Abstract
To investigate the effects of surfactant proteins A and D (SP-A, SP-D) in urinary tract infection (UTI), SP-A and SP-D double knockout (SP-A/D KO) and wild type (WT) C57BL/6 female mice were infected with uropathogenic Escherichia coli by intravesical inoculation. Compared with WT mice SP-A/D KO mice showed increased susceptibility to UTI as evidenced by higher bacterial CFU, more infiltrating neutrophils and severe pathological changes. Keratinocyte-derived chemokine increased in the kidney of WT mice but not in SP-A/D KO mice 24 h post-infection. Compared to control, level of IL-17 was elevated in the kidney of infected WT and SP-A/D KO mice and the level of IL-17 was higher in the infected SP-A/D KO mice than infected WT mice 24 and 48 h post-infection. Basal level of p38 MAPK phosphorylation in SP-A/D KO mice was higher compared to WT mice. Phosphorylated-p38 level was elevated in the kidney of WT mice post-infection but not in SP-A/D KO mice. Furthermore, in vitro growth of uropathogenic E. coli was inhibited by SP-A and SP-D. We conclude that SP-A and SP-D function as mediators of innate immunity by inhibiting bacterial growth and modulating renal inflammation in part by regulating p38 MAPK-related pathway in murine UTI.
Keywords: Innate immunity, Surfactant protein A, Surfactant protein D, urinary tract infection, uropathogenic Escherichia coli
Introduction
Urinary tract infection (UTI) is one of the most common infectious diseases, causing significant morbidity and mortality 1. Approximately 40% of women and 12% of men experience at least one symptomatic UTI in their lifetime. As many as 25% of women experience recurrent UTI within 6 to 12 months 2. UTI causes more than 11 million physician visits and almost half a million hospitalizations, resulting in an estimated cost of 3.5 billion dollars annually in the US alone 2. Uropathogenic Escherichia coli (UPEC) is the most frequent pathogen of asymptomatic bacteriuria and symptomatic UTIs 3.
Recent studies highlight the importance of innate immunity in the development of UTI 4–6. When E. coli and other pathogens overcome various physical barriers by adhering to the epithelium, a robust innate immune response in the epithelial cells is generated 2, 7–8. The effectors of this response include host defense proteins, antimicrobial peptides, cytokines and chemokines that attract phagocytes to the threatened site and enhance their microbicidal capacity, and phagocytosis 9.
Surfactant proteins A and D (SP-A and SP-D) are members of the C-type lectin family that share a collagen-like region and a calcium-dependent globular carbohydrate-recognition domain (CRD) 10. SP-A and SP-D play an important role in the pulmonary innate immune system and protect the lung against various pathogens 11–12. They interact directly with a variety of pathogens, inhibit their growth and enhance clearance by phagocytic cells 13, including E. coli K12 14, Mycoplasma pneumonia, Klebsiella pneumoniae, and Histoplasma capsulatum 15–16.
SP-A and SP-D function as pattern recognition molecules 17 by binding to receptors on the surface of epithelial and phagocytic cells, thereby modulating inflammatory processes via inflammatory signaling pathways like NF-κB pathway 18. Under normal environmental conditions in the lung, SP-A and SP-D bind to signal regulatory protein α (SIRPα) by their globular CRD heads that inhibits p38 mitogen-activated protein kinase (p38 MAPK) activation and attenuates the production of various inflammatory mediators 18. In the presence of microbes, the globular CRD domains of SP-A and SP-D interact with molecules on the surface of the microbes 19 and the collagenous tails of SP-A and SP-D bind to calreticulin/CD91 of cells, thereby stimulating p38 MAPK phosphorylation and NF-κB activation and resulting in increased cytokine and chemokine production (e.g. TNF-α, IL-8, and MCP-1) 18. SP-A and SP-D also interact with TLRs (TLR2 and TLR4) and TLR4 adaptor MD-2 via their CRD domains 15, thus interfering with the interaction of pathogens and TLR receptors 20.
SP-A knockout (SP-A KO) mice and SP-D knockout (SP-D KO) mice have shown increased susceptibility to lung infection by various pathogens, including Pseudomonas aeruginosa, respiratory syncytical virus, Mycoplasma pneumonia, Klebsiella pneumoniae and Pneumocystis Carinii 17, 21. Since there is functional similarity and overlap between SP-A and SP-D in host defense, SP-A and SP-D double knockout (SP-A/D KO) mice were generated 22. SP-A/D KO mice are more susceptible to Pseudomonas aeruginosa lung infection compared with wild type (WT), single gene SP-A KO, and SP-D KO mice 23.
The expression of SP-A and SP-D has been observed in the mucosal surface of the lung and several extrapulmonary organs, including kidney 24–27. Mucosal epithelial cells and surfactant defense proteins form a physical barrier in the lung and urinary tract to prevent pathogens from entering the body. Decreased levels of urinary SP-A and SP-D were recently associated with recurrent UTIs in females 28. We previously showed that SP-D functions as an innate immune factor and modulates inflammation in renal tubular epithelial cells in vitro 26. Recently, it has been shown that SP-D protein could inhibit adherence of UPEC to bladder epithelial cells and the bacterium-induced cytotoxicity 29. The current study examines the role of SP-A and SP-D in murine UTI and provides evidence that SP-A and SP-D function as mediators of innate immunity by inhibiting bacterial growth and modulating renal inflammation in part by regulating p38 MAPK-related pathways in murine UTI.
Materials and Methods
Mice
Female mice (8 to 10 weeks old) were used in this study. Original SP-A/D KO mice with C57BL/6 background were generated by Dr. Hawgood’s group 22 and SP-A/D KO mice were bred in the animal core facility at SUNY Upstate Medical University and maintained under pathogen-free conditions. Age-matched wild-type C57BL/6 mice (WT) were purchased from Jackson Laboratories (ME, USA). All animal experiments were conducted with an approved protocol (IACUC #253) in accordance with IACUC guidelines of SUNY Upstate Medical University and the National Institutes of Health guidelines on the use of laboratory animals.
Bacterial strain and mouse UTI model
UPEC (strain CFT073) was purchased from the American Type Culture Collection (ATCC, VA, USA). Serial passages of static growth of E. coli (CFT073) were made in lysogeny broth (LB) at 37°C, by which the expression of type 1 fimbrae was increased. Bacteria were harvested by centrifugation at 2,000xg for 10 min at 4°C and resuspended in PBS buffer. The bacterial solution was adjusted to OD600=0.5 with PBS buffer. UTI was induced as previously described 30 with some modifications. In brief, mice were anestheytized by intraperitoneal injection with ketamine/xylazine (90 mg/kg of ketamine and 10 mg/kg of xylazine), and were gently massaged and pushed down on the bladder to expel urine. Then bacterial solution (OD600=0.5, 50 μl/mouse) was delivered transurethrally using a sterile 0.28 mm inner diameter polyethylene catheter. Control mice underwent a sham procedure with administration of 50 μl of sterile PBS instead of bacterial suspension. In a pilot study the peak of bacterial load in the kidneys was found to be around 24 hrs after infection. Therefore, mice were sacrificed two time points e.g. 24 hrs or 48 hrs post-infection under anesthesia condition with intraperitoneal ketamine/xylazine. Tissue samples (kidneys) were excised and either immediately frozen in liquid nitrogen or placed in 10% neutral formalin for subsequent histological analysis. Sections were stained with haematoxylin and eosin in a standard fashion and assessed quantitatively the inflammatory score by two experienced investigators. Neutrophils in urine were quantified with countess automatic cell counter (Life Technologies, NY, USA) and were further confirmed using side by side comparisons with trypan blue straining in haemocytometer, and with strained cytospin slides examined by light microscopy. Previous studies have shown that 99% of the infiltrated inflammatory cells were neutrophils 31.
