Abstract
One paradigmatic example of "irrational" bias in human economic decision-making—known as the “reflection effect”—is a tendency to prefer sure amounts over risky gambles in situations involving potential gain, but to prefer risky gambles over sure amounts in situations involving potential loss. To date, there is no causal evidence regarding the neural basis of the reflection effect. The ventromedial prefrontal cortex (vmPFC) is believed to play a critical role in mediating value-based decision-making. In this study, we administered a behavioral test of the reflection effect to three groups of subjects: neurosurgical patients with focal bilateral vmPFC lesions, neurosurgical patients with lesions outside vmPFC, and neurologically healthy adults. Subjects made a series of choices between a sure amount (e.g., gain of $50) and a gamble (e.g., 50% chance of gaining $100, 50% chance of gaining $0). Half the trials featured potential gains while the other half featured potential losses. The sure amounts varied across trials. Relative to the two comparison groups, the vmPFC lesion patients exhibited a significantly greater reflection effect; more gambles selected in the loss condition and fewer gambles selected in the gain condition. This finding demonstrates a critical role for vmPFC in governing susceptibility to bias in decision-making.
Keywords: Neuroeconomics, Prefrontal Cortex, Decision-making, Lesion
INTRODUCTION
Human decision-making is susceptible to influence by a number of cognitive and affective biases that yield systematic deviation from the ostensibly rational (i.e., financially optimal) choice. The “reflection effect” is a classic example of such bias (Kahneman and Tversky, 1979; Tversky and Kahneman, 1981). In this paradigm, which features a choice between a sure outcome and a risky gamble, individuals are more likely to gamble when the choices are prospective losses, as compared to when mathematically equivalent choices are prospective gains1. This pattern of choices violates “expected utility” models of decision-making and demonstrates that economic prospects are evaluated differently when conceived as gains versus losses. The reflection effect has been invoked to explain real-world choices deviating from expected utility, as commonly observed in casino gambling, financial investing, and insurance markets (Camerer, 2001). Identifying the brain regions responsible for the reflection effect would thus help illuminate the neuropsychological mechanisms governing pivotal aspects of human choice behavior. Although functional imaging studies have correlated activity in certain brain areas with the degree of rationality across individuals, there has not yet been any demonstration that a particular brain region plays a causal role in mediating the reflection effect. In this study, we investigate this causal brain-behavior relationship through the study of neurological lesion patients. To test this prediction, we administered a behavioral test of the reflection effect to a sample of neurosurgical patients with focal, bilateral vmPFC lesions.
MATERIALS AND METHODS
Participants
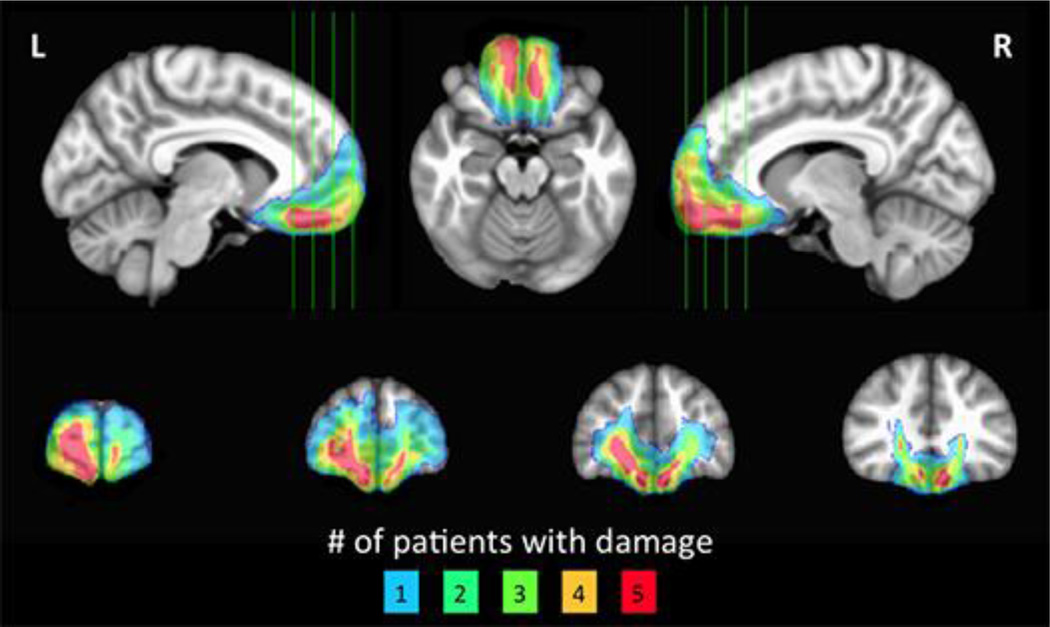
