Abstract
Depression, anxiety, and posttraumatic stress disorder are linked to altered limbic morphology, dysregulated neuroendocrine function, and heightened amygdala responses to salient social cues. Oxytocin appears to be a potent modulator of amygdala reactivity and neuroendocrine responses to psychosocial stress. Given these stress regulatory effects, there is increasing interest in understanding the role of oxytocin in vulnerability to stress-related clinical disorders. The present study examines the impact of a common functional variant within the oxytocin receptor (OXTR) gene (rs2254298) on structure and function of the amygdala in a high-risk sample of urban, low-income, minority youth with a high incidence of early life stress (ELS). Compared to G/G homozygotes, youth carrying the OXTR A-allele showed increased amygdala volume, reduced behavioral performance, and heightened amygdala response during two functional magnetic resonance imaging (fMRI) tasks that involved viewing socially-relevant face stimuli. Higher amygdala response was related to ELS in A-alleles carriers but not G/G homozygotes. These findings underscore a series of relationships among a common oxytocin system gene variant, ELS exposure, and structure and function of the amygdala in early life. Heightened amygdala response to salient social cues in OXTR A-allele carriers may elevate risk for emotional psychopathology by increasing amygdala involvement in disambiguating environmental cues, particularly for individuals with ELS.
Keywords: Limbic, genetics, early stress, depression, Stroop
1. Introduction
In addition to its well-known role in promoting pro-social behavior, the neuropeptide oxytocin is increasingly recognized for its ability to attenuate anxiety and stress reactivity. Oxytocin administration reduces self-reported anxiety (Bartz & Hollander, 2006) and levels of cortisol (Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003), the hormonal end product of the hypothalamic-pituitary-adrenal (HPA) axis. Thus, there is a growing interest in understanding the role of oxytocin in stress-related clinical disorders, such as anxiety, depression, and posttraumatic stress disorder (PTSD).
Variation in the oxytocin system may be an important factor in predicting risk for the development of psychiatric disorders, particularly in the context of early adversity. For example, children and adolescents exposed to early life stress (ELS), one of the most significant predictors of psychiatric illness (Green, et al., 2010), exhibit lower levels of peripheral oxytocin following physical contact with their mothers (Wismer Fries, Ziegler, Kurian, Jacoris, & Pollak, 2005). Further, a common variant in the oxytocin receptor gene (OXTR; rs2254298) has been found to interact with ELS to predict symptoms of anxiety and depression in young participants (Thompson, Parker, Hallmayer, Waugh, & Gotlib, 2011). Given that sensitivity of the oxytocin system appears to be set in early life (Meinlschmidt & Heim, 2007), there is a critical need to better understand the role of oxytocin in mediating psychiatric risk during childhood and adolescence, when stress-related clinical disorders frequently emerge.
In the brain, oxytocin has marked inhibitory effects on the amygdala (Bale, Davis, Auger, Dorsa, & McCarthy, 2001; in adult males), a limbic region that plays a critical role in biasing information processing by orienting attention to salient, emotionally-laden, and biologically-relevant stimuli in the environment (LeDoux, 1998). The amygdala is also posited to govern the processing of potentially-relevant but ambiguous information in the environment, such as faces lacking interpretable emotional content (Wright & Liu, 2006). Higher amygdala response to ambiguous faces is thought to reflect a greater tendency to perceive these cues as threatening (Forbes, Phillips, Silk, Ryan, & Dahl, 2011), and heightened amygdala responses to ambiguous faces are reported in individuals with anxiety (Cooney, Atlas, Joormann, Eugene, & Gotlib, 2006) and in youth at risk for depression (Dearing & Gotlib, 2009). These findings suggest that altered sensitivity of the amygdala to socially-relevant cues might contribute to the functional pathophysiology of these conditions (LeDoux, 1998).
Neuroimaging studies report that intranasal oxytocin administration dampens amygdala reactivity and reduces coupling of the amygdala to brainstem regions implicated in autonomic and behavioral manifestations of fear (Domes, et al., 2007; Kirsch, et al., 2005; in adult males but not females). Thus, it is possible that individual variation in the oxytocin system may impact amygdala sensitivity, which may, in turn, alter the way information in the environment is processed. Consistent with this notion, adult (Inoue, et al., 2010) and adolescent (Furman, Chen, & Gotlib, 2011) carriers of the rs2254298 OXTR A-allele variant are reported to have increased amygdala volume, a neural marker thought to confer elevated emotional reactivity and anxiety (Holmes, et al., 2012). These findings highlight the amygdala as an important neural substrate for OXTR-mediated risk for emotional psychopathology. However, it is unknown whether there is an association between oxytocin and amygdala function in early life. It is possible that increased sensitivity of the amygdala in childhood may contribute to larger amygdala volumes observed in individuals carrying the rs2254298 OXTR A-allele.
The present study tests the effects of the OXTR polymorphism rs2254298 on amygdala volume and functional responses in youth. We evaluated amygdala function using two tasks that involve processing socially-relevant face stimuli to examine the generalizability of effects across tasks. Amygdala responses to social ambiguity and during conditions of varying cognitive load were evaluated. We predicted that A-allele carriers would show heightened amygdala responses to ambiguous social cues and a lower ability to dampen amygdala responses during higher cognitive load. We also predicted that A-allele carriers would show increased amygdala volume, as observed in prior work.
Current theory suggests that A-allele carriers may be more sensitive to environmental exposures and thus more vulnerable to the harmful effects of ELS (Brune, 2012). Emerging empirical data in youth support this notion, showing greater increases in anxiety and depressive symptoms in OXTR rs2254298 A-alleles than in youth with a G/G genotype following exposure to ELS (Thompson, et al., 2011). Given research showing that ELS is associated with enhanced amygdala reactivity (Marusak, Martin, Etkin, & Thomason, 2014), we evaluated the hypothesis that young A-allele carriers are more sensitive to the effects of ELS on amygdala function. Here, we investigate interactive effect of ELS and OXTR genotype on amygdala activity. We do so in a sample of high-risk (i.e., urban, low-income, minority) youth with a high prevalence of ELS. This demographic was selected for several reasons. First, prior research shows not only that trauma frequency is more extreme in African Americans living in impoverished urban areas, but also that the negative consequences of trauma may be more severe (Alim, Charney, & Mellman, 2006). For instance, African American urban residents who experience trauma are nearly two times more likely to develop PTSD than their lower risk counterparts (Goldmann, et al., 2011). Second, lower income is a significant predictor of more severe emotional psychopathology following trauma (Lowe, Galea, Uddin, & Koenen, 2014). Thus, the present sample is considered high-risk due to additive effects of trauma frequency and stress burden.
