Abstract
Background
The annual incidence of Clostridium difficile infection (CDI) in the United States is estimated to be 330,000 cases. We evaluated the impact of using a launderable mattress and bed deck cover on the incidence of hospital-onset CDI in two long-term acute care hospitals (LTACH)s.
Methods
Two LTACH hospitals began using a launderable mattress and bed deck cover on beds starting in May of 2013. One facility had 74 beds and the other had 30 beds. Covers were changed after every patient. The covers were laundered using hot water, detergent, and chlorine. Rates for CDIs were compared using Poisson regression between the 16 months before use of the launderable cover and the 14 months after the cover started being used.
Results
At Hospital A, the use of bedcovers reduced the rate of infections by 47.8% (95% CI 47.1 – 48.6), controlling for the rate of hand washing compliance and length of stay in days. At Hospital B, the use of bedcovers reduced the rate of infections by 50% (95% CI 47.5 – 52.7), controlling for the rate of hand washing compliance and length of stay in days.
Conclusions
The use of a launderable cover for mattresses and bed decks of hospital beds was associated with a decreased rate of healthcare associated CDIs in two LTACHs.
INTRODUCTION
Hospital associated infections (HAIs) are a major source of morbidity and mortality in the United States (US). While many HAIs have decreased in recent years, the incidence and severity of Clostridium difficile infections (CDI) has remained problematic.(1) The most recent estimates indicate that there are 453,000 CDIs in the US each year, with 29,300 deaths.(2) It has been estimated that the additional cost of care for these infections may be as high as $3.2 billion.(3)
There is a large body of evidence showing that patients acquire infections from the hospital room. Studies have shown that patients who are placed in a room previously occupied by a patient who was contaminated with methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), or Clostridium difficile (C diff) are at increased risk of acquiring these infections.(4-7)
In order to provide a clean environment for subsequent patients, the typical hospital room undergoes “Terminal Cleaning” after the previous patient is discharged. During the terminal cleaning process, the entire room is cleaned and disinfected using chemical cleaners. Studies have shown that, even after terminal cleaning, the major touch points in the room (bed, mattress, hand rails, toilet, side table) are still contaminated.(8-12) Previous research has linked many outbreaks of HAIs back to hospital mattresses.(13-18) A recent study questioned the efficacy of detergent wipes for cleaning.(19) The Food and Drug Administration has warned that worn or damaged mattresses may be putting patients at risk of infection.(20)
In order to ensure the mattress is clean, a launderable cover for the mattress and bed deck was developed. Because the cover is removed and laundered with hot water, chlorine, and detergent, it has been shown to provide a significantly cleaner surface for patients than can be provided with terminal cleaning.(21)
There are no published studies examining the clinical efficacy of using a launderable cover. The purpose of the current research is to evaluate if the use of a launderable cover would decrease the incidence of hospital-onset (HO) Clostridium difficile infections in hospitals. CDIs were chosen over other HAIs because of the high incidence of these infections within the hospital environment, the existence of surveillance procedures for CDIs, and the significant clinical and financial impact of these infections to patients and hospitals. It is also important to note that CDIs have continued to increase in frequency while many other HAI rates have decreased.
MATERIALS AND METHODS
In May of 2013, two Long Term Acute Care Hospitals (LTACH) purchased launderable mattress and bed deck covers (Trinity Bed Protection System®) and began using them routinely on almost all patient beds. The launderable cover was manufactured using material similar to that found in high-end bed mattresses, and it also encompasses the bed deck, which is the metal surface upon which the mattress rests (Trinity Guardion; Batesville, Indiana). The covers are manufactured to fit specific models of hospital beds, and a small number of hospital beds cannot, because of their design, be fitted with a cover. Hospital beds used with covers in the current study included VersaCare®, TotalCare®, BurkeBariatric®, Advanta®, Advanta® 2, CamTec®, and Stryker Secure II®. Mattresses used included low airloss, micro-climate and weight distribution.
