Abstract
Bone erosion is a sign of severe rheumatoid arthritis and osteoclasts play a major role in the bone resorption. Recently, myeloid-derived suppressor cells (MDSC) has been reported to be increased in collagen-induced arthritis (CIA). The number of circulating MDSCs is shown to correlate with rheumatoid arthritis. These findings suggest that MDSCs are precursor cells involved in bone erosion. In this study, MDSCs isolated from mice with CIA stimulated with M-CSF and RANKL in vitro expressed osteoclast markers and acquired osteoclast bone resorption function. MDSCs sorted from CIA mice were transferred into the tibia of normal DBA/J1 mice and bones were subjected to histological and Micro CT analyses. The transferred CIA-MDSCs were shown to differentiate into TRAP+ osteoclasts that were capable of bone resorption in vivo. MDSCs isolated from normal mice had more potent suppressor activity and much less capability to differentiate to osteoclast. Additional experiments showed that NF-κB inhibitor Bay 11-7082 or IκB inhibitor peptide blocked the differentiation of MDSCs to osteoclast and bone resorption. IL-1Ra also blocked this differentiation. In contrast, the addition of IL-1α further enhanced osteoclast differentiation and bone resorption. These results suggest that MDSCs are a source of osteoclast precursors and inflammatory cytokines such as IL-1, contributing significantly to erosive changes seen in rheumatoid arthritis and related disorders.
Keywords: MDSC, osteoclast precursor, osteoclast, bone destruction, collagen-induced arthritis and rheumatoid arthritis
1. INTRUCTION
Rheumatoid arthritis (RA) is an autoimmune disease characterized by inflammatory synovitis and subsequent cartilage and bone destruction (erosive changes) leading to severe disabilities [1, 2]. Bone erosions have been reported to be present in 45% of patients with early rheumatoid arthritis [3]. Bone erosion involves osteoclastic bone resorption [4]. Osteoclasts are multinucleated polykaryons commonly derived from monocyte/macrophage lineage. Their major function is bone absorption [5]. Monocytes/macrophages in the presence of monocyte colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL) differentiate into osteoclasts [6]. Over production of osteoclast or increased osteoclast activity often leads to cartilage and bone destruction [7]. In RA, a large number of osteoclasts accumulate in the synovial tissue, causing resorption pits and local bone destruction in the joint [8]. However, the origin of the precursors of these osteoclasts remains obscure [9].
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells with immunosuppressive functions. They accumulate in the spleen and bone marrow in a variety of pathologic conditions [10]. In mice, MDSCs are characterized by the expression of cell surface markers CD11b and Gr-1 and are further defined into CD11b+Ly6ChighLy6G− or CD11b+Ly6ClowLy6G+ subset [11]. The number of MDSCs was reported to increase in autoimmune diseases [12–15]. Recent reports from us and other investigators [15, 16] showed that MDSCs are increased in CIA and that the depletion of MDSC diminished joint swelling and bony destruction. In RA patients, there was a correlation between DAS 28 and the number of circulating MDSC. These observations suggest that MDSCs act as osteoclast precursors in both CIA and rheumatoid arthritis. In this study, MDSCs isolated from mice with CIA were shown to differentiate to osteoclasts with the ability to cause bone resorption both in vitro and in vivo.
2. MATERIALS AND METHODS
2.1. Mice
Eight week old male DBA/1J mice were purchased from SLAC Laboratory Animal Centre (Shanghai, China) and kept in specific pathogen-free conditions in Sun Yat-sen University Animal Center. Chow and water were supplied ad libitum. All animal procedures were approved by the ethical committee of the First Affiliated Hospital, Sun Yat-sen University and performed in accordance with the guidelines provided by the National Institute of Health Guide for Care and Use of Animals.
2.2. Collagen-induced arthritis
Collagen-induced arthritis (CIA) was carried out as previously described [17]. Bovine type II collagen (CII, Chondrex, USA) was emulsified with Freund’s complete adjuvant (Chondrex, USA) at an equal volume. 100μl emulsion containing 100 μg of CII was injected into mice intradermally at the base of the tail on day 0. The mice received a booster challenge of CII emulsified with Freund’s incomplete adjuvant on day 21. Mice were monitored by two blinded examiners every two days for signs of arthritis onset and for arthritis scoring.
2.3. Single cell suspension preparation
Mice were sacrificed by cardiac puncture after they were anaesthetized with chloral hydrate. Long bones and spleens were collected. PBS (3×1ml) was injected into the cavities of long bones to flush out the marrow content. Collected cell suspensions were filtered through a nylon filter. Spleens were teased and cell suspensions were collected after filtering through a nylon mesh. Red cells were lysed by red cell lysing buffer (Sigma, USA).
2.4. Immunosuppressive assay
Bone marrow cells isolated from CIA mice on day 35 after the first immunization were stained with FITC-anti-Gr-1 and PE-anti-CD11b antibodies. CD11b+Gr-1+ MDSCs from bone marrow cells of CIA or normal mice were isolated by flow cytometry (BD influx, USA). The purity of cells was confirmed > 95% by flow analysis. Isolated splenocytes labeled with 5, 6-carboxyfluorescein diacetatesuccinimidyl ester (CFSE) (Invitrogen, USA) according to the manufacture’s instruction. 5×105 splenocytes were co-cultured with sorted MDSCs in 96-well culture plates in the presence of 1μg/ml of anti-CD3/CD28 antibodies (Biolegend, USA) at a ratio of 1:1. After 72 hours of stimulation, cells were collected and stained with APC-anti-CD4 antibody (BD Pharmingen, USA). The proliferation of CD4+ T cells was calculated according to the dilution of CFSE [18].
