Abstract
β-cyclodextrin (β-CD), with a lipophilic inner cavity and hydrophilic outer surface, interacts with a large variety of non-polar guest molecules to form non-covalent inclusion complexes. Conjugation of β-CD onto biomacromolecules can form physically-crosslinked hydrogel networks upon mixing with a guest molecule. Herein describes the development and characterization of self-healing, thermo-responsive hydrogels, based on host-guest inclusion complexes between alginate-graft-β-CD and Pluronic® F108 (poly(ethylene glycol)-b-poly(propylene glycol)-b-poly(ethylene glycol)). The mechanics, flow characteristics, and thermal response were contingent on the polymer concentrations, and the host-guest molar ratio. Transient and reversible physical crosslinking between host and guest polymers governed self-assembly, allowing flow under shear stress, and facilitating complete recovery of the material properties within a few seconds of unloading. The mechanical properties of the dual-crosslinked, multi-stimuli responsive hydrogels were tuned as high as 30 kPa at body temperature, and are advantageous for biomedical applications such as drug delivery and cell transplantation.
Keywords: cyclodextrin, injectable hydrogel, self-healing, supramolecular chemistry, thermo-responsive
1. Introduction
Polymeric hydrogels are porous, 3-D networks of crosslinked macromolecules, able to retain large amounts of water.1 Injectable polymeric hydrogels, which are crosslinked and solidify in situ,2 are advantageous for drug and cell delivery, and tissue engineering applications.2-4 Many existing injectable hydrogel systems polymerize to form covalent crosslinks, through the use of photo-initiated systems, autonomous redox reactions, or Michael Addition chemistries,5 which can affect cytocompatibility6 and protein bioactivity.7 Furthermore, covalently crosslinked hydrogels are unable to undergo reversible solid-liquid transitions.8 In contrast, the dynamic and reversible nature of non-covalent interactions, such as physical crosslinks, demonstrate variable mechanical properties and stimuli responses which lead to unique and programmable modifications in the network structure.9
Indeed, physically-crosslinked hydrogels avoid the limitations associated with permanently crosslinked hydrogel networks.10 Gelation conditions are relatively mild, and reversible physical crosslinks allow hydrogels to re-assemble, or self-heal, after deformation or a disruption in the network.11-13 In addition, physically-crosslinked hydrogels offer a new route toward innocuous, stable materials and components in vivo.14 Recently, the utilization of supramolecular polymer chemistry, specifically host-guest chemistry, has enabled the design of more sophisticated multi-functional materials.15-18,5, 10, 19, 20 Such hydrogels, which mimic strain and stress-responsive tissues, are now being considered for biomedical applications.21
Several macrocyclic host-guest inclusion complexes are reported in the literature, including crown ethers, cyclophanes, ctananes, and cavitands (such as cyclodextrins, calix[n]arenes and cucurbit[n]urils).1 Of particular interest is β-cyclodextrin (β-CD), which is easily grafted onto polymer chains, exhibits negligible cytotoxic effects, and is an important attribute in the pharmaceutical and food industries.22 The covalent conjugation of β-CD onto large biomacromolecules, such as alginate, increases the functionality of the large polymer.23-25 Alginate is a plant-derived polysaccharide, and alginate hydrogels have served as scaffolds for tissue engineering, drug delivery vehicles, and models of extracellular matrices for biological studies.26-29 Alginate hydrogel properties, including stiffness, swelling, degradation, cell attachment, and binding or release of bioactive molecules, can be optimized through the chemical or physical modification of the polysaccharide itself or the inclusion of alginate into a hydrogel network.30, 31
β-CD, with a lipophilic inner cavity and hydrophilic outer surface, interacts with a large variety of non-polar guest molecules to form physical inclusion complexes.22, 32 The hydrophobic guest molecule is held within the cavity of β-CD and the main driving force of complex formation is the release of enthalpy-rich water molecules from the cavity.22, 32 The binding activities between host and guest molecules are not fixed, but rather self-assemble in a dynamic manner.22 Several guest molecules are reported for β-CD, including adamantine,5, 33, 34 cholesterol,35 and other custom-designed molecular recognition compounds.36 Macromolecules such as poly(propylene glycol), PPG, have also been investigated as guest molecules for β-CD.15, 37-40 Difunctional block copolymers, such as PEG-b-PPG-b-PEG (i.e., Pluronic® F108), are utilized in drug delivery systems and cell culture due to low cytotoxicity and innate thermo-responsive properties.41-48
The inclusion of hydrophobic PPG chain segments into the inner cavity of β-CD affords a high binding affinity to form a hydrogel network, while mobile PEG end-blocks entrap water molecules and provide an innocuous environment for cells.49-52 In addition, the thermo-responsive property of Pluronic® F108 is advantageous for in vivo applications due to the presence of PPG in the block copolymer - PPG is water-soluble at low temperatures and reverts into an insoluble form at higher temperatures.45, 53, 54 This behavior is similar to poly(N-isopropyl acrylamide) (PNIPAAm), a commonly synthesized thermo-responsive polymer.16, 53, 55 However, the non-biodegradability and relatively weak mechanical properties limit the wide application of PNIPAAm.56
The goal of this study was to create a host biomacromolecule, alginate-graft-β-CD, and incorporate a difunctional guest molecule, Pluronic® F108, to create physically-crosslinked, moderately stiff hydrogels. The hydrophobic guest molecule is held within the cavity of β-CD and the main driving force of complex formation is the release of enthalpy-rich water molecules from the cavity.22, 32 We hypothesized that the supramolecular inclusion complex formation between alginate-graft-β-CD and Pluronic® F108 will generate non-cytotoxic, dual-crosslinked, multi-stimuli responsive hydrogels with physiologically relevant mechanical properties for biomedical applications.56
2. Experimental Section
Materials
Sodium alginate (PROTANAL® LF200 FTS, Mv = 67-142 kg/mol) was generously donated by FMC BioPolymer. Beta-cyclodextrin (β-CD), ρ-toluenesulfonyl chloride (TosCl), acetonitrile, acetone, 1,6-hexanediamine (HDA), diethyl ether, dimethylformamide (DMF), (benzotriazol-1-yloxy)tris-(dimethylamino) phophonium hexafluorophosphate (BOP), dimethyl sulfoxide (DMSO), ethylenediamine (EDA), and deuterium oxide (D2O) were purchased from Acros Organics. Sodium hydroxide, ammonium chloride, hydrogen chloride (HCl), ethanol, tetrabuylammonium fluoride (TBAF), phosphate buffered saline (PBS), 2-morpholinoethanesulfonic acid (MES) buffer, alpha-modified eagle medium (α-MEM, Hyclone), and bovine serum albumin (BSA) were purchased from Thermo Fisher Scientific. Human mesenchymal stem cell-screened fetal bovine serum (FBS) was purchased from Atlanta Biologics. Penicillin, streptomycin, and trypsin ethylenediaminetetraacetic acid (EDTA) were purchased from Corning Cellgro. Tetrabutylammonium hydroxide (TBAOH), N-ethyl-N’(3-dimethylaminopropyl) carbodiimide hydrochloric acid (EDC), N-hydroxysuccinimide (NHS), Pluronic® F108 (Mn ≈ 14,600 g/mol), and an In Vitro Toxicology Assay Kit (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, i.e., MTT-based) were purchased from Sigma Aldrich. BCA® Protein Assay Kit was purchased from Life Technologies Inc.
