or lack of knowledge for each aspect of care. PCPs then discuss challenging cases, during which the experts address barriers identified, using motivational interviewing as needed, to facilitate behavioral change in applying guideline-concordant care (Hettema, Ernst, Williams, & Miller, 2014; Hettema, Sorensen, Uy, & Jain, 2009). Utilizing open-ended questions, reflections, and summaries, the experts elicit “change talk” from PCPs in situations where PCPs are not adhering to guidelines in their practice.
Outcome measures
Administrative data
Our primary outcomes are 1) PCP adherence to chronic opioid therapy guidelines, defined as whether the patient has ever signed an controlled substance agreement (note that an agreement completed in the EMR prior to the study period would be considered sufficient) (Hariharan, Lamb, & Neuner, 2007) and urine drug testing (at least one completed urine drug test for controlled substances or illicit substances during the one-year study period) (Starrels, et al., 2011); signed controlled substance agreement and performance of urine drug testing are two co-primary variables; and 2) opioid misuse by patients. We define opioid misuse as two or more early refills. To identify early refills of opioid prescriptions, we will calculate the duration of a prescription based on the number of pills dispensed and the directions, conservatively assuming that the patient took the medication at the maximal prescribed rate. We define an early refill as being written at least three days before the previous prescription for the same medication should have been finished. We will calculate the “days early” for each prescription by first calculating the days supplied from the number of pills in the prescription divided by the maximum number of pills per day, interpreted from the PCPs’ instructions on the specific prescription. The days supplied will then be subtracted from the days between prescriptions to give the number of days early. We will also perform sensitivity analyses, employing other definitions of early refills (e.g. 4-7 days early). Our definition of early refills will permit up to one early refill as in previous studies (Reid et al., 2002).
Each outcome is a dichotomous variable assessed one year after enrollment. Our definitions are based on outcomes from our prior studies and from other published literature (Hariharan, et al., 2007; Khalid et al., 2014; Lange et al., 2015; Reid, et al., 2002; Starrels, et al., 2011). A blinded data analyst will extract all study outcomes and exploratory variables from a clinical data warehouse.
Secondary outcomes include substance abuse (urine drug tests where there is an illicit substance or something other than the prescribed medication present), possible opioid diversion (urine drug tests where the prescribed opioid is absent), and “questionable activity” (patients with missed urine drug screens i.e. urine test ordered but not done). We will also measure opioid risk in the following ways: 1) proportion of patients on high dose (> 100 mg/day) of morphine equivalent, 2) mean total dose of opioid prescribed, per patient, at end of study period; 3) Mean change in opioid dose, per patient, over the study period. Measures of health care utilization include number of emergency department and PCP visits over the study period.
All patient-level outcomes will be extracted from the clinical data warehouse; we are not distributing surveys to patients.
PCP survey data
At baseline we surveyed PCPs regarding their demographics and administered the Learning Organization Survey which asks about organizational learning characteristics related to study implementation (Garvin, Edmondson, & Gino, 2008). At baseline and one year we survey PCPs regarding their attitudes toward treating chronic nonmalignant pain (Fox, Kunins, & Starrels, 2012), use of pill counts for patients on opioids, termination of opioids, and use of the Massachusetts PMP. At one year we also ask PCPs about their use of the electronic tools on www.mytopcare.org. Table 1 summarizes how the survey items map to the different domains of the Chronic Care Model.