RT-PCR
Total RNA was isolated from the kidney and lung of mouse using the RNA-Bee reagent (Tel-test, Friendswood, TX) according to the manufacturer’s instructions. cDNA was synthesized from 1 μg of total RNA with oligo-dT primer using the superscript III First-strand synthesis system (Invitrogen, Carlsbad, CA). PCR was performed with primers for SP-A (sense primer: GTGTGCGGGGATCTGAAGTTG and antisense primer: CCGGCTCTGGTACACATCTC), SP-D (sense primer: GCTGGGCCCAAAGGAGAAGTAGGT and antisense primer: TAACAAGGCGCTGCTCTCCACAAG), β-actin (sense primer: GGGAATGGGTCAGAAGGACT and antisense primer TTTGATGTCACGCACGATTT) respectively. To avoid any genomic DNA contamination in the PCR amplification the primers of each pair are located in the different exons of SP-A, or SP-D, or β-actin gene. The PCR products were examined by 1.2% agarose-gel electrophoresis.
Histology and Immunohistochemistry (IHC)
The kidney was fixed in 10% neutral formalin and embedded in paraffin. Sections were cut and stained with haematoxylin and eosin. The inflammatory scores in the bladders and kidneys were determined in a blinded fashion using the histologic grading criteria as previously described 32. In brief, Grade 0 = Normal; 1 = Focal inflammation; 2 = Focal inflammation (more severe) from pelvis to medulla, with and without moderate edema; 3 = Multifocal inflammation and cells from pelvis to medulla; 4 = Extensive segmental inflammation and necrosis evident from pelvis to cortex; 5 = Diffuse tissue necrosis and inflammatory cell infiltration extending from pelvis to cortex. IHC was performed as our previously protocol 33. Sections were incubated with antibodies against mouse SP-A (gift from Dr. McCormack of the University of Cincinnati, OH), mouse SP-D (gift from Dr. Wright of Duke University Medical Center, Durham, NC) or neutrophil marker NIMP-R14 (1:200, Hycult biotech, Uden, Netherlands), overnight at 4°C, followed by biotinylated secondary antibody and avidin-biotin peroxidase complex (Vector Laboratories, CA, USA). Peroxidase activity was visualized by DAB Kit (Vector Laboratories, CA, USA), and the sections were then counter-stained with haematoxylin. Negative controls were performed by nonimmune serum instead of first antibody. To quantitate filtrating neutrophils in the slides of each sample, neutrophils were counted in a total of 20 fields per sample (400X).
Determination of bacterial loads
Kidney tissue was homogenized with an Ultra Turrax T8 homogenizer (IKA Labortechnik, Staufen, Germany) in 0.5 ml sterile PBS. Homogenates were serially diluted in PBS, 100 μL aliquots of kidney homogenate or urine were plated onto LB agar plates, and the number of colonies in kidney or urine was determined after overnight (14–16 hr) culture at 37°C. CFU were enumerated using the Quantity One colony-counting software (Bio-Rad, CA, USA). Quantitative cultures were expressed as CFUs per mg of kidney tissue or CFUs/mL of urine.
ELISA
Samples of mouse kidneys were homogenized in RIPA buffer. The levels of keratinocyte-derived chemokine (KC, the functional homolog of human IL-8) and IL-17 in kidney homogenates were measured by KC Assay Kit (SABiosciences Corporation, MD, USA) according to manufacturer’ instructions. Total protein content of the samples was determined by using BCA protein assay kit (Pierce Biochemicals, FL, USA).
Western blotting analysis
Samples of mouse kidney and lung were homogenized in RIPA buffer containing a cocktail of protein inhibitors and phosphatase inhibitors (Roche Molecular Biochemicals, IN, USA). Protein samples were subjected to electrophoresis with Tris-glycine gel and transferred to PVDF membranes. Membranes were probed with antibodies against mouse SP-A, mouse SP-D, p38 MAPK (Santa Cruz Biotechnology, CA, USA), phosphorylated p38 MAPK (Santa Cruz Biotechnology, CA, USA) or β-actin (Santa Cruz Biotechnology, CA, USA). Bands were detected using ECL method (Pierce Biochemicals, FL, USA).
Preparation of SP-A and SP-D proteins
Native human SP-A was purified from bronchoalveolar lavage fluid (BALF) as described previously 34. Human SP-D was purified from stably transfected Chinese hamster ovary (CHO) cell lines with the pEE14-hSP-D construct as described 26. SP-D was purified from the conditioned culture medium using maltose-affinity chromatography according to the previous method 35. The purity of the SP-A and SP-D preparation were verified by SDS-PAGE followed by silver staining. All proteins were filtered through a 0.2-micron filter to remove any potential contamination.
In vitro study of bacterial growth
For in vitro growth inhibition with SP-A and SP-D, UPEC CFT 073 was grown in 5 ml of LB medium at 37 °C overnight. Bacterial cells were harvested by centrifugation at 2000xg rpm for 10 min. Bacteria were resuspended and diluted to a final OD at 600 nm of approximately 0.2 in sterile TBS (20 mM Tris/140 mM NaCl, 2.5 mM Ca++, pH7.4) 36. In dose-dependent experiments, 80 μl of diluted CFT 073 suspensions was mixed with SP-A at concentrations ranging from 0 to 40 μg/ml of SP-A or SP-D at concentrations ranging from 0 to 20 μg/ml of SP-D, and then cultured for 5 hrs. In time-course experiments, diluted CFT073 solution was mixed with SP-A (final concentration at 40 μg/ml of SP-A) or SP-D (final concentration at 20 μg/ml of SP-D) and then cultured for analysis. Bacterial density was monitored by measuring at OD600 at 1-hour intervals. Bovine serum albumin (BSA) protein was used as negative control 14. After 5 hrs of incubation, samples were diluted to 106 times with TBS and TBS plus 2 mM EDTA. Of the diluted suspensions, 100 μl was cultured on LB Agar plate. Plates were incubated overnight at 37°C and then colonies were counted.
Statistical analysis
Data were expressed as means ± SEM, and statistical analyses were performed using SigmaStat 3.5 (Jandel Scientific, CA, USA). T-test or Mann–Whitney U-test was performed to assess the statistical significance of differences. p value <0.05 was considered statistically significant.
Results
SP-A and SP-D expression in the kidney of WT mice
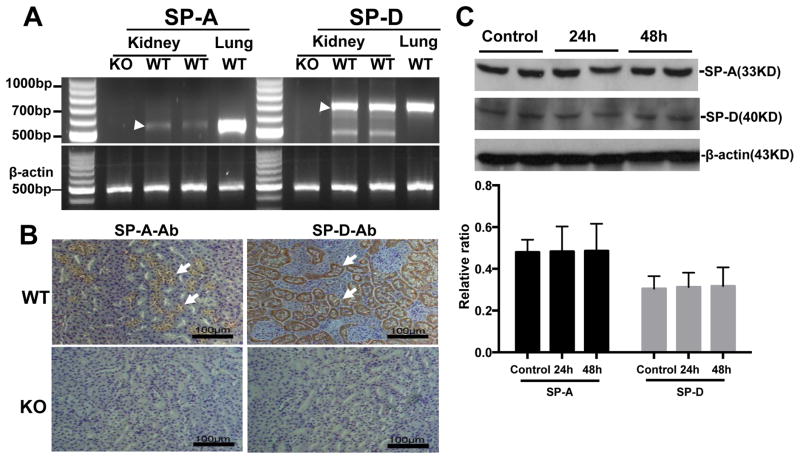
To examine the expression and distribution of SP-A and SP-D in mouse kidney, RT-PCR, immunohistochemistry (IHC), and Western blot analysis were performed. SP-A and SP-D mRNA and protein expression was detected in the kidney of WT mice but not SP-A/D KO mice (Fig. 1). The IHC showed that immunoreactivity for SP-A and SP-D is present in the proximal tubules and medullary collecting tubules of WT mice, but not in the SP-A/D KO mice (Fig. 1B). Furthermore, no significant difference of SP-A and SP-D expression before and after infection was observed in WT mice (Fig. 1C).
