Abstract
Prenatal development is recognized as a critical period in the etiology of obesity and cardiometabolic disease. Potential strategies to reduce maternal obesity-induced risk later in life have been largely overlooked. In this paper, we first propose a conceptual framework for the role of public health and preventive medicine in mitigating the effects of fetal programming. Second, we review a small but growing body of research (through August 2015) that examines interactive effects of maternal obesity and two public health foci – diet and physical activity – in the offspring. Results of the review support the hypothesis that diet and physical activity after early life can attenuate disease susceptibility induced by maternal obesity, but human evidence is scant. Based on the review, we identify major gaps relevant for prevention research, such as characterizing the type and dose response of dietary and physical activity exposures that modify the adverse effects of maternal obesity in the offspring. Third, we discuss potential implications of interactions between maternal obesity and postnatal dietary and physical activity exposures for interventions to mitigate maternal obesity-induced risk among children. Our conceptual framework, evidence review, and future research directions offer a platform to develop, test, and implement fetal programming mitigation strategies for the current and future generations of children.
Keywords: Prenatal Exposure Delayed Effects, obesity, physical activity, nutrition, prevention, second hit
Introduction
An increasing proportion of U.S. children are born to obese mothers, reaching 20% in 2009 (Fisher et al., 2013). The offspring have a three-fold greater risk of obesity (Yu et al., 2013) suggesting that we are in the midst of an escalating intergenerational obesity cycle (Dabelea and Crume, 2011). Existing strategies to curb intergenerational obesity focus on pregnancy and infant health (Nader et al., 2012; Perez-Escamilla and Kac, 2013). In this paper, we evaluate an emerging complimentary strategy: mitigation of maternal obesity-induced chronic disease susceptibility in childhood and adolescence, prior to reproduction.
Maternal obesity induces alterations in prenatal development that “programs” increased susceptibility to obesity and cardiometabolic conditions in offspring (Frias and Grove, 2012; Lawlor et al., 2012). Because of the powerful associations between adverse intrauterine exposures and later disease in offspring, it is tempting to assume a fatalistic interpretation in which programmed risk is perceived as irreversible after early life (Skogen and Overland, 2012; Vickers and Sloboda, 2012). However, programmed disease manifests primarily in the context of adverse exposures encountered in childhood, adolescence, or adulthood (Bagby, 2007). These exposures may include factors such as physical inactivity, poor diet, smoking, or psychosocial stress. Reducing postnatal adverse exposures represents a potential opportunity to mitigate the adverse intrauterine effects of maternal obesity.
In this paper, we first describe a conceptual framework for the role of public health and preventive medicine in mitigating the intergenerational cycle of obesity by preventing key postnatal exposures. Second, we review animal and human studies that consider how maternal obesity interacts with diet or physical activity in childhood, adolescence, or adulthood to alter risk for later disease and identify potential future directions for human research. Third, we discuss implications for interventions seeking to mitigate the deleterious effects of fetal programming in the current and future generations of children.
I. Fetal programming prevention and mitigation in the intergenerational cycle of obesity: conceptual framework
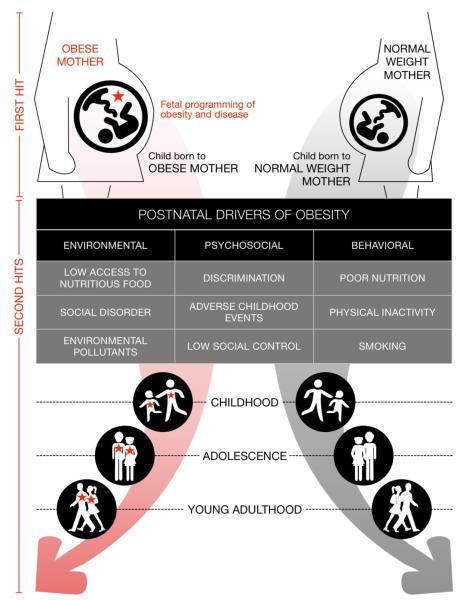
Consider a lineage of disease (Figure 1), starting with the presence or absence of a “first hit” during prenatal development. In this paper, we focus on an individual’s gestation within an obese or normal intrauterine environment. The detrimental effects of maternal obesity on the developing fetus are indicated by a red star. Maternal obesity-induced alterations increase susceptibility to obesity and disease in the offspring (Dabelea and Crume, 2011; Frias and Grove, 2012) which they carry throughout their life. After birth, offspring encounter “second hits” (Bagby, 2007) – postnatal nutritional, environmental, psychosocial, and behavioral factors that drive obesity and disease.
Figure 1.
Public health prevention in the context of fetal programming by maternal obesity
A. Fetal programming prevention: preventing the first hit
Compared with children born to normal weight mothers, those born to mothers who are obese have greater risk of obesity and cardiometabolic disease (Boney et al., 2005; Dabelea et al., 2008; Lau et al., 2014; Yu et al., 2013). Infants born to obese, nondiabetic mothers have elevated adiposity and insulin resistance emerging as early as birth (Catalano et al., 2009). Experimental animal research is consistent with human studies and has begun to provide understanding of the biologic mechanisms underlying intergenerational patterns (Ainge et al., 2011). Examples of these mechanisms include impaired appetite regulation (Breton, 2013; Ferezou-Viala et al., 2007; Kirk et al., 2009), low skeletal muscle mass with impaired glucose regulation (Bayol et al., 2014; Du et al., 2010), and dysfunctional lipid metabolism (McCurdy et al., 2009; Pruis et al., 2014).
Interventions in girls and women who are pregnant or about to become pregnant offer hope for preventing fetal programming (Macaulay et al., 2014; Nader et al., 2012; Perez-Escamilla and Kac, 2013) (the first hit) and reducing the number of individuals in the left side of Figure 1 (obese mother). Promising approaches include preconception weight loss (Catalano and deMouzon, 2015) among women who are obese prior to pregnancy and, among all women, appropriate gestational weight gain, and healthy diet and physical activity before and during pregnancy (McDonald et al., 2015; Muktabhant et al., 2015; Rooney and Ozanne, 2011). Perinatal interventions are active areas of research, but to date have yielded only modest improvements in maternal health (Catalano and deMouzon, 2015; Guelinckx et al., 2010; Kinnunen et al., 2007; Poston et al., 2013; Wolff et al., 2008) and it is too soon to evaluate their effects on long-term offspring health.
B. Fetal programming mitigation: preventing the second hit
While preventing fetal programming exposures (first hit) remains an important area of practice and research, we propose that the course of intergenerational obesity has progressed beyond the stage in which we can focus solely on prenatal health. In 2009, 20.5% of children were born to women who were obese, with greater proportions in minority groups (e.g., 29.2% in African Americans) (Fisher et al., 2013). Resolving the obesity epidemic requires mitigation of programmed susceptibility in children who have already experienced a maternal obesity first hit, which comprises an increasing proportion of all births (Fisher et al., 2013).
We define the term fetal programming mitigation as the attenuation of disease processes after fetal programming has occurred. Mitigation concerns infants and children as they grow into adolescents and adults on the left side of Figure 1 (obese mothers). We hypothesize that preventing second hits can minimize clinical risk factors (e.g., insulin resistance) and prevent or delay disease progression into clinical disease (e.g., diabetes). Fetal programming mitigation would thus reduce the manifestation of programmed risk in any given generation and, upon childbearing, prevent a first hit in the subsequent generation.
While healthy infant feeding practices such as breastfeeding and optimal complimentary feeding (Thompson, 2013) offer early opportunity for reducing obesity risk, less attention has been paid to strategies designed to prevent the second hit in childhood or beyond. Pharmacologic or nutritional therapeutic agents that mitigate fetal programming effects are another critical topic (Chen et al., 2014; Chen et al., 2012) that has been reviewed previously (IOM (Institute of Medicine), 2015; Vickers and Sloboda, 2012). In this paper, we focus on fetal programming mitigation by lifestyle behaviors in childhood, adolescence, and young adulthood.
Traditional public health and preventive medicine: a starting point for fetal programming mitigation
Prevention research has generated vast knowledge about the types, dose, and timing of childhood, adolescent, and adult dietary and physical activity exposures that prevent or promote obesity and disease. However, these studies have been largely conducted without consideration of heterogeneity in effects according to the occurrence of a “first hit”. That is, knowledge about obesity and chronic disease prevention does not distinguish between the right and left sides of Figure 1. Prevention among all offspring remains important for promoting health in the current generation and preventing first hits in the next generation. However, as the prevalence of maternal obesity has increased over time (Fisher et al., 2013), increasing numbers of individuals fall into the left side of Figure 1 (obese mothers) and carry high risk of propagating first hits in their offspring.
