Abstract
Background
As adult brain structure is primarily established in early life, genetic and environmental exposures in infancy and childhood influence risk for Alzheimer Disease (AD). In this systematic review, we identify several early life risk factors and discuss the evidence and underlying mechanism for each.
Summary
Early risk factors for AD may alter brain anatomy, causing vulnerability to AD-related dementia later in life. In the perinatal period, both genes and learning disabilities have been associated with the development of distinct AD phenotypes. During early childhood, education and intellect as well as body growth may predispose to AD through alterations in cognitive and brain reserve, though the specific mediators of neural injury are disputed. Childhood socioeconomic status may predispose to AD by influencing adult socioeconomic status and cognition. Association of these risk factors with underlying AD pathology (rather than just clinical diagnosis) has not been sufficiently examined.
Key messages
Factors that impede or alter brain growth during early life could render certain brain regions or networks selectively vulnerable to the onset accumulation or spread of AD-related pathology during late-life. Careful life-course epidemiology could provide clues as to why the brain systematically degenerates during AD.
Introduction
Adult brain structure is primarily established in early life1 and is a major determinant of an individual’s susceptibility to Alzheimer Disease (AD)2. By altering both the anatomical (number and connectivity of neurons) and functional (ability to engage alternative brain networks) organization of the adult brain, early life exposures influence the vulnerability of brain regions to AD pathology and the ability of the brain to compensate in the presence of disease3. Because AD is diagnosed clinically long after early life, and the onset of pathology, the links between early exposures, premorbid brain structure, and AD pathology and symptomatology remain unclear.
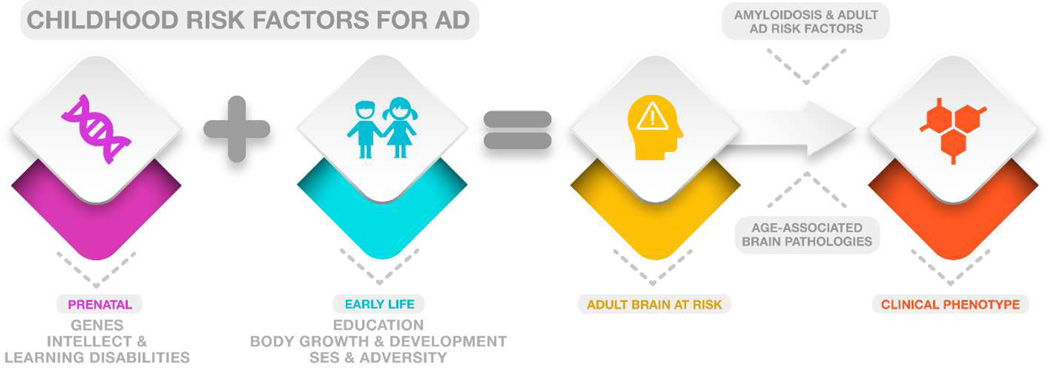
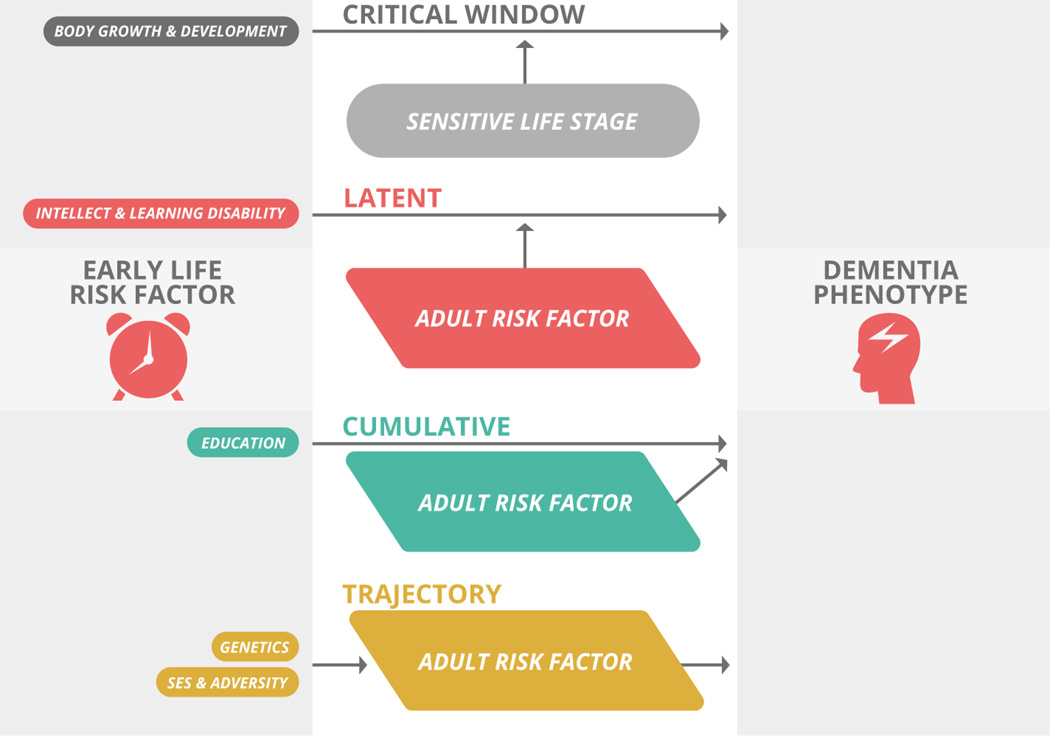
Prior reviews have proposed a developmental basis for AD2, 4–8 and recent studies demonstrate evidence of atypical neurodevelopmental trajectories in people at genetic risk for AD 9, 10 and in people diagnosed with a variant of progressive aphasia (logopenic primary progressive aphasia) associated with AD pathology11. Here, we focus specifically on human epidemiological studies in English that have used incident AD as the primary outcome and excluded studies that used general or all-cause dementia as the outcome measure. Specifically, we discuss the impact of education and intellect, childhood socioeconomic status (SES), body growth, childhood adversity, learning disabilities, and genetics on late-life AD risk, in the context of brain structure and development (Table 2 and Figure 3). We suggest that the mechanisms of these risk factors are best characterized by four models— critical period, latent, cumulative and trajectory 12— that describe the effects of early exposures as timing-specific, clinically unmasked by a stressor later in life, accumulated over time, or determinant of later pathways, respectively (Figure 1). Considered In the context of final achieved adult brain connectivity, these models offer a unified conceptual framework linking early social and biological conditions, premorbid brain structure, and ultimate susceptibility or resilience to AD.