Figure 1. SP-A and SP-D expression in the kidney of WT mice.
Expression of SP-A and SP-D were analyzed in the kidney tissues of WT and SP-A/D KO mice by RT-PCR (A), IHC (B) and Western blot analysis (C).
A: SP-A mRNA (540 bp RT-PCR product, pointed by arrow) and SP-D mRNA (694 bp RT-PCR product, pointed by arrow) expression was detected in the kidney and lung (positive control) of WT mice, but not in SP-A/D KO mice.
B: Positive staining of SP-A and SP-D expression were detected in the tubular epithelial cells (arrows) of WT kidney, but no staining in SP-A/D KO mice.
C: SP-A and SP-D proteins were detected by Western blot analysis but no difference between infected and uninfected WT mice was found. (n = 4–6 mice/group)
Increased susceptibility to UTI in SP-A/D KO mice
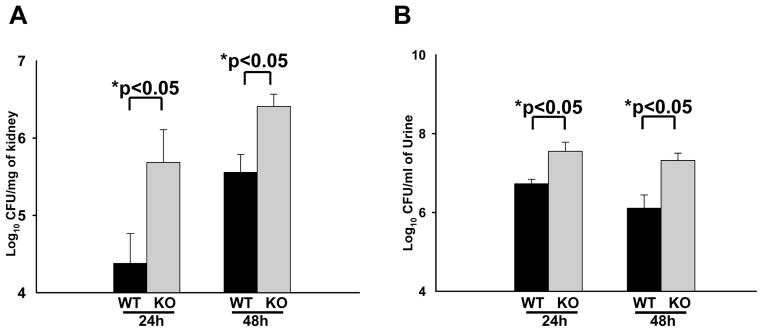
In order to study the effects of SP-A and SP-D in UTI, SP-A/D KO and WT female mice were infected with UPEC CFT073 by intravesical inoculation as previously described 30. Renal and urine bacterial loads in the infected mice were determined 24 hrs and 48 hrs after infection. At both time points, the SP-A/D KO mice demonstrated increased CFUs of E. coli in both the kidney and the urine compared with WT mice (Fig. 2), suggesting that the ability of SP-A/D KO mice to remove and/or kill urinary E. coli is impaired relative to WT mice.
Figure 2. In vivo bacterial loads in WT and SP-A/D KO mice.
CFUs in kidney or urine from infected SP-A/D KO and WT mice were determined by culturing overnight at 37°C. At 24 hrs and 48 hrs after inoculation, SP-A/D KO mice had significantly increased numbers of CFU in both kidney (A) and urine (B) when compared with WT mice. (n = 8–12 mice/group)
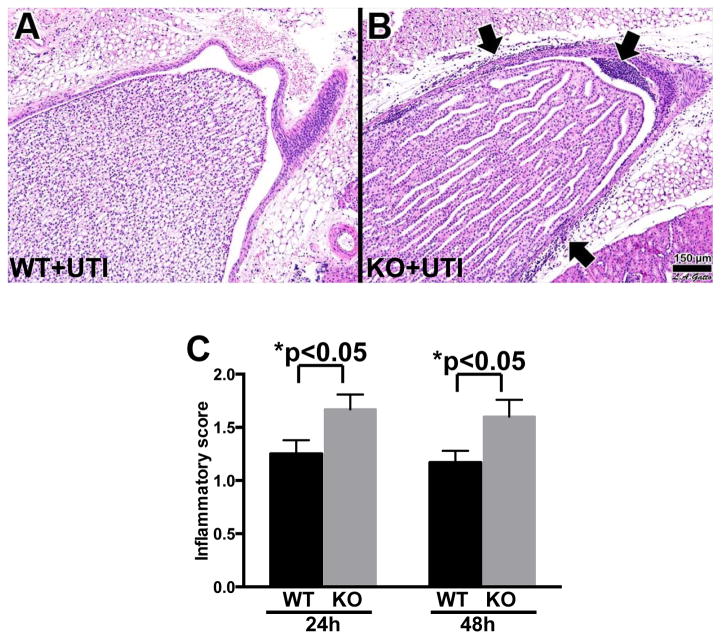
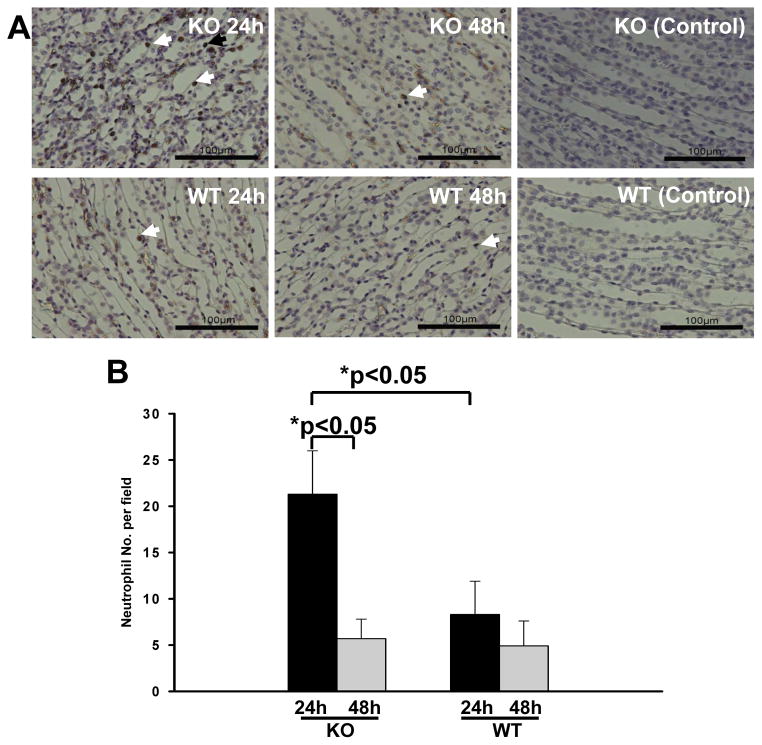
An essential aspect of the host response to invading bacteria is the recruitment of neutrophils to the site of infection. Therefore, renal tissues of the experimental mice were analyzed histologically for the presence of neutrophils. The kidneys of control SP-A/D KO and WT mice were histologically normal, showing no inflammation or other alterations. In contrast, infected WT and SP-A/D KO mice demonstrated inflammatory infiltrates in the medullary region in the kidney, consisting of almost exclusively neutrophils and monocytes (Fig. 3). Kidneys of infected SP-A/D KO mice showed more neutrophil/moncyte infiltration and a few scattered microabscesses when compared with infected WT mice (Fig. 3B). The inflammatory score was higher in the kidney of SP-A/D KO mice compared to WT mice 24 and 48 hrs after infection (Fig. 3C).
Figure 3. Histological analysis of the kidney of infected WT and SP-A/D KO mice.
