Abstract
Objective
To evaluate the temporal course of seizure outcome in children with pathology-confirmed focal cortical dysplasia and to explore predictors of sustained seizure freedom.
Methods
We performed a single-center, retrospective study of children ≤18 years who underwent resective surgery from January 1, 2000, through December 31, 2012, with pathology-proven focal cortical dysplasia. Surgical outcome was classified as seizure free (Engel class I) or seizure recurrence (Engel classes II-IV). Fisher exact and nonparametric Wilcoxon rank sum tests were used, as appropriate. Survival analysis was based on seizure-free outcome. Patients were censored at the time of seizure recurrence or seizure free at last follow-up.
Results
Thirty-eight cases were identified (median age at surgery, 6.5 years; median duration of epilepsy, 3.3 years). Median time to last follow-up was 13.5 months (IQR, 7-41 months). Twenty patients (53%) were seizure free and 26 patients (68%) attained seizure freedom for minimum of three months. Median time to seizure recurrence was 38 months (95% CI, 6-109 months), and the cumulative seizure-free rate was 60% at 12 months (95% CI, 43%-77%). Clinical features associated with seizure freedom at last follow-up included older age at seizure onset (P=.02), older age at surgery (P=.04), absent to mild intellectual disability before surgery (P=.05), and seizure freedom for a minimum of three months (P<.001).
Conclusion
Favorable clinical features associated with sustained seizure freedom included older age at seizure onset, older age at surgery, absent or mild intellectual disability at baseline, and seizure freedom for a minimum of three months.
Keywords: epilepsy surgery, focal cortical dysplasia, neuroimaging, pediatric epilepsy, presurgical evaluation, seizure freedom
Introduction
Focal cortical dysplasia (FCD) represents a subset of malformations of cortical development. First described by Taylor and colleagues in 1971 (1), FCD shows areas of abnormal neuronal migration and organization. It is the most common pathologic finding in pediatric epilepsy surgery and the third most common finding in adult epilepsy surgery (2). Affected patients often present with seizures in childhood, and 70% have daily seizures that are refractory to medical therapy (2).
Epilepsy surgery outcome relies on the preoperative hypothesis of the location of the epileptogenic zone and strongly depends on the presence of a focal anatomic lesion (3). A structural magnetic resonance imaging (MRI) abnormality is present in about 65% of patients with pathology-confirmed FCD (4). Scalp electroencephalography (EEG) correlates with radiographically evident FCD in 49% to 68% of patients (4). In MRI-negative focal epilepsy or in patients with discordant radiographic and neurophysiologic findings, other noninvasive modalities such as fluorodeoxyglucose (FDG), positron emission tomography (PET), singlephoton emission computed tomography (SPECT), and subtraction ictal SPECT coregistered to MRI (SISCOM) may offer additional localizing information (5-8). Invasive EEG monitoring can be performed in MRI-negative cases to define the epileptogenic zone, and in select cases, it may be used to localize areas of the brain that subserve motor, sensory, language, and memory function (the “eloquent cortex”).
Surgical evaluation is an accepted and advocated approach for medically refractory focal epilepsy. Among patients with pathology-confirmed FCD, reported seizure-free outcomes range from 60% to 80% (2,4,9). The best predictor of becoming seizure free after surgery is the removal of the MRI lesion and the epileptogenic zones (ie, the entire lesion) (9). Few studies have examined the temporal course of seizure control in children with pathology-confirmed FCD. The purpose of this study was to evaluate the timing of seizure recurrence after initial seizure-free control and to explore predictors of sustained seizure freedom in children with pathology-confirmed FCD.
Methods
The research protocol was approved by the Mayo Clinic Institutional Review Board.
Patients
This was a single-center, retrospective study of children 18 years and younger with pathology-proven FCD seen at Mayo Clinic (Rochester, Minnesota) from January 1, 2000, through December 31, 2012. Patients were identified via the medical chart search tool, review of 131 resective surgical procedures (excluding corpus callosotomy, vagus nerve stimulator placement) during the study period, and review of pathology results. We excluded three patients: two had a clinical history consistent with tuberous sclerosis and 1 had biopsy-proven FCD without epilepsy surgery.
