Abstract
Focal temporal lobe seizures often cause impaired cortical function and loss of consciousness. Recent work suggests that the mechanism for depressed cortical function during focal seizures may depend on decreased subcortical cholinergic arousal, which leads to a sleep-like state of cortical slow-wave activity. To test this hypothesis, we sought to directly activate subcortical cholinergic neurons during focal limbic seizures to determine the effects on cortical function. Here we used an optogenetic approach to selectively stimulate cholinergic brainstem neurons in the pedunculopontine tegmental nucleus during focal limbic seizures induced in a lightly anesthetized rat model. We found an increase in cortical gamma activity and a decrease in delta activity in response to cholinergic stimulation. These findings support the mechanistic role of reduced subcortical cholinergic arousal in causing cortical dysfunction during seizures. Through further work, electrical or optogenetic stimulation of subcortical arousal networks may ultimately lead to new treatments aimed at preventing cortical dysfunction during seizures.
Keywords: Epilepsy, Consciousness, Optogenetics, Neurostimulation, Acetylcholine, Brainstem
INTRODUCTION
Temporal lobe epilepsy (TLE) is the most common epilepsy of adulthood, with seizures originating from limbic structures, particularly the hippocampus.1 Consistent with the role of the limbic system in memory and emotions, temporal lobe seizures typically cause transient amnesia and emotional changes. However, an additional, puzzling feature is loss of consciousness, occurring even when seizures remain confined to the temporal lobe.2 Why would focal impairment of the temporal lobe cause loss of consciousness? This long-standing mystery is underscored by the fact that even bilateral removal of the temporal lobes does not impair alertness and behavioral responsiveness.
Imaging and intracranial recordings in patients, as well as previous animal model studies, have led to the “network inhibition” hypothesis, in which temporal lobe seizures impair consciousness via inhibition of subcortical arousal circuits.3 Inhibited arousal, in turn, causes a transition of remote brain areas crucial for maintaining consciousness, particularly frontal and parietal cortices, into a sleep-like mode. Consistent with this hypothesis, focal hippocampal seizures cause slow-wave activity on cortical EEG resembling deep sleep.2; 3 Recent data has further shown that subcortical arousal neurons, including cholinergic neurons in the upper brainstem, are suppressed during focal hippocampal seizures.4 Despite these compelling findings, direct evidence for a mechanistic link between inhibited arousal and cortical dysfunction during seizures has been lacking. Crucially, the network inhibition hypothesis predicts that stimulating arousal circuits during focal hippocampal seizures would reverse cortical slowing and restore fast cortical activity. Here, we used recent advances in optogenetics5; 6 to test this prediction, through selective optostimulation of cholinergic neurons of the pedunculo-pontine tegmental nucleus (PPT).
METHODS
Animals
All experimental protocols for this study were approved by the institutional Animal Care and Use Committee and conducted in accordance with the United States Public Health Service's Policy on Humane Care and Use of Laboratory Animals. Male or female rats were used and were 4-16 weeks old at the time of viral injections. Rats expressing Cre-recombinase specifically in cholinergic neurons 5 were bred by mating ChAT-Cre positive breeders to adult wild-type, Long Evans rats (Charles River Laboratories). Pups were genotyped using genomic PCR (Transnetyx).
AAV virus injections
To generate cell-type specific expression of ChR2 in cholinergic neurons, we used a Cre-lox approach, by injecting a Cre-inducible recombinant AAV vector containing ChR2 (pAAV-Ef1α-DIO-hChR2(H134R)-EYFP-WPRE-pA) into rats expressing Cre-recombinase in cholineacetyltransferase (ChAT)-positive neurons (Figure 1A) 5. This viral construct carries an inverted ChR2 fused to the fluorescent marker EYFP, flanked by a pair of canonical loxP sites (loxP) and a pair of mutated loxP sites (lox2272). In the presence of Cre, ChR2-EYFP is inverted into the sense direction and expressed from the EF1-α promoter 6; 7. AAV particles of serotype 5 were produced by the Vector Core Facility at The University of North Carolina at Chapel Hill.
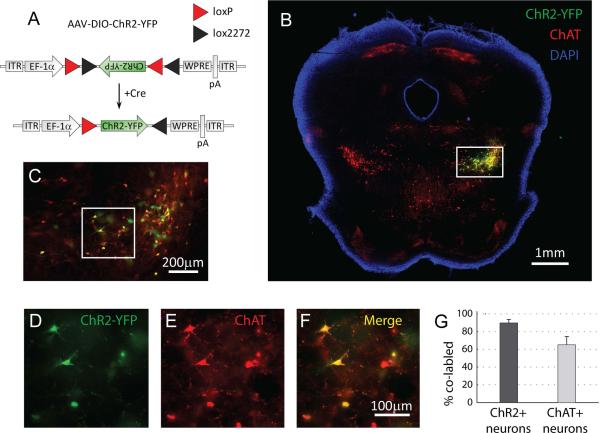
Figure 1. Selective expression of channelrhodopsin-2 in cholinergic brainstem neurons.