2. Material and Methods
2.1 Participants
The present study reports on 55 children and adolescents, ages 7-15, recruited through classified advertisements posted on Craigslist (Metro Detroit), printed flyers, Wayne State University (WSU) community postings, and area pediatric mental health clinics/service providers. Although 61 participants were initially included, 6 were excluded due to image artifacts that prohibited analysis of gray matter volume (GMV), as described below. Study exclusion criteria included history of neurological injury, significant learning disorder, English as a second language, or presence of magnetic resonance imaging (MRI) contraindications. Prior to the scan session, participants and parents were shown a brief video to prepare them for their MRI scan (available at: www.brainnexus.com/links). Full-Scale IQ was determined using the Kaufman Brief Intelligence Test, Second Edition (Kaufman & Kaufman, 2004). Written informed consent and child/adolescent assent were obtained for all participants and their parents as approved by the WSU Institutional Review Board.
2.2 Early Life Stress and Internalizing Symptomology
ELS was measured using the 24-item Traumatic Events Screening Inventory (TESI; Ippen, et al., 2002), a parent report of potential stressors experienced by the child (e.g., assault, witnessing violence, family member arrested). Number of early life stressors was calculated by summing the positively endorsed items on the TESI. Anxiety and depressive symptoms were assessed using the 41-item Screen for Child Anxiety Related Emotional Disorders (SCR; Birmaher, et al., 1997) and the 10-item Children's Depression Inventory (CDI; Kovacs, 1992), respectively. A visual analog scale (VAS) was used to obtain an average rating of fear/anxiety during the MRI visit (repeat measures at 30-minute intervals) as previously described (Thomason, Tocco, Quednau, Bedway, & Carre, 2013).
2.3 Pubertal Development
Pubertal development was assessed with the self-report Tanner stages questionnaire (Marshall & Tanner, 1968). Following prior work (Forbes, et al., 2011), participants were categorized as pre/early (Tanner stages 1-2) or mid/late (stages 3-5) pubertal.
2.4 OXTR Polymorphism Genotyping
Details of genotyping methods are provided in the Supplemental Material. Of the 55 total participants, 34 were carrying two G alleles (G/G homozygotes), 17 were carrying one A and one G allele (A/G), and 4 were carrying two A-alleles (A/A). Individuals heterozygous and homozygous for the A-allele were combined into an ‘A-allele carrier’ group (n = 21). Genetic distribution across the sample was in Hardy-Weinberg equilibrium, Χ2 = 1.163, p = 0.56 (www.ncbi.nlm.nih.gov/snp).
2.5 MRI Data Acquisition
MRI data were acquired with a Siemens 3.0 Tesla MRI scanner (MAGNETOM Verio system, Siemens Medical Solutions) equipped with a 12-channel head coil (WSU School of Medicine MR Research Facility). Blood-oxygen level dependent (BOLD) fMRI data were acquired across the whole brain using a T2*-weighted echo-planar sequence with the following parameters: TR: 2000 ms, TE: 25 ms, 29 axial slices, field of view: 220 × 220 (whole brain coverage), flip angle: 90°, voxel size: 3.44 × 3.44 × 4 mm. High-resolution anatomical images were acquired using a three-dimensional T1 magnetization-prepared rapid gradient-echo (MP-RAGE) sequence with the following parameters: TR: 1680 ms, TE: 3.51 ms, 176 axial slices, field of view: 256 × 256 (whole brain coverage), flip angle: 9°, voxel size: 0.7 × 0.7 × 1.3 mm.
2.6 Structural Image Processing
Prior to analysis, T1-weighted anatomical images were screened for motion artifacts (ghosting, blurring). Individual participant whole-brain intracranial masks were generated and manually corrected for N = 61 anatomical images by a trained rater (N.K.) using the interactive editing tools in the BrainSuite software package (v.13a4; Shattuck & Leahy, 2002; http://brainsuite.org/). Intrarater reliability was tested by generating whole-brain intracranial masks twice for five brains, at least two weeks apart. Intraclass correlation coefficient (ICC) was computed using the total number of voxels in each brain mask; reliability was confirmed by ICC > 0.9. Whole-brain masks and MR image volumes were then processed using BrainSuite to produce participant-specific segmentation of brain areas and derive total GMV of regions of interest (ROIs) in left and right hemispheres (additional detail provided in Supplemental Material; see also Marusak, Kuruvadi, et al., 2015). The amygdala was selected as an a priori ROI. Prior work (Joshi, Leahy, Toga, & Shattuck, 2009; Joshi, Shattuck, Thompson, & Leahy, 2007) shows that segmentation of subcortical brain structures, as performed by BrainSuite software, is highly congruent with manually segmented images drawn by experts at Massachusetts General Hospital (Dice coefficients κ= 0.6474-0.8918). Validation of subcortical segmentation in the latest version of BrainSuite is forthcoming. In order to ensure the accuracy of segmentation results for the current study, segmented image volumes from the automated method were manually inspected by two trained raters (N.K. and H.M.). Data from participants showing poor quality segmentation for ROIs were excluded from corresponding analyses, as detailed below.
2.7 Gray Matter Volume Analyses
Total GMV of left and right amygdala ROIs was computed using BrainSuite software. Outliers that were two standard deviations above or below the mean were removed from analyses. This resulted in the removal of 1 or 2 cases per ROI. Additionally, GMV values were excluded for 3 cases per ROI due to poor quality subcortical segmentation. Upon completion of these quality assurance steps, N = 51 and 50 cases remained for structural analyses of each ROI. Results are reported for quality-assured data, and were further confirmed with the full N = 55 sample by winsorizing outliers to 2 standard deviations above or below the mean and applying whole-sample mean replacement for missing GMV values, as there was no evidence of systematic differences in those with missing values. The primary analysis was a 2×2 ANCOVA for amygdala GMV with gene group as the between-subjects factor (A-alleles carriers, G/G homozygotes) and hemisphere (left, right) as the within-subjects factor. Following prior pediatric studies (Mosconi, et al., 2009), age, IQ, sex, and whole brain volume were entered as nuisance covariates. IQ has been shown to correlate with amygdala volume specifically (Rice, Viscomi, Riggins, & Redcay, 2014) and medial temporal volume more generally (Amat, et al., 2008; Andreasen, et al., 1993; Narr, et al., 2007). Trend-level associations were observed between right amygdala GMV and age (r(50) = 0.27, p = 0.062) and IQ (r(41) = −0.31, p = 0.051). Whole brain volume was not significantly associated with total amygdala volume, r(49) = 0.004, p = 0.98. Because OXTR effects previously reported were bilateral (Furman, et al., 2011), we did not have a priori lateralization hypotheses. OXTR-by-sex and OXTR-by-age interactions were also examined. Few studies to date have evaluated oxytocin genes in African American samples, bringing this understudied group into the wider context. Results in African American participants alone, and in follow-up analyses covarying for race, were consistent with those reported in the full sample.
2.8 fMRI Paradigms
Participants were run through one or two neuroimaging protocols. The same structural MP-RAGE scan was used in both protocols, but they included different fMRI tasks. The tasks involved viewing socially relevant stimuli (faces) and are widely used to probe amygdala sensitivity: (1) a Stroop-like face-categorization task (N = 29; Egner, Etkin, Gale, & Hirsch, 2008), and (2) a face-matching task (N = 35; Hariri, Tessitore, Mattay, Fera, & Weinberger, 2002).
