Abstract
Objective
To test for gender-differences in the relation between mothers’ antenatal anxiety and infants’ body weight during gestation, at birth, and at 1-month of age.
Methods
Two hundred and twelve randomly-recruited women were divided into two groups: Controls (n = 105) and Anxious Group (n = 107) based on a standard cut-off of the Beck Anxiety Inventory. Outcome measures were Fetal Weight derived from biometrics obtained from an ultrasound scan in the 3rd trimester and infants’ weight at birth and at 1-month of age, both obtained from medical records.
Results
Multivariate analyses showed main effects of Gender on infants’ birth weight (P = .001) and on infants’ weight at 1-month of age (P = .004), but no main effects of Anxiety Group at any time-point. Gender x Anxiety Group interactions at all three time points (Fetal weight: P = .05; Birth weight: P = .03; 1-month of age: P = .10) reflected gender differences (males > females) among infants in the anxious group, but not among controls. Distinct trends regarding same sex comparisons across groups (Control vs. Anxiety) were in line with predictions (male controls < male anxious; female controls > females anxious). Controlling for Postpartum Anxiety and Antenatal and Postpartum Depression in the models did not affect primary results.
Conclusion
Gender differences in fetal and birth weight were more substantial among infants of anxious mothers than among controls due to the seemingly accelerated growth of “anxious” males and the diminution of weight among “anxious” females.
Keywords: Antenatal Anxiety, Gender, Fetal programming, Fetal Weight, Sexual Dimorphism
Introduction
It is well documented that pregnancies are more likely to end badly if the baby is a boy [1,2]. As examples, in the 50–70% of pregnancies that fail, the loss of male fetuses exceeds the loss of females [3,4]. Males are 20% more likely to experience a poorer outcome in pregnancies complicated by pre-eclampsia [5] and intrauterine growth restriction (IUGR) or when the mother smokes [6], drinks alcohol, or eats unhealthily during pregnancy [see review in 7]. More males are born preterm [1] and those that survive, have poorer outcomes [8,9,10]. Additionally, several studies demonstrate lower than expected live birth sex ratios (i.e., the ratio of male to female live births) following natural and manmade disasters, such as the terror attacks of September 11, 2001 [11] and periods of economic decline [12].
Mechanistic causes of males’ greater vulnerability than females remain poorly established [7,13,14,15], though accumulating evidence suggests that the increased risk of male fetuses is somehow related to “their” prioritization of growth in the face of challenge [as in 16,171], as induced by pre-eclampsia, maternal asthma, and prematurity [review in 18]. In contrast, the stress-activated mechanisms in females seem to promote the conservation of growth and, with it, reduced size, which could make them less vulnerable to gestational challenge than males. According to recent studies, these gender differences are likely mediated, in part, by the placenta and conferred by sex-specific differences in the regulation and expression of placental genes, proteins, steroids, and structure [18,19]. For males, the strategy is minimalist, with few gene protein or functional changes instituted in the placenta, which ascertains continued growth in less than optimal maternal environments. This male response is associated with bad outcomes such as IUGR, preterm delivery, or death, especially if adversity recurs or is exacerbated [1]. In contrast, the female placenta responds to an adverse maternal environment with multiple placental gene and protein changes that result in a decrease in growth without growth restriction (< 10th weight percentile). These female adjustments predict resilience in the face of additional or recurrent stressors that further compromise nutrient or oxygen supply to the fetus.
The idea of gender-specific stress-effects on fetal development and health also emanates from the influential Trivers and Willard evolution-based model that aims to explain changes in sex ratio in response to environmental challenges [20]. For this, the model assumes that the reproductive success (RS) is more variable and resource-sensitive for males compared to female offspring because males in good condition will reproduce frequently and with many partners, whereas compromised males may not reproduce at all. In contrast, “condition” has less influence on the RS of female offspring because almost all reproduce in their lifetimes. These presumptions predict that mothers will invest more in males when conditions are “good” and more in females when conditions are not good; and correspondingly, that intrauterine challenges will affect males more significantly than females. Assuming maternal mechanisms for the identification and abortion of the frail and weak [20], males in the surviving cohort may be, on average, bigger than usual. At the same time, these survivors may either be vulnerable to pathology because of mothers’ lack of investment or they may show improved development and high indices of good health, in keeping with their size and shown capacity to survive gestational cuts [17]. Evidence to support these predictions among humans comes from studies using a wide range of stressors [e.g., 21,22,23,24,25], although findings from the literature, taken as a whole, are mixed [see review in 26].
Rationale and Hypotheses
The possibility that processes of fetal programming differ for males and females before they are born opens avenues for understanding gender differences in health and development throughout the life span. Essential to advances in this research area is further evidence of gender-distinct responses to antenatal stress during gestation, since at this time there are few prospective studies on humans on which to base conclusions and future hypotheses [review in 27]. On this basis, we aimed to provide new evidence of sex-specific growth in relation to antenatal challenge in the form of maternal anxiety, using estimated fetal weight obtained from an ultrasound scan during the 3rd trimester, birth weights, and weights at 1-month of age as outcome measures and maternal antenatal anxiety as the gestational challenge. Anxiety is appropriate for this because it is an emotional response to stress and is accompanied by a host of physiological responses [see review in 28], such as increased cortisol, a product of the hypothalamic pituitary axis (HPA), that is able to cross the placenta in a limited manner to affect fetal development [29]. Less directly, antenatal anxiety may affect fetal development by altering the barrier enzyme Placental 11b-hydroxysteroid dehydrogenase type 2 (HSD11B2) 11bHSD2 leading to fetuses’ increased exposure to glucocorticoids [30]. Alternatives to an HPA axis-mediated mechanism, such as restricted uterine blood flow [31] and/or immunological mechanisms [32], could affect fetuses’ development and health, as well.
Based on the literature reviewed, we hypothesized that males carried by anxious mothers would weigh the same or more than males in the control group both in utero and at birth, whereas females carried by anxious mothers would weigh less than female controls [17]. In tandem, we expected that males and females in the anxious group would show more substantial differences in weight at each time-point than counterparts in the control group. Finally, for both males and females, we posited that relations between antenatal anxiety, gender, and infant body weight would differ across the three time-points. In this regard, we hypothesized that (a) Gender effects would become more robust with time as the infants grow and develop [33,34], and (b) Gender x Anxiety Group effects would diminish after birth, assuming that a complex array of postpartum environmental variables (e.g., duration of nursing, if at all; schedule of feeding [35]) affect infants’ weight after birth, thus reducing the impact of mothers’ antenatal anxiety.