2.5. Osteoclast differentiation
CD11+bGr-1+ MDSCs from mice with CIA (35 days after the first immunization) or normal mice were sorted by flow cytometry (BD influx, USA). The purification was confirmed by flow cytometry (>95%). 2×105 MDSCs were seeded into 48-well culture plates with or without coverslips in α-MEM (Gibco, USA), 10% heat-inactivated FCS (Hyclone, USA), 50ng/ml of M-CSF and 100ng/ml of RANKL (Both from Peprotech, USA). This culture media is referred to as osteoclast differentiation media. In some experiments, 10ng/ml of IL-1α (Peprotech, USA), 300ng/ml of IL-1 receptor antagonist (IL-1Ra, Prospec, Isreal), 2.5μM of Bay 11-7082 (Sigma, USA) or 200 μM IκB kinase inhibitor peptide (Calbiochem, USA) were included. Culture media was replaced every two days.
Bone marrow derived macrophages that are classical osteoclast precursors were used as positive control. To prepare bone marrow derived macrophages, cells were collected from the long bones of normal DBA/1J mice. Cells were washed twice and let cells to adhere in the dishes. Non-adherent bone marrow cells were collected and cultured in α-MEM containing 10ng/ml of M-CSF (Peprotech, USA). Cells cultured in M-CSF for 2 days were used as bone marrow derived macrophages. Thereafter, cells were cultured with medium with 50ng/ml of M-CSF and 100ng/ml of RANKL [19].
The differentiation of MDSCs to osteoclasts was detected by staining for tartrate-resistant acid phosphatase (TRAP) using a TRAP staining kit (Sigma, USA) according to the manufacturer’s instructions.
2.6. F-actin and bone resorption assays
MDSCs were sorted from CIA bone marrow cells on day 35 and 2×105 cells were cultured under osteoclast differentiation conditions for up to 12 days. Culture media was replaced every two days. Cells were fixed with 4% paraformaldehyde at room temperature for 15 minutes, washed with PBS twice and stained with FITC-phalloidin (Sigma, USA) at 37°C for 30 minutes to detect F-actin. For the bone resorption assay [20], bovine cortical bone slides were layered at the bottom of culture plates. 2×105 of sorted MDSCs were seeded onto the slides and cultured in the osteoclast differentiation media for up to 15 days. Resorption pits on the slides were shown by staining with toluidine blue.
2.7. qPCR
Sorted CD11b+Gr-1+ MDSC cells were cultured under osteoclast differentiation conditions for up to 12 days. Cells were collected from day 6 for the detection of MMP-9, Cathepsin-k (Ctsk) and TRAP by qPCR. The PCR primers were MMP-9 (forward 5′-CTTCTTCTCTGGACGTCAAATG-3′, reverse 5′-CATTTTGGAAACTCACACGCC-3′), Ctsk (forward 5′-GATGCTTACCCATATGTGGGC-3′, reverse 5′-CATATCCTTTGTTTCCCCAGC-3′), TRAP (forward 5′-GCCAAGATGGATTCATGGGTGG-3′, reverse 5′-CAGAGACATGATGAAGTCAGCG-3′) and GAPDH (forward 5′-ACATCATCCCTGCATCCACTG-3′, reverse 5′-TCATTGAGAGCAATGCCAGC-3′). RNA was reverse-transcribed into cDNA using a reverse transcript kit (Fermentas, USA) according to the manufacturer’s instructions. cDNA was amplified by using recombinant Taq DNA polymerase (Fermentas, USA). SYBR green-based quantitative real-time PCR was performed using a Bio-Rad IQ5 (Bio-Rad, USA). The amplification was performed using the following conditions: preheating at 95°C for 10 min and then 35 cycles of denaturing at 95°C for 15 s, annealing and extension at 60°C for 1 min. Results of comparative real-time PCR were analyzed using IQ5 Software (Bio-Rad, USA). GAPDH was used as a control.
2.8. Western blot analysis
Sorted MDSCs were seeded into a 24-well culture plate. Briefly, cells were stimulated with 10ng/ml IL-1α for up to 2 hours. Cytoplasmic and nuclear proteins were extracted by using a nuclear protein extraction kit (Pierce, USA) according to the manufacturer’s instructions. Cytoplasmic protein was for the detection of p-IκB and the nuclear protein was for the detection of NF-κB p65. Proteins were loaded and separated by 10% SDS-polyacrylamide gels. Proteins were electro-transferred onto polyvinylidine difluoride membranes after separation. Blocked with 5% skim milk in TBST, the membranes were incubated with anti-p-IκB (Cell Signaling Technology, USA), anti-NF-κB p65 (Santa, Cruz, USA), anti-GAPDH (Kangcheng, China) or anti-fibrillarin (Santa Cruz, USA) primary antibodies at 4°C overnight. The membranes were then washed with TBST and incubated with horseradish peroxidase conjugated anti-rabbit IgG (Cell Signaling Technology, USA) at room temperature for 60 minutes. The membranes were washed with TBST and the signal was detected by enhanced chemiluminescence (ECL). Semi-quantification of band density was measured by densitometry with image J software.
2.9. Osteoclast differentiation in vivo
MDSCs sorted by flow cytometry were labeled with CFSE. 2×105 labeled MDSCs in PBS were transferred into the proximal tibia of normal male DBA/1J mice. An equal volume of PBS was injected into the tibia as control. Three days later, mice received another injection of 2×105 CFSE labeled MDSCs or PBS. Mice were sacrificed and a single cell suspension was prepared from the long bones. To confirm the differentiation of osteoclasts from MDSC in vivo, CFSE+ cells were collected from bone marrow cells by flow cytometry for the detection of CtsK and TRAP. At the same time, long bones were fixed with 10% formalin for 24 hours and subjected to Micro-CT scan and analyzed by ZKKS-MicroCT 4.1 software (ZKK-MCT-Sharp, China). For TRAP detection and H&E stain, bones were decalcified with 10% EDTA for 4 weeks and embedded in paraffin, sections 4 μm in thickness were stained with H&E and TRAP. For quantification of TRAP+ cells, Zeiss Axio Imager Z1 was used.
