Abstract
Extra-virgin olive oil (EVOO) is one of the main elements of Mediterranean diet. Several studies have suggested that EVOO has several health promoting effects that could protect from and decrease the risk of Alzheimer’s disease (AD). In this study, we investigated the effect of consumption of EVOO-enriched diet on amyloid- and tau- related pathological alterations that are associated with the progression of AD and cerebral amyloid angiopathy (CAA) in TgSwDI mice. Feeding mice with EVOO-enriched diet for 6 months, beginning at an age before amyloid-β (Aβ) accumulation starts, has significantly reduced total Aβ and tau brain levels with a significant improvement in mouse cognitive behavior. This reduction in brain Aβ was explained by the enhanced Aβ clearance pathways and reduced brain production of Aβ via modulation of APP processing. On the other hand, although feeding mice with EVOO-enriched diet for 3 months, beginning at an age after Aβ accumulation starts, showed improved clearance across the BBB and significant reduction in Aβ levels, it did not affect tau levels or improve cognitive functions of TgSwDI mouse. Collectively, results of this study suggest the long-term consumption of EVOO-containing diet starting at early age provides a protective effect against AD and its related disorder CAA.
Keywords: Amyloid-β, blood–brain barrier, clearance, extra-virgin olive oil, Tau
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder of the elderly that afflicts about 30 million patients globally [1]. Although the pathogenesis of AD is complex, it involves two well-defined pathologies, amyloid-β (Aβ) and tau-related neuropathologies [2]. Amyloid-related neuropathological alterations in the brain are due to accumulation and deposition of Aβ peptides [3]. Aβ peptides are derived from proteolytic processing of the amyloid-β precursor protein (APP). Amyloid peptides, mainly Aβ40 and Aβ42, accumulate in the parenchymal tissue and the vasculature of cortical and hippocampal regions of the brain where they assemble and form insoluble plaques, a major hallmark found in the brains of AD patients, as well as soluble oligomers [3,4]. The tau-related neuropathological alterations are stimulated by somatodendritic buildup of hyper-phosphorylated tau, which prevents tau assembly onto microtubules and results in intracellular insoluble neurofibrillary tangles (NFTs) [5].
Dramatic rise in AD prevalence and the lack of options for its effective treatment urge searching for effective strategies for early prevention or delaying the onset of AD. Diet is a probable risk factor that by modification could possibly reduce or delay the onset of AD [6,7]. Several epidemiological and clinical studies suggested that adherence to Mediterranean diet improves cognitive function and slows the progression of AD [6–8]. Daily consumption of extra-virgin olive oil (EVOO) is one of the characteristic elements of a Mediterranean diet [9–11]. Dietary consumption of EVOO ranges from 40 to 50 g/day with the highest daily intake among Greeks [9, 12]. According to European regulations (European commission, 2003), EVOO is defined as a high quality olive oil that is obtained from the first pressing of olive fruit by purely mechanical means. The composition of EVOO is primarily glycerol fraction (~95%) and non-glycerol fraction (~5%) [13]. The glycerol fraction is rich in monounsaturated fatty acids (MUFA) while the non-glycerol fraction contains phenolic compounds that account for EVOO resistance to oxidative rancidity [14]. It has been estimated that the total phenolic content of EVOO is about 500 mg/kg and includes over 30 chemical substances belonging to different classes, such as esters, acids, and volatile compounds [9].
Previous studies demonstrated the ability of EVOO to modify cellular membrane structure and to reduce oxidative modifications [15,16]. In addition, recent human, animal, and in-vitro studies have shown that the phenolic compounds contained in EVOO are antioxidant molecules that are able to scavenge the toxic effects of oxygen metabolism such as free radical formation, thus protecting cells against oxidative damage [15] and contribute significantly to the potential health improving properties of the Mediterranean diet [14].
As a part of Mediterranean diet, several animal studies suggested that consumption of EVOO enhances behavior and cognition of tested animals [17–19]. In addition, animal and in-vitro studies have shown that phenolic compounds of EVOO possess important biological activities against AD [17,18, 20–22]. A recent study reported that feeding SAMP8 mice with EVOO and its phenolic extract was able to improve learning and memory deficits related to the age-related overproduction of Aβ in this mouse model [17]. The authors of this study attributed EVOO beneficial effects on learning and memory deficits to EVOO and its phenolic compounds’ potential to reverse oxidative damage in the brains of SAMP8 mice [17]. In line with their findings, another study showed EVOO consumption to decrease glutathione reductase activity and expression in the brains of aged rats [18]. Moreover, several naturally occurring phenolic compounds that found in the phenolic fraction of EVOO such as oleocanthal and oleuropin have shown to modulate Aβ and tau pathogenesis [20, 23].
The mechanism(s) by which EVOO exert its beneficial effects on cognitive function are still elusive. Thus, in this study, we aimed to assess the effect of chronic consumption of EVOO on AD and CAA progression in TgSwDI mice. To our knowledge, this study is the first to investigate the mechanism by which EVOO exert its beneficial effects on the pathogenesis of AD and cerebral amyloid angiopathy (CAA).
2. Materials and Methods
2.1. Animals
TgSwDI mice were kindly provided by Dr. Jeffrey Keller (Pennington Biomedical Research Center). Mice were housed in plastic cages under standard conditions, 12-h light/dark cycle, 22°C, 35% relative humidity, and ad libitum access to water and food. The TgSwDI mice express human APP under control of Thy 1.2 neuronal promoter harboring double Swedish mutations and the Dutch and Iowa vasculotropic Aβ mutations [24]. In humans, the APP Swedish mutations are responsible for an increased production and deposition of the Aβ40/42 peptides [25], whereas the Dutch and Iowa APP mutations, which occur at positions 22 and 23 of the Aβ peptide, respectively, results in massive accumulation of Aβ in the cerebrovasculature of patients causing vascular fragility with cerebral hemorrhages and dementia [26,27]. In the brain of TgSwDI mice, Aβ begins to accumulate at age 2 to 3 months and deposits extensively at age of 12 months [24]. All animal experiments and procedures were approved by The Institutional Animal Care and Use Committee of the University of Louisiana at Monroe and according to the National Institutes of Health guidelines.
2.2. Animals’ feeding
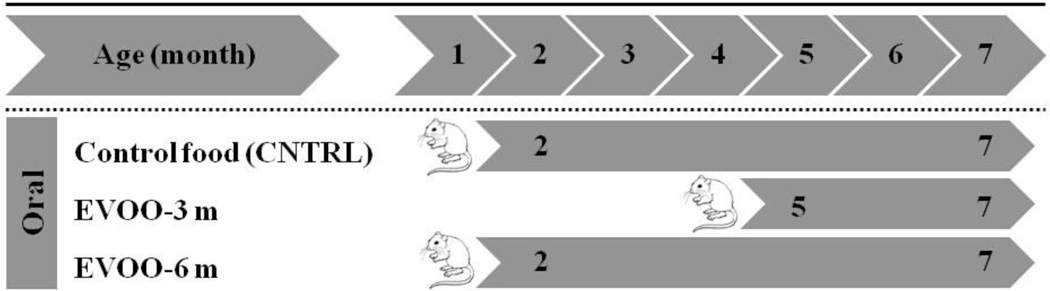
EVOO used in the feeding studies was of the Daily Chef Extra-Virgin Olive Oil from Italy (Lot # L022 RE-56), which contains 71 mg/kg oleocanthal and 3.8 mg/kg oleuropein aglycone as determined by HPLC and Mass spectrometry using a previously reported method [28]. To investigate the effect of dietary consumption of EVOO-enriched food, three groups of TgSwDI mice with 10–12 male mice in each group were assigned to different diets. Scheme 1 summarizes the treatment schedule for all groups. The first group acted as control and fed with regular powdered diet (Teklad Laboratory diets, Harlan Laboratories, Madison, WI; CNTRL group, n=12). The second group fed with EVOO-enriched powdered diet beginning at age of 4 months continuing for 3 months to age 7 months (EVOO-3m group, n=10). The last group fed with EVOO-enriched diet beginning at age 1 month continuing for 6 months to age 7 months (EVOO-6m group, n=12). The EVOO-enriched diet was prepared by mixing EVOO with powdered diet to produce a dose of 0.7 g/day that is equivalent to dietary intake of EVOO in Greek population (50 g/day) [9]. Diet was changed every other day to maintain freshness. The quality of the used EVOO was confirmed using high-resolution ESI-MS experiment that conducted by JEOL JMS-T100 LP AccuTOF LC-Plus, equipped with an ESI source (JEOL Co. Ltd., Tokyo, Japan) (Supp. Fig. 1). During the treatment period with EVOO, the animals were checked for their general health status every day and body weight was measured every 2 weeks. The body weight was not affected by dietary manipulations and were as follows: 27.5±1.1, 28.2±1.3, and 26.9±1.7 gm for CNTRL, EVOO-3m and EVOO-6m mice, respectively, as measured at the end of treatment or feeding. Unchanged body weights between different feeding groups indicate that dietary intake of EVOO-enriched diet did not affect the caloric intake. At the end of treatment period of each group, behavioral testing was performed. After behavioral studies, each treatment group was randomly divided either for brain clearance studies or for immunochemical and molecular analyses.
Scheme 1.
Schematic presentation of the time, age, number and study groups involved in the assessment of the effect of EVOO consumption on Aβ pathology in the brain of TgSwDI mice.