Statistical analysis of administrative and survey data
Based on a two-group study with an alpha of 0.05, 80% power will be achieved with a total sample size of 50 PCPs with a 15-percentage point difference of the proportions of having treatment agreement (e.g., adherence as the primary outcome) between the two groups. A design effect has been added to allow for clustering at the PCP level with an intra-class correlation of 0.1. Assuming that each PCP on average will have 24 patients in the study (as observed in our baseline analyses), the design effect of this clustering is 3.3. For our primary aim, this cluster randomized controlled trial design will allow us to evaluate the relative effectiveness of the intervention compared to the control condition on PCP adherence to chronic opioid therapy guidelines. Subsequently, we will assess its relative impact on reducing opioid misuse. We will first examine demographic characteristics of the intervention and control groups to verify that randomization has resulted in groups with similar baseline characteristics. In order to determine the bivariate relationship of whether the intervention condition achieves greater adherence to guidelines and reduced opioid misuse at 12 month follow-up compared to control participants, we will compare the baseline and follow-up measures of the individual patients, stratifying by intervention status and noting differences that are statistically significant at the p=0.05 level. Analyses will be based on intent-to-treat. To control for potential confounders identified in bivariable analyses as well as variables of a priori clinical significance (gender, age, race, ethnicity, and insurance), we will regress the 12-month follow-up outcomes on the intervention status with the adjustment of baseline measures. We will use multiple logistic regression techniques with robust standard error estimates adjusting for the clustering at the PCP levels (Generalized Estimating Equations method). We will utilize odds ratios with 95% confidence intervals to determine the relative magnitude of the adjusted associations for each outcome. Independent variables with high correlations may result in collinearity. To assess the extent of collinearity, we will assess variance inflation factors and the standard errors estimates for the covariates in the model.
Qualitative data
We are observing the organizational contexts in which study implementation is occurring. We are also conducting semi-structured interviews with PCPs in the nurse care management-patient registry-academic detailing-electronic tools study arm about their experiences. In addition, we are directly observing interactions between NCMs and patients, NCMs and PCPs, and PCPs and academic detailing physicians, using standard observation forms for data collection. In gathering these qualitative data, we will examine how the intervention delivery and content maps to the following domains of the Chronic Care Model (health care organization, delivery system design, clinical information systems, decision support, self-management support and community resources). Corresponding to the domain of health care organization, we are noting the extent to which clinical leadership views chronic care as a priority, as well as the extent to which respondents perceive leadership support for the intervention components. For the domain of delivery system design we are looking at how the NCM's role relates to the PCP's role and how they collaborate in patient care. For the domains of clinical information systems and decision support we are examining how the NCMs use the registry and which registry functions have been the most helpful. We are also examining how the PCPs use the electronic tools. In the domain of self-management support we are looking for NCM-delivered patient education and coaching about exercise and medication use as ways for patients to manage their pain. In the domain of community resources we are looking at how the NCM refers patients to these resources. These qualitative data will also enable us to understand and assess the process of implementing the multi-mode intervention. Forms for structured observations and field notes of academic detailing sessions and NCM – patient interactions were developed based on categories observed in pilot observations. Descriptive field notes about initial PCP meetings at new sites and about ad hoc PCP-NCM meetings are also being recorded. All of the qualitative data will be reviewed and coded in order to identify important themes related to the successes and challenges of the different intervention components, particularly the academic detailing and the nurse care management.
The qualitative data will not be used in the outcome analyses. Rather, this data will help us to understand why the intervention was or was not effective. If the intervention was effective, qualitative data will help us to ascertain which component of the intervention may have been the most effective.
Results
Pilot study
Starting in July 2013, we piloted the intervention for five months with two PCPs and their 33 patients on chronic opioid therapy at the urban safety-net hospital based practice. In this initial pilot test, we demonstrated feasibility and acceptability; the intervention was well received by the PCPs and patients. We observed a high frequency of aberrant behaviors among patients, with four of 33 patients having one of the following aberrant behaviors: they had incorrect numbers of opioid pills at pill counts with NCMs, had Tylenol in their opioid pill bottles instead of the prescribed opioid, cocaine on urine drug screens, and were not taking medication as prescribed. We pilot-tested the intervention at CHC #1 with the clinical champion PCP. We learned that the PCPs in that practice preferred to give opioid prescriptions at the time of a visit, rather than having a nurse prepare prescriptions ahead of time. Therefore, we modified the intervention at that site and the NCM does not prepare opioid prescriptions as part of the intervention at that site. We also pilot-tested the intervention with the clinical champion PCP at CHC #2. At this site, we learned that opioid prescriptions were triggered by a patient phone call, and that nurses completed treatment agreements with patients (whereas PCPs completed agreements at the other study sites). Our NCM at that site worked with the practice to change their workflow, encouraging providers to write 28-day prescriptions. At this site, nurses do not print opioid prescriptions, this is done by PCPs.