The target lesion group consisted of five neurosurgical patients with extensive bilateral parenchymal changes, largely confined to the vmPFC, where vmPFC is defined as Brodmann areas 11, 25, 32, and the medial portion of 10 below the level of the genu of the corpus callosum (Mackey and Petrides, 2014) (Fig. 1). All five patients had large anterior cranial fossa meningiomas with vasogenic edema. Their clinical presentations were subtle or obvious personality changes over at least several months preceding surgery. Each patient underwent gross total tumor resection without any intraoperative or postoperative complications. On post-surgical MRI, although vasogenic edema largely resolved, there were persistent circumscribed bilateral vmPFC lesions in each patient.
Figure 1. Lesion overlap of vmPFC patients.
Color indicates the number of overlapping lesions at each voxel.
Five neurosurgical patients who had focal lesions outside of vmPFC comprised a brain-damaged comparison (BDC) group, which included n=2 patients who had undergone tumor resections and n=3 patients who had undergone surgery for aneurysm clipping following subarachnoid hemorrhage. Lesions in the BDC group involved anterior and lateral temporal cortex (n=3) and dorsal frontal cortex (n=2). All vmPFC and BDC patients’ neurosurgeries were performed in adulthood, and all experimental data were collected at least three months after surgery, during the chronic phase of recovery (vmPFC range: 31.9–74.8 months). The inclusion of these BDC patients allowed us to rule out the possibility that the pattern of choices observed in the vmPFC lesion group could be due to anatomically non-specific effects of brain damage or history of related medical issues (e.g., craniotomy, edema, seizure, past medications, etc.). At the time of testing, one BDC patient and two vmPFC lesion patients were on psychoactive medications (one vmPFC patient on SSRI, one vmPFC patient and one BDC patient on anti-seizure medication). All neurosurgical patients (vmPFC and BDC) were recruited through a patient registry established by Drs. Koenigs and Baskaya through the University of Wisconsin Department of Neurological Surgery.
Thirty neurologically healthy adults also participated as a normal comparison (NC) group. NC participants had no history of brain injury, neurological or psychiatric illness, or current use of psychoactive medication. NC participants were between the ages of 54 and 70, matched to the ages of the lesion groups (see Table 1 for group demographic and neuropsychological data). NC participants were recruited through community advertisement. All participants had normal or corrected to normal vision.
Table 1.
Demographic and neuropsychological data
| Group | Age | Sex | Edu | WRAT Reading |
WRAT Arithmetic |
WAIS Digit Span |
WAIS Arithmetic |
|---|---|---|---|---|---|---|---|
| vmPFC (n=5) |
59.8 (5.2) |
3 M 2 F |
15.6 (3.6) |
105.2 (11.2) |
92.6* (10.4) |
13.0 (3.2) |
9.6 (2.9) |
| BDC (n=5) |
60.0 (7.0) |
3 M 2 F |
14.8 (1.8) |
101.2* (8.1) |
100.4 (5.6) |
N/A | N/A |
| NC (n=30) |
62.0 (4.1) |
17 M 13 F |
17.1 (2.6) |
112.5 (6.0) |
106.0 (9.8) |
N/A | N/A |
Age = age of participant at time of testing; Edu = years of education completed; WRAT Reading = scaled score on the WRAT Blue Reading subtest; WRAT Arithmetic = scaled score on the WRAT Blue Arithmetic subtest (for both WRAT tests, standardized mean = 100, SD = 15); WAIS Digit Span = scaled score on the WAIS digit span subtest; WAIS Arithmetic = scaled score on the WAIS arithmetic subtest (for both WAIS tests, standardized mean = 10, SD = 3). For test data, group means are presented with SD in parentheses.