Histological assessment of the kidney pyelum in (A) infected WT mouse, and (B) infected SP-A/D KO mouse at 24 hours after infection. Infected KO mice exhibited prominent inflammatory cell infiltration (arrows). Kidney inflammatory score of SP-A/D KO mice was higher than that of WT mice 24 and 48 hrs after infection (C). Hematoxylin and eosin, bar = 150 μm. (n = 4–6 mice/group)
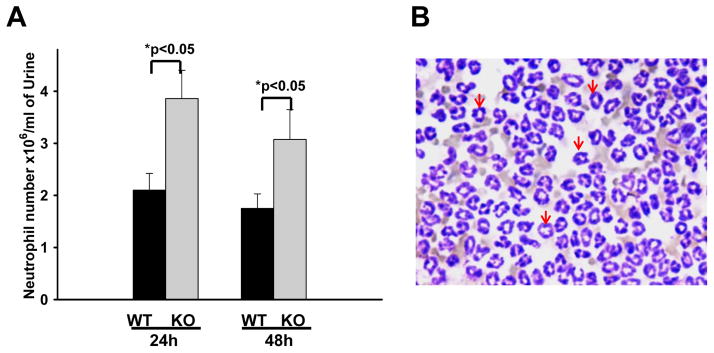
We further quantified the number of neutrophils in the urine collected from infected SP-A/D KO and WT mice. The results indicate that infected SP-A/D KO mice had more neutrophils in the urine 24 hrs and 48 hrs postinfection than infected WT mice (Fig. 4). We also examined the number of monocytes/macrophages in the urine. The results indicate that the number of monosytes/macrophages was less than 1% of neutrophils and there was no significant difference between infected SP-A/D KO and WT mice. Furthermore, neutrophil/monocyte infiltration in the kidneys of infected SP-A/D KO and WT mice was examined by IHC using anti-neutrophil specific antibody NIMP-R14 (Hycult biotech) (Fig. 5). The results in figure 5A indicate that infiltrating neutrophils and monocytes were scattered throughout the collecting tubules in the kidney from both infected SP-A/D KO and WT mice. However, the number of infiltrating neutrophils and monocytes in kidney tissues from infected SP-A/D KO mice is greater than observed in infected WT mice 24 hrs postinfection (p<0.05) (Fig. 5B). Infiltrating neutrophils and moncytes decreased from 24 hrs to 48 hrs postinfection in SP-A/D KO mice (p<0.05). On the basis of these observations, we conclude that SP-A/D KO mice are more susceptible to UTI compared with WT mice, suggesting that SP-A and SP-D are critical effectors of innate immunity in UTI.
Figure 4. Neutrophils in the urine of infected WT and SP-A/D KO mice.
A: Numbers of neutrophils in the urine were significantly increased in infected SP-A/D KO mice compared with WT mice at both 24 hrs and 48 hrs after infection. (n = 5–8 mice/group). B: A representative image of inflammatory cells (neutrophils appointed by arrows) from infected mouse urine.
Figure 5. Neutrophils in the renal tissues of infected SP-A/D KO and WT mice.
A: Neutrophils were detected in kidney tissues by IHC using neutrophil-specific RIMP-R14 antibody. At 24 hrs after infection, increased neutrophils were observed in the infected SP-A/D KO mice compared with the infected WT mice. However, at 48 hrs after infection, a few neutrophils were detected in both the infected SP-A/D KO and infected WT mice. There was no positive staining in the negative control (nonimmune serum instead of first antibody). Bar = 100 μm.
B: The quantitative analysis of neutrophils indicate that Nos. of neutrophils in the kidney were significantly increased in infected SP-A/D KO mice compared with infected WT mice at 24 hrs after infection; and Nos. of neutrophils in the kidney of infected SP-A/D KO mice decreased from 24 hrs to 48 hrs postinfection. (n = 5–8 mice/group)
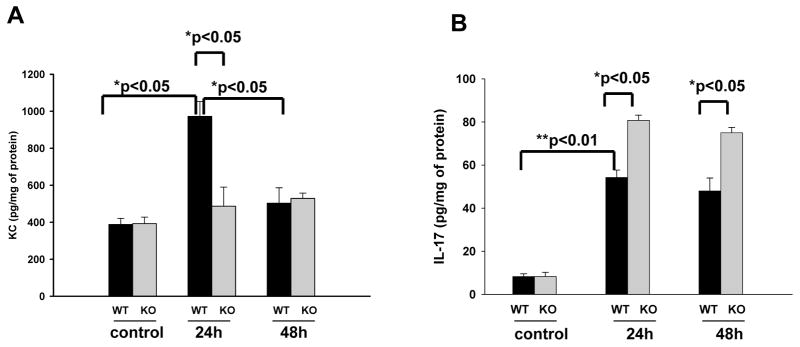
Differential Keratinocyte-derived chemokine (KC) and LI-17 expressions in the infected SP-A/D KO and WT mice
KC (functional homolog of human cytokine IL-8) is an important inflammatory mediator in the pathogenesis of kidney infection. Both SP-A and SP-D have been shown to regulate inflammatory mediator expression in the infected tissues. We therefore examined KC expression in the kidney of infected SP-A/D KO and WT mice. Basal levels of KC in both WT and SP-A/D KO mice were comparable. KC levels in WT mice increased significantly 24 hrs after infection and decreased to near baseline level at 48 hrs (Fig. 6A). However, in the SP-A/D KO mice KC level failed to rise after infection (Fig. 6A). These results suggest that mice lacking SP-A and SP-D do not efficiently upregulate the kidney KC expression in the murine UTI model. Furthermore, we examined IL-17 expression in this study. Compared to control, the level of IL-17 was elevated in the kidney of infected WT and SP-A/D KO mice (Fig. 6B; p<0.01); and the infected SP-A/D KO mice had higher IL-17 expression than the infected WT mice 24 and 48 hrs post-infection (Fig. 6B; p<0.05), suggesting the immunoregulatory effects of SP-A and SP-D in the UTI.
Figure 6. Renal KC and IL-17 levels in the infected WT and SP-A/D KO mice.
KC and IL-17 levels in the kidney tissues of WT and SP-A/D KO mice were measured by ELISA. The data of KC and IL-17 levels were expressed as pg/mg of total protein. Basal levels of KC and IL-17 were determined with uninfected mice. Panel A shows that the level of renal KC in SP-A/D KO mice showed no significant changes after infection, but the KC level of infected WT mice increased significantly at 24 hrs when compared with infected SP-A/D KO mice, or the basal level of WT mice. The KC level of infected WT mice decreased to near baseline at 48 hrs after infection. Panel B depicts IL-17 expression in the kidney of WT and SP-A/D KO mice. Compared to control, the level of IL-17 was elevated in the kidney of infected WT and SP-A/D KO mice (p<0.01); and the infected SP-A/D KO mice had higher IL-17 expression than the infected WT mice 24 and 48 hrs post-infection (p<0.05). (n = 4–6 mice/group)
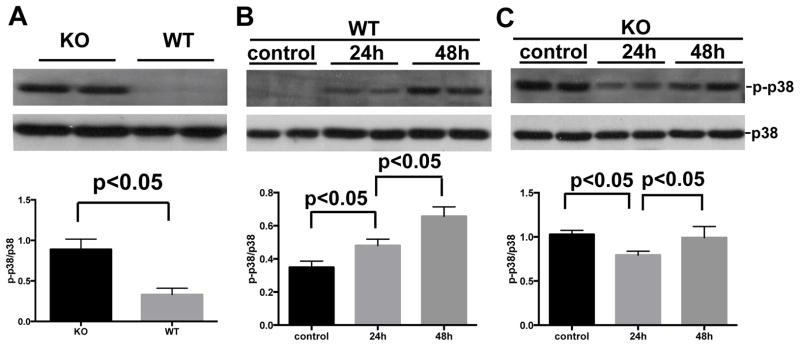
p38 MAPK phosphorylation in kidney tissue
Previous in vitro study has shown that SP-A and SP-D could regulate p38 MAPK phosphorylation and its downstream activation of the signaling pathway 18. Therefore, we examined levels of p38 MAPK phosphorylation in the kidneys of SP-A/D KO mice and WT mice by Western blot with p-p38 MAPK specific antibody. Interestingly, basal level of p38 MAPK phosphorylation was significantly increased in kidney from healthy SP-A/D KO mice relative to WT control mice (Fig. 7A). The increased level of phosphorylated p38 MAPK was observed in the kidney of infected WT mice 24 and 48 hrs after infection (Fig. 7B), but the level of phosphorylated p38 MAPK decreased in the infected SP-A/D KO mice 24 hrs after infection compared to uninfected mice (control), and then increased to similar level as control 48 hrs after infection (Fig. 7C).