(A) We used a Cre-loxP technology to deliver the light-activated protein channelrhodopsin-2 (ChR2) into the pedunculo-pontine tegmental nucleus (PPT) of rats. We injected an adeno-associated virus (AAV) containing ChR2 and yellow-fluorescent protein (YFP) into ChAT-Cre transgenic rats expressing Cre-recombinase specifically in cholinergic neurons. In the presence of Cre-recombinase, ChR2 and YFP are flipped from their anti-sense encoding into the functional right-sense direction and then expressed under the control of the strong promoter EF-1α. ITR: inverted terminal repeat; pA: poly(A); WPRE: woodchuck hepatitis B virus posttranscriptional element; DIO: double-floxed inverted open reading frame. (B-F) Coronal brainstem slice (approximately −7.8mm relative to bregma) 21 days after targeted virus injection into the right-side pedunculo-pontine tegmental nucleus (PPT). Box in B shows area enlarged for C, and box in C shows area enlarged for D-F. Correct targeting of ChR2-YFP is confirmed by the overlap (yellow) between virus expression (ChR2-YFP, green) and immunoreactivity for choline-acetyltransferase (ChAT, red) which delineates the PPT and marks cholinergic neurons. The nuclear stain DAPI was used to highlight the slice margins (B). (G) Quantification at a cellular level indicates that ChR2-YFP was selectively expressed in ChAT+ neurons (89.7 ± 3.9% of CHR2-YFP neurons co-labeled with ChAT, n=5). In addition, a large fraction of ChAT+ neurons in the PPT on the injected side of the brain expressed ChR2-YFP (65.0 ± 9.5%, n=5).
Rats were deeply anesthetized using ketamine/xylazine (90/15 mg/kg, i.m.) and virus was delivered with a Quintessential Stereotaxic Injector (53311, Stoelting) via a pulled glass micropipette (21-175B, Fisher Scientific) lowered through a small durotomy to 6.2mm below the cortical surface. A bolus of 0.8μl of virus (4×1012 viral molecules per ml) was injected into the PPT (coordinates below) at 0.1μl/min. The pipette was held in place for 5min after the injection before being removed. Animals were maintained for 2-4 weeks to allow ChR2 expression prior to experiments.
Electrophysiology
Animals were anesthetized with ketamine/xylazine (90/15 mg/kg, i.m. for surgical procedures, lightened to 40/7 mg/kg for seizure induction) and recordings of multiunit activity (MUA) and local field potentials (LFP) performed as described previously 4; 8; 9. Briefly, a bipolar LFP electrode was placed in the dorsal hippocampus (AP −3.8; ML 2.5; SI 2.6) and a high-impedance MUA/LFP microelectrode placed in orbitofrontal cortex (AP 4.2; ML 2.2; SI 2.4) or in PPT (AP −7.8; ML 2.0; SI 7.0) (coordinates in mm relative to Bregma 10). Recordings were filtered to obtain LFP (0.1–100Hz) and MUA (0.4-10kHz), digitized at 1 kHz for LFP and 20 kHz for MUA. For respiratory measurements, a nasal thermistor (BAT-12 Microprobe Thermistor, Physitemp) with a type T thermocouple copper-constantan sensor (RET-3, Physitemp) was placed into the rat's nose 11. Temperature fluctuations associated with inspiration and expiration were used to determine breathing frequency.
Seizures were induced by a 60 Hz, 2sec train of square-wave biphasic pulses (1ms/phase) to the hippocampus, titrated by ~200μA steps to the lowest intensity (range 200-1000μA) producing a seizure >30s long4; 8; 9. Animals were perfused and brains collected to histologically verify all electrode locations as previously described 12.
Light stimulation
Light stimulation was delivered by a 473nm laser (OptoEngine, MBL-III-473) with computer-controlled pulses. 20-80mW/mm2 was delivered via a 200μm diameter unjacketed optical fiber (M51L01, CFM12L10, Thorlabs) placed in the PPT (AP −7.8; ML 2.0; SI 7.0). For initial calibration experiments (Supplementary Figure S1), light pulse duration and frequency varied. For seizure experiments, light pulses were given at 40ms at 10Hz, with 10s train duration, and stimulation started 10s after seizure onset.