2.3. Extraction of Aβ from the brain
To quantitatively extract total Aβ from the brain tissues, a three-step serial extraction procedure was used as descried previously [29]. Briefly, 150 mg of brain tissue was polytron homogenized in DEA buffer (50 mM NaCl, 0.2% diethylamine, with complete mammalian protease inhibitor (Sigma-Aldrich, MO) and centrifuged at 21,000×g for 45 min at 4°C. Supernatant (DEA fraction) was collected and the pellet was re-extracted with 0.5 ml of 2% sodium dodecylphosphate (SDS; Sigma-Aldrich) and centrifuged as described above. Again, the supernatant (SDS fraction) was collected and pellet was re-extracted with 0.5 ml of 70% formic acid (FA) and centrifuged for 2 h at 21,000×g at 4°C. The supernatant (FA fraction) was collected without disrupting the pellet or floating lipid layer. All fractions were stored at −80°C until the time of analysis. The total amount of Aβ in the brain was calculated by summing the amount of Aβ in all fractions.
2.4. Human Aβ40 and Aβ42 specific ELISA
Human Aβ40 and Aβ42 levels (pmol/g tissue) were determined in each fraction by two-site sandwich ELISA. Before ELISA assay, the DEA fraction was neutralized (1:10) with 0.5 M Tris-HCl buffer (pH 6.2). The SDS fraction was diluted (1:20) with antigen capture buffer (0.02 M sodium phosphate buffer (pH 7), 0.4 M NaCl, 2 mM EDTA, 0.4% Block Ace (Serotec, NC), 0.2% BSA, 0.05% CHAPS, and 0.05% NaN3). FA fraction was neutralized by 1:20 dilution in TP buffer (1 M Tris base, 0.5 M Na2HPO4), followed by further dilution (1:1) in antigen capture buffer. For ELISA analyses, rabbit anti-Aβ40 (Millipore, CA, Cat# AB5737) and rabbit anti-Aβ42 (Calbiochem, CA, Cat# PC150) monoclonal antibodies specific against the C-termini of human Aβ40 and Aβ42, respectively, were used as the capturing antibodies. Antibodies were coated at 5 µg/ml (100 ng/well) on a Maxisorp ELISA plate (Thermo Scientific, IL) to capture Aβ. Detection was achieved with HRP-conjugated 6E10 (Covance Research Products, MA) monoclonal antibody specific against N-terminus of human Aβ (aa. 3–8 human Aβ sequence) at 1 µg/ml. Standard curves of either Aβ40 or Aβ42 were prepared in the same buffer as the samples. The assessment of Aβ oligomers in DEA and SDS fractions, all-size oligomers sandwich ELISA assay was used as described previously [30]. 6E10 antibody was used to capture Aβ oligomers at 5 µg/ml concentration. Detection was performed by HRP-conjugated 6E10 antibody at 1 µg/ml. In this assay, soluble Aβ oligomers captured by 6E10 can be detected by HRP-conjugated 6E10 only if there is at least one more accessible epitope for the detecting HRP-conjugated 6E10 antibody on Aβ oligomers. All standards and samples were run at least in triplicate.
2.5. Immunohistochemical analyses
For detection of total Aβ load, formaldehyde-fixed cryostat brain slices (15 µm) were treated with 70% formic acid for 10 min at room temperature and blocked for 1 h with 10% normal donkey serum in PBS containing 0.1% Triton X-20. For total Aβ detection brain slices were immunostained with 6E10 human-specific anti-Aβ antibody at 1:200 dilution followed by fluorescin-conjugated donkey anti-mouse IgG (Santa Cruz Biotechnology Inc., TX). For detection of Aβ-plaque load in hippocampus, the brain tissue sections were stained with a filtered 1% Thioflavin-S (Sigma-Aldrich, MO) solution in 80% ethanol for 15 min as described previously [31]. Images were captured using Nikon Eclipse Ti-S inverted fluorescence microscope (Norcross, GA) at total magnification of 40×. For each treatment, images acquisition was performed in 6 groups of tissue sections spanning the hippocampus, each separated by 150 µm and each containing three 15-µm sections (total of 18 sections per mouse). Quantification of total Aβ load and Aβ-plaque load in the hippocampus was performed using ImageJ version 1.44 software after adjusting for threshold (Research Services Branch, NIMH/NIH, Bethesda, MD). Total Aβ load in the hippocampus was measured as a percentage of Aβ-covered area and Aβ-plaque load was expressed as the total number of Aβ-plaque.
To assess co-localization of Aβ immunoreactivity with brain microvessels, hippocampal brain sections were fixed and blocked, as described above, then probed by dual immunohistochemical staining for collagen-IV and Aβ with rabbit anti-collagen-IV antibody (Millipore, CA) at 1:200 dilution and 6E10 at 1:200, respectively. The secondary antibodies used were CFL594-conjugated donkey anti-rabbit IgG (Santa Cruz Biotechnology, Inc.) and fluorescein-conjugated donkey anti-mouse IgG for collagen-IV and Aβ, respectively. Images were captured as described above but at a total magnification of 100×. Quantification of colocalization of Aβ with collagen-VI was performed using ImageJ after adjusting for threshold of each fluorophore. Cerebrovascular deposition of Aβ in hippocampus was expressed as a % of Aβ-immunoreactive microvessels [32].
2.6. Brain clearance of 125I-Aβ40
In-vivo 125I-Aβ40 clearance was investigated using the brain clearance index (BCI) method as described previously [33]. Because Aβ40 and Aβ42 share the same clearance pathways [34,35], in this study, Aβ40 was used in the BCI experiments for practicality reasons as it has much faster clearance rate than Aβ42 [36]. In brief, a stainless steel guide cannula was implanted stereotaxically into the right caudate nucleus of mice brains that had been anesthetized with IP xylazine and ketamine (20 and 125mg/kg, respectively) (Henry Schein Inc., NY). A tracer fluid (0.5 µl) containing 125I-Aβ40 (30 nM, PerkinElmer, MA) and 14C-inulin (0.02 mCi, American Radiolabeled Chemicals, MO) prepared in extracellular fluid buffer (ECF) was micro injected. Thirty minutes later, brains were rapidly collected. One hemisphere of the brain was used for 125I-Aβ40 analysis and the second hemisphere was used for microvessel isolation as described below. Calculations of 125I-Aβ40 clearance were performed as described previously [33]. Using trichloroacetic acid (TCA) precipitation assay, intact (precipitate) and degraded (supernatant) 125I-Aβ40 were determined in brain tissues using a Wallac 1470 Wizard Gamma Counter (PerkinElmer). 14C-inulin in the precipitate and supernatant were also determined using a Wallac 1414 WinSpectral Counter (PerkinElmer). The 125I-Aβ40 Brain clearance index (BCITotal(%)), Clearance of 125I-Aβ40 across BBB (BCIBBB(%)), and brain degradation (BCIDegradation(%)) were determined as described previously [33].
2.7. Brain microvessels isolation
Brain microvessels were isolated as described previously [37]. Each brain hemisphere was homogenized in ice-cold DPBS followed by the addition of one volume of 30% Ficoll 400 (Sigma-Aldrich). Homogenates were centrifuged at 8000×g for 10 min and the resulting pellets were suspended in ice-cold DPBS containing 1% BSA and passed over a glass bead column to collect microvessels adhering to the glass beads. Isolated microvessels were used to determine P-glycoprotein (P-gp) and low density lipoprotein receptor-related protein-1 (LRP1) expressions by western blot.
2.8. Western blot analysis
Protein extracts were prepared from brain microvessels (n=4–6 mice), or brain tissues (n=4–6 mice) with RIPA buffer containing 1× complete mammalian protease inhibitor mixture followed by centrifugation at 21,000×g for 1 h at 4°C. The supernatant was collected as protein extract and stored at −80°C to the time of analysis. Protein concentrations were determined by the BCA method. For western blot analysis, 25 µg of protein was resolved on 8% bis-tris gels in 3-(N-morpholino) propanesulfonic acid buffer system and electrotransferred onto a 0.45 µm nitrocellulose membrane. Membranes were blocked with 2% BSA and incubated overnight with monoclonal antibodies for P-gp (C-219; Covance Research Products, MA), LRP1 (Calbiochem, CA), IDE, NEP, ABCA1, ApoE, LXR, RXR, PPARγ or GAPDH (Santa Cruz, TX). Specific antibodies against sAPPα and sAPPβ were obtained from immune-Biological laboratories (IBL, MN). Tau Ab-2 antibody (clone Tau-5; Thermo Scientific, IL) was used for total Tau detection. Phosphospecific antibodies were used for detection of Tau phosphorylated at serine-214, serine-262, threonine-212 and threonine-231 (Signalway, MD). For detection, the membranes were washed free of primary antibody and incubated with HRP-labeled secondary IgG anti-mouse antibody for P-gp, ABCA1, Tau Ab-2, sAPPα, and GAPDH (Santa Cruz, TX); anti-rabbit antibody for LRP1, NEP, LXR, RXR, PPARγ, sAPPβ, and phosphorylated Tau (Santa Cruz, TX); and anti-goat antibody for ApoE and IDE (Santa Cruz, TX). The bands were visualized using a Pierce chemiluminescence detection kit (Thermo Scientific, IL). Quantitative analysis of the immunoreactive bands was performed using Li-Core luminescent image analyzer (LI-COR Biotechnology, NE) and band intensity was measured by densitometric analysis. Three independent Western blotting experiments were carried out for each treatment group.