Figure 7. Level of p38 MAPK phosphorylation in the kidney.
Total protein (20 μg) of kidney tissue was subjected to electrophoresis and then transferred onto a PVDF membrane. Phosphorylated p38 MAPK (p-p38 MAPK) and p38 MAPK (unphosphoryated) were detected with specific p-p38 MAPK antibody and unphosphorylated p38 MAPK antibody, respectively. Relative level of p-p38 MAPK was normalized to unphosphorylated p38 MAPK. Higher p38 MAPK phosphorylation level in the kidney of SP-A/D KO mice was observed compared to WT mice (A). The level of p-p38 MAPK increased 24 and 48 hrs postinfection in WT mice (B); but the level of p-p38 MAPK decreased in infected SP-A/D KO mice 24 hrs postinfection, and then increased to similar level as control 48 hrs postinfection (Fig. 7C). (n = 4–6 mice/group)
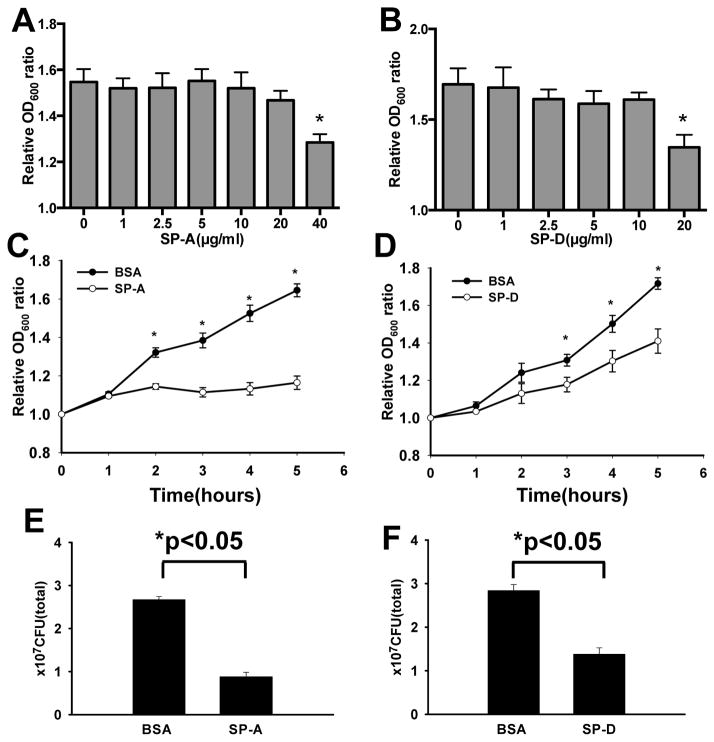
Effects of SP-A and SP-D on the inhibition of in vitro growth of UPEC
The effects of SP-A and SP-D on in vitro growth of E. coli were measured by monitoring the optical density (OD600) of cultured bacteria in TBS buffer containing 2.5 mM Ca++, with or without SP-A or SP-D, and final colony-forming units (CFU) of the culture were quantified. In the absence of SP-A or SP-D, E. coli growth and the OD600 increased steadily from 1 to 5 hrs cultivation. The results from dose-course experiments indicated that 40 μg/ml of SP-A or 20 μg/ml of SP-D could significantly inhibit the growth of UPEC (Fig. 8A and B). Therefore, these concentrations of SP-A and SP-D were used in the following experiments. The results indicated that, in the presence of SP-A (40 μg/ml) or SP-D (20 μg/ml), bacterial growth was markedly inhibited from 2 to 5 hrs for SP-A (p<0.05) (Fig. 8C) and from 3 to 5 hrs for SP-D (p<0.05) compared with only BSA (Fig. 8D). To further examine the effects of SP-A and SP-D on bacterial viability, the final CFUs of the culture were determined by colony-count assays. The results showed that E. coli treated with SP-A (40 μg/ml) (Fig. 8E) or SP-D (20 μg/ml) (Fig. 8F) resulted in significantly lower CFUs (p<0.05) compared to the BSA controls. Additionally, to eliminate the effect of BSA on the growth of the bacteria, we also cultured bacteria in TBS buffer without BSA as control; the results of the bacterial growth were similar to those with BSA. These results suggest that both SP-A and SP-D may inhibit the growth of UPEC, or are bactericidal under these experimental conditions.
Figure 8. Effects of SP-A and SP-D on the UPEC growth inhibition in vitro.
For dose-dependent experiments, UPEC (strain CFT 073) bacterial solutions in TBS were added with SP-A at concentrations ranging from 0 to 40 μg/ml (A) or of SP-D at concentrations ranging from 0 to 20 μg/ml (B), and then were incubated at 37°C for 5 hrs. For time-course experiments, bacterial solutions in TBS were added with 40 μg/ml of SP-A (C), or 20 μg/ml of SP-D (D), or BSA (control) and then incubated to 5 hrs. Bacterial growth was monitored by measuring optical density at 600 nm. Relative OD600 value was used to represent bacterial growth. CFUs of the bacterial culture were determined after mixing SP-A or SP-D for 5 hrs. The results showed bacterial growth was inhibited 3 fold by 40 μg/ml of SP-A (E) and 2 fold by 20 μg/ml of SP-D (F). (n = three independent experiments) *p <0.05.
Discussion
UTI is one of the most common infections in women. Numerous host factors have been implicated in the defense mechanisms against UTI induced by UPEC 6, 37. In a previous study, we found decreased levels of SP-A and SP-D in urine from female patients with recurrent UTI 28, suggesting that SP-A and SP-D, two important innate immune proteins, might be involved in the host defense against UTI. To better understand the mechanisms underlying SP-A and SP-D role in UTI, SP-A/D KO and age-matched female WT mice were studied in a murine model of UPEC -induced UTI. This is the first study to investigate the roles of SP-A and SP-D in an experimental model of UTI. Our results provide evidence that SP-A/D KO mice are more susceptible to uropaothgenic E. coli infection, showing increased bacterial loads and more infiltrating neutrophils in the kidney, the bladder and the urine, as well as severe histopathological changes with higher inflammatory score in the kidney, when compared to WT mice. Of interest, we found increased basal levels of p38 MAPK phosphorylation in SP-A/D KO mice but lack response to infection in KC expression in the SP-A/D KO mice. Furthermore, UPEC growth was inhibited by SP-A and SP-D in vitro. These observations suggest that SP-A and SP-D exerted a direct antibacterial effect and modulated inflammatory response to UTI.