PCPs
Table 2 lists the baseline characteristics of the intervention and control groups; randomization resulted in groups with similar baseline characteristics. PCPs were represented across a spectrum of ages, including those who have recently completed residency training and those who have been in practice for over 25 years. Approximately two-thirds of PCPs were female; most PCPs were white (67%), with the remainder Asian (19%), African American or black (9%), and of other race (4%). None of the participating PCPs were of Hispanic ethnicity. Most PCPs were physicians (91%); with the remainder nurse practitioners (8%) and physician assistants (2%). Nearly one-third of PCPs had completed training in prescription of buprenorphine.
Table 2.
| Characteristic | Intervention, % (n= 25) | Control, % (n= 28) | P value |
|---|---|---|---|
| Age group, y | |||
| 25-35 | 28 | 29 | 0.56 |
| 36-40 | 20 | 7 | |
| 41-45 | 20 | 14 | |
| 46-50 | 8 | 14 | |
| ≥ 51 | 24 | 36 | |
| Sex | |||
| Female | 68 | 60 | 0.58 |
| Race | |||
| White | 64 | 68 | 0.86 |
| African American/Black | 12 | 7 | |
| Asian | 20 | 18 | |
| Other | 4 | 7 | |
| Clinician type | |||
| Physician | 96 | 86 | 0.40 |
| Physician's assistant | 0 | 4 | |
| Nurse practitioner | 4 | 11 | |
| Trained to prescribe buprenorphine | 32 | 29 | 0.62 |
PCPs include physicians, nurse practitioners, and physician assistants.
Totals may not equal 100 due to rounding.
Patients
Baseline characteristics of patients at three of the four participating sites have been published elsewhere (Khalid, et al., 2014; Lange, et al., 2015). At the adult primary care practice at Boston Medical Center, 42% of patients had ever had a controlled substance agreement, 64% had any urine drug testing in the past year, and 32% had at least two early refills of opioids in the past year. At CHC #1, 45% of patients had ever had a controlled substance agreement, 24% had any urine drug testing in the past year, and 39% had at least two early refills of opioids in the past year. Similarly, at CHC #2, 49% of patients had ever had a controlled substance agreement, 37% had any urine drug testing in the past year, and 34% had at least two early refills of opioids in the past year.
Discussion
We are implementing a highly innovative redesign of primary care delivery to improve quality of care received by patients with chronic non-malignant pain on chronic opioid therapy. Our study extends implementation of the chronic care model beyond conditions such as diabetes, depression and asthma. Our use of addiction and pain specialists to perform academic detailing, as well as our website www.mytopcare.org, are other innovations.
As is not uncommon in implementation research, we have encountered several challenges in the intervention rollout. First, one of our community health center study sites hired a NCM to perform an identical role to that of our study NCM. Therefore, we excluded that study site (prior to enrolling PCPs) and recruited an additional community health center site. Second, we had hoped to obtain “batch” data on intervention patients from the Massachusetts PMP. However, such data has been unavailable, and until March, 2015, NCMs were not able to serve as “delegates” to look up PMP data on patients. We have advocated with the Massachusetts Department of Health to enable delegation of this work to non-physician members of the health care team. Third, at two of the community health centers we found that urine test results were not populating the registry. At one site this was due to a preexisting patient privacy data policy, requiring the NCM to look up results manually, patient by patient, and enter the results into the registry. Eventually, these concerns were alleviated from consultation with a local thought leader in the field of opioid prescribing. At the second site the problem was due to incomplete data labeling, which we are addressing.
