Abstract
Neuroimmune and inflammatory processes have been locally associated with the amygdala in alcohol exposure and withdrawal. We and others have suggested that this inflammation in the amygdala may cause disturbance of neural function observed as anxiety and autonomic distress in withdrawal. Despite the potential importance of the robust neuroinflammatory response, the mechanisms contributing to this response are not well understood. We review literature that suggests the effects of alcohol, and other substances of abuse, cause dysbiosis of the gut microbiome. This peripheral response may modulate neuroprotective vagal afferent signaling that permits and exacerbates a neuroinflammatory response in the amygdala. We will examine the mounting evidence that suggests that (1) gut dysbiosis contributes to neuroinflammation, especially in the context of alcohol exposure and withdrawal, (2) the neuroinflammation in the amygdala involves the microglia and astrocytes and their effect on neural cells, and (3) amygdala neuroinflammation itself contributes directly to withdrawal behavior and symptoms. The contribution of the gut to an anxiogenic response is a promising therapeutic target for patients suffering with withdrawal symptoms given the safe and well-established methods of modulating the gut microbiome.
Keywords: Gut Microbiome, Gut-Brain Axis, Neuroinflammation, Vagus Nerve, Withdrawal
1. Introduction
The high prevalence of substance use disorders (SUDs), including alcoholism, motivates the need for increased understanding of physiological mechanisms accompanying addiction and withdrawal. Both addiction and withdrawal have pathologies and comorbidities that are associated with distinct changes in brain function and physiology. In previous work we found the status of alcohol withdrawal produces a robust and sustained neuroinflammatory response in the central nucleus of the amygdala (CeA), a structure strongly associated with regulation of emotion (Freeman et al. 2012, 2013). We and others have suggested that the effects of inflammation on neuronal function drive some of the autonomic disturbances, anxiety, and other negative feelings associated with withdrawal (Retson et al. 2014). These neuroimmune processes appear to involve the activation of microglia and disturbances of the blood brain barrier. The source of this neuroinflammation is not yet understood, and our purpose in the present review is to consider the evidence that changes in the state of the gut microbiome may contribute to neuroimmune processes.
Recent investigations are rapidly uncovering a series of compelling connections between the gut and brain, where each seems able to drive substantial changes in the other (Klarer et al. 2014; Tillisch et al. 2013; Hsiao et al. 2013; Bruce-Keller et al. 2014). While it is unsurprising that the brain may control function of the gut, evidence is accumulating to suggest that the gut may reciprocally be able to influence function of the brain. A major driver of this gut to brain control behavior in humans is derived from the state of the gut microbiome (Foster and McVey Neufeld 2013; Tillisch et al. 2013). Logically, the transmission of the state of the gut as a signal may be communicated through two routes: the circulatory system and/or through activity of vagal afferent nerve fibers. In light of evidence showing that abuse of alcohol can disrupt the normal balance of bacteria in the gastrointestinal tract, we propose that one driver of neuroinflammation and behavioral change in addiction and withdrawal originates in the gut. By understanding how the gut is perturbed in SUDs, the means by which this change of gut state influences neuroinflammation, and the effect of neuroinflammation on addiction and withdrawal behavior, it may be possible to provide novel targets of intervention to mitigate dysregulation of emotion and autonomic function associated with withdrawal. This review will focus upon one type of SUD, alcoholism, in the hopes of demonstrating how it might mechanistically drive perturbations in the gut microbiome that in turn lead to neuroinflammation and pathological brain function. The present review builds on previous literature studying correlations of the state of the gut microbiome and mental functions by summarizing the mechanistic details in logical sequence along the two putative routes of gut microbial influences on the brain, and specifically on the CeA in alcohol addiction and withdrawal.
2. Changes in the Gut Microbiome Composition Modulate Brain Function
Many reviews have been written concerning correlational studies involving the gut microbiota and brain function (Grenham et al. 2011; Aidy et al. 2014; Dash et al. 2015; Fond et al. 2015). Therefore, only a subset of that literature will be reviewed in order to provide context to the other relevant literature that will be reviewed here. The intention here is to assemble and link the various lines of evidence into a plausible mechanism by which changes to the milieu of intestinal bacteria are capable of driving changes in the central nervous system, especially focusing on changes in behavior including anxiety and depression. Compelling evidence for changes in the gut driving changes in the brain lies in the treatment results for hepatic encephalopathy, which develops as a consequence of the liver’s failure to filter nitrogenous small molecules produced by gut bacteria and results in a build-up of these small molecules in the brain causing astrocytic swelling (Butterworth 2015). Hepatic encephalopathy is treated with lactulose, a laxative, and oral rifaximin, an antibiotic that primary stays in the intestinal lumen and is not widely circulated (Waghray et al. 2014). By treating only the intestine, it is possible to treat central nervous system pathology. More direct evidence of this derives from two independent studies where transplantation of gut contents from one animal to another was able to induce behavioral changes repeatedly (Bercik et al. 2011; Hsiao et al. 2013). Such results involving behavior and gut status hold for mouse models even in strict germ-free environments where commensal bacteria can be more tightly controlled (Nishino et al. 2013). Apart from these examples, there is a growing body of literature to suggest that modulation of the gut microbiome through probiotic treatment and fecal transplant may help treat anxiety, depression, and other forms of mental illness in humans (Paulus and Stein 2010; Foster and McVey Neufeld 2013; Slyepchenko et al. 2014). Many polygenic neurological pathologies are suspected to be influenced by the state of the gut including chronic regional pain syndrome (Reichenberger et al. 2013), Parkinson disease (Scheperjans et al. 2014; Conlon and Bird 2015), schizophrenia (Severance et al. 2014; Nemani et al. 2015), and autism (Toh and Allen-vercoe 2015; Weston et al. 2015), to name a few.
Both addiction and withdrawal states have been shown to arise from changes in neurological function. Given the ability of the gut microbiome to influence central nervous system functions, it is worth investigating pathologies in which neural function and gut dysbiosis are both comorbid, like addiction and withdrawal. Since it has been shown that the use of certain addictive substances has an effect on the gut microbiome, then it follows that investigation into the role of the gut in contributing to the effects of the substance is warranted.