Presurgical Evaluations
A standardized noninvasive presurgical evaluation of all cases included history and neurologic examination by pediatric epileptologists, seizure protocol structural MRI (10), and scalp video-EEG. Presurgical neurologic examination was classified as abnormal if focal deficits were identified by the clinician. Intellectual disability was defined by the developmental quotient (DQ), as estimated from clinical documentation or standard neuropsychometric testing. Intellectual disability was classified as absent (estimated DQ, >70), mild (estimated DQ, 50-70), or moderate to severe (estimated DQ, <50).
Results of presurgical evaluations were determined from multidisciplinary epilepsy surgery conferences and/or available reports of SPECT or SISCOM, FDG-PET, subdural EEG monitoring, and intraoperative electrocorticography.
Electroencephalography
All patients underwent prolonged video-EEG monitoring, recorded by a 32-channel system, by using the modified 10-20 system that included subtemporal electrodes. Extraoperative subdural EEG was performed in patients with conflicting results and also as needed to localize the eloquent cortex. Intraoperative functional mapping and electrocorticography (ECog) were also used as necessary. All EEG reports included information about the background and interictal and ictal states; interictal epileptiform abnormalities and ictal EEG onset were categorized as localizing if a single focal abnormality was identified. Epileptogenic areas were delineated from the EEG report and supported by the hospital progress notes for those who underwent extraoperative subdural EEG monitoring.
Neuroimaging
All patients underwent a seizure protocol structural MRI (10). Because the shift from 1.5 T to 3 T MRI may have improved MRI detection of cortical malformation, images from nonlesional and nonspecific lesional MRI studies (20 cases) were reviewed again. MRI images were scored by a neuroradiologist (R.J.W.) as being consistent with FCD (MRI+FCD), normal (MRI− FCD), or showing other structural abnormalities (MRI+ lesional). MRI features of MRI+ FCD included cortical thickening, blurring of the grey-white junction, and presence of a linear area of abnormal signal in the white matter extending from the cortex to the ventricle (transmantle sign).
Neuroimaging studies with SPECT or SISCOM and FDG-PET were obtained in selected cases. The ictal SPECT injection of technetium-99 radioisotope was performed by trained EEG technicians during the prolonged scalp video-EEG monitoring, as previously described (11-14). An interictal SPECT was performed after the patient was seizure-free for at least 24 hours. SPECT images were acquired within two to three hours after injection of radioisotopes for ictal and interictal studies. Interictal images were subtracted from the ictal SPECT and then coregistered with the MRI. PET imaging abnormality was defined as focal glucose hypometabolism. SISCOM and PET results were classified as localizing if there was a single focal abnormality or nonlocalizing otherwise. Various neuroimaging modalities along with the EEG were considered concordant if localizing to the same foci.
Surgery Type, FCD Subtypes, and Outcome
All patients underwent tailored surgical resections aimed to remove the epileptogenic zone that was defined by clinical, neuroimaging, and electrophysiologic studies. FCD subtype was based on a review of the pathology report and classified according to International League Against Epilepsy 2011 criteria (15). Cases in which the FCD subtype could not be determined from the pathology report were classified as FCD not specified. Completeness of anatomic resection was determined by review of available postoperative MRI compared with the preoperative MRI in MRI+ FCD cases. Completeness of the physiologic epileptogenic zone was ascertained from operative report or hospital progress notes when ECog or invasive subdural monitoring was performed. Surgical lateralization and extent of resection (corticectomy, lobar resection, multilobar resection) were determined from operative reports. Surgical outcome was graded according to Engel classification: class I, seizure-free, auras only, single seizure with antiepileptic drug withdrawal; class II, rare, nondisabling seizure (>90% reduction in seizures); class III, worthwhile improvement (80%-90% reduction in seizures); class IV, no worthwhile improvement (<80% reduction in seizures). Early remission was defined as seizure freedom for at least three months after surgical intervention. Early recurrence was defined as seizure recurrence within three months but outside of the immediate one-month postoperative period.