Preventing the second hit
As conceptualized here, fetal programming mitigation requires prevention or reduction of second hits that exacerbate first hit effects (lower left quadrant of Figure 1). We postulate that postnatal exposures exert different effects among those with a first hit, compared to those without a first hit.
That is, fetal programming leads to elevated disease risk in the offspring as a result of physiological alterations that occur during fetal development and persist thereafter (Gluckman et al., 2008). These same physiological effects may alter how postnatal exposures lead to the development of obesity and related disease. For example, in clinical research, children born to an obese mother are vulnerable for early onset reductions in insulin sensitivity (Catalano et al., 2009). Physical activity may be one of few mechanisms through which progression to diabetes can be delayed in these individuals. As a second example, in animal studies, offspring of obese mothers have low levels of skeletal muscle mass, which may have impaired glucose regulation (Bayol et al., 2014; Du et al., 2010); thus, adequate glucose control in children with a first hit may require a more severe reduction in dietary glycemic load. In general, programmed alterations induce early onset or accelerated disease processes, suggesting that critical periods of exposure may be earlier in those with a first hit.
However, characterization of postnatal second hit exposures that translate the effects of maternal obesity-induced fetal programming into disease is largely unexplored. Understanding of second hit exposures is needed to identify behavioral targets for fetal programming mitigation strategies. In Section II, we review animal and human evidence about the second hit effects of two traditional obesity-related exposures – Western-type diet and physical inactivity – in offspring with a maternal obesity-related first hit. In Section III, we discuss implications for interventions, with special consideration of challenges of promoting dramatic dietary or physical activity changes in vulnerable populations.
II. Western diet and physical inactivity as second hits to maternal obesity: initial evidence and future research directions
A. Literature review methods
The objective of this review is to summarize and integrate animal and human evidence of the interactive effects of maternal obesity (first hit) and postnatal diet and physical inactivity (second hits) in the offspring. Because this line of inquiry is emerging and largely based on animal studies, we focused on translating interdisciplinary evidence into potential implications for prevention rather than conducting a systematic review. We report our literature search strategy in the Appendix.
B. Post-weaning diet effects
The vast majority of second hit evidence related to post-weaning diet is based on animal studies, which typically use maternal and post-weaning offspring diets designed to align with Western-type diets that predict obesity and disease in human populations (Ambrosini, 2014; Barbaresko et al., 2013; Oddy et al., 2013). These experimental diets are high in fat, specifically saturated fat (e.g., (Ainge et al., 2011; Fan et al., 2013; Page et al., 2009; Tamashiro et al., 2009; White et al., 2009)) and in some studies, also high in sugar (e.g., (Couvreur et al., 2011; Khanal et al., 2015)). Therefore, our discussion focuses on maternal or offspring “Western diet”, defined as a diet high in saturated fats and/or sugars.
Animal evidence
A substantial body of animal studies examined effects of maternal obesity followed by offspring post-weaning Western-type (compared to healthy) diet on offspring weight, adiposity, metabolic, or cardiovascular outcomes. Of 39 studies reviewed, 35 were rodent studies, with others using pig (Arentson-Lantz et al., 2014), sheep (Khanal et al., 2014; Wallace et al., 2012), or non-human primate (Fan et al., 2013; Thorn et al., 2014) animal models. In all but two studies (Li et al., 2013; Vido et al., 2014), maternal obesity was induced by Western-type maternal diets. Findings were similar according to maternal treatment, except Western-type maternal diet tended to induce weaker effects on offspring when control diets were matched for energy (White et al., 2009) or micronutrient (Zhang et al., 2013) content. In general, offspring experimental diets were applied at weaning through the end of the study period; based on the small number exceptions (Arentson-Lantz et al., 2014; Brenseke et al., 2015; Couvreur et al., 2011; Khanal et al., 2014; Llopis et al., 2014; Ong and Muhlhausler, 2014; Vido et al., 2014; Wallace et al., 2012), we observed no remarkable patterns with regard to the post-gestational timing of exposure to experimental offspring diets.
Compared to a healthy offspring diet, a post-weaning Western-type offspring diet exacerbated the effects of the mother’s obesity on weight gain or adiposity (Bayol et al., 2007; Chen et al., 2009; Ong and Muhlhausler, 2014; White et al., 2009), glucose/insulin regulation (Arentson-Lantz et al., 2014; Benkalfat et al., 2011; Chen et al., 2009; Flynn et al., 2013; Khanal et al., 2014; Li et al., 2013; Page et al., 2009; Rajia et al., 2010; Shalev et al., 2010; Srinivasan et al., 2006; Volpato et al., 2012), cardiovascular measures (Elahi et al., 2009; Fan et al., 2013; Turdi et al., 2013), or fatty liver (Bouanane et al., 2010; Bruce et al., 2009; Hellgren et al., 2014; Li et al., 2013; Mouralidarane et al., 2013; Pruis et al., 2014; Zhang et al., 2013) in the offspring in most studies. For example, Chen and colleagues report that homeostatic model assessment of insulin resistance (HOMA-IR) was similar among rat offspring with high fat prenatal diet alone (0.46) compared to control prenatal and post-weaning diet (0.35); elevated among those with control prenatal and high fat post-weaning diet (0.62); and dramatically elevated among those with high fat control and post-weaning diet combined (1.15; p for interaction>0.05) (Chen et al., 2009). However, many studies did not report interaction significance. Evidence was more consistent for liver fat and insulin/glucose regulation than for body weight or adiposity.
In other cases, effects of post-weaning diet were more severe than the effects of maternal diet (Akyol et al., 2012; King et al., 2014), or the adverse effects of maternal obesity were observed regardless of post-weaning diet (Bayol et al., 2010; Thorn et al., 2014). In six studies, maternal high fat diet protected against the adverse effects of a postnatal Western-type diet with regard to weight gain (Couvreur et al., 2011; Ferezou-Viala et al., 2007; Howie et al., 2009; Mucellini et al., 2014), insulin sensitivity (Ferezou-Viala et al., 2007; Mucellini et al., 2014), lipid levels (Shankar et al., 2008), or blood pressure (Elahi et al., 2009). The potential explanations for these unexpected findings include impacts of maternal obesity on energy homeostasis regulated by the liver and the hypothalamus (Brenseke et al., 2015; Couvreur et al., 2011); nevertheless these studies may be regarded as outliers with no known explanation.
Human evidence
Few human studies have examined how prenatal developmental stressors interact with postnatal dietary exposures. In those that did, birth weight was used as a marker for prenatal development, as opposed to maternal diet or weight status. Furthermore, these studies focused on low birth weight rather than high birth weight (macrosomia). Birth weight is a weak proxy for maternal obesity, yet we present low birth weight studies because they offer initial evidence of prenatal-postnatal interactions in humans. In addition, maternal obesity leads to an elevated risk of both very low birth weight (McDonald et al., 2010) and macrosomia (Yu et al., 2013); therefore, both are pertinent to maternal obesity first hits.
Studies in Danish men compared the outcomes of a high fat overfeeding challenge (60% calories from fat, 1/3 monounsaturated, 1/3 polyunsaturated, 1/3 saturated fatty acids with a 5 day cross-over design) according to birthweight category (Brons et al., 2012; Brons et al., 2010; Gillberg et al., 2014; Jacobsen et al., 2014; Ribel-Madsen et al., 2011; Vienberg et al., 2012). High fat overfeeding induced insulin resistance at the end of the overfeeding period in men born at low birth weight, but not those born with normal weight (Brons et al., 2012; Brons et al., 2010). The mechanisms underlying these birth weight-specific effects may involve differential methylation or expression of genes involved in glucose or lipid metabolism (Brons et al., 2010; Gillberg et al., 2014; Jacobsen et al., 2014) or oxidative stress resulting from greater fat oxidation in low birth weight men (Brons et al., 2012; Brons et al., 2010).
Similarly, in an observational study of older adults in the Helsinki Birth Cohort Study, greater salt intake was associated with higher systolic blood pressure only in those born low (≤3,050 gm) birth weight (versus >3,050 gm) (Perala et al., 2011); the observed effect modification by birth weight is consistent with alterations to kidney structure that places offspring at high risk for hypertension (Bagby, 2007). In a similar cross-sectional analysis of a Dutch cohort, birth weight did not interact with simple carbohydrate or lipid intake in relation to metabolic syndrome (te Velde et al., 2005).
