Abstract
L2pB1 cells are a subpopulation of B-1a B cells that express PD-L2 (programmed death ligand 2) as their unique cell surface marker. In mice, about 50% of peritoneal B-1a cells are L2pB1 cells. The remaining B-1a cells are L2nB1 (PD-L2−) B-1a cells. L2pB1 cells differ from L2nB1 cells in their immunoglobulin repertoire, expression of interleukin 10, and their capacity to phagocytose phosphatidylcholine (PtC). The physiological roles of L2pB1 cells have not been investigated owing to the lack of an animal model that allows for specific depletion of L2pB1 cells. Our data showed that depletion of L2pB1 cells significantly reduces serum anti-phosphorylcholine (PC) IgM level as well as IL-10 expression in the peritoneal cavity. This animal model provides an unequivocal tool for the study of the immune regulatory functions of L2pB1 cells in health and diseases.
Keywords: L2pB1 cells, monoallelic expression, ZsGreen, TdTomato, diphtheria toxin receptor
Introduction
PD-L2 (CD273, B7-DC) is one of the ligands for immune-suppressive receptor programmed death–1 (PD-1, CD279).1 Initially, PD-L2 was found only on macrophages and dendritic cells upon activation. We first reported that, among resting peritoneal B-1a B cells, 50–70% express PD-L2.2 These PD-L2+ B-1a B cells were termed L2pB1 cells, whereas the rest of the B-1a cells were termed L2nB1 cells.1 L2pB1 cells have enriched self-reactivity and a unique immunoglobulin repertoire that is distinct from that of L2nB1 cells.2, 3 Anti-phosphatidylcholine (PtC) antibody, a classical B-1 cell–derived antibody, is exclusively expressed by L2pB1 cells.2 Among the reported features of B-1 B cells are potent antigen presentation, a self-reactive antibody repertoire, and expression of interleukin 10 (IL-10), all essential for the immunoregulatory functions of B-1 B cells. We have previously reported that L2pB1 cells have potent antigen-presentation capacity and a self-reactive antibody repertoire significantly greater than other B-1 B cells. We also showed that, among all B cells, L2pB1 cells produce the most IL-10, both constitutively and inducibly. Despite the in vitro evidence suggesting that L2pB1 cells are the subpopulation that carries out most of the known regulatory functions of B-1 B cells,4 the physiological relevance of L2pB1 cells in health and disease is difficult to prove due to the lack of unique cell-type markers and specific animal models.
The molecular and cellular functions of PD-L2 on L2pB1 cells are currently unclear. However, antigen-presenting cells from PD-L2–deficient mice were shown to display enhanced T cell–activating potential both in vitro and in vivo.5 In an experimental autoimmune encephalitis (EAE) animal model, disease severity was increased when PD-L2 was blocked at either the initiation or chronic phase of the disease.6, 7 These reports suggest that PD-L2 has a physiological role in dampening and regulating T cell–mediated inflammatory immune responses. A recent study also showed that PD-L2 inhibits human T cell activation through engaging PD-1.8 Thus, PD-L2 expression on L2pB1 cells potentially carries important regulatory functions.4 Since L2pB1 cells share most of the surface markers with L2nB1 cells except for PD-L2, it is very difficult to sort L2pB1 cells without using anti–PD-L2 antibody, which blocks and interferes with PD-L2 physiological function. Acid elution of the surface-bound antibody after sorting greatly stresses cells and triggers their non-physiological activation. In order to avoid these problems, an endogenous indicator system for tracking and sorting L2pB1 cells is highly desirable.
PD-L2 expression on L2pB1 cells appears to be intrinsic to L2pB1 cells, as L2nB1 cells, even when activated, do not upregulate expression of PD-L2, which suggests that L2pB1 cells do not originate from L2nB1 cells activated to express PD-L2.2 Thus, unlike on macrophages and dendritic cells, PD-L2 is not a postactivation marker on L2pB1 cells, suggesting that PD-L2 is regulated differently in different cell types. Supporting this, Kaku et al. reported that PD-L2 expression in L2pB1 cells is regulated by a novel intronic promoter located between exons 1 and 2, resulting in a transcription start site that is different from that used in macrophages and dendritic cells.9 Despite the differential regulation in PD-L2 expression, macrophages and dendritic cells still share the same PD-L2 gene (Pdcd1lg2) with L2pB1 cells. Thus, just as a conventional antibody-mediated cell depletion method cannot give definitive answers regarding PD-L2+ cell type–specific functions, neither will targeting PD-L2 gene provide information of L2pB1 cell–specific physiological functions.