2.9. Behavioral testing
At the end of treatment period, TgSwDI mice of all groups were subjected to burrowing and nest construction behavioral testing as described previously [38]. For the burrowing test, a burrow was assembled from plastic downpipe, 68 mm diameter, cut into 20 cm long length, sealed from one end by plastic cap and elevated 3 cm off the floor from the other end by machine screws. Burrow was filled with 200 gm of standard food pellets and placed in a cage. One mouse was placed in the cage with the burrow at 4 pm and allowed to burrow overnight. The weight of the remaining food pellets inside the burrow was measured to calculate the weight of burrowed food pellets. Two experiments 48 h apart were performed for each mouse.
To assess nest construction, mice were individually placed in their home cages with a “Nestlet”, a 5 cm square of pressed cotton batting about one hour before the dark phase. The nests were assessed the next morning on a 5-point scale based on assigned nest construction score as reported previously [39]. Similar to the burrowing test, two experiments 48 h apart were performed for each mouse.
2.10. Statistical analysis
Unless otherwise indicated, the data were expressed as mean ± SEM. The experimental results were statistically analyzed for significant difference using two-tailed Student’s t-test for 2 groups, and one-way analysis of variance (ANOVA) for more than two group analysis. Values of P<0.05 were considered statistically significant.
3. Results
3.1. EVOO consumption slows Aβ pathology in TgSwDI mice
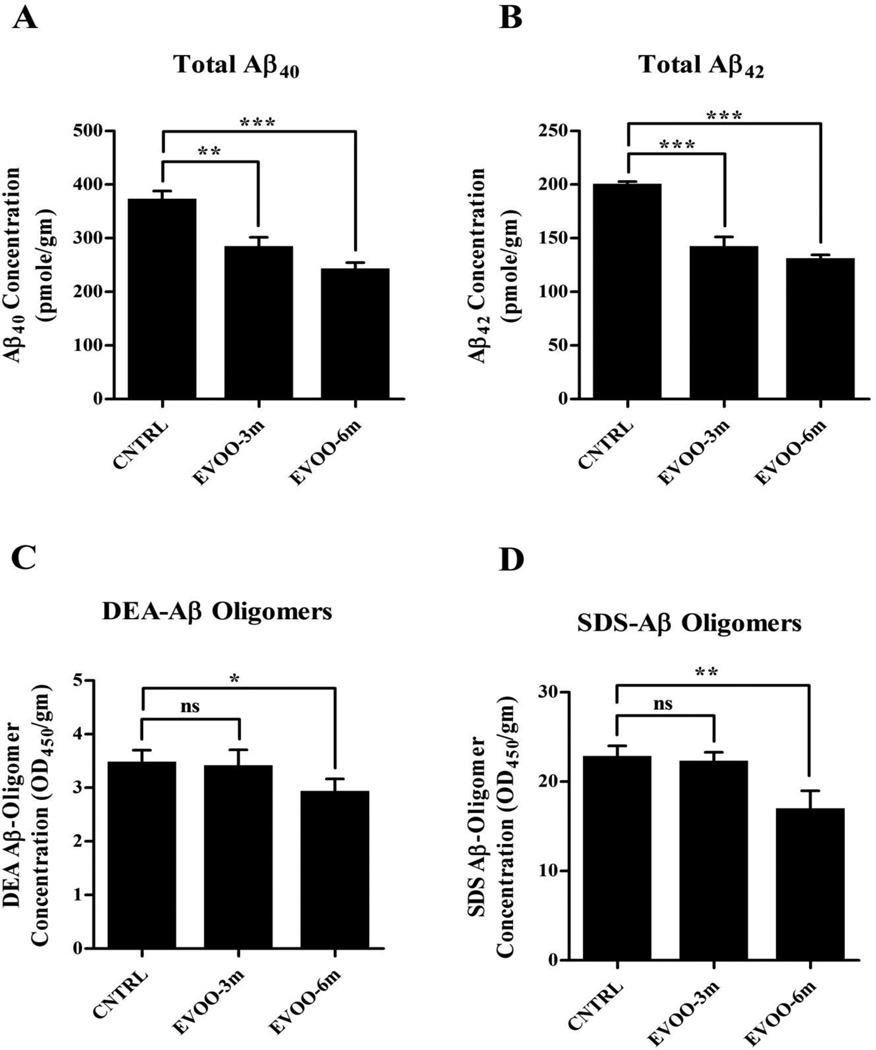
Figure 1A and B show that compared to control diet, three and six months of EVOO consumption resulted in a significant reduction in Aβ burden in the mice brains. Six months treatment with EVOO (EVOO-6m group) significantly reduced total (i.e. DEA+SDS+FA) Aβ40 and Aβ42 by 34% and 36%, respectively. Similarly, total Aβ40 and Aβ42 levels in the brains of EVOO-3m group reduced significantly by 24% and 29%, respectively. Reduction in Aβ40 and Aβ42 levels was mainly associated with a decrease in the formic acid fraction content of Aβ in both EVOO-enriched diet groups (Supp. Fig. 2). However, only EVOO-6m showed a significant reduction in the DEA and SDS fractions content of Aβ40 (27% and 36% respectively, Supp. Fig. 2A and C) and Aβ42 (59% and 36% respectively, Supp. Fig. 2A and D). Consistent with these results, only DEA and SDS fractions obtained from EVOO-6m mice contained significantly less Aβ oligomers (by 15% and 26%, respectively) compared to control mice (Fig. 1C and D).
Figure 1.
EVOO reduces amyloid burden in the brains of TgSwDI. ELISA quantitative analysis of the levels of human Aβ40 (A) and Aβ42 (B) in the brain of TgSwDI mice after consumption of EVOO. Consumption of EVOO-enriched diet for 3 months beginning at age 4 months or for 6 months beginning at age of 1 month significantly reduced total Aβ40 and Aβ42 levels in the brain of TgSwDI mice. All-size Aβ-oligomer ELISA analysis of DEA (C) and SDS (D) fractions show a significant reduction of Aβ oligomers content of both fractions only in EVOO-6m group. Data is presented as mean±SEM of 4–6 mice in each group (ns, not significant; * P<0.05, ** P<0.01 and *** P<0.001).
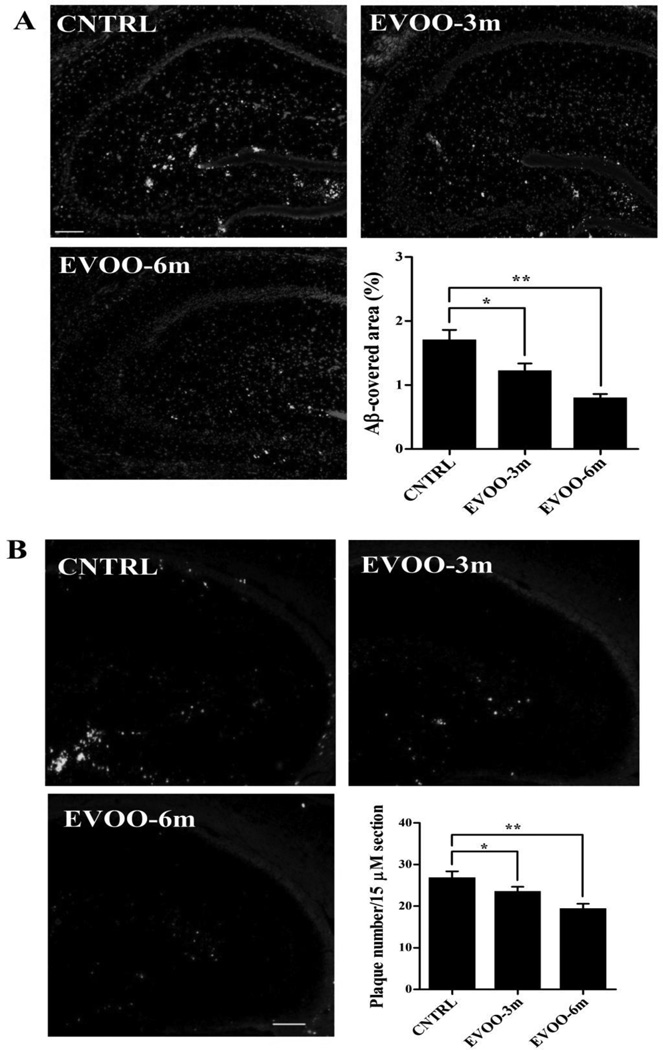
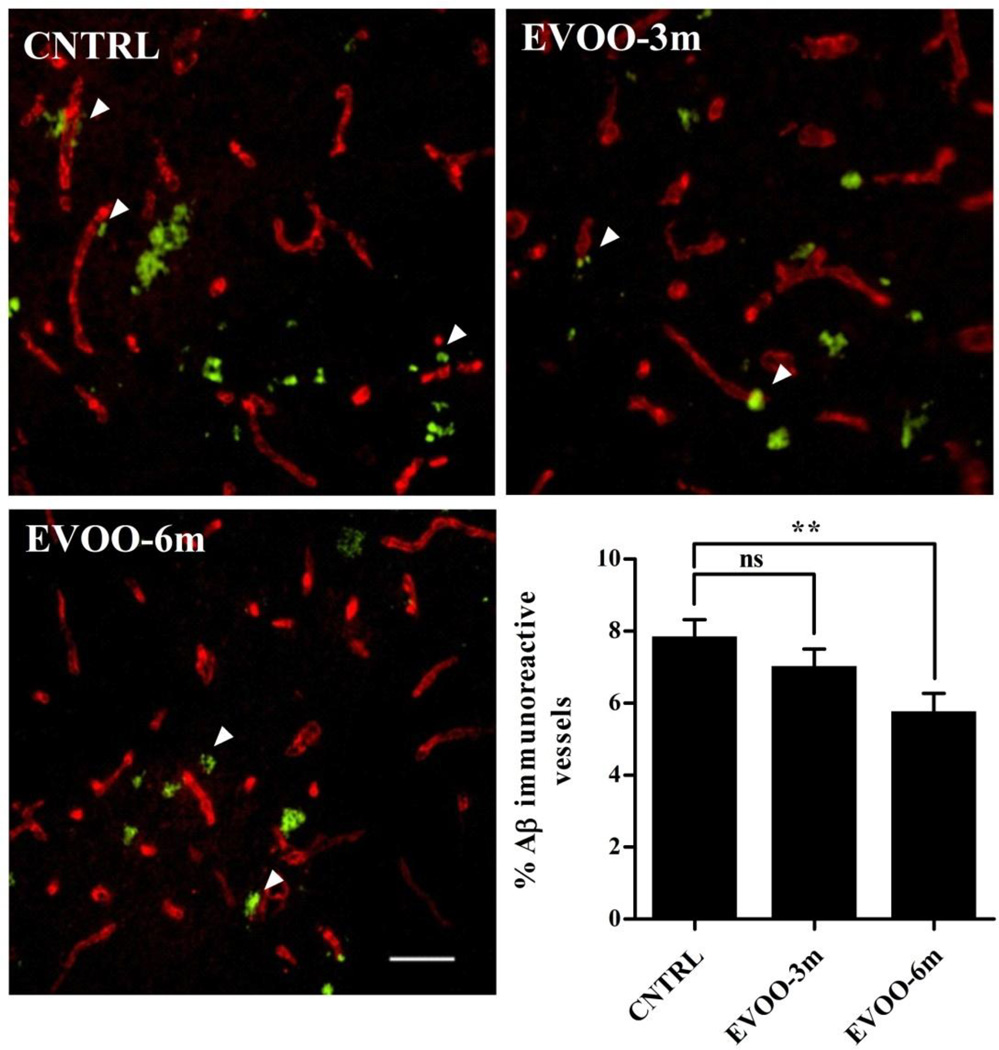