Statistical Analysis
Descriptive statistics included means and standard deviations or medians and ranges, as appropriate. We also classified surgical outcome as favorable (Engel class I/II) or unfavorable (Engel class III/IV) and as sustained seizure freedom (>3 months of seizure freedom) versus early recurrence (≤3 months of seizure freedom). Comparisons between patients with 1) seizure freedom or recurrence at last follow-up; 2) favorable versus unfavorable outcome at last follow-up; and 3) initial seizure freedom at three months versus early recurrence were made using t tests and Wilcoxon rank sum tests for parametric and nonparametric continuous variables, respectively, and by the Fisher exact test for dichotomous variables. Analysis was performed using the JMP statistical software package (version 9; SAS Institute Inc). P values ≤.05 were considered statistically significant. Kaplan-Meier survival analysis was based on seizure-free outcome. Patients were censored at the time of seizure recurrence or seizure free at last follow-up. For those patients who had repeated surgery during the study period, surgical outcome was unfavorable for the initial surgical intervention.
Results
Patient Characteristics
Thirty-eight pathology-confirmed FCD cases were identified, including two patients who had repeat surgery during the study period. Characteristics of the cohort are summarized in Table 1. At the time of presurgical evaluation, focal seizures were present in 36 patients (95%), with secondary generalization in 15 (39%). Two patients (5%) had epileptic spasms only. Most patients had daily seizures (n=30 [79%]) and all had been treated unsuccessfully with at least two antiepileptic medications (median, 5; IQR [interquartile range], 5-8).
Table 1.
Patient Characteristics (N=38)
| Characteristic | Value |
|---|---|
| Male, No. (%) | 26 (68) |
| Age at seizure onset, median (IQR), mo | 11.0 (1-51) |
| Age at surgery, median (IQR), y | 6.5 (1-13) |
| Duration of epilepsy, median (IQR), y | 3.3 (1-8) |
| Seizure onset, No. (%) | |
| Focal | 36 (95) |
| Secondarily generalized | 15 (39) |
| Spasmsa | 8 (21) |
| History of febrile seizure, No. (%) | 4 (11) |
| Number of trialed antiepileptic drugs, median (IQR) | 5 (5-8) |
| Ketogenic diet, No. (%) | 8 (21) |
| Vagal nerve stimulator, No. (%) | 4 (11) |
| Normal neurologic examination findings, No. (%) | 17 (45) |
| Absent or mild intellectual disability, No. (%) | 20 (53) |
| MRI, No. (%) | |
| Positive for FCD | 26 (68) |
| Negative for FCD | 12 (32) |
| EEG findings, No. (%) | |
| Focal interictal abnormality | 11 (29) |
| Focal ictal onset | 20 (53) |
| Intracranial monitoring, No. (%) | 14 (37) |
| Electrocorticography alone, No. (%) | 10 (26) |
| SISCOM, No. (%) | |
| Concordant with MRI+ FCD | 10 (26) |
| Concordant with focal ictal onset EEG | 12 (32) |
| Surgery type, No. (%) | |
| Lesionectomy | 23 (61) |
| Lobectomy | 10 (26) |
| Multilobar resection | 5 (13) |
| Surgery lateralization (right hemisphere), No. (%) | 22 (58) |
| Surgery location (temporal), No. (%) | 11 (29) |
Abbreviations: EEG , electroencephalography; FCD, focal cortical dysplasia; IQR, interquartile range; MRI, magnetic resonance imaging; SISCOM, subtraction ictal SPECT co-registered to MRI.
Two patients (5%) had epileptic spasms only.
During the initial evaluation by an epileptologist, only 17 children (45%) had normal findings upon neurologic examination. Notable findings included hemiparesis (n=12 [32%]), tone abnormalities (n=10 [26%]), facial palsy (n=10 [26%]), hemianopia (n=7 [18%]), and extremity posturing and brisk reflexes with extensor plantar response (n=7; [18%]). Six patients (16%) had normal baseline cognitive status; 14 (37%) showed mild intellectual disability, 15 (39%) had moderate to severe intellectual disability, and three (8%) did not have cognitive status documented. Among the 18 patients (47%) who underwent formal neuropsychometric testing, the median full-scale DQ was 76.5 (IQR, 66-87).
Presurgical Evaluation
Prolonged scalp video-EEG showed focal interictal epileptiform abnormalities in 11 (29%), and focal ictal onset in 20 (53%) patients.
MRI showed findings specific to FCD (MRI+ FCD) in 26 (68%), normal findings (MRI− FCD) in 4 (11%), and other lesional MRI abnormalities (MRI+ lesional) in 8 (21%) (2 with hemimegalencephaly, 2 with polymicrogyria, and 1 each with gliosis due to ischemia acquired during early development, amygdala enlargement, cortical mass, and postoperative changes from prior epilepsy surgery performed elsewhere).