Summary
A growing body of animal research provides evidence that post-weaning Western-type diet comprises a second hit that exacerbates the adverse effects of maternal obesity. Human studies provide initial evidence that interactions between prenatal and postnatal exposures occur in humans, but are limited to examination of interactions with low birth weight, largely in men.
C. Physical inactivity effects
A small body of research on physical inactivity as a second hit includes experimental animal and human research of the interactive effects of adverse prenatal environment and sedentary or physical activity.
Animal evidence
Five rat studies contrasted the effects of maternal diet-induced obesity as a first hit followed by a physical inactivity as the second hit (compared to normal physical activity) (Bahari et al., 2013; Caruso et al., 2013; Rajia et al., 2013; Sun et al., 2013). Physical inactivity was experimentally assigned by withholding a running wheel in the offsprings’ cage, typically starting at the weaning period. Compared to rat offspring who were provided a running wheel, lack of exercise resulted in greater adiposity, metabolic dysregulation (Bahari et al., 2013; Caruso et al., 2013; Rajia et al., 2013), or lower leptin sensitivity (Sun et al., 2013) in offspring of high fat-fed mothers. Beneficial effects of exercise were attributed to increased energy expenditure and improved appetite regulation, which is impaired by maternal obesity (Breton, 2013; Ferezou-Viala et al., 2007; Kirk et al., 2009). Two studies imposed a sedentary period from weaning until later in life: exercise introduced after a 7-week sedentary period (Bahari et al., 2013) reduced maternal obesity effects, but exercise introduced 45-weeks post-weaning did not reduce adiposity (Santos et al., 2015). These studies suggest that physical activity, if introduced early enough, can normalize some of the physiologic deficiencies in offspring that result from maternal obesity in rats.
However, second hit effects of physical activity were not as strong as effects of post-weaning Western-type diet. In studies explicitly examining combinations of post-weaning Western-type (versus control) and sedentary (versus control) (Bahari et al., 2013; Caruso et al., 2013; Rajia et al., 2013), only rats with both exercise and control diets achieved outcomes comparable with the offspring of lean mothers.
Human evidence
Similar to dietary second hits, human studies assessing the relationship between prenatal development and physical activity rely on birth weight as an indicator of fetal development. In a related series of studies in Danish men (see post-weaning diet effects), a 9-day period of bed rest (physical inactivity) induced insulin resistance in both low and normal birth weight men (Alibegovic et al., 2010; Friedrichsen et al., 2012; Mortensen et al., 2013; Sonne et al., 2011), yet specific physiologic responses differed by birth weight. For example, Mortensen and colleagues found greater detrimental reductions in muscle insulin signaling following bed rest in men born at low versus normal birth weight (Mortensen et al., 2014). In the same study population, low birth weight men exhibited stronger skeletal muscle adaptations to exercise (Mortensen et al., 2013). Notably, in a 6-week bicycle intervention in rural Indian men, Madsen and colleagues found greater improvements in fitness, fat free mass, and fat mass:fat free mass ratio in low birth weight versus normal birth weight men (Madsen et al., 2015); improvements in fasting insulin and insulin sensitivity were similar in low and normal birth weight men. We found no experimental studies that examined altered effects of exercise or inactivity in females, or those born with high birth weight or to obese mothers.
Observational evidence is largely based on cross-sectional analysis of European cohorts, three studies in adolescents (from two cohorts) (Labayen et al., 2013; Ortega et al., 2011; Ridgway et al., 2011a) and two in adults (Eriksson et al., 2004; Laaksonen et al., 2003; te Velde et al., 2005). These studies show stronger associations between physical activity and lower insulin resistance (Ortega et al., 2011), glucose intolerance (Eriksson et al., 2004), leptin (Labayen et al., 2013), and metabolic syndrome (Laaksonen et al., 2003) in participants born with lower birth weight, except in one study examining interactive effects on adiposity (Ridgway et al., 2011a). In a cross-sectional sample of Japanese adults, higher birth weight and higher fitness was associated with lower insulin resistance, but birth weight and fitness did not interact (Aoyama et al., 2013). In a cross-sectional study of U.S. adolescents, we found that high birth weight was associated with a greater body mass index, but only among girls did this difference diminish with greater levels of physical activity; physical activity did not interact with low birth weight (Boone-Heinonen et al., 2015). We did not find any relevant longitudinal observational studies. No human studies examining the outcome of combined postnatal physical inactivity and Western diet following a first hit have been reported.
Summary
A small body of animal and human evidence suggests that low physical activity induces second hit effects following adverse prenatal environment. Human evidence is limited and does not address low physical activity following maternal obesity first hits.
D. Future directions for prevention research
The reviewed studies represent an emerging body of evidence suggesting that post-weaning Western-style diet and physical inactivity are important second hits that exacerbate adverse effects of being born to an obese mother. Here, we outline future directions for second hit research in human populations.
1. Improved measures of gestational conditions (first hits)
In the small body of human research investigating interactions between prenatal and postnatal factors, most studies have focused on low birth weight as a proxy for adverse fetal programming. However, high birth weight (macrosomia) is more strongly associated with maternal obesity (Ehrenberg et al., 2004; Gaudet et al., 2014). Furthermore, proxy measures of fetal programming risk such as birth weight can be misleading because maternal obesity induces adverse fetal changes, yet is associated with the full spectrum of birth size (Gaudet et al., 2014; McDonald et al., 2010). Therefore, there is a need for human second hit research that uses more direct measures of first hits, such as maternal adiposity, diet, or physical activity; placental measures (Barker and Thornburg, 2013); or epigenetic biomarkers (Hogg et al., 2012).
Similarly, much of what we know about the physiologic differences between offspring of obese versus lean mothers is based on a relatively homogeneous model of high fat diet-induced maternal obesity (Ainge et al., 2011) typically used in animal research. The degree to which multifaceted, heterogeneous causes of maternal obesity found in humans (Yang et al., 2013) exert the same fetal programming effects, and by extension, the same interactions with postnatal exposures, is largely unexplored. This knowledge gap further emphasizes the need for more prenatal-postnatal interaction studies that examine specific maternal exposures that may induce first hits in the offspring.
2. Characterization of the type and dose response of dietary and physical activity exposures (second hits) among individuals with first hits
Among existing studies examining interactions between first hits and postnatal dietary and physical inactivity, second hit exposures were relatively homogeneous. Experimental postnatal diets were typically high in total or saturated fat. Two human studies examined sodium (Perala et al., 2011), simple sugar, or fat (te Velde et al., 2005) intake. Future research identifying specific dietary exposures that exert the most potent second hit effects in humans is needed. For example, sugar sweetened beverages may exacerbate physiologic vulnerabilities in individuals with maternal obesity-induced fetal programming.
In animals, their natural propensity to exercise was suppressed by withholding a running wheel. In most human studies, participants underwent experimentally assigned bed rest. Two exercise interventions were tested in low and normal birth weight men (Madsen et al., 2015; Mortensen et al., 2013) but second hit effects of physical activity of different types, intensity, frequency, or duration have only begun to be explored in observational research (e.g., (Ortega et al., 2011)).
3. Understanding the role of second hit exposures outside the traditional domain of obesity prevention research
Other potential second hit exposures, such as psychosocial stress, smoking, alcohol consumption, and environmental pollutants (Figure 1) are emerging (Auten et al., 2012; Montgomery and Ekbom, 2002; Pascuan et al., 2014; Perera et al., 2009; Schopper et al., 2012; Van den Hove et al., 2014; Xu et al., 2014) and co-occur with poor diet and physical inactivity. How this complex constellation of second hit exposures interacts with adverse prenatal development is unknown. Other second hit exposures could contribute cumulative, synergistic, or antagonistic effects with dietary or physical inactivity exposures. Understanding these other second hit exposures is critical for understanding how to mitigate fetal programming effects in human populations and requires research that crosses traditionally defined health domains.
4. Characterizing critical periods of development and the durations of dietary and physical inactivity that become second hits
Sensitive periods of developmental plasticity are well known: infancy, adiposity rebound at approximately age five, and adolescence are key periods in which hormonal and developmental changes have long-lasting health effects (Dietz, 1994). However, the degree to which sensitive periods for specific dietary or physical activity exposures vary in those with or without a maternal obesity first hit is unknown.
