Abstract
Interleukin-1β (IL-1β) is a pleotropic cytokine known to influence the central nervous system (CNS) responses to injury or infection. IL-1β also directly induces astrocytic expression of tissue inhibitor of metalloproteinases (TIMP)-1, a potent trophic factor and regulator of matrix metalloproteinase activity. In this study we examined the functional relationship between IL-1β and TIMP-1 and determined that the behavior of astrocytes in response to IL-1β is determined by TIMP-1 expression. Using primary astrocytes from C57Bl/6 mice, we found astrocytes from wildtype (Wt) mice exhibited a robust wound healing response to a scratch wound that was arrested in response to IL-1β. In contrast, TIMP-1 knockout (TIMP-1KO) astrocytes, exhibited minimal response to the scratch wound but an accelerated response following IL-1β-treatment. We also determined that the scratch wound effect in Wt cultures was attenuated by inhibition of Rho kinase but amplified in the TIMP-1KO cultures. We propose that the specific induction of TIMP-1 from astrocytes in response to IL-1β reflects a previously unrecognized physiological relationship where the directionality of astrocytic behavior is determined by the actions of TIMP-1. These findings may provide additional insight into glial responses in the context of neuropathology where expression of TIMP-1 may vary and astrocytic responses may be impacted by the inflammatory milieu of the CNS.
Keywords: Astrocyte, TIMP-1, IL-1β, ROCK, MMP
1. Introduction
Astrocytes are known to promote cellular differentiation, regulate synapse function, and promote axonal regeneration[1–3]. A prominent feature of many neurological diseases and injuries is 'reactive astrogliosis', which is defined by proliferation, astrocytic phenotype changes, changes in morphology and gene expression that is believed to reflect pathological changes in astrocytic functions[4, 5]. The mechanisms regulating astrogliosis are not fully understood.
Reactive gliosis can be modeled in vitro by stimulating astrocytes with pro-inflammatory factors, such as interleukin 1 beta (IL-1β). IL-1β replicates features observed with reactive gliosis in vivo, including upregulated glial fibrillary acidic protein (GFAP) gene expression and cellular hypotrophy[6, 7]. Importantly, IL-1β induction of reactive astrogliosis has been shown to be due to deactivation of the Rho kinase (ROCK) signaling pathway; constitutively active ROCK was found to eliminate the impaired astrocytic response induced by IL-1β[8]. In addition, it has been shown that this IL-1β effect is partially due to the effect of the extracellular matrix and cross talk between additional signaling cascades such as ERK1/2, leaving an area of interest with regard to regulation of IL-1β effects on astrocytes[9]. Interestingly, the ROCK pathway has been implicated in a variety of CNS diseases including stroke and Alzheimer’s disease (AD) where ROCK inhibitors are potential therapeutic agents[10]. Astrogliosis is also a hallmark feature of these diseases, suggesting that pathological changes in ROCK pathway regulation may affect astrocyte functions in disease.
IL-1β is also known to modify the behavioral response of astrocytes to injury[11], in part, through altering the astrocyte secretome[12]. Tissue Inhibitor of Metalloproteinase (TIMP)-1 is a highly inducible secreted protein produced by astrocytes after CNS infection, inflammation, or injury[4, 5, 13]. TIMP-1 expression is also directly regulated by IL-1β[14–16]. We have recently determined that reactive gliosis is greatly diminished in the absence of TIMP-1[2], and that TIMP-1 is a potent activator of astrocytes[2]. Given the ubiquitous induction of TIMP-1 with acute brain injuries in association with astrogliosis[13], and the pleiotropic nature of TIMP-1 function[2], we hypothesized that TIMP-1 may impart physiological responses to astrocytes resulting from IL-1β exposure.
Herein, we report that the astrocyte responses to IL-1β are determined by the production of TIMP-1 as it regulates the functional effect of by modulating injury-induced activation of ROCK pathway. These findings provide new information on the functions of astrocytes that relate to pathology in many CNS diseases.
2. Materials and Methods
2.1 Primary Astrocyte Cultures
Cultures were developed from cerebral cortices of neonatal C57BL/6 wildtype or TIMP-1 deficient (KO) mouse pups (P0–P3) using a neural tissue dissociation kit (Miltenyi Biotec)[11, 17]. Cells were plated in T175 flasks for 2 weeks before detachment using trypsin (Sigma) and re-plating into 24-well plates onto laminin-coated coverglass (Ln, 10 µg/µL; Sigma Aldrich) The purity of each culture system (GFAP+ cells) was consistent with previous reports[2, 18], as verified by immunocytochemistry (ICC) for GFAP (1:1000, Chemicon), and, Iba-1 to identify microglia (1:1000, WAKO).