SP-A and SP-D were originally identified as surfactant-associated proteins, dominantly expressed in lung epithelial cells 11. SP-A and SP-D expression was subsequently observed in several extrapulmonary organs including kidney, salivary gland, prostate gland, pancreas, intestine, stomach and female reproductive tract 24–25, 27, 38–39. Several studies demonstrated that both SP-A and SP-D proteins play roles not only in the respiratory system 13, but also in the gastrointestinal tract 40 and the reproductive system 41. Urinary tract system is exposed to various environmental factors including pathogens and requires multiple host defense mechanisms against environmental insults, including agents of the innate immunity such as SP-A and SP-D.
The immunoregulatory roles of SP-A and SP-D have recently received more attention 13, 20. SP-A/D KO mice were shown to be more susceptible to lung infection with P. aeruginosa, when compared to single gene knockout e.g. SP-A KO, or SP-D KO, and wild type mice 23. Although SP-A and SP-D have shown the ability to inhibit the growth of nonpathogenic E. coli K12 14, it was unclear whether they would have the same effects on the growth of pathogenic E. coli since pathogenic strains usually have different composition/structure of endotoxin (LPS) from nonpathogenic strains. SP-A and SP-D can bind directly to rough LPS but not to smooth LPS from E. coli and other gram-negative bacteria 14, 42–43. Recent report indicates that SP-D has the effects on the inhibition of UPEC adherence to bladder epithelial cells and the bacterium-induced cytotoxicity 29. This study demonstrated that both SP-A and SP-D could inhibit the growth of UPEC in vitro, but at relative higher concentrations compared to nonpathogenic E. coli strain 14, which may reflect difference between pathogenic and nonpathogenic strains. More effective agglutination of nonpathogenic E. coli K12 mediated by SP-A and SP-D were observed compared to pathogenic E. coli CFT073 in this study (data not shown). Purified SP-A and SP-D proteins from BAL fluid of patients with alveolar proteinosis or recombinant mammalian cell expression systems have been widely used for functional studies in vitro and in vivo 14, 42, 44. The biological roles of SP-A and SP-D on the inhibition of bacterial growth and adherence serve as an effective innate immune mechanism against UTI. The notion is further confirmed by the results from the in vivo UTI, where SP-A/D KO mice, compared to WT mice, had higher bacterial loads in the infected kidney and the urine, indicating their decreased ability to remove E. coli in this UTI model.
The p38 MAPK signaling pathway plays a central role in the regulation of proinflammatory cytokine and chemokine production, because p38 MAPK activation can regulate several downstream pathways involved in proinflammatory cytokine expression 45. Our previous study also found SP-A/D KO mice had higher basal level of autophagy in liver and a more pronounced inflammatory response to sepsis than WT mice 46,47. Of interest, in the present study, we observed uninfected SP-A/D KO mice exhibit significant higher basal levels of p38 MAPK phosphorylation compared with uninfected WT mice. Previous studies have shown that p38 MAPK is phosphorylated in response to bacterial infection and regulates the activation of nuclear transcription factors including NF-κB in UTI 48–49. Based on the observations from this study and other published information 18, 44, 49, SP-A and SP-D are two important mediators of innate immunity in the pathogenesis of UTI. SP-A and SP-D proteins can bind to E. coli and epithelial cell receptors 50, like TLR2 and TLR4 through their CRD domain. When the urinary tract is infected, the CRD domain of SP-A and SP-D first binds to bacteria (UPEC), and thus the collagen-like region of SP-A and SP-D interacts with CD91/calreticulin receptor in the epithelial cells of the kidney 51, by which p38 MAPK signaling pathway could be activated and the expression of inflammatory mediators could be upregulated. In contrast, the level of p38 MAPK phosphorylation decreased 24 hr postinfection and the level of KC expression showed no response to UTI due to the lack of SP-A and SP-D expression in the SP-A/D KO mice. This model also explain, at least in part, the increased NF-κB activity observed in lung tissue from uninfected SP-D KO mice 52.
Neutrophils are the most abundant and rapid cellular responders to the infected urinary tract 53. Neutrophils also represent a major cause of renal scarring, and are implicated in the antibacterial defense of the urinary tract system 53. In the present UTI model, more bacteria and neutrophil infiltration were observed in kidney and urine of the infected SP-A/D KO mice than those in the infected WT mice. One possible mechanism is the lack of the effects of SP-A and SP-D host defense. As an unexpected finding, no cytokine KC response but higher IL-17 expression to bacterial infection at 24 and 48 hrs time points in the infected SP-A/D KO mice were observed in this study, it is possible that a) IL-17 and other cytokines or chemokines, like MIP-2, MCP-1, GM-CSF and CCL5 may replace the effects of KC in the recruitment of neutrophils in the UTI model. For example, the increased IL-17 and GM-CSF levels were found in uninfected SP-A/D KO mice 54 and in UPEC-induced UTI animals 55, which may have an impact on the recruitment of neutrophils to infectious site. Future studies are needed to explore the mechanisms of p38 MAPK activation response to UTI and one comprehensive evaluation of cytokine and chemokine expression in SP-A/D KO mice. Epithelial cells of the upper urinary tract not only form a barrier to prevent pathogens, but also produce a variety of cytokines and chemokines in response to bacterial stimulation 56. Murine KC is a functional homolog of human cytokine IL-8. IL-8 expression has been observed to be significantly increased in the bladder and kidney of human patients with UTI 57. Human IL-8 production in the urinary tract epithelial cells is dependent on the p38 MAPK-mediated NF-κB activation 58. The lack of KC response to E. coli infection in SP-A/D KO mice may be caused by the absence of interactions between SP-A, SP-D and cell receptors, as well as the activation of the p38 MAPK signaling pathway. Previous studies found SP-A and SP-D can interact with the various receptors, resulting in increased phosphorylation of p38 MAPK and upregulation of the NF-κB pathway with enhanced expression of inflammatory factors in the infected lung 18, 44, 59. In the present study, the phosphorylation of p38 MAPK in response to bacterial infection was observed in WT mice but not SP-A/D KO mice. The process of p38 MAPK phosphorylation is a rapid process in the cells after stimulation/infection 49, 58. Changes in abundance or activity of p38 activation or deactivation by phosphatases (phosphorylation/dephosphorylation) represent potential mechanisms. In addition, monocytes are known to arrive at the sites of infection in UTI model 60. Monocytes can further differentiate into macrophages and clean up dying neutrophils. Both SP-A and SP-D can interact with receptors on the surface of macrophages and promote the clearance of dying cells 13. SP-A and SP-D also play roles in the regulation of inflammatory cells and removal of dying cells in the UTI. However, compared to neutrophils, only a few monocytes and macrophages were determined in the urea if infected mice in the present study.
In summary, our results suggest that the protective effects of SP-A and SP-D in UTI are potentially mediated by two different mechanisms: 1) Direct inhibition of UPEC growth and 2) SP-A and SP-D modulate inflammatory processes through regulation of p38 MAPK phosphorylation and its downstream signaling pathways (e.g. NF-κB activation). Collectively, our results provide evidence SP-A and SP-D function as critically important innate immune molecules in the urinary tract. Additional studies are needed to further elucidate the mechanisms by which SP-A and SP-D impact bacterial growth and aggregation of uropathogenic E. coli and tissue inflammatory responses in UTI, as well as the correlation between human SP-A and SP-D genetic variants and individual susceptibility to UTI using SP-A or SP-D single knockout, and humanized transgenic mice.
Acknowledgments
This work was supported by NIH grant HL096007 and grants from the National Science Foundation of China (30670985, 81070556, 81300617).