In sum, our aim was to compare the (body) weight of fetuses/infants born to mothers with and without significant anxiety symptoms in order to test for gender-related responses to challenge (maternal anxiety) during gestation and at and after delivery. As such, this is the first study to examine the issue longitudinally by tracking infants’ weights from gestation to after birth. Results favoring our hypotheses would constitute new evidence of the effects of antenatal anxiety on fetal and infant development and could further understanding of the differential effects that some gestational challenges have on males and females before and after they are born. Finally, the study of risks related to antenatal anxiety is relevant because of the high prevalence rate of maternal anxiety symptoms, even in low risk samples (e.g., 35.8% assessed by the Hospital Anxiety and Depression Scale [36]; 18.6% assessed by BAI, with cut-off score 10 [37].
Methods
Participants
The final sample comprised 212 infants and their mothers. As shown in Table I, the women were on average well-educated, mature, and married. The families’ median monthly salary was approximately $3,000/month, which is about average by Israeli standards [38].
Table I.
Means (M) and standard deviations (SD) of demographics/background and symptomology scores; stratified by Group and Gender.
| Total | Controls | Anxiety | |||||
|---|---|---|---|---|---|---|---|
| N= 212 | Boys (n = 67) | Girls (n = 67) | Total (n = 134) | Boys (n = 38) | Girls (n = 40) | Total (n = 78) | |
| BACKGROUND | |||||||
| Mother Age (M, SD) | 28.75 (4.90) | 28.57 (5.34) | 29.62 (5.32) | 29.06 (5.34) | 28.08 (3.74) | 28.37 (4.28) | 28.23 (4.00) |
| Mother Education (M, SD) | 15.43 (2.23) | 15.52 (2.14) | 15.67 (1.92) | 15.59 (2.03) | 15.25 (3.03) | 15.05 (3.06) | 15.14 (2.53) |
| Mother BMI (M, SD) | 23.64 (4.28) | 23.64 (4.64) | 23.52 (4.27) | 23.58 (4.44) | 22.72 (3.63) | 24.70 (4.16) | 23.74 (4.04) |
| Mother. Parity (%)1 | 38.73 | 30.40 | 32.80 | 31.23 | 44.44 | 35.92 | 38.78 |
| Infant GA birth (M, SD) | 39.65 (1.14) | 39.65 (1.14) | 39.60 (1.14) | 39.63 (1.11) | 39.67 (1.09) | 39.68 (1.32) | 39.68 (1.21) |
| Infant gender (% boys) | 50.6 | 51.5 | 48.54 | x | 44.90 | 55.10 | x |
| SYMPTOM SCORES | |||||||
| BAI- 3rd 22 | 7.20 (6.65) | 3.14 (2.40) | 3.34 (2.2) | 3.23 (2.3) | 13.69 (5.23) | 14.39 (6.86) | 14.05 (6.09) |
| BAI- PP3 | 4.65 (6.12) | 3.01 (3.76) | 4.25 (7.19) | 3.60 (5.65) | 5.78 (6.17) | 7.18 (6.81) | 6.51 (6.51) |
| BDI- 3rd 4 | 7.62 (5.59) | 5.63 (4.93) | 5.82 (3.50) | 5.72 (4.32) | 9.13 (5.41) | 12.55 (6.21) | 10.86 (6.04) |
| BDI- PP 5 | 6.74 (6.11) | 5.27 (5.00) | 5.22 (4.16) | 5.25 (4.61) | 7.60 (5.58) | 10.98 (8.55) | 9.35 (7.43) |
Percent primipare;
BAI-3rd - Beck Anxiety Inventory score during 3rd trimester pregnancy;
BAI-PP- Postpartum Beck Anxiety score;
BDI-3rd - Beck Depression Inventory score during 3rd trimester;
BDI-PP- Postpartum Beck Depression Inventory score
Procedures
Procedures were approved by institutional review boards. Pregnant women living throughout Israel were recruited from 04/09 to 12/10 by advertisements in newspapers and posters in health clinics. The data were taken from a study on stress and maternal health, motivated by the gender differences revealed in preplanned preliminary analyses of the data. Only pertinent aspects of the study are described here.
In stage 1, women received an explanation of the study by phone, and those who agreed to participate were screened at 22–30 weeks gestation (mean (M) = 28.68 weeks; standard deviation (SD) = 2.84) for chronic illnesses and pregnancy complications that served as exclusionary criteria (see below). In the following stage, 32–36 weeks into pregnancy (M = 34.17, SD = 1.29), women were examined by ultrasound (see below) to obtain biometrics, asked about health issues that may have arisen since stage 1, and filled out anxiety and depression symptomology questionnaires. At 1–3 months postpartum (M = 9.8 weeks, SD = 2.29), the women were visited in their home, where they again filled out questionnaires on symptomology. At that time, the women provided us with a copy of the infants’ well-baby records, which included infants’ weight at 1-month of age. The women also provided written permission to access their medical records from the hospital where they gave birth.
Exclusion and attrition
Prior to childbirth, exclusion criteria included: (a) confirmed pregnancy with multiple fetuses, (b) smoking or drinking during pregnancy, and/or (c) documented chronic illnesses or medical complications during pregnancy (e.g., maternal hypertension, diabetes mellitus, and fetal growth restriction) that were deemed dangerous to fetus and/or mother. These exclusionary criteria were applied because they refer to conditions or behaviors that increase the risk for atypical fetal growth and early deliveries [39]. Medical decisions were made on a case-by-case basis by Dr. D. Mankuta, head of delivery and labor rooms in Hadassah Medical Center, Jerusalem, Israel.
Following acceptance into the study (N = 309), 29 mothers and their infants were excluded due to pregnancy complications reported after enrollment (e.g., high blood pressure) or recommended bed-stay. Twenty-two women refused to travel to the hospital in their 3rd trimester. Another 20 were excluded because of technical problems with the ultrasound scanner, difficulties in scheduling an ultrasound or home visit (n = 23), and/or failure to obtain medical records (n= 3).
Tools and Measures
Ultrasound, biometrics, and fetal weight
All women in the final sample underwent a 3rd trimester ultrasound, free of charge, at Hadassah Medical Center, Jerusalem, Israel. The scans were undertaken by certified and experienced sonographers or physicians, who were blind to the aim of the study or to group placement. Professor EJH Mulder (Department of Obstetrics, University Medical Center Utrecht, Utrecht, The Netherlands) provided supervision in designing protocol, and all of the sonographers and physicians involved in the project were instructed in the (sonographic) protocol of this study in a two day training course led (in person) by Professor JIP de Vries (Department of Obstetrics and Gynecology, University Hospital, Groningen, The Netherlands). Ultrasound sessions were attended at regular intervals by project coordinators to assure that procedures were standard across sessions. Only one ultrasound machine, certified by the Israel Ministry of Health, was used to obtain fetal weights for the study.