Table 2.
Mechanistic models describing the effects of early life risk factors on ad risk
| Risk Factor | Model | Mechanism |
|---|---|---|
| Childhood SES | Trajectory | Gradual accrual of risk or protection over time |
| Body Growth | Critical Window | Timing-specific risk or protection |
|
Intellect & Learning Disability |
Latent | Initial factor unmasked by a later factor |
| Genetics | Trajectory | Initial factor determines later development |
| Childhood Adversity | Latent | Initial factor unmasked by a later factor |
Figure 3.
Conceptual model of relationship between early-life risk factors and adult AD pathology. In this model, genetically and environmentally influenced childhood brain structure determines adult brain structure and vulnerability. Subsequently, cumulative age-related brain pathologies (including cerebrovascular disease, synucleinopathy and TDP-43) and other adult risk factors influence when and whether neurodegeneration begins in the selectively vulnerable region. Interventions to address childhood risk factors for AD would work by influencing childhood brain development.
Figure 1.
Models of early life risk factors for AD.
We discuss the findings in the context of the hypothesis that genetic and environmental factors that cause atypical brain development may lead to an adult brain that is more susceptible to neurodegenerative pathology in late life. Ultimately, increased awareness of the early life factors associated with risk for AD could lead not only to a better understanding of the causes of AD but also to earlier identification of at-risk individuals and more timely access to future preventative interventions.
Materials & Methods
References for this review were identified by searches of PubMed between March 1970 and October 2014, including references from relevant articles. The following search terms and their synonyms were used to identify the initial list of abstracts: Alzheimer’s Disease, Childhood, Socioeconomic Status, adversity, nutrition, head circumference, stature, arm length, leg length, and learning disability. We searched for articles published in English between January 1st, 1970 and December 31st, 2014. These terms were drawn from previous reviews of the literature on early life epidemiology of cognition2, 8. Of the 2711 abstracts initially identified as potentially relevant, a total of 436 were selected for review of the full article. Of the 436 full articles reviewed for this study, a total of 43 were selected for final inclusion. We only included studies of late-onset Alzheimer’s Disease, as the early onset form has a different molecular pathogenesis and is likely affected by different risk factors. Studies in which the primary outcome included final adult cognition but not Alzheimer’s Disease diagnosis were not included. Studies in which the primary outcome included episodic memory difficulty and/or decline were retained in the final list because the most common cause of episodic memory trouble in late life is Alzheimer’s Disease.
Results
Myriad cross-sectional and longitudinal epidemiological studies were identified linking early life factors to adult brain structure and AD risk, including a few studies in which pathology-supported criteria was used for AD diagnosis. Table 1 lists the characteristics of each study in further detail. Below, we discuss the studies in order of risk factor and classified by the study design.
Table 1.