As one solution to this this problem, we devevloped a PD-L2–ZsGreen–TdTomato–diphtheria toxin receptor (PZTD) knock-in and inducible knockout mouse model to specifically track and inducibly deplete L2pB1 cells without affecting macrophages and dendritic cells. In this mouse, all the PD-L2–expressing cells are green, whereas any cell type of interest with PD-L2 expression can switch off the green but turn on a red fluorescence signal upon crossing to cell type–specific Cre transgenic mice. Moreover, the red PD-L2+ cells of interest can be depleted in vivo with diphtheria toxin. This color-toggling indicator mouse is the first of its kind, and the methodology is generally applicable to studying other genes.
Results and discussion
The design of an L2pB1 indicator knock-in and inducible knockout mouse model
Currently, sorting out L2pB1 cells requires using an antibody specific for PD-L2. In order to avoid interfering with PD-L2 function on L2pB1 cells, a transgenic mouse expressing fluorescent protein was created to specifically tag PD-L2 only in L2pB1 cells. To achieve this, a ZsGreen fluorescent protein gene was first inserted downstream of the Pdcd1lg2 coding region after the stop codon in exon 5 (Fig. 1). In the targeted allele, an internal ribosome entry site (IRES) sequence links Pdcd1lg2 and the ZsGreen gene so that ZsGreen is expressed whenever PD-L2 is expressed. Therefore, all the cells that express PD-L2, including L2pB1 cells, activated dendritic cells, and macrophages, are labeled with green fluorescence in this mouse. The ZsGreen gene and a neomycin-resistance gene are flanked by two LoxP sites, so that upon crossing with a B cell–specific CD19-driven Cre recombinase transgenic mouse, the ZsGreen genes are permanently deleted only in CD19+ B cells, while PD-L2–expressing macrophages and dendritic cells still express ZsGreen.
Figure 1.
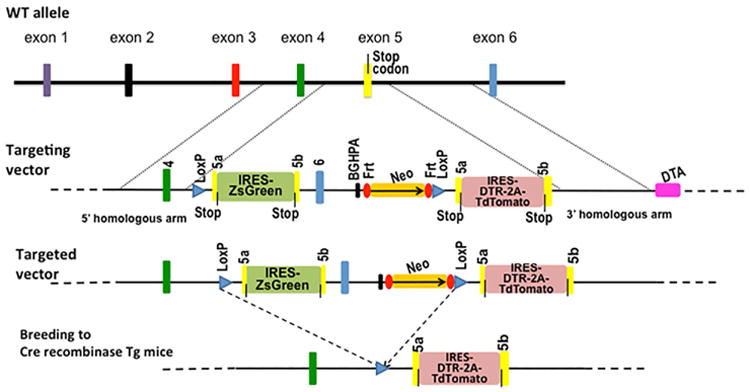
Genetic targeting strategy. A cDNA copy of ZsGreen, a green fluorescent protein, was inserted after the stop codon in exon 5 (yellow bar) of the PD-L2 gene, separated by an internal ribosome entry site (IRES). A neomycin-resistance gene (Neo) and a BGHPA sequence were inserted after exon 6 (blue bar). All these insertions were flanked by two LoxP sequences (blue triangles). A duplication of exon 5 was inserted after the 3′-end LoxP sequence. An IRES and a cDNA copy of diphtheria toxin receptor (DTR) were inserted after the stop codon in the duplicate exon 5 followed by a cDNA copy of the red fluorescent protein TdTomato. The DTR and TdTomato genes were separated by a DNA sequence coding a 2A self-cleavage peptide. After crossing to a Cre recombinase transgenic mouse, the sequence flanked by LoxP sites will be removed permanently, leaving only the IRES–DTR–2A–TdTomato sequence.
In order to inducibly deplete L2pB1 cells, a diphtheria toxin receptor (DTR) gene was inserted in a duplicated exon 5 downstream of the 3′ LoxP site (Fig. 1). DTR will not be expressed until the floxed region is removed by Cre recombinase. Upon deletion of the floxed region by Cre recombinase, the PD-L2 gene now ends at the stop codon of the duplicated exon 5, followed by the IRES-linked DTR gene. Consequently, PD-L2+ cells of interest are now highly susceptible to diphtheria toxin.
In order to continue labeling L2pB1 cells with fluorescent protein after the depletion of the ZsGreen gene in the floxed region, a cDNA copy of the red fluorescent protein TdTomato was inserted downstream of the DTR gene, linked in frame by a self-cleaving 2A peptide sequence from foot-and-mouth disease virus (FMDV).10, 11 As a result, after Cre excision of the floxed region, PD-L2, DTR, and TdTomato are expressed simultaneously but independently, as they are separated by the IRES between the PD-L2 and DTR genes, and by the 2A sequence between the DTR and TdTomato genes (Fig. 1).