2.8.1 Face-Categorization Stroop Task
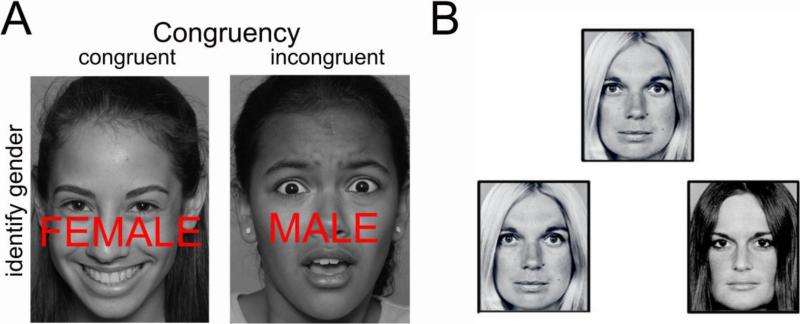
The Stroop task enabled us to examine the impact of high vs. low cognitive demand in the context of high arousal emotional faces. During the task (Figure 1A), participants were instructed to identify the gender of the face stimuli with a button press response, while ignoring the task-irrelevant gender word label. Face stimuli consisted of either happy or fearful expressions. Neurobehavioral responses during trials where the word label was incongruent with the face gender were compared with trials where the word label and gender were congruent. This contrast enabled us to permute cognitive demand. Prior evidence from similar Stroop (McKenna, 1986) and emotion dot probe (Armony & Dolan, 2002) studies show that stimuli with emotional valence require more attentional resources than do neutral stimuli.
Figure 1.
Experimental paradigms used to examine amygdala responses to social cues. (A) Face-categorization Stroop experimental task. Participants were instructed to indicate the gender of the face, while ignoring the overlying gender distracter word. Face stimuli included fearful and happy expressions. Trials varied such that word stimuli were either congruent or incongruent with the gender of the face. This enabled us to evaluate amygdala response while varying cognitive demand. (B) Face-matching task. Participants were instructed to indicate which of the two faces on the bottom of the screen matched the target face at the top of the screen. Trials were presented in emotion blocks (neutral, fearful, angry, and happy). Primary analyses focused on neutral facial expressions, given prior research showing that ambiguous faces engage variable amygdala response in youth. Gene effects on other facial expressions were also examined.
2.8.2 Face-Matching Task
The second task involved matching neutral faces (Figure 1B). A number of studies have shown that neutral faces are not processed as emotionally neutral, especially as perceived by youth. In fact, a still face with direct eye contact is currently the favored approach for evoking the stress response in infants (Mesman, van Ijzendoorn, & Bakermans-Kranenburg, 2009). Indeed, neutral faces elicit threat-related brain responses in youth (Forbes, et al., 2011; Thomas, et al., 2001). Amygdala response to neutral faces (particularly of adults) in children is comparable to activity elicited by negatively valenced faces in adults (Lobaugh, Gibson, & Taylor, 2006; Marusak, Carre, & Thomason, 2013; Thomas, et al., 2001). This observation may be due to greater perception of threat and/or increased ambiguity of neutral faces in children, as threat and ambiguity are both known to engage the amygdala (LeDoux, 1998). Thus, for children, neutral faces are less commonly used as baseline stimuli. Increasingly, abstract neutral stimuli, such as shapes, are providing more appropriate baseline (Herba & Phillips, 2004). Here, we focused on neutral faces for their ability to elicit variable amygdala response in youth, which may reveal individual differences in negativity biases. Elliptical shapes served as the baseline sensorimotor condition. Neutral Ekman face stimuli are provided in Figure S1. Effects of OXTR on processing non-neutral (fearful, happy, and angry expressions) were examined in follow-up analyses, given that oxytocin administration studies in adults have not consistently shown selective effects for neutral faces.
2.9 Functional Image Processing and Motion
During acquisition, head movements were corrected online using Siemens MRI motion correction (MoCo) software, which compares successively acquired volumes and prospectively adjusts subsequent slice acquisitions. MoCo also retrospectively corrects the time series for motion that occurred before the online correction is applied. All fMRI data reported here utilized the MoCo-corrected timeseries, and both tasks were processed using the same procedures unless otherwise noted. Data were processed with the SPM8 software package (Statistical Parametric Mapping; www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (Mathworks, Inc., Natick, Mass.). The first 4 EPI volumes were discarded to allow for system stabilization. Images were realigned to correct for motion, and the 6 motion parameters were included in participant-level models as regressors of no interest. Participants with movement exceeding 3.15 mm or 3 degrees in any direction were excluded from respective analyses (n = 9 participants total). This resulted in a final sample of 29 participants for the face-categorization task (G/G, n = 17; A-alleles, n = 12), and 35 for the face-matching task (G/G, n = 23; A-alleles, n = 12). 20 participants provided data from both tasks. For remaining participants, average movement and root-mean-square (RMS) head position change was computed across translational (x, y, z) and rotational (roll, pitch, yaw) directions. Overall, movement was well within accepted standards (< 1.5 mm RMS; Fair, et al., 2012). Movement parameters were compared between OXTR groups for each task using two-sample t-tests and a p ≤ 0.05 (two-tailed) significance level, and reported in Results.
Images were slice-time corrected, spatially transformed to the Montreal Neurological Institute (MNI) coordinate system, and spatially smoothed with a Gaussian kernel (6 mm FWHM for the face-categorization task, 8 mm FWHM for face-matching task). Data were not resampled during normalization, thus retained the native resolution (3.44 × 3.44 × 4 mm). A 128-second temporal high-pass filter was applied, and temporal autocorrelation was estimated using a first-order autoregressive model.
2.9.1 Face-Categorization Stroop Task
Task overview and timing is provided in Supplemental Material. Participant-level models were created, as previously described (Marusak, Etkin, & Thomason, 2015). In brief, separate regressors for stimulus events were created for incongruent and congruent trials, using 1 s boxcar functions convolved with a canonical hemodynamic response function. Error and post-error trials were modeled separately. Participant level contrasts isolated effects of conflict by contrasting incongruent - congruent trials. Average signal change was extracted for each participant from anatomically-defined left and right amygdala ROIs, using masks defined by FSL FIRST segmentation tool (Patenaude, Smith, Kennedy, & Jenkinson, 2011). A 2×2 ANOVA was used to test for OXTR effects on amygdala response to incongruent-congruent trials, with gene group as the between-subjects factor and hemisphere (left, right) as the within-subjects factor. Behavioral performance for incongruent vs. congruent trials was calculated and compared between groups using two-sample t-tests. Behavioral and neural effects were considered significant at p ≤ 0.05 (two-tailed).