The ultrasound scan consisted of a 1-hour transabdominal (ultrasound) study that included a systematic structural survey of the fetus that focused on the provision of biophysical scores, blood flow velocimetry of umbilical and middle cerebral arteries, and a clear view of the fetus for the coding of fetal movements (data not included here). During the scan, a total of three sets of measurements: (a) biparietal distance [BPD] and head circumference [HC], (b) abdominal circumference [AC], and (c) femur length [FL]; were calculated (in mm) for each woman. As recommended by the International Society of Ultrasound in Obstetrics & Gynecology [40], the BPD was measured from the proximal echo of the fetal skull to the proximal edge of the deep border at the level of the cavum Speti Pellicidi. The HC was measured as an ellipse around the perimeter of the fetal skull. The AC was measured in the transverse plane, when the umbilical vein is positioned in the first third of the fetal abdomen and the stomach bubble is in the same plane. The FL was obtained in a horizontal view, measuring the full femur excluding the epiphysis from one end of the diaphysis to the other. Estimated fetal weight (EFW), was calculated from measurements of AC and FL, using the Hadlock formula (Log10 EFW = 1.304 + (0.05281 × AC) + (0.1938 × FL) − (0.004 × AC × FL) [41].
Infants’ birth weight (BW) and weight at 1-month of age
BW was measured using electronic scales and recorded by midwives immediately after birth. Infants’ weight at the 1-month exam was taken from the mothers’ well baby file and confirmed by their mothers during the home visit.
Anxiety Symptomology
The Beck Anxiety Inventory (BAI) [42] provides a measure of anxiety severity during the past week. The BAI consists of 21 disorder-related symptoms, rated in severity, 0–3, and summed to obtain a final score. According to established thresholds, scores 0–7 are considered “normal” and a score of 8 or higher is considered outside of normal range (i.e., score 0–7 no anxiety, 8–15 minimum anxiety, 16–25 moderate anxiety; 26–63 severe anxiety). The inventory has good psychometric properties, including discriminative validity with depression [43]. In this study, Cronbach α was .85.
Covariates
Gestational Age (GA) at birth
GA was read from the medical records and cross-checked with our own calculations using last menstrual period (LMP) confirmed by 1st trimester ultrasound or the earliest ultrasound available. The date was then corrected if the LMP deviated significantly (> 7 days) from the early ultrasound, if available (n = 170, 70.2%).
Pre pregnancy Body Mass Index (BMI)
BMI was derived from mothers’ reports of her prepregnancy weight and calculated by the standard equation: BMI = Weight (kg) /Height (m)2. Mothers’ pre pregnancy BMI has been correlated with estimated weights of fetuses and infants’ birth weight [35].
Demographics
Personal and family demographics, including mothers’ age, education, and parity (primipare vs. multiparae), were obtained by interview. Women also were asked specific questions about their infant’s history of feeding (breast feeding, formula, or combination) since birth and whether solids had been introduced. From these data, we calculated a measure Duration of Breast-feeding. This information was considered relevant because breast fed infants may weigh less than bottle fed infants [35], especially when infants are very young and milk constitutes almost all of their diet.
Beck Depression Inventory (BDI) [44]
Depression symptomology was assessed by women’s ratings on the BDI, which consists of 21 disorder-related symptoms (rated in severity, 0–3). Categories of severity are: scores 14–19, mild depression; 20–28, moderate depression; 29–64, severe depression. Psychometrics are acceptable [45], and in this study, Cronbach α was .85.
Data Reduction: Anxiety Groups
Women were categorized into anxious vs. non anxious/control groups using a BAI cut off score of 7/8 [42]. We did not further subdivide women in the Anxious Group by levels of severity because only 23 women scored above the cut off for moderate anxiety (BAI score ≥ 16), and only 5 of those gave birth to sons.
Statistical Analyses
Data for all variables were screened for missing values, normality and outliers. Weights were normally distributed and were entered into analyses as raw values. Homogeneity of variance between groups was assessed by the Levene test for equality of error variances.
In preliminary analyses, we tested for potential biases due to attrition by comparing women who completed the study vs. women who did not on demographic variables and outcome measures. We also ran simple correlations between outcome measures and between outcomes and sample demographics to test for inter relations/covariation between focal measures.
The primary analyses comprised three general linear models (GLM) analyses of variance (ANOVA), with Gender and Anxiety Group (Controls vs. Anxious) as between-group variables and Weight at one time-point as the outcome measure. Univariate Analyses of Variances (ANOVAs) were used to explore significant interaction effects.
In supplemental analyses, we reran the three ANOVA models and included Antenatal Depression as a covariate in order to partial out its contribution to weight measures. We also controlled for postpartum depression and anxiety (in separate analyses) in the models with infants’ weight at 1-month postpartum as the dependent variable. Control of Depression was warranted because the incidence rate of anxiety and comorbid depression is high [37,46], and the presence of both disorders predicts different and/or more extreme outcomes than either disorder on its own [47].
In all analyses, mothers’ age, education, parity (primipare vs. multiparae), mothers’ BMI prior to pregnancy, duration of breast feeding (weeks, including 0 for none), and gestational age of infant at birth and (if different) infants’ age at the time of assessment were considered potential covariates. To begin, we entered all candidate covariates simultaneously into each model and then removed them, one at a time, starting with the one with the largest p value and stopping when the remaining predictors were at least marginally significant (p < .10).
Results
Preliminary Analyses
Women in our sample scored between 0 – 35 and 0 – 46 on the BAI during pregnancy and at the time of the home visit, respectively. Scores on the antenatal and post partum BDI ranged between 0 – 32 and 0 – 35, respectively. The prevalence rate of antenatal anxiety symptoms (> score 7) was 36.79%. Differences in scores between mothers of boys and girls were not significant (ps > .22). Approximately half (50.4%) of the women gave birth to girl infants. Eighty-three percent delivered vaginally and 17% by C-section (3.3% planned, 13.7% unplanned).
One infant (female from the anxious group) was born prematurely (35.0 weeks GA); six infants (2 boys and 3 girls from control group; one girl from the anxious group) were born at low weight (< 2500 grams); and 18 infants were born heavier than normal (> 4000 g; 8 boys and 4 girls from the control group, 5 boys and 1 girl from the anxious group). All of the infants scored within normal range on the Apgar scale (scores 8 –10). As shown in Table II, fetal, birth, and 1-month weights were correlated, but there were a few significant correlations between weights and background/demographic variables used in this study.
Table II.