L2pB1 cells in heterozygous and homozygous PZTD mice
Both heterozygous and homozygous PZTD littermates revealed the expected genotype (Fig. 2A) and phenotype (Fig. 2B). Fluorescence-activated cell sorting (FACS) analysis of peritoneal lymphocytes in naive wild-type, heterozygous, and homozygous littermates revealed specific expression of ZsGreen fluorescent protein only in PD-L2–expressing B-1a cells, while expression was not seen in T cells, B-2 B cells, or other cells (green arrows in Fig. 2B). In naive mice, no PD-L2–expressing macrophages were observed. A small number of L2pB1 cells were detected outside the peritoneal cavity, including in the spleen and thymus, as expected (data not shown).
Figure 2.
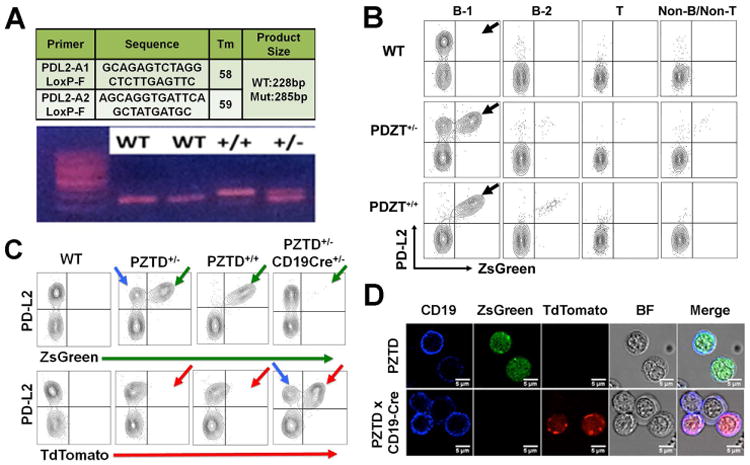
Genotype and phenotype of PZTD mice. (A) Tail DNA was extracted for PCR amplification. A fragment of 228 bp was amplified from a wild-type (−/−) mouse and a 285-bp fragment was amplified from a homozygous PZTD (+/+) mouse. Heterozygous mouse samples show both bands. (B) Peritoneal cavity washout cells were isolated from wild-type (−/−), heterozygous (+/−), and homozygous (+/+) PZTD littermates. Peritoneal T cells, B-1 B cells, and B2 B cells were gated according to B220, CD5, and IgM expression. Red arrows indicate the PD-L2+ ZsGreen+ population. (C) PZTD mice were crossed to CD19–Cre knock-in mice. FACS analysis indicated the loss of ZsGreen+ L2pB1 cells (green arrows) and gain of TdTomato+ L2pB1 cells (red arrows). Blue arrows indicate the wild-type cells in heterozygous mice that neither express ZsGreen nor TdTomato. (D) Microscopic analysis of peritoneal CD19+ B cells (blue) and ZsGreen+ cells (green) in PZTD mice (top panel). After crossing with CD19–Cre knock-in mice, no ZsGreen-expressing CD19+ B cells were seen. Instead CD19+ TdTomato+ cells were observed (Bottom panel).
It is interesting to note that PD-L2–ZsGreen expression is monoallelic, as shown in heterozygous mice (Fig. 2B). At the single-cell level, either the knock-in allele or the wild-type allele is expressed, but both are not expressed at the same time in the same cell (Fig. 2B). Similar monoallelic expression patterns have been reported for many cytokine genes.12–15 This would be problematic in cytokine knock-in reporter mice in which the reporter gene replaces the cytokine gene; in such cases, there would be false positive or false negative cells in heterozygous mice. When a single cell expresses the wild-type cytokine allele but not the reporter gene allele, it will be considered negative despite the expression of the cytokine allele. On the other hand, when a single cell chooses to express the reporter gene allele, but not the wild-type cytokine gene allele, it will be considered positive despite possessing no actual cytokine expression. However, such false positive and negative instances will not occur in our animal model, as the PD-L2 gene is not replaced by the ZsGreen gene as in a traditional knock-in mouse. In our model, both PD-L2 and ZsGreen are co-expressed by the knock-in allele. Thus, whenever there is ZsGreen expression, there must be PD-L2 expression. This is the advantage of our design over traditional knock-in reporter mouse models for monoallelic genes.
Moreover, monoallelic expression is a mechanism for certain immune cell receptors to regulate their signaling strength through gene dosage. For example, natural killer (NK) cells achieve self-tolerance through the expression of self-MHC–specific inhibitory receptors, such as members of the Ly49 and CD94/NKG2 families. All known MHC-specific receptors on mouse NK cells (i.e., individual Ly49 proteins, as well as CD94/NKG2A) are all expressed in a monoallelic fashion to govern gene dosage and signal strength.16 Our finding that PD-L2 is expressed in a similar monoallelic manner in L2pB1 cells further supports the view that PD-L2 may deliver delicate signaling to its cognate receptor PD-1 on activated lymphocytes. Sorting and adoptive transfer of L2pB1 cells covered by anti–PD-L2 antibodies could likely affect their physiological functions in vivo. Our PZTD knock-in indicator mouse is thus an ideal model for faithfully studying L2pB1 cell functions in vivo.