3. Substance Use Disorders and the Microbiome
While relatively few studies to date examine the effects of substance abuse on the composition of the gut microbiome or consequent inflammation, those that have been completed consistently demonstrate dysbiosis and increased levels of inflammation. Most of these studies have examined the changes in the gut microbiome in the context of alcohol dependence. Increased abundance of pro-inflammatory gut microbes, like Proteobacteria species, coupled with decreased abundance of normal commensal bacteria has been reported in both alcoholics and murine models of chronic alcohol exposure (Mutlu et al. 2012; Bull-Otterson et al. 2013; Kakiyama et al. 2014; Malaguarnera 2014). For example, in all cases of dysbiosis seen in alcoholic patients, commensal members of the Bacteroidetes species had significantly lower abundance than healthy controls. It seems that alcohol exposure in mice takes about 3 weeks before large changes are seen in the gut composition, particularly with an upregulation of Actinobacteria species and simultaneous increases in pH (Bull-Otterson et al. 2013). The effects on plasma and pH were attenuated with co-administration of probiotics (L. rhamnosus) in this study. From an analysis of correlational network connectivity between bacterial taxa, the dysbiotic gut has a significantly lower connectivity, which is taken by the study to mean that it is less metabolically robust and its ability to respond to external stressors is impaired (Mutlu et al. 2012). As one potential mechanism by which dysbiosis leads to inflammation lies in certain gut bacteria that process primary bile acids to inflammatory types of secondary bile acids, leading to increased gut inflammation as well as serum inflammation (Kakiyama et al. 2014). All of these data point in the same direction, that alcohol abuse leads to gut dysbiosis and peripheral inflammation. We will later discuss how such changes in gut composition and peripheral inflammatory response can lead to neuroinflammation and thus pathological neural outcomes.
4. Interoception and Communication of Gut State by Vagal Afferents
There is a long-established body of literature demonstrating that the state of the gut organs, including the immune system, communicates to the brain via vagal afferents (Goehler et al. 2000). The nodose ganglion contains the gut sensory afferents of the vagus nerve, whose central terminals end on neurons of the nucleus tractus solitarius (NTS). The phenomenology of alterations in gut bacteria affecting neuronal function and neurochemical phenotype is becoming increasingly well-established, yet many of the actual effectors of these changes remain opaque. One such effector, cholecystokinin (CCK), has been shown to alter the neurochemical phenotype of vagal afferents directly (Dockray 2009a; Dockray and Burdyga 2011). Even though there aren’t an overwhelming number of studies examining other such mechanisms specifically, there are some that are compelling and promote hope that with more effort, the mechanisms can become more transparent. Vagal gut afferents modulate innate anxiety and the learned fear response as shown through behavioral studies in rats. In these studies subdiaphragmatic deafferentation led to concomitant region-specific changes in gamma amino butyric acid (GABA) and norepinephrine (NE) levels (Klarer et al. 2014). The gut microbiome has been shown to affect this pathway with ingestion of Lactobacillus rhamnosus regulating central GABA receptor expression with consequent effects on emotional behavior and response to stress in mice, an effect that was eliminated in vagotomized animals (Bravo et al. 2011). Further, the same strain of bacteria administered directly into the gut lumen causes increased vagal afferent activity that was semi-dependent upon activation of stretch receptors, meaning that the bacteria seemed to prime the neurons to be more reactive to stretch stimuli (Perez-Burgos et al. 2013). It appeared that the increased activity was driven by the same neurons firing with greater frequency rather than the recruitment of more neurons. Results obtained from administration of Lactobacillus reuteri in rats has demonstrated inhibition of calcium-dependent potassium channels opening in colonic enteric sensory neurons, having the net effect of increasing excitability of these neurons (Kunze et al. 2009). In spite of this effect stemming from a different Lactobacillus species and being found in enteric sensory neurons and not vagal afferents, it does add one potential mechanism by which gut microbiota directly affect neuronal function. There are many other potential pathways driven by gut microbial alterations; relative amounts of metabolites can act directly on vagal afferent neurons or through cascades with downstream effects on these neurons (Wikoff et al. 2009).
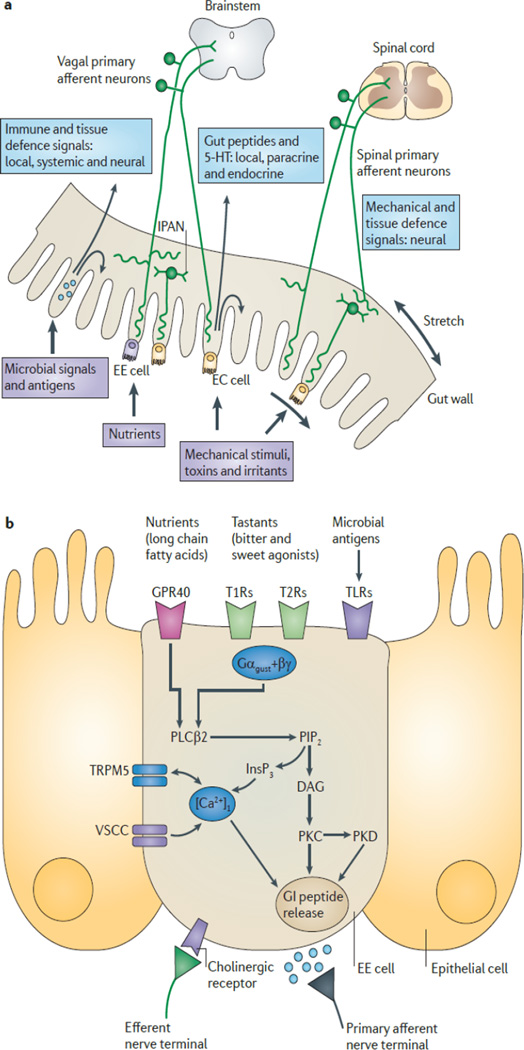
How might changes to relative bacterial abundance physically be sensed by the vagal afferents? As alluded to previously, one route of communication may be through direct interactions between vagal afferent neurons and bacteria or their metabolic products. Another route includes effects of paracrine signaling from enterochromaffin (EC) cells and from enteroendocrine (EE) cells (Figure 1; Mayer 2011) that interact with the gut lumen causing secretion of factors that affect nearby cells in the gut wall (Rhee et al. 2009). These paracrine factors may also interact with local circuit neurons of the enteric nervous system known as intrinsic primary afferent neurons (IPAN). Both IPAN and extrinsic afferents of the vagal or splanchnic systems send projections that richly innervate myenteric plexuses, allowing for some paracrine type or other means of communication (Mayer 2011). It is also possible that secretion of normal gut factors, like cholecystokinin (CCK), which are known to signal the state of the gut to the brain via the vagus nerve, can be altered in dysbiosis (Dockray 2014). Adjustments of this nature can change vagal neuron signaling, altering peripheral feedback effects of vagal efferents (Dockray and Burdyga 2011). Regardless of which mechanisms drive these effects, they are reliably observed. It is clear that a better characterization of how vagal activity is altered and precisely what this means for vagal projections that influence regions of the brain will remain important topics of inquiry for some time.
Figure 1.