Immunohistochemistry
100μm sections (Leica VT1000S vibratome) were incubated overnight at room temperature in 5% donkey serum and PBS with 0.3% Triton X-100 with anti-Chat primary antibody (Millipore, AP144, 1:250) and anti-YFP (Life Technologies, A21311, 1:1000), washed and then incubated for 2h at room temperature in secondary antibody (Life Technologies, A11058, 1:500), washed and then mounted on glass slides with Vectashield with DAPI (Vector Laboratories, H-1200). ChR2-YFP expression was scored by hand through examination of every 100μm coronal section (n=5 rats) for quantification of co-labelling of ChR2-YFP and ChAT using a Zeiss AxioImager Z2 microscope (Carl Zeiss).
Data and statistical analysis
LFP power was analyzed in 10s epochs before and after effects of optogenetic stimulation. Analysis windows were selected based on the slight delay in the effects of optogenetic stimulation (see Supplementary Figures S2, S3), so that both the baseline and stimulated analysis window were shifted forward in time. Thus we used 5-15s after seizure onset as baseline, and 18-28s after seizure onset (8-18s after optogenetic stimulus onset) as the optogenetic stimulation analysis window. Any seizures exhibiting secondary generalization based on poly-spike discharges in the frontal cortical LFP 9 during the analysis epochs were excluded. Data are presented as mean±SEM. Changes in LFP power were analyzed in Spike2 and MATLAB with two-tailed t test significance threshold P<0.05.
RESULTS
Histological analysis confirmed selective expression of ChR2 in cholinergic neurons of the PPT (Figure 1). To test the functionality of ChR2 in the PPT, we conducted in vivo recordings from the PPT during optical stimulation and optimized stimulation parameters to maximize ChR2-mediated firing of PPT neurons (Supplementary Figure S1). These measurements led us to use a light pulse duration of 40ms and stimulation at 10Hz for subsequent experiments. Importantly, we never observed changes in PPT neuronal firing in control optical stimulation experiments with the same parameters in ChAT-Cre rats which had been injected with an AAV construct containing YFP but not ChR2 (n=4, data not shown).
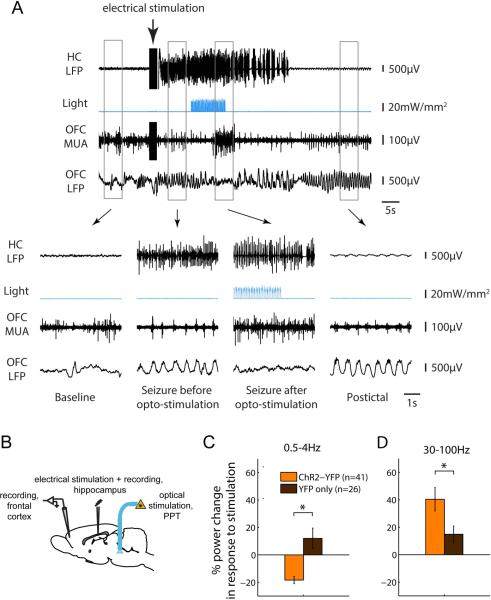
Next, we applied our fine-tuned stimulation paradigm to the question at hand, namely the role of subcortical arousal networks in controlling the cortical state and slow-wave activity during focal limbic seizures induced in an established rat model under light anesthesia 4; 8; 9. We found that optogenetic stimulation of cholinergic PPT neurons led to a marked reduction in frontal cortical slow-wave activity during limbic seizures (Figure 2A, B). Specifically, frontal cortical delta power (0.5-4 Hz) was reduced by 18.4 ± 2.7 % in ChR2-YFP experiments, compared to an increase of 12.0 ± 7.52 % in YFP control experiments (p<0.001; Figure 2C) (n=41 seizures in 8 animals for ChR2-YFP experiments; n=26 seizures in 4 animals for YFP controls). At the same time, frontal cortical gamma power (30-100 Hz), a marker of cortical arousal 13 was enhanced by 40.4 ± 8.52 % in ChR2-YFP experiments compared to 14.8 ± 6.1% in YFP control experiments (p<0.033; Figure 2D). Changes in cortical physiology occurred with a slight delay after onset of optogenetic stimulation in PPT (Figure 2A, B; see also Supplementary Figures S2, S3). Interestingly, breathing frequency, which may provide another marker for increased arousal, also increased in response to optogenetic stimulation in ChR2-injected animals (9.0 +/− 2.4%) compared to YFP-injected controls (0.2 +/− 1.9%; p=0.01).
Figure 2. Optogenetic stimulation of the PPT reduces cortical slow wave activity during seizures.