Cross-sectional and longitudinal epidemiological studies of early life risk factors and Alzheimer’s Disease
| Marker | Ref. | Sampling Method | Study Design | Strengths | Co-variates | Outcome Measure for AD Diagnosis |
Results from fully adjusted model (95% CI) |
Key Limitations |
|---|---|---|---|---|---|---|---|---|
| Genetics | ||||||||
| Cross-Sectional | ||||||||
| White matter myelin water fraction and gray matter volume |
9 | Community- based |
Cross-sectional | APO-E4 carrier and non-carrier groups matched for age, gestational duration, birth weight, sex ratio, maternal age, education, and socioeconomic status. |
Age, gestational duration, birth weight, maternal age and SES |
Positive APOE4 genotype |
Infants carrying APOE4 had lower white matter myelin water fraction and gray matter volume than noncarriers (p<0.05) |
Unclear whether these metrics are also lower in APOE4 carriers after infancy (perhaps a temporary effect only) |
| Standardized achievement tests and R-O complex figure administered to children |
16 | Community- based |
Retrospective cohort |
Analyzed effects of APOE4 status on cognition in children |
Age | Positive APOE4 genotype and positive family history of AD |
Children with both an APOE4 allele and +FH scored significantly lower on reading (p=0.032), language (p=0.044), and the R-O complex figure test (p=0.015) |
Small sample size (n=109) |
| The SORL1 gene and convergent neural risk for Alzheimer’s disease across the human lifespan. |
10 | Community- based |
Cross-sectional | Analyzed white matter microstructure |
Age, APOE4 status, sex |
SORL1 SNP rs689021 |
Lower frontotemporal white matter fractional anisotropy in carriers of the SORL1 SNP (p=0.008) |
Only one metric of white matter integrity used |
| Thickness of left entorhinal cortex in adolescence |
13 | Community- based |
Cross-sectional | Use of specific cortical region for thickness measurements |
Age, sex, race | Positive APOE4 genotype |
APOE4 carriers had thicker left entorhinal cortex (3.79 mm) than non-carriers (3.94 mm) (p=0.03) |
High SES of participants could bias results |
| Right hippocampal volume |
14 | Clinic-based | Cross-sectional | Use of extensive testing to rule out AD symptoms |
Age, sex, education |
Positive APOE4 genotype |
APOE4 carriers had smaller right hippocampi (P=0.09) |
Hippocampal volume measured in adulthood |
| Mitochondrial activity in posterior cingulate cortex |
15 | Population- based |
Case-control | Use of pathological histology for mitochondrial activity |
Age at death and postmortem interval |
Positive APOE4 genotype |
APOE4 carriers had reduced posterior cingulate mitochondria activity (p=0.009) |
None noted. |
| Bilateral hippocampal volume in young adults |
18 | Population- based |
Cross-sectional | Large, population- based sample |
Age, sex, total brain volume |
AD-associated SORL1 SNPs |
Individuals with AD-associated SORL1 SNPs had smaller bilateral hippocampal volumes (p=0.01) |
Relatively homogenous Netherlandish population. |
| Learning Disability | ||||||||
| Cross-Sectional | ||||||||
| Self-report: Family history of learning disability |
27 | Community- based |
Case-control | None | McKhann, Neary and Mesulam criteria including consensus of neurologist and a neuropsychologist. |
16% of PPA (of any type) vs. 6% of behavioral variant, 7% typical AD, 5% controls. |
||
| Self-reported personal history of delay in speaking or reading |
11 | Clinic-based | Consecutive clinicopathological series |
Age, gender, handedness, scanner and total intracranial volume |
Consensus diagnostic criteria for PPA supported by imaging. |
25% of logopenic PPA patients vs. 3% of semantic and 3% of non-fluent PPA. |
||
| Educational and developmental history from neuropsychological evaluation |
30 | Clinic-based | Restrospective case-control |
First to examine connection between LD and atypical dementia |
Age, gender, handedness, education and symptom duration |
Consensus diagnosis of PPA and AD |
Patients with probable learning disability 13 times more likely to be diagnosed with dementia (OR 13.1 95% CI 1.3–128.4) |
Uncertainty of learning disability presence due to self-report of symptoms |
| Longitudinal | ||||||||
| Self-report: Family history of learning disability |
29 | Clinic-based | Case-control | Use of pathological criteria for AD and FTLD |
Not discussed. | Autopsy-based diagnoses |
LD prevalence in PPA was about 50% with no difference between PPA from AD and FTLD. |
Small sample size and only estimated prevalence. |
| Education & Intellect | ||||||||
| Cross-Sectional | ||||||||
| Self-assessed school performance (“below” or “above” average) |
23 |
Population based |
Case-control | Large sample size; adjusted for presence of APOE4 |
Age, gender, race, presence of APOE4 |
NINCDS / ADRDA criteria and DSM III and IV criteria |
Participants with “below average” self-assessed school performance were more likely to have AD (OR 4.0; 1.2–14 95% CI) |
|
| Longitudinal | ||||||||
| Young adult (∼22 years) linguistic ability (idea density and grammatical complexity) |
25 |
Community based |
Longitudinal cohort |
Use of neuropathology to confirm AD pathology Dissociation of idea content and grammar |
Age, education occupation |
AD neuropathology at autopsy |
Nuns with AD had decreased idea density (P<0.001) but not decreased grammatical complexity in early writings (P=0.61) |
Catholic nuns are well-educated and thus may not represent the premorbid mental abilities of the general population |
| Idea density in handwritten autobiographies from 19 to 32 years old |
24 | Community- based |
Longitudinal | Use of childhood written accounts (rather than self- report) and autopsy pathology |