Ictal scalp EEG was localizing in 15 of 26 patients (58%) with MRI+ FCD and was concordant with the radiographic location of FCD in 11 (42%). Ictal scalp EEG was localizing in four MRI+ lesional patients and one MRI− FCD patient.
Focal SISCOM abnormality was present in 23 of 25 patients in whom SISCOM was obtained. SISCOM abnormality was concordant with the anatomic abnormality in 10 of 26 patients with MRI+ FCD. SISCOM provided additional localizing information in three of four patients with MRI– FCD and in 12 of 18 patients with nonlocalizing ictal EEG findings.
FDG-PET was performed in only six patients. All showed focal hypometabolism abnormality and provided additional information in potential seizure focus for two of two patients with nonlocalizing SISCOM, two of four patients with MRI– FCD, and five of 18 patients with nonlocalizing ictal scalp video-EEG findings.
Twelve patients underwent resective surgery without invasive monitoring (MRI+ FCD, eight cases; MRI+ lesional, four cases). Extraoperative subdural invasive EEG monitoring was performed in 14 patients (seven with MRI+ FCD, all four MRI– FCD, three with MRI+ lesional), and 10 (26%) had intraoperative ECog only (nine with MRI+ FCD and one with MRI+ lesional). One patient underwent repeat extraoperative subdural EEG monitoring for repeat surgery. Another patient had intraoperative ECog for the first resection, with subsequent surgery delineated by extraoperative subdural EEG monitoring.
Surgery and FCD Classification
Most patients underwent corticectomy or lobectomy (n=33 [87%]), and multilobar resection was performed in five patients (13%). Corticectomy was more likely to be performed in children who underwent intraoperative ECog (P=.05). Of the 10 patients with single lobar resection, seven resections (70%) were extratemporal in location (frontal lobectomy in six, occipital lobectomy in one child with preexisting hemianopia). Among the 26 MRI+ FCD cases, 16 (62%) had complete resection of the anatomic and physiologic epileptogenic zone delineated by ECog or invasive monitoring. Complete resection of the anatomic MRI abnormality was performed in eight MRI+ FCD cases without ECog or invasive monitoring. Two cases had incomplete anatomic resection of MRI+ FCD. Among the four MRI− FCD cases, all had resection of the physiologic epileptogenic zone delineated by ECog or invasive monitoring. Among the eight MRI+ lesional cases, six had resection of the anatomic and physiologic epileptogenic zone delineated by ECog or invasive monitoring. The remaining patients included one whose epileptogenic zone may not have been entirely delineated, given that ictal involvement occurred at the edge of intracranial coverage. Another patient had focal resective surgery with hemispheric abnormality.
FCD subtypes included five with FCD I, nine with FCD IIA, and 13 with FCD IIB. There was a single case of FCD IIID (FCD adjacent to ischemic stroke acquired during early life). The FCD subtype was not specified in eight cases (MRI+ FCD, 7; MRI+ lesional, 1).
Surgical Outcome
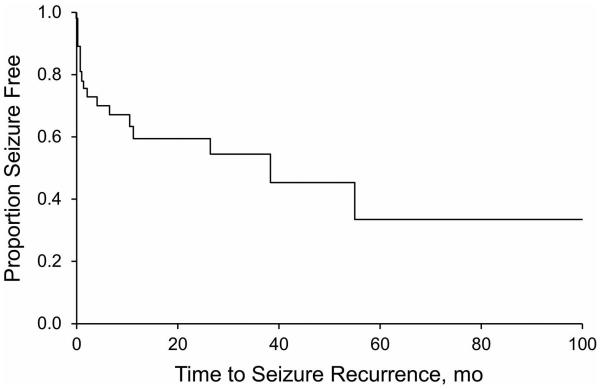
Twenty patients (53%) were seizure free at last follow-up (median follow-up, 13.5 months [IQR, 7-41 months]). In 18 patients (47%) with seizure recurrence at last follow-up, outcomes were favorable (Engel I/II) for eight and unfavorable (Engel III/IV) for 10. Median time to seizure recurrence was 38 months (95% CI, 6-109 months); the cumulative seizure-free outcome rate at 12 months was 60% (95% CI, 43%-77%) (Figure).