Signaling pathways that permit the state of the gut to influence primary vagal afferent neurons through direct interactions and EE cells.
Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Neuroscience (Mayer 2011)
5. Vagal Afferents Modulate Neuroinflammation in the Limbic System
Stimulation of vagal afferents has been clinically used to treat neurological and psychiatric disorders including depression (Shah et al. 2014), drug-resistant epilepsy (Fraschini et al. 2014), and Alzheimer’s disease (Vonck et al. 2014). Vagal stimulation has also been used in animal models of neuropathic pain (Oshinsky et al. 2014) and of schizophrenia (Perez et al. 2014). Although some studies listed above also suggest mechanisms involving dopaminergic signaling and network reorganization, much of the experimental work on vagal afferent signaling and its effect on the brain has dealt with the anti-inflammatory pathways. Stimulation of the vagal afferents limits synaptic hyperexcitability induced by lipopolysaccharide (LPS) driven IL-6 production (Cunningham et al. 2008; Garcia-Oscos et al. 2015). Vagal stimulation also seems to be protective against neuroinflammation that is induced in the context of stroke through activation of specific noradrenergic neurons (Mravec 2010). Increasing the amount of norepinephrine present in the brain, e.g. cortex and hippocampus, has been shown to mitigate expression of cytokines, adhesion molecules, and other pro-inflammatory markers (O’Sullivan et al. 2010). This sets up an anti-inflammatory pathway whereby the vagal afferents activate noradrenergic neurons of the nucleus of the solitary tract (NTS), which then project to the central amygdala and provide a noradrenergic milieu that mitigates neuroinflammation (O’Sullivan et al. 2010). Similarly, peripheral vagus nerve stimulation has been shown to induce production of anti-inflammatory cytokines in the neurovasculature of animals treated with LPS injection, suggesting that the activity of vagal afferents is able to modulate the brain’s protective immune response (Mihaylova et al. 2012). Even cytokines and inflammation in the peripheral circulation have been shown to activate vagal afferents and modulate anti-inflammatory effects in the central nervous system (CNS) (Maier et al. 1998). It is therefore plausible that the humoral communication of cytokines to the brain is potentiated or permitted in the face of altered vagal signaling from the gut due to microbial imbalance.
Having seen the potential anti-inflammatory role of the vagus, how then might gut microbiome signaling through the vagus have a pro-inflammatory effect? The state of the gut microbiome is reported to the brain via vagal projections to the NTS (Maier et al. 1998; Goehler et al. 2000; Bravo et al. 2011) by changes in the activity of the vagus nerve (Perez-Burgos et al. 2013) and in the neurochemical phenotype of the vagal afferent neurons in the nodose ganglia and their targets in the NTS (Dockray 2009b; Burdyga et al. 2010; Dockray and Burdyga 2011). The NTS in turn has viscerosensory projections to the central nucleus of the amygdala (CeA) that involves NE (Reyes and Van Bockstaele 2006; Rinaman 2011). Decreased activity of these projections would lead to a loss of its anti-inflammatory activity as described above, mediating stress and anxiety. More recently neurons expressing glucagon-like peptide 1 (GLP-1) and glutamate have also been described to participate in these NTS viscerosensory projections to the CeA in a proposed anxiogenic GLP-1 pathway to the corticotropin releasing factor (CRF) neurons in the CeA (Maniscalco et al. 2012; Zheng et al. 2014). The CeA contains a dense population of CRF neurons that co-express GABA and a dense plexus of CRF terminals. CRF is a key neuropeptide mediator of stress and neuroinflammatory responses linked to withdrawal (Zorrilla et al. 2014; Koob 2010; Gilpin 2012; Weiss et al. 2001; Retson et al. 2014). Thus increased activity of the NTS GLP-1 neurons by vagal inputs may well be pro-inflammatory and anxiogenic in the CeA (Sakanaka et al. 1986; Phelix et al. 1994; Koob 1999; Asan et al. 2005).
As a demonstration of the concept of gut microbial driver of anxiety, probiotic treatment with Lactobacillus rhamnosus resulted in decreased anxiety-like behavior and changes in GABA receptor expression indicative of a less anxious state in mice when the vagus was intact (Bravo et al. 2011). Vagal integrity has also proved necessary for the increase in anxiety-like behavior eight hours following Campylobacter jejuni (C. jejuni) infection (Goehler et al. 2008). There is a small but growing literature on visceral interoceptive effects of the NTS on the CeA strongly impacting emotional and higher processes, including those reporting the state of the gut microbiome. Vagally mediated increases in c-Fos expression in NTS and CeA following C. jejuni infection were taken to suggest that this neuronal route may play a key role in the induced anxiety-like behavior driven by gut dysbiosis (Gaykema et al. 2004; Goehler et al. 2005, 2008).
Dysregulating or altering the vagal afferents via the gut microbiome may interact with this system and promote neuroinflammation. We hypothesis that the largest effects of vagal signaling may not be seen on a chronic basis due to adaptive processes and compensation, but instead manifest upon sudden changes that happen faster than compensatory mechanisms can respond. This may contribute to the increase in neuroinflammation seen upon withdrawal that was present at a lower state during chronic exposure to alcohol (Freeman et al. 2012).
6. Humoral Factors from the Gut and Neuroinflammation
Apart from the vagal route of gut-to-brain communication, it is also possible that inflammatory signaling molecules and/or bacterial toxins in the circulation can act as a means to drive neuroinflammation through direct interaction in the brain. The best characterized mechanism for this starts with the “leaky gut” concept, wherein inflammation leads to a breakdown of lymphatic duct lining and endothelial cell junctions which permits inflammatory molecules to leak outside of the gut into the bloodstream or surrounding adipocytes (Keita and Söderholm 2010). Once in the blood stream, it is possible for peripheral cytokines to enter the brain through active transport with the potential to induce their own synthesis, even with an intact blood brain barrier (Skinner et al. 2009; Banks et al. 1995; Downs et al., 2014). In one study, it was shown directly in nonhuman primates that endotoxin-induced inflammation in the periphery was able to activate microglia and thus precipitate a neuroinflammatory response (Hannestad et al. 2012). This means that the precipitating neuroinflammation that is seen in exposure and withdrawal can indeed come first from the periphery; it doesn’t require that the blood brain barrier be disturbed as a prerequisite to allow entry of inflammatory molecules. We hypothesize that the vagus nerve driven neural circuit would have the fastest influences on central inflammation, but that a blood borne route may follow, producing interactions of the two routes of influence. However, to our knowledge, fine-grained time series or other studies aiming to further pick this mechanism apart have not been performed in the context of substance use or withdrawal. Once the blood brain barrier is disturbed, presumably through inflammatory processes that promote breakdown of the tight junctions in the neurovascular endothelial cells, peripheral cytokines and toxins may further promote inflammation in a positive feedback loop. Taking this premise, the question then becomes, what sort of peripheral inflammatory response can arise from substance use and withdrawal?