Significant difference from NC group (p <0.05). All subjects scored in the “average” range or better on all WRAT and WAIS tests.
Lesion Segmentation and Image Normalization
vmPFC patients’ lesions were visually identified and manually segmented on a high-resolution (1 mm3) T1-weighted anatomical MRI image. Lesion boundaries were drawn to include areas with evidence of gross tissue damage or abnormal signal characteristics. A T2*-weighted FLAIR anatomical image was used to identify additional damage surrounding the core lesion area not apparent on the T1-weighted image (tissue with signal characteristics differing from healthy grey or white matter, e.g., hyperintensity). All structural MRI data were obtained at least three months after surgery (range: 13.6–55.5 months). T1-weighted anatomical images were preprocessed with AFNI (Cox, 1996) to remove non-brain tissue. The resulting skull-stripped anatomical images were diffeomorphically aligned to the Montreal Neurological Institute (MNI) coordinate system using a Symmetric Normalization algorithm (Avants and Gee, 2004) with constrained cost-function masking to prevent warping of tissue within the lesion mask (Brett et al., 2001). A lesion overlap map (Fig. 1) was created by computing the sum of lesion masks for all subjects in MNI space.
Decision-Making Task
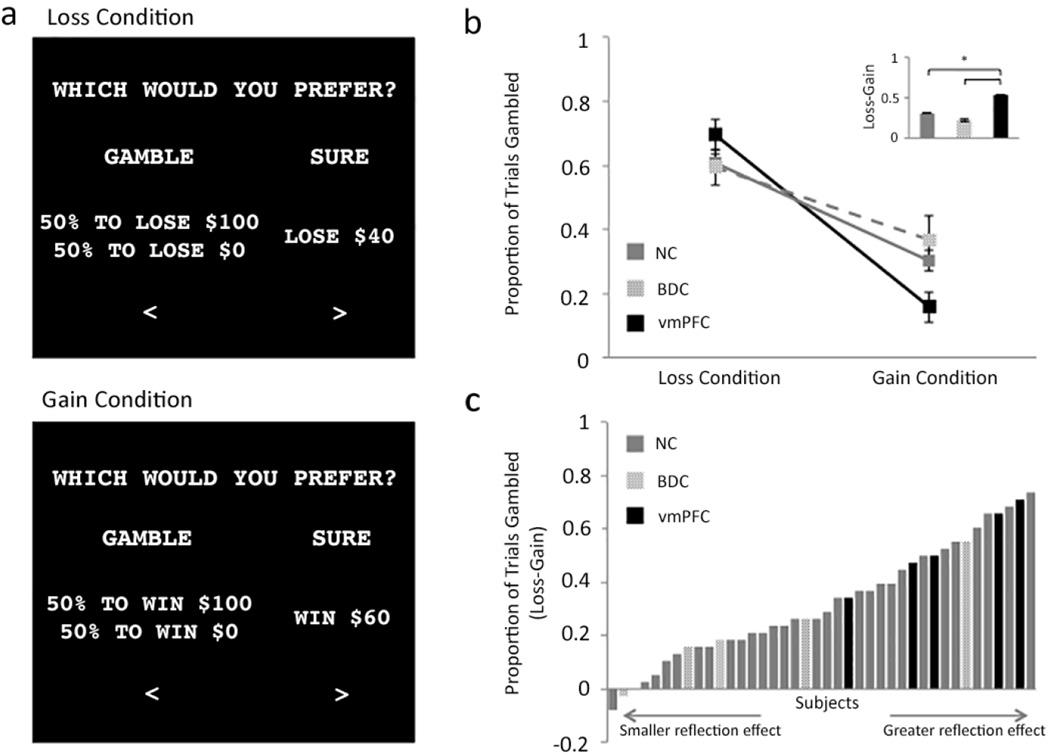
Participants performed a financial decision-making test adapted from original tests of Prospect Theory (Kahneman and Tversky, 1979; Tversky and Kahneman, 1981) (Fig. 2a). At the beginning of each trial, participants saw a fixation cross (+) in the center of the screen for two seconds followed by the question “Which would you prefer?”. Underneath this question, two hypothetical options were presented: a sure option and a gamble. Sure options ranged from gains or losses of $5 to $95, in increments of $5. Each sure value was presented twice for both the gain and loss conditions, for a total of 76 trials. The sure value was presented next to a gamble for a 50% chance to win or lose $100, depending on the condition (i.e., sure gains presented alongside a gamble to win $0 or $100 and sure losses presented alongside a gamble to lose $0 or $100). To ensure that all participants understood the task structure and the stakes of the gambles, practice trials involving the $5 and $95 sure values were presented for each gain/loss condition for a total of four practice trials. Participants were instructed to press the left or right arrow keys to choose one of the two options and to treat each trial independently of the other trials. There was no limit on decision time. Participants did not see feedback of their gains and losses following their choice. Trial presentation was pseudorandomized such that all participants saw the same randomized trial order. The position of the sure option (left or right side of screen) was counterbalanced across trials.