Alginate-Tetrabutylammonium (Alg-TBA) Synthesis
Sodium alginate (Na-Alg, 2 g) was added to a mixture of HCl (0.6 N, 30 mL) and ethanol (30 mL) and stirred overnight at 4 °C. After filtering under vacuum with filter paper and washing with ethanol and acetone, pure alginic acid was obtained and dried overnight. The dried powder was dispersed in DI water (100 mL). Aqueous TBAOH was added slowly under continuous stirring and the pH was adjusted to between 7.0 and 10.0. The solution was dialyzed and lyophilized to yield white Alg-TBA powder.57-61
β-CD-TosCl Synthesis
β-CD (20 g, 17.62 mM, 1 molar equivalent) was suspended in 125 mL ice DI water, and TosCl (4.2 g) was dissolved in minimum acetonitrile (~10 mL) and added drop wise to the aqueous phase. The reaction was stirred vigorously for 2 h at room temperature. Sodium hydroxide (2.18 g) was dissolved in DI water (~10 mL) and added drop wise. After 30 min of stirring at room temperature, solid ammonium chloride was added to adjust the pH to 8.5 and the solution was cooled on ice to collect precipitants. The product was washed with cold DI water and acetone 3 times respectively and dried under vacuum; 6-o-monotosyl-6-deoxy-β-CD was obtained (~25%).5, 62, 63
β-CD-HDA Synthesis
β-CD-TosCl (5 g) was dissolved in DMF (25 mL) with 1,6-hexanediamine (20 g) and stirred under nitrogen at 80 °C for 24 h using a condenser. The product was precipitated out of solution using cold acetone (5 × 500 mL), washed with cold diethyl ether (2 × 100 mL), and dried under vacuum to afford the final product mono-6-deoxy-6-aminohexaneamino-β-CD).5, 64
β-CD-EDA Synthesis
6-(6-aminohyxyl)amino-6-deoxy-β-cyclodextrin (1.5 g) was added to EDA (5 mL). The reaction was performed at 60 °C for 24 h and cooled to room temperature. The precipitant was collected from a large amount of ethanol and dried under vacuum to afford the final product (mono-6-deoxy-6-aminoethylamino-β-CD).65
Alginate-graft-C6-Cyclodextrin (Alg-C6) Synthesis
TBAF (10 g) was dissolved in DMSO (100 mL) to afford a 10% (w/v) solution. Alg-TBA (2 g, 1 molar equivalent) and β-CD-HDA (2.96 g, 0.5 molar equivalent) were added to the mixture and stirred at room temperature under nitrogen flow until a clear solution was obtained.58 BOP (1.06 g, 0.5 molar equivalent) was dissolved in minimal DMSO (5 mL) and added via syringe. The reaction was carried out at room temperature under vigorous mixing for 24 h and then dialyzed against DI water (× 3), followed by 0.05 M sodium phosphate dibasic solution until fully dissolved in water, and finally DI water (× 2). The final product was frozen and lyophilized. The theoretical modification was 50%.5
Alginate-graft-C2-Cyclodextrin (Alg-C2) Synthesis
Sodium alginate (2.73 g) was dissolved in 0.1 M MES buffer (pH 5.6, 150 mL) to which EDC (2 g) and NHS (1.2 g) were added. After mixing for 30 min at room temperature, β-CD-EDA (4.5 g) was added under vigorous mixing at room temperature for 1 day. The alginate solution was dialyzed against DI water for 3 days, frozen, and lyophilized to afford dry polymer.65
1H-NMR Spectroscopy
To qualitatively verify the successful synthesis of Alg-C6 and Alg-C2, lyophilized polymer was dissolved in D2O and the result was confirmed via 1H-NMR (Bruker AVANCE III 500 MHz high-field NMR spectrometer). In addition to standard 1H-NMR, solvent (water) suppression 1H-NMR, as well as diffusion edited 1H-NMR at 95% strength (programmed in Bruker NMR spectrometer), were also performed for alginate, Alg-C2, and Alg-C6, to confirm the covalent conjugation of β-CD onto alginate. The detailed 1H-NMR spectra are provided in the supplemental data.
Alginate Hydrogel Formation and Erosion
Alg-C2 and Alg-C6 hydrogels were prepared from solutions of the individual polymers in PBS at desired concentrations. 4 and 6% (w/v) Alg-C2 and Alg-C6 solutions were prepared in 10 mL syringes. Pluronic® F108 crystals were dissolved in DI water (10% w/v), frozen at −80 °C, and lyophilized to obtain a white powder, which was added to the Alg-g-CD solutions at Pluronic® F108:β-CD ratios of 1:4 and 1:2, respectively. Hydrogels and single host and guest polymer solutions were injected into glass vials; the glass vials were stored at either 25 or 37 °C. Optical images were taken at each time point after the injection to analyze hydrogel stability. To qualitatively analyze hydrolytic erosion of the hydrogels, images were taken of the hydrogels after adding a known amount of PBS on top of each hydrogel to qualitatively visualize hydrogel surface erosion over a period of 14 days at 37 °C under gentle agitation in a shaking incubator (see additional Supplemental Materials). Samples were sealed with parafilm to eliminate water evaporation.
Rheological Characterization
All experiments were performed using an AR2000 stress-controlled rheometer (TA Instruments) fitted with a 40 mm diameter 1°59’47’’ steel cone geometry and 27 μm gap at 37 °C, however, the temperature sweep study included a temperature range. Oscillatory time sweeps for single polymer constituents (e.g., 4% (w/v) Alg-g-CD, 10% Pluronic® F108) and hydrogels were deformed at 1% strain and 10 Hz over 250 s. Oscillatory frequency sweeps were performed at 0.5% strain with increasing frequency from 0.1 to 100 Hz. Continuous flow experiments used a shear rate linearly ramped from 0 to 1 s−1. Oscillatory strain sweeps were performed at 10 Hz with increasing radial strain from 0.01 to 500%. Dynamic shear strain tests were performed at high (250%) and low (0.5%) strains at 1 Hz and 37 °C for certain lengths of time and the cycles were repeated three times to test the self-healing and recovery properties of the hydrogels. Temperature sweeps were performed at 1 Hz and 1% strain, with a heating rate of 0.5 °C*min−1 from 25 to 37 °C.
In Vitro Cytotoxicity Assay
Materials for the in vitro cell study were lyophilized and exposed to UV light overnight. The hydrogels and polymer solutions were prepared with sterile PBS using similar protocols stated above. Primary human mesenchymal stem cells (MSCs) were purchased from Rooster Bio. MSCs (passage 4) were seeded in 48-well tissue culture polystyrene (TCPS) plates at a density of 20,000 cells/well in 500 μL/well of standard MSC growth medium (α-MEM, 10% FBS, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin) and allowed to adhere for 24 h. Cells were incubated in the presence of Alg-C6 hydrogels with ratios of 1:4 and 1:2 and Alg-C2 hydrogels with ratios of 1:4 and 1:2. In addition, cells were incubated without hydrogels under the same culture conditions as control group. After 24 h of incubation, media containing the hydrogel and polymer solutions was removed, and cells were rinsed two times in sterile PBS then analyzed using a MTT-based In Vitro Toxicology Assay Kit following the manufacturer's protocol. The optical density was measured at 570 nm using a BioTek plate reader. Background absorbance at 690 nm was subtracted from the measured absorbance. Absorbance values for the experimental and control samples were normalized to non-modified TCPS controls.66
In Vitro BSA Release
BSA (10 mg) was added to 1 mL of different hydrogel solutions (prepared as stated above) and injected into 24-well plates. The plates were incubated at 37 °C overnight to ensure gelation complete. One mL of PBS was added to each well and the plate was gently agitated at 37 °C in a shaker incubator. At each time point, 100 μL sample aliquots were removed and replaced with 100 μL fresh PBS. The protein concentration was determined using a BCA Protein Assay Kit according to the manufacture's protocol. The optical density was measured at 562 nm using a BioTek plate reader. Background absorbance of non-modified TCPS controls was subtracted from the measured absorbance; absorbance values for the experimental, blank (i.e., containing no BSA), and control samples were reported.
Statistical Methods
All experiments were performed in triplicate; results are reported as mean ± standard deviation. Statistical analysis was performed on the storage moduli for 25 and 37 °C between each of the treatment groups and in vitro cytotoxicity data respectively, using one-way ANOVA with Tukey multiple comparisons (α = 0.05) via the SAS statistics program in the GLM procedure as the post-test to compare all of the groups. P < 0.05 was considered significantly different.