The leaky gut hypothesis has been shown to have merit in several studies wherein pro-inflammatory gut contents are able to pass into the blood stream (reviewed in van Hemert et al. 2014; Anders et al. 2013). The mechanistic underpinning of this lies in effects on the epithelial cells either from the pro-inflammatory molecules or, in the case of alcohol, from the substance of abuse itself (Asai et al. 2003). Given that bacterial translocation into the portal circulation due to a breakdown of intestinal epithelial lining also strongly affects the state of the liver in patients with chronic alcohol abuse (Hartmann et al. 2012), it is no surprise that alcoholics have been shown to have serum gram negative bacterial endotoxin levels (e.g. LPS) that are significantly higher than in people that consume alcohol casually (Mutlu et al. 2012). Levels of certain microbial species are known to influence leaky gut, for example, Butyricicoccus pullicaecorum has been shown to play an essential role in protecting against increased epithelial permeability in inflammatory bowel diseases (Eeckhaut et al. 2013), but few gut microbes have yet to be examined in alcohol addiction or withdrawal.
7. Neuroinflammation Influences Withdrawal Behavior
The idea that neuroinflammation contributes to the symptoms seen in mental illnesses including depression, anxiety, and withdrawal behavior is not new, but direct mechanistic evidence that this is actually the case is still under development. Emotional dysregulation is a significant aspect of alcohol withdrawal behavior in human patients (Koob 2015). It appears likely that one component of relapse is the attempted avoidance of negative affective symptoms like depression and anxiety. Such mental states may reflect neural function under the influence of inflammation. Not only is there an extensive body of literature demonstrating the high correlation between a systemic inflammatory response and major depressive disorder (Reviewed in Berk et al. 2013), but there is also evidence to suggest that higher levels of certain inflammatory cytokines are a risk factor for the development of depression in individuals that were not previously diagnosed with depression (Dowlati et al. 2010). Clinical data have also shown that humans receiving vaccinations, such as those for S typhi, can induce behavioral changes that track with peripheral cytokine levels (Miller et al. 2009). Alternatively, selective serotonin reuptake inhibitors (SSRIs), which have been successfully used to treat depression in many individuals, have recently been found to have strong anti-inflammatory properties with serious consideration as to what clinical role this added mechanism of action plays in the treatment of depression (Reviewed in Walker 2013). In their review, Jones & Thomsen suggest three main lines of clinical evidence in support of inflammation being a major contributor of depressive symptoms: induction of depression with cytokine therapies, increased incidence of depression in patients with autoimmune disorders, and increased levels of inflammatory biomarkers in patients with depression (Jones and Thomsen 2013).
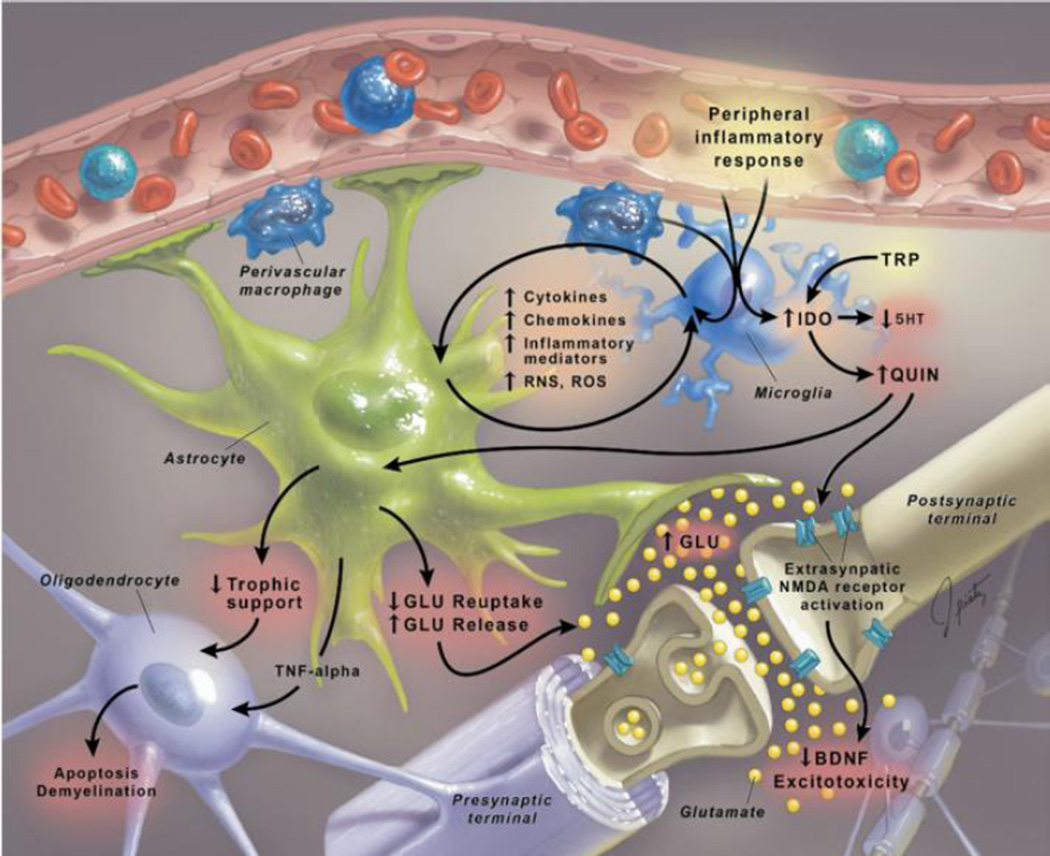
There are certain inflammatory molecules that induce behavioral changes when present in the brain. The literature implicates both the innate and acquired immune response pathways (Mössner et al. 2007), although such results do not point to one neuronal mechanism over another. For example, anhedonic stress-induced behavior in mice has been mitigated by intracerebroventricular infusion of the IL-1β receptor antagonist IL-1Ra (Goshen et al. 2008; Koo and Duman 2009). Both IL-1β and TNFα have been implicated in altering glutamatergic signaling due to production of quinolinic acid (an NMDA receptor agonist) and downregulation of the glutamate transporter EAAT on astrocytes (Khairova et al. 2009). IL-1β and TNFα in the brain can come from the periphery or be induced by the inflammatory molecules that infiltrate from the periphery into the CNS. Either way these results demonstrate a mechanism by which neuroinflammation could promote some of the depressive-like behaviors seen in withdrawal. Other mechanisms include interaction with the non-neuronal cell types in the CNS, like microglia and astrocytes (see Figure 2). It is likely that any pro-inflammatory pathway within the CNS will involve activation of microglia, the brain’s resident immune cells that are of bone-marrow derived monocyte lineage (González et al. 2014). This is supported by studies showing increased brain levels of CD11b, a monocyte-specific marker, during stress-induced depression (Farooq et al. 2012). The evidence taken as a whole suggest that if something were to induce peripheral inflammation, then it would indeed be possible for there to be an effect on behavior, especially if certain brain regions, such as the amygdala and NTS, were affected more severely than others.