2.10. Statistical analyses
Data were expressed as the means ± SD. Statistical analysis was performed using SPSS 13.0. The differences were assessed by t test, or one way ANOVA with or without repeated measurements followed by Bonferroni’s multiple comparison post test as appropriate. Two-tailed p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Increased MDSCs are associated with bone destruction in mice with CIA
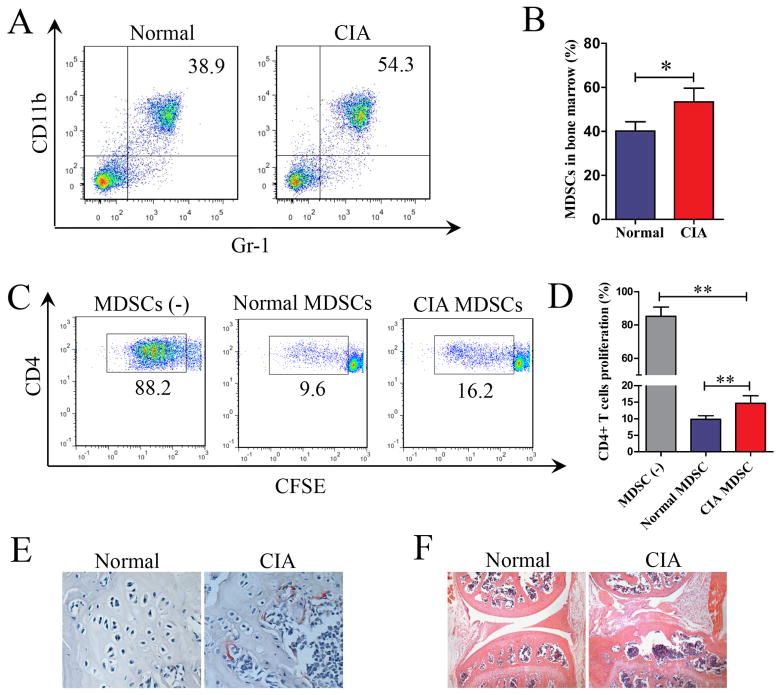
Single cell suspension of bone marrow cells from mice with CIA (35 days after the first immunization with CII) or from normal mice were analyzed for MDSC by flow cytometry. The number of MDSCs in the bone marrow of mice with CIA increased significantly compared to that of normal mice (Figure 1A and 1B).
Figure 1. Expansion of MDSCs in bone marrow and bone destruction in CIA.
(A) Mice with CIA were sacrificed on day 35 and age-matched normal mice were used as controls. Bone marrow cells were stained with anti-CD11b and anti-GR-1 mAbs. Data were acquired by flow cytometry and representative dot plots are presented. (B) The frequency of MDSCs in the bone marrow of normal or CIA mice. Six mice in each group were tested. (C) Splenocytes from normal mice were labeled with CFSE and co-cultured with or without 5×105 MDSCs from CIA or normal mice in anti-CD3/CD28 bound culture plates. Cells were collected and stained with APC-anti-CD4 mAb after 72 hours stimulation. Data was analyzed by flow cytometry and representative dot plots are presented. (D) The proliferation rates of CD4 T+ cells. Data were acquired by flow cytometry and summarized from three independent experiments. (E, F) Mice with CIA were sacrificed on day 35. Bones were fixed and decalcified with EDTA. Knee joints section were stained for TRAP (E) and with H&E (F). Six mice were tested for each group and representative images are presented. Data in B and D are shown as mean ± SD. *p<0.05, **p <0.01.
Splenocytes (5×105 cells) were labeled with CFSE and stimulated with anti-CD3/CD28 mAbs in the presence or absence of an equal number of MDSCs isolated from mice with CIA or normal mice. After 72 hours of culturing, cells were collected and stained with APC-anti-CD4 antibody. As shown in Figure 1C and 1D, MDSCs from CIA or normal mice suppressed the proliferation of CD4+ T cells. MDSCs from CIA mice in comparison to MDSCs from normal mice were less effective in the suppression assay. As shown in Figure 1E and 1F, marked increased osteoclast formation and bone destruction in mice with CIA were evident by TRAP and H&E stains of the knee joint sections.
3.2. MDSCs from CIA mice can differentiate into TRAP+ osteoclasts and are capable of bone resorption in vitro
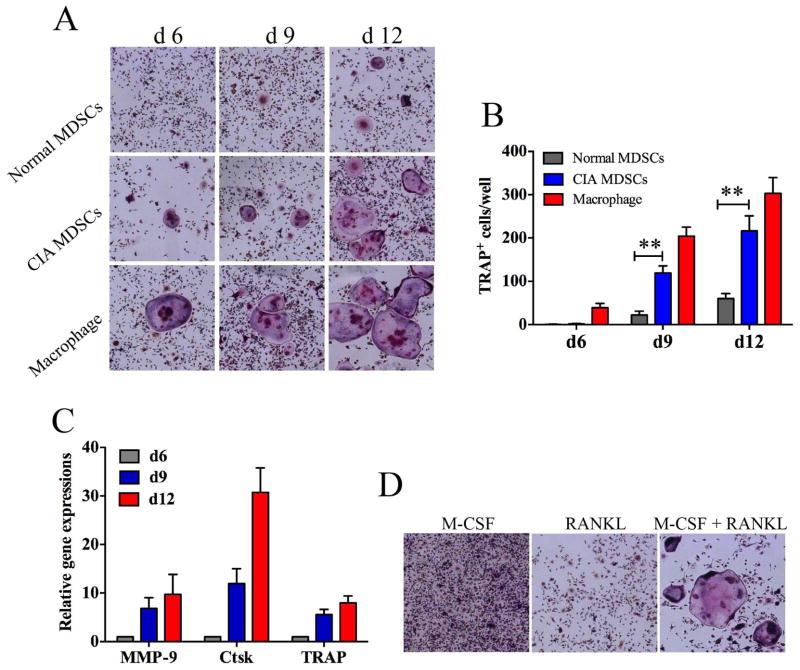
MDSCs from bone marrow of mice with CIA or normal mice were cultured in 48-well culture plates with M-CSF and RANKL. As a positive control, bone marrow derived macrophages were cultured under similar conditions. As shown in Figure 2A and 2B, very few TRAP+ multinuclear osteoclasts were observed on day 6 in the culture of MDSCs. In contrast TRAP+ multinuclear osteoclasts were readily observed in the culture of bone marrow derived macrophages MDSCs. However, on day 9 and on day 12, TRAP+ multinuclear osteoclasts were observed readily in the cultures of MDSCs. In addition, MDSCs from CIA mice formed significantly more osteoclast compared to MDSCs from normal mice. The quantitative aspect of these experiments is shown in Figure 2B. MDSC-derived osteoclasts expressed high levels of MMP-9, CtsK and TRAP as determined by qPCR (Figure 2C). An additional control is shown in Figure 2D. M-CSF or RANKL alone did not stimulate the differentiation of osteoclasts from MDSCs
Figure 2. Differentiation of MDSCs and bone marrow-derived macrophages into TRAP+ osteoclasts.