2.9.2 Face-Matching Task
Task overview and timing is provided in Supplemental Material. Participant-level models were created, as previously described (Marusak, et al., 2013). In brief, emotion blocks (neutral, fearful, angry, and happy) and baseline blocks (shapes) were modeled using convolved boxcar functions (42 s blocks). Participant level contrasts focused on blocks of neutral face-matching vs. shapes-matching. Average signal change was extracted from anatomically-defined left and right amygdala ROIs, as described above, and compared between OXTR groups using a group × hemisphere ANOVA. We also tested for OXTR effects on behavioral performance, and on amygdala responses to other face emotions (fear, angry, happy). Analyses were re-run excluding 2 participants with less than 60% overall task accuracy, yielding no significant changes to reported results.
2.9.3 Localization of amygdala effects
The amygdala consists of functionally distinct nuclei that can be broadly divided into three main subdivisions: basolateral, centromedial, and superficial. We conducted follow-up exploratory analyses on voxel-wise data to test whether whole-amygdala functional effects could be localized to specific amygdala subdivision(s). Bilateral amygdala subdivision masks were generated from 3D probabilistic cytoarchitectonic maps (Amunts, et al., 2005) and localization was evaluated by examining each subdivision mask for voxels surviving small-volume correction, at a family-wise error corrected threshold of pFWE = 0.05.
2.9.4 Whole-brain effects
While primary analyses focused on group difference in amygdala response, we also report results of exploratory whole-brain voxel-wise analyses at a threshold of p < 0.005, cluster minimum = 10 voxels.
2.10 Relation to Early Stress Exposure
Given prior work showing elevated levels of anxiety and depression in OXTR A-allele carriers in the context of ELS (Thompson, et al., 2011), we tested for interactive effects of ELS and OXTR on amygdala volume and reactivity. Correlation analyses were first used to test for relationships between ELS and amygdala volume/reactivity, using a p ≤ 0.05 threshold, adjusted for multiple comparisons (volume and 2 fMRI tasks: p ≤ 0.0167). For significant effects, PROCESS software (v.2.11; www.processmacro.org/) implemented in IBM SPSS v.21 was then used to test if ELS moderates the relationship between OXTR genotype and amygdala volume/reactivity.
3. Results
3.1 Participant Characteristics
OXTR groups did not differ in age, IQ, sex, income, race, pubertal development, anxiety or depressive symptoms, or whole brain volume (Table 1). OXTR groups also did not differ in fear/anxiety (VAS) reported during the MRI visit, t(53) = 0.48, p = 0.63. Effects observed are thus not likely influenced by group differences in stress responsivity during MRI scanning (Thomason, et al., 2013). ELS was highly prevalent across the sample, with 96% reporting witnessing family threats and 32% witnessing domestic violence. Similar rates were observed within the subset of fMRI participants (see Table S2). Number of early life stressors endorsed did not differ between OXTR groups (Table 1). Overall, 26 participants were pre/early pubertal (Tanner stages 1-2), and 29 were mid/late pubertal (Tanner stages 3-5), Χ2(1) = 0.16, p = 0.68. Males and females did not differ on pubertal maturation, though there was a trend for females to be more mature, Χ2(1) = 2.92, p = 0.076.
Table 1.
Demographic and Clinical Characteristics by OXTR Group.
| Variable | OXTR G/G (n = 34) | OXTR A-Alleles (n = 21) | Group difference |
|---|---|---|---|
| Age, m (SD) | 12.11 (2.42) | 11.05 (2.59) | ns |
| Sex (Females), n (%) | 23 (67.65) | 11 (52.38) | ns |
| IQ, m (SD) | 104.04 (12.33) | 100.72 (16.1) | ns |
| Pubertal Development Group (Tanner Staging), n (%) | ns | ||
| Pre/Early Pubertal | 14 (41.18) | 12 (57.14) | |
| Mid/Late Pubertal | 20 (58.82) | 9 (42.86) | |
| Ethnicity/Race, n (%) | ns | ||
| African American | 12 (35.3) | 11 (52.3) | |
| Caucasian | 12 (35.3) | 6 (28.6) | |
| Mixed | 5 (14.7) | 2 (9.5) | |
| Hispanic | 1 (2.9) | 1 (4.8) | |
| Not reported | 4 (11.8) | 1 (4.8) | |
| Annual Household Income, n (%) | ns | ||
| Less than $40,000 | 16 (47.1) | 14 (66.7) | |
| $40,000 - $60,000 | 9 (26.5) | 2 (9.5) | |
| $60,000 - $80,000 | 3 (8.8) | 2 (9.5) | |
| $80,000 - $100,000 | 2 (5.9) | 0 | |
| Over $100,000 | 3 (8.8) | 3 (14.3) | |
| Not Reported | 1 (2.9) | 0 | |
| Whole Brain Volume (mm3), m (SD) | 1378701.18 (140652.9) | 1364428.1 (106421.75) | ns |
| Number of Early Life Stressors (TESI), m (SD) | 2.82 (2.02) | 2.05 (1.1) | ns |
| Anxiety Symptoms (SCR), m (SD) | 16.32 (12.06) | 14.95 (12.27) | ns |
| Depressive Symptoms (CDI), m (SD) | 2.94 (3.43) | 1.81 (2.79) | ns |
Group difference tested using t-tests (age, IQ, anxiety), chi-square tests (sex, race), or Mann-Whitney U tests (income).
Abbreviations: mean, m; not significant, ns; Intelligence quotient, IQ; Screen for Child Anxiety Related Emotional Disorders, SCR; Children's Depression Inventory, CDI: Traumatic Events Screening Inventory, TESI.
Demographics for the 29 participants in the face-categorization Stroop task and the 35 participants in the face-matching task are provided in Table S1. For both fMRI tasks, OXTR groups did not differ in age, IQ, income, race, pubertal development, anxiety or depressive symptoms, whole-brain volume, or movement. There was a higher proportion of female participants in the G/G homozygote group than in the A-allele group for the face-categorization task, Χ2(1) = 5.15, p = 0.033. Follow-up analyses controlling for sex yielded no changes to results reported.
3.2 Amygdala Volume
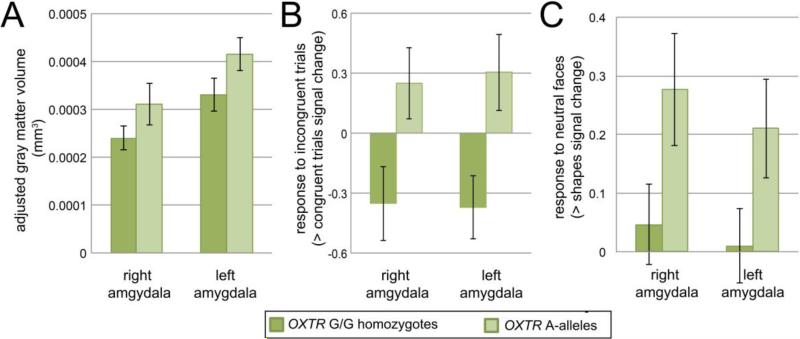
ANCOVA controlling for age, IQ, sex, and whole brain volume revealed a main effect of OXTR on amygdala volume, F(1, 44) = 4.15, p = 0.048, ηp2= 0.086. As predicted, this effect was driven by higher GMV in A-alleles compared to G/G homozygotes (see Figure 2A). The main effect of hemisphere, main effect of sex, and hemisphere × OXTR interaction were not significant, ps > 0.2. The main effect of OXTR on amygdala GMV remained significant when all covariates except IQ were removed, F(1,37) = 5.32, p = 0.027, and became F(1,46) = 3.85, p = 0.056 when all except age were removed.