Depletion of L2pB1 cells using diphtheria toxin
After crossing PZTD+/− mice with CD19–Cre knock-in mice, ZsGreen expression (green arrows in Fig. 2C) was switched off and the TdTomato gene was turned on specifically in L2pB1 cells (red arrows in Fig. 2C). The wild-type allele (blue arrows in Fig. 2C) is expressed in some cells of the heterozygous mice owing to monoalleleic expression. The TdTomato+ L2pB1 cells in CD19–Cre PZTD+/− mice now express DTR on their surfaces.
While humans and monkeys are susceptible to diphtheria toxin (DT), mice and rats are resistant to DT owing to the sequence difference of the DTR, the membrane-anchored form of the heparin-binding EGF-like growth factor (HB-EGF).17 The expression of a human DTR on the surface of a mouse cell allows for ablation of specific cell type through the well-established mechanism of diphtheria toxin–mediated cell death.18, 19 DT acts by inhibiting protein synthesis and causing apoptosis of the mouse cells expressing human DTR, whereas the wild-type cells remain insensitive.17 Demircik et al. showed successful depletion of B cells in a CD19-Cre transgenic mouse that was crossed with the iDTR mouse.20
To deplete TdTomato-expressing L2pB1 cells in CD19–Cre PZTD mice, 25 ng of DT per gram of body weight was administered per injection to each mouse over 4 consecutive days. FACS data showed that, in the heterozygous mice, wild-type L2pB1 cells (blue arrows in Fig. 3A) are not depleted, indicating the specificity of DT-mediated depletion. More than 80% of L2pB1 cells can be depleted after a single DT injection. After four injections, more than 95% of L2pB1 cells can be depleted (red arrows in Fig. 3A). For long-term experiments, a booster DT injection is needed to deplete any L2pB1 cells that might expand from the residual L2pB1 cells that are not depleted during the first round of depletion.
Figure 3.

Reduction of serum anti-PC IgM upon L2pB1 cell depletion. (A) CD19-Cre PZTD heterozygous mice were injected intraperitoneally. with 25ng/g body weight DT for 4 consecutive days. Peritoneal cavity washout (PCW) cells were collected at day 0, 1, 2, 3, and 4 for FACS analysis. Blue arrows indicate that the wild-type L2pB1 cells in the heterozygous mice were not deleted by DT injection, whereas red arrows indicate the knock-in cells that were being depleted after 4-day injections. (B) CD19-Cre PZTD homozygous mice were injected with DT for 4 days. PCW samples were analyzed and B220intCD5+ B-1a cells were gated. About 50% of B1a cells in control mice are double positive for PD-L2 and TdTomato (top right), whereas in DT-injected mice, less than 1% of double-positive cells were detected (bottom right). (C) Percentages of L2pB1 cells (TdTomato+ cells) in total peritoneal B1a cells in DT-injected mice are significantly reduced compared to control mice (P < 0.05, n = 6 for control group, n = 5 for DT group). (D) Serum anti-PC IgM level was analyzed by ELISA. ELISA readings of days 4 and 7 post–DT injection were compared to the day 0 level of the same individual mouse. After 4 days following the first DT injection, the DT group (n = 5) show significant reduction of serum anti-PC IgM levels as compared to the control group (n = 5). The levels were further reduced at day 7 post DT injection. * P = 0.001; ** P = 0.0019; *** P = 0.0004.