MDSCs from the bone marrow of mice with CIA or normal mice were sorted by flow cytometry and cultured in the presence of 50ng/ml M-CSF and 100ng/ml RANKL. Bone marrow derived macrophages were cultured under similar conditions as positive controls. (A) Cells recovered at different time points were stained for TRAP. Representative images are presented. (B) The numbers of TRAP+ osteoclasts in each well from cultures at different time points are shown. (C) mRNA expressions of MMP-9, CasK and TRAP by cultured MDSCs were determined by qPCR. (D) MDSCs were cultured for 12 days and stained with TRAP, representative images are presented. Data in B and C are shown as mean ± SD. The results represent three independent experiments., **p <0.01.
3.3. MDSC derived osteoclasts form podosomes and have bone resorption function
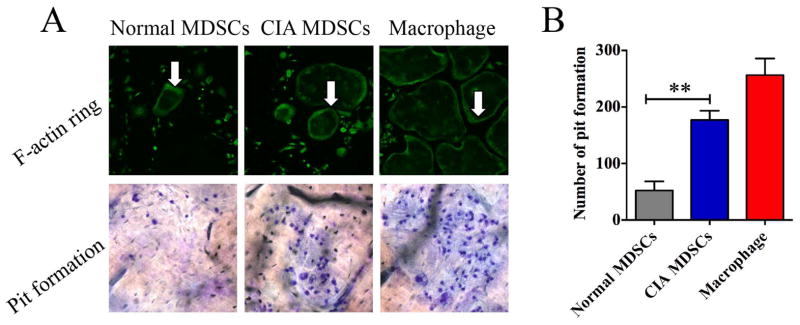
The osteoclasts derived from MDSCs from bone marrow of mice with CIA mice were characterized further. The expression of a band of F-actin containing podosomes in osteoclasts has been shown to be involved in bone resorption [21] Thus MDSC derived osteoclasts were stained with FITC-phalloidin. Bands of F-actin containing podosomes were detected in both MDSC and bone marrow derived macrophage cultures (Figure 3A). This implies that MDSC derived osteoclasts are capable of causing bone erosion.
Figure 3. Bone resorption by osteoclasts derived from MDSC isolated from mice with CIA and bone marrow derived macrophages.
(A) MDSCs isolated from mice with CIA or normal mice were cultured in the presence of 50ng/ml M-CSF and 100ng/ml RANKL for 12 days. Cells were fixed and stained with FITC-phalloidin (white arrows). For the bone resorption analysis, cells were cultured with bovine cortical slides layered at the bottom of culture plate for 15 days and the cortical slides were stained with toluidine blue. Representative images are presented. (B) Pit formation on each slide was counted. Bone marrow derived macrophages were used as positive controls. Data are shown as mean ± SD. The results represent three independent experiments. **p <0.01.
To demonstrate directly the capacity of MDSC derived osteoclasts to resorb bone, bovine cortical slides were layered at the bottom of the culture plates and the cells were seeded onto the slides. The cultures were kept for 15 days. The slides were then fixed and stained with toluidine blue. Pit formation appeared both in the slides cultured with MDSCs and in slides cultured with bone marrow derived macrophages (Figure 3A). The quantitative aspect of these experiments is summarized in Figure 3B. MDSCs from CIA mice were more readily to differentiate to osteoclast and cause bone resorption compared to those from normal mice.
3.4. MDSCs differentiated into osteoclasts capable of causing bone resorption in vivo
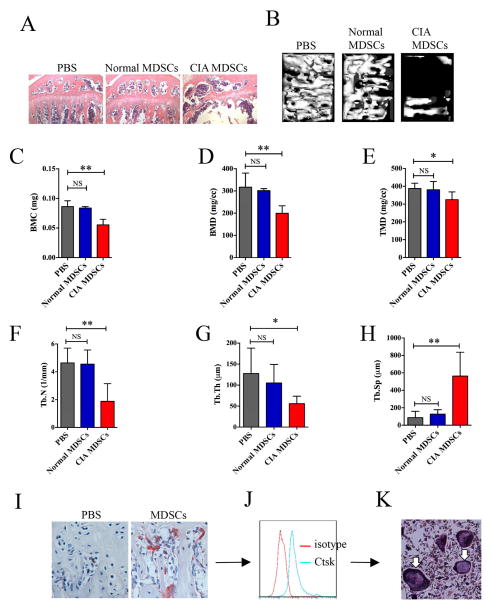
MDSCs were isolated from mice with CIA or normal mice, 2×105 MDSCs or an equal volume of PBS were injected into the tibia of normal male DBA/1J mice for 2 times at a 3-day interval. Mice were sacrificed on day 12. Bone sections from mice that received MDSC from mice with CIA (CIA-MDSCs) showed significant bone destruction as shown by H&E staining (Figure 4A). No obvious bone destruction was observed in control mice injected with either normal-MDSCs or PBS. Micro-CT scan of the tibia showed that the number of trabeculae was significantly lower in mice receiving CIA-MDSCs from CIA mice in comparison with those seen in control (Figure 4B). The bone mineral content, bone mineral density, and tissue mineral density were significantly lower in the tibia of mice receiving CIA-MDSCs (Figure 4C, 4D and 4E). Trabecular number and trabecular thickness were also significantly decreased in mice injected with CIA-MDSCs (Figure 4F and 4G). The distance between trabecules (trabecular separation) was significantly longer (Figure 4H). In addition, CIA-MDSCs injected mice had much more osteoclast formation in the tibia (Figure 4I). In this experiment, mice received normal-MDSCs had mild bone destruction. However, none of the indexes reached a statistically significant difference compared to the mice that received PBS injection. To confirm that the increased osteoclasts were derived from transferred MDSCs, CIA-MDSCs were stained with CFSE and injected into the tibia of the mice. CFSE+ cells were isolated from the bone marrow on day 12 by flow cytometry. Flow cytometry data showed that CFSE+ cells expressed CtsK, an specific marker for osteoclast (Figure 4J). The sorted CFSE+ cells expressed TRAP after culturing with M-CSF and RANKL for 24 hours (Figure 4K). These results confirm the osteoclast precursor role of MDSCs from mice with CIA and their capability of differentiation into osteoclasts capable of causing bone resorption in vivo.