Most of the reviewed animal studies examined second hit exposures applied at weaning and continued through the end of the study period. In human studies, second hit exposures were applied for a short duration or examined at a single time point. The effects of second hit exposures applied at different life stages and for varying durations have not been studied in humans. For example, whether it is possible to slow or reverse programmed disease processes already underway by transitioning offspring from a high fat diet to a healthy diet is unknown.
Early intervention is likely needed due to the difficulty of losing weight after obesity onset (Dietz et al., 2015); it is likely to be even more difficult in fetal-programmed individuals, but this has not been studied. Furthermore, the age at which excess adiposity can most readily be reversed, optimal interventions at various ages, and the extent to which these vary between those with or without a first hit is unknown.
5. Studies of the environmental influences of diet and physical activity behavior in children with a first hit
Experimental evidence of developmental remediation by post-weaning diet is, with two exceptions (Brenseke et al., 2015; Ong and Muhlhausler, 2014), based on provision of ad libitum access to post-weaning intervention versus control diet: animals controlled the amount, but not the type of food consumed. A healthy diet appears to be critical for mitigation of maternal obesity effects, but may be difficult to achieve within the Western food environment. That is, maternal obesity affects offspring appetite and food preference (Breton, 2013; Ferezou-Viala et al., 2007; Kirk et al., 2009; Ventura and Worobey, 2013) such that offspring are likely to be more responsive to environmental exposures such as visual cues from food marketing, and frequent reinforcement by widespread availability of foods that are low cost and manufactured to be highly palatable. However, the impacts of fetal programming on behavioral and physiological response to food choice and food cues are largely unexplored.
With regard to physical activity, the impacts of maternal obesity on offspring physical activity are mixed. In animals, maternal obesity may lead to increased (Bahari et al., 2013) or decreased (Samuelsson et al., 2008) physical activity in the offspring. In humans, the relationship between birth weight and subsequent physical activity is unclear (Ridgway et al., 2011b), but these associations may be confounded by availability of environmental and social supports, and the association between maternal obesity and offspring physical activity is unknown.
III. Considerations for public health and preventive medicine interventions
In this section, we consider potential implications for interventions aiming to prevent diet and physical inactivity second hits as a way to mitigate risk among children vulnerable as a result of a first hit.
Targeted interventions: children with a first hit as a vulnerable population
Public health and preventive medicine involves population-wide interventions in conjunction with greater support for racial or ethnic minorities, low income families, and other vulnerable groups in order to overcome high disease risk and socioeconomic barriers (Centers for Disease Control and Prevention).
We propose that individuals who are likely to have maternal obesity-induced fetal programming represent a vulnerable subgroup. They are at high risk for not only developing obesity and disease in their own lifetimes, but also propagating that risk into the next generation. Moreover, vulnerability due to fetal programming likely overlaps with vulnerability related to socioeconomic disadvantage (Messer et al., 2015). That is, low income and racial/ethnic minority groups experience greater unhealthy dietary and physical inactivity exposures (Gordon-Larsen et al., 1999; Kirkpatrick et al., 2012; Marshall et al., 2007) and greater risk of low birth weight and other neonatal health indicators (Blumenshine et al., 2010; Culhane and Goldenberg, 2011) that predict future disease risk and are, potentially, indicative of fetal programming (Kuzawa and Sweet, 2009). In addition, first hit individuals may need special attention also because they have different psychological drivers for appetite and may be more likely to eat foods that are advertised as highly palatable but have low nutritional value.
Females also comprise a vulnerable, high priority population for two primary reasons. First, adolescent females experience greater weight gain as they transition into adulthood (Gordon-Larsen et al., 2004), lower physical activity levels (Troiano et al., 2008), and more dramatic racial and ethnic disparities in obesity prevalence (Flegal et al., 2010). Second, maternal health and nutrition status determines the intrauterine environment, a substantial contributor to fetal programming effects (Aiken and Ozanne, 2014). On the other hand, both males and females contribute to and benefit from broad societal change in food policy and supports for active lifestyles that are needed to improve maternal obesity-related risk factors (Swinburn et al., 2011). Additionally, spouses and partners are emerging as a critical factor in promoting healthy diet (Lawrence and Barker, 2009) and other behaviors (Jackson et al., 2015). Therefore targeted social, economic, and environmental support of healthy lifestyle in girls and women, alongside ongoing support for boys and men is needed to improve intergenerational health.
Tailored interventions
Interventions are frequently tailored to social or cultural needs of vulnerable populations, or to the specific needs of those who have already developed clinical disease, such as severe obesity or Type 2 Diabetes (TODAY Study Group, 2010). Interventions may also need to be tailored to recognize specific physiologic vulnerabilities in children with adverse fetal programming.
Tailoring may include different prioritization of behavioral targets (e.g., physical activity as a critical component), different recommendations for optimal levels of behaviors (e.g., more drastic reductions in intake of refined sugars), or differences in optimal timing of interventions (e.g., early and sustained intervention). Notably, those with a first hit may require greater intervention intensity to address three factors. First, more frequent or personalized contact is likely needed to support more dramatic improvements in diet or physical activity required by individuals with a first hit. Second, due to accelerated disease progression, individuals with a first hit may begin interventions with poorer baseline health that has been present for a longer duration than children without a first hit. Third, because individuals with a first hit may be more likely to be low income or minority race/ethnicity, interventions may need to address social and behavioral barriers. On the other hand, the potential benefits of diet and physical activity interventions may be amplified in subpopulations with adverse fetal programming characteristics and are critical for reducing health disparities.
Greater coordination between public health and preventive medicine
Analogous to other applications of personalized prevention (Konstantinidou et al., 2014), individualized recommendations may be required to address specific vulnerabilities resulting from fetal programming. Fetal programming mitigation likely requires personalized prevention approaches placed alongside population-wide public health policy and targeted interventions to support healthy lifestyles and interventions for socially or economically disadvantaged communities.
Conclusion: the promise of mitigation
In this paper, we present emerging evidence that the adverse effects of maternal obesity on offspring health can potentially be ameliorated by healthy diet and physical activity. Rather than conceptualizing obesity and disease prevention as if everyone starts with a blank slate, evidence suggests that many individuals begin life on a healthy or unhealthy trajectory. We offer a framework for reconceptualizing prevention as not just preventing new adverse exposures, but also remediating the multigenerational effects of historical adverse exposures. The question is: to what extent do prevention strategies need to target specific groups, as opposed to the traditional one size fits all approach? Clarification of the types and timing of second hit exposures are needed to develop, test, and implement fetal programming mitigation interventions for current and future generations of children.
Supplementary Material
HIGHLIGHTS.
We propose a conceptual framework for mitigating the effects of fetal programming.
We reviewed how maternal obesity interacts with offspring diet or physical activity.
Obesogenic offspring behavior intensified maternal obesity effects in animals.
Relevant human research is emerging but scant.
We present future directions for human research on fetal programming mitigation.
ACKNOWLEDGEMENTS
The project described was funded by the Office of Research in Women’s Health and the National Institute of Child Health and Human Development, Oregon BIRCWH Award Number K12HD043488-01 (JBH). KLT is supported by 2PO1HD34430 and the OHSU Edwards Endowment. We thank Jenny Marx for her information design expertise and creation of Figure 1, and Laura Zeigen for her library science expertise.