We would like to thank Dr. Francis X. McCormack of the University of Cincinnati for kindly providing human BAL fluid from AP patients and mouse SP-A antibody, Dr. Erika Crouch of the School of Medicine of Washington University in St. Louis for detailed protocol of SP-D purification, and Dr. Jo Rae Wright of the Duke University Medical Center for mouse SP-D antibody.
Footnotes
Disclosure
All the authors declared no competing interests.
References
- 1.Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infectious disease clinics of North America. 2014;28(1):1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Nielubowicz GR, Mobley HL. Host-pathogen interactions in urinary tract infection. Nat Rev Urol. 2010;7(8):430–441. doi: 10.1038/nrurol.2010.101. [DOI] [PubMed] [Google Scholar]
- 3.Svanborg C, Godaly G. Bacterial virulence in urinary tract infection. Infect Dis Clin North Am. 1997;11(3):513–529. doi: 10.1016/s0891-5520(05)70371-8. [DOI] [PubMed] [Google Scholar]
- 4.Ragnarsdottir B, Lutay N, Gronberg-Hernandez J, Koves B, Svanborg C. Genetics of innate immunity and UTI susceptibility. Nat Rev Urol. 2011;8(8):449–468. doi: 10.1038/nrurol.2011.100. [DOI] [PubMed] [Google Scholar]
- 5.Jaillon S, Moalli F, Ragnarsdottir B, Bonavita E, Puthia M, Riva F, et al. The Humoral Pattern Recognition Molecule PTX3 Is a Key Component of Innate Immunity against Urinary Tract Infection. Immunity. 2014;40(4):621–632. doi: 10.1016/j.immuni.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Ulett GC, Totsika M, Schaale K, Carey AJ, Sweet MJ, Schembri MA. Uropathogenic Escherichia coli virulence and innate immune responses during urinary tract infection. Current opinion in microbiology. 2013;16(1):100–107. doi: 10.1016/j.mib.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Duell BL, Carey AJ, Tan CK, Cui X, Webb RI, Totsika M, et al. Innate transcriptional networks activated in bladder in response to uropathogenic Escherichia coli drive diverse biological pathways and rapid synthesis of IL-10 for defense against bacterial urinary tract infection. J Immunol. 2012;188(2):781–792. doi: 10.4049/jimmunol.1101231. [DOI] [PubMed] [Google Scholar]
- 8.Reigstad CS, Hultgren SJ, Gordon JI. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. The Journal of biological chemistry. 2007;282(29):21259–21267. doi: 10.1074/jbc.M611502200. [DOI] [PubMed] [Google Scholar]
- 9.Zasloff M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. Journal of the American Society of Nephrology: JASN. 2007;18(11):2810–2816. doi: 10.1681/ASN.2007050611. [DOI] [PubMed] [Google Scholar]
- 10.Seaton BA, Crouch EC, McCormack FX, Head JF, Hartshorn KL, Mendelsohn R. Review: Structural determinants of pattern recognition by lung collectins. Innate Immun. 2010;16(3):143–150. doi: 10.1177/1753425910368716. [DOI] [PubMed] [Google Scholar]
- 11.Crouch E, Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 12.Douda DN, Jackson R, Grasemann H, Palaniyar N. Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. J Immunol. 2011;187(4):1856–1865. doi: 10.4049/jimmunol.1004201. [DOI] [PubMed] [Google Scholar]
- 13.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5(1):58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 14.Wu H, Kuzmenko A, Wan S, Schaffer L, Weiss A, Fisher JH, et al. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest. 2003;111(10):1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol. 2007;9(8):1871–1879. doi: 10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 16.Awasthi S. Surfactant protein (SP)-A and SP-D as antimicrobial and immunotherapeutic agents. Recent Pat Antiinfect Drug Discov. 2010;5(2):115–123. doi: 10.2174/157489110791233559. [DOI] [PubMed] [Google Scholar]
- 17.Waters P, Vaid M, Kishore U, Madan T. Lung surfactant proteins A and D as pattern recognition proteins. Adv Exp Med Biol. 2009;653:74–97. doi: 10.1007/978-1-4419-0901-5_6. [DOI] [PubMed] [Google Scholar]
- 18.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, et al. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115(1):13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 19.Ogasawara Y, McCormack FX, Mason RJ, Voelker DR. Chimeras of surfactant proteins A and D identify the carbohydrate recognition domains as essential for phospholipid interaction. J Biol Chem. 1994;269(47):29785–29792. [PubMed] [Google Scholar]
- 20.Chroneos ZC, Sever-Chroneos Z, Shepherd VL. Pulmonary surfactant: an immunological perspective. Cell Physiol Biochem. 2010;25(1):13–26. doi: 10.1159/000272047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, et al. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43(9):1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Hawgood S, Ochs M, Jung A, Akiyama J, Allen L, Brown C, et al. Sequential targeted deficiency of SP-A and -D leads to progressive alveolar lipoproteinosis and emphysema. Am J Physiol Lung Cell Mol Physiol. 2002;283(5):L1002–1010. doi: 10.1152/ajplung.00118.2002. [DOI] [PubMed] [Google Scholar]
- 23.Giannoni E, Sawa T, Allen L, Wiener-Kronish J, Hawgood S. Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 2006;34(6):704–710. doi: 10.1165/rcmb.2005-0461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stahlman MT, Gray ME, Hull WM, Whitsett JA. Immunolocalization of surfactant protein-D (SP-D) in human fetal, newborn, and adult tissues. J Histochem Cytochem. 2002;50(5):651–660. doi: 10.1177/002215540205000506. [DOI] [PubMed] [Google Scholar]
- 25.Madsen J, Kliem A, Tornoe I, Skjodt K, Koch C, Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol. 2000;164(11):5866–5870. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- 26.Hu F, Liang W, Ren Z, Wang G, Ding G. Surfactant protein D inhibits lipopolysaccharide-induced monocyte chemoattractant protein-1 expression in human renal tubular epithelial cells: implication for tubulointerstitial fibrosis. Clin Exp Immunol. 2012;167(3):514–522. doi: 10.1111/j.1365-2249.2011.04521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Hu F, Wang G, Zhou Q, Ding G. Lipopolysaccharide-induced expression of surfactant proteins A1 and A2 in human renal tubular epithelial cells. Journal of inflammation. 2013;10(1):2. doi: 10.1186/1476-9255-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Hu F, Liang W, Wang G, Singhal PC, Ding G. Polymorphisms in the surfactant protein a gene are associated with the susceptibility to recurrent urinary tract infection in chinese women. The Tohoku journal of experimental medicine. 2010;221(1):35–42. doi: 10.1620/tjem.221.35. [DOI] [PubMed] [Google Scholar]
- 29.Kurimura Y, Nishitani C, Ariki S, Saito A, Hasegawa Y, Takahashi M, et al. Surfactant protein D inhibits adherence of uropathogenic Escherichia coli to the bladder epithelial cells and the bacterium-induced cytotoxicity: a possible function in urinary tract. The Journal of biological chemistry. 2012;287(47):39578–39588. doi: 10.1074/jbc.M112.380287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung CS, Dodson KW, Hultgren SJ. A murine model of urinary tract infection. Nat Protoc. 2009;4(8):1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olszyna DP, Florquin S, Sewnath M, Branger J, Speelman P, van Deventer SJ, et al. CXC chemokine receptor 2 contributes to host defense in murine urinary tract infection. J Infect Dis. 2001;184(3):301–307. doi: 10.1086/322030. [DOI] [PubMed] [Google Scholar]