Age at death and location of convent (population of nuns) |
Neurofibrillary tangle and senile plaque count in frontal, temporal, and parietal lobes |
Greater idea density in childhood writing inversely correlated with neurofibrillary tangle and senile plaque count at autopsy (∼-0.5 for tangles and ∼-0.3 for plaques) (p<0.0001 for tangles and p<0.001 for plaques) |
Only used text analysis for idea density and not for other linguistic or writing measures |
| SES | ||||||||
| Cross-Sectional | ||||||||
| Self-reported childhood rural residence |
32 | Community- based |
Case-Control | Randomized sample | Age, gender, education |
NINCDS-ADRDA criteria |
OR 6.5 (2.6 to 16.7) for low education/rural residence vs. high education/urban residence |
Not adjusted for major medical comorbidities (as would be expected in rural and urban populations). Cutoff for “low education” (grade 6 or lower) may be problematic as effect of education was only seen in rural residents. |
| Informant reported mother’s age at subject’s birth, birth order, sibship size, and area of residence before the age of 18 years |
34 | Community- based |
case-control | Stratified by presence of APOE4 Significant linear trend for number of siblings |
Age, gender, education, APOE |
NINCDS/ADRDA or Definite AD by neuropathologic criteria |
OR 1.4 (1.0 to 2.0) for sibship size of five or more; OR 0.5 (0.3 to 0.8) for suburban childhood residence |
Proxies used for both case and control interviews, potentially producing misclassification of information. Greater response rate from cases. |
| Father’s occupation, parents’ age, household size, birth order, sibship size, and home ownership |
33 | Community- based |
Case control | Stratified by presence of APOE4. Use of objective data (census and birth certificates) rather than interviews. |
Age, gender, education, APOE |
NINCDS / ADRDA criteria. |
OR 1.8 (1.2 to 2.7). Strong interaction with APOE4. |
Use of father’s occupation as a surrogate for quality of early home environment is limited (analysis did not include maternal occupation, area of residence, etc). |
| Longitudinal | ||||||||
| Self-reported parental highest years of schooling, paternal occupational prestige, family financial status and cognitive milieu |
35 | Population- based |
Longitudinal Cohort | Longitudinal 5 year follow-up time |
Age, gender, race, education |
Symbol Digit Modalities Test, East Boston Story, MMSE |
No association with cognitive change over time (beta −0.005, p<0.10) |
Self-reported recall of childhood cognitive milieu Even though the follow-up was five years, perhaps a longer period is necessary to show significant decline |
| Self-reported parental education, occupational prestige, sibship size |
36 | Community- based |
Longitudinal clinic- pathologic |
Age, gender, education, county-level SES |
NINCDS / ADRDA criteria. |
No association: RR 1.1 (0.9 to 1.4) for higher household SES composite score. |
||
| Paternal occupation, number of public rooms in childhood home, and number of people in home per sanitation facility |
37 |
Community based |
Cross-sectional and longitudinal |
Analysis of HPC using volumetric MRI |
Mental ability at 11 years old adult SES gender education |
Hippocampal volume from MRI |
Low childhood SES is associated with lower adult hippocampal volume (p=0.032) |
Individuals with higher mental ability at 11 years selectively participated possibly leading to a systematic bias |
| Reading level and early SES |
38 |
Community based |
Prospective cohort |
Adjusted for APOE4 status Use of resilience metric, rather than AD clinical or pathological criteria only |
Age, gender education |
AD pathology at autopsy Cognitive testing for memory Disparity between metrics = “resilience” |
Adult reading level associated with greater resilience (p<0.0001) and accounts for effects of early life SES |
Study population (Caucasian volunteers agreeing to post-mortem examination) may not represent entire aging population |
| Body Growth | ||||||||
| Cross-sectional | ||||||||
| Arm and leg length |
41 | Populatio n-based, |
Case-control | Collected culturally relevant measures of early life environment; MMSE & KDRS scores from t-2 years for 64% of current sample (paired assessment). Observed sex differences in APO-E4 effect: Intrasex group risk difference (inc risk of dementia w e4) for men; intra-sex group risk difference was the opposite for women with e4 providing a protecting factor. Inclusive sampling source; cases and controls screened prior to inclusion. |
Age, gender, education, menarche, menopause |
NINCDS-ADRDA criteria, using two independent teams |
In women only: OR 2.5 (1.6 to 4.0) for 5cm decrease. |
Variable methods for measuring leg length (gold standard?). No assoc of sitting height with dementia. Anthropometric measures were not treated as continuous variables, why? In models adjusted for age, education, female gender, risk of Vas dementia 4x that of men, yet hypertension and diabetes were not associated w risk of dementia in this sample. Report of AD diagnosis: No formal investigation of APO-E4 status, limb length and AD. Reported higher rate of AD than population estimates for East Asia. |
| Intracranial area by CT scan |
43 | Clinic- based |
Retrospective case series |
Gender-specific, brain- imaging (MRI/ CT scans) for confirmation of Probable AD. CT scan suitability determined by location of anatomical structures. Brain size correlated positively with age at first symptom. |
Education, height ethnic group |
Self-reported date of onset of symptoms of AD (diagnosed using NINCDS- ADRDA criteria using consensus panel) |
Correlation 0.48, p 0.009 between head size and age of onset |