Depletion of L2pB1 cells significantly affects serum anti-PC IgM levels
One of the physiological functions of B-1 B cells is to produce natural IgM antibodies (nAbs).21 These nAbs are polyreactive IgM antibodies that recognize common bacterial structures as well as self-antigens, such as cell membrane phospholipids and oxidized lipoprotein. Recent studies have highlighted that B-1 B cell–derived natural antibodies are essential in the maintenance of tissue homeostasis via clearance of apoptotic and altered cells, inhibition of inflammation, removal of misfolded proteins, and regulation of pathogenic autoantibody-producing conventional B-2 B cells.22
Anti-phosphorylcholine (PC) and anti-phosphatidylcholine (PtC) IgMs are two well-studied B-1 B cell–derived nAbs. In mice, about 1% of B-1 B cells produce anti-PC nAbs and up to 10% of B-1 B cells produce anti-PtC nAbs.23–26 PC is the polar head group of PtC, which is a major phospholipid component of the cell membrane. Interestingly, anti-PC IgM produced by B-1 B cells also recognizes oxidized low-density lipoprotein (OxLDL) and is atheroprotective.27 Reconstitution of anti-PtC IgM in IgM-deficient mice substantially reduced the bacterial load in a cecal ligation and puncture model.28 In addition to bacteria, B-1 B cell–derived nAbs also play an important role in immune defense against influenza virus.29 In humans, 85% of sera from clinically healthy subjects contain IgM anti-PtC, none of which is IgG.30
To test whether L2pB1 cell depletion affects serum anti-PC IgM levels, blood from CD19–Cre PZTD mice at days 0, 4, and 7 following DT injections was collected for anti-PC IgM enzyme-linked immunosorbent assay (ELISA). The half-life of therapeutic IgM antibodies ranges from hours to 2 days, whereas it is generally believed that endogenous IgM has a half-life of about 5 days.31, 32 The ELISA result showed a significant reduction of anti-PC IgM in the blood by day 4 after the first DT injection (Fig. 3). A more significant reduction of over 50% is seen by day 7 after the first DT injection (Fig. 3). Possible sources of remaining anti-PC IgM may include residual undeleted L2pB1 cells (< 5%), memory cells, or plasma cells that may not be depleted. L2nB1 cells may also produce some alternative PC-binding antibodies with different affinity and VH usage. As previously mentioned, for experiments longer than 3 weeks, a DT injection booster is needed every 2–3 weeks to maintain L2pB1 cell depletion greater than 95%. In summary, L2pB1 cells contribute significantly to anti-PC IgM antibody in the circulation.
Depletion of L2pB1 cells significantly affects IL-10 expression in the peritoneal cavity
Despite the mounting evidence suggesting that certain B cells have regulatory functions essential for limiting inflammation through the anti-inflammatory cytokine IL-10, the actual identity of such regulatory B (Breg) cells remains elusive. Recent studies attribute Breg cells to a rare (< 1%) splenic B cell population with a CD1dhiCD5+CD19hiCD23− phenotype. Because of the exclusive production of IL-10 by these cells when stimulated by LPS, these Breg cells were termed B10 Breg cells.33, 34 However, IL-10 cannot be directly detected in these B10 cells. Rather, it is only detectable after in vitro stimulation. Thus, they are called IL-10–competent cells. Interestingly, reports have also shown that activated B-1 B cells are also suppressive.35, 36 Early study of CD5+ B lymphomas and peritoneal B-1 B cells showed that peritoneal B-1 B cells secrete significant amounts of IL-10.37
Using IL-10–GFP transgenic mice, we found that L2pB1 cells express significantly more IL-10 than L2nB1 cells, especially after LPS stimulation (Fig. 4A & 4B).
Figure 4.
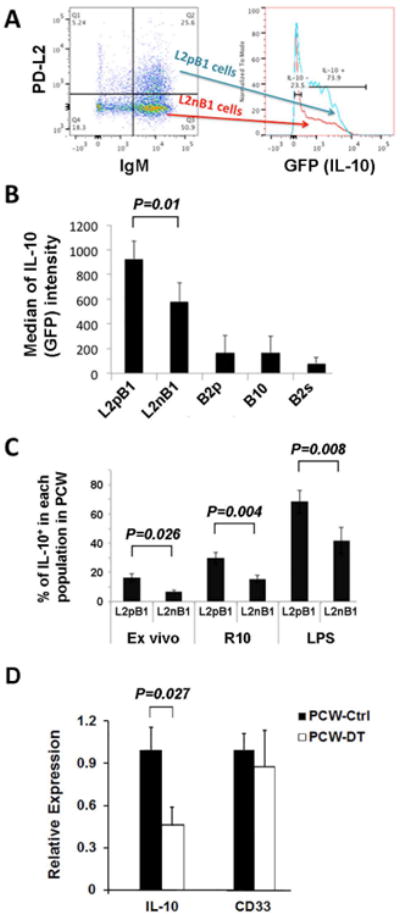
Peritoneal IL-10 gene expression is significantly reduced upon L2pB1 cell depletion. Single-cell suspension was obtained from PCW a day after four consecutive intraperitoneal injections of PBS or DT (one injection per day). RNA was extracted, followed by reverse transcription and rea-time PCR. Relative gene expression levels of IL-10 and CD33 of PBS control (n = 3; dark bar) and DT-treated mice (n = 3; light bar) are normalized against expression of the housekeeping protein GAPDH. Triplicate PCR was performed for each gene from each sample, and the average gene expression level relative to GAPDH is shown with standard deviation. Student's t-test was performed and P values < 0.05 are shown.