Figure 2.
OXTR A-alleles show increased amygdala gray matter volume (GMV) and response when viewing socially-relevant face cues. GMV values are adjusted for whole brain volume. Error bars represent standard error of the mean. Main effect of OXTR, ps < 0.05.
3.3 Amygdala Reactivity
3.3.1 Face-Categorization Stroop Task
Consistent with previous work, reaction time (RT) across the sample was slower for incongruent (mean = 876.3 ms, SD = 232.17 ms) relative to congruent (mean = 842 ms, SD = 211.35 ms) trials, t(27) = 3.67, p = 0.001. Similarly, accuracy was lower for incongruent (mean = 84.67%, SD = 12.17%) than congruent (mean = 88%, SD = 9.67%) trials, t(28) = 2.89, p = 0.007. OXTR groups did not differ in their degree of RT interference, t(26) = 0.9, p = 0.37. Compared to G/G homozygotes, A-alleles showed a greater reduction in accuracy for incongruent relative to congruent trials, t(27) = 2.33, p = 0.027 (see Table S3 for behavioral performance, by group). This behavioral impairment was accompanied by higher average signal in the amygdala in OXTR A-allele carriers during incongruent relative to congruent trials (Figure 2B), F(1, 27) = 7.01, p = 0.013. There was no main effect of hemisphere or hemisphere × OXTR interaction, Fs < 0.1, ps > 0.6. The pattern of increased amygdala response and impaired behavioral performance is consistent with greater interference by incongruent stimuli in A-allele carriers. Post hoc within-group hemisphere × trial-type (congruent, incongruent; beta weights) ANOVAs revealed a main effect of trial-type in G/G homozygotes, F(1,16) = 5.08, p = 0.039, but not in A-allele carriers, F(1,11) = 2.49, p = 0.14. That is, amygdala activity was lower during incongruent relative to congruent trials in G/G homozygotes but not in A-allele carriers. This suggests that the observed higher amygdala response in A-allele carriers was driven by a lower ability to dampen amygdala response during incongruent trials. Evaluation of trial types separately (incongruent, congruent) showed no differences between groups (ps > 0.05). Magnitude of amygdala response was not correlated with any measure of accuracy or RT across participants, or within either gene group (all ps > 0.05).
3.3.2 Face-Matching Task
Neither accuracy nor RT differed between OXTR groups for neutral face-matching, matching other face valences (fearful, angry, happy), for the shapes-matching condition, or for neutral vs. shapes (all ts < 1.3, all ps > 0.2; see Table S3 for behavioral performance by group). Neuroimaging data showed that amygdala response to neutral faces was higher in A-allele carriers than G/G homozygotes, F(1,33) = 4.14, p = 0.05 (Figure 2C). No main effect of hemisphere or hemisphere × OXTR interaction was observed, ps > 0.18. The effect of OXTR was not driven by group differences in amygdala activity during the baseline shapes condition (vs. implicit baseline), F(1, 33) = 0.14, p = 0.7. To test whether effects of OXTR genotype were specific to ambiguous faces, we conducted a 3-way (hemisphere × emotion × gene-group) ANOVA to examine amygdala responses to other face emotions, i.e., fearful, happy, and angry. No main effects or interactions were observed (ps > 0.2). Amygdala response was not correlated with accuracy or RT during the neutral face condition across the sample (accuracy: r(34) = −0.19, p = 0.3; RT: r(34) = −0.15, p = 0.43) or within either group (ps > 0.16).
3.3.3 Localization of functional responses in the amygdala
Follow-up exploratory analyses on voxel-wise data evaluated whether group differences in whole-amygdala response could be localized to specific subregion(s). For the face-categorization conflict task, OXTR group differences were significant in basolateral amygdala, pFWE = 0.011, Zmax = 3.63, × = −30, y = −4, z = −34 (MNI). For the face-matching task, OXTR effects on neutral face-matching could not be unequivocally localized to a specific subregion, pFWEs 0.054-0.063.
3.3.4 Whole-brain results
Results of exploratory whole-brain fMRI analyses are provided in Table S4. Briefly, A-allele carriers showed higher activation to conflict (incongruent-congruent trials) than G/G homozygotes in posterior insula, amygdala, hippocampus, midbrain, and brainstem. There were no regions in which activity to conflict was elevated in G/G homozygotes relative to A-alleles. For neutral face-matching, A-alleles showed elevated response in amygdala, posterior insula, parahippocampal gyrus, middle temporal gyrus, and inferior frontal gyrus. G/G homozygotes had greater activity in striatum, superior temporal gyrus, and inferior parietal lobe.
3.4 Relation to Early Stress Exposure
3.4.1 Amygdala volume
Neither left nor right amygdala volume was related to number of early stressors in either gene group or across the sample, rs < |0.15|, ps > 0.6.
3.4.2 Amygdala reactivity
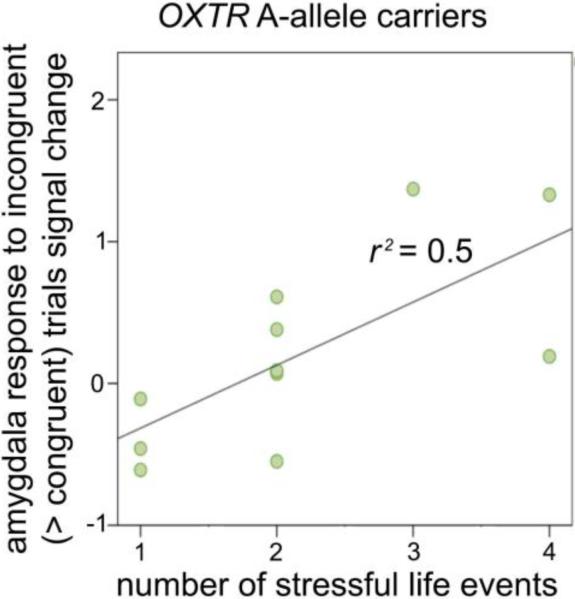
During the face-categorization task, we found a positive association between right amygdala response and number of early life stressors in A-allele carriers, r(11) = 0.705, p = 0.015 (Figure 3). The correlation remained significant if nonparametric tests were applied, rs(11) = 0.749, p = 0.008. The association between ELS and amygdala response was not significant for left amygdala, r(11) = 0.32, p = 0.34, or in G/G homozygotes (left: r(22) = −0.08, p = 0.71; right: r(22) = 0.06, p = 0.79). Correlation between right amygdala response during the face-categorization task and ELS was significantly higher among A-allele carriers than among G/G homozygotes, Z = 1.94, p = 0.05. Moderation analyses confirmed that ELS moderates the relationship between OXTR genotype and right amygdala reactivity, ΔR2 = 0.36, ΔF(1,24) = 3.93, p = 0.02, b = 0.48, t(24) = 2.46, p = 0.02. ELS was not correlated with amygdala response to neutral faces across the sample (left: r(33) = −0.13, p = 0.47; right: r(33) = −0.25, p = 0.15) or in either gene group (ps > 0.4).