Use of brain imaging at diagnosis for proxy of pre-morbid brain size; age at first symptom was imputed with no indication of a statistical measure). No accounting for height, weight, or overall volume with respect to brain size. Aside: Wouldn’t bigger brains just produce more plagues and neurofibrillary tangles?? |
| Head circumference |
52 | Commun ity-based |
Case-control | sampling from multiple cognitive categories for balancing |
Age, gender, education |
NINCDS-ADRDA criteria |
OR 0.9 (0.3 to 1.9) for HC (treated linearly) |
Statistical manipulation of head circumference and unbalanced weighting of categories, renders findings questionable. `Specific population; Japanese-American. Accuracy of head circumference measurements at birth. Head circumference was not a significant predictor of AD for prevalent AD. Incorrect assumption of head circumference as proxy for cognitive reserve. No adjustment for height or weight. Sub-sample analyses among patients diagnosed with probable AD didn’t attenuate the effect of “THC with CASI score” when adjusted for height. |
| Arm length | 42 | Populatio n-based |
cross- sectional |
Population based sample; multi-tier diagnosis of dementia based on cognitive tests and blinded neurological assessments. |
Age, gender, education, smoking, alcohol consumption, pulse pressure, hypertension and diabetes |
NINCDS-ADRDA criteria using consensus between a physician and neurologist; also change in Korean MMSE over three years |
OR 1.2 (1.0 to 1.3) for 1cm decrease in arm length |
None other than weakness of causal or correlative evidence |
| Head circumference |
44 | Populatio n-based |
Case-control | Height, weight, education, APOE |
NINCDS-ADRDA criteria |
OR 2.9 (1.4 to 6.1) for women and 2.3 (0.6 to 9.8) for men for lowest quintile |
||
| Intracranial volume by CT scan |
54 | Clinic- based |
Clinic-based, case-control |
Use of gold-standard measure of premorbid brain size: total intracranial volume |
Gender | NINCDS-ADRDA criteria with consensus |
No significant differences in intracranial volume |
Why is brain size a proxy for cognitive reserve? While women with AD had smaller head size on average in comparison to female controls, controlling for years of education, decreased that difference. The finding of no association between APO-E4, age, birth year and TIV. Even lowest tertiles of TIV not predictive of AD. |
| Intracranial volume by CT scan |
53 | Clinic based |
case-control study |
Matched controls, blinded MRI image analysis, results assessed for inter-tester variability |
Age at scan, gender, familial vs. sporadic AD |
NINCDS-ADRDA criteria |
No significant differences in intracranial volume |
None noted. |
| Prenatal sex hormone exposure (measured through 2D:4D length ratio proxy) |
45 | Clinic- based |
Case-control | Gender-specific; determined that high estrogen:testosterone ratios are a risk factor in men but protective in women. Ability to estimate prenatal hormone exposure |
Age and years of education |
NINCDS-ADRDA criteria (consensus diagnosis) |
AD males had higher 2D:4D ratio (high E:T) than controls (p<0.001) AD females had lower 2D:4D ratios (low E:T) than controls (p<0.001) |
Accuracy of 2D:4D ratio as a proxy for prenatal hormone exposure Could not describe effects of estrogen and testosterone individually |
| Longitudinal | ||||||||
| Height | 47 | Commun ity-based |
Case-control | Longitudinal data on height. |
Age, body mass index, years of childhood lived in Japan, level of education and father’s occupation |
NINCDS-ADRDA criteria using consensus panel (study neurologist and two other dementia experts) |
In men only: Prevalence of AD higher (4.7% vs. 2.9%, p =0.18) in men shorter than 154cm |
Standing height at baseline used as anthropometric measure, with no adjustment for age related changes in height; Japanese heritage vs. multiracial status not addressed. No report on standardization of height for race. |
| Head circumference |
55 | Community- based |
Retrospective cohort |
Apoe4 | Age, education, gender |
NINCDS-ADRDA criteria |
Combination of small head circumference and APOE4 positivity predicted earlier onset of AD (p=0.0007) |
|
| Head circumference |
56 | Community based |
Prospective Cohort study |
Apoe4; enhanced follow-up for subjects with marked cognitive changes (CASI ≤87) |
Head circumference, height, verbal IQ, income, education, age at growth cessation, household characteristics seated BP, anthropometrics |
APOE 4 Hetero- and homozygosity had a differential effect on AD for men vs women: HRheterozygosity men= 1.9 (95% CI 0.7–5.4) vs HRheterozygositywomen= 4.2 (95% CI 2.1–8.6); HRhomozygositymen = 5.3 (95% CI 0.7–41) vs HRhomozygositywomen = 18.3 (95% CI 2.3–144) |
||
| Height | 48 | Community- based, |
Nested case- control |
Blind confirmatory diagnoses of dementia by neurologists + Consensus diagnoses (by a blinded 2nd neurologist) |
Age, gender, education, occupation, and area of birth |
NINCDS-ADRDA criteria |
OR 0.6 (0.4–0.9) for highest quartile vs. lowest quartile |
TICS-m used for initial dementia diagnosis; Healthy volunteer effect (1999 sample overall had lower risks factors) |
| BMI and HOMA- IR |
98 | Clinic-based | Prospective cohort | Use of serum-based biomarkers of AD: |
Age, gender, fasting lipid panel, glucose, and WBC |
Aβ-42 and PSEN1 |
RR: 7.1, p-value= 0.002 for Aβ- 42 |
|
| Arm length | 49 | Population- based |
Longitudinal (prospective cohort) |
Gold standard assessment tools: MRI, Genetic testing MMSE and 3MSE; Representative cohort (across race and age) |
Age, gender, ethnicity, education, income, self-reported health, APOE4 status |
NINCDS-ADRDA criteria with consensus (one neurologist and one psychiatrist) |