We induced focal seizures by injecting a brief (2sec) electric current via a bipolar electrode implanted into the hippocampus (see part B for schematic). The same electrode was used for recording hippocampal local field potential (LFP). 10s after seizure onset, we delivered a 10s-long train of blue light pulses (473nm; pulse duration 40ms, frequency 10Hz) into the PPT. We also recorded LFP and multiunit activity from the orbitofrontal cortex (OFC). In control seizures (not shown), in which YFP but no ChR2 had been injected into the PPT, cortical slow wave activity persisted throughout the seizure as previously described 8; 9. (A) Optogenetic stimulation of the PPT following ChR2 injection resulted in a transition of the cortical LFP from slow waves to low voltage fast activity. (B) Schematic of seizure experimental setup. (C-D) Changes in cortical LFP delta (0.5-4Hz) and gamma (30-100Hz) power in response to optogenetic stimulation. LFP Power was quantified shortly after seizure onset and following optogenetic stimulation (5-15s and 18-28s after seizure onset, respectively), and the percent change in power between the two time windows was averaged across seizures. * P<0.05.
DISCUSSION
Impaired consciousness during focal temporal lobe seizures has long puzzled clinicians and basic researchers, given that the temporal lobe per se is not required for maintaining consciousness. Physiologically, impaired consciousness is characterized by prominent slow-wave activity in the fronto-parietal cortical network 2. Here, we demonstrate that optogenetic stimulation of cholinergic neurons in the upper brainstem during focal hippocampal seizures in rats reduces cortical slow-wave activity. These findings provide the first direct mechanistic link between suppressed subcortical arousal during seizures and neocortical dysfunction as observed both in patients and in animal models of TLE.3
We focused our experiments on the cholinergic system due to (1) its well established role in arousal 14; (2) previous data from our lab specifically implicating the cholinergic system during seizures 4; and (3) the availability of genetic-molecular tools to manipulate cholinergic neurons in the rat 5. Importantly, many other neurotransmitter systems play essential roles in arousal 15 and are likely modulated during seizures as well. The relative contribution of these neurotransmitter systems to impaired behavioral responsiveness during seizures remains to be determined.
Loss of consciousness increases the risk of injury in epilepsy patients, and limits their ability to engage in normal activities such as driving. In cases when seizures cannot be fully controlled by medication or surgery, treatments to reduce the severity of seizures and to prevent loss of consciousness may offer a promising avenue to ameliorate the quality of life of patients. Whereas optogenetic stimulation remains purely a research tool for investigating brain circuits in animal models, other clinical treatments, particularly electrical deep brain stimulation, may be applied for restoring consciousness during seizures based on better understanding of the underlying networks.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH/NINDS R01 NS066974, R21 NS083783, P30 NS052519, the Swebilius Trust, the Loughridge Williams Foundation, and by the Betsy and Jonathan Blattmachr family.
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST
None of the authors has any conflict of interest to disclose.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Williamson PD, Thadani VM, French JA, et al. Medial temporal lobe epilepsy: videotape analysis of objective clinical seizure characteristics. Epilepsia. 1998;39:1182–1188. doi: 10.1111/j.1528-1157.1998.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 2.Englot DJ, Yang L, Hamid H, et al. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133:3764–3777. doi: 10.1093/brain/awq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenfeld H. Impaired consciousness in epilepsy. Lancet Neurology. 2012;11:814–826. doi: 10.1016/S1474-4422(12)70188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motelow JE, Li W, Zhan Q, et al. Decreased subcortical cholinergic arousal in focal seizures. Neuron. 2015;85:561–572. doi: 10.1016/j.neuron.2014.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witten IB, Steinberg EE, Lee SY, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardin JA, Carlen M, Meletis K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. %U http://dx.doi.org/610.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atasoy D, Aponte Y, Su HH, et al. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. Journal of Neuroscience. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Englot DJ, Modi B, Mishra AM, et al. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci. 2009;29:13006–13018. doi: 10.1523/JNEUROSCI.3846-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Englot DJ, Mishra AM, Mansuripur PK, et al. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28:9066–9081. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- 11.Wesson DW. Sniffing behavior communicates social hierarchy. Current Biology. 2013;23:575–580. doi: 10.1016/j.cub.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Gummadavelli A, Motelow JE, Smith N, et al. Thalamic stimulation to improve level of consciousness after seizures: evaluation of electrophysiology and behavior. Epilepsia. 2015;56:114–124. doi: 10.1111/epi.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cape EG, Jones BE. Differential modulation of high-frequency gamma-electroencephalogram activity and sleep-wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J Neurosci. 1998;18:2653–2666. doi: 10.1523/JNEUROSCI.18-07-02653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones BE. Modulation of Cortical Activation and Behavioral Arousal by Cholinergic and Orexinergic Systems. Annals of the New York Academy of Sciences. 2008;1129:26–34. doi: 10.1196/annals.1417.026. [DOI] [PubMed] [Google Scholar]
- 15.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.