Immunostaining analysis of total Aβ was performed using 6E10 antibody, and Aβ plaques using Thioflavin-S. Consistent with the ELISA results of Aβ in whole brain, significant reductions in total Aβ by 29% and 53%, and in Aβ plaques number by 15% and 30% were observed in the hippocampi of EVOO-3m and EVOO-6m mice, respectively (Fig. 2). In addition, immunohistochemical analysis of Aβ co-localization with collagen-IV (a marker of microvessels) in the hippocampus revealed a significant reduction in the percentage of Aβ-immunoreactive microvessels by 26% in EVOO-6m mice (Fig. 3). However, while the EVOO-3m mice showed a trend toward reduction in Aβ-immunoreactive microvessels, the difference between control and EVOO-3m groups failed to reach statistical significance (Fig. 3).
Figure 2.
EVOO consumption reduces Aβ burden in the hippocampus of TgSwDI mice. (A) Representative hippocampus sections stained with 6E10 (green) antibody against Aβ to detect Aβ load and DAPI (blue) to stain nuclei. (B) Representative hippocampus sections stained with thioflavin-S to detect Aβ-plaque burden. Quantitative analysis of total Aβ load (A) and Aβ plaque load (B) showed a significant reduction in Aβ load (measured as a % of Aβ-covered area) and Aβ plaque load (measured as number of the plaque per 15 µm section) in the hippocampus of all EVOO-fed mice. Data is presented as mean±SEM of 4–6 mice in each group (* P<0.05 and ** P<0.01). Scale bar, 50 µm.
Figure 3.
Immunohistochemical analysis of cerebrovascular Aβ deposition in the hippocampus of CNTRL (control), EVOO-3m and EVOO-6m TgSwDI mice. Representative hippocampus sections from all treatment groups were double-immunostained with 6E10 for detection of Aβ (Green) and collagen-VI to identify brain microvessels (Red). Quantification of cerebrovascular Aβ deposition (CAA pathogenesis) as a % of Aβ-immunoreactive vessels demonstrated a significant reduction in microvascular Aβ deposits only in the hippocampus of EVOO-6m group. Data is presented as mean±SEM of 4–6 mice in each group. Scale bar, 50 µm (ns, not significant; ** P<0.01).
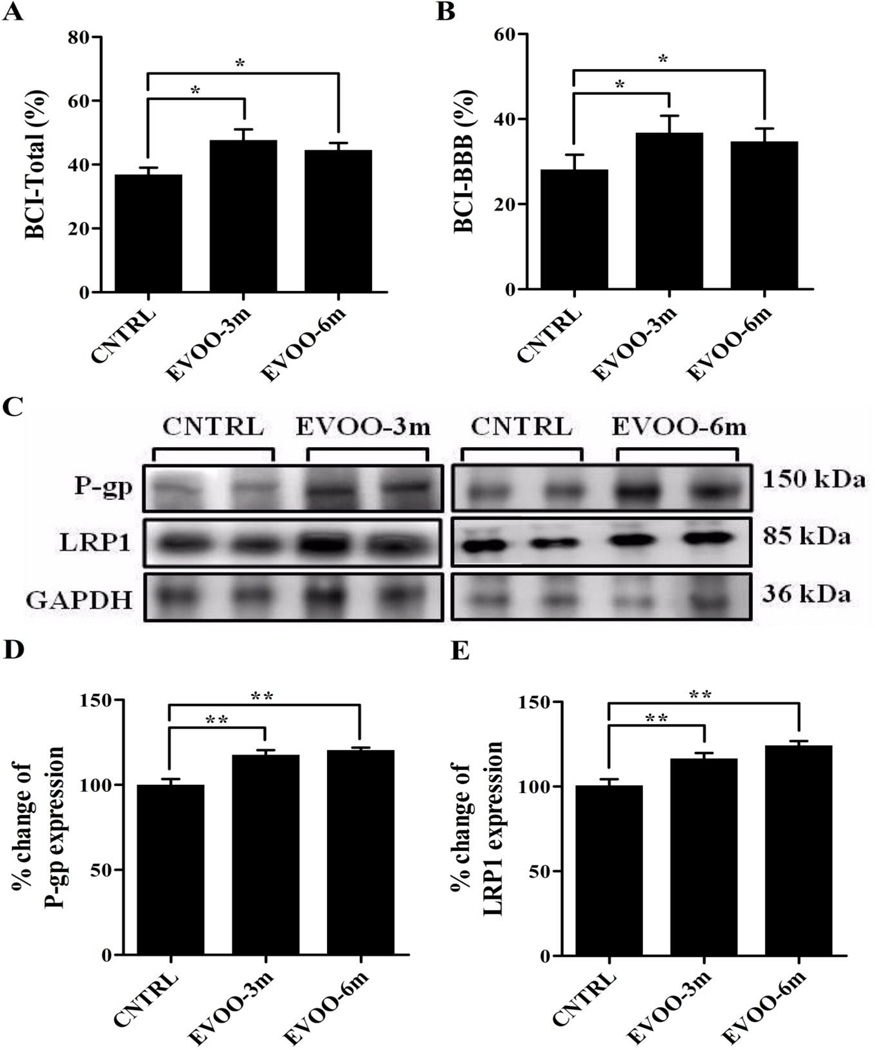
3.2. EVOO-enriched diet enhances 125I-Aβ40 clearance across the BBB
BCI studies revealed significant increase in brain 125I-Aβ40 clearance in EVOO-3m and EVOO-6m groups (Fig. 4). The increase in total Aβ clearance (BCITotal(%); Fig. 4A) was in part due to enhanced removal of Aβ across the BBB where mice treatments caused a significant increase in 125I-Aβ40 clearance across the BBB (BCIBBB(%)) by ~12% (Fig. 4B); while brain 125I-Aβ40 degradation (BCIDegradation(%)) was comparable for all groups (Supp. Fig. 3A). To explain the enhanced clearance of 125I-Aβ40 across the BBB, P-gp and LRP1 proteins expression were examined in the capillaries of the mice brains. Consistent with increased 125I-Aβ40 clearance across the BBB, a significant increase over controls in the expressions of P-gp and LRP1 was observed in both feeding groups. Figures 4C–E show a significant increase by 18% and 20% in P-gp expression, and 16% and 24% in LRP1 expression in the brain capillaries of EVOO-3m and EVOO-6m mice, respectively.
Figure 4.
In-vivo clearance of 125I-Aβ40 from brain of TgSwDI mice. Enhanced total brain clearance (BCI-Total (%)) (A) and BBB clearance (BCI-BBB (%)) (B) of 125I-Aβ40 was observed in all EVOO-fed mice. Increase in the brain clearance of 125I-Aβ40 was associated with up-regulation in the expressions of P-gp and LRP1 in the brains’ microvessels as shown in the representative blots (C) and densitometry analyses (D and E). Data is presented as mean±SEM of 4–6 mice in each group (* P<0.05 and ** P<0.01).