Footnotes
CONFLICT OF INTEREST
The authors declare there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aiken CE, Ozanne SE. Transgenerational developmental programming. Hum Reprod Update. 2014;20:63–75. doi: 10.1093/humupd/dmt043. [DOI] [PubMed] [Google Scholar]
- Ainge H, Thompson C, Ozanne SE, Rooney KB. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes (Lond) 2011;35:325–35. doi: 10.1038/ijo.2010.149. [DOI] [PubMed] [Google Scholar]
- Akyol A, McMullen S, Langley-Evans SC. Glucose intolerance associated with early-life exposure to maternal cafeteria feeding is dependent upon post-weaning diet. Br J Nutr. 2012;107:964–78. doi: 10.1017/S0007114511003916. [DOI] [PubMed] [Google Scholar]
- Alibegovic AC, Hojbjerre L, Sonne MP, van Hall G, Alsted TJ, Kiens B, Stallknecht B, Dela F, Vaag A. Increased rate of whole body lipolysis before and after 9 days of bed rest in healthy young men born with low birth weight. Am J Physiol Endocrinol Metab. 2010;298:E555–64. doi: 10.1152/ajpendo.00223.2009. [DOI] [PubMed] [Google Scholar]
- Ambrosini GL. Childhood dietary patterns and later obesity: a review of the evidence. Proc Nutr Soc. 2014;73:137–46. doi: 10.1017/S0029665113003765. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Tsushita K, Miyatake N, Numata T, Miyachi M, Tabata I, Cao ZB, Sakamoto S, Higuchi M. Does cardiorespiratory fitness modify the association between birth weight and insulin resistance in adult life? PLoS One. 2013;8:e73967. doi: 10.1371/journal.pone.0073967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentson-Lantz EJ, Buhman KK, Ajuwon K, Donkin SS. Excess pregnancy weight gain leads to early indications of metabolic syndrome in a swine model of fetal programming. Nutr Res. 2014;34:241–9. doi: 10.1016/j.nutres.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Auten RL, Gilmour MI, Krantz QT, Potts EN, Mason SN, Foster WM. Maternal diesel inhalation increases airway hyperreactivity in ozone-exposed offspring. Am J Respir Cell Mol Biol. 2012;46:454–60. doi: 10.1165/rcmb.2011-0256OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bagby SP. Maternal nutrition, low nephron number, and hypertension in later life: pathways of nutritional programming. J Nutr. 2007;137:1066–72. doi: 10.1093/jn/137.4.1066. [DOI] [PubMed] [Google Scholar]
- Bahari H, Caruso V, Morris MJ. Late-onset exercise in female rat offspring ameliorates the detrimental metabolic impact of maternal obesity. Endocrinology. 2013;154:3610–21. doi: 10.1210/en.2013-1059. [DOI] [PubMed] [Google Scholar]
- Barbaresko J, Koch M, Schulze MB, Nothlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71:511–27. doi: 10.1111/nure.12035. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Thornburg KL. Placental programming of chronic diseases, cancer and lifespan: a review. Placenta. 2013;34:841–5. doi: 10.1016/j.placenta.2013.07.063. [DOI] [PubMed] [Google Scholar]
- Bayol SA, Bruce CR, Wadley GD. Growing healthy muscles to optimise metabolic health into adult life. J Dev Orig Health Dis. 2014;1:15. doi: 10.1017/S2040174414000452. [DOI] [PubMed] [Google Scholar]
- Bayol SA, Farrington SJ, Stickland NC. A maternal 'junk food' diet in pregnancy and lactation promotes an exacerbated taste for 'junk food' and a greater propensity for obesity in rat offspring. Br J Nutr. 2007;98:843–51. doi: 10.1017/S0007114507812037. [DOI] [PubMed] [Google Scholar]
- Bayol SA, Simbi BH, Fowkes RC, Stickland NC. A maternal “junk food” diet in pregnancy and lactation promotes nonalcoholic Fatty liver disease in rat offspring. Endocrinology. 2010;151:1451–61. doi: 10.1210/en.2009-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkalfat NB, Merzouk H, Bouanane S, Merzouk SA, Bellenger J, Gresti J, Tessier C, Narce M. Altered adipose tissue metabolism in offspring of dietary obese rat dams. Clin Sci (Lond) 2011;121:19–28. doi: 10.1042/CS20100534. [DOI] [PubMed] [Google Scholar]
- Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am J Prev Med. 2010;39:263–72. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–6. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- Boone-Heinonen J, Markwardt S, Fortmann SP, Thornburg KL. Overcoming birth weight: can physical activity mitigate birth weight-related differences in adiposity? Pediatr Obes. 2015 doi: 10.1111/ijpo.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouanane S, Merzouk H, Benkalfat NB, Soulimane N, Merzouk SA, Gresti J, Tessier C, Narce M. Hepatic and very low-density lipoprotein fatty acids in obese offspring of overfed dams. Metabolism. 2010;59:1701–9. doi: 10.1016/j.metabol.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Brenseke B, Bahamonde J, Talanian M, Kornfeind E, Daly J, Cobb G, Zhang J, Prater MR, Davis GC, et al. Mitigating or exacerbating effects of maternal-fetal programming of female mice through the food choice environment. Endocrinology. 2015;156:182–92. doi: 10.1210/en.2014-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton C. The hypothalamus-adipose axis is a key target of developmental programming by maternal nutritional manipulation. J Endocrinol. 2013;216:R19–31. doi: 10.1530/JOE-12-0157. [DOI] [PubMed] [Google Scholar]
- Brons C, Jacobsen S, Hiscock N, White A, Nilsson E, Dunger D, Astrup A, Quistorff B, Vaag A. Effects of high-fat overfeeding on mitochondrial function, glucose and fat metabolism, and adipokine levels in low-birth-weight subjects. Am J Physiol Endocrinol Metab. 2012;302:E43–51. doi: 10.1152/ajpendo.00095.2011. [DOI] [PubMed] [Google Scholar]
- Brons C, Jacobsen S, Nilsson E, Ronn T, Jensen CB, Storgaard H, Poulsen P, Groop L, Ling C, et al. Deoxyribonucleic acid methylation and gene expression of PPARGC1A in human muscle is influenced by high-fat overfeeding in a birth-weight-dependent manner. J Clin Endocrinol Metab. 2010;95:3048–56. doi: 10.1210/jc.2009-2413. [DOI] [PubMed] [Google Scholar]
- Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, Bateman AC, Clough GF, Poston L, et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50:1796–808. doi: 10.1002/hep.23205. [DOI] [PubMed] [Google Scholar]
- Caruso V, Bahari H, Morris MJ. The beneficial effects of early short-term exercise in the offspring of obese mothers are accompanied by alterations in the hypothalamic gene expression of appetite regulators and FTO (fat mass and obesity associated) gene. J Neuroendocrinol. 2013;25:742–52. doi: 10.1111/jne.12053. [DOI] [PubMed] [Google Scholar]
- Catalano P, deMouzon SH. Maternal obesity and metabolic risk to the offspring: why lifestyle interventions may have not achieved the desired outcomes. Int J Obes (Lond) 2015;39:642–9. doi: 10.1038/ijo.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32:1076–80. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Minority Health. Available at: http://www.cdc.gov/minorityhealth/populations.html.