Figure.
Probability of seizure freedom after surgical resection of pathologyconfirmed focal cortical dysplasia in children.
Twenty-six patients (68%) attained early remission with seizure freedom for a minimum of three months. Among those who attained early remission, six patients had recurrence, with the median time to seizure of 54 months (95% CI, 37-109 months). In children who were seizure-free at three months, continued seizure freedom at 12 and 36 months was seen in 85% and 79%, respectively.
Clinical Factors Predictive of Seizure-Free Outcome
Clinical features associated with seizure freedom at last follow-up included older age at seizure onset (P=.02), older age at surgery (P=.04), absent to mild intellectual disability before surgery (P=.05), and early remission of three months (P<.001) (Table 2).
Table 2.
Surgical Outcome Categorized by Epilepsy History and Presurgical Evaluations
| Characteristic | Seizure Free (n=20) |
Seizure Recurrence (n=18) |
P
Value |
Initial Seizure Freedom (n=26) |
Early Seizure Recurrence (n=12) |
P
Value |
|---|---|---|---|---|---|---|
| Male, No. (%) | 16 (80) | 10 (56) | .20 | 21 (81) | 5 (42) | .02 |
| Age at seizure onset, median (IQR), mo |
18.0 (5-80) | 5.0 (1-13) | .02 | 11.5 (1-62) | 8.0 (1-14) | .40 |
| Age at surgery, median (IQR), y |
10 (3.5-14) | 2.5 (1-11) | .04 | 7.5 (1-13) | 5.5 (1-13) | .70 |
| Duration of epilepsy, median (IQR), y |
4.0 (2-10) | 1.1 (0.5-8) | .20 | 3.0 (0.5-8) | 4.0 (0.5-11) | .70 |
| Seizure onset, No. (%) Focal |
19 (95) | 17 (94) | 1.00 | 25 (96) | 11 (92) | .60 |
| Secondarily generalized |
6 (30) | 9 (50) | .30 | 9 (35) | 6 (50) | .40 |
| Spasms | 3 (15) | 5 (28) | .40 | 4 (15) | 4 (33) | .20 |
| History of febrile seizure, No. (%) |
1 (5) | 3 (17) | .30 | 2 (8) | 2 (17) | .40 |
| Normal neurologic examination, No. (%) |
11 (55) | 6 (33) | .20 | 14 (54) | 3 (25) | .09 |
| Absent or mild intellectual disability, No. (%) |
14 (70) | 6 (33) | .05 | 16 (62) | 4 (33) | .20 |
| Lesional MRI (MRI+
FCD), No. (%) |
12 (60) | 14 (78) | .30 | 16 (62) | 10 (83) | .30 |
| SISCOM concordant with MRI, No. (%) |
8 (40) | 6 (33) | .70 | 11 (42) | 5 (42) | .30 |
| Intracranial monitoring, No. (%) |
8 (40) | 6 (33) | .70 | 10 (38) | 4 (33) | 1.00 |
| Electrocorticography alone, No. (%) |
5 (25) | 5 (28) | 1.00 | 5 (19) | 5 (42) | .20 |
| Type of surgery (lesionectomy), No. (%) |
12 (60) | 11 (61) | 1.00 | 14 (54) | 9 (75) | .30 |
| Location of surgery (temporal), No. (%) |
7 (35) | 4 (22) | .07 | 7 (27) | 4 (33) | .07 |
Abbreviations: FLD, focal cortical dysplasia; IQR, interquartile range; MRI, magnetic resonance imaging; SISCOM, subtraction ictal SPECT coregistered to MRI.
Neurologic examination findings were not predictive of seizure-free outcome (P=.20). Intracranial monitoring was performed in 14 patients (37%), including seven with MRI+ FCD, four with MRI– FCD, and three with MRI+ lesional. There was no difference in grid placement rates in temporal (zero of four patients) versus extratemporal (10 of 30 patients) lesional MRI studies (P=.30). Surgical outcome did not differ between children with and without intracranial monitoring.