Figure 4. Osteoclasts derived from MDSCs isolated from mice with CIA are capable of bone resorption in vivo.
(A) Isolated MDSCs from mice with CIA or normal mice (CIA-MDSCs or Normal-MCSDs) were injected into the tibia of normal DBA/1J mice on day 0 and day 3. PBS was injected in the third group (PBS) as an additional control. Mice were sacrificed 12 days later. Tibia sections were stained by H&E. Representative images are presented. (B) Micro-CT showed trabecula in the tibia and representative images are presented. (C–H) Micro-CT analysis of 1.1mm × 0.8mm × 0.3mm bone tissue under the grow plate line of tibia. (C) BMC, bone mineral content. (D) BMD, bone mineral density. (E) TMD, tissue mineral density. (F) Tb.N, trabecular number. (G) Tb.Th, trabecular thickness. (H) Tb.Sp, trabecular separation. In (I) to (J), CFSE-labeled CIA-MDSCs were injected into the tibia of normal mice. Tibia slides were stained for TRAP. Representative images were presented (J). CFSE+ bone marrow cells were sorted and stained for Ctsk and analyzed by flow cytometry 12 days after injection (K). Sorted CFSE+ bone marrow cells were cultured in a medium containing 50ng/ml M-CSF and 100ng/ml RANKL for 24 hours. Osteoclast formation was confirmed by TRAP staining (white arrows). Data are shown as mean ± SD. Data are summarized from 5 mice of each group. *p<0.05, ** p<0.01.
3.5. NF-κB pathway plays a critical role in the differentiation of MDSCs to osteoclasts
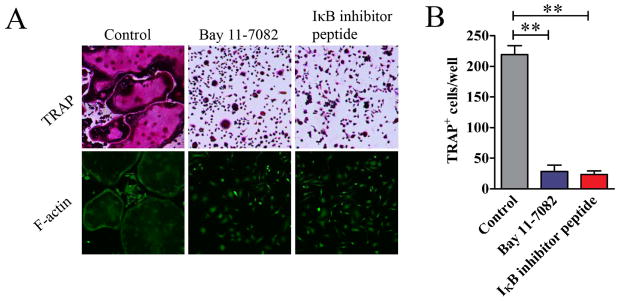
The requirement of RANKL, receptor activator of nuclear factor κB for osteoclast differentiation, suggests that the NF-κB pathway plays a critical role in the differentiation of osteoclasts. To directly test whether this pathway is essential for MDSC differentiation to osteoclasts, MDSCs from CIA mice were cultured in the presence of NF-κB inhibitor Bay 11-7082. Bay 11-7082 significantly inhibited the differentiation of MDSCs to osteoclasts as shown by the marked decrease in staining for TRAP (Figure 5A) and the decrease in TRAP+ cells in the culture (Figure 5B). In addition, no F-actin ring was observed in Bay 11-7082-treated cells (Figure 5A). Similarly, an IκB kinase inhibitor peptide, which has been reported to inhibit NF-κB signal pathway specifically [22, 23], inhibited the differentiation of MDSCs to osteoclasts. These results imply that NF-κB pathway is essential in the differentiation of MDSCs to osteoclasts and the function of MDSC-derived osteoclast.
Figure 5. NF-κB signaling pathway in the differentiation of osteoclast from MDSCs.
