Abstract
The majority of applications in countercurrent and centrifugal partition chromatography, collectively known as countercurrent separation, are dedicated to medicinal plant and natural product research. In countercurrent separation, the selection of the appropriate solvent system is of utmost importance as it is the equivalent to the simultaneous choice of column and eluent in liquid chromatography. However, solvent system selection is often laborious, involving extensive partition and/or analytical trials. Therefore, simplified solvent system selection strategies that predict the partition coefficients and, thus, analyte behavior are in high demand and may advance both the science of countercurrent separation and its applications. The last decade of solvent system selection theory and applications are critically reviewed, and strategies are classified according to their data input requirements. This offers the practitioner an up-to-date overview of rationales and methods for choosing an efficient solvent system, provides a perspective regarding their accuracy, reliability, and practicality, and discusses the possibility of combining multiple methods for enhanced prediction power.
Keywords: countercurrent separation, countercurrent chromatography, centrifugal partition chromatography, solvent system selection
Introduction
Nature creates abundant molecular structures with diverse pharmacological activities. Bioactive molecules are often present at the level of parts per million in the raw plant material. Countercurrent chromatography and centrifugal partition chromatography, collectively known as counter-current separation (CCS, syn. CS), provide orthogonal and scalable analytical capabilities and, thus, are important chromatography techniques for natural product chemists. Being a liquid-only technique, CCS avoids the loss by degradation or absorption of analytes on a solid support. It is characterized by high recovery and reproducibility and has demonstrated its usefulness in the natural product laboratory [1–3].
Because the stationary phase is a liquid in CCS, the separation resolution of an analyte depends on its partition coefficient (K), i.e., its relative distribution between the mobile and stationary phases of the solvent system. Thus, K is an invariant parameter of any analyte in a particular solvent system and an important characteristic parameter in a CCS experiment. If the analyte greatly prefers the mobile phase, it will be eluted in the solvent front, but if it strongly favors the stationary phase, the analyte will stay in the column, and may be extruded – a mechanism unavailable to solid phase-based LC. However, neither of these extreme K values (K ≅ 0 or K ≅ ∞) are likely to provide efficient analyte separation. An ideal solvent system has to deliver an analyte into a distinctive K value range, referred to as the “sweet spot”. This range is generally from K = 0.25 to K = 16 [4].
As most solvent system families used in CCS are ternary or quaternary solvent systems, the names of the multicomponent mixtures are cumbersome. Therefore, an abbreviated naming system for CCS solvent systems (Table 1 S, Supporting Information) was developed which allows for the assembly of pronounceable names that rapidly identify the system.
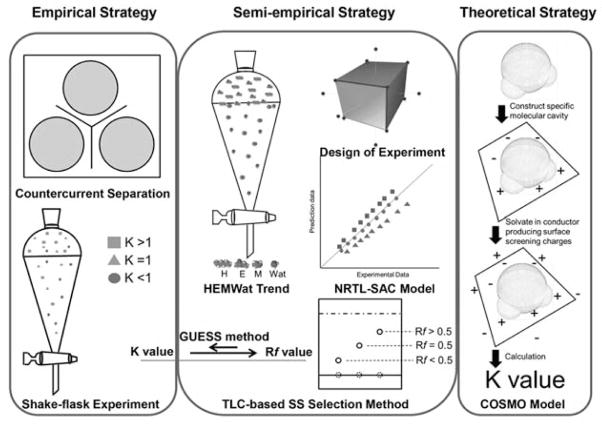
In CCS practice, solvent system selection may occupy 90% of the time taken in an entire CCS operation [5]. Thus, in the past decade, CCS researchers have sought new methods or models to reduce the effort of determining the appropriate solvent system and have introduced several selection strategies. In the present review, these strategies are classified into, and presented as, three main classes: empirical, semiempirical, and theoretical. Fig. 1 offers a comparison of the primary features of the three different approaches to solvent system selection. The possibility of a fully predictive means of solvent system selection is discussed at the end of the review.
Fig. 1.
A diagrammatic sketch of solvent system selection strategies in CCS. The empirical strategies are comprised of an analytical CCS and a partitioning experiment. The semiempirical strategies involve a TLC-based method, the general HEMWat family trend, three-dimensional K value maps, and the NRTL-SAC model. One representative theoretical strategy is the COSMO-RS model. (Color figure available online only.)
Empirical Solvent System Selection Strategy
Empirical methods require the practitioner to choose the solvent system composition, the solvent system family, and the constituent proportions of the initial solvent system(s). Typical applications may be divided into three categories: 1) the isolation of a single target analyte; 2) the separation and isolation of a group of analytes; and 3) the distribution of a complex mixture in which the target analytes are not explicitly known. As the desired outcomes vary accordingly, researchers attempt to discover a solvent system where: 1) the target analyte has a K value near unity; 2) the target compounds have K values in the sweet spot with a desirable resolution; and 3) an optimal distribution of mass and/or biological activity is observed.
Empirical solvent system selection is an iterative process. Once the results are known for the first set of trials, the second set of trials is planned. Sometimes the optimal solvent may be discovered by adjusting the solvent ratio within a solvent system family (a group of solvent systems that are mixtures of the same solvents). For example, if the target analyte has a high affinity for the organic upper phase of a hexane-ethyl acetate-methanol-water (HEMWat) solvent system, the proportions of hexane and/or methanol relative to ethyl acetate and/or water may be increased in the next set of trials. On the other hand, a solvent system with a different composition than the original solvent system may be attempted if a) there is not a good polarity and/or solubility match between the original solvent system and the target analytes, b) the target analytes are poorly resolved in several formulations of the original solvent system family, c) emulsions form with the extract that may result in poor countercurrent separation behavior. Overall, this approach requires considerable skill and experience on the part of the practitioner to determine what the next steps of the iterative process should be, as well as knowing when the desired objective has been sufficiently attained.
Partitioning experiments
The use of partitioning experiments (also known as “shake flask” experiments) relies on the fact that the partition coefficient (K) obtained by the static equilibrium of an analyte between two phases of a biphasic solvent system will coincide with the K of the analyte in the same solvent system produced in a dynamic CCS experiment. The K is, therefore, a constant parameter to describe the distribution of a given analyte in a specific solvent system. In the partitioning experiment, the K value is calculated by the following equation (Eq. 1):
| Eq 1 |
where [C]upper phase is the concentration of the analyte in the upper phase and [C]lower phase is the concentration of the analyte in the lower phase. This distribution corresponds to the dynamic K value obtained from a CCS experiment run with the upper phase stationary (in practise, the CCS-based determination of K values has to be done manually and is unsupported by most CCS instruments). If the lower phase is the stationary phase in the CCS experiment, the ratio must be inverted.
Analytes with different K values in a solvent system may be separated by CCS with that solvent system [5]. The loading material may be a standard sample [6–8] or a crude material [9–16]. Such experiments have been described in detail [6,16]. Simply, the material is distributed to equilibrium, by vigorous shaking, between the two phases of a small amount of the solvent system. This may be done in a separatory funnel or in a vial. Samples of each phase are drawn off and assayed to quantitate the concentration of the desired analyte(s) in the upper and lower phases. The quantitative method is most often HPLC with UV-vis detection [17–19]. However, a wide range of analytical methods have been reported, such as thin-layer chromatography (TLC) with chemical revelation [20], TLC coupled with fluorimetry [21], GC-MS [22], LC-MS [6], and quantitative 1H NMR (qHNMR) [23, 24]. The choice of quantitation method often reflects the properties of the natural product(s) being targeted, the complexity of the sample, and/or targeting of single vs. multiple analytes, as well as the preferences of the researcher. If the analyte is unknown in an experiment to discover the bioactive component in a crude mixture, K-by-bioactivity is a practical method to correlate the distribution of the bioactive principle(s) to the sweet spot of a CCS chromatogram [25].