Pearson correlations between select demographics and symptomology measures in continuous and categorical form with outcome (weight) variables
| Weights | 1 | 2 | 3 | M. age | M. Ed | 1 M. Parity | 2M. BMI | 3 M. BAI-3rd | 4 M. BAI-PP | 5 M. BDI-3rd | 6 M. BDI-PP | 7 Group | 7 Group | I. Gender | I. GA birth | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | FW | x | .42*** | .34*** | −.06 | −.12 | .067 | .23** | .09 | −.05 | .07 | .008 | .07 | −.06 | .016 | −.006 |
| 2 | BW | x | .70*** | .02 | .02 | .05 | .13t | .01 | .02 | .04 | .04 | .03 | .06 | −.11 | .35*** | |
| 3 | 1-m | x | −.05 | −.03 | .04 | .09 | .02 | .02 | .03 | −.06 | .07 | .04 | −.16* | .23** |
<.10;
< .0005;
< .01;
< .05
Parity categorized as 0- primipare, 1-multiparae
M. BAI-3rd – mothers’ continuous Beck Anxiety scores 3rd trimester pregnancy
M. BAI-PP- mothers’ continuous Beck Depression scores 1-month postpartum
M. BDI-3rd – mothers’ continuous Beck Depression scores 3rd trimester pregnancy
M. BDI-PP- mothers’ continuous Beck Depression scores 1-month postpartum
Group - Controls vs. Anxious (point biserial correlation)
Abbreviations: M - mother, I - Infant, BMI- body mass index, FW- estimated fetal weight., BW- birth weight, GA birth- Gestational age at birth.
In separate analyses, we confirmed that there were no differences between the women who completed the study and those who did not on demographics (age, education, and parity), anxiety symptomology scores, or infants’ weights at any of the three time-points.
Primary Analyses
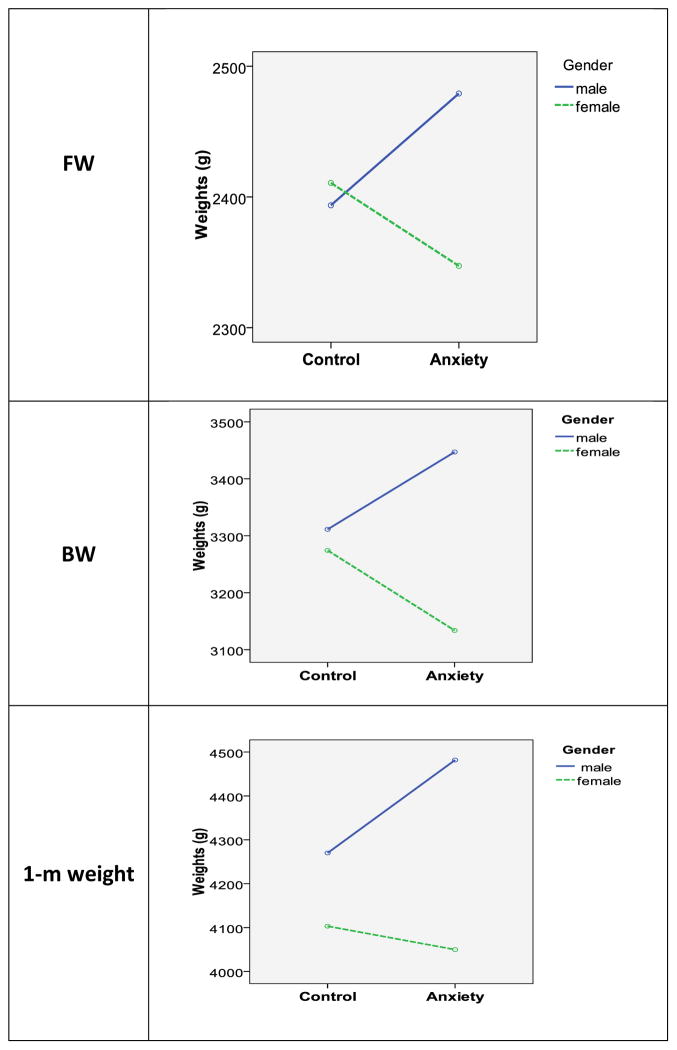
Results of the multivariate analyses are illustrated in Fig 1 (see below, page 13).
Figure 1.
Weights of fetuses during 3rd trimester (FW), at birth (BW), and at 1-month post delivery. Shown are weights of infants (males and females) of anxious and control mothers
Results of the primary analyses revealed:
Main effects of Gender (males > females) on BW (F(1, 206) = 10.55, p = .001, partial eta squared [ηp2] = .06) and on infants’ weights at 1-month of age (F (1, 206) = 8.66, p = .004, ηp2 = .07), but not on fetal weights (F (1, 206) = 2.21, p = .14, ηp2 =.01).
No main effect of Anxiety group at any of the three time-points (EFW: F (1, 206) = .082, p = .78, ηp2 = .007; BW: (F (1, 206) = .002, p = .97, ηp2 = .02), 1-month (F(1, 206) = 1.99, p = .16, ηp2 = .005).
Significant interactions between Gender and Anxiety Group in relation to EFW (F (2, 206) = 3.74, p = .05, ηp2 = .02) and BW (F (1, 206) = 6.57, p =.01, ηp2 = .03), as well as a distinct trend in relation to infants’ weight at 1-month of age (F (1, 206) = 2.35, p = .10, ηp2 = .02).
Subsequent posthoc tests were aimed at deciphering the core of significant interactions. Analyses revealed that, at the 3rd trimester time-point, male fetuses in the anxious group weighed more than females in the anxious group (F (1, 74) = 4.66, p = .03, ηp2 = .06), but the same gender difference was not evident among fetuses of controls (F (1, 130) = .11, p = .75, ηp2 = .001). Further, male fetuses of anxious mothers weighed (numerically, though not significantly) more than male fetuses of controls (F (1, 101) = 2.00, p = .16, ηp2 = .02); and the opposite was the case for females (F (1, 103) = 1.68, p = .20, ηp2 = .02). Covariates included in these models were GA at the time of weight assessment and mothers’ BMI prior to pregnancy.
At the time of birth, sons of anxious mothers again weighed significantly more than daughters of anxious mothers (F (1, 74) = 17.99, p = .0001, ηp2 = .20), and there was no comparable gender difference between infants of controls (F (1, 130) = .27, p = .60, ηp2 =.002). In addition, there was a significant weight difference between daughters of anxious vs. controls mothers (F (1, 103) = 4.73, p = .03, ηp2 = .044) and a strong tendency for sons of anxious mothers to weigh more than sons of controls (F (1, 101) = 2.56, p = .11, ηp2 = .03). Covariates in these models were GA at birth and mothers’ BMI prior to pregnancy.