Prior to use, each cover is laundered according to the CDC and manufacturers standards including 180°F water, detergent, use of chlorine, and drying at temperatures exceeding 160°F. The cover was light table inspected after each laundering, and if damaged, it was patched using a thermal patch. If the damage cannot be repaired, the cover is taken out of service. Each cover is then reverse rolled to prevent contamination of the patient surface by any bacteria that might still be present on the bed after terminal cleaning. All covers are color coded to facilitate correct cover use by housekeeping staff. After training, all environmental service employees could install the covers in approximately 2 minutes. After discharge, the cover is rolled up on itself in order not to transmit any pathogens to the underlying mattress.
The LTACHs were both in Indiana and had all private rooms. In Facility A, which had 74 beds and was built 10 years ago, covers were changed after patient discharge or after 30 days, and a new cover was placed after terminal cleaning and patient admission. At facility B, which had 30 beds and was built 75 years ago, the covers were also changed every two weeks for patients who remained hospitalized. The period from January 1, 2012 until April 30, 2013 served as the baseline period for establishing the rate of CDIs. The period for the new launderable cover started in May of 2013 and ran through June of 2014. All CDIs were calculated based on the actual number of hospital associated CDIs divided by the number of patient days for that month. Both facilities utilized nucleic acid amplification test (NAAT)-based assay for Clostridium difficile detection during both time periods of the study. All data was collected retrospectively from the infection control reports of both facilities for 2013 and 2014. Hand washing data were unavailable for one month in the pre-intervention period for Hospital A and for two month in the pre-intervention period for Hospital B. These data were left missing and the Poisson regression models were calculated without these data. All other analyses, as well as all tables and figures contain complete data. For 2012, laboratory reports of CDIs were analyzed using the recommended procedures from the CDC.(22) The launderable cover could be utilized on 95% of the beds at both facilities. All beds, even those in beds without covers, were included with calculating CDI rates.
Although the two facilities used different cleaning companies, there was no change during the study periods. The methods of cleaning and chemicals used were unchanged during the two time periods. Infection control surveillance was the same during both study periods and there were no other infection control interventions initiated. Both facilities use bleach when a room has been contaminated with C. diff. Facility A uses a quaternary ammonium compound by Johnson Diversey. Facility B uses a phenol cleaner, Wex-Cide®. All covers used at both facilities were laundered at the same laundry. Ten different administrative persons perform 3 observations each month (30 total observations monthly) to determine hand-washing compliance in both facilities. To get credit for handwashing, the employee must wash their hands both as they enter and exit the patient room.
A significant concern in LTACHs is the development of pressure ulcers. Introduction of the launderable cover between the patient and the mattress created, at least, the hypothetical concern that this new interface could have a detrimental effect on the development of pressure ulcers at these LTACHs. This issue is in part addressed by the fact that the launderable cover is made of similar material as the permanent mattress cover, they are constructed to allow for vapor-moisture transmission. This design feature is intended to prevent the development of pressure ulcers by allowing moisture to move through the cover and away from the patient. In order to evaluate any effect that the launderable cover usage may have had on the development of pressure ulcers, we reported the number of stage II pressure ulcers that occurred at each facility during both study periods.
Definitions
The hospital onset CDIs were identified according to the CDC’s National Healthcare Safety Network (NHSN) definitions. A hospital onset (HO) CDI is defined as an infection starting on day four or later of hospital admission or within 4 weeks following discharge.
Data Analysis
Descriptive statistics were used to report the number of infections, number of patient days, hand-washing compliance, length of stay, acuity (Case Mix Index (CMI)), and the rate of CDI per 10,000 patient days. The CMI is calculated by taking the total of all patient’s diagnosis related group weights and dividing it by the total number of patients). Poisson Regression was used to compare the monthly counts of CDI, adjusted for patient days, at both facilities during the two study periods. The rate of CDIs, hand-washing compliance, acuity, and average length were included in the analysis. All data analyses were performed using SPSS 22.0 (IBM Corporation, Armonk, NY). Graphics were produced using SPSS and R (2.15.3).
Human Studies
The study was reviewed and approved by the Institutional Review Board of Saint Vincent Health.
RESULTS
Hospital A
There were 35 HO CDIs and 29,747 patient days in the pre-intervention period and 15 HO CDIs and 26,083 patient days in the post-intervention period. The mean age pre-intervention was 66 years (SD 1) and post-intervention it was 65 years (SD 2). The median hand washing compliance rate was 97% pre-intervention (range 72% - 100% and was 91% post-intervention (range 64% - 100%). Descriptive statistics for hand washing rates, acuity and length of stay for the pre- and post-intervention periods are reported in Table 1.