3. Result & Discussion
3.1 Dual Strategies for Synthesizing Alginate-graft-Cyclodextrin (Alg-g-CD)
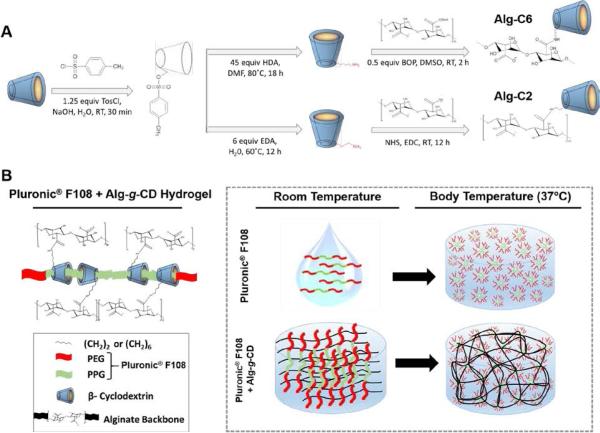
Two strategies were employed to synthesize Alg-g-CD (i.e., host macromolecules) using a short-chain methylene group to conjugate β-CD onto the alginate backbone, providing mobility and thus crosslinking efficiency. While one reaction was performed in an organic solvent with six methylene groups (-CH2-) connecting the alginate backbone to β-CD (Alg-C6),12 the other reaction was performed in aqueous solution with two methylene groups (-CH2-) connecting the alginate backbone to β-CD (Alg-C2).65 The detailed chemical synthesis for both reactions are presented in Figure 1A. Briefly, β-CD was first reacted with TosCl to obtain β-CD-TosCl. In one reaction scheme, β-CD-TosCl was reacted with HDA in DMF at 80 °C for 18 h. Amine groups reacted with carboxyl groups (pre-neutralized with TBA salt) on alginate in DMSO in the presence of BOP. Alternatively in the aqueous solution method, β-CD-TosCl was reacted with EDA at 60 °C for 12 h to obtain β-CD-EDA. The standard 1H-NMR spectra for the final products, Alg-C6 and Alg-C2, are provided in supplemental materials.
Figure 1.
A) Schematic of Alg-g-CD synthesis using organic solvents or aqueous-based solutions. Alg-C6 was the product of the organic synthesis (top), and Alg-C2 was the product of the aqueous synthesis (bottom). B) Schematic of physical crosslinking between Alg-g-CD macromolecules and Pluronic® F108, and the effect of Pluronic® F108 on the thermo-response of the hydrogel network; Pluronic® F108 forms micelles and self-crosslinks at body temperature due to the triblock structure of PEG-b-PPG-b-PEG. β-CD conjugated onto the alginate backbone served as the host (Alg-g-CD), which formed a physically-crosslinked supramolecular inclusion complex with the guest, the PPG component (green) of Pluronic® F108.
In addition to standard 1H-NMR, solvent suppression (water) 1H-NMR as well as diffusion edited 1H-NMR at 95% strength for alginate, Alg-C2 and Alg-C6, were performed to confirm the covalent conjugation of β-CD onto alginate. The detailed 1H-NMR spectra are provided in the supplemental data. The spectra indicated that β-CD was covalently conjugated onto alginate in both Alg-C2 and Alg-C6. By comparing alginate, Alg-C2 and Alg-C6 spectra, peaks between 1 – 3 ppm were only presented in the final product of Alg-C2 and Alg-C6 rather than alginate, suggesting the covalent conjugation of methylene groups, as linker between β-CD and alginate. The degree of β-CD modification, i.e., β-CD density, may generate an effect on the hydrogel physical and mechanical properties.12 The theoretical reaction ratio of β-CD to alginate repeat units (C6H7NaO6)n was 50%. However, by calculating the ratio of hydrogen integration of sugar units (both on alginate backbone repeat units and on β-CD repeat units), and hydrogen integration of the methylene groups on the linker between the backbone and β-CD,65 the actual β-CD modification for both reaction methods was 28% and 30%, respectively for Alg-C2 and Alg-C6, indicating no significant differences in alginate modification for the different Alg-g-CD reaction schemes. There are limitations with using 1H-NMR to calculate the β-CD degree of modification due to the high molecular weight and polydispersity of the alginate (Mv 67-142 kg/mol) starting material; however, using either standard 1H-NMR or diffusion edited 1H-NMR is common in this type of polymer chemistry characterization.5, 12, 48
3.2 Hydrogel Formation and Rheological Analysis
A self-assembled hydrogel network formed almost immediately upon mixing Alg-g-CD and Pluronic® F108 solutions (quantity based on constituent molar ratios). The formation of a pseudo-plastic hydrogel occurred through the complexation of host-guest moieties, i.e., β-CD conjugated alginate (Alg-g-CD) and PPG, as part of the Pluronic® F108 structure. In addition to the supramolecular inclusion complex formation between Alg-g-CD and Pluronic® F108, a dual-crosslinked hydrogel was created via the innate thermo-response of Pluronic® F108. Such dual-crosslinking capabilities significantly increased the storage moduli compared to a single physical crosslinking technique based on host-guest chemistry alone (Figure 1B).
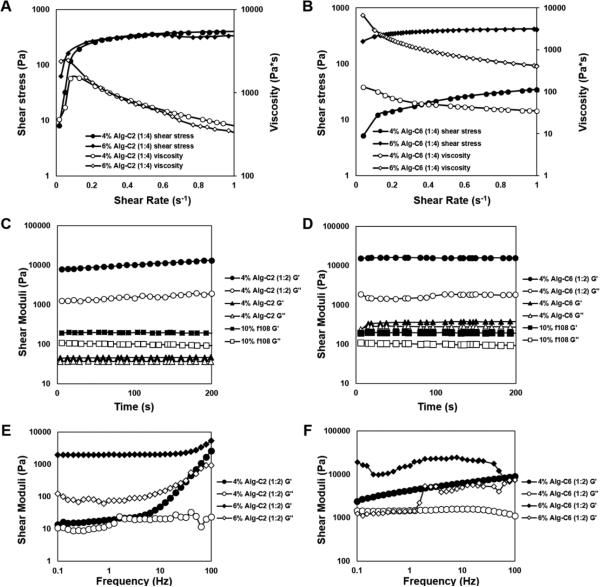
The formation and physical integrity of the supramolecular inclusion complex hydrogels were quantified using rheometry. Hydrogels and their pre-curser solutions were exposed to varying shear forces to examine steady-state shear moduli with time, the effect of frequency on shear moduli, and the effect of shear rate on the shear stress and viscosity of hydrogel pre-curser solutions. To examine the hydrogels in their least compliant states, experiments were performed at 37 °C. In Figure 2A, the shear rate was linearly ramped from 0 to 1 s−1 to investigate the effect of shear rate on viscosity and resultant shear stress; the Alg-C2 group generated an increase in shear stress from approximately 40 Pa to 400 Pa while shear rates increased from 0 to 0.3 s−1 and then plateaued until 1 s−1. The viscosity decreased from near 3500 to 300 Pa*s while shear rates increased from 0 to 0.7 s−1. For the Alg-C6 hydrogels (Figure 2B), the shear stress increased from approximately 5 to 50 Pa for 4% (w/v) Alg-C6 hydrogels and from 100 to 400 Pa for 6% and 8% Alg-C6 hydrogels. While maintaining the same molar ratio between Alg-g-CD and Pluronic® F108, a more distinct concentration-dependent behavior was seen in the Alg-C6 groups compared to the Alg-C2 groups. This may be due to the self-crosslinking of the Alg-C2 polymers, as the material properties were less dependent on polymer concentration, as the β-CD conjugation for both materials was ~30%.65
Figure 2.