HR 1.7 (1.1–2.6) in women vs. 0.9 (0.8–1.0) in men |
Potential misclassification (non- differential/differential?), Conclusion of lower knee height and arm spans associated with increased risk of dementia troublesome bc: phenomenon seen only among women for knee height and when assessed for lowest quartile of knee height, found not significant. However, arm span was significantly associated with dementia (men and women) and AD (women only) No record of assessment of childhood nutritional deficiencies though! |
| Fetal head circumference and adult head circumference |
50 | Community- based |
Prospective case control study |
Comparison of cognitive function at study enrollment and at 3.5 year follow-up |
Age, sex, education, social class at birth, history of cerebrovascular disease, Nottingham Health Profile emotion subscale score, gestational age |
AH4 Intelligence Test, Logical Memory subtest of the Wechsler Memory Scale |
OR for delayed recall 0.3 (0.1 to 0.9) for highest quartile |
Accuracy of childhood head circumference measurements. Categorized adult head circumference and used the lowest quartile as the reference group for effects of the measure on the Logical memory test; once again due to nonstandardization of anthropometric measurements. When comparing furthermore sample size for observed decline in WLM were far to small and unmatched for comparisons. |
| Intracranial volume (ICV) and total brain volume (TBV) from MRI |
51 | Clinic-based | Longitudinal cohort | ICV is better predictor of premorbid brain size than head circumference Use of APOE status |
Age, gender, education APOE genotype, CV disease presence |
NINCDS-ADRDA criteria for AD MMSE, ADAS- cog, and CDR scores for longitudinal follow-up |
Atrophy and APOE4 allele had reduced impact on cognitive and clinical decline in MCI with larger ICV (p<0.05) |
ICV measurements taken after diagnosis (not in childhood or mid- adulthood) ICV is an imperfect approximation of premorbid brain size |
| Childhood Adversity | ||||||||
| Longitudinal | ||||||||
| Early parental death and remarriage of widowed parents |
58 |
Population based |
Prospective cohort |
Use of consensus AD diagnosis Large, population based sample |
Age, gender and education |
NINCDS-ADRDA criteria; consensus diagnosis |
Maternal death from age 11 17 associated with 2x risk of AD |
Adjustment for later SES from parental death was difficult due to missing data |
Genetics
Genes are major determinants of brain development and adult neuroanatomy and modify risk for AD later in life. Many studies have shown that brain network structure and function develop differently during early childhood and adolescence in individuals with genetic risk for AD, particularly carriers of the late-onset risk genes APOE4 and SORL19, 10. Infant carriers of APOE4 develop less white and gray matter volume during the first three years of life than non-carriers in temporal, parietal, and cingulate regions preferentially affected by AD9. Similarly, the left entorhinal cortex is significantly thinner in healthy children and adolescents carrying APOE4 compared to non-carriers13 and symptomatic APOE4 carriers have smaller hippocampi, with differences most pronounced prior to age 6514. In young healthy adults, APOE4 is also associated with abnormal white matter microstructure and with reduced posterior cingulate mitochondrial activity, without differences in soluble or insoluble amyloid15. Remarkably, APOE4 positive children with a family history of AD show impairment on tests of reading and language, suggesting that the early structural changes from APOE4 may cause functional impairment long before AD pathology begins16. Together, these findings indicate that the presence of the APOE4 allele significantly reduces cortical volume and connectivity in a distributed network later affected by AD and may impair cognition throughout the lifespan.
Other genetic elements may also alter brain anatomy and contribute to AD risk. Mutations in the APOE receptor SORL1 have also been linked to late-onset AD and alter neural structure in AD-affected networks17. SORL1 variant children show white matter microstructural abnormalities, and the underexpression of SORL1 mRNA in brain occurs specifically during childhood and adolescence10. Similarly, SORL1 polymorphisms, including the AD-associated SNP rs668387, were also associated with reduced hippocampal volume in a 936 healthy Caucasian young persons aged 18 to 36 years18. Though late-onset AD genes like APOE4 and SORL1 have been the focus of most studies, the early-onset AD genes presenilin 1, presenilin 2, and amyloid precursor protein are also neurodevelopmental genes that may cause similar abnormalities19,20. . Consistent with a trajectory model, AD-associated genetic and epigenetic alterations contribute not only to the molecular pathology of AD but also cause aberrant structural and functional development that may make the brain anatomically susceptible to AD.
Childhood Intellect & Learning Disabilities
Here we discuss studies of innate intellect or learning disabilities, as opposed to educational levels, and late-life AD risk. Higher education has been consistently associated with reduced risk for AD in many cross-sectional and longitudinal studies, and two recent meta-analyses both found that the incidence of AD is inversely proportional to level of education21, 22. Full discussion of this extensively studied topic3 is beyond the scope of this review and thus studies on education and AD risk were not included in the final list of studies, but the mechanisms of the protective effects of education are discussed in the discussion section in the context of childhood SES.