- 32.Hopkins WJ, Gendron-Fitzpatrick A, Balish E, Uehling DT. Time course and host responses to Escherichia coli urinary tract infection in genetically distinct mouse strains. Infection and immunity. 1998;66(6):2798–2802. doi: 10.1128/iai.66.6.2798-2802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang W, Chen C, Shi J, Ren Z, Hu F, van Goor H, et al. Disparate effects of eplerenone, amlodipine and telmisartan on podocyte injury in aldosterone-infused rats. Nephrol Dial Transplant. 2011;26(3):789–799. doi: 10.1093/ndt/gfq514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang G, Phelps DS, Umstead TM, Floros J. Human SP-A protein variants derived from one or both genes stimulate TNF-alpha production in the THP-1 cell line. Am J Physiol Lung Cell Mol Physiol. 2000;278(5):L946–954. doi: 10.1152/ajplung.2000.278.5.L946. [DOI] [PubMed] [Google Scholar]
- 35.Wang G, Umstead TM, Phelps DS, Al-Mondhiry H, Floros J. The effect of ozone exposure on the ability of human surfactant protein a variants to stimulate cytokine production. Environ Health Perspect. 2002;110(1):79–84. doi: 10.1289/ehp.0211079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang G, Myers C, Mikerov A, Floros J. Effect of cysteine 85 on biochemical properties and biological function of human surfactant protein A variants. Biochemistry. 2007;46(28):8425–8435. doi: 10.1021/bi7004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sivick KE, Mobley HL. Waging war against uropathogenic Escherichia coli: winning back the urinary tract. Infect Immun. 2010;78(2):568–585. doi: 10.1128/IAI.01000-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oberley RE, Goss KL, Hoffmann DS, Ault KA, Neff TL, Ramsey KH, et al. Regulation of surfactant protein D in the mouse female reproductive tract in vivo. Mol Hum Reprod. 2007;13(12):863–868. doi: 10.1093/molehr/gam074. [DOI] [PubMed] [Google Scholar]
- 39.Oberley RE, Goss KL, Dahmoush L, Ault KA, Crouch EC, Snyder JM. A role for surfactant protein D in innate immunity of the human prostate. Prostate. 2005;65(3):241–251. doi: 10.1002/pros.20292. [DOI] [PubMed] [Google Scholar]
- 40.Bourbon JR, Chailley-Heu B. Surfactant proteins in the digestive tract, mesentery, and other organs: evolutionary significance. Comp Biochem Physiol A Mol Integr Physiol. 2001;129(1):151–161. doi: 10.1016/s1095-6433(01)00312-9. [DOI] [PubMed] [Google Scholar]
- 41.Yadav AK, Madan T, Bernal AL. Surfactant proteins A and D in pregnancy and parturition. Front Biosci (Elite Ed) 2011;3:291–300. doi: 10.2741/e244. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Verdugo I, Wang G, Floros J, Casals C. Structural analysis and lipid-binding properties of recombinant human surfactant protein a derived from one or both genes. Biochemistry. 2002;41(47):14041–14053. doi: 10.1021/bi026540l. [DOI] [PubMed] [Google Scholar]
- 43.Kuan SF, Rust K, Crouch E. Interactions of surfactant protein D with bacterial lipopolysaccharides. Surfactant protein D is an Escherichia coli-binding protein in bronchoalveolar lavage. J Clin Invest. 1992;90(1):97–106. doi: 10.1172/JCI115861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, et al. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169(7):3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 45.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429(3):403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 46.Tang Z, Ni L, Javidiparsijani S, Hu F, Gatto LA, Cooney R, et al. Enhanced liver autophagic activity improves survival of septic mice lacking surfactant proteins A and D. The Tohoku journal of experimental medicine. 2013;231(2):127–138. doi: 10.1620/tjem.231.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, Abdel-Razek O, Liu Z, Hu F, Zhou Q, Cooney RN, et al. Role of surfactant proteins a and d in sepsis-induced acute kidney injury. Shock. 2015;43(1):31–38. doi: 10.1097/SHK.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischer H, Lutay N, Ragnarsdottir B, Yadav M, Jonsson K, Urbano A, et al. Pathogen specific, IRF3-dependent signaling and innate resistance to human kidney infection. PLoS Pathog. 2010;6(9):e1001109. doi: 10.1371/journal.ppat.1001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chassin C, Goujon JM, Darche S, du Merle L, Bens M, Cluzeaud F, et al. Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J Immunol. 2006;177(7):4773–4784. doi: 10.4049/jimmunol.177.7.4773. [DOI] [PubMed] [Google Scholar]
- 50.Kurihara H, Harita Y, Ichimura K, Hattori S, Sakai T. SIRP-alpha-CD47 system functions as an intercellular signal in the renal glomerulus. Am J Physiol Renal Physiol. 2010;299(3):F517–527. doi: 10.1152/ajprenal.00571.2009. [DOI] [PubMed] [Google Scholar]
- 51.Prakoura N, Politis PK, Ihara Y, Michalak M, Charonis AS. Epithelial calreticulin up-regulation promotes profibrotic responses and tubulointerstitial fibrosis development. The American journal of pathology. 2013;183(5):1474–1487. doi: 10.1016/j.ajpath.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida M, Korfhagen TR, Whitsett JA. Surfactant protein D regulates NF-kappa B and matrix metalloproteinase production in alveolar macrophages via oxidant-sensitive pathways. J Immunol. 2001;166(12):7514–7519. doi: 10.4049/jimmunol.166.12.7514. [DOI] [PubMed] [Google Scholar]
- 53.Haraoka M, Hang L, Frendeus B, Godaly G, Burdick M, Strieter R, et al. Neutrophil recruitment and resistance to urinary tract infection. J Infect Dis. 1999;180(4):1220–1229. doi: 10.1086/315006. [DOI] [PubMed] [Google Scholar]
- 54.Gram K, Yang S, Steiner M, Somani A, Hawgood S, Blazar BR, et al. Simultaneous absence of surfactant proteins A and D increases lung inflammation and injury after allogeneic HSCT in mice. Am J Physiol Lung Cell Mol Physiol. 2009;296(2):L167–175. doi: 10.1152/ajplung.90253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan CK, Carey AJ, Cui X, Webb RI, Ipe D, Crowley M, et al. Genome-wide mapping of cystitis due to Streptococcus agalactiae and Escherichia coli in mice identifies a unique bladder transcriptome that signifies pathogen-specific antimicrobial defense against urinary tract infection. Infection and immunity. 2012;80(9):3145–3160. doi: 10.1128/IAI.00023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100(1):6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol. 2001;166(2):1148–1155. doi: 10.4049/jimmunol.166.2.1148. [DOI] [PubMed] [Google Scholar]
- 58.Tsai KW, Lai HT, Tsai TC, Wu YC, Yang YT, Chen KY, et al. Difference in the regulation of IL-8 expression induced by uropathogenic E. coli between two kinds of urinary tract epithelial cells. J Biomed Sci. 2009;16:91. doi: 10.1186/1423-0127-16-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo CJ, Atochina-Vasserman EN, Abramova E, Foley JP, Zaman A, Crouch E, et al. S-nitrosylation of surfactant protein-D controls inflammatory function. PLoS Biol. 2008;6(11):e266. doi: 10.1371/journal.pbio.0060266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang C, Mendonsa GR, Symington JW, Zhang Q, Cadwell K, Virgin HW, et al. Atg16L1 deficiency confers protection from uropathogenic Escherichia coli infection in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(27):11008–11013. doi: 10.1073/pnas.1203952109. [DOI] [PMC free article] [PubMed] [Google Scholar]