FACS analysis showed that about 20% of ex vivo L2pB1 cells spontaneously express IL-10 without external stimulation (Fig. 4C). Upon overnight culture, the percentage of IL-10+ L2pB1 cells increased by threefold over the ex vivo level. With LPS stimulation, more than three-fourths of L2pB1 cells express IL-10 (Fig. 4B & 4C). We found that, upon culture, IL-10 production in L2nB1 cells was also increased. However, the percentage of IL-10+ L2pB1 cells in the L2pB1 cell population was significantly higher than that of IL-10+ L2nB1 cells in the L2nB1 population. We conclude that L2pB1 cells are a major contributor of IL-10 among peritoneal lymphocytes. To further test this, we measured IL-10 mRNA levels in total peritoneal cells in L2pB1 cell–depleted (PCW-DT) and non-depleted (PCW-Ctrl) CD19–Cre PZTD mice (Fig. 4D). Real-time polymerase chain reaction (PCR) results show that, after L2pB1 cell depletion, there is a significant decrease in IL-10 levels as compared to non-depleted control mice, whereas the levels of a control mRNA (CD33) are comparable between these two groups (Fig. 4D).
The spontaneous secretion of IL-10 in the absence of stimulation distinguishes L2pB1 cells from other IL-10–producing B cells. With the significant amount of IL-10 expressed by L2pB1 cells, it is very likely that L2pB1 cells are the key lymphocytes that prevent tissue from inflammation on a regular basis. L2pB1 cells may also be the first responders to rapidly increase IL-10 secretion upon the detection of tissue damage and inflammation.
In summary, we have successfully established a mouse model in which PD-L2–expressing cells can be tracked by the expression of the green fluorescent protein ZsGreen. Upon breeding to CD19–Cre knock-in mice, PD-L2–expressing L2pB1 B cells can be tracked by the red fluorescent protein TdTomato, and over 95% of L2pB1 cells can be depleted by DT injection. Depletion of L2pB1 cells significantly reduces serum anti-PC natural IgM levels and IL-10 expression in the peritoneal cavity. Further investigations are currently underway to study the long-term physiological consequences of L2pB1 cell depletion in various disease models. The PZTD mouse model can also be used for studying other PD-L2–expressing cells, such as macrophages and dendritic cells, under disease conditions by breeding to macrophage- or dendritic cell–specific Cre transgenic mice. Our color-toggling mouse model provides researchers with ample manipulation ability for studying the in vivo functions of genes and host cells in the context of a potentially complicated expression profile that crosses multiple cell types.
Materials and methods
Mice
PZTD+/+ and CD-19–Cre+/− PZTD+/+ mice on a C57BL/6 background were generated and bred in the animal facility at Boston University School of Medicine. IL-10–GFP transgenic mice on a C57BL/6 background were purchased from the Jackson Laboratory (MN) and bred at the animal facility at Boston University School of Medicine. All procedures involving animal use were approved by the Institutional Animal Care and Use Committee of the Boston University School of Medicine.
Mouse tail DNA extraction and PCR genotyping
DNA Lysis Buffer (Antagen Pharmaceuticals, Inc., MA) was used according to the manufacturer's manual. Briefly, 200 μL of the lysis buffer was added to each tail sample followed by boiling for 30 min. One μL of the lysate was then directly used for PCR amplification. PCR was performed in 20 μL reaction with Taq B DNA polymerase (Enzymatics, MA). Two pairs of primers were used. The primers for the PD-L2 gene are listed in Figure 1A. The primers for Cre recombinase are: cre5 (GCGGTCTGGCAGTAAAAACTATC) and cre3 (TAACCCTGATCCTGGCAATTTC). The reactions were heated to 95 °C for 5 min followed by 40 cycles of 95 °C for 20 s, 60 °C for 25 s, and 72 °C for 40 s.
Diphtheria toxin injection
Mice received intraperitoneal injection of 25 ng of DT (Sigma-Aldrich, St. Louis, MO) per gram of body weight per day for 4 consecutive days. Control mice received Ca2+/Mg2+-free PBS at a volume proportional to their bodyweight.
Anti-PC IgM ELISA
ELISA plates were coated with PC-BSA (BioSearch Technologies, CA) overnight, then blocked with PBS supplemented with 2% BSA-TBS and washed with PBS with 0.5% Tween. Serum samples were diluted at 1:50 or as indicated with blocking buffer and incubated for 1 h at 37 °C. Goat anti-mouse IgM (μ-chain specific)-HRP (Sigma, MO) was used as secondary antibody, incubated at 37 °C for 30 min, washed, and followed by addition of 1-Step Ultra TMB-ELISA substrate solution (ThermoFischer, NH). After a 15-min incubation, stop solution (BioLegend, CA) was added and samples were read at 405 nm.
Peritoneal cell isolation
Peritoneal cavity washout (PCW) was collected with HBSS, 2% FBS. Briefly, 10 ml was injected into abdominal cavity and was retrieved with a syringe. Total PCW cells were then washed, counted, and either stained for FACS analysis or proceeded to tissue culture.