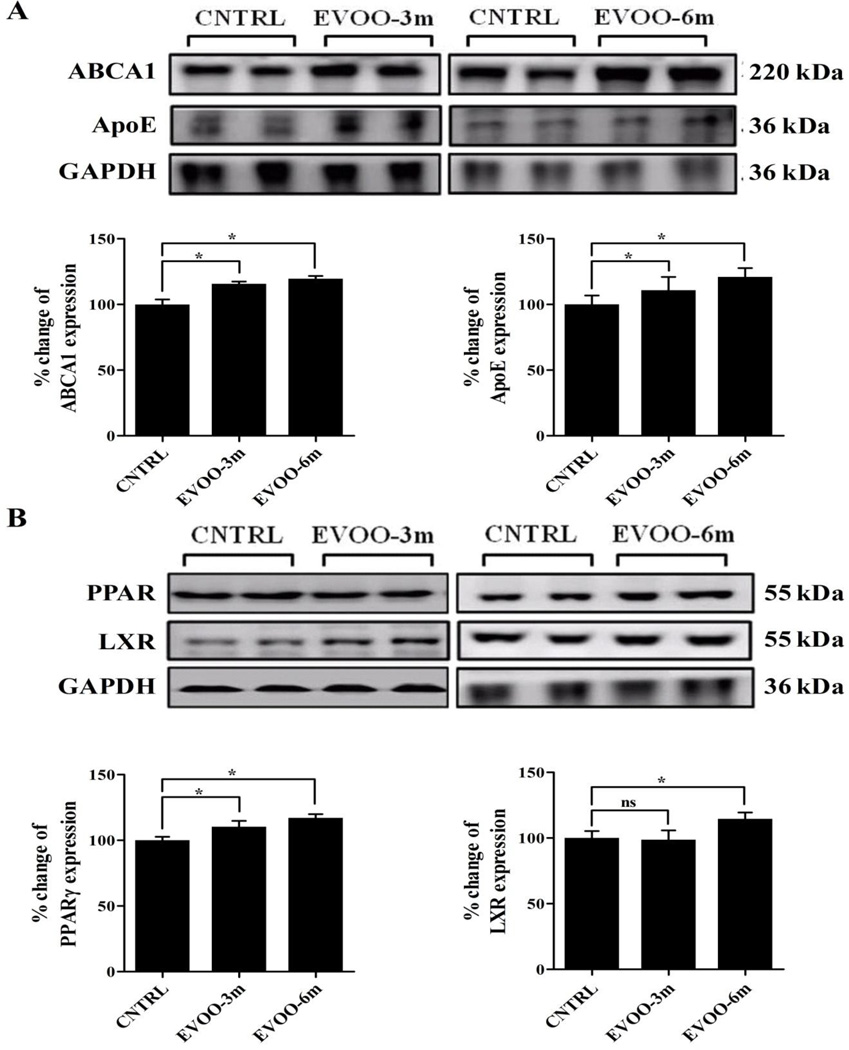
3.3. EVOO-enriched diet enhances ApoE-dependent pathway of Aβ clearance
Mice fed with EVOO-enriched diet showed elevated brain levels of ABCA1 and ApoE. ABCA1 expression increased by 15% and 20% in the brains of EVOO-3m and EVOO-6m mice, respectively (Fig. 5A). In addition to ABCA1, significant 12% and 21% increases in ApoE levels were observed in the brains EVOO-3m and EVOO-6m, respectively (Fig. 5A).
Figure 5.
EVOO-enriched diet stimulated the ApoE-dependent clearance pathway of Aβ through the activation of PPARγ. (A) Representative blots and densitometry analysis of ABCA1 and ApoE showed significant up-regulation in their expressions in the brain tissue of TgSwDI mice after consumption of EVOO-enriched diet. (B) Representative blot and densitometry analysis show significant induction in the expression of PPARγ in both groups while expression of LXR was enhanced only in EVOO-6m group. Data is presented as mean±SEM of 4–6 mice in each group (ns, not significant; and * P<0.05).
To explore the mechanism underlying these increase in the expression of ABCA1 and ApoE, we examined the expression of the ligand-activated nuclear receptors, peroxisome proliferator-activated receptor gamma (PPARγ) and liver-X receptors (LXRs). PPARγ and LXR act in a feed-forward manner to induce the expression of ABCA1 and ApoE [40]. Our results demonstrated significant increase in the expression of PPARγ by 12 and 18% in EVOO-3m and EVOO-6m, respectively (Fig. 5B). Only the EVOO-6m group showed a significant increase by 15% in the brain expression of LXR (Fig. 5B). Finally, we assessed the effect of the treatments on the expression of retinoid-X receptor (RXR). RXR is a nuclear receptor that forms obligate heterodimers with PPARγ and LXR [41]. In contrast to PPARγ and LXR, RXR brain level was not altered by any feeding paradigm (Supp. Fig. 3C).
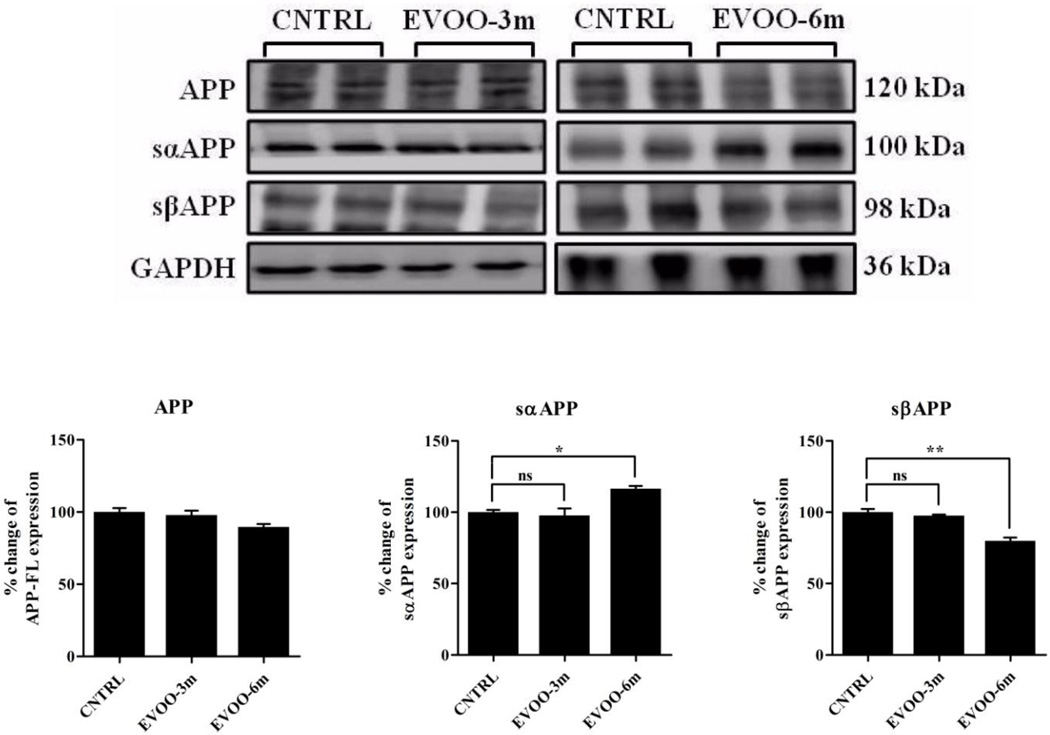
3.4. Consumption of EVOO-enriched diet for 6 months modulates APP processing
Effect of treatment on APP processing in the brains of TgSwDI mice was assessed by Western blot analysis. A significant increase by 18% and decrease by 21% in the levels of sAPPα and sAPPβ, respectively, were only observed in the EVOO-6m group (Fig. 6).
Figure 6.
Effect of EVOO consumption for 3 or 6 months on APP processing. Representative blots and densitometry analysis of full length APP (APP-FL), sAPPα and sAPPβ. EVOO-6m group demonstrated a significant modulation in the processing of APP with a reduction trend in the expression of full length APP, significant increase in sAPPα and significant decrease in sAPPβ. Data is presented as mean±SEM of 4–6 mice in each group (ns, not significant; * P<0.05 and ** P<0.01).