- Chen H, Simar D, Morris MJ. Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: interaction with postnatal nutritional environment. PLoS One. 2009;4:e6259. doi: 10.1371/journal.pone.0006259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Simar D, Pegg K, Saad S, Palmer C, Morris MJ. Exendin-4 is effective against metabolic disorders induced by intrauterine and postnatal overnutrition in rodents. Diabetologia. 2014;57:614–22. doi: 10.1007/s00125-013-3132-5. [DOI] [PubMed] [Google Scholar]
- Chen H, Simar D, Ting JH, Erkelens JR, Morris MJ. Leucine improves glucose and lipid status in offspring from obese dams, dependent on diet type, but not caloric intake. J Neuroendocrinol. 2012;24:1356–64. doi: 10.1111/j.1365-2826.2012.02339.x. [DOI] [PubMed] [Google Scholar]
- Couvreur O, Ferezou J, Gripois D, Serougne C, Crepin D, Aubourg A, Gertler A, Vacher CM, Taouis M. Unexpected long-term protection of adult offspring born to high-fat fed dams against obesity induced by a sucrose-rich diet. PLoS One. 2011;6:e18043. doi: 10.1371/journal.pone.0018043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culhane JF, Goldenberg RL. Racial disparities in preterm birth. Semin Perinatol. 2011;35:234–9. doi: 10.1053/j.semperi.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Crume T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes. 2011;60:1849–55. doi: 10.2337/db11-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabelea D, Mayer-Davis EJ, Lamichhane AP, D'Agostino RB, Jr., Liese AD, Vehik KS, Narayan KM, Zeitler P, Hamman RF. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care. 2008;31:1422–6. doi: 10.2337/dc07-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59:955–9. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- Dietz WH, Baur LA, Hall K, Puhl RM, Taveras EM, Uauy R, Kopelman P. Management of obesity: improvement of health-care training and systems for prevention and care. Lancet. 2015 doi: 10.1016/S0140-6736(14)61748-7. [DOI] [PubMed] [Google Scholar]
- Du M, Yan X, Tong JF, Zhao J, Zhu MJ. Maternal obesity, inflammation, and fetal skeletal muscle development. Biol Reprod. 2010;82:4–12. doi: 10.1095/biolreprod.109.077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191:964–8. doi: 10.1016/j.ajog.2004.05.052. [DOI] [PubMed] [Google Scholar]
- Elahi MM, Cagampang FR, Mukhtar D, Anthony FW, Ohri SK, Hanson MA. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br J Nutr. 2009;102:514–9. doi: 10.1017/S000711450820749X. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Yliharsila H, Forsen T, Osmond C, Barker DJ. Exercise protects against glucose intolerance in individuals with a small body size at birth. Prev Med. 2004;39:164–7. doi: 10.1016/j.ypmed.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Fan L, Lindsley SR, Comstock SM, Takahashi DL, Evans AE, He GW, Thornburg KL, Grove KL. Maternal high-fat diet impacts endothelial function in nonhuman primate offspring. Int J Obes (Lond) 2013;37:254–62. doi: 10.1038/ijo.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou-Viala J, Roy AF, Serougne C, Gripois D, Parquet M, Bailleux V, Gertler A, Delplanque B, Djiane J, et al. Long-term consequences of maternal high-fat feeding on hypothalamic leptin sensitivity and diet-induced obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1056–62. doi: 10.1152/ajpregu.00117.2007. [DOI] [PubMed] [Google Scholar]
- Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003-2009. Prev Med. 2013;56:372–8. doi: 10.1016/j.ypmed.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. Jama. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Flynn ER, Alexander BT, Lee J, Hutchens ZM, Jr., Maric-Bilkan C. High-fat/fructose feeding during prenatal and postnatal development in female rats increases susceptibility to renal and metabolic injury later in life. Am J Physiol Regul Integr Comp Physiol. 2013;304:R278–85. doi: 10.1152/ajpregu.00433.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias AE, Grove KL. Obesity: a transgenerational problem linked to nutrition during pregnancy. Semin Reprod Med. 2012;30:472–8. doi: 10.1055/s-0032-1328875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichsen M, Ribel-Madsen R, Mortensen B, Hansen CN, Alibegovic AC, Hojbjerre L, Sonne MP, Wojtaszewski JF, Stallknecht B, et al. Muscle inflammatory signaling in response to 9 days of physical inactivity in young men with low compared with normal birth weight. Eur J Endocrinol. 2012;167:829–38. doi: 10.1530/EJE-12-0498. [DOI] [PubMed] [Google Scholar]
- Gaudet L, Ferraro ZM, Wen SW, Walker M. Maternal obesity and occurrence of fetal macrosomia: a systematic review and meta-analysis. Biomed Res Int. 2014;2014:640291. doi: 10.1155/2014/640291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg L, Jacobsen SC, Ronn T, Brons C, Vaag A. PPARGC1A DNA methylation in subcutaneous adipose tissue in low birth weight subjects--impact of 5 days of high-fat overfeeding. Metabolism. 2014;63:263–71. doi: 10.1016/j.metabol.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Larsen P, Adair LS, Nelson MC, Popkin BM. Five-Year obesity incidence in the transition period between adolescence and adulthood: the National Longitudinal Study of Adolescent Health. Am J Clin Nutr. 2004;80:569–75. doi: 10.1093/ajcn/80.3.569. [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen P, McMurray RG, Popkin BM. Adolescent physical activity and inactivity vary by ethnicity: The National Longitudinal Study of Adolescent Health. J Pediatr. 1999;135:301–06. doi: 10.1016/s0022-3476(99)70124-1. [DOI] [PubMed] [Google Scholar]
- Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr. 2010;91:373–80. doi: 10.3945/ajcn.2009.28166. [DOI] [PubMed] [Google Scholar]
- Hellgren LI, Jensen RI, Waterstradt MS, Quistorff B, Lauritzen L. Acute and perinatal programming effects of a fat-rich diet on rat muscle mitochondrial function and hepatic lipid accumulation. Acta Obstet Gynecol Scand. 2014;93:1170–80. doi: 10.1111/aogs.12458. [DOI] [PubMed] [Google Scholar]
- Hogg K, Price EM, Hanna CW, Robinson WP. Prenatal and perinatal environmental influences on the human fetal and placental epigenome. Clin Pharmacol Ther. 2012;92:716–26. doi: 10.1038/clpt.2012.141. [DOI] [PubMed] [Google Scholar]
- Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol. 2009;587:905–15. doi: 10.1113/jphysiol.2008.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) Examining a developmental approach to childhood obesityL the fetal and early childhood years: workshop summary. The National Academies Press; Washington, DC: 2015. [PubMed] [Google Scholar]
- Jackson SE, Steptoe A, Wardle J. The influence of partner's behavior on health behavior change: the English Longitudinal Study of Ageing. JAMA Intern Med. 2015;175:385–92. doi: 10.1001/jamainternmed.2014.7554. [DOI] [PubMed] [Google Scholar]
- Jacobsen SC, Gillberg L, Bork-Jensen J, Ribel-Madsen R, Lara E, Calvanese V, Ling C, Fernandez AF, Fraga MF, et al. Young men with low birthweight exhibit decreased plasticity of genome-wide muscle DNA methylation by high-fat overfeeding. Diabetologia. 2014;57:1154–8. doi: 10.1007/s00125-014-3198-8. [DOI] [PubMed] [Google Scholar]
- Khanal P, Axel AM, Kongsted AH, Husted SV, Johnsen L, Pandey D, Pedersen KL, Birtwistle M, Markussen B, et al. Late gestation under- and overnutrition have differential impacts when combined with a post-natal obesogenic diet on glucose-lactate-insulin adaptations during metabolic challenges in adolescent sheep. Acta Physiol (Oxf) 2015;213:519–36. doi: 10.1111/apha.12391. [DOI] [PubMed] [Google Scholar]
- Khanal P, Husted SV, Axel AM, Johnsen L, Pedersen KL, Mortensen MS, Kongsted AH, Nielsen MO. Late gestation over- and undernutrition predispose for visceral adiposity in response to a post-natal obesogenic diet, but with differential impacts on glucose-insulin adaptations during fasting in lambs. Acta Physiol (Oxf) 2014;210:110–26. doi: 10.1111/apha.12129. [DOI] [PubMed] [Google Scholar]
- King V, Norman JE, Seckl JR, Drake AJ. Post-weaning diet determines metabolic risk in mice exposed to overnutrition in early life. Reprod Biol Endocrinol. 2014;12:73. doi: 10.1186/1477-7827-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen TI, Pasanen M, Aittasalo M, Fogelholm M, Hilakivi-Clarke L, Weiderpass E, Luoto R. Preventing excessive weight gain during pregnancy - a controlled trial in primary health care. Eur J Clin Nutr. 2007;61:884–91. doi: 10.1038/sj.ejcn.1602602. [DOI] [PubMed] [Google Scholar]
- Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, Poston L, Taylor PD, Coen CW. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS One. 2009;4:e5870. doi: 10.1371/journal.pone.0005870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick SI, Dodd KW, Reedy J, Krebs-Smith SM. Income and race/ethnicity are associated with adherence to food-based dietary guidance among US adults and children. J Acad Nutr Diet. 2012;112:624–35. e6. doi: 10.1016/j.jand.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidou V, Ruiz LA, Ordovas JM. Personalized nutrition and cardiovascular disease prevention: From Framingham to PREDIMED. Adv Nutr. 2014;5:368S–71S. doi: 10.3945/an.113.005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW, Sweet E. Epigenetics and the embodiment of race: developmental origins of US racial disparities in cardiovascular health. Am J Hum Biol. 2009;21:2–15. doi: 10.1002/ajhb.20822. [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Lakka HM, Lynch J, Lakka TA, Niskanen L, Rauramaa R, Salonen JT, Kauhanen J. Cardiorespiratory fitness and vigorous leisure-time physical activity modify the association of small size at birth with the metabolic syndrome. Diabetes Care. 2003;26:2156–64. doi: 10.2337/diacare.26.7.2156. [DOI] [PubMed] [Google Scholar]
- Labayen I, Ortega FB, Moreno LA, Gonzalez-Gross M, Jimenez-Pavon D, Martinez-Gomez D, Breidenassel C, Marcos A, Molnar D, et al. Physical activity attenuates the negative effect of low birth weight on leptin levels in European adolescents; the HELENA study. Nutr Metab Cardiovasc Dis. 2013;23:344–9. doi: 10.1016/j.numecd.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Lau EY, Liu J, Archer E, McDonald SM. Maternal weight gain in pregnancy and risk of obesity among offspring: a systematic review. J Obes. 2014;2014:524939. doi: 10.1155/2014/524939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Relton C, Sattar N, Nelson SM. Maternal adiposity--a determinant of perinatal and offspring outcomes? Nat Rev Endocrinol. 2012;8:679–88. doi: 10.1038/nrendo.2012.176. [DOI] [PubMed] [Google Scholar]
- Lawrence W, Barker M. A review of factors affecting the food choices of disadvantaged women. Proc Nutr Soc. 2009;68:189–94. doi: 10.1017/S0029665109001013. [DOI] [PubMed] [Google Scholar]
- Li CC, Young PE, Maloney CA, Eaton SA, Cowley MJ, Buckland ME, Preiss T, Henstridge DC, Cooney GJ, et al. Maternal obesity and diabetes induces latent metabolic defects and widespread epigenetic changes in isogenic mice. Epigenetics. 2013;8:602–11. doi: 10.4161/epi.24656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llopis M, Sanchez J, Priego T, Palou A, Pico C. Maternal fat supplementation during late pregnancy and lactation influences the development of hepatic steatosis in offspring depending on the fat source. J Agric Food Chem. 2014;62:1590–601. doi: 10.1021/jf405161e. [DOI] [PubMed] [Google Scholar]
- Macaulay EC, Donovan EL, Leask MP, Bloomfield FH, Vickers MH, Dearden PK, Baker PN. The importance of early life in childhood obesity and related diseases: a report from the 2014 Gravida Strategic Summit. J Dev Orig Health Dis. 2014;5:398–407. doi: 10.1017/S2040174414000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen C, Mogensen P, Thomas N, Christensen DL, Bygbjerg IC, Mohan V, Inbakumari M, Nadig SV, Alex R, et al. Effects of an outdoor bicycle-based intervention in healthy rural Indian men with normal and low birth weight. J Dev Orig Health Dis. 2015;6:27–37. doi: 10.1017/S2040174414000609. [DOI] [PubMed] [Google Scholar]
- Marshall SJ, Jones DA, Ainsworth BE, Reis JP, Levy SS, Macera CA. Race/ethnicity, social class, and leisure-time physical inactivity. Med Sci Sports Exerc. 2007;39:44–51. doi: 10.1249/01.mss.0000239401.16381.37. [DOI] [PubMed] [Google Scholar]
- McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–35. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SD, Han Z, Mulla S, Beyene J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. Bmj. 2010;341:c3428. doi: 10.1136/bmj.c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SM, Liu J, Wilcox S, Lau EY, Archer E. Does dose matter in reducing gestational weight gain in exercise interventions? A systematic review of literature. J Sci Med Sport. 2015 doi: 10.1016/j.jsams.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer LC, Boone-Heinonen J, Mponwane L, Wallack L, Thornburg KL. Developmental programming: priming disease susceptibility for subsequent generations. Current Epidemiology Reports. 2015;2 doi: 10.1007/s40471-014-0033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SM, Ekbom A. Smoking during pregnancy and diabetes mellitus in a British longitudinal birth cohort. Bmj. 2002;324:26–7. doi: 10.1136/bmj.324.7328.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen B, Friedrichsen M, Andersen NR, Alibegovic AC, Hojbjerre L, Sonne MP, Stallknecht B, Dela F, Wojtaszewski JF, et al. Physical inactivity affects skeletal muscle insulin signaling in a birth weight-dependent manner. J Diabetes Complications. 2014;28:71–8. doi: 10.1016/j.jdiacomp.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Mortensen B, Hingst JR, Frederiksen N, Hansen RW, Christiansen CS, Iversen N, Friedrichsen M, Birk JB, Pilegaard H, et al. Effect of birth weight and 12 weeks of exercise training on exercise-induced AMPK signaling in human skeletal muscle. Am J Physiol Endocrinol Metab. 2013;304:E1379–90. doi: 10.1152/ajpendo.00295.2012. [DOI] [PubMed] [Google Scholar]
- Mouralidarane A, Soeda J, Visconti-Pugmire C, Samuelsson AM, Pombo J, Maragkoudaki X, Butt A, Saraswati R, Novelli M, et al. Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology. 2013;58:128–38. doi: 10.1002/hep.26248. [DOI] [PubMed] [Google Scholar]
- Mucellini AB, Goularte JF, de Araujo da Cunha AC, Caceres RC, Noschang C, da Silva Benetti C, Silveira PP, Sanvitto GL. Effects of exposure to a cafeteria diet during gestation and after weaning on the metabolism and body weight of adult male offspring in rats. Br J Nutr. 2014;111:1499–506. doi: 10.1017/S0007114513003838. [DOI] [PubMed] [Google Scholar]
- Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015;6:CD007145. doi: 10.1002/14651858.CD007145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader PR, Huang T, Gahagan S, Kumanyika S, Hammond RA, Christoffel KK. Next steps in obesity prevention: altering early life systems to support healthy parents, infants, and toddlers. Childhood Obesity. 2012;8:195–204. doi: 10.1089/chi.2012.0004. [DOI] [PubMed] [Google Scholar]
- Oddy WH, Herbison CE, Jacoby P, Ambrosini GL, O'Sullivan TA, Ayonrinde OT, Olynyk JK, Black LJ, Beilin LJ, et al. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am J Gastroenterol. 2013;108:778–85. doi: 10.1038/ajg.2013.95. [DOI] [PubMed] [Google Scholar]
- Ong ZY, Muhlhausler BS. Consuming a low-fat diet from weaning to adulthood reverses the programming of food preferences in male, but not in female, offspring of 'junk food'-fed rat dams. Acta Physiol (Oxf) 2014;210:127–41. doi: 10.1111/apha.12132. [DOI] [PubMed] [Google Scholar]
- Ortega FB, Ruiz JR, Hurtig-Wennlof A, Meirhaeghe A, Gonzalez-Gross M, Moreno LA, Molnar D, Kafatos A, Gottrand F, et al. Physical activity attenuates the effect of low birth weight on insulin resistance in adolescents: findings from two observational studies. Diabetes. 2011;60:2295–9. doi: 10.2337/db10-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KC, Malik RE, Ripple JA, Anday EK. Maternal and postweaning diet interaction alters hypothalamic gene expression and modulates response to a high-fat diet in male offspring. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1049–57. doi: 10.1152/ajpregu.90585.2008. [DOI] [PubMed] [Google Scholar]
- Pascuan CG, Rubinstein MR, Palumbo ML, Genaro AM. Prenatal stress induces up-regulation of glucocorticoid receptors on lymphoid cells modifying the T-cell response after acute stress exposure in the adult life. Physiol Behav. 2014;128:141–7. doi: 10.1016/j.physbeh.2014.01.040. [DOI] [PubMed] [Google Scholar]
- Perala MM, Moltchanova E, Kaartinen NE, Mannisto S, Kajantie E, Osmond C, Barker DJ, Valsta LM, Eriksson JG. The association between salt intake and adult systolic blood pressure is modified by birth weight. Am J Clin Nutr. 2011;93:422–6. doi: 10.3945/ajcn.2010.30022. [DOI] [PubMed] [Google Scholar]
- Perera FP, Li Z, Whyatt R, Hoepner L, Wang S, Camann D, Rauh V. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009;124:e195–202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Escamilla R, Kac G. Childhood obesity prevention: a life-course framework. Int J Obes Suppl. 2013;3:S3–S5. doi: 10.1038/ijosup.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston L, Briley AL, Barr S, Bell R, Croker H, Coxon K, Essex HN, Hunt C, Hayes L, et al. Developing a complex intervention for diet and activity behaviour change in obese pregnant women (the UPBEAT trial); assessment of behavioural change and process evaluation in a pilot randomised controlled trial. BMC Pregnancy Childbirth. 2013;13:148. doi: 10.1186/1471-2393-13-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruis MG, Lendvai A, Bloks VW, Zwier MV, Baller JF, de Bruin A, Groen AK, Plosch T. Maternal western diet primes non-alcoholic fatty liver disease in adult mouse offspring. Acta Physiol (Oxf) 2014;210:215–27. doi: 10.1111/apha.12197. [DOI] [PubMed] [Google Scholar]