Flow cytometry and immunofluorescent microscopy
Cells were incubated with Fc Blocker (BioLegend, CA) for 15 min and then stained with the following antibodies for 45 min: B220–PerCp–Cy5.5, CD5–PECy7, Cd11b–e780 or CD11b APC–Cy7, CD19–e450, PD-L2–PE (eBioscience, CA), Ghost Dye Violet 510 (Tonbo Biosciences, CA), IgM–AF350 (Life Technologies, CA), or IgM–APC (eBioscience, CA). Cells were then washed three times by centrifugation and resuspended in Live Cell Imaging Solution (Life Technologies, CA). FACS data were collected with BD LSRII and analyzed using FlowJo software (TreeStar, OR). Fluorescent images were taken using a 63× oil-immersion objective with a Leica TCS SP5 microscope.
Real-time PCR
Real-time PCR was performed according to the manufacturer's manual. RNA was extracted from total peritoneal washout cells using Trizol (Life Technologies, NY). cDNA was made using ProtoScript® II Reverse Transcriptase (NEB, MA). PCR master mix was purchased from iQ Multiplex Powermix (BioRad, CA). PCR primers and probe sets were purchased from Ab Applied Biosystems (Life Technologies, NY). The catalog numbers of primer/probe set for IL-10 was Mm439616_m1; for CD33, Mm00491152_m1; and for GAPDH, Mm99999915_g1. Data analysis was performed using the ΔΔC(t) method. Statistics analysis was performed with Student's t-test.
Statistics
Statistical significance was calculated using a Student's t-test. P values < 0.05 were considered statistically significant.
Supplementary Material
Figure S1. DT injection only affects L2pB1 cells but not other immune cells. (A). CD19-Cre PZTD mice received intraperitoneal injection of either PBS or DT for 4 consecutive days. Cells were then harvested from PCW (n = 4 per group), thymus (n = 5 per group), spleen (n = 5 per group), and bone marrow (n = 5 per group). Representative FACS results from each tissue with live and single-cell gating are shown. L2pB1 cells are shown as TdTomato+ cells in the B-1 cell gate (CD5+CD19+IgM+CD11b+). All other cell types are gated from total live single cells. Black arrows indicate the missing L2pB1 cells in the DT-injected group in the same gating of PBS-injected control group.
(B). Percentages of each immune cell population in peritoneal cavity washout cells are compared in PBS-injected and DT-injected groups. Percentage is of total PCW cells unless otherwise indicated. Four mice (n=4) were in each group. Student's t-test was performed. The P value of * is 0.01, ** is < 0.000013, and *** is < 0.000006.
Acknowledgments
The authors thank Biocytogen (Boston, MA) for their service of vector and ES cell cloning. We also thank the transgenic core at Boston University School of Medicine for ES cell microinjection and obtaining germ line–transmitting mice. Our research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number 5R21AR063387 to Dr. Zhong. The research is also supported by a pilot grant to Dr. Zhong co-funded by the Department of Medicine and Clinical and Translational Science Institute (CTSI) supported by an award under NIH Award Number UL1RR025771.
References
- 1.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 2.Zhong X, et al. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol. 2007;37:2405–2410. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
- 3.Zhong X, et al. A novel subpopulation of B-1 cells is enriched with autoreactivity in normal and lupus-prone mice. Arthritis Rheum. 2009;60:3734–3743. doi: 10.1002/art.25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klinker MW, Lundy SK. Multiple mechanisms of immune suppression by B lymphocytes. Mol Med. 2012;18:123–137. doi: 10.2119/molmed.2011.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, et al. Regulation of T cell activation and tolerance by PDL2. Proc Natl Acad Sci U S A. 2006;103:11695–11700. doi: 10.1073/pnas.0601347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salama AD, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198:71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu B, et al. Differential role of programmed death-ligand 1 [corrected] and programmed death-ligand 2 [corrected] in regulating the susceptibility and chronic progression of experimental autoimmune encephalomyelitis. J Immunol. 2006;176:3480–3489. doi: 10.4049/jimmunol.176.6.3480. [DOI] [PubMed] [Google Scholar]
- 8.Pfistershammer K, et al. No evidence for dualism in function and receptors: PD-L2/B7-DC is an inhibitory regulator of human T cell activation. Eur J Immunol. 2006;36:1104–1113. doi: 10.1002/eji.200535344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaku H, Rothstein TL. Octamer binding protein 2 (Oct2) regulates PD-L2 gene expression in B-1 cells through lineage-specific activity of a unique, intronic promoter. Genes Immun. 2010;11:55–66. doi: 10.1038/gene.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szymczak AL, et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 11.Yen HH, Scheerlinck JP. Biological activity of ovine IL-23 expressed using a foot-and-mouth disease virus 2A self-cleaving peptide. Cytokine. 2013;61:744–746. doi: 10.1016/j.cyto.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Hollander GA, et al. Monoallelic expression of the interleukin-2 locus. Science. 1998;279:2118–2121. doi: 10.1126/science.279.5359.2118. [DOI] [PubMed] [Google Scholar]