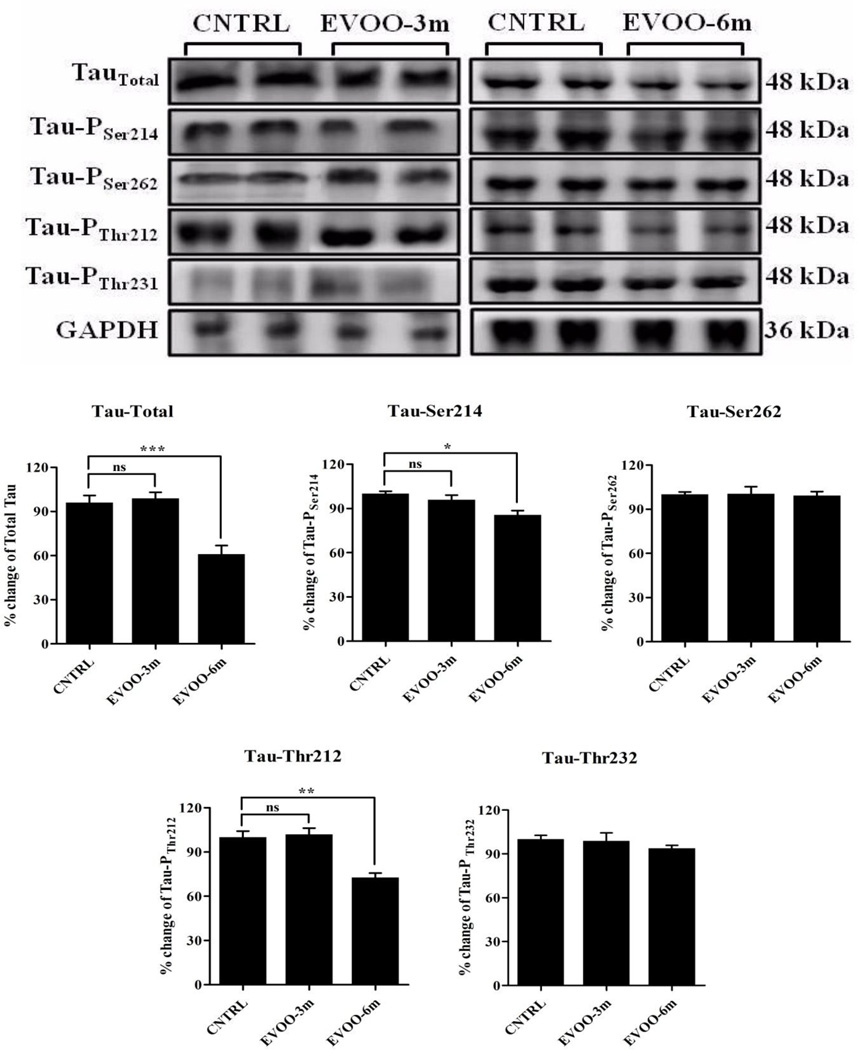
3.5. Effect of EVOO on tau expression and phosphorylation
Besides Aβ pathology, we investigated the effect of EVOO consumption on tau expression and phosphorylation in the brains of TgSwDI mice by Western blot using antibodies against total tau and different phosphorylation sites of tau, including serine-214, serine-262, threonine-212, and threonine-231. The results showed a significant reduction in total tau by 38%, and in phosphorylation by 15% and 27% at serine-214 and threonine-212 epitopes, respectively, in the brains of EVOO-6m group (Fig. 7). However, phosphorylation of tau at serine-262 and threonine-231 epitopes was not altered in the brains of EVOO-6m group (Fig. 7). EVOO-3m feeding has no effect on total tau expression and phosphorylation at the aforementioned epitopes (Fig. 7).
Figure 7.
Representative blots and densitometry analysis of total tau and tau phosphorylation at amino acid residues serine 214 and 262 and threonine 212 and 231. Significant reduction in total tau and its phosphorylation at amino acid residues serine 214 and threonine 212 were observed in EVOO-6m mice. Data is presented as mean±SEM of 4–6 mice in each group (ns, not significant; * P<0.05; ** P<0.01 and *** P<0.001).
3.6. Improvement in the hippocampal-dependent behavioral tests of EVOO-6m group
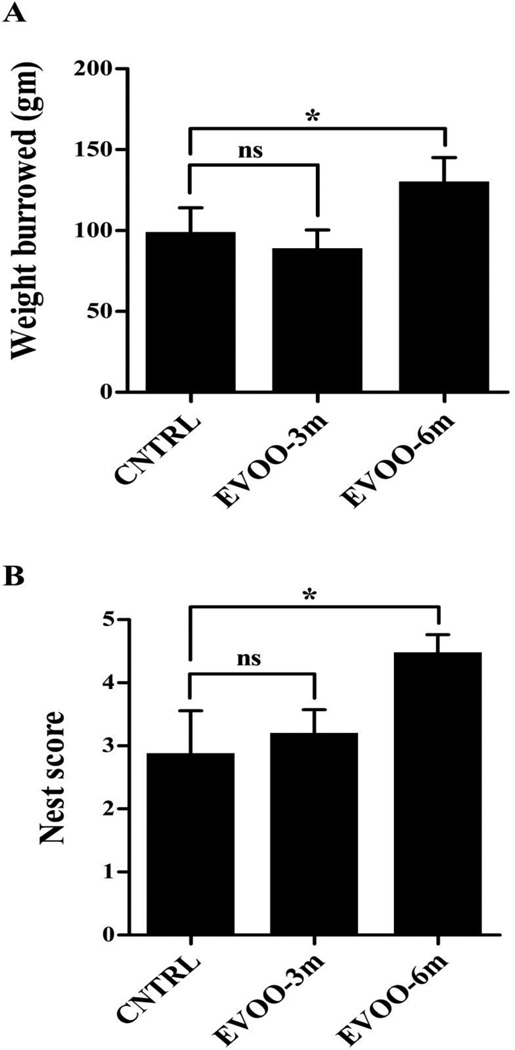
Consistent with global improvement of Aβ and tau related neuropathological alterations in the brains of EVOO-6m group, significant progress in behavioral performance of this group was observed in hippocampal-dependent behavioral tests. The Burrowing test showed that mice burrowed more food pellets (131.4 ± 4.3 gm) compared to the control group (99.3 ± 5.7 gm, p<0.05, Fig. 8A). In addition, the nest construction test showed a significantly higher score for mice of EVOO-6m group compared to the control group (4.5 ± 1.2 vs 2.8 ± 0.7, p<0.05, Fig. 8B). On the other hand, EVOO-3m groups did not show any improvement in the burrowing and nest construction tests compared to control groups (Fig. 8A and B).
Figure 8.
Burrowing (A) and nest construction (B) behavioral studies demonstrated positive effect of EVOO consumption in TgSwDI mice of EVOO-6m group only while EVOO-3mfeeding paradigm failed to rescue behavioral deterioration in TgSwDI mice. Data is presented as mean±SEM of 10–12 mice in each group (ns, not significant; * P<0.05 and ** P<0.01).
4. Discussion
The Mediterranean diet has been associated with lower risk of AD and other related cerebrovascular diseases [42, 43]. Available reports suggested that greater adherence to the Mediterranean diet enhanced cognitive function, reduced the risk of developing MCI, and reduced the risk of MCI conversion to AD [8]. Various aspects of Mediterranean diet have been hypothesized as possible determinants of these effects; however, one of the characteristic dietary habits in the Mediterranean is the daily consumption of EVOO [19, 44]. EVOO contains more than 30 phenolic compounds including oleocanthal and oleuropein aglycone that possess beneficial affects against both Aβ and tau pathologies [16, 20–23, 45]. Unlike EVOO, during the refining process to produce the refined olive oil, these phenolic compounds are usually lost [46,47]. Thus, in the current study, to clarify the mechanism(s) by which EVOO exert its beneficial effects on cognitive function we assessed the effect of EVOO consumption on the progression of amyloid and tau pathologies and cognitive functions in TgSwDI mice. TgSwDI mouse is a model of AD and CAA that exhibits early onset and robust Aβ pathology accompanied with cerebrovascular changes [24]. The effects of EVOO-enriched dietary modifications on Aβ and tau pathological alterations in the brains of TgSwDI mice were evaluated following two strategies. The first, namely EVOO-3m, involved initiating EVOO consumption at 4 months of age, when Aβ pathogenesis is invariably evident in the brains of TgSwDI mice [24]. The central purpose of this strategy was to assess the ability of EVOO consumption to reverse Aβ pathogenesis. In the second strategy, EVOO-6m, the effect of long-term consumption of EVOO started at an early age (1 month), before Aβ accumulation, was investigated as a strategy for early prevention or delayed onset of Aβ pathogenesis.
Our findings revealed that EVOO consumption in EVOO-3m and EVOO-6m groups have significantly reduced total Aβ40 and Aβ42 loads in the brains of TgSwDI mice. In the EVOO-6m group, EVOO consumption significantly decreased Aβ loads in all fractions, while in the EVOO-3m group animals Aβ levels were only reduced in the FA fraction. Consistent with reduction in total and least-soluble Aβ fractions (FA-fraction), mice treatment with EVOO for 3 and 6 months resulted in a significant reduction in hippocampal total and Aβ plaque loads. Interestingly, analysis of Aβ load within DEA and SDS fractions in the brains of EVOO-6m showed a significant reduction in Aβ oligomers within these fractions. This reduction in Aβ oligomers could relate, at least in part, to the enhanced Aβ clearance from the brain of TgSwDI mice as suggested by the BCI studies, and to the oligomer-forming inhibitory effect of EVOO and its phenolic compounds as suggested by a previous in-vitro study [48]. Although Aβ plaques are the most visible and well characterized amyloid-associated changes in AD and CAA brains, plaque loads correlate poorly with cognitive decline [49], with many elderly brains in postmortem showing Aβ plaque accumulation without cognitive decline before death [50]. More recent studies, however, reported Aβ oligomers as a central player in causing neurotoxicity and initiating pathological and cognitive disturbances that are consistent with those observed in AD [30, 51]. Collectively, these results suggest that EVOO could exert its protective effect against Aβ pathology by reducing the levels of toxic soluble Aβ oligomers and the amounts of various forms of insoluble Aβ in the brains of TgSwDI mice.