Figure 2.
Signaling responses in the brain to peripheral inflammation demonstrating negative interacting effects on multiple cell types.
Reprinted with permission from (Miller et al. 2009)
8. Proposed Mechanism for Gut-Derived Neuroinflammatory Withdrawal Response
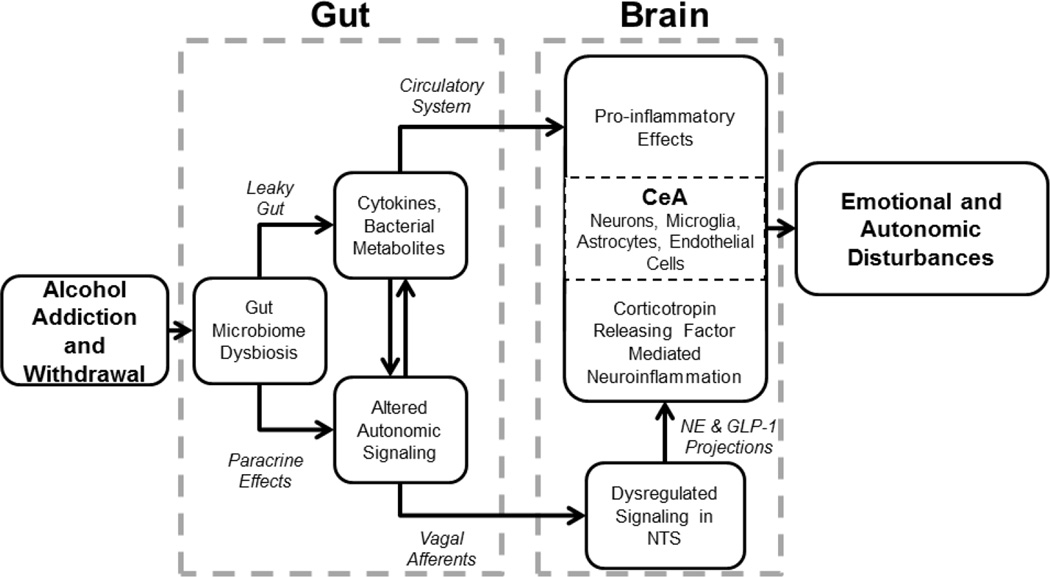
The literature reviewed here suggests a mechanism by which the gut microbial balance, when perturbed by SUDs such as alcoholism, contributes to neuroinflammatory effects on emotion. Starting with the gut, alcohol addiction or withdrawal may lead to a decrease in the protective colonies or an increase in the pathogenic colonies, both of which can drive changes in inflammatory signaling and cytokine release. Such alterations may prime the vagus nerve to withdraw or alter its neuroprotective afferent signals or promote vagal signals that are in some way harmful. With the brain primed by vagal afferent alterations, it may be more susceptible to influence from the peripheral inflammatory response that occurs in response to substance use and/or withdrawal. Neuroinflammation in regions like the CeA and others can contribute to emotional dysregulation seen in withdrawal, summarized in Figure 3. We acknowledge that feedback from the brain (e.g. CeA) to the gut that participates in the mechanisms outlined here, but our present focus is on the routes of influence from gut to brain.
Figure 3.
Summary of routes by which dysbiotic gut from alcohol addiction and withdrawal drives neuroinflammation in the central nucleus of the amygdala (CeA) via vagal afferent signaling acting on neurons in the nucleus of the solitary tract (NTS) as well as via humoral factors in the circulatory system.
From this mechanistic perspective, it is possible to formulate several predictions that can be tested to clarify and validate the ideas presented here. It would be expected that withdrawal behavior could be mitigated through manipulation of the gut microbiome. This could be in the form of pre-, pro-, or post-biotics administered both with and without addiction therapeutics or even through fecal transplant. It would also be interesting to see what occurs when a fecal transplant is performed from an animal in withdrawal to a naïve animal. While the effects may not recapitulate full blown withdrawal behavior, it would be expected that some symptoms would arise from this treatment, further validating the mechanistic predictions here. While it would be less specific, an examination of vagal stimulation treatment for alcohol withdrawal symptoms would also have the potential to show a mitigating effect. Part of the mechanism discussed here is the withdrawal of protective vagal signals that permit a neuroinflammatory response. It is possible that by stimulating the vagal afferents from the gut that some of this signaling may be restored. Perhaps more simply, we would suspect that treatment with anti-inflammatory agents or certain oral antibiotics would have an effect on withdrawal symptoms through alterations of the neuroinflammation and gut microbial balance. The bacterial constituents that are driving dysbiosis in this context are not all characterized, so it is difficult to know which, if any, oral antibiotics that stay within the gut would be harmful or beneficial. Given the complex network of interacting factors, we believe that a systems approach needs to be taken in order to disentangle the mechanisms that are driving the changes found in the gut, the periphery, and in the brain.
9. Implications
In spite of many medical and nonmedical interventions, alcoholism and other SUDs are still a vexing problem. Patients have widely heterogeneous responses to pharmaceutical therapies that help to break addictions and similarly heterogeneous responses to support groups and other approaches. This suggests that there is more to the addiction mechanism that has yet to be addressed by any current interventions. Demonstration of the effects of gut dysbiosis in withdrawal in human patients is accessible with the collection of blood and fecal samples both being relatively noninvasive and can be done in conjunction with addiction therapy. This research may lead to low-cost, ready-made treatments with real benefits to the patients battling alcoholism or other SUDs. While gut dysbiosis is not likely the one silver bullet, it may turn out to be the one additional component that can tip the balance in favor of recovery from addiction in those suffering from withdrawal symptoms. This is enticing because of the ease with which intervention can occur in human patients. Manipulation of the gut microbiome through antibiotics, probiotics, and even fecal transplant are all techniques that generally have excellent safety profiles and are well-established. At this point, we lack mechanistic understanding of precisely how gut dysbiosis may affect the brain in alcohol addiction and withdrawal and therefore the ability to design interventions to address it. We have, however, outlined several lines of causal reasoning that demand further inquiry into this effect that would further elucidate these mechanisms, providing insight into effective treatment.