Post hoc examination of data obtained postpartum revealed a robust gender effect among infants of anxious mothers (F (1, 74) = 7.48, p = .008, ηp2 = .09) and a weak trend among infants of controls (F (1, 130) = 2.08, p = .15, ηp2 = .02). At least in part, the gender difference shown by infants from the anxious group is explained by the near-significant Group difference in 1-month weight for boys (Controls < Anxious Group, F (1, 101) = 2.80, p = .097, .03), but not for girls (F (1, 103) = .05, p = .82, ηp2 =.001). As before, we controlled GA at birth and mothers’ BMI prior to pregnancy in these analyses.
Finally, the repeated measure analysis designed to test for change over time (time-point 1: gestation, time-point 2: birth, time-point 3: 1-month post birth) yielded between subject effects of Gender (F (1, 206) = 10.20, p =.002, ηp2 = .06) and Gender x Anxiety Group = (F (1, 206) = 2.81, p = .05; ηp2 = .02), and no effect of Anxiety Group (1, 206) = .86, p = .36; ηp2 = .005). Additionally, within-subject effects (indicating change over time) included a significant Time-point x Gender interaction (F (2, 414) = 3.84, p = .022, ηp2 = .02) and a distinct trend in relation to the Time-point x Gender x Anxiety Group interaction (F (2, 414) = 2.45, p = .11, ηp2 = .014). Of interest here- these latter results reflect the more substantial Gender x Anxiety interaction at time-point 1 and 2, compared to time-point 3 and a significant gender difference at time-point 2 and 3, but not at time-point 1. Covariates in these models were Gestational Ae at birth and mothers’ BMI.
Supplemental Analyses
a. Controlling postpartum anxiety
The expanded ANOVAs, with Postpartum Anxiety included as a covariate, showed no main effect of that measure on infants’ 1-month weight (F(1, 205) = .70, p = .40, ηp2 = .004) and, as in the primary tests, null findings for Antenatal Anxiety (F (1, 205) = .13, p = .72, ηp2 = .001), a significant effect of Gender: (F (1, 205) = 10.46, p = .001, ηp2 = .0.06); and a nonsignificant trend in the Gender x Anxiety interaction (F (1, 205) = 1.87, p = .15, ηp2 = .013).
b. Controlling antenatal and postpartum depression
Results of the analyses with inclusion of antenatal BDI scores as a covariate revealed no significant contribution of the variable on infants’ weight at any time-point (3rd trimester: F (1, 205) = .34, p = .56, ηp2 = .002; BW: F (1, 205) = 2.08, p = .15, ηp2 = .01; 1-month: F (1, 205) = .001, p = .98, ηp2 = .0001), and results of the expanded models were essentially the same as those obtained from the primary analyses. Likewise, inclusion of postpartum depression scores in the model predicting infants’ 1-month weights revealed no main effect of Depression (F (1, 205) = .47, p = .49, ηp2 = .003) and the results were similar to those obtained from the primary analyses.
c. Distribution of infant gender among mothers with moderate anxiety (BAI ≥ 16)
As noted, of the 23 women who scored higher than the cut off for moderate anxiety, only 5 delivered sons. According the binomial probability test, this finding is significant, meaning that more daughters than sons were born to mothers with high anxiety.
d. Sample limited to full term infants
We repeated primary analyses without the one premature infant, and our findings were essentially the same as those based on the whole sample, with the infant included.
Discussion
We examined the hypothesis that male and female fetuses “respond” differently to gestational challenge, defined here as maternal anxiety. Evidence in favor of the hypothesis entails gender differences in weight among infants of anxious mothers but not among infants of controls at all three time-points. Concurrently, sons of anxious mothers tended to weigh more than sons of controls at birth and at 1-month of age, and daughters of anxious mothers weighed less than daughters of controls at the time of birth. Further and in line with predictions, the significant repeated measure Time x Gender x Anxiety Group interaction showed a diminution of Anxiety x Gender effects at time point 3 compared to time point 2, which is likely due to the complex array of environmental variables that can contribute to infants’ weight after birth [e.g., 35]. Notably, none of the results were substantially altered by the inclusion of Depression (antenatal or postnatal BDI scores) or Postpartum Anxiety as covariates in separate statistical models.
Though effects sizes were uniformly small, the general direction of the results concurs with the idea that males and females fetuses “respond” differently to some antenatal challenges, with a tendency for females to constrain or sustain growth and a tendency for males to accelerate growth, at least under conditions of mild challenge [also see 17,48]. As such, these results provide a new example of sexual dimorphism in the context of rather mild antenatal anxiety and other indices of stress and stand out from existing evidence which has focused almost entirely on outcomes related to substantial stressors and derived at the time of birth (e.g., placenta dimensions or vascularization at birth [18], infants’ birth weight or gestational age at birth [49] or afterwards (e.g., motor development [50], prevalence of affective problems and autism [51], learning problems [14]). The present study adds to these findings by providing a picture of sex-specific anxiety-related growth derived from multiple estimates of weight from 3rd trimester gestation to 1-month postpartum using a sample of low risk and healthy women [also see 34].
Given the novelty of the findings, the relatively small sample, and the generally small size effect sizes, it will be important to replicate our findings. Nonetheless, as they stand now, they are consistent with hypotheses and make sense from an evolutionary point of view, assuming that a stressful intrauterine environment forecasts birth into stressful extra uterine surrounds. Under these circumstances, accelerated growth among males would help them compete strenuously for resources and mates [52]; whereas females would probably benefit more from protecting their “innards” so to increase their chances of reproducing healthy offspring in the future.
Another perspective of the results rests on the assumption that the mild to moderate anxiety, as reported by women in our Anxious Group, was within the range (i.e., not too low and not too high) that can benefit fetal (and infant) maturation via accelerated neural, physical, and psychological development and not perturb fetal development as do higher levels of anxiety/stress [48,53,54,55,56]. As such, mild to moderately stressful uterine conditions could be considered “good conditions”, as defined by Trivers and Willard [20]; and according to their model, could prompt mothers to preferentially channel resources to males/sons, thus accelerating their growth and channeling fewer resources to females.
A final explanation of our results rests on findings of antenatal sex differences in the trajectory of products of complex physiologic processes that underlie gestation and which could differentially influence fetal development of males and females [reviews in 7,15,27,57]. Such differences include the trajectory of cortisol release during gestation, which can have gender-specific effects on fetal behavior [50,58] and neuromaturation [50]. Also, gender differences in placenta responsivitiy to stress [59], immune and hormonal changes during gestation (e.g., indexed by levels of proinflammatory and regulatory cytokines and proangiogenic growth factors [60]), or the reprogramming of imprinted genes expression [reviews in 61,62,] could contribute to gender-related responses to challenge during gestation.