2.2 Scratch Injury model
A scratch injury ~600µm in diameter across a confluent astrocyte monolayer was made using a sterile P200 pipette tip[11, 19]. At varying times after injury, cells were fixed and ICC performed. Treatments included: IL-1β (10 ng/ml; Peprotech)[8, 11]; rm-TIMP-1 (10ng/mL; R&D) or the TIMP-1 C-terminal domain peptide (amino acids 126–184; Anaspec Inc.)[2]; GM6001 (12.5 µmol/L; Calbiochem)[20]; ROCK-inhibitor, Y-27632 (10 µM; Fisher)[8]. Scratch injuries were measured perpendicular to the longitudinal axis of the scratch at a minimum of three points spanning the width of the scratch. Measurements were then used to determine the amount of recovery relative to baseline (i.e. wound diameter at time of the scratch, or t=0) for each sample and treatment. The average of each technical replicate was then compared across biological replicates to assess variability, though all data points were included in the final analyses.
2.3 ELISA
A TIMP-1 ELISA (Duoset; R&D Systems) was performed on conditioned media samples according to manufacturer’s protocol, as previously described[2].
2.4 Immunocytochemistry (ICC)
ICC was performed as previously described[2]. Cultures were fixed in 4% paraformaldehyde, washed and incubated with primary fluorescent conjugated antisera for Glial Fibrillary Acidic Protein (GFAP-Cy3; 1:1000, Sigma). 4',6-diamidino-2-phenylindole (DAPI) was added after incubation to counterstain nuclei. Immunoreactivity was visualized by fluorescence microscopy (Olympus, IX71) and representative images acquired using image analysis software (Empix Imaging).
2.5 Rho-associated Kinase (ROCK) Activity Assay
was performed according to manufacturer’s protocol (Cell Biolabs, Inc.). Briefly, protein lysates were collected from astrocyte cultures and placed in MYPT-1 coated wells; the level of ROCK activity was measured by optical density to quantify Thr696 phosphorylation of MYPT-1[21].
2.6 Statistics
Experiments were performed in quadruplicate replicates and then repeated in triplicate. Comparisons between treatments were made using t-tests, or, where appropriate, a two-way ANOVA with Bonferroni post-hoc tests was used to evaluate response differences between genotypes with treatments using GraphPad Prism software (La Jolla, CA). Data are presented as mean±SEM. The null hypothesis for all experiments was P<0.05.
3. Results
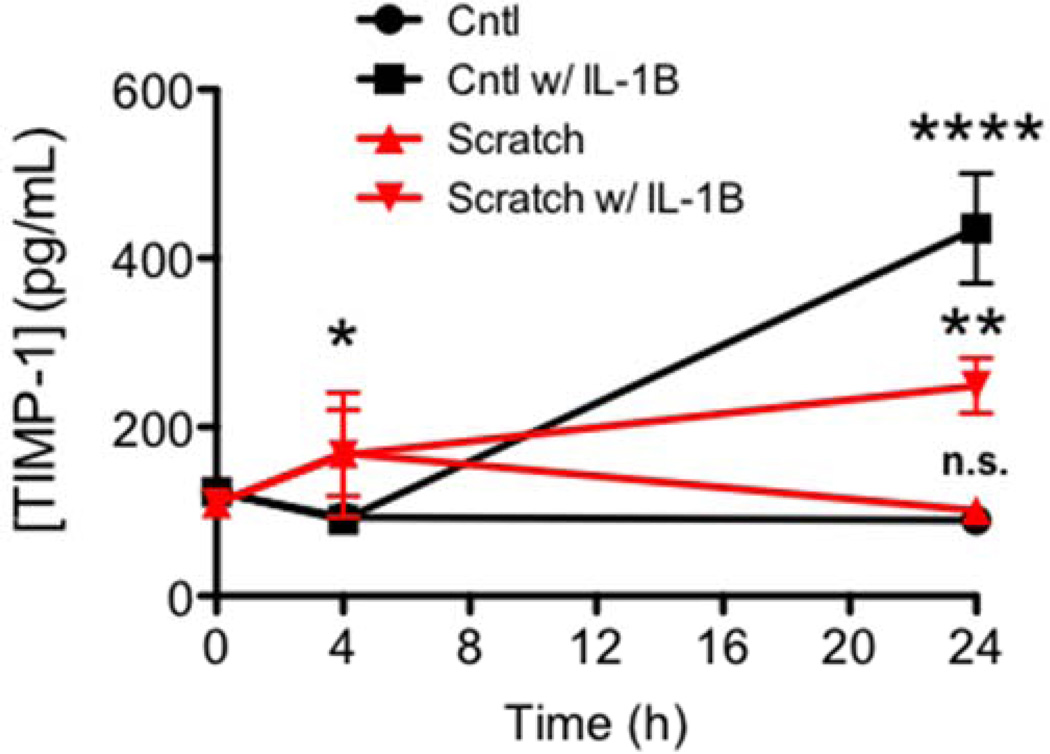
To understand the dynamics of TIMP-1 expression in culture and to determine whether its expression was altered by a mechanical wound in vitro, we measured levels of TIMP-1 in conditioned media from Wt cultures either at the time of the scratch (t0), 4 or 24 hours later. We found that production of TIMP-1 was induced over time by the scratch wound without any additional stimulus, where TIMP-1 levels were significantly elevated by 4 hours post injury, and subsequently returned to baseline levels by 24 hours (Figure 1). No significant changes in TIMP-1 production was found in untreated, unscratched cultures over the same 24 hr time course (Figure 1). Consistent with previous findings we found IL-1β induced a significant increase in TIMP-1 protein levels in the conditioned media from Wt cultures (Figure 1). We next determined what effect mechanical wound stress would have on induction of TIMP-1 in response to IL-1β treatment. Interestingly, when compared with either scratched or IL-1β treatment alone, TIMP-1 production in the scratched astrocyte cultures was delayed and significantly lessened in scratched cultures that were also treated with IL-1β (Figure 1). These data pointed to a previously unrecognized complexity in the relationship between IL-1β, TIMP-1 and mechanical wound stress in astrocytes.