Acknowledgements
We would like to thank James Park and Daniel Cook for comments on the manuscript, and acknowledge Kate Freeman, D. Craig Hooper, Beverly Reyes, Elisabeth Van Bockstaele, and Sean O’Sullivan for their thoughts and contributions specifically with respect to alcohol and also in the context of ongoing closely parallel work with the amygdala, neuroimmune processes, opioid withdrawal, and the gut microbiome. This work was supported by R21 DA036372-01; R01 HL111621-01A1; and T32 AA007463-28.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aidy SEl, Dinan TG, Cryan JF. Immune modulation of the brain-gut-microbe axis. Front Microbiol. 2014 Apr;5:3–6. doi: 10.3389/fmicb.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders H-J, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int [Internet] 2013;83(6):1010–1016. doi: 10.1038/ki.2012.440. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23325079. [DOI] [PubMed] [Google Scholar]
- Asai K, Buurman WA, Reutelingsperger CPM, Schutte B, Kaminishi M. Low concentrations of ethanol induce apoptosis in human intestinal cells. Scand J Gastroenterol. Norway. 2003 Nov;38(11):1154–1161. doi: 10.1080/00365520310006252. [DOI] [PubMed] [Google Scholar]
- Asan E, Yilmazer-Hanke DM, Eliava M, Hantsch M, Lesch KP, Schmitt a. The Corticotropin-Releasing Factor (CRF)-system and monoaminergic afferents in the central amygdala: Investigations in different mouse strains and comparison with the rat. Neuroscience. 2005;131:953–967. doi: 10.1016/j.neuroscience.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Banks W, Plotkin S, Kastin A. Permiability of the blood-brain barrier to soluable cytokine receptors. Neuroimmunomodulation. 1995;2(3):161–165. doi: 10.1159/000096887. [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco Ja, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med [Internet] BMC Medicine. 2013;11(1):200. doi: 10.1186/1741-7015-11-200. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3846682&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011 Sep;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, et al. Metagenomic Analyses of Alcohol Induced Pathogenic Alterations in the Intestinal Microbiome and the Effect of Lactobacillus rhamnosus GG Treatment. PLoS One. 2013;8(1):4–13. doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons: kinetics and role in influencing neurochemical phenotype. Am J Physiol Gastrointest Liver Physiol. 2010 Jul;299(1):G63–G59. doi: 10.1152/ajpgi.00059.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth RF. Pathogenesis of Hepatic Encephalopathy and Brain Edema in Acute Liver Failure. J Clin Exp Hepatol [Internet]. Elsevier Ltd. 2015 Mar;5:S96–S103. doi: 10.1016/j.jceh.2014.02.004. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0973688314000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon Ma, Bird AR. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. 2015:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JT, Mifflin SW, Gould GG, Frazer A. Induction of c-Fos and DeltaFosB immunoreactivity in rat brain by Vagal nerve stimulation. [cited 2014 Jul 31];Neuropsychopharmacology [Internet] 2008 Jul;33(8):1884–1895. doi: 10.1038/sj.npp.1301570. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17957222. [DOI] [PubMed] [Google Scholar]
- Dash S, Clarke G, Berk M, Jacka FN. The gut microbiome and diet in psychiatry. Curr Opin Psychiatry [Internet] 2015;28:1–6. doi: 10.1097/YCO.0000000000000117. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00001504-201501000-00002. [DOI] [PubMed] [Google Scholar]
- Dockray GJ. Cholecystokinin and gut-brain signalling. Regul Pept [Internet]. Elsevier B.V. 2009a;155(1–3):6–10. doi: 10.1016/j.regpep.2009.03.015. Available from: http://dx.doi.org/10.1016/j.regpep.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Dockray GJ. The versatility of the vagus. Physiol Behav. 2009b Jul;97(5):531–536. doi: 10.1016/j.physbeh.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Dockray GJ. Gastrointestinal hormones and the dialogue between gut and brain. J Physiol [Internet] 2014:1–15. doi: 10.1113/jphysiol.2014.270850. 00(July 2013) Available from: http://www.ncbi.nlm.nih.gov/pubmed/24566540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray GJ, Burdyga G. Plasticity in vagal afferent neurones during feeding and fasting: mechanisms and significance. Acta Physiol (Oxf) 2011 Mar;201(3):313–321. doi: 10.1111/j.1748-1716.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A Meta-Analysis of Cytokines in Major Depression. Biol Psychiatry [Internet]. Elsevier Inc. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. Available from: http://dx.doi.org/10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Eeckhaut V, Machiels K, Perrier C, Romero C, Maes S, Flahou B, et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut [Internet] 2013;62(12):1745–1752. doi: 10.1136/gutjnl-2012-303611. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23263527. [DOI] [PubMed] [Google Scholar]
- Farooq RK, Isingrini E, Tanti A, Le Guisquet AM, Arlicot N, Minier F, et al. Is unpredictable chronic mild stress (UCMS) a reliable model to study depression-induced neuroinflammation? Behav Brain Res [Internet]. Elsevier B.V. 2012;231(1):130–137. doi: 10.1016/j.bbr.2012.03.020. Available from: http://dx.doi.org/10.1016/j.bbr.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Fond G, Boukouaci W, Chevalier G, Regnault A, Eberl G, Hamdani N, et al. The “psychomicrobiotic”: Targeting microbiota in major psychiatric disorders: A systematic review. Pathol. Biol. (Paris) 2015:35–42. doi: 10.1016/j.patbio.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Foster Ja, McVey Neufeld KA. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci [Internet]. Elsevier Ltd. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. Available from: http://dx.doi.org/10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Fraschini M, Demuru M, Puligheddu M, Floridia S, Polizzi L, Maleci A, et al. The re-organization of functional brain networks in pharmaco-resistant epileptic patients who respond to VNS. Neurosci Lett [Internet]. Elsevier Ireland Ltd. 2014;580:153–157. doi: 10.1016/j.neulet.2014.08.010. Available from: http://www.sciencedirect.com/science/article/pii/S030439401400651X. [DOI] [PubMed] [Google Scholar]