Timing of Seizure Recurrence and Postoperative Complications
Of the 18 patients with seizure recurrence, eight (44%) had favorable outcomes (Engel class I/II) and 10 (56%) had unfavorable outcomes (Engel class III/IV) at the last follow-up visit. Twelve patients (67%) had early recurrence (≤3 months of seizure freedom). About 90% of children with seizure recurrence still had worthwhile improvement (Engel class II or III), and only two had Engel class IV outcomes at the last follow-up visit.
Postsurgical neurologic deficit was present in 11 patients (29%) and had been anticipated in seven of these patients because of involvement of the eloquent cortex. The deficit was transient in three patients (8%) and permanent in the remaining eight (21%). Of the 11 patients with postsurgical neurologic deficits, nine (82%) were seizure free (P=.03). The most common deficit attributed to surgical resection was hemiparesis (75%) and homonymous hemianopia (25%).
Additionally, three patients (8%) had complications associated with surgery: two had wound infection or seroma and one had pseudomeningocele.
The median number of antiepileptic drugs used before surgery was two (IQR, 2-4). Postoperatively, the median number of antiepileptic drugs used was one (IQR, 1-3), including the six patients who remained seizure free without antiepileptic medication.
Discussion
In our cohort, 68% of children with pathology-confirmed FCD achieved early remission, and most continued to be seizure free at one (60%) and three years (55%) after surgery. Overall, sustained seizure freedom was achieved in 20 children (53%) at a median follow-up of 13.5 months (IQR, 4-71 months). These results are comparable to other studies reporting seizure-free outcomes after epilepsy surgery, which ranged from 50% to 60% (and as high as 82% in one series) (4). For patients with recurrent seizures, they occurred within three months of surgery for two-thirds of patients, and approximately 90% had seizure reduction without a return to baseline seizure frequency.
Additional favorable clinical features associated with sustained seizure freedom included older age at seizure onset, older age at surgery, and normal cognitive status at baseline. Previous work has also suggested higher chances of recurrence in children with earlier-onset seizures; this association is perhaps explained by early and more widespread establishment of the epileptogenic network, with larger distributions of cortical malformation and the difficulties in adequately characterizing the extent of malformation in infants (3). In contrast, studies have shown short duration from epilepsy onset to surgery or early age at epilepsy surgery to be associated with favorable surgical outcomes (16,17). We were unable to replicate those results, and in fact, later age at surgery was associated with higher chance of sustained seizure freedom. The favorable association of older age at surgery and normal baseline cognitive status with sustained seizure freedom may be explained by a lower preoperative seizure burden, which resulted in fewer cognitive effects and allowed surgical resection at a later age (18).
Presurgical evaluations that were routinely performed in our study included seizure protocol structural MRI and prolonged scalp video-EEG. MRI findings were abnormal in more than 90% of patients, with findings specific to FCD in 26 patients (68%), a rate comparable to those reported in other studies (4,19-22). Ictal findings localized to one region on prolonged scalp video-EEG in 20 patients (53%). Similar findings were reported in recent publications, with localizing value of ictal EEG ranging from 42% to 77% (4,19-22).
Additional presurgical evaluation is institutional specific. SISCOM was concordant and confirmed the localization in 10 of 26 MRI+ FCD but did not provide additional localizing information. Of the remaining 12 patients with MRI+ lesional and MRI– FCD, SISCOM provided localizing information for six of the seven patients (86%) without focal ictal EEG findings. Among those patients without concordant noninvasive evaluations, imaging with PET showed additional localizing information for three patients (43%).
Focal or lobar resection is the preferred surgical approach, and it is performed in approximately 70% of patients, similar to what we observed in our cohort (4). Limited resection may lead to incomplete removal of microscopic dysplasia that is not evident in the MRI. The extent of surgery may be limited by proximity to the eloquent cortex. Because a high percentage of FCDs are located at or near eloquent cortices, our patients did have a relatively higher rate of permanent neurologic deficits compared with other reports (21% versus 2%) (4).
The number of patients not taking antiepilepsy drugs one to two years after surgery varies from 14% to 41% (4,20). In our cohort, 16% of children were not taking medication at a median follow-up of 13.5 months. The smaller percentage of patients not receiving antiepileptic medications may be attributable to the shorter follow-up time and our standard institutional approach of discontinuing medications only after one or two years of seizure freedom.