Rheological experiments were performed at 37 °C to verify formation and physical integrity of the supramolecular alginate network. (A, B) Continuous, increasing shear rates allowed for the determination of viscosity and shear stress of formed hydrogels; A) Alg-C2 and B) Alg-C6. The shear stress increased with an increase in shear rate from 0 to 1 s−1, demonstrating viscoelastic behavior. (C, D) Oscillatory time sweep experiments for hydrogel pre-curser solutions, Alg-g-CD and Pluronic® F108, and formed hydrogels at 1% strain, 10 Hz, 37 °C; C) Alg-C2 and D) Alg-C6. The storage moduli increased from 100 Pa for single polymer constituents to 10 kPa for 4% (w/v) Pluronic® F108:Alg-g-CD hydrogels. (E, F) Oscillatory frequency sweeps were performed at 0.5% radial strain; E) Alg-C2 and F) Alg-C6.
Shear storage and loss moduli were measured to both verify and quantify the formation of the supramolecular inclusion complex hydrogel from independent measurements of pre-curser solutions. Oscillatory rheology confirmed that the individual Pluronic® F108 and Alg-g-CD precursers were solutions at high frequencies and that an increase in moduli of several orders of magnitude occurred immediately upon mixing of the two components. While the individual polymer solutions (i.e., hydrogel constituents) displayed viscous behaviors associated with intermolecular entanglement in solution, the formed supramolecular inclusion complex hydrogel was stable during a qualitative inversion test. Pluronic® F108 remained unstable and free flowing at room temperature and formed small micelles (size ~20nm) at body temperature.67, 68 The micelles were inter-connected with each other and therefore, higher concentrations of Pluronic® F108 formed a soft network in solution, also shown by the slight increase in viscosity. For the Alg-C2, the G′ increased from 300 Pa to 12 kPa (Figure 2C). The multi-arm structure of EDA on β-CD may also react with various carboxyl groups on neighboring alginate molecules, to create an intramolecular-crosslinked network beyond the host-guest interaction. Previous literature has shown the concept that such crosslinking will increase the stiffness of the hydrogel, although only slightly.[32] For Alg-C6, the G′ increased from 300 Pa for the polymer solution, to near 10 kPa for the Pluronic® F108:Alg-g-CD hydrogel (1:2), and G″ increased from 50 Pa for the polymer solution to 10 kPa for the Pluronic® F108:Alg-g-CD hydrogel. The large increase in shear moduli that occurred after mixing the two polymer constituents verified that an interaction took place to form a more permanent network, compared to polymer solutions alone; the formation of the supramolecular inclusion complex resulted in the 100-fold increase in shear moduli (Figure 2C,D). Thus, the bulk material response indicated that the chemical modification of alginate was successful, through the formation of a stiff hydrogel network upon mixing with Pluronic® F108.
Shear storage and loss moduli were also measured to determine effect of frequency during dynamic shear application. Oscillatory frequency sweeps were performed on 4% Alg-C6 and Alg-C2 hydrogels with a ratio Pluronic® F108:Alg-g-CD 1:4 at 0.5% radial strain and 37 °C. The hydrogel exhibited steady-state behavior up to 10 Hz, after which the moduli increased in response to the increasing shear rate. The effect followed an exponential trend for frequencies greater than 10 Hz for the Alg-C2 hydrogels (Figure 2E). In addition, the hydrogels were stable at lower frequencies and not responsive to frequency as an input parameter. The Alg-C6 hydrogels displayed noisy data, which suggested a less stable network, especially at increasing frequencies (Figure 2F).
3.3 Strain Responsive Properties and Self-Healing
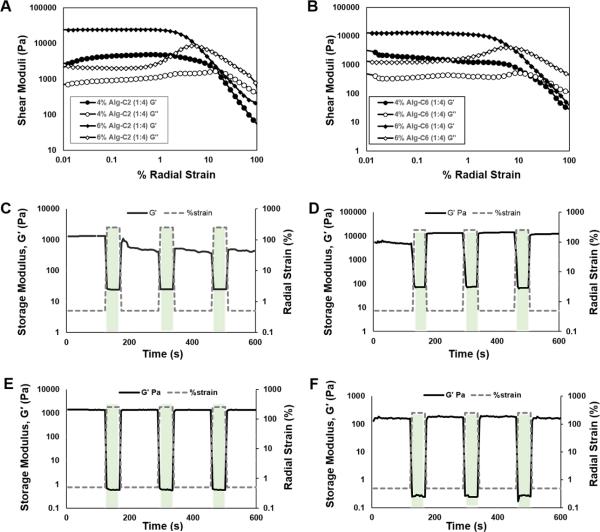
Multi-chain entanglements of macromolecular guest polymers create a unique structure upon crosslinking which affords a high modulus hydrogel with shear-thinning behaviors. Reversible, dynamic physical crosslinks allow supramolecular inclusion complex hydrogels to exhibit flow and recovery characteristics under mechanical radial strain. To validate and optimize the shear-shinning properties of the injectable delivery systems, hydrogels were tested using oscillatory strain sweeps from 0.01% to 500% radial strain at a frequency of 10 Hz. Both Alg-C2 and Alg-C6 (Figure 3A, B) groups were more elastic at low strains, with storage moduli (G') greater than the loss moduli (G”). At 1% strain, the G' and G” curves for Alg-C2 began to cross over, indicating a transition from an elastic solid material to a compliant, viscous liquid. The crossover of G' and G” for Alg-C6 hydrogels occurred near 10% strain, suggesting that the inter-molecular crosslinking density for Alg-C6 was higher compared to Alg-C2; this may be attributed to a longer amine chain, which may have provided more mobility to allow Pluronic® F108 to enter the β-CD cavity. The shear-thinning characteristics were also dependent on the de-association of host-guest polymers. Alg-C2 hydrogel networks were more easily strained to failure compared to Alg-C6 hydrogels, and the Alg-C2 hydrogels exhibited a lower transition strain (%) compared to Alg-C6 hydrogels.
Figure 3.
(A, B) Oscillatory strain sweeps were performed at 10 Hz and 37 °C using a 40 mm 1°59'47” steel cone geometry on F108:Alg-g-CD hydrogels: A) Alg-C2 and B) Alg-C6 hydrogels. The storage moduli (G') and loss moduli (G”) of the hydrogels crossed at high strains, demonstrating a solid-liquid transition. (C – F) Dynamic shear testing of hydrogels was performed to demonstrate a self-healing, physically crosslinked network. Dashed gray lines represent the radial strain (%) input parameters and the solid black lines represent shear storage moduli (G') results. All of the groups tested demonstrated a repeatable ability to deform and reassemble upon loading and un-loading, resulting in radial shear deformations. Hydrogels consisting of 4% (w/v) Alg-g-CD were analyzed at various ratios of Pluronic® F108:Alg-g-CD; C) Alg-C2 (1:2), D) Alg-C6 (1:2), E) Alg-C2 (1:4), F) Alg-C6 (1:4). The hydrogels exhibited higher storage moduli values at 0.5% strain while exhibiting lower G' values at 250% strain. Compared to alginate supramolecular inclusion complexes formed with β-CD, the novel Pluronic® F108:Alg-g-CD hydrogels presented here are shear-thinning, and amendable to injectable biomaterials applications.25
The reversibility and repeatability of the self-healing properties of the hydrogels were independent of host:guest ratios or polymer concentration. To assess material recovery, hydrogels were subjected to cycles of high amplitude oscillatory strain (250%) followed by low amplitude oscillatory strain (0.5%) at 1 Hz. Storage moduli (G') decreased 1/100 fold with a change from low to high amplitude strain (i.e., each time step with strain variation). A decline in moduli was concurrent with the presence of solid-liquid transitions during the application of high strain. During transitions from high to low strains, the initial mechanical properties were recovered in a short time period of 10 s. These results indicate that the novel hydrogels exhibit shear-shinning behavior, recovering mechanical properties immediately upon un-loading. Various hydrogel formulations demonstrated a variety of strain-induced shear moduli values: D) Alg-C6 (1:2) (Figure 3D) and Alg-C2 (1:4) (Figure 3E) hydrogels exhibited a change in G' over 3 orders of magnitude, whereas the Alg-C2 (1:2) (Figure 3C) and Alg-C6 (1:4) (Figure 3F) exhibited a change in G' by 2 orders of magnitude. Despite that the crossover transient points for both Alg-C2 and Alg-C6 upon strain changes in strain were relatively low, the mechanical properties were as high as 10 kPa, indicating a dynamic material with a range of achievable moduli.