Table 1 describes 73 compounds belonging to 10 different classes that were isolated and purified using solvent systems selected by partitioning experiments. Terpenoids, flavonoids, and alkaloids are the major target natural product classes. The most commonly selected solvent system is HEMWat (used for 18 of the 73 natural products), because this solvent system provides two-phase systems over a wide polarity range [26].
Table 1.
Overview of the analytes and their respective solvent systems that were selected through extensive partitioning experiments.
| Class and references | Analytes | Selected SS (v/v) |
|---|---|---|
| Terpenoids [12, 24,59, 60] | Bilobalide, ginkgolides A, B, C, and J | ChMWat (10: 7: 3) and HEMWatSo (4: 6: 4: 6: 0.5%) |
| 2,3-O-acetylshengmanol-3-O-β-D-xylopyranoside, cimiracemoside D, 2,5-O-acetylcimige-nol-3-O-β-D-xylopyranoside, and aglycone cimigenol | HAtEIsoEtWat (3.5 :1 :2 :1 :0.5 : 2) | |
| Triptonide, isoneotriptophenolide, hypolide, triptophenolide, and triptonoterpene methyl ether VI | HEMWat (3 :2 :3 :2) | |
| Eriocalyxin B | HEMWat (1 :1 :1 :1) | |
| Flavonoids [7, 8,13, 16,61] | Tephrosin, 4′,5′-dimethoxy-6,6-dimethylpyranoisoflavone, deguelin, and 6a,12a-dehydro-deguelin | HEMWat (1 :0.8 : 1: 0.6) |
| Amentoflavone, robustaflavone, bilobetin, hinokiflavone, isocryptomerin and apigenin-diglucoside | HepEMWat (2 :3 :2 :3) | |
| Procyanidins | Methyl Acetate-Water (1 :1) | |
| Anthocyanins | BuWat (1 :1) | |
| Catechins | terAcWat (TFA) (2 :2: 3: 0.1%) | |
| Alkaloids [14, 23] | Daurisolin, dauricine, daurinoline, and dauricicoline | PetEEtWat (1 :2 :1 :2) |
| Ansamitocin P-3 | HEMWat (0.6 : 1: 0.6: 1) | |
| Ginkgotoxin | ChMWat (10: 5: 5) | |
| Lignans [10, 15,62] | 3′-Formylhonokiol, 5-formylhonokiol, and 3′,5-diformylhonokiol | HEMWat (1 :0.4 : 1: 0.4) |
| Honokiol and magnolol | HEMWat (1 :0.4 : 1: 0.4) | |
| Quinones [9] | Tanshinone IIA | HDiMWat (4: 0.75: 4: 1) and HEtWat (4: 2: 2) |
| Organic acids [63–65] | (±)-Cyclohexylmandelic acid | HterWat (9: 1: 10) with chiral selectors |
| Caftaric acid, coutaric acid, and fertaric acid | terAcBuWat (TFA) (2: 2: 1: 5: 0.5%) | |
| 20 Fatty acids | HMWat (350: 175: 2) | |
| Sterols [66] | Sitostanol and β-sitosterol | HMWat (silver nitrate) (34: 24: 1%) |
| Coumarins [67] | Notoptol and divaricatol | HEMWat (1 :1 :1 :1) |
| Peptides [6] | Enramycin A and B | HBuWat (TFA) (43 :7 :50: 0.05%) |
| Curcuminoids [17] | Curcumin, demethoxycurcumin and bisdemethoxycurcumin | HChMWat (5 :10 :7.5 : 2.5) |
Although the predetermination of K before a CCS instrument operation avoids solvent waste, an examination of several solvent systems takes a large amount of extra experimental effort. In one study, as many as 47 candidate solvent systems have been examined [17]. Automation, with a liquid handling robot, may ease the burden [19], but most laboratories do not have this capability.
Analytical countercurrent separation
Countercurrent separation chromatograms are linearly scalable, providing identical separations (K values) with similar efficiency from 20 mL to multi-liter capacity instruments [27–29]. Furthermore, with the development of analytical CCS instruments (Table 2 S, Supporting Information), the runtime has been shortened from days or hours to minutes [30]. Thus, analytical CCS may be used to test different solvent systems and directly observe K values, separation resolution, and stationary phase retention. Table 2 provides details of the use of analytical CCS for the separation of 44 natural products from eight classes. HEMWat and chloroform-methanol-water (ChMWat) systems are the main selected solvent systems used for 15 and 9 of the 44 compounds, respectively.
Table 2.
Overview of the analytes and their respective solvent systems that were selected and/or isolated by analytical scale CCS instruments.
| Class and references | Analytes | Selected SS (v/v) |
|---|---|---|
| Terpenoids [68, 69] | Linalool, terpinen-4-ol, α-terpineol, and p-anisaldehyde | HEMWat (5: 5: 2: 2) |
| Anethole and foeniculin | HM (1: 1) | |
| Lycopene | HDiAc (10 :3.5 : 6.5) | |
| Flavonoids [70–73] | Hispidulin, nepetin, homoplantaginin and nepetin-7-glucoside | HChMWat (0.5: 4: 3: 2) and ChMWat (4:3 :2) |
| Patuletin-3-O-glucoside, hyperoside, 6-methoxykaempferol-3-O-gal-actoside, and astragalin | EMWat (10: 1: 10) | |
| Quercetin, kaempferol, and isorhamnetin | DiMWat (5: 3: 2) | |
| Amygdalin | EBuWat (5: 2: 5), (5: 1: 5), (10 :1 :10), (50: 1: 50) and BuWat (1: 1) | |
| Taxifolin-3-O-glucoside and hyperoside | EMWat (25: 1: 25), (10: 1: 10) and (5: 1: 5) | |
| Baicalein-7-O-diglucoside, baicalein-7-O-glucoside, baicalein and chrysin | HEMWat (1: 1.2: 1: 1) | |
| Isoflavan glycoside and pterocarpan glycoside | EEtWatAa (4 :1 :5 :0.25) | |
| Alkaloids [74–77] | Betalain | ProAcWat-(sat’d(NH4)2SO4) (1 :0.5 :1 :1.2) and terBuAcWat (HFBA) (2: 2: 1: 5: 0.7) |
| Betacyanin | terBuAcWat (HFBA) (1: 3: 1: 5: 0.7) | |
| Dehydrocavidine | ChMWat (0.3 M HCl) (4: 0.5 :2) | |
| Lappaconitine, ranaconitine, N-deacetyllappaconitine and N-deacetylranaconitine | ChMWat (0.3 M or 0.2 M HCl) (4: 1.5: 2) | |
| Phenylpropanoids [78,79] | Salvianolic acid B | HEMWatAa (1: 5: 1.5: 5: 0. 596%) |
| Eleutheroside E | ChMIsoWat (5: 6: 1: 4) | |
| Coumarins [80,81] | Imperatorin, oxypeucedanin and isoimperatorin | HEMWat (1: 1: 1: 1) and (5 :5 :4.5 :5.5) |
| Osthol and xanthotoxol | HEMWat (1: 1: 1: 1) and (5 :5 :6: 4) | |
| Iridoids [82] | Geniposide | EBuWat (2: 1.5: 3) |
| Anthraquinones [83] | Rhein | HEMWat (3: 7: 5: 5) |
| Benzoquinones [84] | Coenzyme Q10 | HepDiAc (12 :7 :14) |
Evaluation of empirical strategies
Empirical solvent system selection strategies have a reputation of being labor intensive and tedious. However, they have several advantages, such as flexibility in the source material used for solvent system selection. Any source material may be employed for these experiments from commercial standards to crude extracts. Depending on the method of analysis, the K values of many different analytes in a particular solvent system may be determined simultaneously. In addition, the flexibility of analytical methods to quantitate the composition of upper and lower phases includes the assessment of biological activity [25] and the acquisition of structural data by NMR [24]. Finally, the results from empirical methods transfer well into the larger scale separations in terms of K value, resolution, and solvent system suitability for the dynamic CCS process.