Native intelligence may influence risk for AD. In a population-based, case-control study, participants rated their childhood school performance; “below average” performance was correlated with a fourfold higher incidence of AD23. Similarly, two longitudinal studies of adolescent handwritten memoirs from cloistered nuns found that lower idea density in the memoirs predicted greater neurofibrillary tangle and senile plaque pathology at autopsy and an increased incidence of clinical diagnosis of AD24, 25. There was no association between grammaticality and AD, indicating that the effect of idea density on AD pathology is unrelated to general writing ability developed in school.
For people with atypical neurodevelopmental trajectories, developmental or acquired alterations in the language network may lead to selective vulnerability of the language cortex to neurodegenerative pathology in later life26. Four studies have examined the association of developmental learning disabilities and specific types of dementia, particularly the atypical AD variant of logopenic-type primary progressive aphasia (PPA). Logopenic PPA from AD has the same molecular pathology as hippocampal (“typical”) AD and is affected by the same early life risk factors; we discuss it specifically in this section because a history of learning disability appears to predispose individuals not just to AD pathology but also to the unique anatomical involvement and clinical presentation seen in logopenic PPA from AD.
Cross-sectional Studies
In a study of over 600 subjects from the Northwestern Alzheimer’s Disease Center registry, 16% of the 108 individuals with PPA (of any type) and 32% of their first degree family members answered affirmatively to having a history of learning disability27. These frequencies were significantly higher than those noted in control subjects without AD, subjects with typical amnestic AD and subjects with behavioral variant FTLD. Among the families of PPA probands, remarkable clusters of learning disabilities, particularly developmental dyslexia, were noted.
A second study used a more specific breakdown of the type of PPA, with imaging-supported classification11. Specifically, 8% of all PPA subjects from the University of California San Francisco Memory and Aging Center had self- or informant-based report personal history of delay in speaking or reading at baseline medical interview. This was driven by a particularly high prevalence (25%) in the 48 subjects with logopenic PPA. This finding is important because although logopenic-type PPA is often caused by underlying FTLD pathology, it is frequently associated with AD pathology28. Logopenic PPA subjects with learning disability were younger at onset and showed atrophy in the areas affected by developmental dyslexia (posterior middle and superior temporal gyri).
The authors of the initial study demonstrating higher rates of self-reported LD in people with PPA recently published a follow-up study using pathology-supported diagnoses to attempt to address the question of whether PPA patients reporting prior LD in fact had AD or FTLD pathology. The results showed both AD and FTLD pathology were represented equally in the PPA patients reporting childhood LD, suggesting that the relationship between LD and neurodegenerative disease is not specific to neurodegeneration due to AD.29 One major caveat is that this study used family history as the proxy for LD; although LD does run in families, a family history of LD does not equate with a personal history of LD.
Finally, our group recently compared the frequency of self-reported learning disabilities in 68 typical AD cases, vs. 17 atypical AD cases (Posterior Cortical Atrophy or PCA, Logopenic type PPA, and Dysexecutive AD; we demonstrated a 13-fold higher risk of self-reported LD in the atypical group, after adjusting for demographics and disease severity30. The type of learning disability was different for PPA vs. PCA cases.
In summary, native intellect, including atypical neurodevelopment as seen in learning disabilities, likely predisposes either to AD risk/resilience or to atypical phenotypes of AD. The factor(s) that determine which people with learning disabilities develop atypical dementias remain unknown. However, it is striking that the types of learning disabilities may in fact segregate with subtypes of AD phenotypes; this would be consistent with latent model in which early changes in a specific anatomical region predispose to AD pathology in that region, rather than serving as a systemic risk factor.
Early Life Socioeconomic Status
Several studies have examined the association between early life SES and incidence of late-life AD; markers of early life SES include place of birth, literacy and education, sibship size, birth order, and parental occupation and education. Though multiple studies agree that a lower early-life SES is associated with reduced late-life cognitive ability, evidence for an association with late-life AD is equivocal. In general, SES would be expected to influence late-life AD risk via a social trajectory model as early SES has a major impact on adult health and cognition31.
Cross-sectional Studies
Various cross-sectional studies have reported that early residence, parental occupation, and sibship size increase risk for AD, though these factors interact with education level and the APOE4 genotype. In 2,212 African Americans 65 years of age or older drawn from a community-based prevalence study of AD from 29 census tracts in Indianapolis, the combination of living in a rural residence and having less than six years of schooling had the strongest effect size in increasing risk for AD32. The authors concluded that low education serves as a marker for other factors related to low SES that may increase risk for AD in late-life. In a case control study of over 700 participants of the Genetic Differences in AD study from the University of Washington Alzheimer’s Disease Patient Registry, subjects with a higher sibship size or a father in a manual occupation had a higher risk for AD but the effect was only significant in the presence of APOE433. In a community-based, case control study of over 700 individuals recruited from a health maintenance organization in the Seattle region, area of residence before age 18 years and number of siblings were associated with clinical or pathological diagnosis of AD34.