Figure 1. TIMP-1 induction in response to mechanical injury and inflammation.
Analysis of TIMP-1 production in primary cultures 0, 4, and 24 hrs in response to control (squares) or IL-1β treatment (triangles) and scratch wound (red lines). 2-way ANOVA, P<0.0002; where ****, P<0.0001 Cntl w/ IL-1β vs. Cntl; **P<0.01 Scratch w/ IL-1β vs. Cntl w/ IL-1β, and * P<0.05, Scratch w/ IL-1β or Scratch vs Cntl.
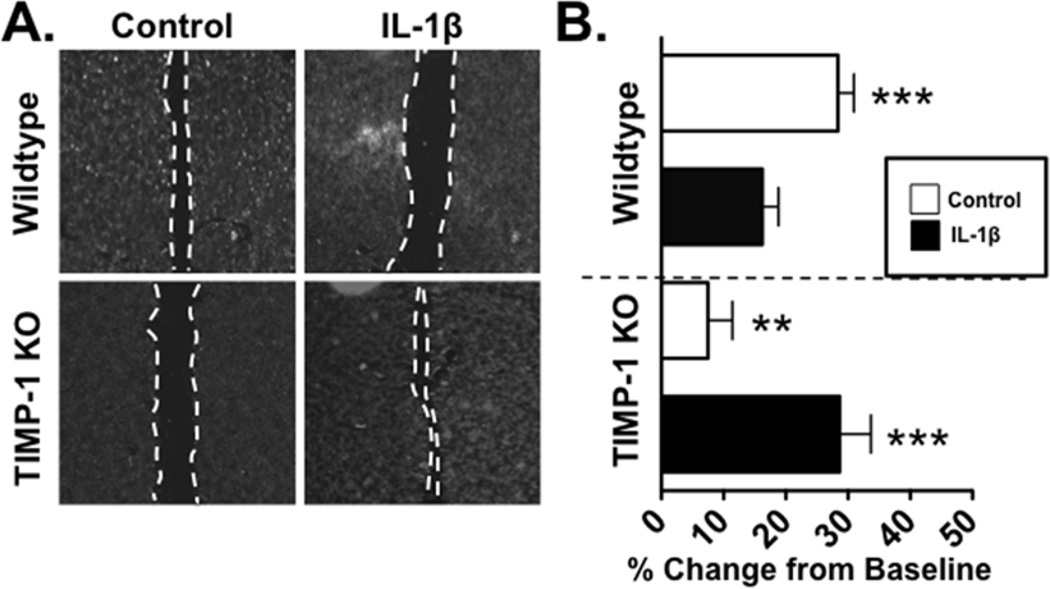
Our previous studies had determined that TIMP-1 can directly activate astrocytes[2], but the functional implications of this activation had not been previously addressed. To examine how TIMP-1 influences the behavior of primary astrocytes in culture, we compared confluent primary Wt and TIMP-1KO astrocyte cultures and assessed their response to a scratch wound[11]. We found significant recovery in Wt astrocytes by 24 hours after the scratch (Figure 2), however, TIMP-1KO astrocytes exhibited minimal recovery (Figure 2). Since (a) IL-1β induces TIMP-1 expression, (b) IL-1β treatment attenuated the production of TIMP-1 when the culture was scratched, and (c) IL-1β treatment is also known to arrest scratch recovery in astrocyte cultures[8, 11], we next examined the effect IL-1β on scratch recovery in TIMP-1KO astrocytes. Consistent with previous work[8, 11], we found that administration of IL-1β (10ng/mL) was sufficient to arrest recovery in Wt glial cells, whereas application of IL-1β was found to increase the rate of scratch wound recovery in TIMP-1KO cultures (Figure 2). Thus, the physiological response of astrocytes to IL-1B was inverted in the absence of TIMP-1.
Figure 2. The physiological effect of IL-1β on astrocytes is inverted in TIMP-1KO cultures.