- Freeman K, Brureau A, Vadigepalli R, Staehle MM, Brureau MM, Gonye GE, et al. Temporal changes in innate immune signals in a rat model of alcohol withdrawal in emotional and cardiorespiratory homeostatic nuclei. [cited 2014 Jun 3];J Neuroinflammation [Internet] 2012 Jan;9(1):97. doi: 10.1186/1742-2094-9-97. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3411448&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman K, Staehle MM, Vadigepalli R, Gonye GE, Ogunnaike Ba, Hoek JB, et al. Coordinated Dynamic Gene Expression Changes in the Central Nucleus of the Amygdala During Alcohol Withdrawal. [cited 2014 Jun 3];Alcohol Clin Exp Res [Internet] 2013 Jan;37(SUPPL.1):E88–E100. doi: 10.1111/j.1530-0277.2012.01910.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22827539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Oscos F, Peña D, Housini M, Cheng D, Lopez D, Borland MS, et al. Vagal nerve stimulation blocks interleukin 6-dependent synaptic hyperexcitability induced by lipopolysaccharide-induced acute stress in the rodent prefrontal cortex. Brain Behav Immun [Internet]. Elsevier Inc. 2015;43:149–158. doi: 10.1016/j.bbi.2014.07.020. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0889159114004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema RPA, Goehler LE, Lyte M. Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain Behav Immun. 2004 May;18(3):238–245. doi: 10.1016/j.bbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Gilpin NW. Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): effects on inhibitory transmission in central amygdala, and anxiety- & alcohol-related behaviors. Alcohol. 2012 Jun;46(4):329–337. doi: 10.1016/j.alcohol.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPa, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: A visceral chemosensory pathway. Auton Neurosci Basic Clin. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005 Jul;19(4):334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RPA. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: Possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav Immun. 2008;22(3):354–366. doi: 10.1016/j.bbi.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González H, Elgueta D, Montoya A, Pacheco R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J Neuroimmunol [Internet]. Elsevier B.V. 2014;274(1–2):1–13. doi: 10.1016/j.jneuroim.2014.07.012. Available from: http://dx.doi.org/10.1016/j.jneuroim.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011 Dec;2:1–15. doi: 10.3389/fphys.2011.00094. DEC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, Gallezot JD, Schafbauer T, Lim K, Kloczynski T, Morris ED, et al. Endotoxin-induced systemic inflammation activates microglia: [11C]PBR28 positron emission tomography in nonhuman primates. Neuroimage [Internet]. Elsevier Inc. 2012;63(1):232–239. doi: 10.1016/j.neuroimage.2012.06.055. Available from: http://dx.doi.org/10.1016/j.neuroimage.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann P, Chen WC, Schnabl B. The intestinal microbiome and the leaky gut as therapeutic targets in alcoholic liver disease. Front Physiol. 2012 Oct;3:1–10. doi: 10.3389/fphys.2012.00402. OCT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hemert S, Breedveld AC, Rovers JörgenMP, Vermeiden JPW, Witteman BJM, Smits MG, et al. Migraine Associated with Gastrointestinal Disorders: Review of the Literature and Clinical Implications. Front Neurol [Internet] 2014 Nov;5:1–7. doi: 10.3389/fneur.2014.00241. Available from: http://journal.frontiersin.org/journal/10.3389/fneur.2014.00241/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell [Internet]. Elsevier Inc. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. Available from: http://dx.doi.org/10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones Ka, Thomsen C. The role of the innate immune system in psychiatric disorders. Mol Cell Neurosci [Internet]. Elsevier Inc. 2013;53:52–62. doi: 10.1016/j.mcn.2012.10.002. Available from: http://dx.doi.org/10.1016/j.mcn.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Kakiyama G, Hylemon PB, Zhou H, Pandak WM, Heuman DM, Kang DJ, et al. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. AJP Gastrointest Liver Physiol [Internet] 2014;306:G929–G937. doi: 10.1152/ajpgi.00315.2013. Available from: http://ajpgi.physiology.org/cgi/doi/10.1152/ajpgi.00315.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita AV, Söderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil. 2010;22:718–733. doi: 10.1111/j.1365-2982.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- Khairova Ra, Machado-Vieira R, Du J, Manji HK. A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2009;12:561–578. doi: 10.1017/S1461145709009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarer M, Arnold M, Gunther L, Winter C, Langhans W, Meyer U. Gut Vagal Afferents Differentially Modulate Innate Anxiety and Learned Fear. J Neurosci [Internet] 2014;34(21):7067–7076. doi: 10.1523/JNEUROSCI.0252-14.2014. Available from: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.0252-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. Evidence for IL-1 receptor blockade as a therapeutic strategy for the treatment of depression. Curr Opin Investig Drugs. 2009;10(7):664–671. [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. Elsevier B.V. 2010 Feb;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The dark side of emotion : The addiction perspective. Eur J Pharmacol. 2015;753:73–87. doi: 10.1016/j.ejphar.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze Wa, Mao YK, Wang B, Huizinga JD, Ma X, Forsythe P, et al. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med. 2009;13(8):2261–2270. doi: 10.1111/j.1582-4934.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci. UNITED STATES. 1998 May;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- Malaguarnera G. Gut microbiota in alcoholic liver disease: Pathogenetic role and therapeutic perspectives. World J Gastroenterol [Internet] 2014;20(44):16639. doi: 10.3748/wjg.v20.i44.16639. Available from: http://www.wjgnet.com/1007-9327/full/v20/i44/16639.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco JW, Kreisler AD, Rinaman L. Satiation and stress-induced hypophagia: Examining the role of hindbrain neurons expressing prolactin-releasing peptide or glucagon-like peptide 1. Front Neurosci. 2012 Jan;6:1–17. doi: 10.3389/fnins.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer Ea. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci [Internet]. Nature Publishing Group. 2011 Aug;12:453–466. doi: 10.1038/nrn3071. Available from: http://dx.doi.org/10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova S, Killian A, Mayer K, Pullamsetti SS, Schermuly R, Rosengarten B. Effects of antiinflammatory vagus nerve stimulation on the cerebral microcirculation in endotoxinemic rats. J Neuroinflammation [Internet] 2012;9(1):183. doi: 10.1186/1742-2094-9-183. Available from: http://www.jneuroinflammation.com/content/9/1/183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol Psychiatry [Internet]. Society of Biological Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. Available from: http://dx.doi.org/10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mössner R, Mikova O, Koutsilieri E, Saoud M, Ehlis A-C, Müller N, et al. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World J Biol Psychiatry. 2007;8(3):141–174. doi: 10.1080/15622970701263303. [DOI] [PubMed] [Google Scholar]
- Mravec B. The role of the vagus nerve in stroke. Auton Neurosci Basic Clin [Internet] Elsevier B.V. 2010;158(1–2):8–12. doi: 10.1016/j.autneu.2010.08.009. Available from: http://dx.doi.org/10.1016/j.autneu.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Mutlu Ea, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi a, Engen Pa, et al. Colonic microbiome is altered in alcoholism. AJP Gastrointest Liver Physiol. 2012;302:G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani K, Hosseini Ghomi R, McCormick B, Fan X. Schizophrenia and the gut-brain axis. Prog Neuro-Psychopharmacology Biol Psychiatry [Internet]. Elsevier Inc. 2015;56:155–160. doi: 10.1016/j.pnpbp.2014.08.018. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0278584614001687. [DOI] [PubMed] [Google Scholar]