Limitations
Our primary study outcomes are proximal to the “harder outcomes” of morbidity and mortality. We considered outcomes such as opioid overdoses and overdose-related deaths. However, these outcomes would be too rare in a sample of 1200 patients. Another study limitation is that it will not be possible to determine the individual effect of each intervention component on quantitative study outcomes. Rather, we are only able to test the effectiveness of the entire, four-component intervention package against the electronic tools-only control condition. Early refills are a proxy for opioid misuse and are not a direct measure of misuse. We have previously demonstrated that 20% of early refills are prescribed for legitimate reasons (e.g., for vacation supply or dose escalation (Khalid, et al., 2014)); using early refills as a proxy for opioid misuse may overestimate the magnitude of such misuse. To better estimate aberrant early refills versus refills generated from closer monitoring with shorter prescriptions (e.g. 14 vs. 28 day prescriptions), we have developed algorithms that take into account the length of time of the prescription. While each early refill may not indicate misuse, it does create an opportunity for misuse or diversion to occur. We will not have information about early refills provided by prescribers outside of the primary care practices studied. Thus, the prevalence of early refills in our study is likely an underestimate.
Potential implications
Our study may improve monitoring of patients with chronic pain on opioids, thus potentially preventing the urgent public health issues of opioid diversion and misuse. If this study demonstrates the effectiveness of the intervention components on increasing PCP adherence to chronic opioid therapy guidelines and reducing opioid misuse among patients, there is the opportunity to transform the way in which care is currently delivered to patients with chronic pain on chronic opioid therapy.
Highlights: We will.
Present the protocol of a cluster randomized controlled trial testing the effectiveness of nurse care management, use of a patient registry, academic detailing, and electronic tools, relative to electronic tools only, to promote PCP adherence to chronic opioid therapy guidelines and reduce patient opioid misuse
Present baseline data on PCPs enrolled to date
Acknowledgments
Funding Source: This research is supported by a grant from the National Institute on Drug Abuse R01DA034252
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: The protocol for this study is registered with clinicaltrials.gov (registration number: NCT01909076).
References
- Alford DP, LaBelle CT, Kretsch N, Bergeron A, Winter M, Botticelli M, Samet JH. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Archives of internal medicine. 2011;171(5):425–431. doi: 10.1001/archinternmed.2010.541. doi: 10.1001/archinternmed.2010.541. [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S.].
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. The New England journal of medicine. 2003;349(20):1943–1953. doi: 10.1056/NEJMra025411. doi: 10.1056/NEJMra025411. [Research Support, U.S. Gov't, P.H.S. Review].
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. Jama. 2002;288(14):1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- Carter A, Hall W. Informed consent to opioid agonist maintenance treatment: recommended ethical guidelines. The International journal on drug policy. 2008;19(1):79–89. doi: 10.1016/j.drugpo.2007.09.007. doi: 10.1016/j.drugpo.2007.09.007. [Research Support, Non-U.S. Gov't].
- Chou R FG, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O'Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbeej N, Berman AH, Gumpert CH, Palmstierna T, Kristiansson M, Alm C. Validation of the Alcohol Use Disorders Identification Test and the Drug Use Disorders Identification Test in a Swedish sample of suspected offenders with signs of mental health problems: results from the Mental Disorder, Substance Abuse and Crime study. Journal of substance abuse treatment. 2010;39(4):364–377. doi: 10.1016/j.jsat.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Figlie NB, Pillon SC, Dunn J, Laranjeira R. The frequency of smoking and problem drinking among general hospital inpatients in Brazil - using the AUDIT and Fagerstrom questionnaires. Sao Paulo medical journal = Revista paulista de medicina. 2000;118(5):139–143. doi: 10.1590/S1516-31802000000500005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AD, Kunins HV, Starrels JL. Which skills are associated with residents' sense of preparedness to manage chronic pain? Journal of opioid management. 2012;8(5):328–336. doi: 10.5055/jom.2012.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin DA, Edmondson AC, Gino F. Is yours a learning organization? Harv Bus Rev. 2008;86(3):109–116. [PubMed] [Google Scholar]
- Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6(2):107–112. doi: 10.1111/j.1526-4637.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, Donaldson C. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health technology assessment. 2004;8(6):iii–iv. 1–72. doi: 10.3310/hta8060. [Research Support, Non-U.S. Gov't Review].