3.4 Thermo-Responsive Properties
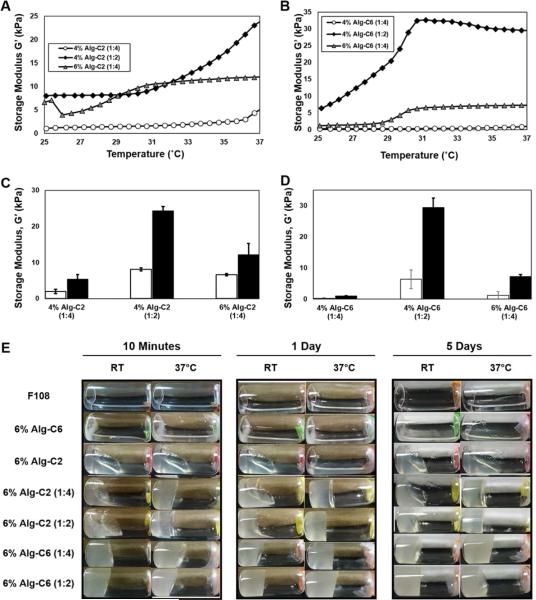
The alginate-based supramolecular inclusion complex hydrogels are thermo-responsive and exhibit secondary crosslinking effects with increasing temperature, due to the incorporation of Pluronic® F108. To demonstrate the thermo-responsive properties of the Alg-g-CD and Pluronic® F108 hydrogels, temperature sweeps were performed from 25 to 37 °C at a heating rate of 0.5 °C min−1 (1% strain; 1 Hz). The significant transition temperatures occurred near 30 °C. For all hydrogel groups tested, the storage moduli (G') increased with increasing temperature (Figure 4A,B). The shear moduli for all hydrogel samples were statistically different between 25 and 37 °C for all samples within the same group (Figure 4C,D), with G' values significantly higher at body temperature compared to room temperature. Independent of polymer concentration, hydrogels fabricated using Pluronic® F108:Alg-g-CD ratios of 1:2 were significantly stiffer compared to ratios of 1:4 at both 25 and 37 °C. The differences in G' within the same group at the different temperatures were elevated due to the addition of Pluronic® F108 in the hydrogel system, indicating that Pluronic® F108 played a significant role in the thermo-response of the host-guest hydrogels. Furthermore, considering only hydrogels with a Pluronic® F108:Alg-g-CD ratio of 1:4, 6% (w/v) Alg-g-CD hydrogels were significant higher compared to 4% hydrogels, for both Alg-C2 and Alg-C6 formulations. Alg-C6 (1:2) hydrogels exhibited G' values near 30 kPa at 37 °C, which corresponded to a ~3% increase in stiffness compared to hydrogels at 25 °C (< 10 kPa). The thermo-transition from room temperature to body temperature was markedly higher compared to existing literature,69 suggesting potential in vivo applications of the thermal-responsive hydrogel in drug delivery and tissue engineering. The novel Pluronic® F108:Alg-g-CD hydrogels were strain-responsive, i.e., shear-thinning, in addition to exhibiting thermo-responsive behavior. Strain-responsive alginate-based supramolecular inclusion complexes presented in the literature do not exhibit thermo-responsive behavior.70 Likewise, thermo-responsive alginate hydrogels presented in the literature do not exhibit shear-thinning and self-healing behavior.71-73
Figure 4.
(A, B) Oscillatory temperature sweeps were performed at 1 Hz and 1% radial strain on Alg-g-CD hydrogels: A) Alg-C2 and B) Alg-C6. (C, D) Shear storage moduli (G') for Alg-g-CD hydrogels at 25 °C (white bars) and 37 °C (black bars) (n = 3, average ± standard deviation), specifically C) Alg-C2 and D) Alg-C6. The ratio of Alg-g-CD to Pluronic® F108 was critical to the magnitude of the thermo-responsive properties. The greater the ratio of Pluronic® F108, the stiffer the hydrogel and more responsive the thermal behavior. Significant differences, of the same hydrogel sample between 25 and 37 °C, were observed in both Alg-g-CD hydrogel formulations. E) Images of Alg-g-CD and Pluronic® F108 solutions, and Alg-g-CD hydrogels formed after mixing the two pre-cursers and waiting 10 min, 1 day, and 5 days. Images were taken of hydrogels incubated at room temperature (RT) and 37 °C, respectively.
3.5 Hydrogel Stability
The physical integrity of the hydrogel network (i.e., supramolecular inclusion complex) was visually analyzed by examining hydrogel stability over 5 days at both room temperature and 37 °C. The 4% (w/v) Alg-g-CD hydrogels, with Pluronic® F108:Alg-g-CD ratios of 1:4 and 1:2, solidified (i.e., physically crosslinked) quicker when exposed to 37 °C compared to those hydrogels exposed to room temperature. For 4% Alg-C2 (1:4 and 1:2) and 4% Alg-when C6 (1:4) formulations, a solid hydrogel was only formed at 37 °C rather than at room temperature, indicating the significant impact of the secondary physical crosslinking arising from the micelle formation of Pluronic® F108. This effect was evident for hydrogels formed from lower concentration solutions (supplemental information 8). For higher concentration solutions, the hydrogel formed upon mixing of the pre-curser solutions at room temperature (Figure 4E). In summary, hydrogels remained stable for up to 5 days after initial formation of the supramolecular inclusion complex, in a non-disturbed, static state. Further observation was performed of Alg-C2 and Alg-C6 hydrogels (see supplemental material) at room temperature for over 6 months (sealed with parafilm to eliminate water evaporation); optical photographs are shown in supplemental information 9 under “day 0”. They remained solid and crosslinked, demonstrating the stability of the hydrogels in air. However, in order to assess the stability of the hydrogels under physiological conditions, a degradation or erosion experiment must also be performed.
3.6 Hydrogel Erosion
To visualize hydrolytic erosion of Alg-C2 and Alg-C6 supramolecular inclusion complex hydrogels, a qualitative surface erosion study was performed. Freshly prepared hydrogels were injected into glass vials, the hydrogels flowed out of the needle to take the shape of the container before completion of crosslinked, and solidified at 37 °C. PBS was added to the vials, and the vials were inverted days 1 – 7, followed by static incubation for an additional 7 days. The surface erosion of 4% and 6% Alg-C6 (1:4) hydrogels was observed as the height of the solidified hydrogels getting shorter until day 14, which was different compared to the response of the Alg-C2 hydrogels. The 4% (w/v) Alg-C2 hydrogels exhibited decreased stability and less resistance to flow after 3 days of culture in PBS during physical inversion; however, when the vials were returned to a static state, the hydrogels solidified; after day 3, the Alg-C2 hydrogels appeared to reach a swollen equilibrium state and remained at the bottom of the vial until day 14 (supplemental information 9 under newly injected from day 0 with PBS to day 14). Over time the hydrogels appeared to continue to crosslink, indicative of self-healing characteristics, which were quantified above. Interestingly, the 6% Alg-C6 (1:2) hydrogel swelled and incorporated the PBS into the network, and solidified the solution. This was likely due to surface erosion releasing Alg-C6 and Pluronic® F108 polymers into the PBS. In addition, the Alg-C6 hydrogels may have inherently enabled more flexibility to the CD functional group compared to the Alg-C2 due to the longer methylene group. The recovery and stability characteristics of the Alg-C6 hydrogels were more evident compared to the Alg-C2 over the 14 day period.