MDSCs from the bone marrow of mice with CIA or normal mice were cultured in the presence of 50ng/ml M-CSF and 100ng/ml RANKL with or without Bay 11-7082 or IκB kinase inhibitor peptide for 12 days. (A) Cells were stained for TRAP or with FITC-phalloidin. Representative images are presented. (B) TRAP+ osteoclasts in each well were quantified and are shown as mean ± SD. The results represent three independent experiments. **p<0.01
3.6. IL-1 signal promotes osteoclast differentiation from MDSC via activating NF-κB pathway
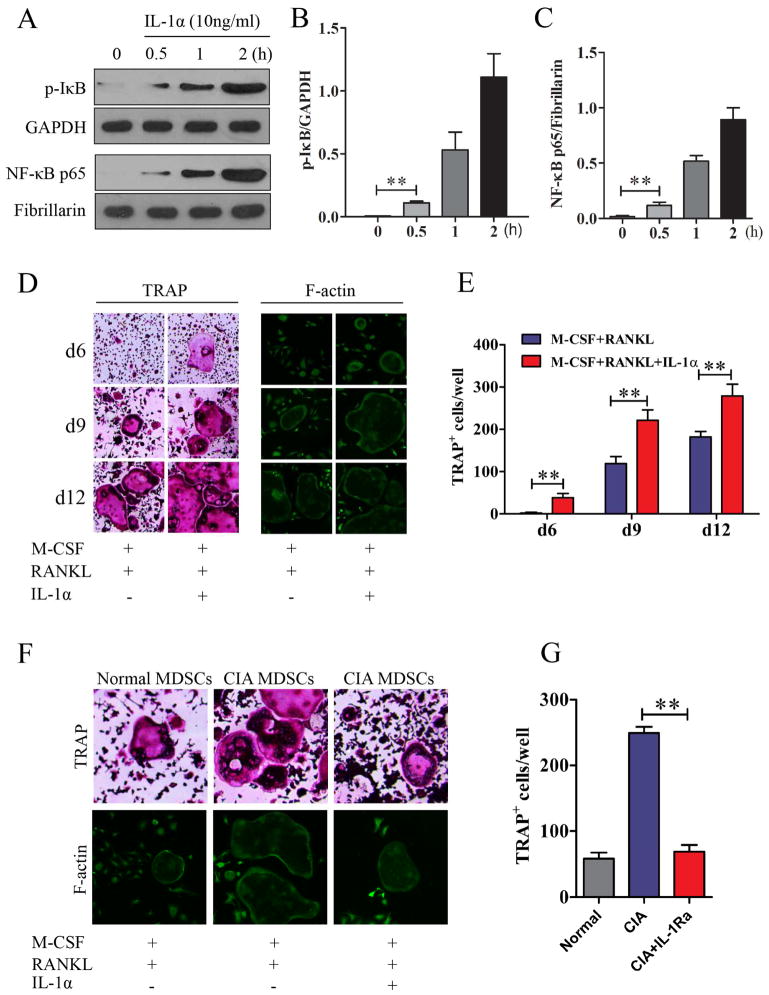
Our previous studies and those of others showed that MDSC from CIA mice produced more IL-1 compared to those from normal mice [15, 16]. In addition, IL-1 has been shown to be correlated with joint damage index [24] and blocking IL-1 has been demonstrated to protect bone and cartilage in the RA animal model and patients [25]. To study the role of IL-1 in the differentiation of MDSCs to osteoclasts, IL-1α (10ng/ml) stimulated MDSCs were lysed. Extracted cytoplasmic and nuclear proteins were subjected to western blot analysis respectively. There was a significant increase in the phosphorylation of IκB (Figure 6A and 6B). In addition, the translocation of NF-κB p65 into the nucleus was also markedly increased compared to that in the control group (Figure 6A and 6C). The addition of IL-1α (10ng/ml) into the osteoclast differentiation culture medium further enhanced the differentiation of MDSCs to osteoclasts as evident by the earlier appearance of TRAP and the increased number of TRAP+ cells (Figure 6D and 6E). In addition, IL-1α also increased the expression of F-actin ring (Figure 6D), indicating that IL-1 also enhanced the bone resorption function. Furthermore, the differentiation of MDSCs to osteoclasts in the presence of M-CSF and RANKL without exogenous source of IL-1 was inhibited by the addition of IL-1Ra (Figure 6F and 6G). This further confirms a significant role of IL-1 in the differentiation of MDSCs to osteoclasts. It is of interest in Figure 6F to note that the in vitro differentiation of MDSCs from mice with CIA to osteoclasts was much more robust in comparison with those from normal mice.
Figure 6. Promotion of osteoclast differentiation from MDSC by IL-1α through activating NF-κB pathway.
(A)–(C). Activation of NF-κB signal pathway by IL-1α. MDSCs isolated from mice with CIA were cultured with 10ng/ml IL-1α. At 0, 0.5, 1.0 and 2.0 hours, cytoplasmic and nuclear proteins were extracted for the detection of p-IκB and NF-κB p65 by western blot respectively (A). Quantitative data are shown in (B) and (C). Isolated MCSDs were cultured in the presence of 50ng/ml M-CSF and 100ng/ml RANKL with or without 10ng/ml IL-1α. Cells were stained for TRAP and with FITC-phalloidin. Representative images are presented in (D). TRAP+ osteoclasts under different culture conditions were quantified and are presented in (E). Isolated MDSCs from normal mice or mice with CIA were cultured in the presence of 50ng/ml M-CSF and 100ng/ml RANKL with or without 300ng/ml IL-1Ra for 12 days. Cells were stained for TRAP or with FITC-phalloidin (F) and TRAP+ osteoclasts in each well were quantified (G). Data are shown as mean ± SD. The results represent three independent experiments. **p <0.01.
4. DISCUSSION
It has been well documented that erosions are found by magnetic resonance imaging (MRI) in a significant number of patients with early rheumatoid arthritis (3). Osteoclastic bone resorption has been implicated in both rheumatoid arthritis and CIA, an experimental model of rheumatoid arthritis [4, 26]. Although osteoclasts have been shown to be derived from hematopoietic stem cells and specifically from the monocyte/macrophage lineage [4, 27], the precursors of osteoclasts in rheumatoid arthritis and in CIA have not been identified. In this study, MDSCs were shown to be osteoclast precursor cells. These precursors can differentiate to osteoclasts both in vitro and in vivo. The induced osteoclasts express characteristic biomarkers such as TRAP [25] and podosomes [19, 26]. They also express high levels of MMP-9 and CtsK [27]. The MDSC derived osteoclasts are shown to have bone resorption capability by both in vitro and in vivo assays. It is of interest to note that MDSCs from mice with CIA have significantly more potential to differentiate to osteoclasts in comparison to those from normal mice. Since most erosive lesions in RA and in CIA are detected in small joints where hematopoiesis is not likely to be active, the precursors of osteoclasts are likely derived from circulating cells. The results from this study identify the source of the osteoclast precursors in MDSCs that are likely to circulate in diseased mice. Thus MDSCs are involved in the bone erosion of CIA. In addition, MDSCs are also likely to be implicated in bone erosion in rheumatoid arthritis.