(A). Representative images of WT and TIMP-1KO astrocytes treated with vehicle or IL-1β. (B). Quantitative analysis of scratch recovery (in percent) from baseline scratch wound in confluent Wt and TIMP-1KO astrocyte cultures treated with vehicle or IL-1β (10 ng/ml).ANOVA, P<0.0001; **, P<0.01 TIMP-1KO Cntl vs. Wt Cntl; ***, P<0.001, Wt Cntl vs TIMP-1KO Cntl or Wt IL-1β, and P<0.002 TIMP-1KO IL-1β vs. TIMP-1KO Cntl. Scale bar =
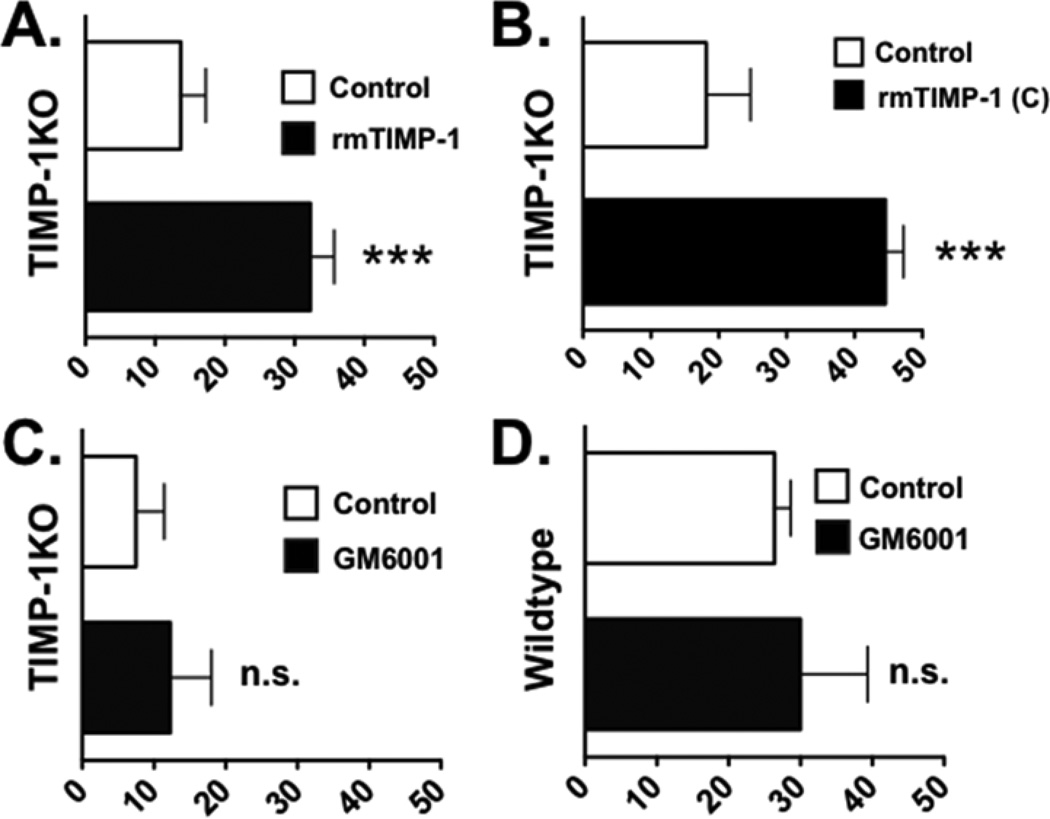
To confirm that TIMP-1 was responsible for these differences in the astrocytic response to the scratch injury, we examined whether we could recover a "wildtype" phenotype by introducing TIMP-1 to the TIMP-1KO astrocytes. Application of recombinant murine (rm)-TIMP-1 protein was found to significantly improve wound recovery (P=0.0017, Figure 3A). TIMP-1 is well-characterized as a dual function protein, with both metalloproteinase-inhibitory function and a receptor-mediated growth factor function. To determine what function of TIMP-1 mediated the scratch recovery, we then applied a recombinant peptide of TIMP-1 which encompassed only the trophic factor domain and found that it significantly improved scratch wound recovery (Figure 3B). When we applied GM6001 (12.5uM), a non-selective broad spectrum MMP inhibitor compound, to either TIMP-1KO cultures (Figure 3C) or Wt cultures (Figure 3D) and measured the scratch response, we found that GM6001 did not significantly alter the scratch wound response of either genotype (P=0.3369; Figure 3C,D). This result indicated that deficit in scratch wound response in TIMP-1KO astrocyte cultures was a result of a trophic factor action of TIMP-1 and not via inhibition of MMPs.
Figure 3. Introduction of recombinant TIMP-1 restores Wt-like phenotype response to TIMP-1KO cultures.
(A) Quantification of scratch recovery (in percent from baseline) in TIMP-1KO astrocyte cultures treated with either vehicle or rm-TIMP-1 (10 ng/ml). (B). Quantification of scratch recovery in TIMP-1 KO astrocyte cultures treated with a C-terminal peptide of TIMP-1 [TIMP-1(C); 10 ng/ml]. (C,D) Quantification of scratch recovery in TIMP-1KO and wildtype astrocyte cultures treated broad spectrum MMP-inhibitor, GM6001, or vehicle (control). Student's t test:; ***, P=0.001.