Table 1.
Descriptive statistics for hand washing, acuity, and length of stay by hospital
| Pre-Intervention | Post-Intervention | |||||
|---|---|---|---|---|---|---|
| Median | IQR | Range | Median | IQR | Range | |
| Hospital A | ||||||
| Hand washing compliance rate | 97 | 93-98 | 73-100 | 91 | 88-100 | 64-100 |
| Acuity | 1.47 | 1.40-1.52 | 1.15-1.73 | 1.51 | 1.46-1.56 | 1.37-.1.70 |
| Length of stay (days) | 31 | 29-33 | 27-40 | 34 | 33-40 | 31-43 |
|
| ||||||
| Hospital B | ||||||
| Hand washing compliance rate | 96 | 94-100 | 90-100 | 99 | 96-100 | 81-100 |
| Acuity | 1.34 | 1.21-1.45 | 1.05-1.96 | 1.17 | 1.11-1.27 | 0.99-1.68 |
| Length of stay (days) | 31 | 26-34 | 18-42 | 30 | 27-36 | 23-42 |
Poisson regression results indicated that the use of bedcovers reduced the rate of infections by 47.8% (95% CI 0 47.1 – 48.6), controlling for the rate of hand washing compliance and length of stay in days. The rate of hand washing compliance was a statistically significant contributor to the model, though the effect size was small (IRR 0.995, 95% CI 0.994 – 0.996). Length of stay was also a statistically significant contributor to the model, though the effect size was small (IRR 0.995, 95% CI 0.993 – 0.997) (Table 2, Figure 1). Acuity was not a significant predictor and was removed from the model.
Table 2.
Parameter estimates for Poisson regression models by hospital
| Parameter | Coeff. | SE | 95% CI | Hypothesis Test | Exp(B) | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Wald Chi-Square | df | Sig. | Lower | Upper | ||||
| Hospital A | ||||||||||
| (Intercept) | 1.455 | 0.051 | 1.356 | 1.554 | 823.996 | 1 | <0.0001 | 4.285 | 3.880 | 4.732 |
| Bed cover | -0.737 | 0.008 | -0.754 | -0.721 | 7683.707 | 1 | <0.0001 | 0.478 | 0.471 | 0.486 |
| Hand washing compliance rate | -0.005 | 0.000 | -0.006 | -0.004 | 145.485 | 1 | <0.0001 | 0.995 | 0.994 | 0.996 |
| Length of stay (days) | -0.005 | 0.001 | -0.007 | -0.003 | 29.166 | 1 | <0.0001 | 0.995 | 0.993 | 0.997 |
|
| ||||||||||
| Hospital B | ||||||||||
| (Intercept) | -18.353 | 0.485 | -19.304 | -17.403 | 1432.926 | 1 | <0.0001 | 0.000 | 0.000 | 0.000 |
| Bed cover | -0.692 | 0.026 | -0.743 | -0.641 | 700.369 | 1 | <0.0001 | 0.500 | 0.475 | 0.527 |
| Hand washing compliance rate | 0.167 | 0.005 | 0.157 | 0.176 | 1113.763 | 1 | <0.0001 | 1.181 | 1.170 | 1.193 |
| Length of stay (days) | 0.053 | 0.002 | 0.049 | 0.057 | 667.882 | 1 | <0.0001 | 1.055 | 1.051 | 1.059 |
Figure 1.
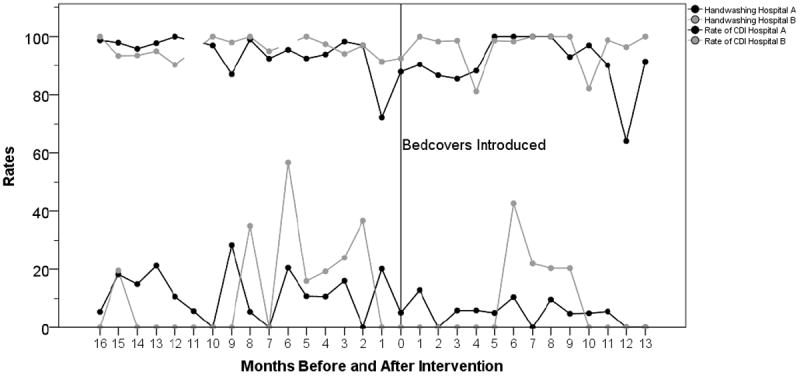
There were 14 Stage II Ulcers in the pre-intervention period (median rate per 1,000 patient days per month 0%, range 0% to 2%) and 10 Stage II Ulcers in the post-intervention period (median rate per 1,000 patient days per month 0%, range 0% to 1%).