- Nishino R, Mikami K, Takahashi H, Tomonaga S, Furuse M, Hiramoto T, et al. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culturebased methods. Neurogastroenterol Motil. 2013 Jun;25(6):521–528. doi: 10.1111/nmo.12110. [DOI] [PubMed] [Google Scholar]
- O’Sullivan JB, Ryan KM, Harkin A, Connor TJ. Noradrenaline reuptake inhibitors inhibit expression of chemokines IP-10 and RANTES and cell adhesion molecules VCAM-1 and ICAM-1 in the CNS following a systemic inflammatory challenge. J Neuroimmunol [Internet]. Elsevier B.V. 2010;220(1–2):34–42. doi: 10.1016/j.jneuroim.2009.12.007. Available from: http://dx.doi.org/10.1016/j.jneuroim.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Oshinsky ML, Murphy AL, Hekierski H, Cooper M, Simon BJ. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain [Internet] [cited 2014 Sep 9];International Association for the Study of Pain. 2014 May;155(5):1037–1042. doi: 10.1016/j.pain.2014.02.009. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24530613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010:1–13. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SM, Carreno FR, Frazer a, Lodge DJ. Vagal Nerve Stimulation Reverses Aberrant Dopamine System Function in the Methylazoxymethanol Acetate Rodent Model of Schizophrenia. J Neurosci [Internet] 2014;34(28):9261–9267. doi: 10.1523/JNEUROSCI.0588-14.2014. Available from: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.0588-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Burgos A, Wang B, Mao Y-K, Mistry B, McVey Neufeld K-A, Bienenstock J, et al. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2013 Jan;304(2):G211–G220. doi: 10.1152/ajpgi.00128.2012. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Catecholamine-CRF synaptic interaction in a septal bed nucleus: afferents of neurons in the bed nucleus of the stria terminalis. Brain Res Bull. UNITED STATES. 1994;33(1):109–119. doi: 10.1016/0361-9230(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Reichenberger ER, Alexander GM, Perreault MJ, Russell Ja, Schwartzman RJ, Hershberg U, et al. Establishing a relationship between bacteria in the human gut and Complex Regional Pain Syndrome. Brain Behav Immun [Internet] 2013;29:62–69. doi: 10.1016/j.bbi.2012.12.005. Available from: http://dx.doi.org/10.1016/j.bbi.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Retson Ta, Hoek JB, Sterling RC, Van Bockstaele EJ. Amygdalar neuronal plasticity and the interactions of alcohol sex stress. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BaS, Van Bockstaele EJ. Divergent projections of catecholaminergic neurons in the nucleus of the solitary tract to limbic forebrain and medullary autonomic brain regions. Brain Res. 2006;1117(1):69–79. doi: 10.1016/j.brainres.2006.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Pothoulakis C, Mayer Ea. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6(5):306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300:R222–R235. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. The Corticotropin-Releasing Factor (CRF)-system and monoaminergic afferents in the central amygdala: Investigations in different mouse strains and comparison with the rat. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Aho V, Pereira PaB, Koskinen K, Paulin L, Pekkonen E, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord [Internet] 2014 doi: 10.1002/mds.26069. Available from: http://doi.wiley.com/10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- Severance EG, Yolken RH, Eaton WW. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: More than a gut feeling. Schizophr Res [Internet]. Elsevier B.V. 2014 doi: 10.1016/j.schres.2014.06.027. Available from: http://dx.doi.org/10.1016/j.schres.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Carreno FR, Frazer A. Therapeutic Modalities for Treatment Resistant Depression : Focus on Vagal Nerve Stimulation and Ketamine. 2014;12(2):83–93. doi: 10.9758/cpn.2014.12.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner Ra, Gibson RM, Rothwell NJ, Pinteaux E, Penny JI. Transport of interleukin-1 across cerebromicrovascular endothelial cells. Br J Pharmacol. 2009;156:1115–1123. doi: 10.1111/j.1476-5381.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyepchenko A, Carvalho AF, Cha DS, Kasper S, McIntyre RS. Gut emotions - mechanisms of action of probiotics as novel therapeutic targets for depression and anxiety disorders. CNS Neurol Disord Drug Targets. United Arab Emirates. 2014;13(10):1770–1786. doi: 10.2174/1871527313666141130205242. [DOI] [PubMed] [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology [Internet]. Elsevier Inc. 2013;144(7):1394–1401. e4. doi: 10.1053/j.gastro.2013.02.043. Available from: http://dx.doi.org/10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh MC, Allen-vercoe E. The human gut microbiota with reference to autism spectrum disorder: considering the whole as more than a sum of its parts. Microb Ecol Health Dis. 2015;1:1–7. doi: 10.3402/mehd.v26.26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonck K, Raedt R, Naulaerts J, De Vogelaere F, Thiery E, Van Roost D, et al. Vagus nerve stimulation…25 years later! What do we know about the effects on cognition? Neurosci Biobehav Rev [Internet]. Elsevier Ltd. 2014;45C:63–71. doi: 10.1016/j.neubiorev.2014.05.005. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24858008. [DOI] [PubMed] [Google Scholar]
- Waghray A, Waghray N, Kanna S, Mullen K. Optimal treatment of hepatic encephalopathy. Minerva Gastroenterol Dietol. Italy. 2014 Mar;60(1):55–70. [PubMed] [Google Scholar]
- Walker FR. A critical review of the mechanism of action for the selective serotonin reuptake inhibitors: Do these drugs possess anti-inflammatory properties and how relevant is this in the treatment of depression? Neuropharmacology [Internet]. Elsevier Ltd. 2013;67:304–317. doi: 10.1016/j.neuropharm.2012.10.002. Available from: http://dx.doi.org/10.1016/j.neuropharm.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Weston B, Fogal B, Cook D, Dhurjati P. An agent-based modeling framework for evaluating hypotheses on risks for developing autism: Effects of the gut microbial environment. Med Hypotheses. 2015 Jan; doi: 10.1016/j.mehy.2015.01.027. [DOI] [PubMed] [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley Sa, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106(10):3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Cai L, Rinaman L. Distribution of glucagon-like peptide 1-immunopositive neurons in human caudal medulla. Brain Struct Funct. 2014:1–7. doi: 10.1007/s00429-014-0714-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol. 2014 Apr;35(2):234–244. doi: 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]