Our study had several limitations, including the retrospective design, limited follow-up in some patients, and smaller sample size that may have limited study power. We also lacked information on histopathologic classification, which was used in other studies to correlate presurgical evaluations, surgical outcome, and neuropsychological status. Additionally, standardized surgical reports about partial versus full resection were lacking, and interpretation bias may have been present. Lastly, postsurgical follow-up with standardized neuropsychometric testing was inconsistent and did not allow rigorous comparison of cognitive development before and after surgery.
In summary, our retrospective study showed that surgery is a valuable option to treat children with intractable focal epilepsy, with 53% of children being free of disabling seizures and an additional 21% having a favorable outcome at a median follow up of 13.5 months (IQR, 4-71 months). Clinical features associated with sustained freedom from seizures included older age at seizure onset, older age at surgery, and normal cognitive status at baseline.
Acknowledgment
This project was supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Role of the Funding Source
This study was not externally funded. NCATS had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest: None.
Contributor Information
Dr Anna Mrelashvili, Division of Child and Adolescent Neurology, Mayo Clinic, Rochester, Minnesota.
Dr Robert J. Witte, Department of Radiology, Mayo Clinic, Rochester, Minnesota.
Dr Elaine C. Wirrell, Division of Child and Adolescent Neurology, Mayo Clinic, Rochester, Minnesota.
Dr Katherine C. Nickels, Division of Child and Adolescent Neurology, Mayo Clinic, Rochester, Minnesota.
Dr Lily C. Wong-Kisiel, Division of Child and Adolescent Neurology, Mayo Clinic, Rochester, Minnesota.
References
- 1.Taylor DC, Falconer MA, Bruton CJ, Corsellis JA. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry. 1971 Aug;34(4):369–87. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauptman JS, Mathern GW. Surgical treatment of epilepsy associated with cortical dysplasia: 2012 update. Epilepsia. 2012 Sep;53(Suppl 4):98–104. doi: 10.1111/j.1528-1167.2012.03619.x. [DOI] [PubMed] [Google Scholar]
- 3.Cossu M, Lo Russo G, Francione S, Mai R, Nobili L, Sartori I, et al. Epilepsy surgery in children: results and predictors of outcome on seizures. Epilepsia. 2008 Jan;49(1):65–72. doi: 10.1111/j.1528-1167.2007.01207.x. Epub 2007 Jul 21. [DOI] [PubMed] [Google Scholar]
- 4.Lerner JT, Salamon N, Hauptman JS, Velasco TR, Hemb M, Wu JY, et al. Assessment and surgical outcomes for mild type I and severe type II cortical dysplasia: a critical review and the UCLA experience. Epilepsia. 2009 2009 Jun;Jan;50(6):1310–35. 21. doi: 10.1111/j.1528-1167.2008.01998.x. Epub. [DOI] [PubMed] [Google Scholar]
- 5.van’t Klooster MA, Huiskamp G, Zijlmans M, Debets RM, Comans EF, Bouvard S, et al. Can we increase the yield of FDG-PET in the preoperative work-up for epilepsy surgery? Epilepsy Res. 2014 2014 Aug;May;108(6):1095–105. 13. doi: 10.1016/j.eplepsyres.2014.04.011. Epub. [DOI] [PubMed] [Google Scholar]
- 6.Chassoux F, Rodrigo S, Semah F, Beuvon F, Landre E, Devaux B, et al. FDG-PET improves surgical outcome in negative MRI Taylor-type focal cortical dysplasias. Neurology. 2010 Dec 14;75(24):2168–75. doi: 10.1212/WNL.0b013e31820203a9. [DOI] [PubMed] [Google Scholar]
- 7.Sulc V, Stykel S, Hanson DP, Brinkmann BH, Jones DT, Holmes DR, 3rd, et al. Statistical SPECT processing in MRI-negative epilepsy surgery. Neurology. 2014 Mar 18;82(11):932–9. doi: 10.1212/WNL.0000000000000209. Epub 2014 Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krsek P, Kudr M, Jahodova A, Komarek V, Maton B, Malone S, et al. Localizing value of ictal SPECT is comparable to MRI and EEG in children with focal cortical dysplasia. Epilepsia. 2013 Feb;54(2):351–8. doi: 10.1111/epi.12059. Epub 2013 Jan 7. [DOI] [PubMed] [Google Scholar]