Semiempirical Solvent System Selection Strategies
Semiempirical solvent system selection strategies attempt to reduce the number of partitioning experiments. They can identify a suitable solvent system by replacing some or all of the partitioning experiments with a simple method of predicting K values and/or using mathematical relationships to utilize a small number of experimentally determined K values to predict additional data points.
Thin-layer chromatography-based and reversed-phase high-performance liquid chromatography-based solvent system selection
TLC separation depends on two mechanisms, principally absorption and, to a lesser extent, partitioning. Although the CCS mechanism is only based on partitioning, both systems provide separations coordinated with analyte polarities. This is demonstrated by the commonly used TLC analysis of CCS fractions, which shows the distribution of analytes as strongly polarity related [31]. This general observation inspired the development of a TLC-based solvent system selection methodology. Marston and Hostettmann proposed that the upper and lower phases of a partitioning experiment be developed on silica gel TLC with the upper phase as the mobile phase [32]. In this way, both the partitioning and the suitability of the compound for a particular solvent system may be assessed.
The Generally Useful Estimate of Solvent Systems (GUESS) method [33] discusses the relationship between the TLC Rf value and the CCS K value. A mixture of 21 natural products with widely varying polarities (the GUESSmix) was prepared [4,33]. The K values were determined by partitioning experiments in both HEMWat and ChMWat solvent systems. These were compared with Rf values from TLC plates developed with the organic phase of that solvent system and/or a simplified formulation of the organic phase [33]. This study demonstrated a relationship between Rf values and K values such that Rf values between 0.29 and 0.71 (optimal value 0.5) could be correlated with K values from 0.4 to 2.5 (optimal value 1).
Representing an exemplary application of the GUESS method, ginkgotoxin, at the parts per million level [23], was enriched from Ginkgo biloba L. (Ginkgoaceae) seeds using GUESS-based CCS combined with qHNMR. In another example, the EBuWat (2:1:6, v/v) solvent system, predicted by TLC, led to the separation of isorhamnetin-3-O-gentiobioside, rutin, and narcissin [34]. Thus, mobility on TLC may be used as an indicator of CCS selectivity, and the TLC-based GUESS method provides a convenient means of estimating the K value in a solvent system. TLC-based methodology has the added advantage that it may be combined with a bioautography-based evaluation of bioactivity [35]. While the GUESS method simplifies the empirical methodology, its establishment and extension still requires the same iterative process exemplified by partitioning experiments.
Wagenaar et al. described the correlation of synthetic compound groups (libraries) with RP-HPLC (acetonitrile with 0.1% TFA and water gradient) and CCS retention times in selected HEMWat solvent systems [18]. There appears to be a nearly linear correlation between closely related compounds. However, the methodology has not been extended to natural products or beyond the HEM-Wat solvent system.
K value prediction within the HEMWat family
One outcome of the previously mentioned GUESS article was the observation that the logarithmic K value of an analyte, such as umbelliferone, has a linear relationship in 16 predefined HEM-Wat solvent systems [33]. This creates the scenario where if the K value in one HEMWat solvent system is known, the corresponding K value in a different HEMWat solvent system may be predicted. Ignatova et al. also observed a nearly linear correlation between the LogK values of pharmaceuticals and the respective HEMWat solvent system number [36]. The use of the HEMWat solvent system as a test case for solvent system selection methodologies arises, in part, because of the predominance of HEMWat in reported CCS applications [37]. For example, Tables 1 and 2 include a total of 33 compounds from eight different classes that were separated by CCS with a HEMWat solvent system. A significant feature of the HEMWat solvent system is that a wide range of polarities may be covered by the systematic modification of the hexane and ethyl acetate proportions relative to the methanol and water proportions between HEMWat 10:0:10:0 on the nonpolar extremity and HEMWat 0:10:0:10 on the polar extremity.
Han et al. described a method for predicting K values for a series of HMWat or HEMWat solvent systems by methodically modifying a pair of parameters. For example, the K value of an analyte may be mapped in the HMWat solvent system family by following the formula 5:x:10-x, where “x” is the volume of methanol in the range from 0 to 10. The K values of four formulas created a map that may be described by a mathematical equation (y = axb) that may be used to predict K values. The authors also investigated the families created by HEMWat 5:5:x:10-x (“x” again being the volume of methanol) and HEMWat x:10-x:5:5 (“x” being the volume of hexane). The method was applied to determine that HEMWat 5:5:5:5 was the optimal two-phase solvent system for the CCS preparation of pseudolaric acid from Pseudolarix kaempferi (Lindl.) Gordon (Pinaceae) [38].
Zhang et al. reported a solvent system selection method that was guided by the calculation of the average polarity (P’) of 36 solvent systems. The calculations were based on the solvent proportions and their Rohyschneider Snyder P′ solvent polarity values. The method was employed to isolate racemic tetrahydropalmatine from Corydalis yanhusuo W.T. Wang ex Z.Y. Su & C.Y. Wu (Papaveraceae) with HEMWat (4:6:5:5) [39]. Table 3 lists the defined HEMWat solvent systems and those used for the calculation of average polarity.
Table 3.
The surveyed HEMWat trends and polarities. The polarity gradient and HEMWat family trend may be obtained by experimental methods (e.g., the GUESS method [33]) or by calculation of the average of polarity [39].
| Defined HEMWat SSs HEMWat SS No. |
HEMWat volume ratios (v/v) | HEMWat SSs vs. average polarities HEMWat volume ratios (v/v) |
Polarity |
|---|---|---|---|
| HEMWat − 7 | HEMWat (9: 1: 9:1) | – | – |
| – | – | HEMWat (5: 1: 5: 1) | 3.38 |
| HEMWat − 6 | HEMWat (8: 2: 8:2) | – | – |
| HEMWat − 5 | HEMWat (7: 3: 7:3) | – | – |
| HEMWat − 4 | HEMWat (7: 3: 6:4) | – | – |
| – | – | HEMWat (6: 4: 5: 3) | 4.13 |
| HEMWat − 3 | HEMWat (6: 4: 6:4) | ||
| – | – | HEMWat (5: 4: 5: 4) | 4.69 |
| HEMWat − 2 | HEMWat (7: 3: 5:5) | – | – |
| HEMWat − 1 | HEMWat (6: 4: 5:5) | 4.74 | |
| HEMWat 0 | HEMWat (5: 5: 5:5) | 4.95 | |
| HEMWat + 1 | HEMWat (4: 6: 5:5) | 5.16 | |
| HEMWat + 2 | HEMWat (3: 7: 5:5) | 5.38 | |
| HEMWat + 3 | HEMWat (4: 6: 4:6) | – | – |
| – | – | HEMWat (3: 5: 3: 5) | 5.54 |
| HEMWat + 4 | HEMWat (3: 7: 4:6) | – | – |
| – | – | HEMWat (1: 2: 1: 2) | 5.73 |
| HEMWat + 5 | HEMWat (3: 7: 3:7) | – | – |
| – | – | HEMWat (2: 5: 2: 5) | 5.96 |
| – | – | HEMWat (3: 10: 3: 10) | 6.22 |
| HEMWat + 6 | HEMWat (2: 8: 2:8) | – | – |
| – | – | HEMWat (1: 5: 1: 5) | 6.52 |
| HEMWat + 7 | HEMWat (1: 9: 1:9) | – | – |
| HEMWat + 8 | HEMWat (0: 10:0 :10) | – | – |
Three-dimensional K value maps
Dubant et al. described a solvent system screening method that created a three-dimensional map correlating the composition of the upper and lower phases with the partition coefficient of a target analyte [40]. Creating the map required performing partitioning experiments to determine the partition coefficients of the target analyte in at least nine carefully selected solvent systems. Proof of principle was done with a mixture of seven pharmaceutical analytes: reserpine, ninhydrin, chloropropamide, dipropyl phthalate, methyl prednisolone, cortisone, and lidocaine. Further applications have not been reported, and future work will be needed to demonstrate that the primary screening of the K values with single factor experiments can be applied more generally.