Figure 3.
Relation between amygdala reactivity and early life stressors (ELS) present in OXTR A-allele carriers but not G/G homozygotes.
4. Discussion
Emerging data suggest that genetic variation in OXTR modulates emotion processing and regulation, and thus likely plays a role in susceptibility to psychopathology. The present study demonstrates novel links among OXTR genotype, ELS exposure, and structure and function of the amygdala in early life. We report that amygdala GMV and responses to social cues shift with oxytocin receptor gene variant rs2254298 in a sample of high-risk children and adolescents. Specifically, compared to youth with the G/G genotype, youth carrying the A-allele showed increased volume and higher response of the amygdala across two tasks that required viewing socially-relevant face cues. Increased amygdala responses in A-allele carriers corresponded to poorer task performance during the face-categorization Stroop task. The link between OXTR genotype and amygdala reactivity was moderated by ELS exposure, such that the magnitude of amygdala response was associated with ELS in A-allele carriers but not G/G homozygotes. These findings provide empirical support for recent hypotheses that OXTR rs2254298 A-allele carriers are more susceptible to the deleterious effects of adverse environmental exposures compared to their G/G counterparts (Brune, 2012).
Consistent with prior findings in adults (Inoue, et al., 2010) and adolescents (Furman, et al., 2011), we observed increased amygdala GMV in OXTR rs2254298 A-allele carriers. We augment these prior studies by demonstrating that amygdala function is also altered in youth carrying the A-allele. Given the amygdala's critical role in detecting the salience, significance, ambiguity, and personal relevance of environmental stimuli (LeDoux, 1998), higher amygdala responsivity may reflect greater biological tuning to socially-relevant information. While increased attentional capture of relevant social information, such as faces, may be adaptive for survival, it may also interfere with cognitive function if task demands are high. In particular, because processing capacity of the brain is limited, emotion processing must be dampened to effectively carry out higher-order cognitive processes. For instance, regional blood flow of the amygdala decreases during cognitively demanding tasks, whereas it increases during specific emotion-related tasks (Drevets & Raichle, 1998). In line with this framework, we observed that higher amygdala response, likely reflecting emotional processing, led to impaired task performance in A-allele carriers when cognitive demands were high (i.e., during the face-categorization Stroop task). In contrast, when tasks demands were low (i.e., during the face-matching task), elevated amygdala activity observed in A-allele carriers did not predict lower performance. This dissociation is consistent with conceptual models proposing that emotion processing can hinder or facilitate ongoing processing depending on the level of cognitive demand (Pessoa, 2009).
Follow-up voxel-wise analyses indicated that OXTR effects on amygdala response during the face-categorization task were likely localized to the basolateral amygdala subdivision. Basolateral amygdala is a main target of sensory afferents to the amygdala (McDonald, 1998) and plays an important role in social cue processing (Adolphs, 2001). Receiving highly processed sensory information enables basolateral amygdala to detect biologically relevant stimuli in the environment (Sah, Faber, Lopez De Armentia, & Power, 2003). Importantly, administration of intranasal oxytocin appears to alter activity in the basolateral amygdala (Gamer, Zurowski, & Büchel, 2010), which may partially explain oxytocin's implicated role in attenuating fear responses. In this light, lower ability to dampen basolateral amygdala response to conflict may reflect lower filtering of sensory information in OXTR A-allele carriers. Consistent with this hypothesis, elevated neural activity was accompanied with greater performance decrements to conflict in A-alleles.
Prior neuroimaging studies show that oxytocin administration dampens amygdala responses to salient social cues (Domes, et al., 2007; in adult males). By altering oxytocin levels or receptor binding/density, the OXTR polymorphism may similarly modulate the tuning of the amygdala to salient social information. Here, we found OXTR effects on amygdala activity during face processing in youth. We did not observe OXTR effects during processing of fearful, angry, or happy expressions, but found response to neutral faces to differ by OXTR genotype. This suggests that, for children, the OXTR polymorphism affects sensitivity to ambiguous social threat cues, but not to signals with clearer biological relevance (e.g., an angry face signals direct threat to the perceiver). As noted above (see Methods), elevated amygdala response to facial expressions of neutral valence may correspond to a negative interpretation of these stimuli in youth. Future studies will be needed to determine if the observed increase in amygdala response in A-alleles coincides with a greater perception of threat, negativity, or ambiguity in these cues.
Heightened perceptual processing of ambiguous social cues may increase risk for disorder in youth who are predisposed to interpret these cues as threatening, such as those reared in adverse environments. In accord with this notion, we discovered an association between amygdala reactivity and ELS exposure in OXTR A-allele carriers. Youth with ELS carrying the OXTR A-allele appeared to manifest increased correlates of risk for internalizing conditions; they had greater amygdala responses to salient social cues. It is possible that those with both genetic susceptibility and ELS exposure not only exhibit increased sensitivity to conflicting or ambiguous stimuli, but also process them more deeply, thereby increasing susceptibility to negative rumination. This interpretation is consistent with research indicating that youth at risk for depression have a higher tendency to perceive ambiguous stimuli as threatening (Dearing & Gotlib, 2009), and fits with cognitive theories of depression which hold that greater tendency to interpret ambiguous situations as negative precipitates negative mood states (Foland-Ross & Gotlib, 2012). And indeed, elevated anxiety and depressive symptoms are reported in OXTR A-alleles in the context of ELS (Thompson, et al., 2011).
Given that impairments in social behavior are ubiquitous in neuropsychiatric disorders, the observed effects of the OXTR polymorphism on amygdala processing of social cues have relevance for disease. These findings may also have implications for adolescent development outside the realm of psychopathology. In particular, higher amygdala reactivity to ambiguous social stimuli in OXTR A-allele carriers could hamper development in the social domain, as these individuals may be less likely to engage in social risk-taking and exploration or to initiate new peer relationships. On the other hand, such a behavioral pattern might also serve a protective function by increasing wariness even in the absence of overt threat cues. These predictions are supported by research showing that intranasal oxytocin administration in humans increases willingness to accept social risks arising through interpersonal interactions (Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005), as well as animal literature highlighting oxytocin's effects on pro-social approach behavior (Lukas, et al., 2011).