- Hariharan J, Lamb GC, Neuner JM. Long-term opioid contract use for chronic pain management in primary care practice. A five year experience. [Comparative Study]. Journal of general internal medicine. 2007;22(4):485–490. doi: 10.1007/s11606-006-0084-1. doi: 10.1007/s11606-006-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JE, Ernst D, Williams JR, Miller KJ. Parallel processes: using motivational interviewing as an implementation coaching strategy. J Behav Health Serv Res. 2014;41(3):324–336. doi: 10.1007/s11414-013-9381-8. [DOI] [PubMed] [Google Scholar]
- Hettema JE, Sorensen JL, Uy M, Jain S. Motivational enhancement therapy to increase resident physician engagement in substance abuse education. Subst Abus. 2009;30(3):244–247. doi: 10.1080/08897070903041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Lee A, Vinson D, Seale JP. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcoholism, clinical and experimental research. 2013;37(1):1530–0277. doi: 10.1111/j.1530-0277.2012.01898.x. [DOI] [PubMed] [Google Scholar]
- Katz N, Panas L, Kim M, Audet AD, Bilansky A, Eadie J, Carrow G. Usefulness of prescription monitoring programs for surveillance--analysis of Schedule II opioid prescription data in Massachusetts, 1996-2006. Pharmacoepidemiology and drug safety. 2010;19(2):115–123. doi: 10.1002/pds.1878. doi: 10.1002/pds.1878. [Historical Article Research Support, U.S. Gov't, Non-P.H.S.].
- Khalid L, Liebschutz JM, Xuan Z, Dossabhoy S, Kim Y, Crooks D, Lasser KE. Adherence to Prescription Opioid Monitoring Guidelines among Residents and Attending Physicians in the Primary Care Setting. Pain Med. 2014;19(10):12602. doi: 10.1111/pme.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu J, Sutherland JM, Kroenke K. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. Journal of general internal medicine. 2009;24(6):733–738. doi: 10.1007/s11606-009-0981-1. doi: 10.1007/s11606-009-0981-1. [Comparative Study Research Support, N.I.H., Extramural, U.S. Gov't, Non-P.H.S. Validation Studies].
- Kroenke K, Bair MJ, Damush TM, Wu J, Hoke S, Sutherland J, Tu W. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA. 2009;301(20):2099–2110. doi: 10.1001/jama.2009.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Krebs EE, Wu J, Yu Z, Chumbler NR, Bair MJ. Telecare collaborative management of chronic pain in primary care: a randomized clinical trial. JAMA. 2014;312(3):240–248. doi: 10.1001/jama.2014.7689. [DOI] [PubMed] [Google Scholar]
- Landon BE, Hicks LS, O'Malley AJ, Lieu TA, Keegan T, McNeil BJ, Guadagnoli E. Improving the management of chronic disease at community health centers. The New England journal of medicine. 2007;356(9):921–934. doi: 10.1056/NEJMsa062860. doi: 10.1056/NEJMsa062860. [Evaluation Studies Research Support, U.S. Gov't, P.H.S.].
- Lange A, Lasser KE, Xuan Z, Khalid L, Beers D, Heymann OD, Liebschutz JM. Variability in opioid prescription monitoring and evidence of aberrant medication taking behaviors in urban safety-net clinics. Pain. 2015;156(2):335–340. doi: 10.1097/01.j.pain.0000460314.73358.ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin A, Hallgren M, Balliu N, Forsell Y. The Use of Alcohol Use Disorders Identification Test (AUDIT) in Detecting Alcohol Use Disorder and Risk Drinking in the General Population: Validation of AUDIT Using Schedules for Clinical Assessment in Neuropsychiatry. Alcoholism, clinical and experimental research. 2015;39(1):158–165. doi: 10.1111/acer.12593. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Manchukonda R, Pampati V, Damron KS, Brandon DE, Cash KA, McManus CD. Does random urine drug testing reduce illicit drug use in chronic pain patients receiving opioids? Pain physician. 2006;9(2):123–129. [Comparative Study Randomized Controlled Trial].