- 13.Bayley JP, et al. Allele-specific expression of the IL-1 alpha gene in human CD4+ T cell clones. J Immunol. 2003;171:2349–2353. doi: 10.4049/jimmunol.171.5.2349. [DOI] [PubMed] [Google Scholar]
- 14.Mostoslavsky R, Alt FW, Rajewsky K. The lingering enigma of the allelic exclusion mechanism. Cell. 2004;118:539–544. doi: 10.1016/j.cell.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Bouabe H. Cytokine reporter mice: the special case of IL-10. Scand J Immunol. 2012;75:553–567. doi: 10.1111/j.1365-3083.2012.02695.x. [DOI] [PubMed] [Google Scholar]
- 16.Vance RE, et al. Implications of CD94 deficiency and monoallelic NKG2A expression for natural killer cell development and repertoire formation. Proc Natl Acad Sci U S A. 2002;99:868–873. doi: 10.1073/pnas.022500599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito M, et al. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 18.Buch T, et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- 19.Mitamura T, et al. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem. 1995;270:1015–1019. doi: 10.1074/jbc.270.3.1015. [DOI] [PubMed] [Google Scholar]
- 20.Demircik F, Buch T, Waisman A. Efficient B cell depletion via diphtheria toxin in CD19-Cre/iDTR mice. PLoS One. 2013;8:e60643. doi: 10.1371/journal.pone.0060643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 22.Gronwall C, Vas J, Silverman GJ. Protective Roles of Natural IgM Antibodies. Front Immunol. 2012;3:66. doi: 10.3389/fimmu.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmack CE, et al. Rearrangement and selection of VH11 in the Ly-1 B cell lineage. J Exp Med. 1990;172:371–374. doi: 10.1084/jem.172.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mercolino TJ, et al. Restricted immunoglobulin variable region gene usage by normal Ly-1 (CD5+) B cells that recognize phosphatidyl choline. J Exp Med. 1989;169:1869–1877. doi: 10.1084/jem.169.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercolino TJ, et al. Normal mouse peritoneum contains a large population of Ly-1+ (CD5) B cells that recognize phosphatidyl choline. Relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J Exp Med. 1988;168:687–698. doi: 10.1084/jem.168.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw PX, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw PX, et al. The autoreactivity of anti-phosphorylcholine antibodies for atherosclerosis-associated neo-antigens and apoptotic cells. J Immunol. 2003;170:6151–6157. doi: 10.4049/jimmunol.170.12.6151. [DOI] [PubMed] [Google Scholar]
- 28.Boes M, et al. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumgarth N, et al. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabiedes J, et al. Characterization of anti-phosphatidylcholine polyreactive natural autoantibodies from normal human subjects. J Autoimmun. 2002;18:181–190. doi: 10.1006/jaut.2001.0575. [DOI] [PubMed] [Google Scholar]
- 31.Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988;18:313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- 32.Brekke OH, Sandlie I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nat Rev Drug Discov. 2003;2:52–62. doi: 10.1038/nrd984. [DOI] [PubMed] [Google Scholar]
- 33.Matsushita T, et al. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 35.Paciorkowski N, Shultz LD, Rajan TV. Primed peritoneal B lymphocytes are sufficient to transfer protection against Brugia pahangi infection in mice. Infect Immun. 2003;71:1370–1378. doi: 10.1128/IAI.71.3.1370-1378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lampropoulou V, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 37.O'Garra A, et al. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. DT injection only affects L2pB1 cells but not other immune cells. (A). CD19-Cre PZTD mice received intraperitoneal injection of either PBS or DT for 4 consecutive days. Cells were then harvested from PCW (n = 4 per group), thymus (n = 5 per group), spleen (n = 5 per group), and bone marrow (n = 5 per group). Representative FACS results from each tissue with live and single-cell gating are shown. L2pB1 cells are shown as TdTomato+ cells in the B-1 cell gate (CD5+CD19+IgM+CD11b+). All other cell types are gated from total live single cells. Black arrows indicate the missing L2pB1 cells in the DT-injected group in the same gating of PBS-injected control group.
(B). Percentages of each immune cell population in peritoneal cavity washout cells are compared in PBS-injected and DT-injected groups. Percentage is of total PCW cells unless otherwise indicated. Four mice (n=4) were in each group. Student's t-test was performed. The P value of * is 0.01, ** is < 0.000013, and *** is < 0.000006.