Osteoclasts are terminally differentiated cells derived from monocyte/macrophage lineages and hematopoietic stem cells. The differentiation of osteoclast precursors into functional osteoclasts is essential for bone resorption as well as for maintaining bone structure, function and remodeling [5]. However, exaggerated expansion of osteoclast precursors can lead to formation of excessive osteoclasts, resulting in bone destruction. It has been reported that the number of osteoclast precursors in psoriasis arthritis patients was increased [28] and was associated with enhanced bone erosion [29]. Similarly, this phenomenon has also been observed in CIA mice [26]. In agreement with these studies, the number of osteoclast precursors identified in the present study was significantly increased in the bone marrow of mice with CIA.
NF-κB is a critical transcription factor in the process of differentiation of hematopoietic cells [30, 31]. NF-κB deficient mice failed to generate mature osteoclasts [32]. In addition, the NF-κB signal is involved in the activation and survival of osteoclasts [33]. In the present study, osteoclast formation was markedly reduced by NF-κB inhibitor Bay 11-7082 and IκB kinase inhibitor peptide, indicating that NF-κB plays an important role in the differentiation of osteoclast from MDSC. In addition, the roles of other proinflammatory cytokines in the differentiation of osteoclast need to be emphasized [34]. It has been shown that TNF-α stimulates osteoclast differentiation in a RANKL-RANK independent mechanism [35]. IL-6 produced by osteoblast promotes the differentiation of osteoclast from osteoclast presursors and increased osteoclast function [36]. IL-17 and IL-23 produced by lymphocytes aslo promoted osteoclast differentiation [37].
It has been reported that IL-1 signal induced osteoclast activation and differentiation from bone marrow-derived macrophages through activating NF-κB [33, 38]. In the presence of TNF-α and IL-1, osteoclasts are shown to be activated in rheumatoid arthritis in a manner independent of RANKL [39]. The source of IL-1 remains to be ascertained. In this study, IL-1 is shown to be involved in the differentiation of MDSCs to osteoclasts. The addition of IL-1 significantly increased the number of TRAP+ osteoclasts in cultured MDSCs in the presence of M-CSF and RANKL with enhanced bone resorption ability. In addition, the differentiation of MDSCs isolated from mice with CIA to osteoclasts was inhibited by IL-1Ra. Our previous study [15] showed that MDSCs isolated from mice with CIA make significant amounts of IL-1. Thus MDSCs is the source of endogenous IL-1 that is needed for the differentiation of MDSCs isolated from mice with CIA to osteoclasts. It remains to be determined whether some of the osteoclast precursors in MDSCs in arthritic mice can be directly activated by IL-1 alone or in conjunction with TNFα. Nevertheless, these data allow us to conclude that IL-1, a major cytokine in the pathogenesis of rheumatoid arthritis, plays a significant role in bone erosion. Our results may explain the slowing of progressive bone damage when patients were treated with IL-1Ra [40].
It has been reported that the numbers of circulating MDSCs correlate with the numbers of circulating Th17 cells [15, 16]. Furthermore, disease activity as measured with DAS28 is correlative with the numbers of circulating MDSCs [15]. In view of the observation that MDSCs isolated from mice with CIA have greater potential to differentiate to osteoclasts, it would be of interest to determine whether erosive and other joint changes detected by MRI correlate with the numbers of circulating MDSCs. If this is indeed the case, circulating MDSCs would be considered as a biomarker for erosive rheumatoid arthritis. This biomarker may also be useful in monitoring patients’ responses to therapy.
5. Conclusions
Our results demonstrate that MDSCs expand in the bone marrow of CIA mice and are associated with bone destruction. Isolated MDSCs from CIA mice can differentiate into bone destruction osteoclast when stimulated with M-CSF and RANKL in vitro. Further results show that MDSCs can differentiate into osteoclast and cause bone resorption in vivo. MDSCs differentiate into osteoclast depends on NF-κB and IL-1 signal pathways. In conclusion, MDSCs are source of osteoclast precursor and contribute to bone destruction in CIA.
Myeloid-derived suppressor cells (MDSC) expand and in associate with bone destruction in collagen-induced arthritis.
MDSC can differentiate into functional osteoclast in vitro and in vivo.
MDSC differentiate into osteoclast depends on NF-κB and IL-1 signals.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (81273278 and 81471598), the PhD Program Foundation of Ministry of Education of China (20120171110064), Guangdong Natural Science Foundation (S2012010008780, and S2011010004578), and the Guangzhou Science and Technology Planning Program (2012J4100085). SMF and FG were supported by grants from the National Institutes of Health (R01-AR047988 and R01-AR049449) and a grant from the Alliance for Lupus Research.