In an associated editorial, Knopman suggests that the Mediterranean diet may exert beneficial effects on maintenance of normal cognition through cerebrovascular mechanisms [52]. Results from current study suggest that chronic and early onset dietary intake of EVOO reduces Aβ deposition on the microvessels of the brain of TgSwDI, which could provide a mechanistic explanation for improvement in the cerebrovascular function that was associated with the Mediterranean diet. Cerebral levels of Aβ are regulated by the balance of brain production and clearance [53]. Although production of Aβ increases significantly in early-onset familial AD, mounting evidence suggests that Aβ accumulation in the brains of late-onset AD patients is related to its impaired clearance from the brain [54]. On the other hand, mutations in Aβ sequence, such as the Dutch and Iowa mutations, increase Aβ propensity to aggregate specifically on microvessels and decrease its cerebral clearance in patients with familial CAA [24, 55]. Therefore, reduction in cerebral clearance of Aβ is considered an important component accounting for its accumulation and the subsequent development of AD and CAA. Clearance of Aβ from the brain takes place by three pathways, transport across the BBB, brain degradation, and bulk flow of cerebrospinal fluid [56]. The contribution of the BBB was reported as the most significant pathway and was estimated to be 62% in mice [33]. Given this major contribution of the BBB to the total clearance of Aβ, enhancement of cerebral Aβ clearance across the BBB could effectively prevent Aβ brain accumulation and associated neuropathological alterations. This hypothesis was supported by findings of the BCI study where EVOO and oleocanthal treatments have significantly enhanced 125IAβ40 clearance across the BBB, which could, in part, explain the reduced Aβ load in the brains of TgSwDI mice. This increase in Aβ clearance was concomitant with a significant increase in P-gp and LRP1 levels expressed in the microvessels isolated from treated mice brains, which was also associated with reduced cerebrovascular deposition of Aβ. Interestingly, EVOO treatment was able to reduce brain levels of Dutch- and Iowa-mutated Aβ that have been recognized for their slow brain clearance [24, 55], which extends a beneficial effect not only for sporadic but also for familial AD and CAA.
ApoE-dependent Aβ clearance is a well-established pathway known for its high efficiency in cerebral Aβ removal [57, 58]. Recent study has shown the therapeutic agent bexarotene to modulate Aβ clearance in an apoE-dependent manner [59]. To investigate the effect of EVOO consumption on ApoE pathway, ApoE, ABCA1 and their regulatory receptors were quantified and compared to control group. ApoE and ABCA1 are transcriptionally regulated by the ligand-activated nuclear receptors, PPARγ and LXRs, which form obligate heterodimers with RXR [40]. Our findings demonstrated a significant increase in the expressions of ApoE, ABCA1 and PPARγ providing an additional mechanism that contributes to the decreased levels of Aβ in the brains of TgSwDI mice. These results are consistent with the findings of Zolezzi et al who reported that activation of PPARγ reduces Aβ levels and improves cognitive function in mouse models of AD [60], and with those of Cramer et al who reported that enhanced expression of PPARγ stimulates ApoE and ABCA1 expression and thus Aβ clearance [59]. The possible mechanism by which ApoE-mediated pathway enhances Aβ clearance could be related to activation of Aβ phagocytosis by macrophages and microglial cells and/or other clearance pathways [59, 60]. In addition to PPARγ up-regulation, EVOO-6m mice showed a significant increase in the expression of LXR, which is another stimulator for ApoE-dependent Aβ clearance pathway [59].
Besides the improved clearance of Aβ, APP processing was also modulated in the brains of EVOO-6m mice where EVOO treatment for 6 months was able to reduce sAPPβ, and increase sAPPα expressions demonstrating the stimulatory effect of EVOO on the non-pathogenic processing pathway of APP over its pathogenic pathway [1, 3]. This effect, however, was not observed with EVOO-3m group, which could be related to the short treatment period and/or the time at which treatment was initiated (4 months, after AD pathology is evident).
The levels of tau and its phosphorylation at specific sites were also examined. Tau is a microtubule-associated protein that accumulates in an abnormally hyper-phosphorylated state forming intracellular filamentous deposits in AD [5]. Tau promotes the assembly of tubulin into microtubules and stabilizes the microtubule structure that supports axoplasmic transport [5]. In AD, hyper-phosphorylation of tau at serine and threonine by several protein kinases decreases its interaction with microtubules, promoting their neurotoxic effects [5]. Our results revealed that, EVOO consumption for 6 months beginning at age of 1 month reduced total tau steady-state levels and reduced its hyperphosphorylation at the crucial epitopes serine-214 and threonine-212 in the brains of TgSwDI mice. Previous studies demonstrated a hierarchical relationship between Aβ and tau pathologies, with Aβ causing tau to accumulate and to undergo phosphorylation [61]. Therefore, the reduction observed here in the steady-state levels of tau and its phosphorylation in the brain of EVOO-6m mice could be closely related to the reduction seen in cerebral Aβ levels.
Consistent with the significant reduction in Aβ and tau levels, EVOO-6m mice showed significantly improved cognitive functions. It has been reported that mice with hippocampal dysfunction fail to burrow or to construct their own nest [39, 62], and our current findings indicated that feeding TgSwDI mice with EVOO supplemented diet at early age was able to slow the deterioration in their cognitive function. The burrowing and nest construction behavioral activities of EVOO-6m mice at the age of 7 months were comparable to those of untreated mice at 4 months age and were significantly better than the untreated control group of same age (7 month) suggesting that EVOO may prevent and/or slow the disease progression.
5. Conclusions
In conclusion, the results of this study suggest that EVOO consumption could provide a protective effect and/or slow AD pathology and behavioral progression when given at early age and for long time. According to our data, EVOO consumption exerts its positive effect via reduction of Aβ production, enhancement of Aβ clearance, and decrease of parenchymal and vascular deposits of Aβ as well as total tau and phosphorylation, which collectively could provide a protection mechanism against AD and CAA in humans. However, the possibility of using EVOO-rich diet to modulate human cognitive function remains to be confirmed in clinical studies.
Supplementary Material
Acknowledgments
This project was supported by a grant from the National Institute of General Medical Sciences (P20GM103424) from the National Institutes of Health.
Abbreviations
- AD
Alzheimer’s disease
- Aβ
amyloid-β
- APP
amyloid-β precursor protein
- BBB
blood-brain barrier
- DEA
diethylamine
- EVOO
extra-virgin olive oil
- IDE
insulin degrading enzyme
- LRP1
low density lipoprotein receptor-related protein-1
- MUFA
monounsaturated fatty acids
- NEP
neprilysin
- NFT
neurofibrillary tangles
- P-gp
P-glycoprotein
- RAGE
receptor for advanced glycation end products
- SDS
sodium dodecylphosphate
- TCA
trichloroacetic acid
- ThS
thioflavin-S
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have declared that no conflict interests exist.
References
- 1.Citron M. Alzheimer's disease: strategies for disease modification. Nat Rev Drug Discov. 2010;9:387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 4.Lehman EJ, Kulnane LS, Lamb BT. Alterations in beta-amyloid production and deposition in brain regions of two transgenic models. Neurobiol Aging. 2003;24:645–653. doi: 10.1016/s0197-4580(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 5.Iqbal K, Liu F, Gong CX, Alonso Adel C, Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009;118:53–69. doi: 10.1007/s00401-009-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu Y, Nieves JW, Stern Y, Luchsinger JA, Scarmeas N. Food combination and Alzheimer disease risk: a protective diet. Arch Neurol. 2010;67:699–706. doi: 10.1001/archneurol.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuck KL, Hayball PJ. Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem. 2002;13:636–644. doi: 10.1016/s0955-2863(02)00229-2. [DOI] [PubMed] [Google Scholar]
- 10.Salvini S, Sera F, Caruso D, Giovannelli L, Visioli F, Saieva C, et al. Daily consumption of a high-phenol extra-virgin olive oil reduces oxidative DNA damage in postmenopausal women. Br J Nutr. 2006;95:742–751. doi: 10.1079/bjn20051674. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra A, Maruthappu M, Stephenson T. Healthy eating: an NHS priority A sure way to improve health outcomes for NHS staff and the public. Postgrad Med J. 2014 doi: 10.1136/postgradmedj-2014-133103. [DOI] [PubMed] [Google Scholar]
- 12.Corona G, Spencer JP, Dessi MA. Extra virgin olive oil phenolics: absorption, metabolism, and biological activities in the GI tract. Toxicol Ind Health. 2009;25:285–293. doi: 10.1177/0748233709102951. [DOI] [PubMed] [Google Scholar]
- 13.Tripoli E, Giammanco M, Tabacchi G, Di Majo D, Giammanco S, La Guardia M. The phenolic compounds of olive oil: structure, biological activity and beneficial effects on human health. Nutr Res Rev. 2005;18:98–112. doi: 10.1079/NRR200495. [DOI] [PubMed] [Google Scholar]
- 14.Cicerale S, Lucas L, Keast R. Biological activities of phenolic compounds present in virgin olive oil. Int J Mol Sci. 2010;11:458–479. doi: 10.3390/ijms11020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vissers MN, Zock PL, Katan MB. Bioavailability and antioxidant effects of olive oil phenols in humans: a review. Eur J Clin Nutr. 2004;58:955–965. doi: 10.1038/sj.ejcn.1601917. [DOI] [PubMed] [Google Scholar]