Figure 2. Example trials and summary data.
(A) Examples of trials from the loss condition (top panel) and gain condition (bottom panel). In both examples, the difference between the gamble and the sure option is mathematically equivalent. The reflection effect is demonstrated by a greater likelihood to choose the gamble in the loss condition than in the gain condition. (B) Comparison of gamble frequency for the loss condition and gain condition, for each group. Error bars indicate standard error. The bar graph inset shows the difference in gamble frequency between the loss and gain conditions for each group. *P<0.05. (C) Each vertical bar represents the magnitude of reflection effect for an individual subject.
Supplementary Cognitive Tasks
All vmPFC patients completed several tests to ensure intact basic elements of cognitive function germane to the demands of the decision-making task. vmPFC patients completed the Wechsler Adult Intelligence Scale-IV (WAIS) Working Memory Index (Wechsler, 2008), which consists of Digit Span and Arithmetic subtests to measure attention, concentration, mental control, and concentration while performing math problems. All participants completed the Wide Range Achievement Test (WRAT) 4 blue arithmetic test (Wilkinson and Robertson, 2006), a measure of basic arithmetic abilities. All vmPFC patients exhibited normal performance on each of these tests (Table 1).
Statistical Analysis
We performed non-parametric statistical tests because of the small sample sizes of vmPFC lesion patients (n=5) and BDC patients (n=5) using SPSS. We used a two-tailed Wilcoxon signed-rank test for paired data to compare the proportion of gambles selected for the loss condition as compared to the gain condition, within each group. For between-group analyses we used a two-tailed Kruskal-Wallis test along with two-tailed Mann-Whitney U tests for pairwise comparisons. Because these tests collapse across all sure amount values, they are extremely conservative estimates of our within- and between-group reflection effects. We therefore also ran a mixed effects logistic regression using the R statistical package (https://www.R-project.org) that allowed us to test for an overall interaction of group (vmPFC, NC, BDC) and condition (gain, loss), controlling for the effect of different levels of sure value (e.g., $5–$95), with respect to the subject’s preference for the gamble versus the sure option.
RESULTS
As expected, the NC subjects exhibited a significant difference between conditions (reflection effect), selecting the gamble more frequently overall in the loss condition (61.0%; SD: 15.5%) than in the gain condition (30.3%; SD: 17.5%) (Wilcoxon Z=−4.64, P=3×10−6) (Fig. 2b). As the key test of the study hypothesis, we compared the strength of the reflection effect (i.e., the difference between the proportion of trials gambled in the loss condition and the gain condition) between groups. Collapsing across all trials within each condition, the vmPFC patients (69.5% gamble frequency in loss condition; SD: 10.6%, 15.8% gamble frequency in gain condition; SD: 10.9%) exhibited a significantly stronger reflection effect than NC subjects (Mann-Whitney U=29.50, P=0.032) (Fig. 2b,c). This enhanced reflection effect cannot be attributed to non-specific effects of brain damage, as the BDC group exhibited a similar magnitude reflection effect to the NC group (59.5% gamble frequency in loss condition; SD: 12.6%, 36.8% gamble frequency in gain condition; SD: 16.4%) (Mann-Whitney U=57.50, P=0.41), but a smaller reflection effect than the vmPFC group (Mann-Whitney U=3.0, P=0.05). Nor can the group differences in the reflection effect be attributed to overall differences in gambling rates (Kruskal-Wallis χ2=0.61, P=0.74) or reaction times (Kruskal-Wallis χ2=1.77, P=0.41).