To our knowledge, this is the first study to examine neural variation associated with the OXTR polymorphism rs2254298 in a predominantly African American sample. By replicating effects observed in other demographic samples (Furman, et al., 2011; Inoue, et al., 2010) this work extends findings to wider ethnic populations. We found that amygdala structural and functional neurobiology varies with rs2254298 genotype in youth. Although mixed-race samples may improve generalizability of findings, results may be biased by population substructure and allelic frequencies may differ by population. The current analysis was sensitive to these concerns. First, allele frequencies in the sample were in line with expected frequencies for the racial majority group (African Americans). Second, although genomic control or ancestry informative marker methods were unavailable here, we did attempt to rule out population stratification by reanalyzing the data controlling for self-reported race/ethnicity, which shows high correspondence with genotyped markers (Divers, et al., 2011; Levran, Awolesi, Shen, Adelson, & Kreek, 2012). Additionally, OXTR groups did not differ on race, and sub-analyses in African American participants (the racial majority) were consistent with results reported in the full sample. Findings of these control analyses are consistent with a lack of confounding population substructure. Nonetheless, our sample of urban, low-income, minority youth represents an under-studied population at high risk for ELS and associated adverse outcomes (e.g., depression, PTSD, substance use disorders). Thus, research in this area may be useful for informing broader public health policies and practices. These results should nevertheless be considered preliminary and replication in other samples is needed.
We note several limitations of the current study. First, the functionality of the OXTR polymorphism rs2254298 is currently unknown. While higher peripheral oxytocin levels have been reported in OXTR rs2254298 A-allele carriers compared to G/G homozygotes (Apter-Levy, Feldman, Vakart, Ebstein, & Feldman, 2013), how this variant translates into the actual availability or binding of oxytocin in the brain is unclear. Next, prior studies in adults using acute oxytocin administration have identified sex differences (Domes, et al., 2010; Weisman, Zagoory-Sharon, Schneiderman, Gordon, & Feldman, 2013) that were not replicated here. This may be the result of immature sexual dimorphism in childhood, because this was a gene variant rather than an acute administration study, or because this interaction may have been underpowered. These possibilities cannot be resolved here, but lack of sex differences have been observed in adult studies that evaluated the gene variant investigated here (Inoue, et al., 2010). This introduces the idea that acute effects of oxytocin administration differ from more stable effects of functional gene polymorphisms. In addition, sex distribution did not differ between groups for volumetric analyses or for the face-matching task. For the face-categorization task, there was a higher proportion of female participants in the G/G homozygote group relative to the A-allele carrier group. However, a converging pattern of results across tasks, namely higher amygdala reactivity in A-alleles, suggests that observed effects are not driven by sex. Nonetheless, our power to detect OXTR-by-sex interactions was limited and we urge future studies with larger samples to address this important question. Third, rs2254298 is one of two widely studied oxytocin-system related polymorphisms. We did not examine effects of other common OXTR variants, which may also modulate oxytocin system function. Here, we chose to focus on rs2254298 to extend previous work documenting effects of this polymorphism on amygdala volume and emotional symptomology in a separate sample of mixed-race youth (Thompson, et al., 2011). A recent meta-analysis (Bakermans-Kranenburg & van IJzendoorn, 2014) found null effects of both OXTR rs2254298 and OXTR rs53576 on psychopathology and social behavior. The current study, as well as prior work in adolescents (Thompson, et al., 2011), demonstrate that genotype alone may not be sufficient to produce psychopathology. Rather, a stress diathesis model may be more appropriate for understanding the downstream consequences of genetic variation in the OXTR gene.
Given what appears to be a critical developmental period for solidifying the relation between genotype and amygdala structure and function in the context of ELS, regulation of amygdala sensitivity may be a promising target for preventive interventions in early life before morphological changes become hardwired in the brain or before clinically-significant conditions emerge. For instance, selective serotonin reuptake inhibitors, often used to treat anxiety and depression, are effective at attenuating amygdala responses. Likewise, oxytocin administration has been shown to dampen amygdala responses, and is emerging as a potential therapeutic for stress-related clinical conditions. Given evidence that amygdala responsivity is under control of both oxytocin and serotonin (Mottolese, Redoute, Costes, Le Bars, & Sirigu, 2014), interventions should take into account individual variation in both systems.
Supplementary Material
Highlights.
Amygdala volume and responses to social cues vary with an OXTR gene variant
Amygdala response was related to early stress in youth A-allele carriers
Increased amygdala response in youth may convey higher risk of psychopathology
Acknowledgements
The authors thank Zahid Latif and Yashwanth Katkuri of Wayne State University (WSU) for their assistance in neuroimaging data acquisition, Kayla Martin, Rita Elias, Gregory H. Baldwin, Melissa Youmans, Mallory Gardner, Amy Katherine Swartz, Timothy Lozon, Berta Rihan, and Ali Daher of WSU for assistance in participant recruitment and data collection, Ana Daughtery for recommendations on anatomical tracing reliability procedures, Julianne Facca, Matthew Hess, and Susan Land of the WSU Applied Genomics Technology Center for assistance with genetic analyses, and the children and families who volunteered to participate in this research.
Research reported in this publication was supported, in part, by the Merrill Palmer Skillman Institute and the Department of Pediatrics, Wayne State University (WSU) School of Medicine, a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (MET), NIH National Institute of Environmental Health Sciences awards P30 ES020957 and R21 ES026022 (MET), and the NIH National Institute of Neurological Disorders And Stroke award R01 NS074980 (DWS and AAJ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The WSU Genomics Core is supported, in part, by NIH National Cancer Institute award P30 CA022453.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Alim TN, Charney DS, Mellman TA. An overview of posttraumatic stress disorder in African Americans. J Clin Psychol. 2006;62:801–813. doi: 10.1002/jclp.20280. [DOI] [PubMed] [Google Scholar]
- Amat JA, Bansal R, Whiteman R, Haggerty R, Royal J, Peterson BS. Correlates of intellectual ability with morphology of the hippocampus and amygdala in healthy adults. Brain Cogn. 2008;66:105–114. doi: 10.1016/j.bandc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Swayze V, 2nd, O'Leary DS, Alliger R, Cohen G, Ehrhardt J, Yuh WT. Intelligence and brain structure in normal individuals. Am J Psychiatry. 1993;150:130–134. doi: 10.1176/ajp.150.1.130. [DOI] [PubMed] [Google Scholar]
- Apter-Levy Y, Feldman M, Vakart A, Ebstein RP, Feldman R. Impact of maternal depression across the first 6 years of life on the child's mental health, social engagement, and empathy: The moderating role of oxytocin. Am J Psychiatry. 2013;170:1161–1168. doi: 10.1176/appi.ajp.2013.12121597. [DOI] [PubMed] [Google Scholar]
- Armony JL, Dolan RJ. Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsychologia. 2002;40:817–826. doi: 10.1016/s0028-3932(01)00178-6. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. A sociability gene? Meta-analysis of oxytocin receptor genotype effects in humans. Psychiatric Genetics. 2014;24:45–51. doi: 10.1097/YPG.0b013e3283643684. [DOI] [PubMed] [Google Scholar]