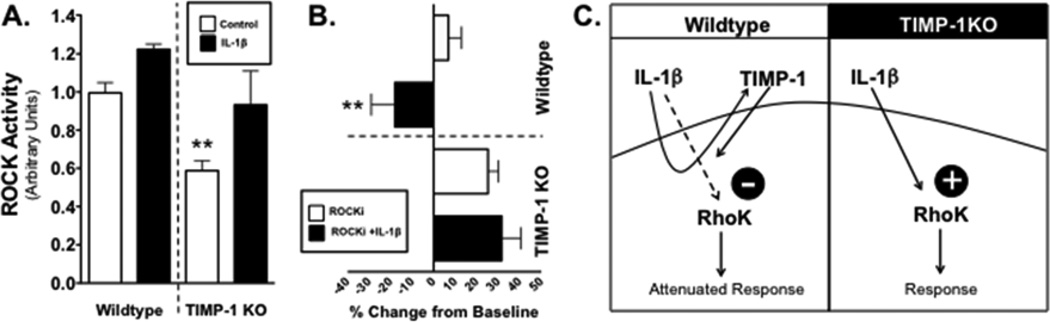
To determine why the physiological response of astrocytes to IL-1β is inverted in TIMP-1KO astrocytes, we examined how TIMP-1 may influence IL-1β regulation of Rho GTPases[8]. The ROCK pathway is important for cell shape, motility, and migration[22]. ROCK activity was measured in cell lysates from Wt and TIMP-1KO cultures that had been either treated with IL-1β, or vehicle, and either a ROCK inhibitor (ROCKi, Y-27632; 10 µM), or vehicle control. Basal ROCK activity in TIMP-1KO astrocytes was significantly lower than basal levels measured in Wt cultures (P<0.01, Figure 4A). Treatment with IL-1β increased ROCK activity in both Wt and TIMP-1 KO cultures (Figure 4A), with the increase in IL-1β induced ROCK activity in TIMP-1 KO cultures equal to levels in untreated Wt cultures (P>0.05).
Figure 4. Diminished astrocytic Rho kinase activity in absence of TIMP-1.
(A). ROCK activity measured in Wt and TIMP-1KO astrocytes treated with either IL-1β or vehicle (control). Two-way ANOVA; **, P=0.007 for genotype, P<0.018 for treatment effect. (B). Quantification of scratch recovery in Wt and TIMP-1KO astrocyte cultures (in percent from baseline scratch) when treated with either IL-1β or IL-1β and Rho kinase (ROCK) inhibitor. Two-way ANOVA, ** P<0.0009 for genotype interaction, and P<0.01 for IL-1β treatment. (C) Hypothesized IL-1β pathway regulation of RhoK and the influence of TIMP-1 where the absence of receptor-mediated signaling via TIMP-1 (i.e. TIMP-1KO) modifies the physiological response of astrocytes to IL-1β.
To evaluate the functional impact of these differences in ROCK activity, we performed the scratch assay on Wt and TIMP-1KO astrocyte cultures using ROCKi and IL-1β (Figure 4B). Addition of ROCKi to Wt cultures dropped ROCK activity to levels equal to basal levels in TIMP-1KO cultures (not shown), and there was a significant decrease in the ability of the wt astrocytes to recover from the injury (Figure 4B). Addition of IL-1β to ROCKi treated Wt cultures significantly impaired wound healing resulting in negative recovery (i.e. wound widening). In contrast, application of the ROCKi to TIMP-1KO astrocyte improved the scratch wound response compared to baseline scratch response in these cultures (ANOVA, P<0.005; Figure 4B), and, there was no measurable effect of IL-1β over that of ROCKi treatment in the knockout cultures (Figure 4B)
4. Discussion
Our data confirm previous findings that ROCK activation is important for recovery from a scratch injury in Wt astrocytes[8]. However, our data also indicate that inhibition of ROCK in TIMP-1KO astrocytes elicited an opposite response to Wt cultures. These data imply that the physiological impact of IL-1β on astrocytes may be determined, in part, by the production and ability of astrocytes to express TIMP-1. TIMP-1 is a top candidate gene upregulated in two models of astrogliosis[5], and we have previously shown that TIMP-1 is important for astrocyte activity in vivo[2]. There is also an emerging understanding that TIMP-1 is dynamically regulated and that dysregulation of TIMP-1 expression by astrocytes may contribute to context under which astrocytes respond in a variety of neurological diseases. We postulate that many of the actions of TIMP-1 in this context are not MMP-mediated but rather through a receptor-mediated signaling mechanism. Previous studies have reported TIMP-1 activation of PI3K and Akt signaling in non-neural cells[23], though these process have not yet been directly confirmed in CNS cell types[24]. Future studies on the trophic actions of TIMP-1 may provide important insights into the acute effect of inflammation on astrocytes in disease, where a rapid elevation in TIMP-1 have been associated with CNS repair. In contrast, elevated expression of TIMP-1 is not always observed in several chronic inflammatory neurodegenerative diseases, including HIV-encephalomyelitis and multiple sclerosis (MS)[25–27], suggesting that a reduced propensity of astrocytes to express TIMP-1 may result from chronic inflammation and then significantly impact how astrocytes function/respond in these chronic disease states.