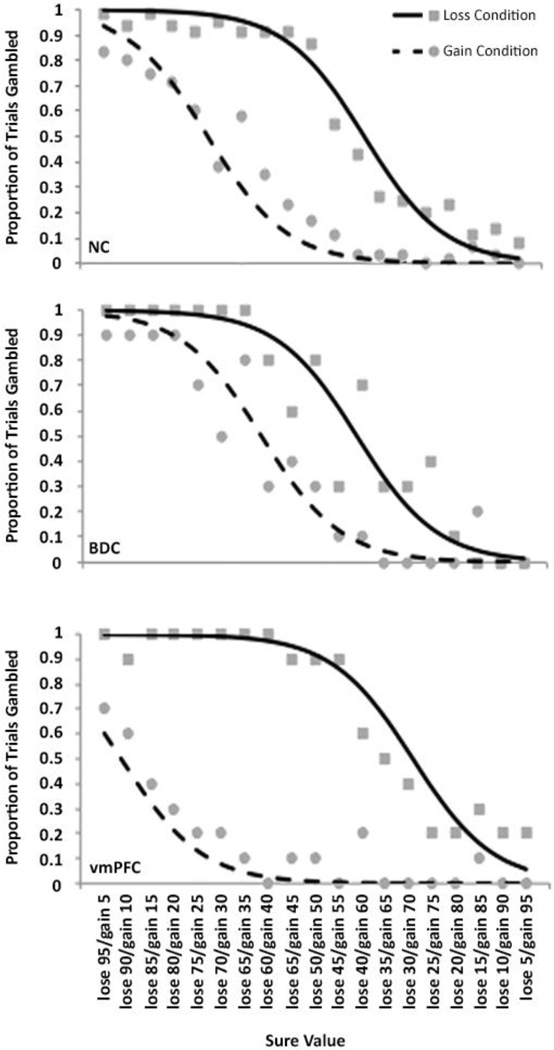
We confirmed the significant group difference in reflection effect using a mixed effects logistic regression that accounted for variable non-independent choices across the different levels of sure values and group differences in standardized WRAT arithmetic scores (Fig. 3). This analysis demonstrated a significant overall group by condition interaction (χ2=31.22, P=1.66×10−7), which held for specific comparisons between the NC and vmPFC groups (Z=3.28, P=8.00×10−7) as well as between the BDC and vmPFC groups (Z=4.83, P=1.02×10−6).
Figure 3. Reflection effect data for each sure option amount within each group.
Each data point represents the overall proportion of selected gambles for each condition, for a given sure value amount. The x-axis indicates the sure value amounts (each column represents mathematically equivalent choices as either gains or losses). The lines represent the smoothed estimated probability of gambling for each condition, corresponding to the logistic regression analysis.
We conducted post-hoc Mann-Whitney U and logistic regression analyses to examine choices for the gain and loss conditions separately. Between the NC and vmPFC groups, there was no significant difference in gambling for the loss condition (Mann-Whitney U=41.5, P=0.11; logistic regression: Z=−1.01, P=0.78) but a trending significant difference for the gain condition (Mann-Whitney U=36.5, P=0.07; logistic regression: Z=2.26, P=0.11). Between the BDC and vmPFC group, there was no significant difference in gambling for the loss condition (Mann-Whitney U=7.0, P=0.25; logistic regression: Z=1.34, P=0.73) but a significant difference for the gain condition (Mann-Whitney U=3.5, P=0.06; logistic regression: Z=−3.49, P=0.03). Between the NC and BDC groups, there was no difference in gambling for the loss condition (Mann-Whitney U=70.5, P=0.83; logistic regression: Z=0.32, P=0.99) or for the gain condition (Mann-Whitney U=63.0, P=0.57; logistic regression: Z=−1.22, P=0.76).