Hospital B
There were 11 HO CDIs and 8,466 patient days in the pre-intervention period and 5 HO CDIs and 6,767 patient days in the post-intervention period. The mean age pre-intervention was 65 years (SD 2) and post-intervention it was 65 years (SD 3). The median hand washing compliance rate was 96% pre-intervention (range 90% - 100% and was 99% post-intervention (range 71% - 100%). Descriptive statistics for hand washing rates, acuity and length of stay for the pre- and post-intervention periods are reported in Table 1.
In the second hospital, Poisson regression results again indicated that the use of bedcovers reduced the rate of infections by 50% (95% CI 47.5 – 52.7), controlling for the rate of hand washing compliance and length of stay in days. The rate of hand washing compliance was a statistically significant contributor to the model, though in a different direction than for Hospital A (IRR 1.181, 95% CI 1.170 – 1.193). Length of stay was also a statistically significant contributor to the model, though again in a different direction than Hospital A. Again, the effect size was small (IRR 1.055, 95% CI 1.051 – 1.059) (Table 2, Figure 1). Acuity was not a significant predictor and was removed from the model.
There was 1 Stage II Ulcer in the pre-intervention period and 2 Stage II Ulcers in the post-intervention period.
DISCUSSION
The use of a launderable cover for mattresses and bed decks of hospital beds was associated with a significantly decreased rates of healthcare onset CDIs by 50% (50% in one facility and 47.8% in the other) in two LTACHs. The covers were applied to 95% of the beds at the facilities, can be utilized on most commercially available hospital beds, and require minimal training of nursing and environmental services staff.
The hospital mattress is clearly one of the highest touch points for patients when they are in a hospital room. As such, these must be adequately decontaminated between patients. Unfortunately, multiple studies have shown that current processes of cleaning after each patient (terminal cleaning) does not adequately protect future patients.(4-7) Currently, most hospitals utilize EPA registered disinfectants that were not approved for use on soft surfaces (like mattresses). Additionally, most top hospitals do not actually follow the manufacturers recommendations for use of these chemicals.(23) A recent study showed that, although these disinfectants did decrease levels of bacterial contamination on bedrails by 99%, bacteria survived and the levels of contamination rebounded by 30% in only 6.5 hours.(24)
Although there were no formal time and motion studies done, the use of the launderable covers should improve room turnover times because bed surface is no longer grossly contaminated and there is not time required to remove blood and organic material from the mattress. The contaminated launderable cover is simply removed from the bed and sent to the laundry, which makes for a more standardized process. Also, damage to mattresses is a common problem and can lead to contamination and HAIs.(18) The Food and Drug Administration (FDA) has warned that the use of disinfectants can result in damage to the mattresses and cause them to become fluid permeable.(20) Use of the launderable cover should prevent damage to the underlying mattress, which can cost up to $5,000 to replace, if damaged.
The current study only examined CDIs because the overall rate of those was high enough for the study to be done in a feasible time frame. However, it is a logical conclusion that the use of the launderable cover should help decrease other HAIs that have been linked to environmental transmission, such as MRSA and VRE.
The prevention of CDIs in the hospital can help prevent morbidity and mortality among hospitalized patients. Recent studies have shown that CDIs have a mortality rate of 9.3% and that attributable costs for a HO-CDI may be as high as $15,397.(2,25) It is important for hospitals to find ways to decrease the chance of acquiring CDI, and improved environmental cleaning, along with improved hand washing and antibiotic stewardship, is an important part of any effective strategy.