Several points regarding more general themes also are worthy of mention. First, our data suggest that even mild anxiety influences fetal growth, thus showing again that stress-related modulation of fetal growth is not limited to severe conditions [63]. Second, our results suggest that fetal development can be accelerated in the context of mild challenge as has been reported in a few previous studies [48,52,64]. Third, it is important to point out that although effect sizes were small; even subtle deviations in infants’ weight at birth can have significant long term detrimental effects on development, including cognitive development and educational achievement during childhood and on adults’ response to stress and physical and mental health up to and beyond middle-age [65,66,67; reviews in 68,69,70]. Fourth, it is interesting that so few boy infants were born to mothers with moderate anxiety, defined as a BAI score of 16 or higher, and it is conceivable that this reflects the higher risk of male fetuses compared to female fetuses in the context of considerable antenatal challenges [e.g., 71,72]. Finally, our results support the call for including gender into models predicting stress-related changes in fetal and infant development (e.g., 60,73]. As shown here, combining data from males and females could cancel out sex-related repercussions, thereby obscuring positive findings.
Before closing, we note several caveats. First, anxiety was assessed once during pregnancy, during the third trimester, so we cannot know whether that reading represents the level of symptoms experienced by women in the sample during the course of their pregnancy. Second, our sample was relatively small and some of group differences showed notable trends, but did not reach significance, This could be due to the sample size together with small size effects. Third, though a number of covariates were entered into statistical models in order to account for their contribution; it is possible that unmeasured confounds could account for significant variance in results. Finally, according to the literature, our findings may not be generalizable to more heterogeneous samples or to samples comprising women with more severe anxiety symptoms [74].
These issues, not withstanding, our findings are novel and provide some of the first evidence of gender-related responses to stress in general and anxiety in specific among humans during gestation. Accordingly, the results underscore the importance of considering gender in research on fetal (and infant) development. They also suggest that mild to moderate anxiety during pregnancy can impact important parameters of infants’ growth during gestation, but differently for boys and girls.
Highlights.
We tested for gender-related differences in effects of mothers’ antenatal anxiety.
Fetal weight and weights at birth and at 1-month were outcome measures.
Gender effects in weight were more substantial among anxious than control groups.
Results show challenge-related sexual dimorphism in early growth of humans.
Acknowledgments
We thank the project coordinators (Nomi Ban, Sara Burstin, Polly Hyams Sherman, Mika Inbar) and the interviewers and recruiters (Linor Tzumer, Nurit Almagor, Noa Bar Ziv, Ortal Bhoknik, Noa Gohar, Irit Goldner, Yael Hassid, Yael Millgram, Dana Nozik, Avital Tessler, Amit Yehudian) who worked on the project. We appreciate the assistance of the sonographers Miriam Cohen, Libit Shpitzer, Avital Tauber, and Maya Spielman and the administrative assistance of Nathan Bellinson, Gail DePalma, Ruth Fisch, Shelly Friedman, Gal Nassi-Abrass, and Elinor Slater. Many thanks to Professor EJH Mulder for his helpful, professional guidance and supervision and to Professor JIP de Vries (Department of Obstetrics and Gynecology, University Hospital, Groningen, The Netherlands) for her advice and for training the project-sonographers. We are indebted to the women who participated in the study.
Funding
This study was supported by a grant from the National Institute of Child Health and Development (NICHD) (R01HD053586, 2009 - 2013) to SV Faraone and M Kaitz. NICHD played no role in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Study reports preliminary findings on birth weight.
All of the authors contributed equally to the study:
M.K.: contributed to the conception and design of the study, the acquisition of funding, data, managed literature searches, organized and conducted the statistical analyses, wrote the first draft of the manuscript.
D.M.: contributed to the conception and design of the study, was responsible for medical decisions regarding women’s health and their exclusion/inclusion into the study, oversaw standardization of ultrasound procedures, and revised the manuscript critically for important intellectual content.
A.M.R.: contributed to the conception and design of the study, coordinated the running of the study, carried out literature searchers, oversaw standardization of daily protocol, revised the first draft of manuscript, and interviewed participants in the study.
S.V.F: contributed to the conception and design of the study, acquisition of funding, contributed substantially to analyses and interpretation of the results, revised the manuscript critically for important intellectual content.
Conflict of interest
In the past year, Dr. Faraone received income, travel expenses, and/or research support from and/or has been on an advisory board for Pfizer, Ironshore, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA Pharma, Neurovance, Impax, and NeuroLifeSciences and has received research support from the National Institutes of Health (NIH); his institution is seeking a patent for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD; in previous years, he received consulting fees or was on advisory boards or participated in continuing medical education programs sponsored by Shire, Alcobra, Otsuka, McNeil, Janssen, Novartis, Pfizer, and Eli Lilly; and he receives royalties from books published by Guilford Press and Oxford University Press. In previous years, Dr. Kaitz, Dr. Mankuta, and Ms. Rokem received support from NICHD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marsha Kaitz, Email: msmarsha@mscc.huji.ac.il.
David Mankuta, Email: mankutad@gmail.com.
Ann Marie Rokem, Email: amrokem@gmail.com.
Stephen V. Faraone, Email: sfaraone@childpsychresearch.org.