- 9.Rowland NC, Englot DJ, Cage TA, Sughrue ME, Barbaro NM, Chang EF. A meta-analysis of predictors of seizure freedom in the surgical management of focal cortical dysplasia. J Neurosurg. 2012 May;116(5):1035–41. doi: 10.3171/2012.1.JNS111105. Epub 2012 Feb 10. [DOI] [PubMed] [Google Scholar]
- 10.Jack CR., Jr. Magnetic resonance imaging: neuroimaging and anatomy. Neuroimaging Clin N Am. 1995 Nov;5(4):597–622. [PubMed] [Google Scholar]
- 11.O’Brien TJ, O’Connor MK, Mullan BP, Brinkmann BH, Hanson D, Jack CR, et al. Subtraction ictal SPET co-registered to MRI in partial epilepsy: description and technical validation of the method with phantom and patient studies. Nucl Med Commun. 1998 Jan;19(1):31–45. doi: 10.1097/00006231-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Bohnen NI, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology. 1998 Feb;50(2):445–54. doi: 10.1212/wnl.50.2.445. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Jack CR Jr, et al. Subtraction SPECT co-registered to MRI improves postictal SPECT localization of seizure foci. Neurology. 1999 Jan 1;52(1):137–46. doi: 10.1212/wnl.52.1.137. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien TJ, Zupanc ML, Mullan BP, O’Connor MK, Brinkmann BH, Cicora KM, et al. The practical utility of performing peri-ictal SPECT in the evaluation of children with partial epilepsy. Pediatr Neurol. 1998 Jul;19(1):15–22. doi: 10.1016/s0887-8994(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 15.Blumcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011 Jan;52(1):158–74. doi: 10.1111/j.1528-1167.2010.02777.x. Epub 2010 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otsuki T, Honda R, Takahashi A, Kaido T, Kaneko Y, Nakai T, et al. Surgical management of cortical dysplasia in infancy and early childhood. Brain Dev. 2013 Sep;35(8):802–9. doi: 10.1016/j.braindev.2013.04.008. Epub 2013 May 18. [DOI] [PubMed] [Google Scholar]
- 17.Fauser S, Essang C, Altenmüller DM, Staack AM, Steinhoff BJ, Strobl K, et al. Long-term seizure outcome in 211 patients with focal cortical dysplasia. Epilepsia. 2015 Jan;56(1):66–76. doi: 10.1111/epi.12876. Epub 2014 Dec 13. [DOI] [PubMed] [Google Scholar]
- 18.Baca CB, Vickrey BG, Vassar S, Hauptman JS, Dadour A, Oh T, et al. Time to pediatric epilepsy surgery is related to disease severity and nonclinical factors. Neurology. 2013 Mar 26;80(13):1231–9. doi: 10.1212/WNL.0b013e3182897082. Epub 2013 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YH, Kang HC, Kim DS, Kim SH, Shim KW, Kim HD, et al. Neuroimaging in identifying focal cortical dysplasia and prognostic factors in pediatric and adolescent epilepsy surgery. Epilepsia. 2011 Apr;52(4):722–7. doi: 10.1111/j.1528-1167.2010.02950.x. Epub 2011 Jan 28. [DOI] [PubMed] [Google Scholar]
- 20.Kral T, von Lehe M, Podlogar M, Clusmann H, Süssmann P, Kurthen M, et al. Focal cortical dysplasia: long term seizure outcome after surgical treatment. J Neurol Neurosurg Psychiatry. 2007 Aug;78(8):853–6. doi: 10.1136/jnnp.2006.105361. Epub 2007 Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krsek P, Maton B, Korman B, Pacheco-Jacome E, Jayakar P, Dunoyer C, et al. Different features of histopathological subtypes of pediatric focal cortical dysplasia. Ann Neurol. 2008 Jun;63(6):758–69. doi: 10.1002/ana.21398. [DOI] [PubMed] [Google Scholar]
- 22.Widdess-Walsh P, Kellinghaus C, Jeha L, Kotagal P, Prayson R, Bingaman W, et al. Electro-clinical and imaging characteristics of focal cortical dysplasia: correlation with pathological subtypes. Epilepsy Res. 2005 Oct-Nov;67(1-2):25–33. doi: 10.1016/j.eplepsyres.2005.07.013. Epub 2005 Sep 21. [DOI] [PubMed] [Google Scholar]