Predictive models using empirical data to define descriptors
Ren et al. developed a K value prediction process employing a NonRandom Two-Liquid Segment Activity Coefficient (NRTL-SAC) model [41] for representing liquid-liquid equilibria. NRTL-SAC relies on solvent and analyte descriptors that define the liquid non-ideality of analytes and solvent molecules in terms of interactions among three pairwise interacting conceptual segments: the hydrophobic segment (molecular surface area that is adverse to hydrogen bonding), the polar segment (molecular surface area with interactions characteristic of an electron donor or acceptor), and the hydrophilic segment (molecular surface area with interactions characteristic of a hydrogen-bond donor or acceptor). The descriptors for the most common solvents are already known [42]. The K value of an analyte may be calculated as follows (Eq. 2) [43]:
| Eq. 2 |
where X = hydrophobic segment, Y− = polar-attractive segment, Y+ = polar-repulsive segment, and Z = hydrophilic segment. The liquid phase equilibrium composition of a selected solvent system is represented by PE.
It was previously shown that NRTL provided the best thermodynamic equation for the investigation of the K value of an analyte [44]. The NRTL-SAC model has been developed for solvent prediction based on a few partitioning experiment results. In order to evaluate the precision of the NRTL-SAC model, acetaminophen, sulfadiazine, cimetidine, sulfamerazine; magnolol, honokiol, 3-hydrophloridzin, and phloridzin were selected as standard analytes [43,45,46].
Another application of using descriptors to predict partition coefficients was reported by Qian and Poole. They determined the partition coefficients for 86 analytes in ChMWat 8:4:3, representing the solvent system for the Folch partition. The empirical data was used to determine the coefficients for a series of five solute descriptors based on excess molar refraction, polarizability, hydrogen-bond acidity, hydrogen-bond basicity, and McGowan’s characteristic volume [47].
Other semiempirical strategies
Both step gradients [20] and continuous gradients [36] have been successfully employed for the isolation of selected analytes by CCS. However, the use of gradient CCS chromatography as a solvent system selection technique is still in its infancy. More likely, the solvent system selection approach may induce the researcher to attempt a gradient as the best means of achieving a particular separation goal.
Evaluation of semiempirical strategies
Semiempirical strategies reduce, but do not entirely replace, the need for multiple partitioning experiments for solvent system selection. This reduces not only the workload but also the required expertise of the researcher. The iterative process of partitioning experiments is replaced by allowing the researcher to evaluate K values from many different solvent systems simultaneously. The choice of solvent system composition must still be determined by the researcher. However, the exploration of suitability of a particular formulation within the chosen solvent system family is facilitated by semiempirical methods. It may still be necessary to verify the resulting solvent system choice by either an equilibrium partitioning (“shake flask”) experiment or by analytical CCS before committing the experimental sample to the appropriate CCS scale. In addition, the general applicability of semiempirical methods outside of the common solvent system families has not been explored in most cases.
Theoretical Strategies
Theoretical strategies attempt to preclude the need for partitioning experiments altogether by combining solvent descriptors with similar properties attributed to an analyte in order to predict relative solubility. Predictive methods to determine the partition coefficient of a given analyte in the octanol/water solvent system are examples of theoretical strategies [48,49]. The main concept is that the solubility of an analyte in the upper and lower phases of a biphasic solvent system may be calculated independently given the structure of the analyte, the structures of the solvents, and the solvent composition of the phase.
Conductor-like screening model for real solvents
The Conductor-like Screening Model (COSMO) calculates the dielectric screening charges and energies on a van der Waals surface similar to a conductor and optimizes the analyte molecular geometry within a solvent system [50]. COSMO focuses on evaluating a particular analyte’s interaction with a few solvent molecules in order to predict the influence of the rest of the solvent on the analyte by an effective solvent continuum. COSMO for Real Solvents (COSMO-RS) has been developed as a quantum chemical and thermodynamic model [51]. It combines both solvent and analyte descriptors in order to calculate K values based on the structural information of analytes and solvents along with the solvent system phase composition. Two steps of the process include the optimization of the analyte’s molecular geometry as well as the generation of charge density of the analyte’s molecular surface. At this point, the COSMO-RS model is not applicable to ionized analytes. The details on K value calculations by COSMO-RS are described by Hopmann et al. [2,52]. Several case studies have been offered including n-alkylbenzenes in HepMWat solvent systems, steroids in HepEMWat solvent systems, benzyl alcohol in HepEMWat, phenols in HepEMWat, and selected GUESSmix analytes in HEMWat. Furthermore, coumarin, piperine, capsaicin, and dihydrocapsaicin were isolated by solvent systems selected with the COSMO-RS model [52,53].
A recent article employs both the COSMO-RS and the UNIversal quasichemical Functional-group Activity Coefficients (UNIFAC) predictive thermodynamic models in calculating K values. Both COSMO-RS and UNIFAC predictions may be calculated with or without partitioning experiment data to inform the descriptor values. Various combinations of UNIFAC, experimental, and COSMO-RS generated data were compared with the ultimate goal of predicting K values that coincide with experimental values. It was concluded that, in most cases, the values obtained from semiempirical and even theoretical methods was sufficient to recommend one or more solvent systems for further analysis with partitioning experiments. It was proposed that these theoretical predictive models may be employed to explore the suitability of a wide variety of solvent system families for countercurrent separation [54].
Evaluation of theoretical strategies
Analyte solubility behavior in solvent systems may be evaluated by software programs. This shifts the iterative solvent system selection process from the laboratory bench to the computer screen. A large number of solvent system formulations may be systematically investigated with minimal effort once the geometric and thermodynamic characteristics of both solvents and analytes have been established. The complete process from molecular structure to K calculation requires at least three different commercial software platforms, which may somewhat limit the overall applicability of the approach. As with the previous methods, the ability of the researcher to input solvent system families and to intelligently guide an iterative selection process is still needed. Because analyte structural information has to be provided, application in natural product discovery is limited in instances of unknown molecules.