References
- 1.Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gender Med. 2007;4:19–30. doi: 10.1016/s1550-8579(07)80004-0. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol. 2010;22:330–55. doi: 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrne J, Warburton D, Opitz JM, Reynolds JF. Male excess among anatomically normal fetuses in spontaneous abortions. Am J Med Genet. 1987;26:605–11. doi: 10.1002/ajmg.1320260315. [DOI] [PubMed] [Google Scholar]
- 4.Boklage CE. The survival probability of human conceptions from fertilization to term. Int J Fertil. 1990;35:75–9. [PubMed] [Google Scholar]
- 5.Reynolds SA, Roberts JM, Bodnar LM, Haggerty CL, Youk AO, Catov JM. Newborns of preeclamptic women show evidence of sex-specific disparity in fetal growth. Gender Med. 2012;9:424–35. doi: 10.1016/j.genm.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Zarén B, Lindmark G, Bakketeig L. Maternal smoking effects fetal growth more in the male fetus. Paediatr Perinat Epidemiol. 2000;14:118–26. doi: 10.1046/j.1365-3016.2000.00247.x. [DOI] [PubMed] [Google Scholar]
- 7.Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction. 2013;145:R1–13. doi: 10.1530/REP-11-0489. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson DK, Verter J, Fanaroff AA, Oh W, Ehrenkranz RA, Shankaran S, et al. Sex differences in outcomes of very low birth weight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed. 2000;83:F182–5. doi: 10.1136/fn.83.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birth weight infants. Am J Obstet Gynecol. 2007;196:147–e1. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res. 2012;71:305–10. doi: 10.1038/pr.2011.50. [DOI] [PubMed] [Google Scholar]
- 11.Bruckner TA, Catalano R, Ahern J. Male fetal loss in the US following the terrorist attacks of September 11, 2001. BMC Public Health. 2010;10:273–8. doi: 10.1186/1471-2458-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catalano R, Bruckner T, Anderson E, Gould JB. Fetal death sex ratios: a test of the economic stress hypothesis. Intl J Epidemiol. 2005;34:944–8. doi: 10.1093/ije/dyi081. [DOI] [PubMed] [Google Scholar]
- 13.Elsmén E, Steen M, Hellström-Westas L. Sex and gender differences in newborn infants: why are boys at increased risk? J Men Health Gend. 2004;1:303–11. [Google Scholar]
- 14.Glover V, Hill J. Sex differences in the programming effects of prenatal stress on psychopathology and stress responses: An evolutionary perspective. Physiol Behav. 2012;106:736–40. doi: 10.1016/j.physbeh.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Beijers R, Buitelaar JK, de Weerth C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: beyond the HPA axis. Eur Child Adolesc Psy. 2014;23:943–56. doi: 10.1007/s00787-014-0566-3. [DOI] [PubMed] [Google Scholar]
- 16.Tegethoff M, Greene N, Olsen J, Meyer AH, Meinlschmidt G. Maternal psychosocial adversity during pregnancy is associated with length of gestation and offspring size at birth: evidence from a population- based cohort study. J Psychosom Med. 2010;72:419–26. doi: 10.1097/PSY.0b013e3181d2f0b0. [DOI] [PubMed] [Google Scholar]
- 17.Kaitz M, Mankuta D, Rokem AM, Faraone SV. Moderate antenatal anxiety symptoms and birth outcomes of boys and girls. J Psychosom Obst Gyn. 2014;35:116–23. doi: 10.3109/0167482X.2014.952279. [DOI] [PubMed] [Google Scholar]
- 18.Clifton VL. Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31:S33–9. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–65. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–2. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- 21.Almond D, Edlund L. Trivers–Willard at birth and one year: evidence from US natality data 1983–2001. P Roy Soc Lond B Bio. 2007;274:2491–9. doi: 10.1098/rspb.2007.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catalano R, Bruckner T, Smith K. Ambient temperature predicts sex ratios and male longevity. P Natl Acad Sci USA. 2008;105:2244–7. doi: 10.1073/pnas.0710711104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norberg K. Partnership status and the human sex ratio at birth. Proc R Soc Lond B: Biol Sci. 2004;271:2403–10. doi: 10.1098/rspb.2004.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuda M, Fukuda K, Shimizu T, Møller Decline in sex ratio at birth after Kobe earthquake. Hum Reprod. 1998;13:2321–2. doi: 10.1093/humrep/13.8.2321. [DOI] [PubMed] [Google Scholar]
- 25.Torche F, Kleinhaus K. Prenatal stress, gestational age and secondary sex ratio: the sex-specific effects of exposure to a natural disaster in early pregnancy. Hum Reprod. 2012;27:558–67. doi: 10.1093/humrep/der390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valente C. Civil conflict, gender-specific fetal loss, and selection: A new test of the Trivers–Willard hypothesis. J Health Econ. 2015;39:31–50. doi: 10.1016/j.jhealeco.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Sandman CA, Glynn LM, Davis EP. Is there a viability–vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res. 2013;75:327–35. doi: 10.1016/j.jpsychores.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connor TG, Monk C, Fitelson EM. Practitioner Review: Maternal mood in pregnancy and child development– implications for child psychology and psychiatry. J Child Psychol Psyc. 2014;55:99–111. doi: 10.1111/jcpp.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkar P, Bergman K, O’Connor TG, Glover V. Maternal antenatal anxiety and amniotic fluid cortisol and testosterone: Possible implications for foetal programming. J Neuroendocrinol. 2008;20:489–96. doi: 10.1111/j.1365-2826.2008.01659.x. [DOI] [PubMed] [Google Scholar]
- 30.O’Donnell KJ, Jensen AB, Freeman L, Khalife N, O’Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrino. 2012;37:818–26. doi: 10.1016/j.psyneuen.2011.09.014. 2012. [DOI] [PubMed] [Google Scholar]
- 31.Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: Cohort based study. BJM. 1999;318:153–7. doi: 10.1136/bmj.318.7177.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O’Connor TJ. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosom Med. 2011;73:656–63. doi: 10.1097/PSY.0b013e31822fc277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6–15. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henrichs J, Schenk JJ, Roza SJ, Van den Berg MP, Schmidt HG, Steegers EAP, et al. Maternal psychological distress and fetal growth trajectories: the Generation R Study. Psychol Med. 2010;40:633–43. doi: 10.1017/S0033291709990894. [DOI] [PubMed] [Google Scholar]
- 35.Baker JL, Michaelsen KF, Rasmussen KM, Sørensen TI. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr. 2004;80:1579–88. doi: 10.1093/ajcn/80.6.1579. [DOI] [PubMed] [Google Scholar]
- 36.Lee AM, Lam SK, Lau SMSM, Chong CSY, Chui HW, Fong DYT. Prevalence, course, and risk factors for antenatal anxiety and depression. Obstet Gynecol. 2007;110:1102–12. doi: 10.1097/01.AOG.0000287065.59491.70. [DOI] [PubMed] [Google Scholar]
- 37.Stuart S, Couser G, Schilder K, O’Hara MW, Gorman L. Postpartum anxiety and depression: onset and comorbidity in a community sample. J Nerv Ment Dis. 1998;86:420–4. doi: 10.1097/00005053-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Central Bureau of Statistics. [Accessed December 16, 2014];Israel in Figures 2011. 2012 available from: http://cbs.gov.il/www/publications/isr_in_n11e.pdf.