To examine the vmPFC patients’ pattern of choices in relation to “rational” choice, we calculated the average cumulative hypothetical earnings for each group across all trials. For this calculation we used the expected value of the gamble for any trial in which the gamble was selected (i.e., the product of the potential outcome and the probability of that outcome; $50 for the gain condition or −$50 for the loss condition). A purely “rational” actor who always selects the greater (or less negative) option between the expected value of the gamble and the sure amount would finish this task with a net balance of $900. The average hypothetical ending balance for the vmPFC group ($480, SD: $190.53) trended toward a significantly lower value than the NC group ($638.50, SD: $207.49; Mann-Whitney U=37.5, P=0.08) and the BDC group ($718.00, SD: $125.62; Mann-Whitney U=4.0, P = 0.10). There was no significant difference between the NC and BDC groups (Mann-Whitney U=64.5, P=0.63).
To further examine the choice behavior of each group, we calculated the sure value amounts at which the choice probability for each group was equal to 0.5. These values serve as an index of subjective equality (indifference) between the sure and gamble options. In the gain condition, the indifference point for the vmPFC lesion group ($8.25) was lower than either comparison group (NC: $27.73; BDC: $38.30), consistent with the selection of fewer gambles. In the loss condition, the indifference point for the vmPFC lesion group was less negative (−$29.60) than either comparison group (NC: −$38.31; BDC: −$41.08), consistent with the selection of more gambles.
Finally, we found no significant group by condition interaction for reaction times (χ2=2.25, p=0.32; Fig. 4).
Figure 4. Reaction time data for each sure option amount within each group.
Each data point represents the average reaction time for each condition, for a given sure value amount. The x-axis indicates the sure value amounts (each column represents mathematically equivalent choices as either gains or losses). The lines represent the smoothed estimated reaction time for each condition, corresponding to the logistic regression analysis.
DISCUSSION
The difference in risk-taking for prospective gains relative to losses is one of the seminal demonstrations of irrational bias in human decision-making. This study is the first to identify a brain region that plays a causal role in moderating this effect. Furthermore, this study provides novel evidence regarding the function of vmPFC, which is a key node in the brain network underlying value-based decision-making. Human functional imaging research has shown that, across a wide variety of experimental stimuli, tasks, and outcomes, vmPFC activity is commonly linked to reward and subjective value (Knutson et al., 2003; Grabenhorst and Rolls, 2011; Liu et al., 2011; Levy and Glimcher, 2012). Moreover, vmPFC damage has been associated with impairments in real-world decision-making (Blumer and Benson, 1975; Eslinger and Damasio, 1985; Barrash et al., 2000), as well as in laboratory paradigms involving risky gambles (Bechara et al., 1997; Camille et al., 2004), moral judgment (Ciaramelli et al., 2007; Koenigs et al., 2007; Young et al., 2010), probabilistic reinforcement learning (Fellows and Farah, 2003; Wheeler and Fellows, 2008), economic exchange (Koenigs and Tranel, 2007; Krajbich et al., 2009), and simple binary item preference (Henri-Bhargava et al., 2012). Despite the well-established role for vmPFC in value-based decision-making, the specific cognitive and affective functions subserved by this brain area are still a matter of debate and inquiry.
The reflection effect can be interpreted in terms of a dual-process model of decision-making, where one process is intuitive, affective, fast, heuristic, and automatic, whereas the other process is deliberative, effortful, slow, analytical, and based on conscious reasoning (Kahneman, 2003). It is assumed that the reflection effect is based on a relative predominance of the former process over the latter. Previous studies have suggested that vmPFC mediates the intuitive/affective contribution to value-based decision-making (Damasio, 1996; Bechara et al., 1997; Greene, 2007; Koenigs et al., 2007). The present results challenge this interpretation, as damage to the vmPFC resulted in a putatively less rational pattern of choices. It is important to note that the vmPFC patients’ choices were not simply more erratic or less consistent, as has been shown in simple preference studies (Fellows and Farah, 2007; Henri-Bhargava et al., 2012). As can be seen in Figure 3, the vmPFC patients exhibited choice functions (proportions of trials gambled across different sure amounts) that were at least as “smooth” or consistent as the choice functions of the NC and BDC groups, in that the proportion of gambles for a given sure gain amount was almost always greater than or equal to the proportion of gambles for smaller sure gain amounts, and almost always less than or equal to the proportion of gambles for larger sure gain amounts (and vice versa for sure loss amounts). Nor were vmPFC patients simply more apt to gamble, regardless of condition. Rather, the vmPFC patients evinced a systematically enhanced reflection effect, indicating a more complicated, multi-faceted role for vmPFC in decision-making.