- Rajia S, Chen H, Morris MJ. Maternal overnutrition impacts offspring adiposity and brain appetite markers-modulation by postweaning diet. J Neuroendocrinol. 2010;22:905–14. doi: 10.1111/j.1365-2826.2010.02005.x. [DOI] [PubMed] [Google Scholar]
- Rajia S, Chen H, Morris MJ. Voluntary post weaning exercise restores metabolic homeostasis in offspring of obese rats. Nutr Metab Cardiovasc Dis. 2013;23:574–81. doi: 10.1016/j.numecd.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Ribel-Madsen R, Brons C, Friedrichsen M, Poulsen P, Vaag A. Retinol-binding protein 4 in young men with low versus normal birth weight: physiological response to short-term overfeeding. Obesity (Silver Spring) 2011;19:1304–6. doi: 10.1038/oby.2010.311. [DOI] [PubMed] [Google Scholar]
- Ridgway CL, Brage S, Anderssen SA, Sardinha LB, Andersen LB, Ekelund U. Do physical activity and aerobic fitness moderate the association between birth weight and metabolic risk in youth?: the European Youth Heart Study. Diabetes Care. 2011a;34:187–92. doi: 10.2337/dc10-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway CL, Brage S, Sharp SJ, Corder K, Westgate KL, van Sluijs EM, Goodyer IM, Hallal PC, Anderssen SA, et al. Does birth weight influence physical activity in youth? A combined analysis of four studies using objectively measured physical activity. PLoS One. 2011b;6:e16125. doi: 10.1371/journal.pone.0016125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney K, Ozanne SE. Maternal over-nutrition and offspring obesity predisposition: targets for preventative interventions. Int J Obes (Lond) 2011;35:883–90. doi: 10.1038/ijo.2011.96. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–92. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- Santos M, Rodriguez-Gonzalez GL, Ibanez C, Vega CC, Nathanielsz PW, Zambrano E. Adult exercise effects on oxidative stress and reproductive programming in male offspring of obese rats. Am J Physiol Regul Integr Comp Physiol. 2015;308:R219–25. doi: 10.1152/ajpregu.00398.2014. [DOI] [PubMed] [Google Scholar]
- Schopper H, Palme R, Ruf T, Huber S. Effects of prenatal stress on hypothalamic-pituitary-adrenal (HPA) axis function over two generations of guinea pigs (Cavia aperea f. porcellus) Gen Comp Endocrinol. 2012;176:18–27. doi: 10.1016/j.ygcen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Shalev U, Tylor A, Schuster K, Frate C, Tobin S, Woodside B. Long-term physiological and behavioral effects of exposure to a highly palatable diet during the perinatal and post-weaning periods. Physiol Behav. 2010;101:494–502. doi: 10.1016/j.physbeh.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2008;294:R528–38. doi: 10.1152/ajpregu.00316.2007. [DOI] [PubMed] [Google Scholar]
- Skogen JC, Overland S. The fetal origins of adult disease: a narrative review of the epidemiological literature. JRSM Short Rep. 2012;3:59. doi: 10.1258/shorts.2012.012048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonne MP, Hojbjerre L, Alibegovic AC, Nielsen LB, Stallknecht B, Vaag AA, Dela F. Endothelial function after 10 days of bed rest in individuals at risk for type 2 diabetes and cardiovascular disease. Exp Physiol. 2011;96:1000–9. doi: 10.1113/expphysiol.2011.058511. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol Endocrinol Metab. 2006;291:E792–9. doi: 10.1152/ajpendo.00078.2006. [DOI] [PubMed] [Google Scholar]
- Sun B, Liang NC, Ewald ER, Purcell RH, Boersma GJ, Yan J, Moran TH, Tamashiro KL. Early postweaning exercise improves central leptin sensitivity in offspring of rat dams fed high-fat diet during pregnancy and lactation. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1076–84. doi: 10.1152/ajpregu.00566.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–14. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes. 2009;58:1116–25. doi: 10.2337/db08-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde SJ, Twisk JW, van Mechelen W, Kemper HC. A birth-weight questionnaire indicated that life style modifies the birth weight and metabolic syndrome relationship at age 36. J Clin Epidemiol. 2005;58:1172–9. doi: 10.1016/j.jclinepi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Thompson AL. Intergenerational impact of maternal obesity and postnatal feeding practices on pediatric obesity. Nutr Rev. 2013;71(Suppl 1):S55–61. doi: 10.1111/nure.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn SR, Baquero KC, Newsom SA, El Kasmi KC, Bergman BC, Shulman GI, Grove KL, Friedman JE. Early life exposure to maternal insulin resistance has persistent effects on hepatic NAFLD in juvenile nonhuman primates. Diabetes. 2014;63:2702–13. doi: 10.2337/db14-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODAY Study Group Design of a family-based lifestyle intervention for youth with type 2 diabetes: the TODAY study. Int J Obes (Lond) 2010;34:217–26. doi: 10.1038/ijo.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Turdi S, Ge W, Hu N, Bradley KM, Wang X, Ren J. Interaction between maternal and postnatal high fat diet leads to a greater risk of myocardial dysfunction in offspring via enhanced lipotoxicity, IRS-1 serine phosphorylation and mitochondrial defects. J Mol Cell Cardiol. 2013;55:117–29. doi: 10.1016/j.yjmcc.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Van den Hove DL, Leibold NK, Strackx E, Martinez-Claros M, Lesch KP, Steinbusch HW, Schruers KR, Prickaerts J. Prenatal stress and subsequent exposure to chronic mild stress in rats; interdependent effects on emotional behavior and the serotonergic system. Eur Neuropsychopharmacol. 2014;24:595–607. doi: 10.1016/j.euroneuro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Ventura AK, Worobey J. Early influences on the development of food preferences. Curr Biol. 2013;23:R401–8. doi: 10.1016/j.cub.2013.02.037. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Sloboda DM. Strategies for reversing the effects of metabolic disorders induced as a consequence of developmental programming. Front Physiol. 2012;3:242. doi: 10.3389/fphys.2012.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vido DS, Nejm MB, Silva NR, Silva SM, Cravo SL, Luz J. Maternal obesity and late effects on offspring metabolism. Arq Bras Endocrinol Metabol. 2014;58:301–7. doi: 10.1590/0004-2730000003043. [DOI] [PubMed] [Google Scholar]
- Vienberg SG, Brons C, Nilsson E, Astrup A, Vaag A, Andersen B. Impact of short-term high-fat feeding and insulin-stimulated FGF21 levels in subjects with low birth weight and controls. Eur J Endocrinol. 2012;167:49–57. doi: 10.1530/EJE-12-0039. [DOI] [PubMed] [Google Scholar]
- Volpato AM, Schultz A, Magalhaes-da-Costa E, Correia ML, Aguila MB, Mandarim-de-Lacerda CA. Maternal high-fat diet programs for metabolic disturbances in offspring despite leptin sensitivity. Neuroendocrinology. 2012;96:272–84. doi: 10.1159/000336377. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Milne JS, Adam CL, Aitken RP. Adverse metabolic phenotype in low-birth-weight lambs and its modification by postnatal nutrition. Br J Nutr. 2012;107:510–22. doi: 10.1017/S0007114511003175. [DOI] [PubMed] [Google Scholar]
- White CL, Purpera MN, Morrison CD. Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1464–72. doi: 10.1152/ajpregu.91015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes (Lond) 2008;32:495–501. doi: 10.1038/sj.ijo.0803710. [DOI] [PubMed] [Google Scholar]
- Xu L, Sun Y, Gao L, Cai YY, Shi SX. Prenatal restraint stress is associated with demethylation of corticotrophin releasing hormone (CRH) promoter and enhances CRH transcriptional responses to stress in adolescent rats. Neurochem Res. 2014;39:1193–8. doi: 10.1007/s11064-014-1296-0. [DOI] [PubMed] [Google Scholar]
- Yang N, Ginsburg GS, Simmons LA. Personalized medicine in women's obesity prevention and treatment: implications for research, policy and practice. Obes Rev. 2013;14:145–61. doi: 10.1111/j.1467-789X.2012.01048.x. [DOI] [PubMed] [Google Scholar]
- Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8:e61627. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Dai YB, Wang HN, Wang MW. Supplementation of the maternal diet during pregnancy with chocolate and fructose interacts with the high-fat diet of the young to facilitate the onset of metabolic disorders in rat offspring. Clin Exp Pharmacol Physiol. 2013;40:652–61. doi: 10.1111/1440-1681.12147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.