In this study, we wanted to further elucidate the contribution and function of astrocytes in response to IL-1β, which has been shown to be important for the formation of the glial scar because the mechanism that generates the astroglial phenotype of the reactive astrocytes is still not fully understood. A better understanding of the regulation of astrocyte reactivity could provide a beneficial insight into astroglial heterogeneity in function and phenotype in disease. In particular, the role of TIMP-1 and the ability of astrocytes to react to external stimulation. These previous observations have lead to our study to understand a potential mechanism for TIMP1 regulation of astrocyte activity. On a cellular level, our data are also consistent with primary human astrocyte cultures from MS lesion biopsies, which have been reported to exhibit increased proliferation and migratory capacity when compared with cultures derived from non-diseased brain or spinal cord regions[28]. Although measurement of TIMP-1 produced in the context of human disease-specific astrocyte cultures has not yet been reported, our findings would suggest that the behavior of astrocytes may strongly influenced by TIMP-1 expression and this would change over time as a result of the chronic inflammation during disease.
We have found that the astrocyte response to IL-1β is linked to the presence of TIMP-1, and that this response is coupled to the activity of the ROCK pathway. The ROCK pathway has been implicated in many cell functions including migration. It is important to highlight that the cellular response to a scratch wound is complex and is known to engage many signaling pathways related to cell proliferation, migration and cell death[29]. For instance, cdc42, another GTPase shown to impact astrocyte behavior in the scratch assay[19], also interacts with ROCK signaling[30]. Based on our scratch assay data (Figure 4), the absence of TIMP-1 dramatically influenced the magnitude of scratch wound recovery by regulating ROCK activity in response to IL-1β. It is possible that a mechanism for TIMP-1 regulation of astrocyte responses to IL-1β may involve signaling molecules such as cdc42. Interestingly, another upregulated gene in response to astrogliosis, is lipocalin 2 (lcn2)[5], which also has been previously shown to play an important role in astrocytic reactivity is also positively regulated by ROCK pathway signaling[31]. Previous studies have found that stimulating lcn2 can act to polarize the function of astrocytes[32] by activating the ROCK pathway and stimulating reactive gliosis[31]. If TIMP-1 is found to regulate lcn2 expression, future studies may provide a plausible ROCK-mediated mechanism by which astrocytic reactivity, astrogliosis, and wound healing may be managed TIMP-1.
Dysfunction of astrocytes is recognized as an underlying etiology of a number of neurological diseases including: MS, AD, and amyotrophic lateral sclerosis (ALS)[2, 13, 33–37]. Reactive astrocytes surround MS plaques and are thought to impair remyelination[38], which suggests an important role for astrocytes in the pathology of MS. In AD, activated astrocytes have been shown to release pro-inflammatory factors that promote inflammation and foster Aβ deposition[36]. Astrocytes are thought to cause of motor neuron degeneration in ALS[37, 39]. TIMP-1 has been implicated in all of these, and other, neurodegenerative diseases. Therefore, our findings may have broad implications for understanding astrocytic functions in a variety of neurodegenerative diseases[13].
5. Conclusions
Astrocyte responses to IL-1β are profoundly influenced by the production of TIMP-1 through modulation of the ROCK pathway. These findings provide new information on how TIMP-1 may control astrocyte function and impact astrocyte function(s) in a variety of CNS diseases.
Highlights.
TIMP-1KO astrocytes exhibited an arrested response to a scratch wound.
IL-1β treatment, which arrested scratch response in Wt cultures, instead evoked a robust recovery to scratch in TIMP-1KO astrocyte cultures.
Inhibition of Rho kinase in Wt cultures arrested scratch response to IL-1β, but enhanced scratch response in TIMP-1KO cultures.
Astrocytic expression of TIMP-1 strongly influences the response of astrocytes to IL-1β
Acknowledgments
We thank Sophie Gysling and Cory Willis for technical assistance. This work was supported by grants (to S.J.C.) from National Institutes of Health (NS078392), National Multiple Sclerosis Society (RG5001-A-3), and CT Innovations (SCA-06-011).