Abbreviations
- MDSC
Myeloid-derived suppressor cell
- RA
Rheumatoid arthritis
- M-CSF
Macrophage Colony-stimulating Factor
- RANKL
Receptor activator of NF-κB ligand
- CIA
Collagen-induced arthritis
- CtsK
Cathepsin-K
- IL-1Ra
IL-1 receptor antagonist
- TRAP
tartrate-resistant acid phosphatase
- ECL
enhanced chemiluminescence
- MRI
Magnetic resonance imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis. 2002;61(Suppl 2):ii84–6. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 3.McQueen FM, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan PL, et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals a high prevalence of erosions at four months after symptom onset. Ann Rheum Dis. 1998;57:350–6. doi: 10.1136/ard.57.6.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, Goldring SR. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998;152:943–51. [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 6.Quinn JM, Elliott J, Gillespie MT, Martin TJ. A combination of osteoclast differentiation factor and macrophage-colony stimulating factor is sufficient for both human and mouse osteoclast formation in vitro. Endocrinology. 1998;139:4424–7. doi: 10.1210/endo.139.10.6331. [DOI] [PubMed] [Google Scholar]
- 7.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–14. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 8.Schett G. Cells of the synovium in rheumatoid arthritis. Osteoclasts Arthritis Res Ther. 2007;9:203. doi: 10.1186/ar2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamopoulos IE, Mellins ED. Alternative pathways of osteoclastogenesis in inflammatory arthritis. Nat Rev Rheumatol. 2015;11:189–94. doi: 10.1038/nrrheum.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–44. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Haile LA, von Wasielewski R, Gamrekelashvili J, Kruger C, Bachmann O, Westendorf AM, et al. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135:871–81. 81 e1–5. doi: 10.1053/j.gastro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Trigunaite A, Khan A, Der E, Song A, Varikuti S, Jorgensen TN. Gr-1(high) CD11b+ cells suppress B cell differentiation and lupus-like disease in lupus-prone male mice. Arthritis Rheum. 2013;65:2392–402. doi: 10.1002/art.38048. [DOI] [PubMed] [Google Scholar]
- 14.Yi H, Guo C, Yu X, Zuo D, Wang XY. Mouse CD11b+Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis. J Immunol. 2012;189:4295–304. doi: 10.4049/jimmunol.1200086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Wang S, Huang Y, Wang H, Zhao J, Gaskin F, et al. Myeloid-derived suppressor cells are proinflammatory and regulate collagen-induced arthritis through manipulating Th17 cell differentiation. Clin Immunol. 2015 doi: 10.1016/j.clim.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo C, Hu F, Yi H, Feng Z, Li C, Shi L, et al. Myeloid-derived suppressor cells have a proinflammatory role in the pathogenesis of autoimmune arthritis. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Zhao J, Zhang H, Huang Y, Wang S, Tu Q, et al. CARD11 blockade suppresses murine collagen-induced arthritis via inhibiting CARD11/Bcl10 assembly and T helper type 17 response. Clin Exp Immunol. 2014;176:238–45. doi: 10.1111/cei.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–6. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 19.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 20.Scholtysek C, Krönke G, Schett G. Bone Resorption Assay. Bio-protocol. 2014;4(14):e1187. [Google Scholar]
- 21.Kanehisa J, Yamanaka T, Doi S, Turksen K, Heersche JN, Aubin JE, et al. A band of F-actin containing podosomes is involved in bone resorption by osteoclasts. Bone. 1990;11:287–93. doi: 10.1016/8756-3282(90)90082-a. [DOI] [PubMed] [Google Scholar]
- 22.Swaroop N, Chen F, Wang L, Dokka S, Toledo D, Rojanasakul Y. Inhibition of nuclear transcription factor-kappaB by specific IkappaB kinase peptide inhibitor. Pharm Res. 2001;18:1631–3. doi: 10.1023/a:1013051019098. [DOI] [PubMed] [Google Scholar]
- 23.Dai S, Hirayama T, Abbas S, Abu-Amer Y. The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J Biol Chem. 2004;279:37219–22. doi: 10.1074/jbc.C400258200. [DOI] [PubMed] [Google Scholar]
- 24.Eastgate JA, Symons JA, Wood NC, Grinlinton FM, di Giovine FS, Duff GW. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988;2:706–9. doi: 10.1016/s0140-6736(88)90185-7. [DOI] [PubMed] [Google Scholar]
- 25.Abramson SB, Amin A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology (Oxford) 2002;41:972–80. doi: 10.1093/rheumatology/41.9.972. [DOI] [PubMed] [Google Scholar]
- 26.De Klerck B, Carpentier I, Lories RJ, Habraken Y, Piette J, Carmeliet G, et al. Enhanced osteoclast development in collagen-induced arthritis in interferon-gamma receptor knock-out mice as related to increased splenic CD11b+ myelopoiesis. Arthritis Res Ther. 2004;6:R220–31. doi: 10.1186/ar1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 28.Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003;111:821–31. doi: 10.1172/JCI16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu YG, Shao T, Feng C, Mensah KA, Thullen M, Schwarz EM, et al. CD16 (FcRgammaIII) as a potential marker of osteoclast precursors in psoriatic arthritis. Arthritis Res Ther. 2010;12:R14. doi: 10.1186/ar2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denk A, Wirth T, Baumann B. NF-kappaB transcription factors: critical regulators of hematopoiesis and neuronal survival. Cytokine Growth F R. 2000;11:303–20. doi: 10.1016/s1359-6101(00)00009-5. [DOI] [PubMed] [Google Scholar]
- 31.Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80:331–40. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 32.Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–96. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyazaki T, Katagiri H, Kanegae Y, Takayanagi H, Sawada Y, Yamamoto A, et al. Reciprocal role of ERK and NF-kappaB pathways in survival and activation of osteoclasts. J Cell Biol. 2000;148:333–42. doi: 10.1083/jcb.148.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth F R. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–86. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe E, Nakamura Y, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–303. [PubMed] [Google Scholar]
- 37.Yago T, Nanke Y, Kawamoto M, Furuya T, Kobashigawa T, Kamatani N, et al. IL-23 induces human osteoclastogenesis via IL-17 in vitro, and anti-IL-23 antibody attenuates collagen-induced arthritis in rats. Arthritis Res Ther. 2007;9:R96. doi: 10.1186/ar2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JH, Jin HM, Kim K, Song I, Youn BU, Matsuo K, et al. The mechanism of osteoclast differentiation induced by IL-1. J Immunol. 2009;183:1862–70. doi: 10.4049/jimmunol.0803007. [DOI] [PubMed] [Google Scholar]
- 39.DOG, Ireland D, Bord S, Compston JE. Joint erosion in rheumatoid arthritis: interactions between tumour necrosis factor alpha, interleukin 1, and receptor activator of nuclear factor kappaB ligand (RANKL) regulate osteoclasts. Ann Rheum Dis. 2004;63:354–9. doi: 10.1136/ard.2003.008458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Y, Genant HK, Watt I, Cobby M, Bresnihan B, Aitchison R, et al. A multicenter, double-blind, dose-ranging, randomized, placebo-controlled study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: radiologic progression and correlation of Genant and Larsen scores. Arthritis Rheum. 2000;43:1001–9. doi: 10.1002/1529-0131(200005)43:5<1001::AID-ANR7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]