- Mdege ND, Lang J. Screening instruments for detecting illicit drug use/abuse that could be useful in general hospital wards: A systematic review. Addictive behaviors. 2011;36(12):1111–1119. doi: 10.1016/j.addbeh.2011.07.007. doi: http://dx.doi.org/10.1016/j.addbeh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Meneses-Gaya C, Zuardi AW, Loureiro SR, Hallak JE, Trzesniak C, de Azevedo Marques JM, Crippa JA. Is the full version of the AUDIT really necessary? Study of the validity and internal construct of its abbreviated versions. Alcoholism, clinical and experimental research. 2010;34(8):1417–1424. doi: 10.1111/j.1530-0277.2010.01225.x. doi: 10.1111/j.1530-0277.2010.01225.x. [Comparative Study Research Support, Non-U.S. Gov't Validation Studies].
- Milette K, Hudson M, Baron M, Thombs BD. Comparison of the PHQ-9 and CES-D depression scales in systemic sclerosis: internal consistency reliability, convergent validity and clinical correlates. Rheumatology. 2010;49(4):789–796. doi: 10.1093/rheumatology/kep443. doi: 10.1093/rheumatology/kep443. [Multicenter Study Research Support, Non-U.S. Gov't].
- Morasco BJ, Duckart JP, Dobscha SK. Adherence to clinical guidelines for opioid therapy for chronic pain in patients with substance use disorder. Journal of general internal medicine. 2011;26(9):965–971. doi: 10.1007/s11606-011-1734-5. doi: 10.1007/s11606-011-1734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard-Jensen J, Kristoffersen DT, Harvey EL. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane database of systematic reviews. 2007;(4):CD000409. doi: 10.1002/14651858.CD000409.pub2. [Meta-Analysis Review]. doi: 10.1002/14651858.CD000409.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passik SD, Kirsh KL. Assessing aberrant drug-taking behaviors in the patient with chronic pain. Curr Pain Headache Rep. 2004;8(4):289–294. doi: 10.1007/s11916-004-0010-3. [DOI] [PubMed] [Google Scholar]
- Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O'Connor PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. Journal of general internal medicine. 2002;17(3):173–179. doi: 10.1046/j.1525-1497.2002.10435.x. [Research Support, U.S. Gov't, Non-P.H.S.].
- Shojania KG, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane database of systematic reviews. 2009;(3):CD001096. doi: 10.1002/14651858.CD001096.pub2. [Meta-Analysis Review] doi: 10.1002/14651858.CD001096.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon DH, Van Houten L, Glynn RJ, Baden L, Curtis K, Schrager H, Avorn J. Academic detailing to improve use of broad-spectrum antibiotics at an academic medical center. Archives of internal medicine. 2001;161(15):1897–1902. doi: 10.1001/archinte.161.15.1897. [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov't].
- Soumerai SB, Salem-Schatz S, Avorn J, Casteris CS, Ross-Degnan D, Popovsky MA. A controlled trial of educational outreach to improve blood transfusion practice. JAMA : the journal of the American Medical Association. 1993;270(8):961–966. [Clinical Trial Multicenter Study Randomized Controlled Trial Research Support, U.S. Gov't, P.H.S.].
- Starrels JL, Becker WC, Weiner MG, Li X, Heo M, Turner BJ. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. Journal of general internal medicine. 2011;26(9):958–964. doi: 10.1007/s11606-011-1648-2. doi: 10.1007/s11606-011-1648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration Results from the 2006 National Survey on Drug Use and Health: national Findings. 2007 [Google Scholar]
- Wang J, Christo PJ. The influence of prescription monitoring programs on chronic pain management. Pain physician. 2009;12(3):507–515. [PubMed] [Google Scholar]
- Zgierska A, Miller M, Rabago D. Patient Satisfaction, Prescription Drug Abuse, and Potential Unintended Consequences. Journal of the American Medical Association. 2012;307(13) doi: 10.1001/jama.2012.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuithoff NP, Vergouwe Y, King M, Nazareth I, van Wezep MJ, Moons KG, Geerlings MI. The Patient Health Questionnaire-9 for detection of major depressive disorder in primary care: consequences of current thresholds in a crosssectional study. BMC family practice. 2010;11:98. doi: 10.1186/1471-2296-11-98. doi: 10.1186/1471-2296-11-98. [Research Support, Non-U.S. Gov't Validation Studies].