- 39.Nordentoft M, Lou HC, Hansen D, Nim J, Pryds O, Rubin P, Hemmingsen R. Intrauterine growth retardation and premature delivery: the influence of maternal smoking and psychosocial factors. Am J Public Health. 1996;86:347–54. doi: 10.2105/ajph.86.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) 2013 http://www.isuog.org/StandardsAndGuidelines/Statements+and+Guidelines/Practice+Guidelines/ [cited on December 18, 2014]
- 41.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body and femur measurements—A prospective stud. Am J Obstet Gynecol. 1985;151:333–7. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 42.Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: Harcourt, Brace, & Co; 1993. [Google Scholar]
- 43.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psych. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 44.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiat. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 45.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 46.Wenzel A, Haugen EN, Jackson LC, Brendle JR. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19:295–311. doi: 10.1016/j.janxdis.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Field T, Diego M, Hernandez-Reif M, Figueiredo B, Deeds O, Ascencio A, et al. Comorbid depression and anxiety effects on pregnancy and neonatal outcome. Infant Behav Dev. 2010;33:23–9. doi: 10.1016/j.infbeh.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DiPietro JA, Novak MF, Costigan KA, Atella LD, Reusing SP. Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev. 2006;77:573–87. doi: 10.1111/j.1467-8624.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 49.Colman I, Ataullahjan A, Naicker K, Van Kueshout RJ. Birthweight, stress, and symptoms of depression in adolescence: Evidence of fetal programming in a national Canadian Cohort. Can J Psychiat. 2012;57:422–28. doi: 10.1177/070674371205700705. [DOI] [PubMed] [Google Scholar]
- 50.Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50:232–41. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davis EP, Pfaff D. Sexually dimorphic responses to early adversity: Implications for affective problems and autism spectrum disorder. Psychoneuroendocrino. 2014;49:11–25. doi: 10.1016/j.psyneuen.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amiel-Tison C, Cabrol D, Denver R, Jarreau PH, Papiernik E, Piazza PV. Fetal adaptation to stress: Part I: Acceleration of fetal maturation and earlier birth triggered by placental insufficiency in humans. Early Hum Dev. 2004;78:15–27. doi: 10.1016/j.earlhumdev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 53.DiPietro JA, Kivlighan KT, Costigan KA, Rubin SE, Shiffler DE, Henderson JL, et al. Prenatal antecedents of newborn neurological maturation. Child Dev. 2010;81:115–30. doi: 10.1111/j.1467-8624.2009.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Del Giudice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neurosc Biohav R. 2011;35:1562–92. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fairbanks LA, Hinde K. Behavioral response of mothers and infants to variation in maternal condition: Adaptation, compensation and resilience. In: Clancy KBH, Hinde K, Rutherford JN, editors. Building babies: Primate developmental trajectories in proximate and ultimate perspectives. New York: Springer; 2013. pp. 281–302. [Google Scholar]
- 56.Maestripieri D, Klimczuk A. Prenatal and maternal psychosocial stress in primates: Adaptive plasticity or vulnerability to pathology? In: Laviola G, Macrì S, editors. Adaptive and Maladaptive Aspects of Developmental Stress. New York: Springer; 2013. pp. 45–64. [Google Scholar]
- 57.Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res. 2007;32:1730–40. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- 58.DiPietro JA, Kivlighan KT, Costigan KA, Laudenslager ML. Fetal motor activity and maternal cortisol. Dev Psychobiol. 2009;51:505–12. doi: 10.1002/dev.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Bruijn AT, van Bakel HJ, van Baar AL. Sex differences in the relation between prenatal maternal emotional complaints and child outcome. Early Hum Dev. 2009;85:319–24. doi: 10.1016/j.earlhumdev.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 60.Bale TL. Neuroendocrine and immune influences on the CNS: it’s a matter of sex. Neuron. 2009;64:13–16. doi: 10.1016/j.neuron.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 61.Morgan HD, Santos F, Green K, Dean W, Reik W. Epigenetic reprogramming in mammals. Hum Mol Genet. 2005;14(suppl 1):R47–58. doi: 10.1093/hmg/ddi114. [DOI] [PubMed] [Google Scholar]
- 62.Gabory A, Attig L, Junien C. Sexual dimorphism in environmental epigenetic programming. Mol Cell Endocrinol. 2009;304:8–18. doi: 10.1016/j.mce.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 63.Coe CL, Lubach GR. Fetal programming prenatal origins of health and illness. Curr Dir Psychol Sci. 2008;17:36–41. [Google Scholar]
- 64.DiPietro JA. Maternal stress in pregnancy: considerations for fetal development. J Adolescent Health. 2012;51:S3–S8. doi: 10.1016/j.jadohealth.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nilsson PM, Nyberg P, Östergren PO. Increased susceptibility to stress at a psychological assessment of stress tolerance is associated with impaired fetal growth. Int J Epidemiol. 2001;30:75–80. doi: 10.1093/ije/30.1.75. [DOI] [PubMed] [Google Scholar]
- 66.Cheung YB, Khoo KS, Karlberg J, Machin D. Association between psychological symptoms in adults and growth in early life: longitudinal follow up study. BMJ. 2002;325:749–53. doi: 10.1136/bmj.325.7367.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walhovd KB, Fjell AM, Brown TT, Kuperman JM, Chung Y, Hagler DJ, et al. Long-term influence of normal variation in neonatal characteristics on human brain development. Proc Natl Acad Sci USA. 2012;109:20089–94. doi: 10.1073/pnas.1208180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glover V. Annual research review: prenatal stress and the origins of psychopathology: an evolutionary perspective. J Child Psychol Psyc. 2011;52:356–67. doi: 10.1111/j.1469-7610.2011.02371.x. [DOI] [PubMed] [Google Scholar]
- 69.development: a systematic review. Child Psychiat Hum D. 2012;43:683–714. doi: 10.1007/s10578-012-0291-4. [DOI] [PubMed] [Google Scholar]
- 70.Van den Bergh BR, Loomans EM, Mennes M. Perinatal Programming of Neurodevelopment. New York: Springer; 2015. Early life influences on cognition, behavior, and emotion in humans: From birth to age 20; pp. 315–31. [DOI] [PubMed] [Google Scholar]
- 71.Kaitz M, Mankuta D, Rokem AM, Faraone S. Moderate antenatal anxiety symptoms and birth outcomes of boys and girls. J Psychosom Obst Gyn. 2014;35:116–123. doi: 10.3109/0167482X.2014.952279. [DOI] [PubMed] [Google Scholar]
- 72.Bruckner T, Catalano R. The sex ratio and age-specific male mortality: Evidence for culling in utero. Am J Hum Biol. 2007;19:763–73. doi: 10.1002/ajhb.20636. [DOI] [PubMed] [Google Scholar]
- 73.Glezerman M. Discrimination by good intention: gender-based medicine. Isr Med Assoc J. 2009;11:39–41. [PubMed] [Google Scholar]
- 74.Maina G, Saracco P, Giolito MR, Danelon D, Bogetto F, Todros T. Impact of maternal psychological distress on fetal weight, prematurity and intrauterine growth retardation. J Affect Disorders. 2008;111:214–20. doi: 10.1016/j.jad.2008.02.017. [DOI] [PubMed] [Google Scholar]