One possibility is that vmPFC plays a critical role in triggering emotional responses to imagined, hypothetical gambles. It has been proposed that affective responses to hypothetical outcomes are critical input during value-based decision-making (Damasio, 1996; Bechara et al., 1997). For example, imagining winning money in a gamble could engender a positive emotional response (thereby making the gamble an attractive option), whereas imagining losing money in a gamble could engender a negative emotional response (thereby making the gamble an unattractive option). A previous study found that vmPFC patients were emotionally insensitive (in terms of subjective ratings and skin conductance responses) to the results of hypothetical gambles that they could have (but did not) engage in (Camille et al., 2004). If the vmPFC patients in the present study were similarly insensitive to the hypothetical outcomes of the gamble options, then their choice behavior would be driven predominantly by their reactions to the sure options. The vmPFC patients would exhibit reduced attraction to the potential gamble gains relative to the sure gains (i.e., fewer gambles chosen in the gain condition) and reduced aversion to the potential gamble losses relative to the sure losses (i.e., more gambles chosen in the loss condition)—in other words, an abnormally large reflection effect. We therefore see our results as consistent with the general idea that vmPFC contributes to value-based decision-making by triggering affective responses to hypothetical risks and rewards (Bechara et al., 2003). However, it should be noted that all options in the task (sure amounts and gambles) were hypothetical, so this interpretation presumes that the gamble options require one to compare and contrast multiple uncertain hypothetical outcomes in a way that the sure options do not. Future studies that compare responses for real, immediate gains/losses as opposed to hypothetical or distant gains/losses could more definitively test this interpretation.
The paradigm used in this study to examine the reflection effect is similar, conceptually and methodologically, to paradigms used to examine the framing effect. The framing effect and reflection effect were two key initial pillars of empirical support for prospect theory. Both paradigms involve a series of choices between a sure amount and a risky gamble; the main difference is that framing effects refer to a choice between options that have mathematically equivalent outcomes, but are framed differently (i.e., highlighting what may be lost in a particular transaction instead of what may be gained, or vice versa), whereas reflection effects refer to choices in conditions of potential gain versus potential loss. Regardless of this difference, both paradigms have shown that normal individuals are more likely to gamble when considering prospective losses as compared to prospective gains. Consistent with our results, a previous neuroimaging study of the framing effect in healthy individuals showed that vmPFC activity correlated with the “rational” choice (i.e., lower levels of vmPFC activity were associated with larger framing effects) (De Martino et al., 2006).
One feature of the study design that warrants further discussion is the limited sample size of vmPFC lesion patients (n=5). For this study, we employed stringent selection criteria for our target group; lesions had to involve substantial portions of vmPFC bilaterally, but could not extend significantly outside vmPFC. This patient selection strategy is distinct from typical vmPFC lesion studies, which often include patients with lesions that are exclusively or primarily unilateral and/or lesions that extend beyond the boundaries of vmPFC (e.g., into adjacent dorsomedial PFC, lateral PFC, or anterior temporal lobe). Limiting the vmPFC lesion patient group to these more stringent criteria increases lesion homogeneity and reduces the likelihood of preservation of function by a single hemisphere. We believe the uniformity of lesion characteristics in this vmPFC patient sample likely contributes to the remarkable consistency of the individual results. As can be seen in Fig. 2c, the reflection effects for each vmPFC patient were similar to one another, and all well above the median of each comparison group (medianNC=0.26, medianBDC=0.18;, rangevmPFC=0.34–0.71). These results provide novel insight into the neuropsychological mechanisms that instill irrational bias in human decision-making.
Highlights.