Combination of Solvent System Selection Strategies
Navigating the maze of solvent system selection strategies
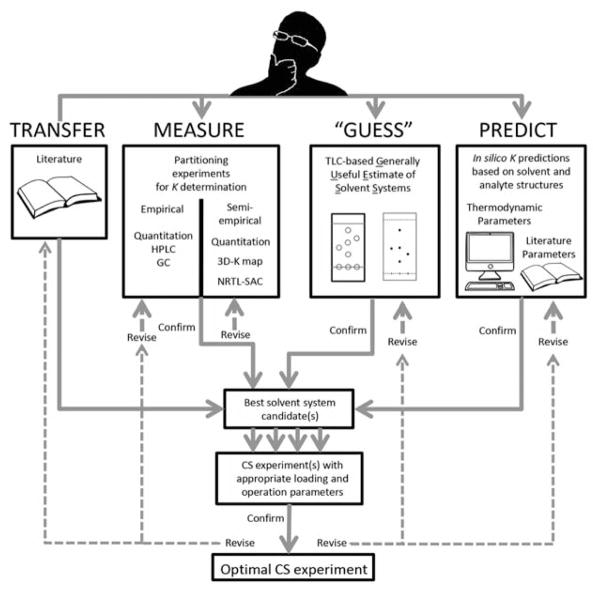
Fig. 2 represents the workflow divisions that belong to different methods of solvent system selection and how they interrelate to each other. The workflow in Fig. 2 emphasizes the iterative nature of the solvent system selection process. There are several decision points that will indicate whether or not the tested solvent system should be carried forward (confirmed) or reformulated (revised) and retested.
Fig. 2.
Workflow highlighting the four methods of solvent system selection. At the top, the chromatographer must decide which solvent system(s) will be tested. On the first level, the researcher may TRANSFER solvent systems from within the CCS literature directly to the second level for best solvent system testing. The researcher may MEASURE the K values of target analytes by a series of partitioning experiments. Partitioning experiments may be performed independently in an empirical manner or they can be organized in a semiempirical fashion to maximize the information generated. The researcher may employ the GUESS method, which relies on TLC Rf values to predict the best solvent system(s) for separation. Finally, the researcher may PREDICT the best solvent system(s) through theoretical modeling of the solubility parameters generated from structural information or gleaned from the literature. The workflow highlights the iterative nature of the solvent system selection process by showing that each solvent system must be evaluated and either confirmed or revised. (Color figure available online only.)
In the beginning, the researcher must decide which solvent system(s) will be attempted. Often, the CCS literature, such as the references in Tables 1 and 2, is helpful to reveal solvent systems that have been successfully employed to isolate certain types of compounds. Another approach is to start with well-known “portal” solvent systems that have established usefulness, such as HEMWat 1:1:1:1 and ChMWat 10:3:7 [32]. In addition, a skilled CCS practitioner may be able to propose a feasible starting point based on the structural characteristics or TLC polarity of the target compound(s).
The solvent system selection method may take four different pathways: “TRANSFER” – the researcher has a high degree of confidence that a literature solvent system will be effective. “MEASURE” – the researcher has a high-throughput quantitative analysis method (chemical or biological) available that may analyze partitioning experiments. Measuring can be approached with “brute force” by testing a large number of solvents systems or a “smart” methodology that uses a limited number of partitioning experiments to predict the analyte(s) K value(s) in other solvent systems. “GUESS” – the compound(s) is readily identifiable by TLC (chemically or biologically), and the researcher prefers to work with TLC data for preliminary testing. “PREDICT” – the structure(s) of the target compound(s) is known and the research team has the hardware as well as software expertise to analyze a large number of potential solvent systems in silico.
Once the preliminary testing has finished, a second level of solvent system testing is warranted. A primary solvent system should be tried on a CCS experiment. This will reveal the performance characteristics of the solvent system and extract combination. Adjustments may need to be made at this level to improve the stationary phase retention volume or reduce column overloading.
The optimal CCS experimental procedure is a culmination of the sample extraction method, preliminary purification, solvent system selection, and appropriate operating conditions which will lead to a desirable separation.
Conclusion
An aspect of solvent system selection in CCS that remains untouched by most solvent system selection strategies is the development of guided decision-making pathways. Current solvent system selection strategies allow the researcher to produce more K value data with less experimental effort, but this still leaves out the question of how to interpret this data. Data interpretation suffers because the comparative polarity and selectivity characteristics of different solvent systems have only been investigated superficially [4,55]. More systematic and in-depth studies of comparative polarity and selectivity characteristics of solvent systems are needed. This is especially important for the development of new solvent system formulations such as the recent work reported on limonene/methanol/water [56] and n-hexane/cyclohexane/tert-butylmethylether/methanol/water solvent systems [57]. In addition, predictive models for the addition of solvent system modifiers such as ionic liquids or inorganic salts have been only sparingly addressed [58].
Of the three classes of solvent system selection strategies, the empirical methods have been most widely used in the literature. However, the semiempirical and theoretical solvent system selection strategies developed recently employ methods or models that show great potential for efficient solvent system selection and guidance to facilitate the CCS purification of natural products. In addition, proper linkage of several solvent system selection strategies may reduce the experimental effort for solvent system selection significantly and lower the experimental burden of CCS applications.
It is important to note that all three types of selection strategies are suitable, in general, as solvent system selection guides for CCS novices. As the semiempirical strategies combine existing expertise with prediction power or recently developed methods, they may offer the best balance of theory and practice in contemporary applications of CCS. As the solvent system selection strategies evolve, such knowledge-based approaches will further simplify the procedure to select the right solvent system or at least provide a starting point with minimal effort.
Supplementary Material
Acknowledgments
The authors acknowledge support for their research involving CCS methodology through grants from the National Institutes of Health (P50 AT 000155, R01 DE 21040, and R44 AT 004534).
Abbreviations
- Aa
acetic acid
- Ac
acetonitrile
- At
acetone
- Bu
n-butanol
- Ch
chloroform
- Di
dichloromethane
- E
ethyl acetate
- Et
ethanol
- H
n-hexane or hexane(s)
- Hep
n-heptane
- HFBA
heptafluorobutyric acid
- Iso
iso-propanol
- M
methanol
- Pet
petroleum ether
- Pro
n-propanol
- So
DMSO
- ter
methyl tert-butylether
- TFA
trifluoroacetic acid
- Wat
water
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supporting information available online at http://www.thieme-connect.de/products
An overview of the abbreviated CCS solvent naming system and examples of contemporary analytical CCS instruments are available as Supporting Information.