3.7 In Vitro BSA Release
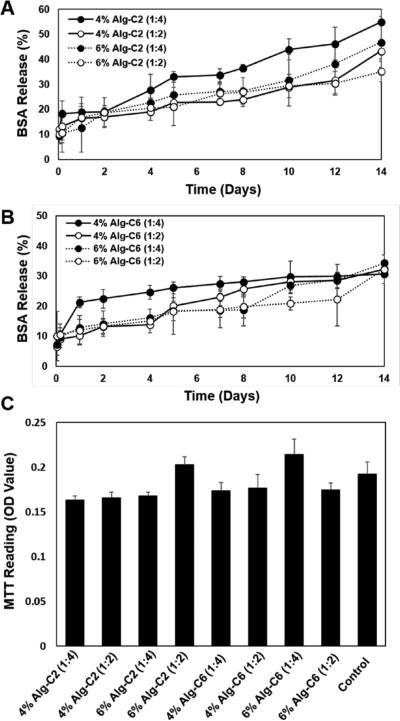
The inner cavity of β-CD undergoes hydrophobic interactions, not only with PPG but with other organic molecules, and such interactions have been extensively employed as drug carriers. Specifically, drug release profiles for supramolecular inclusion network hydrogels utilizing β-CD as a physical crosslinker are of recent interest.5 For this study, BSA, a moderately sized biomolecule (66.5 kDa) with a globular structure, was encapsulated and released from Alg-g-CD hydrogels. BSA release was quantified for up to 14 days for both 4% (w/v) and 6% Alg-C6 and Alg-C2 hydrogels with ratios of 1:2 and 1:4. For each sample, BSA exhibited a sustained release over 14 days to afford a release amount of 40% of the encapsulated drug (Figure 5). A lower amount of BSA was released from hydrogels formulated with a Pluronic® F108:Alg-g-CD ratio of 1:4, suggesting that the reduction in Pluronic® F108 content created a moderately crosslinked hydrogel, resulting in a greater release of BSA. Alg-C2 hydrogels (Figure 5A) released less BSA compared to Alg-C6 hydrogels (Figure 5B) over a period of 14 days, due to a swollen host-guest intermolecular network also observed in the qualitative surface erosion study (supplemental information). Alg-C2 hydrogels swelled after the addition of PBS. Thus, the hydrogel structure was weakened and compromised, resulting an enhanced BSA release compared to Alg-C6 hydrogels, which exhibited primarily surface degradation.
Figure 5.
(A,B) In vitro BSA release from Pluronic® F108:Alg-g-CD hydrogels. Experiments were performed at 37 °C under mild agitation in PBS, pH 7.4, investigating A) Alg-C2 and B) Alg-C6 hydrogels. Black circles represent 6% (w/v) Alg-g-CD solutions, and open circles represent 4% Alg-g-CD solutions. Solid lines represent Pluronic® F108:Alg-g-CD hydrogels with ratios of 1:2, and dashed lines represent hydrogels with ratios of 1:4. C) In vitro MTT-based cytotoxicity assay results for hydrogels cultured with primary human MSCs indicated no toxic effects compared to the non-modified tissue culture polystyrene control group. There were no significant differences between each group.
3.8 In Vitro Cytotoxicity
The safety and efficacy of the alginate-based hydrogels for biomedical applications were tested using a 24 h mitochondrial activity (i.e., MTT content) based assay. 4% (w/v) and 6% Alg-g-CD hydrogels, with Pluronic® F108:Alg-g-CD ratios 1:2 and 1:4, were co-cultured with primary human MSCs for 24 h. An in vitro cytotoxicity assay showed no toxic effects related to the presence of the hydrogels. Alg-C2 hydrogels were slightly less cytotoxic compared to Alg-C6 hydrogels; however, there were no significant differences between experimental groups (Figure 5C) and the control group (non-modified tissue culture polystyrene).
4. Conclusions
In summary, we have developed the first dual-crosslinked, self-healing, and strain and thermo-responsive alginate-based hydrogels with moderate mechanical properties, based on the supramolecular inclusion complex formation between Pluronic® F108 and Alg-g-CD. The intermolecular entanglements of guest polymers (e.g., PPG) and Alg-g-CD host molecules create a unique structure upon crosslinking which affords shear-thinning behavior. Furthermore, Pluronic® F108 affords a thermo-responsive behavior of the injectable hydrogel, generating a dual-crosslinked hydrogel upon an increase to body temperature. Indeed, the shear storage moduli of the hydrogel reached as high as 30 kPa at body temperature, exhibiting biologically-relevant mechanical properties for biomedical applications.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported by an NIH training fellowship for Spencer Fenn (T32 HL076122) and the College of Engineering and Mathematical Sciences at the University of Vermont. The authors gratefully acknowledge Dr. Monika Ivancic for assistance with 1H-NMR data collection and analysis.
Footnotes
ASSOCIATED CONTENT
Supporting information is available from the ACS website. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCE
- 1.Appel EA, del Barrio J, Loh XJ, Scherman OA. Supramolecular polymeric hydrogels. Chemical Society Reviews. 2012;41(18):6195–6214. doi: 10.1039/c2cs35264h. [DOI] [PubMed] [Google Scholar]
- 2.Bae KH, Wang L-S, Kurisawa M. Injectable biodegradable hydrogels: progress and challenges. Journal of Materials Chemistry B. 2013;1(40):5371–5388. doi: 10.1039/c3tb20940g. [DOI] [PubMed] [Google Scholar]
- 3.Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat Commun. 2012;3:1269. doi: 10.1038/ncomms2271. [DOI] [PubMed] [Google Scholar]
- 4.Yang J-A, Yeom J, Hwang BW, Hoffman AS, Hahn SK. In situ-forming injectable hydrogels for regenerative medicine. Progress in Polymer Science. 2014;39(12):1973–1986. [Google Scholar]
- 5.Rodell CB, Kaminski AL, Burdick JA. Rational Design of Network Properties in Guest–Host Assembled and Shear-Thinning Hyaluronic Acid Hydrogels. Biomacromolecules. 2013;14(11):4125–4134. doi: 10.1021/bm401280z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26(11):1211–8. doi: 10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 7.McCall JD, Anseth KS. Thiol–Ene Photopolymerizations Provide a Facile Method To Encapsulate Proteins and Maintain Their Bioactivity. Biomacromolecules. 2012;13(8):2410–2417. doi: 10.1021/bm300671s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. European Journal of Pharmaceutics and Biopharmaceutics. 2004;57(1):19–34. doi: 10.1016/s0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- 9.Yan X, Wang F, Zheng B, Huang F. Stimuli-responsive supramolecular polymeric materials. Chemical Society Reviews. 2012;41(18):6042–6065. doi: 10.1039/c2cs35091b. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Zhu L, Chen H, Yan H, Huang L, Yang J, Zheng J. A Novel Design Strategy for Fully Physically Linked Double Network Hydrogels with Tough, Fatigue Resistant, and Self-Healing Properties. Advanced Functional Materials. 2015;25(10):1598–1607. [Google Scholar]
- 11.Appel EA, Tibbitt MW, Webber MJ, Mattix BA, Veiseh O, Langer R. Self-assembled hydrogels utilizing polymer-nanoparticle interactions. Nat Commun. 2015;6:6295. doi: 10.1038/ncomms7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodell CB, MacArthur JW, Dorsey SM, Wade RJ, Wang LL, Woo YJ, Burdick JA. Shear-Thinning Supramolecular Hydrogels with Secondary Autonomous Covalent Crosslinking to Modulate Viscoelastic Properties In Vivo. Advanced Functional Materials. 2015;25(4):636–644. doi: 10.1002/adfm.201403550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodell CB, Rai R, Faubel S, Burdick JA, Soranno DE. Local immunotherapy via delivery of interleukin-10 and transforming growth factor β antagonist for treatment of chronic kidney disease. Journal of Controlled Release. 2015;206(0):131–139. doi: 10.1016/j.jconrel.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Blaiszik B, Kramer S, Olugebefola S, Moore JS, Sottos NR, White SR. Self-healing polymers and composites. Annual Review of Materials Research. 2010;40:179–211. [Google Scholar]