LIMITATIONS
The current study had a number of limitations. The study was only performed at two facilities within one healthcare system. Many factors can lead to decreased CDIs including antibiotic stewardship, improved handwashing, deceased use of Proton Pump Inhibitors, and improved environmental cleaning. We were unable to quantify any changes in antibiotic usage during the study because data was unavailable; however, there were no initiatives to improve antibiotic stewardship during the study periods. If antibiotic usage did decrease, this may have explained some of the decrease in CDIs that were observed. We were also unable to quantify any changes in use of Proton Pump Inhibitors during the study. There were no other initiatives in place to decrease CDIs during the study period. Hand washing and length of stay had only a small effect on CDI rates at both institution, but the effect was opposite at the two sites. However, using the regression model to control for these differences, the decrease in CDIs was 50 percent at both institutions.
CONCLUSIONS
Control of hospital-acquired infections, especially Clostridium difficile, requires a comprehensive approach including strict hand washing, antimicrobial stewardship, and excellent environmental hygiene. The addition of a launderable mattress and bed deck cover was feasible and was associated with a 50% decrease in HO-CDIs within two LTACHs.
Acknowledgments
This project was supported by an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 8UL1-TR000077.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Edmond A Hooker, Email: ehooker@mac.com, Xavier University, 3800 Victory Parkway, Cincinnati, Ohio 45207-5141, 513-745-4397.
Mark Bochan, Email: mbochan@idipsc.com, Infectious Disease of Indiana, 11455 N. Meridian Street, Suite 200, Carmel, IN 46032.
Troy T. Reiff, Email: ttreiff@stvincent.org, Saint Vincent Health, 8050 Township Line Road, Indianapolis, Indiana 46260, 317-415-8500.
Catherine Blackwell, Email: cablackw@stvincent.org, Saint Vincent Health, 205 Ann Avenue, Pendleton, In 46064, 317-345-3302.
Kevin W. Webb, Email: Kevin.Webb@medxcelfacilities.com, Medxcel Facilities Management, 5451 Lakeview Parkway S. Drive, Indianapolis, IN 46268.
Kimberly W Hart, Email: hartkb@ucmail.uc.edu, 231 Albert Sabin Way, PO Box 670769, Cincinnati, Ohio 45267-0769, 513.558.6028.
References
- 1.Kelly CP, LaMont JT. Clostridium difficile — More Difficult Than Ever. N Engl J Med. 2008;359(18):1932–40. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 2.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile Infection in the United States. N Engl J Med. 2015;372(9):825–34. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghantoji SS, Sail K, Lairson DR, DuPont HL, Garey KW. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect. 2010;74(4):309–18. doi: 10.1016/j.jhin.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Drees M, Snydman DR, Schmid CH, Barefoot L, Hansjosten K, Vue PM, et al. Prior environmental contamination increases the risk of acquisition of vancomycin-resistant enterococci. Clin Infect Dis. 2008;46(5):678. doi: 10.1086/527394. [DOI] [PubMed] [Google Scholar]
- 5.Huang SS, Datta R, Platt R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch Intern Med. 2006;166(18):1945–51. doi: 10.1001/archinte.166.18.1945. [DOI] [PubMed] [Google Scholar]
- 6.Shaughnessy MK, MD, Micielli RL, MD, DePestel DD, PharmD, Arndt J, MS, Strachan CL, MSRN, Welch KB, MS, et al. Evaluation of Hospital Room Assignment and Acquisition of Clostridium difficile Infection. Infect Control Hosp Epidemiol. 2011 Mar 1;32(3):201–6. doi: 10.1086/658669. [DOI] [PubMed] [Google Scholar]
- 7.Kelley R, Wiemken T, Curran D, Khan M, Pacholski E, Carrico R, et al. Risk of Acquiring Carpapenem-resistant Klebsiella Pneumoniae from Bed Contact in a Long-term Acute Care Hospital. Am J Infect Control. 2014;42(6):S30–1. [Google Scholar]
- 8.Hooker EA, Allen SD, Gray LD. Terminal Cleaning of Hospital Bed Mattresses and Bedecks does not eliminate Bacterial Contamination. Am J Infect Control. 2011;39(5):E23–4. [Google Scholar]