References
- 1.Ito Y. High-speed countercurrent chromatography. Nature. 1987;326:419–420. doi: 10.1038/326419a0. [DOI] [PubMed] [Google Scholar]
- 2.Hopmann E, Arlt W, Minceva M. Solvent system selection in counter-current chromatography using conductor-like screening model for real solvents. J Chromatogr A. 2011;1218:242–250. doi: 10.1016/j.chroma.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Eromosele O, Bo S, Jia H, Ping L. Preparative isolation and purification of glyceollins from soy bean elicited with Aspergillus sojae by high-speed countercurrent chromatography. J Chromatogr Sep Technol. 2012;3:1–7. [Google Scholar]
- 4.Friesen JB, Pauli GF. Rational development of solvent system families in counter-current chromatography. J Chromatogr A. 2007;1151:51–59. doi: 10.1016/j.chroma.2007.01.126. [DOI] [PubMed] [Google Scholar]
- 5.Ito Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J Chromatogr A. 2005;1065:145–168. doi: 10.1016/j.chroma.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 6.Inoue K, Hattori Y, Hino T, Oka H. An approach to on-line electrospray mass spectrometric detection of polypeptide antibiotics of enramycin for high-speed counter-current chromatographic separation. J Pharm Biomed Anal. 2010;51:1154–1160. doi: 10.1016/j.jpba.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Yanagida A, Shoji A, Shibusawa Y, Shindo H, Tagashira M, Ikeda M, Ito Y. Analytical separation of tea catechins and food-related polyphenols by high-speed counter-current chromatography. J Chromatogr A. 2006;1112:195–201. doi: 10.1016/j.chroma.2005.09.086. [DOI] [PubMed] [Google Scholar]
- 8.Shibusaw Y, Yanagida A, Isozaki M, Shindo H, Ito Y. Separation of apple procyanidins into different degrees of polymerization by high-speed counter-current chromatography. J Chromatogr A. 2001;915:253–257. doi: 10.1016/s0021-9673(01)00575-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Ignatova S, Hu P, Liang Q, Wang Y, Luo G, Jun FW, Sutherland I. Development of a strategy and process parameters for a green process in counter-current chromatography: purification of tanshinone IIA and cryptotanshinone from Salvia miltiorrhiza Bunge as a case study. J Chromatogr A. 2011;1218:6031–6037. doi: 10.1016/j.chroma.2010.12.118. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Zhang Q, Yang G, Fan L, Tang J, Garrard I, Ignatova S, Fisher D, Sutherland IA. Rapid purification and scale-up of honokiol and magnolol using high-capacity high-speed counter-current chromatography. J Chromatogr A. 2007;1142:115–122. doi: 10.1016/j.chroma.2006.09.098. [DOI] [PubMed] [Google Scholar]
- 11.Luo H, Peng M, Ye H, Chen L, Peng A, Tang M, Zhang F, Shi J. Predictable and linear scale-up of four phenolic alkaloids separation from the roots of Menispermum dauricum using high-performance counter-current chromatography. J Chromatogr B. 2010;878:1929–1933. doi: 10.1016/j.jchromb.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Cicek SS, Schwaiger S, Ellmerer EP, Stuppner H. Development of a fast and convenient method for the isolation of triterpene saponins from Actaea racemosa by high-speed countercurrent chromatography coupled with evaporative light scattering detection. Planta Med. 2010;76:467–473. doi: 10.1055/s-0029-1186236. [DOI] [PubMed] [Google Scholar]
- 13.Yuan Y, Wang B, Chen L, Luo H, Fisher D, Sutherland IA, Wei Y. How to realize the linear scale-up process for rapid purification using high-performance counter-current chromatography. J Chromatogr A. 2008;1194:192–198. doi: 10.1016/j.chroma.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 14.Yao Y, Cheng Z, Ye H, Xie Y, He J, Tang M, Shen T, Wang J, Zhou Y, Lu Z, Luo F, Chen L, Yu L, Yang JL, Peng A, Wei Y. Preparative isolation and purification of anti-tumor agent ansamitocin P-3 from fermentation broth of Actinosynnema pretiosum using high-performance counter-current chromatography. J Sep Sci. 2010;33:1331–1337. doi: 10.1002/jssc.200900746. [DOI] [PubMed] [Google Scholar]
- 15.Luo Y, Xu Y, Chen L, Luo H, Peng C, Fu J, Chen H, Peng A, Ye H, Xie D, Fu A, Shi J, Yang S, Wei Y. Preparative purification of anti-tumor derivatives of honokiol by high-speed counter-current chromatography. J Chromatogr A. 2008;1178:160–165. doi: 10.1016/j.chroma.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 16.Ye H, Chen L, Li Y, Peng A, Fu A, Song H, Tang M, Luo H, Luo Y, Xu Y, Shi J, Wei Y. Preparative isolation and purification of three rotenoids and one isoflavone from the seeds of Millettia pachycarpa Benth by high-speed counter-current chromatography. J Chromatogr A. 2008;1178:101–107. doi: 10.1016/j.chroma.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 17.Inoue K, Nomura C, Ito S, Nagatsu A, Hino T, Oka H. Purification of curcumin, demethoxycurcumin, and bisdemethoxycurcumin by high-speed countercurrent chromatography. J Agric Food Chem. 2008;56:9328–9336. doi: 10.1021/jf801815n. [DOI] [PubMed] [Google Scholar]
- 18.Wagenaar FL, Hochlowski JE, Pan JY, Tu NR, Searle PA. Purification of high-throughput organic synthesis libraries by counter-current chromatography. J Chromatogr A. 2009;1216:4154–4160. doi: 10.1016/j.chroma.2008.11.092. [DOI] [PubMed] [Google Scholar]
- 19.Garrard IJ. Simple approach to the development of a CCC solvent selection protocol suitable for automation. J Liq Chromatogr Relat Technol. 2005;28:1923–1935. [Google Scholar]
- 20.Leitao GG, de Souza PA, Moraes AA, Brown L. Step-gradient CCC separation of phenylpropanoid and iridoid glycosides from roots of Stachytarpheta cayennensis (Rich) vahl. J Liq Chromatogr Relat Technol. 2005;28:2053–2060. [Google Scholar]
- 21.He K, Ye X, Li X, Chen H, Yuan L, Deng Y, Chen X, Li X. Separation of two constituents from purple sweet potato by combination of silica gel column and high-speed counter-current chromatography. J Chromatogr B. 2012;881–882:49–54. doi: 10.1016/j.jchromb.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 22.Dang YY, Li XC, Zhang QW, Li SP, Wang YT. Preparative isolation and purification of six volatile compounds from essential oil of Curcuma wenyujin using high-performance centrifugal partition chromatography. J Sep Sci. 2010;33:1658–1664. doi: 10.1002/jssc.200900453. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Chen SN, McAlpine JB, Klein LL, Friesen JB, Lankin DC, Pauli GF. Quantification of a botanical negative marker without an identical standard: ginkgotoxin in Ginkgo biloba. J Nat Prod. 2014;77:611–617. doi: 10.1021/np400874z. [DOI] [PubMed] [Google Scholar]
- 24.Qiu F, Friesen JB, McAlpine JB, Pauli GF. Design of countercurrent separation of Ginkgo biloba terpene lactones by nuclear magnetic resonance. J Chromatogr A. 2012;1242:26–34. doi: 10.1016/j.chroma.2012.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh JC, Garrard IJ, Cho CW, Annie Bligh SW, Lu GH, Fan TP, Fisher D. Bio-activity-guided fractionation of the volatile oil of Angelica sinensis radix designed to preserve the synergistic effects of the mixture followed by identification of the active principles. J Chromatogr A. 2012;1236:132–138. doi: 10.1016/j.chroma.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Sutherland IA, Fisher D. Role of counter-current chromatography in the modernisation of Chinese herbal medicines. J Chromatogr A. 2009;1216:740–753. doi: 10.1016/j.chroma.2008.11.095. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland IA, Brown L, Graham AS, Guillon GG, Hawes D, Janaway L, Whiteside R, Wood P. Industrial scale-up of countercurrent chromatography: predictive scale-up. J Chromatogr Sci. 2001;39:21–28. doi: 10.1093/chromsci/39.1.21. [DOI] [PubMed] [Google Scholar]