- 15.Harada A, Takashima Y, Yamaguchi H. Cyclodextrin-based supramolecular polymers. Chemical Society Reviews. 2009;38(4):875–882. doi: 10.1039/b705458k. [DOI] [PubMed] [Google Scholar]
- 16.Cai L, Dewi RE, Heilshorn SC. Injectable Hydrogels with In Situ Double Network Formation Enhance Retention of Transplanted Stem Cells. Advanced Functional Materials. 2015;25(9):1344–1351. doi: 10.1002/adfm.201403631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang C-Y, Chu C-C. Synthesis of photoresponsive hybrid alginate hydrogel with photo-controlled release behavior. Carbohydrate Polymers. 2015;119(0):18–25. doi: 10.1016/j.carbpol.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 18.Huang F, Scherman OA. Supramolecular polymers. Chemical Society Reviews. 2012;41(18):5879–5880. doi: 10.1039/c2cs90071h. [DOI] [PubMed] [Google Scholar]
- 19.Guvendiren M, Lu HD, Burdick JA. Shear-thinning hydrogels for biomedical applications. Soft Matter. 2012;8(2):260–272. [Google Scholar]
- 20.Wei Z, Yang JH, Liu ZQ, Xu F, Zhou JX, Zrínyi M, Osada Y, Chen YM. Self-Healing Materials: Novel Biocompatible Polysaccharide-Based Self-Healing Hydrogel (Adv. Funct. Mater. 9/2015). Advanced Functional Materials. 2015;25(9):1471–1471. [Google Scholar]
- 21.Kuhl E. Growing matter: a review of growth in living systems. J Mech Behav Biomed Mater. 2014;29:529–43. doi: 10.1016/j.jmbbm.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Del Valle EMM. Cyclodextrins and their uses: a review. Process Biochemistry. 2004;39(9):1033–1046. [Google Scholar]
- 23.Boekhoven J, Rubert Pérez CM, Sur S, Worthy A, Stupp SI. Dynamic Display of Bioactivity through Host–Guest Chemistry. Angewandte Chemie International Edition. 2013;52(46):12077–12080. doi: 10.1002/anie.201306278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes Fraceto L, Grillo R, Sobarzo-Sanchez E. Cyclodextrin inclusion complexes loaded in particles as drug carrier systems. Current topics in medicinal chemistry. 2014;14(4):518–525. doi: 10.2174/1568026613666131219124847. [DOI] [PubMed] [Google Scholar]
- 25.Tan L, Liu Y, Ha W, Ding L-S, Peng S-L, Zhang S, Li B-J. Stimuli-induced gel-sol transition of multi-sensitive supramolecular [small beta]-cyclodextrin grafted alginate/ferrocene modified pluronic hydrogel. Soft Matter. 2012;8(21):5746–5749. [Google Scholar]
- 26.Ciofani G, Raffa V, Menciassi A, Dario P. Alginate and chitosan particles are drug delivery system for cell therapy. Biomed Microdevices. 2008;10(4):597–600. doi: 10.1007/s10544-007-9118-7. [DOI] [PubMed] [Google Scholar]
- 27.Jay SM, Saltzman WM. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. Journal of Controlled Release. 2009;134(1):26–34. doi: 10.1016/j.jconrel.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jay SM, Shepherd BR, Bertram JP, Pober JS, Saltzman WM. Engineering of multifunctional gels integrating highly efficient growth factor delivery with endothelial cell transplantation. FASEB J. 2008;22(8):2949–56. doi: 10.1096/fj.08-108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemoine D, Wauters F, Bouchend'homme S, Préat V. Preparation and characterization of alginate microspheres containing a model antigen. International Journal of Pharmaceutics. 1998;176(1):9–19. [Google Scholar]
- 30.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromolecular bioscience. 2006;6(8):623–33. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 31.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Progress in polymer science. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Advanced Drug Delivery Reviews. 2007;59(7):645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Paolino M, Ennen F, Lamponi S, Cernescu M, Voit B, Cappelli A, Appelhans D, Komber H. Cyclodextrin-Adamantane Host–Guest Interactions on the Surface of Biocompatible Adamantyl-Modified Glycodendrimers. Macromolecules. 2013;46(9):3215–3227. [Google Scholar]
- 34.Granadero D, Bordello J, Pérez-Alvite MJ, Novo M, Al-Soufi W. Host-Guest Complexation Studied by Fluorescence Correlation Spectroscopy: Adamantane–Cyclodextrin Inclusion. International Journal of Molecular Sciences. 2010;11(1):173–188. doi: 10.3390/ijms11010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Manakker F, Braeckmans K, Morabit N. e., De Smedt SC, van Nostrum CF, Hennink WE. Protein-Release Behavior of Self-Assembled PEG–β-Cyclodextrin/PEG–Cholesterol Hydrogels. Advanced Functional Materials. 2009;19(18):2992–3001. [Google Scholar]
- 36.Yamaguchi H, Kobayashi Y, Kobayashi R, Takashima Y, Hashidzume A, Harada A. Photoswitchable gel assembly based on molecular recognition. Nat Commun. 2012;3:603. doi: 10.1038/ncomms1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pozuelo J, Mendicuti F, Mattice WL. Inclusion Complexes of Chain Molecules with Cycloamyloses III. Molecular Dynamics Simulations of Polyrotaxanes Formed by Poly(propylene glycol) and [beta]-Cyclodextrins. Polym J. 1998;30(6):479–484. [Google Scholar]
- 38.Qin J, Meng X, Li B, Ha W, Yu X, Zhang S. Self-assembly of β-cyclodextrin and pluronic into hollow nanospheres in aqueous solution. Journal of colloid and interface science. 2010;350(2):447–452. doi: 10.1016/j.jcis.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 39.Okada M, Kamachi M, Harada A. Preparation and Characterization of Inclusion Complexes of Poly(propylene glycol) with Methylated Cyclodextrins. The Journal of Physical Chemistry B. 1999;103(14):2607–2613. [Google Scholar]
- 40.Harada A, Okada M, Li J, Kamachi M. Preparation and Characterization of Inclusion Complexes of Poly(propylene glycol) with Cyclodextrins. Macromolecules. 1995;28(24):8406–8411. [Google Scholar]
- 41.Corey JM, Gertz CC, Sutton TJ, Chen Q, Mycek KB, Wang B-S, Martin AA, Johnson SL, Feldman EL. Patterning N-type and S-type neuroblastoma cells with Pluronic F108 and ECM proteins. Journal of Biomedical Materials Research Part A. 2010;93A(2):673–686. doi: 10.1002/jbm.a.32485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tharmalingam T, Goudar CT. Evaluating the impact of high Pluronic® F68 concentrations on antibody producing CHO cell lines. Biotechnology and Bioengineering. 2015;112(4):832–837. doi: 10.1002/bit.25491. [DOI] [PubMed] [Google Scholar]
- 43.Loh XJ, Goh SH, Li J. Hydrolytic degradation and protein release studies of thermogelling polyurethane copolymers consisting of poly[(R)-3-hydroxybutyrate], poly(ethylene glycol), and poly(propylene glycol). Biomaterials. 2007;28(28):4113–4123. doi: 10.1016/j.biomaterials.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 44.Loh XJ, Peh P, Liao S, Sng C, Li J. Controlled drug release from biodegradable thermoresponsive physical hydrogel nanofibers. Journal of Controlled Release. 2010;143(2):175–182. doi: 10.1016/j.jconrel.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 45.Loh XJ, Colin Sng KB, Li J. Synthesis and water-swelling of thermo-responsive poly(ester urethane)s containing poly(ε-caprolactone), poly(ethylene glycol) and poly(propylene glycol). Biomaterials. 2008;29(22):3185–3194. doi: 10.1016/j.biomaterials.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 46.Loh XJ, Tan YX, Li Z, Teo LS, Goh SH, Li J. Biodegradable thermogelling poly(ester urethane)s consisting of poly(lactic acid) – Thermodynamics of micellization and hydrolytic degradation. Biomaterials. 2008;29(14):2164–2172. doi: 10.1016/j.biomaterials.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Pradal C, Jack KS, Grøndahl L, Cooper-White JJ. Gelation Kinetics and Viscoelastic Properties of Pluronic and α-Cyclodextrin-Based Pseudopolyrotaxane Hydrogels. Biomacromolecules. 2013;14(10):3780–3792. doi: 10.1021/bm401168h. [DOI] [PubMed] [Google Scholar]