- 9.Blythe D, Keenlyside D, Dawson SJ, Galloway A. Environmental contamination due to methicillin-resistant Staphylococcus aureus (MRSA) J Hosp Infect. 1998;38(1):67–9. doi: 10.1016/s0195-6701(98)90176-1. [DOI] [PubMed] [Google Scholar]
- 10.Denton M, Wilcox MH, Parnell P, Green D, Keer V, Hawkey PM, et al. Role of environmental cleaning in controlling an outbreak of Acinetobacter baumannii on a neurosurgical intensive care unit. J Hosp Infect. 2004;56(2):106–10. doi: 10.1016/j.jhin.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Andrade D, Angerami ELS, Padovani CR. A bacteriological study of hospital beds before and after disinfection with phenolic disinfectant. Rev Panam Salud Pública. 2000;7:179–84. doi: 10.1590/s1020-49892000000300007. [DOI] [PubMed] [Google Scholar]
- 12.French GL, Otter JA, Shannon KP, Adams NMT, Watling D, Parks MJ. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): a comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J Hosp Infect. 2004;57(1):31–7. doi: 10.1016/j.jhin.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Ndawula EM, Brown L. Mattresses as reservoirs of epidemic methicillin-resistant Staphylococcus aureus. Lancet. 1991;337(8739):488. doi: 10.1016/0140-6736(91)93420-e. [DOI] [PubMed] [Google Scholar]
- 14.Lilly HA, Kidson A, Fujita K. Investigation of hospital infection from a damaged mattress and the demonstration of its mechanism. Burns. 1982;8(6):408–13. doi: 10.1016/0305-4179(82)90112-7. [DOI] [PubMed] [Google Scholar]
- 15.Fujita K, Lilly HA, Kidson A, Ayliffe GA. Gentamicin-resistant Pseudomonas aeruginosa infection from mattresses in a burns unit. Br Med J Clin Res Ed. 1981;283(6285):219. doi: 10.1136/bmj.283.6285.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loomes S. The Journal of Infection Control Nursing. Is it safe to lie down in hospital? Nurs Times. 1988 Dec 7;84(49):63–5. [PubMed] [Google Scholar]
- 17.Creamer E, Humphreys H. The contribution of beds to healthcare-associated infection: the importance of adequate decontamination. J Hosp Infect. 2008;69(1):8–23. doi: 10.1016/j.jhin.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Van der Mee-Marquet N, Girard S, Lagarrigue F, Leroux I, Voyer I, Bloc D, et al. Multiresistant Enterobacter cloacae outbreak in an intensive care unit associated with therapeutic beds. Crit Care Lond Engl. 2006 Feb;10(1):405. doi: 10.1186/cc4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramm L, Siani H, Wesgate R, Maillard J-Y. Pathogen transfer and high variability in pathogen removal by detergent wipes. [2015 Jun 25];Am J Infect Control. doi: 10.1016/j.ajic.2015.03.024. [Internet]. Available from: http://www.sciencedirect.com/science/article/pii/S0196655315001947. [DOI] [PubMed]
- 20.Food and Drug Administration. [2014 Aug 3];Safety Communications - Damaged or Worn Covers for Medical Bed Mattresses Pose Risk of Contamination and Patient Infection: FDA Safety Communication. [Internet]. Available from: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm348016.htm.
- 21.Hooker EA, Allen S, Gray L, Kaufman C. A randomized trial to evaluate a launderable bed protection system for hospital beds. Antimicrob Resist Infect Control. 2012;1(1):1–7. doi: 10.1186/2047-2994-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. [2015 Mar 3];Multidrug-Resistant Organism & Clostridium difficile Infection (MDRO/CDI) Module. [Internet]. Available from: http://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf.
- 23.Hooker E, Leigh Jones K. Cleaning practices for hospital mattresses in top US adult hospitals. Am J Infect Control. 2012;40(5):e43. [Google Scholar]
- 24.Attaway HH, III, Fairey S, Steed LL, Salgado CD, Michels HT, Schmidt MG. Intrinsic bacterial burden associated with intensive care unit hospital beds: effects of disinfection on population recovery and mitigation of potential infection risk. Am J Infect Control. 2012;40(10):907–12. doi: 10.1016/j.ajic.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88–92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]