Abbreviations
- IL-1β
Interleukin-1β
- TIMP-1
Tissue Inhibitor of Metalloproteinase-1
- ROCK
Rho Kinase
- MMP
Matrix Metalloproteinase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Claycomb KI, Johnson KM, Winokur PN, Sacino AV, Crocker SJ. Astrocyte regulation of CNS inflammation and remyelination. Brain sciences. 2013;3:1109–1127. doi: 10.3390/brainsci3031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore CS, Milner R, Nishiyama A, Frausto RF, Serwanski DR, Pagarigan RR, Whitton JL, Miller RH, Crocker SJ. Astrocytic tissue inhibitor of metalloproteinase-1 (TIMP-1) promotes oligodendrocyte differentiation and enhances CNS myelination. The Journal of neuroscience. 2011;31:6247–6254. doi: 10.1523/JNEUROSCI.5474-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen NJ, Barres BA. Neuroscience: Glia - more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- 4.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 5.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. Journal of neuroscience. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sticozzi C, Belmonte G, Meini A, Carbotti P, Grasso G, Palmi M. IL-1beta induces GFAP expression in vitro and in vivo and protects neurons from traumatic injury-associated apoptosis in rat brain striatum via NFkappaB/Ca(2)(+)-calmodulin/ERK mitogen-activated protein kinase signaling pathway. Neuroscience. 2013;252:367–383. doi: 10.1016/j.neuroscience.2013.07.061. [DOI] [PubMed] [Google Scholar]
- 7.John GR, Lee SC, Brosnan CF. Cytokines: powerful regulators of glial cell activation. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2003;9:10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- 8.John GR, Chen L, Rivieccio MA, Melendez-Vasquez CV, Hartley A, Brosnan CF. Interleukin-1beta induces a reactive astroglial phenotype via deactivation of the Rho GTPase-Rock axis. Journal of neuroscience. 2004;24:2837–2845. doi: 10.1523/JNEUROSCI.4789-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Summers L, Kangwantas K, Nguyen L, Kielty C, Pinteaux E. Adhesion to the extracellular matrix is required for interleukin-1 beta actions leading to reactive phenotype in rat astrocytes. Molecular and cellular neurosciences. 2010;44:272–281. doi: 10.1016/j.mcn.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubo T, Yamaguchi A, Iwata N, Yamashita T. The therapeutic effects of Rho-ROCK inhibitors on CNS disorders. Therapeutics and clinical risk management. 2008;4:605–615. doi: 10.2147/tcrm.s2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson KM, Milner R, Crocker SJ. Extracellular matrix composition determines astrocyte responses to mechanical and inflammatory stimuli. Neuroscience letters. 2015 doi: 10.1016/j.neulet.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keene SD, Greco TM, Parastatidis I, Lee SH, Hughes EG, Balice-Gordon RJ, Speicher DW, Ischiropoulos H. Mass spectrometric and computational analysis of cytokine-induced alterations in the astrocyte secretome. Proteomics. 2009;9:768–782. doi: 10.1002/pmic.200800385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crocker SJ, Pagenstecher A, Campbell IL. The TIMPs tango with MMPs and more in the central nervous system. Journal of neuroscience research. 2004;75:1–11. doi: 10.1002/jnr.10836. [DOI] [PubMed] [Google Scholar]
- 14.Crocker SJ, Whitmire JK, Frausto RF, Chertboonmuang P, Soloway PD, Whitton JL, Campbell IL. Persistent macrophage/microglial activation and myelin disruption after experimental autoimmune encephalomyelitis in tissue inhibitor of metalloproteinase-1-deficient mice. The American journal of pathology. 2006;169:2104–2116. doi: 10.2353/ajpath.2006.060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crocker SJ, Milner R, Pham-Mitchell N, Campbell IL. Cell and agonist-specific regulation of genes for matrix metalloproteinases and their tissue inhibitors by primary glial cells. Journal of neurochemistry. 2006;98:812–823. doi: 10.1111/j.1471-4159.2006.03927.x. [DOI] [PubMed] [Google Scholar]
- 16.Welser-Alves JV, Crocker SJ, Milner R. A dual role for microglia in promoting tissue inhibitor of metalloproteinase (TIMP) expression in glial cells in response to neuroinflammatory stimuli. Journal of neuroinflammation. 2011;8:61. doi: 10.1186/1742-2094-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crocker SJ, Frausto RF, Whitton JL, Milner R. A novel method to establish microglia-free astrocyte cultures: comparison of matrix metalloproteinase expression profiles in pure cultures of astrocytes and microglia. Glia. 2008;56:1187–1198. doi: 10.1002/glia.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welser-Alves JV, Boroujerdi A, Tigges U, Milner R. Microglia use multiple mechanisms to mediate interactions with vitronectin; non-essential roles for the highly-expressed alphavbeta3 and alphavbeta5 integrins. Journal of neuroinflammation. 2011;8:157. doi: 10.1186/1742-2094-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robel S, Bardehle S, Lepier A, Brakebusch C, Gotz M. Genetic deletion of cdc42 reveals a crucial role for astrocyte recruitment to the injury site in vitro and in vivo. The Journal of neuroscience. 