- 48.Pradal C, Grondahl L, Cooper-White JJ. Hydrolytically degradable polyrotaxane hydrogels for drug and cell delivery applications. Biomacromolecules. 2015;16(1):389–403. doi: 10.1021/bm501615p. [DOI] [PubMed] [Google Scholar]
- 49.Lin N, Dufresne A. Supramolecular hydrogels from in situ host-guest inclusion between chemically modified cellulose nanocrystals and cyclodextrin. Biomacromolecules. 2013;14(3):871–80. doi: 10.1021/bm301955k. [DOI] [PubMed] [Google Scholar]
- 50.Matthew JE, Nazario YL, Roberts SC, Bhatia SR. Effect of mammalian cell culture medium on the gelation properties of Pluronic® F127. Biomaterials. 2002;23(23):4615–4619. doi: 10.1016/s0142-9612(02)00208-9. [DOI] [PubMed] [Google Scholar]
- 51.Khattak SF, Bhatia SR, Roberts SC. Pluronic F127 as a cell encapsulation material: utilization of membrane-stabilizing agents. Tissue engineering. 2005;11(5-6):974–83. doi: 10.1089/ten.2005.11.974. [DOI] [PubMed] [Google Scholar]
- 52.Hofig I, Atkinson MJ, Mall S, Krackhardt AM, Thirion C, Anastasov N. Poloxamer synperonic F108 improves cellular transduction with lentiviral vectors. The journal of gene medicine. 2012;14(8):549–60. doi: 10.1002/jgm.2653. [DOI] [PubMed] [Google Scholar]
- 53.Badi N, Lutz J-F. PEG-based thermogels: Applicability in physiological media. Journal of Controlled Release. 2009;140(3):224–229. doi: 10.1016/j.jconrel.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Fujita H, Ooya T, Yui N. Thermally-Responsive Properties of a Polyrotaxane Consisting of [beta]-Cyclodextrins and a Poly(ethylene glycol)-Poly(propylene glycol) Triblock-Copolymer. Polym J. 1999;31(11_2):1099–1104. [Google Scholar]
- 55.Guan Y, Zhang Y. PNIPAM microgels for biomedical applications: from dispersed particles to 3D assemblies. Soft Matter. 2011;7(14):6375–6384. [Google Scholar]
- 56.Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications - a review. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2008;68(1):34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Babak VG, Skotnikova EA, Lukina IG, Pelletier S, Hubert P, Dellacherie E. Hydrophobically Associating Alginate Derivatives: Surface Tension Properties of Their Mixed Aqueous Solutions with Oppositely Charged Surfactants. Journal of colloid and interface science. 2000;225(2):505–510. doi: 10.1006/jcis.2000.6788. [DOI] [PubMed] [Google Scholar]
- 58.Pawar SN, Edgar KJ. Chemical Modification of Alginates in Organic Solvent Systems. Biomacromolecules. 2011;12(11):4095–4103. doi: 10.1021/bm201152a. [DOI] [PubMed] [Google Scholar]
- 59.Pawar SN, Edgar KJ. Alginate esters via chemoselective carboxyl group modification. Carbohydrate Polymers. 2013;98(2):1288–1296. doi: 10.1016/j.carbpol.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 60.Schleeh T, Madau M, Roessner D. Synthesis enhancements for generating highly soluble tetrabutylammonium alginates in organic solvents. Carbohydr Polym. 2014;114:493–9. doi: 10.1016/j.carbpol.2014.07.079. [DOI] [PubMed] [Google Scholar]
- 61.Daemi H, Barikani M. Molecular engineering of manipulated alginate-based polyurethanes. Carbohydr Polym. 2014;112:638–47. doi: 10.1016/j.carbpol.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 62.Petter RC, Salek JS, Sikorski CT, Kumaravel G, Lin FT. Cooperative binding by aggregated mono-6-(alkylamino)-.beta.-cyclodextrins. Journal of the American Chemical Society. 1990;112(10):3860–3868. [Google Scholar]
- 63.McNaughton M, Engman L, Birmingham A, Powis G, Cotgreave IA. Cyclodextrin-Derived Diorganyl Tellurides as Glutathione Peroxidase Mimics and Inhibitors of Thioredoxin Reductase and Cancer Cell Growth. Journal of Medicinal Chemistry. 2004;47(1):233–239. doi: 10.1021/jm030916r. [DOI] [PubMed] [Google Scholar]
- 64.Kaya E, Mathias LJ. Synthesis and characterization of physical crosslinking systems based on cyclodextrin inclusion/host-guest complexation. Journal of Polymer Science Part A: Polymer Chemistry. 2010;48(3):581–592. [Google Scholar]
- 65.Izawa H, Kawakami K, Sumita M, Tateyama Y, Hill JP, Ariga K. [small beta]-Cyclodextrincrosslinked alginate gel for patient-controlled drug delivery systems: regulation of host-guest interactions with mechanical stimuli. Journal of Materials Chemistry B. 2013;1(16):2155–2161. doi: 10.1039/c3tb00503h. [DOI] [PubMed] [Google Scholar]
- 66.Miao T, Rao KS, Spees JL, Oldinski RA. Osteogenic differentiation of human mesenchymal stem cells through alginate-graft-poly(ethylene glycol) microsphere-mediated intracellular growth factor delivery. Journal of Controlled Release. 2014;192(0):57–66. doi: 10.1016/j.jconrel.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohn D, Sagiv H, Benyamin A, Lando G. Engineering thermoresponsive polymeric nanoshells. Biomaterials. 2009;30(19):3289–3296. doi: 10.1016/j.biomaterials.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 68.Huang H-Y, Hu S-H, Chian C-S, Chen S-Y, Lai H-Y, Chen Y-Y. Self-assembling PVA-F127 thermosensitive nanocarriers with highly sensitive magnetically-triggered drug release for epilepsy therapy in vivo. Journal of Materials Chemistry. 2012;22(17):8566–8573. [Google Scholar]
- 69.Sinha MK, Gao J, Stowell CET, Wang Y. Synthesis and biocompatibility of a biodegradable and functionalizable thermo-sensitive hydrogel. Regenerative Biomaterials. 2015 doi: 10.1093/rb/rbv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan L, Li J, Liu Y, Zhou H, Zhang Z, Deng L. Synthesis and characterization of β-cyclodextrin-conjugated alginate hydrogel for controlled release of hydrocortisone acetate in response to mechanical stimulation. Journal of Bioactive and Compatible Polymers: Biomedical Applications. 2015 [Google Scholar]
- 71.Vasile C, Nita LE. Novel multi-stimuli responsive sodium alginate-grafted-poly(N-isopropylacrylamide) copolymers: II. Dilute solution properties. Carbohydrate Polymers. 2011;86(1):77–84. [Google Scholar]
- 72.Soledad Lencina MM, Iatridi Z, Villar MA, Tsitsilianis C. Thermoresponsive hydrogels from alginate-based graft copolymers. European Polymer Journal. 2014;61:33–44. [Google Scholar]
- 73.Teodorescu M, Andrei M, Turturicǎ G, Stǎnescu PO, Zaharia A, Sârbu A. Novel Thermoreversible Injectable Hydrogel Formulations Based on Sodium Alginate and Poly(NIsopropylacrylamide). International Journal of Polymeric Materials and Polymeric Biomaterials. 2015;64(15):763–771. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.