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Hollander E. The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Horm Behav. 2006;50:518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Brune M. Does the oxytocin receptor (OXTR) polymorphism (rs2254298) confer ‘vulnerability’ for psychopathology or ‘differential susceptibility’? Insights from evolution. BMC Med. 2012;10:38. doi: 10.1186/1741-7015-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney RE, Atlas LY, Joormann J, Eugene F, Gotlib IH. Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? Psychiatry Res. 2006;148:55–59. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Dearing KF, Gotlib IH. Interpretation of ambiguous information in girls at risk for depression. J Abnorm Child Psychol. 2009;37:79–91. doi: 10.1007/s10802-008-9259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divers J, Redden DT, Rice KM, Vaughan LK, Padilla MA, Allison DB, Bluemke DA, Young HJ, Arnett DK. Comparing self-reported ethnicity to genetic background measures in the context of the Multi-Ethnic Study of Atherosclerosis (MESA). BMC Genet. 2011;12:28. doi: 10.1186/1471-2156-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition and Emotion. 1998;12:353–385. [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, Schlaggar BL, Mennes M, Gutman D, Bangaru S, Buitelaar JK, Dickstein DP, Di Martino A, Kennedy DN, Kelly C, Luna B, Schweitzer JB, Velanova K, Wang YF, Mostofsky S, Castellanos FX, Milham MP. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Gotlib IH. Cognitive and neural aspects of information processing in major depressive disorder: an integrative perspective. Front Psychol. 2012;3:489. doi: 10.3389/fpsyg.2012.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Dev Neuropsychol. 2011;36:429–452. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman DJ, Chen MC, Gotlib IH. Variant in oxytocin receptor gene is associated with amygdala volume. Psychoneuroendocrinology. 2011;36:891–897. doi: 10.1016/j.psyneuen.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci U S A. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann E, Aiello A, Uddin M, Delva J, Koenen K, Gant LM, Galea S. Pervasive exposure to violence and posttraumatic stress disorder in a predominantly African American Urban Community: the Detroit Neighborhood Health Study. J Trauma Stress. 2011;24:747–751. doi: 10.1002/jts.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The Amygdala Response to Emotional Stimuli: A Comparison of Faces and Scenes. NeuroImage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Herba C, Phillips M. Annotation: Development of facial expression recognition from childhood to adolescence: behavioural and neurological perspectives. Journal of Child Psychology and Psychiatry. 2004;45:1185–1198. doi: 10.1111/j.1469-7610.2004.00316.x. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, Buckner RL. Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. J Neurosci. 2012;32:18087–18100. doi: 10.1523/JNEUROSCI.2531-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Yamasue H, Tochigi M, Abe O, Liu X, Kawamura Y, Takei K, Suga M, Yamada H, Rogers MA, Aoki S, Sasaki T, Kasai K. Association between the oxytocin receptor gene and amygdalar volume in healthy adults. Biol Psychiatry. 2010;68:1066–1072. doi: 10.1016/j.biopsych.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Ippen CG, Ford J, Racusin R, Acker M, K. B, Rogers C, Ellis C, Schiffman J, Ribbe D, Cone P, Lukovitz M, Edwards J. Traumatic Events Screening Inventory - Parent Report Revised. Early Trauma Network and the National Center for PTSD Dartmouth Child Trauma Research Group; San Francisco, CA: 2002. [Google Scholar]
- Joshi A, Leahy RM, Toga A, Shattuck DW. A Framework for Brain Registration via Simultaneous Surface and Volume Flow. Information processing in medical imaging : proceedings of the ... conference. 2009;21:576–588. doi: 10.1007/978-3-642-02498-6_48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Shattuck DW, Thompson PM, Leahy RM. Surface-constrained volumetric brain registration using harmonic mappings. IEEE Trans Med Imaging. 2007;26:1657–1669. doi: 10.1109/tmi.2007.901432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test: KBIT 2; Manual. Pearson; 2004. [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children's Depression Inventory: Multi-Health Systems. 1992 Incorporated. [Google Scholar]
- LeDoux J. Fear and the brain: where have we been, and where are we going? Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Levran O, Awolesi O, Shen PH, Adelson M, Kreek MJ. Estimating ancestral proportions in a multi-ethnic US sample: implications for studies of admixed populations. Hum Genomics. 2012;6:2. doi: 10.1186/1479-7364-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobaugh NJ, Gibson E, Taylor MJ. Children recruit distinct neural systems for implicit emotional face processing. Neuroreport. 2006;17:215–219. doi: 10.1097/01.wnr.0000198946.00445.2f. [DOI] [PubMed] [Google Scholar]
- Lowe SR, Galea S, Uddin M, Koenen KC. Trajectories of posttraumatic stress among urban residents. Am J Community Psychol. 2014;53:159–172. doi: 10.1007/s10464-014-9634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- Marusak HA, Carre JM, Thomason ME. The stimuli drive the response: an fMRI study of youth processing adult or child emotional face stimuli. NeuroImage. 2013;83:679–689. doi: 10.1016/j.neuroimage.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Marusak HA, Etkin A, Thomason ME. Disrupted insula-based connectome organization and conflict interference in trauma-exposed youth. NeuroImage: Clinical. 2015 doi: 10.1016/j.nicl.2015.04.007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Kuruvadi N, Vila AM, Shattuck DW, Joshi SH, Joshi AA, Jella PK, Thomason ME. Interactive effects of BDNF Val66Met genotype and trauma on limbic brain anatomy in childhood. Eur Child Adolesc Psychiatry. 2015 doi: 10.1007/s00787-015-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Martin KR, Etkin A, Thomason ME. Childhood trauma exposure disrupts the automatic regulation of emotional processing. 2014 doi: 10.1038/npp.2014.311. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in Neurobiology. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McKenna F. Effects of unattended emotional stimuli on color-naming performance. Current Psychology. 1986;5:3–9. [Google Scholar]
- Meinlschmidt G, Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biol Psychiatry. 2007;61:1109–1111. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Mesman J, van Ijzendoorn MH, Bakermans-Kranenburg MJ. The many faces of the Still-Face Paradigm: A review and meta-analysis. Developmental Review. 2009;29:120–162. [Google Scholar]
- Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry. 2009;66:509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottolese R, Redoute J, Costes N, Le Bars D, Sirigu A. Switching brain serotonin with oxytocin. Proc Natl Acad Sci U S A. 2014;111:8637–8642. doi: 10.1073/pnas.1319810111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice K, Viscomi B, Riggins T, Redcay E. Amygdala volume linked to individual differences in mental state inference in early childhood and adulthood. Developmental Cognitive Neuroscience. 2014;8:153–163. doi: 10.1016/j.dcn.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002;6:129–142. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Tocco MA, Quednau KA, Bedway AR, Carre JM. Idle behaviors of the hippocampus reflect endogenous cortisol levels in youth. J Am Acad Child Adolesc Psychiatry. 2013;52:642–652 e641. doi: 10.1016/j.jaac.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology. 2011;36:144–147. doi: 10.1016/j.psyneuen.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Schneiderman I, Gordon I, Feldman R. Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology. 2013;38:694–701. doi: 10.1016/j.psyneuen.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci U S A. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P, Liu Y. Neutral faces activate the amygdala during identity matching. NeuroImage. 2006;29:628–636. doi: 10.1016/j.neuroimage.2005.07.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.