2011;31:12471–12482. doi: 10.1523/JNEUROSCI.2696-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claycomb KI, Winokur PN, Johnson KM, Nicaise AM, Giampetruzzi AW, Sacino AV, Snyder EY, Barbarese E, Bongarzone ER, Crocker SJ. Aberrant production of tenascin-C in globoid cell leukodystrophy alters psychosine-induced microglial functions. Journal of neuropathology and experimental neurology. 2014;73:964–974. doi: 10.1097/NEN.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barcia C, Ros CM, Annese V, Carrillo-de Sauvage MA, Ros-Bernal F, Gomez A, Yuste JE, Campuzano CM, de Pablos V, Fernandez-Villalba E, Herrero MT. ROCK/Cdc42-mediated microglial motility and gliapse formation lead to phagocytosis of degenerating dopaminergic neurons in vivo. Scientific reports. 2012;2:809. doi: 10.1038/srep00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz AA, Govek EE, Bottner B, Van Aelst L. Rho GTPases: signaling, migration, and invasion. Experimental cell research. 2000;261:1–12. doi: 10.1006/excr.2000.5049. [DOI] [PubMed] [Google Scholar]
- 23.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Science signaling 1. 2008;1(27):re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SY, Kim JM, Cho SY, Kim HS, Shin HS, Jeon JY, Kausar R, Jeong SY, Lee YS, Lee MA. TIMP-1 modulates chemotaxis of human neural stem cells through CD63 and integrin signalling. The Biochemical journal. 2014;459:565–576. doi: 10.1042/BJ20131119. [DOI] [PubMed] [Google Scholar]
- 25.Gardner J, Borgmann K, Deshpande MS, Dhar A, Wu L, Persidsky R, Ghorpade A. Potential mechanisms for astrocyte-TIMP-1 downregulation in chronic inflammatory diseases. Journal of neuroscience research. 2006;83:1281–1292. doi: 10.1002/jnr.20823. [DOI] [PubMed] [Google Scholar]
- 26.Ichiyama T, Kajimoto M, Suenaga N, Maeba S, Matsubara T, Furukawa S. Serum levels of matrix metalloproteinase-9 and its tissue inhibitor (TIMP-1) in acute disseminated encephalomyelitis. Journal of neuroimmunology. 2006;172:182–186. doi: 10.1016/j.jneuroim.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Avolio C, Filippi M, Tortorella C, Rocca MA, Ruggieri M, Agosta F, Tomassini V, Pozzilli C, Stecchi S, Giaquinto P, Livrea P, Trojano M. Serum MMP-9/TIMP-1 and MMP-2/TIMP-2 ratios in multiple sclerosis: relationships with different magnetic resonance imaging measures of disease activity during IFN-beta-1a treatment. Multiple sclerosis. 2005;11:441–446. doi: 10.1191/1352458505ms1193oa. [DOI] [PubMed] [Google Scholar]
- 28.De Groot CJ, Langeveld CH, Jongenelen CA, Montagne L, Van Der Valk P, Dijkstra CD. Establishment of human adult astrocyte cultures derived from postmortem multiple sclerosis and control brain and spinal cord regions: immunophenotypical and functional characterization. Journal of neuroscience research. 1997;49:342–354. doi: 10.1002/(sici)1097-4547(19970801)49:3<342::aid-jnr9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 29.Kornyei Z, Czirok A, Vicsek T, Madarasz E. Proliferative and migratory responses of astrocytes to in vitro injury. Journal of neuroscience research. 2000;61:421–429. doi: 10.1002/1097-4547(20000815)61:4<421::AID-JNR8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton. 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S, Park JY, Lee WH, Kim H, Park HC, Mori K, Suk K. Lipocalin-2 is an autocrine mediator of reactive astrocytosis. The Journal of neuroscience. 2009;29:234–249. doi: 10.1523/JNEUROSCI.5273-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang E, Kim JH, Lee S, Kim JH, Seo JW, Jin M, Lee MG, Jang IS, Lee WH, Suk K. Phenotypic polarization of activated astrocytes: the critical role of lipocalin-2 in the classical inflammatory activation of astrocytes. Journal of immunology. 2013;191:5204–5219. doi: 10.4049/jimmunol.1301637. [DOI] [PubMed] [Google Scholar]
- 33.Moore CS, Abdullah SL, Brown A, Arulpragasam A, Crocker SJ. How factors secreted from astrocytes impact myelin repair. Journal of neuroscience research. 2011;89:13–21. doi: 10.1002/jnr.22482. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 35.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nature neuroscience. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF, Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A. Glial cells in (patho)physiology. Journal of neurochemistry. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lasiene J, Yamanaka K. Glial cells in amyotrophic lateral sclerosis. Neurology research international. 2011;2011:718987. doi: 10.1155/2011/718987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnett SC, Linington C. Myelination: do astrocytes play a role? The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2013;19:442–450. doi: 10.1177/1073858412465655. [DOI] [PubMed] [Google Scholar]
- 39.Morimoto N, Nagai M, Ohta Y, Miyazaki K, Kurata T, Morimoto M, Murakami T, Takehisa Y, Ikeda Y, Kamiya T, Abe K. Increased autophagy in transgenic mice with a G93A mutant SOD1 gene. Brain research. 2007;1167:112–117. doi: 10.1016/j.brainres.2007.06.045. [DOI] [PubMed] [Google Scholar]