- 28.Wood P, Ignatova S, Janaway L, Keay D, Hawes D, Garrard I, Sutherland IA. Counter-current chromatography separation scaled up from an analytical column to a production column. J Chromatogr A. 2007;1151:5–30. doi: 10.1016/j.chroma.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Ignatova S, Wood P, Hawes D, Janaway L, Keay D, Sutherland I. Feasibility of scaling from pilot to process scale. J Chromatogr A. 2007;1151:20–24. doi: 10.1016/j.chroma.2007.02.084. [DOI] [PubMed] [Google Scholar]
- 30.Faure KM, Nazim M, Meucci J, Berthod A. Solvent selection in counter-current chromatography using small-volume hydrostatic columns. LC GC N Am. 2013;31:132–143. [Google Scholar]
- 31.Hostettmann K, Hostettmann-Kaldas M, Sticher O. Application of droplet counter-current chromatography to the isolation of natural products. J Chromatogr A. 1979;186:529–534. [Google Scholar]
- 32.Marston A, Hostettmann K. Developments in the application of counter-current chromatography to plant analysis. J Chromatogr A. 2006;1112:181–194. doi: 10.1016/j.chroma.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 33.Friesen JB, Pauli GF. G.U.E.S.S. – A generally useful estimate of solvent systems for CCC. J Liq Chromatogr Relat Technol. 2005;28:2777–2806. [Google Scholar]
- 34.Yang C, Yang Y, Aisa HA, Xin X, Ma H, Yili A, Zhao Y. Bioassay-guided isolation of antioxidants from Astragalus altaicus by combination of chromatographic techniques. J Sep Sci. 2012;35:977–983. doi: 10.1002/jssc.201101104. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Schwack W. High-performance thin-layer chromatography screening of multi class antibiotics in animal food by bioluminescent bioautography and electrospray ionization mass spectrometry. J Chromatogr A. 2014;1356:249–257. doi: 10.1016/j.chroma.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 36.Ignatova S, Sumner N, Colclough N, Sutherland I. Gradient elution in counter-current chromatography: a new layout for an old path. J Chromatogr A. 2011;1218:6053–6060. doi: 10.1016/j.chroma.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 37.Pauli GF, Pro SM, Friesen JB. Countercurrent separation of natural products. J Nat Prod. 2008;71:1489–1508. doi: 10.1021/np800144q. [DOI] [PubMed] [Google Scholar]
- 38.Han QB, Wong L, Yang NY, Song JZ, Qiao CF, Yiu H, Ito Y, Xu HX. A simple method to optimize the HSCCC two-phase solvent system by predicting the partition coefficient for target compound. J Sep Sci. 2008;31:1189–1194. doi: 10.1002/jssc.200700582. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S, Wang X, Ouyang F, Su Z, Wang C, Gu M. Separation and purification of dl-tetrahydropalmatine from Corydalis yanhusuo W. T. Wang by HSCCC with a new solvent system screening method. J Liq Chromatogr Relat Technol. 2008;31:2632–2642. [Google Scholar]
- 40.Dubant S, Mathews B, Higginson P, Crook R, Snowden M, Mitchell J. Practical solvent system selection for counter-current separation of pharmaceutical compounds. J Chromatogr A. 2008;1207:190–192. doi: 10.1016/j.chroma.2008.08.113. [DOI] [PubMed] [Google Scholar]
- 41.Renon H, Prausnitz JM. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 2004;14:135–144. [Google Scholar]
- 42.Chen CC, Song Y. Solubility modeling with a nonrandom two-liquid segment activity coefficient model. Ind Eng Chem Res. 2004;43:8354–8362. [Google Scholar]
- 43.Chen CC, Crafts PA. Correlation and prediction of drug molecule solubility in mixed solvent systems with the nonrandom two-liquid segment activity coefficient (NRTL–SAC) model. Ind Eng Chem Res. 2006;45:4816–4824. [Google Scholar]
- 44.Chen J, Yu Y, Li Z. Accurate calculation for liquid–liquid equilibria of typical solvent systems used in CCC. J Liq Chromatogr Relat Technol. 2005;28:1937–1946. [Google Scholar]
- 45.Ren DB, Yang ZH, Liang YZ, Ding Q, Chen C, Ouyang ML. Correlation and prediction of partition coefficient using nonrandom two-liquid segment activity coefficient model for solvent system selection in counter-current chromatography separation. J Chromatogr A. 2013;1301:10–18. doi: 10.1016/j.chroma.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 46.Ren DB, Qin YH, Yun YH, Lu HM, Chen XQ, Liang YZ. Using nonrandom two-liquid model for solvent system selection in counter-current chromatography. J Chromatogr A. 2014;1355:80–85. doi: 10.1016/j.chroma.2014.05.080. [DOI] [PubMed] [Google Scholar]
- 47.Qian J, Poole CF. Distribution model for Folch partition. J Sep Sci. 2007;30:2326–2331. doi: 10.1002/jssc.200700175. [DOI] [PubMed] [Google Scholar]
- 48.Ghose AK, Viswanadhan VN, Wendoloski JJ. Prediction of hydrophobic (lipophilic) properties of small organic molecules using fragmental methods: An analysis of ALOGP and CLOGP methods. J Phys Chem A. 1998;102:3762–3772. [Google Scholar]
- 49.Moriguchi I, Hirono S, Liu Q, Nakagome I, Matsushita Y. Simple method of calculating octanol water partition-coefficient. Chem Pharm Bull. 1992;40:127–130. [Google Scholar]
- 50.Stewart JP. MOPAC: a semiempirical molecular orbital program. J Comput Aided Mol Des. 1990;4:1–105. doi: 10.1007/BF00128336. [DOI] [PubMed] [Google Scholar]
- 51.Klamt A. Conductor-like screening model for real solvents: a new approach to the quantitative calculation of solvation phenomena. J Phys Chem A. 1995;99:2224–2235. [Google Scholar]
- 52.Hopmann E, Frey A, Minceva M. A priori selection of the mobile and stationary phase in centrifugal partition chromatography and counter-current chromatography. J Chromatogr A. 2012;1238:68–76. doi: 10.1016/j.chroma.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 53.Goll J, Frey A, Minceva M. Study of the separation limits of continuous solid support free liquid-liquid chromatography: separation of capsaicin and dihydrocapsaicin by centrifugal partition chromatography. J Chromatogr A. 2013;1284:59–68. doi: 10.1016/j.chroma.2013.01.116. [DOI] [PubMed] [Google Scholar]
- 54.Frey A, Hopmann E, Minceva M. Selection of biphasic liquid systems in liquid-liquid chromatography using predictive thermodynamic models. Chem Eng Technol. 2014;37:1663–1674. [Google Scholar]
- 55.Friesen JB, Ahmed S, Pauli GF. Qualitative and quantitative evaluation of solvent systems for countercurrent separation. J Chromatogr A. 2015;1377:55–63. doi: 10.1016/j.chroma.2014.11.085. [DOI] [PubMed] [Google Scholar]
- 56.Faure K, Bouju E, Suchet P, Berthod A. Use of limonene in countercurrent chromatography: a green alkane substitute. Anal Chem. 2013;85:4644–4650. doi: 10.1021/ac4002854. [DOI] [PubMed] [Google Scholar]
- 57.Englert M, Vetter W. Solvent systems with n-hexane and/or cyclohexane in countercurrent chromatography: Physico-chemical parameters and their impact on the separation of alkyl hydroxybenzoates. J Chromatogr A. 2014;1342:54–62. doi: 10.1016/j.chroma.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 58.Romero-Gonzalez RR, Verpoorte R. Salting-out gradients in centrifugal partition chromatography for the isolation of chlorogenic acids from green coffee beans. J Chromatogr A. 2009;1216:4245–4251. doi: 10.1016/j.chroma.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Peng A, Li R, Hu J, Chen L, Zhao X, Luo H, Ye H, Yuan Y, Wei Y. Flow rate gradient high-speed counter-current chromatography separation of five diterpenoids from Triperygium wilfordii and scale-up. J Chromatogr A. 2008;1200:129–135. doi: 10.1016/j.chroma.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 60.Han QB, Yu T, Lai F, Zhou Y, Feng C, Wang WN, Fu XH, Lau CB, Luo KQ, Xu HX, Sun HD, Fung KP, Leung PC. Quick identification of apoptosis inducer from Isodon eriocalyx by a drug discovery platform composed of analytical high-speed counter-current chromatography and the fluorescence-based caspase-3 biosensor detection. Talanta. 2010;82:1521–1527. doi: 10.1016/j.talanta.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 61.Inoue K, Baba E, Hino T, Oka H. A strategy for high-speed countercurrent chromatography purification of specific antioxidants from natural products based on on-line HPLC method with radical scavenging assay. Food Chem. 2012;134:2276–2282. doi: 10.1016/j.foodchem.2012.02.219. [DOI] [PubMed] [Google Scholar]