Reflection effect is a classic example of irrational bias in human decision-making
Choice between sure amounts and risky gambles; gain and loss conditions
vmPFC lesion patients exhibited a greater reflection effect than normal subjects
Less likely to gamble in gain conditions, more likely to gamble in loss conditions
ACKNOWLEDGEMENTS
We thank Julian Motzkin, Ian Carroll, and Varun Razdan for assistance with task design and data collection.
FUNDING
This work was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was funded by a grant from NIMH (MH086787), with additional support from grants T32GM007507 and T32MH018931.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This difference in risk tolerance for gains versus losses (risk aversion for positive prospects but risk seeking for negative prospects) is called the “reflection effect” because the preference reverses around zero, as a mirror image or reflection (Kahneman D, Tversky A (1979) Prospect Theory - Analysis of Decision under Risk. Econometrica 47:263–291.).
The authors declare no competing financial interests.
REFERENCES
- Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape averaging and interpolation. Neuroimage. 2004;23(Suppl 1):S139–S150. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol. 2000;18:355–381. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Ann N Y Acad Sci. 2003;985:356–369. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Blumer D, Benson DF. Personality changes with frontal and temporal lesions. In: Benson DF, Blumer D, editors. Psychiatric Aspects of Neurological Disease. New York: Stratton; 1975. [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Camerer CF. Choices, Values, and Frames. Contemporary Psychology. Washington DC: American Psychological Association; 2001. Prospect Theory in the Wild: Evidence from the Field; pp. 288–300. [Google Scholar]
- Camille N, Coricelli G, Sallet J, Pradat-Diehl P, Duhamel JR, Sirigu A. The involvement of the orbitofrontal cortex in the experience of regret. Science. 2004;304:1167–1170. doi: 10.1126/science.1094550. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Muccioli M, Ladavas E, di Pellegrino G. Selective deficit in personal moral judgment following damage to ventromedial prefrontal cortex. Soc Cogn Affect Neurosci. 2007;2:84–92. doi: 10.1093/scan/nsm001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. The role of ventromedial prefrontal cortex in decision making: judgment under uncertainty or judgment per se? Cereb Cortex. 2007;17:2669–2674. doi: 10.1093/cercor/bhl176. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Greene JD. Why are VMPFC patients more utilitarian? A dual-process theory of moral judgment explains. Trends Cogn Sci. 2007;11:322–323. doi: 10.1016/j.tics.2007.06.004. author reply 323–324. [DOI] [PubMed] [Google Scholar]
- Henri-Bhargava A, Simioni A, Fellows LK. Ventromedial frontal lobe damage disrupts the accuracy, but not the speed, of value-based preference judgments. Neuropsychologia. 2012;50:1536–1542. doi: 10.1016/j.neuropsychologia.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Kahneman D. A perspective on judgment and choice: mapping bounded rationality. The American psychologist. 2003;58:697–720. doi: 10.1037/0003-066X.58.9.697. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect Theory - Analysis of Decision under Risk. Econometrica. 1979;47:263–291. [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. J Neurosci. 2007;27:951–956. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajbich I, Adolphs R, Tranel D, Denburg NL, Camerer CF. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. J Neurosci. 2009;29:2188–2192. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Curr Opin Neurobiol. 2012;22:1027–1038. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and biobehavioral reviews. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S, Petrides M. Architecture and morphology of the human ventromedial prefrontal cortex. Eur J Neurosci. 2014;40:2777–2796. doi: 10.1111/ejn.12654. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. The Framing of Decisions and the Psychology of Choice. Science. 1981;211:453–458. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Fourth Edition. San Antonio, TX: Pearson; 2008. [Google Scholar]
- Wheeler EZ, Fellows LK. The human ventromedial frontal lobe is critical for learning from negative feedback. Brain. 2008;131:1323–1331. doi: 10.1093/brain/awn041. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. WRAT4: Wide Range Achievement Test. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- Young L, Bechara A, Tranel D, Damasio H, Hauser M, Damasio A. Damage to ventromedial prefrontal cortex impairs judgment of harmful intent. Neuron. 2010;65:845–851. doi: 10.1016/j.neuron.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]