- 16.Lopez S, Bermudez B, Montserrat-de la Paz S, Jaramillo S, Varela LM, Ortega-Gomez A, et al. Membrane composition and dynamics: a target of bioactive virgin olive oil constituents. Biochim Biophys Acta. 2014;1838:1638–1656. doi: 10.1016/j.bbamem.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Farr SA, Price TO, Dominguez LJ, Motisi A, Saiano F, Niehoff ML, et al. Extra virgin olive oil improves learning and memory in SAMP8 mice. J Alzheimers Dis. 2012;28:81–92. doi: 10.3233/JAD-2011-110662. [DOI] [PubMed] [Google Scholar]
- 18.Pitozzi V, Jacomelli M, Zaid M, Luceri C, Bigagli E, Lodovici M, et al. Effects of dietary extra-virgin olive oil on behaviour and brain biochemical parameters in ageing rats. Br J Nutr. 2010;103:1674–1683. doi: 10.1017/S0007114509993655. [DOI] [PubMed] [Google Scholar]
- 19.Pitozzi V, Jacomelli M, Catelan D, Servili M, Taticchi A, Biggeri A, et al. Long-term dietary extra-virgin olive oil rich in polyphenols reverses age-related dysfunctions in motor coordination and contextual memory in mice: role of oxidative stress. Rejuvenation Res. 2012;15:601–612. doi: 10.1089/rej.2012.1346. [DOI] [PubMed] [Google Scholar]
- 20.Abuznait AH, Qosa H, Busnena BA, El Sayed KA, Kaddoumi A. Olive-oil-derived oleocanthal enhances beta-amyloid clearance as a potential neuroprotective mechanism against Alzheimer's disease: in vitro and in vivo studies. ACS Chem Neurosci. 2013;4:973–982. doi: 10.1021/cn400024q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossi C, Rigacci S, Ambrosini S, Ed Dami T, Luccarini I, Traini C, et al. The polyphenol oleuropein aglycone protects TgCRND8 mice against Ass plaque pathology. PLoS One. 2013;8:e71702. doi: 10.1371/journal.pone.0071702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luccarini I, Ed Dami T, Grossi C, Rigacci S, Stefani M, Casamenti F. Oleuropein aglycone counteracts Abeta42 toxicity in the rat brain. Neurosci Lett. 2014;558:67–72. doi: 10.1016/j.neulet.2013.10.062. [DOI] [PubMed] [Google Scholar]
- 23.Beauchamp GK, Keast RS, Morel D, Lin J, Pika J, Han Q, et al. Phytochemistry: ibuprofen-like activity in extra-virgin olive oil. Nature. 2005;437:45–46. doi: 10.1038/437045a. [DOI] [PubMed] [Google Scholar]
- 24.Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, et al. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem. 2004;279:20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- 25.Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, et al. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992;1:345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- 26.Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, et al. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 27.Grabowski TJ, Cho HS, Vonsattel JP, Rebeck GW, Greenberg SM. Novel amyloid precursor protein mutation in an Iowa family with dementia and severe cerebral amyloid angiopathy. Ann Neurol. 2001;49:697–705. doi: 10.1002/ana.1009. [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Trujillo M, Gómez-Caravaca AM, Segura-Carretero A, Fernández-Gutiérrez A, Parella T. Separation identification of phenolic compounds of extra virgin olive oil from Olea europaea L. by HPLC-DAD-SPE-NMR/MS. Identification of a new diastereoisomer of the aldehydic form of oleuropein aglycone. J Agric Food Chem. 2010;58:9129–9136. doi: 10.1021/jf101847e. [DOI] [PubMed] [Google Scholar]
- 29.Murphy MP, Beckett TL, Ding Q, Patel E, Markesbery WR, St Clair DK, et al. Abeta solubility and deposition during AD progression and in APPxPS-1 knock-in mice. Neurobiol Dis. 2007;27:301–311. doi: 10.1016/j.nbd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Qosa H, LeVine H, 3rd, Keller JN, Kaddoumi A. Mixed oligomers and monomeric amyloid-beta disrupts endothelial cells integrity and reduces monomeric amyloid-beta transport across hCMEC/D3 cell line as an in vitro blood-brain barrier model. Biochim Biophys Acta. 2014;1842:1806–1815. doi: 10.1016/j.bbadis.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ly PT, Cai F, Song W. Detection of neuritic plaques in Alzheimer's disease mouse model. J Vis Exp. 2011 doi: 10.3791/2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Nostrand WE, Xu F, Rozemuller AJ, Colton CA. Enhanced capillary amyloid angiopathy-associated pathology in Tg-SwDI mice with deleted nitric oxide synthase 2. Stroke. 2010;41:S135–S138. doi: 10.1161/STROKEAHA.110.595272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qosa H, Abuasal BS, Romero IA, Weksler B, Couraud PO, Keller JN, et al. Differences in amyloid-beta clearance across mouse and human blood-brain barrier models: Kinetic analysis and mechanistic modeling. Neuropharmacology. 2014;79C:668–678. doi: 10.1016/j.neuropharm.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhnke D, Jedlitschky G, Grube M, Krohn M, Jucker M, Mosyagin I, et al. MDR1-P-Glycoprotein (ABCB1) Mediates Transport of Alzheimer's amyloid-beta peptides--implications for the mechanisms of Abeta clearance at the blood-brain barrier. Brain Pathol. 2007;17:347–353. doi: 10.1111/j.1750-3639.2007.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, et al. Clearance of Alzheimer's amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zlokovic BV, Yamada S, Holtzman D, Ghiso J, Frangione B. Clearance of amyloid beta-peptide from brain: transport or metabolism? Nat Med. 2000;6:718–719. doi: 10.1038/77397. [DOI] [PubMed] [Google Scholar]
- 37.Qosa H, Abuznait AH, Hill RA, Kaddoumi A. Enhanced brain amyloid-beta clearance by rifampicin and caffeine as a possible protective mechanism against Alzheimer's disease. J Alzheimers Dis. 2012;31:151–165. doi: 10.3233/JAD-2012-120319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deacon R. Assessing burrowing, nest construction, and hoarding in mice. J Vis Exp. 2012:e2607. doi: 10.3791/2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deacon RM. Assessing nest building in mice. Nat Protoc. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- 40.Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 41.Lefebvre P, Benomar Y, Staels B. Retinoid × receptors: common heterodimerization partners with distinct functions. Trends Endocrinol Metab. 2010;21:676–683. doi: 10.1016/j.tem.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63:1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scarmeas N, Luchsinger JA, Stern Y, Gu Y, He J, DeCarli C, et al. Mediterranean diet and magnetic resonance imaging-assessed cerebrovascular disease. Ann Neurol. 2011;69:257–268. doi: 10.1002/ana.22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopez-Miranda J, Perez-Jimenez F, Ros E, De Caterina R, Badimon L, Covas MI, et al. Olive oil and health: summary of the II international conference on olive oil and health consensus report, Jaen and Cordoba (Spain) 2008. Nutr Metab Cardiovasc Dis. 2010;20:284–294. doi: 10.1016/j.numecd.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Monti MC, Margarucci L, Tosco A, Riccio R, Casapullo A. New insights on the interaction mechanism between tau protein and oleocanthal, an extra-virgin olive-oil bioactive component. Food Funct. 2011;2:423–428. doi: 10.1039/c1fo10064e. [DOI] [PubMed] [Google Scholar]
- 46.Ramirez-Tortosa MC, Urbano G, López-Jurado M, Nestares T, Gomez MC, Mir A, Ros E, Mataix J, Gil A. Extra-virgin olive oil increases the resistance of LDL to oxidation more than refined olive oil in free-living men with peripheral vascular disease. J Nutr. 1999;129:2177–2183. doi: 10.1093/jn/129.12.2177. [DOI] [PubMed] [Google Scholar]
- 47.de la Torre-Carbot K1, Chávez-Servín JL, Jaúregui O, Castellote AI, Lamuela-Raventós RM, Nurmi T, Poulsen HE, Gaddi AV, Kaikkonen J, Zunft HF, Kiesewetter H, Fitó M, Covas MI, López-Sabater MC. Elevated circulating LDL phenol levels in men who consumed virgin rather than refined olive oil are associated with less oxidation of plasma LDL. J Nutr. 2010;140:501–508. doi: 10.3945/jn.109.112912. [DOI] [PubMed] [Google Scholar]
- 48.Pitt J, Roth W, Lacor P, Smith AB, 3rd, Blankenship M, Velasco P, et al. Alzheimer's-associated Abeta oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicol Appl Pharmacol. 2009;240:189–197. doi: 10.1016/j.taap.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer's disease from normal and pathologic aging. Exp Neurol. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- 50.Benzing WC, Mufson EJ, Armstrong DM. Alzheimer's disease-like dystrophic neurites characteristically associated with senile plaques are not found within other neurodegenerative diseases unless amyloid beta-protein deposition is present. Brain Res. 1993;606:10–18. doi: 10.1016/0006-8993(93)91563-8. [DOI] [PubMed] [Google Scholar]
- 51.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knopman DS. Mediterranean diet and late-life cognitive impairment: a taste of benefit. JAMA. 2009;302:686–687. doi: 10.1001/jama.2009.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 56.Wang YJ, Zhou HD, Zhou XF. Clearance of amyloid-beta in Alzheimer's disease: progress, problems and perspectives. Drug Discov Today. 2006;11:931–938. doi: 10.1016/j.drudis.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Kline A. Apolipoprotein E, amyloid-ss clearance and therapeutic opportunities in Alzheimer's disease. Alzheimers Res Ther. 2012;4:32. doi: 10.1186/alzrt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wildsmith KR, Holley M, Savage JC, Skerrett R, Landreth GE. Evidence for impaired amyloid beta clearance in Alzheimer's disease. Alzheimers Res Ther. 2013;5:33. doi: 10.1186/alzrt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, et al. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zolezzi JM, Bastias-Candia S, Santos MJ, Inestrosa NC. Alzheimer's disease: relevant molecular and physiopathological events affecting amyloid-beta brain balance and the putative role of PPARs. Front Aging Neurosci. 2014;6:176. doi: 10.3389/fnagi.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Deacon RM. Burrowing: a sensitive behavioural assay, tested in five species of laboratory rodents. Behav Brain Res. 2009;200:128–133. doi: 10.1016/j.bbr.2009.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.