- 62.Wang X, Wang Y, Geng Y, Li F, Zheng C. Isolation and purification of honokiol and magnolol from cortex Magnoliae officinalis by high-speed counter-current chromatography. J Chromatogr A. 2004;1036:171–175. doi: 10.1016/j.chroma.2004.02.073. [DOI] [PubMed] [Google Scholar]
- 63.Tong S, Yan J, Guan YX, Fu Y, Ito Y. Separation of alpha-cyclohexylmandelic acid enantiomers using biphasic chiral recognition high-speed counter-current chromatography. J Chromatogr A. 2010;1217:3044–3052. doi: 10.1016/j.chroma.2010.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maier T, Sanzenbacher S, Kammerer DR, Berardini N, Conrad J, Beifuss U, Carle R, Schieber A. Isolation of hydroxycinnamoyltartaric acids from grape pomace by high-speed counter-current chromatography. J Chromatogr A. 2006;1128:61–67. doi: 10.1016/j.chroma.2006.06.082. [DOI] [PubMed] [Google Scholar]
- 65.Hammann S, Tillmann U, Schröder M, Vetter W. Profiling the fatty acids from a strain of the microalgae Alexandrium tamarense by means of high-speed counter-current chromatography and gas chromatography coupled with mass spectrometry. J Chromatogr A. 2013;1312:93–103. doi: 10.1016/j.chroma.2013.08.090. [DOI] [PubMed] [Google Scholar]
- 66.Schröder M, Vetter W. High-speed counter-current chromatographic separation of phytosterols. Anal Bioanal Chem. 2011;400:3615–3623. doi: 10.1007/s00216-011-4995-2. [DOI] [PubMed] [Google Scholar]
- 67.Skalicka-Wozniak K, Mroczek T, Garrard I, Glowniak K. Isolation of the minor and rare constituents from fruits of Peucedanum alsaticum L. using high-performance counter-current chromatography. J Sep Sci. 2012;35:790–797. doi: 10.1002/jssc.201100815. [DOI] [PubMed] [Google Scholar]
- 68.Skalicka-Wozniak K, Walasek M, Ludwiczuk A, Glowniak K. Isolation of terpenoids from Pimpinella anisum essential oil by high-performance counter-current chromatography. J Sep Sci. 2013;36:2611–2614. doi: 10.1002/jssc.201300407. [DOI] [PubMed] [Google Scholar]
- 69.Wei Y, Zhang T, Xu G, Ito Y. Application of analytical and preparative high-speed counter-current chromatography for separation of lycopene from crude extract of tomato paste. J Chromatogr A. 2001;929:169–173. doi: 10.1016/s0021-9673(01)01177-3. [DOI] [PubMed] [Google Scholar]
- 70.Li J, Zhang X, Yu Q, Fu X, Wang W. One-step separation of four flavonoids from Herba Salviae plbeiae by HSCCC. J Chromatogr Sci. 2014;52:1288–1293. doi: 10.1093/chromsci/bmu007. [DOI] [PubMed] [Google Scholar]
- 71.Wei Y, Xie Q, Fisher D, Sutherland IA. Separation of patuletin-3-O-glucoside, astragalin, quercetin, kaempferol and isorhamnetin from Flaveria bidentis (L) Kuntze by elution-pump-out high-performance counter-current chromatography. J Chromatogr A. 2011;1218:6206–6211. doi: 10.1016/j.chroma.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 72.Wei Y, Xie Q, Ito Y. Preparative separation of axifolin-3-glucoside, hyperoside and amygdalin from plant extracts by high-speed countercur-rent chromatography. J Liq Chromatogr Relat Technol. 2009;32:1010–1022. doi: 10.1080/10826070902790983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen LJ, Song H, Lan XQ, Games DE, Sutherland IA. Comparison of high-speed counter-current chromatography instruments for the separation of the extracts of the seeds of Oroxylum indicum. J Chromatogr A. 2005;1063:241–245. doi: 10.1016/j.chroma.2004.11.072. [DOI] [PubMed] [Google Scholar]
- 74.Sporna-Kucab A, Ignatova S, Garrard I, Wybraniec S. Versatile solvent systems for the separation of betalains from processed Beta vulgaris L. juice using counter-current chromatography. J Chromatogr B. 2013;941:54–61. doi: 10.1016/j.jchromb.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Jerz G, Gebers N, Szot D, Szaleniec M, Winterhalter P, Wybraniec S. Separation of amaranthine-type betacyanins by ion-pair high-speed countercurrent chromatography. J Chromatogr A. 2014;1344:42–50. doi: 10.1016/j.chroma.2014.03.085. [DOI] [PubMed] [Google Scholar]
- 76.Deng J, Xiao X, Li G, Ruan G. Application of microwave-assisted extraction coupled with high-speed counter-current chromatography for separation and purification of dehydrocavidine from Corydalis saxicola Bunting. Phytochem Anal. 2009;20:498–502. doi: 10.1002/pca.1152. [DOI] [PubMed] [Google Scholar]
- 77.Yang F, Ito Y. Preparative separation of lappaconitine, ranaconitine, N-deacetyllappaconitine and N-deacetylranaconitine from crude alkaloids of sample Aconitum sinomontanum Nakai by high-speed counter-current chromatography. J Chromatogr A. 2002;943:219–225. doi: 10.1016/s0021-9673(01)01464-9. [DOI] [PubMed] [Google Scholar]
- 78.Zhang M, Ignatova S, Liang Q, Wu Jun F, Sutherland I, Wang Y, Luo G. Rapid and high-throughput purification of salvianolic acid B from Salvia miltiorrhiza Bunge by high-performance counter-current chromatography. J Chromatogr A. 2009;1216:3869–3873. doi: 10.1016/j.chroma.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 79.Wei Y, Zhang TY, Wu KY. Separation of eleutheroside E from crude extract of Radix Acanthopanacis senticosus by analytical and preparative high-speed countercurrent chromatography. Se Pu. 2002;20:543–545. [PubMed] [Google Scholar]
- 80.Wei Y, Ito Y. Preparative isolation of imperatorin, oxypeucedanin and isoimperatorin from traditional Chinese herb “bai zhi” Angelica dahurica (Fisch. ex Hoffm) Benth. et Hook using multidimensional high-speed counter-current chromatography. J Chromatogr A. 2006;1115:112–117. doi: 10.1016/j.chroma.2006.02.081. [DOI] [PubMed] [Google Scholar]
- 81.Ma X, Tu P, Chen Y, Zhang T, Wei Y, Ito Y. Preparative isolation and purification of isoflavan and pterocarpan glycosides from Astragalus membranaceus Bge. var. mongholicus (Bge) Hsiao by high-speed counter-current chromatography. J Chromatogr A. 2004;1023:311–315. doi: 10.1016/j.chroma.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 82.Zhou T, Fan G, Hong Z, Chai Y, Wu Y. Large-scale isolation and purification of geniposide from the fruit of Gardenia jasminoides Ellis by high-speed counter-current chromatography. J Chromatogr A. 2005;1100:76–80. doi: 10.1016/j.chroma.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 83.Wei Y, Zhang T, Ito Y. Preparative separation of rhein from Chinese traditional herb by repeated high-speed counter-current chromatography. J Chromatogr A. 2003;1017:125–130. doi: 10.1016/j.chroma.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 84.Cao XL, Xu YT, Zhang GM, Xie SM, Dong YM, Ito Y. Purification of coenzyme Q10 from fermentation extract: high-speed counter-current chromatography versus silica gel column chromatography. J Chromatogr A. 2006;1127:92–96. doi: 10.1016/j.chroma.2006.05.083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.