Longitudinal Studies
In contrast to the cross-sectional studies demonstrating possible links between childhood SES and AD risk, two longitudinal studies with approximately five to six years of follow-up showed associations between early life SES and overall late-life cognitive capacity but not rates of AD dementia35, 36. In a population of Chicago adults, a combined measure of parental education, occupation, and financial status showed a small but significant association with cognitive testing at age 65 or older in a population of Chicago adults35. In a study of Catholic clergy members, the socioeconomic level of the participants’ birth county and household correlated with late-life cognitive function; however, there was no association with the development of AD in either study36.
Early SES may influence late-life cognitive function through its effects on adult brain anatomy and cognition. Individuals with lower childhood SES have smaller hippocampi by volumetric MRI37 and one prospective cohort study demonstrated that higher adult reading level predicted a larger discrepancy between evidence of AD at pathology and late-life cognitive testing and thus greater resilience to AD38. Reading level accounted for the effects of early life SES, implying that the effect of childhood SES on clinical AD may be mediated indirectly through adult cognition.
Together, these studies suggest three conclusions. First, some studies have demonstrated a link between childhood SES and late-onset AD, though other results are inconsistent. Second, APOE4 status and other biological risk factors may be critical determinants of the effects of early SES on AD risk. Finally, early SES has been consistently shown to be associated with cognition, brain anatomy and SES in adulthood; any effects of early SES on later AD may thus be mediated through the adult milieu consistent with a trajectory model39. The potential relationship between early life SES and pathologically defined AD, partly addressed in only one study34, requires further exploration.
Body Growth & Development
Of all the childhood risk factors for AD, early life body growth has been studied the most extensively. Adult anthropomorphic measures of early life body development such as leg length, height, head circumference and digit ratios are markers of early life nutrition influenced by a host of early environmental factors, including hormonal exposure, and have been associated with late life cognitive outcomes including clinical diagnoses of AD. As optimal body and brain development is achieved during specific periods of childhood and adolescence40, the effects of body growth on brain size, cognitive reserve, and ultimate symptomatology of AD are best described with a critical window model.
Cross-sectional Studies Included in the Analysis
Most cross-sectional studies suggest that smaller cranial and body measurements are associated with increased risk for AD. In 746 Koreans aged 65 or over, shorter arm and leg length were independently associated with clinical diagnosis of both vascular dementia and AD, but only in women41; in a similar sample, arm length but not body height was associated with cognitive function and clinical diagnosis of AD dementia in both genders42 In a convenience sample of 28 women with clinical diagnosis of Probable AD attending an outpatient memory disorders clinic in New York, lower intracranial area measured on CT scan in adulthood was associated with earlier age of onset of symptoms43. In a large, ethnically diverse, population-based sample from the Northern Manhattan Aging Project, subjects in the smallest quintile of head circumference had a higher risk for prevalent Probable or Possible AD44. Head circumference was not associated with educational attainment or premorbid cognitive capacity. Interestingly, in a small study of 20 adults diagnosed with AD and 20 controls, digit ratio - a marker of prenatal testosterone and estrogen exposure - was associated with AD risk, with opposite patterns for males vs. females (higher digit ratios in AD males and lower digit ratios in AD females)45. Finally, high childhood BMI and concomitant insulin resistance increases circulating levels of the AD-associated proteins amyloid-beta 42 and presenilin-1, suggesting a molecular link between childhood metabolism, body measurements, and later AD46.
Longitudinal Studies
Results from the longitudinal studies largely agree with those of the cross-sectional studies. Two studies have associated shorter body height with increased incidence of AD: the Honolulu Asia Aging study found that AD was significantly more common in men 61 inches or shorter in over 3000 Japanese men47, and the Israeli Ischemic Heart Disease Study found increased risk of AD in individuals with shorter stature48. Other longitudinal studies have focused on more specific measurements., while in the Cardiovascular Health Cognition Study of Medicare recipients the participants in the lowest quartile of arm span had increased risk of incident clinical AD49. Effects were not mediated by education, income or self-reported health.
Though most of these studies indicate that reduced body measurements may predispose to AD, a few studies either qualify or disagree with these results. In 215 men and women aged 66–75 years from England whose head circumference had been recorded at birth and as adults, people who had a larger head circumference as an adult, but not at birth, were less likely to show decline over a 3.5 year period in immediate and delayed recall on the Logical Memory test50. Although diagnosis of AD was not made in this study, impaired delayed recall is specifically associated with AD. Similarly, a study of 674 subjects with normal cognition, amnestic MCI, or AD found that increased intracranial volume attenuated the impact of cortical atrophy from AD on cognition51. Though body measurements are presumably determined in early life, it is possible that growth in adolescence and young adulthood contributes to adult body measurements and the risk for AD. Finally, three other studies showed no association between intracranial volume measurements and clinical diagnosis or age of onset of AD52–54.
Interactions with APOE genotype, gender and other factors may explain some of these discrepancies (although one study showed no interaction with genotype)44. Among a cohort of 1859 Japanese Americans living in Washington, followed prospectively for six years, developmental risk factors were associated with incident AD in APOE4 positive individuals, whereas vascular risk factors were more important for APOE4 negative individuals55. In 59 incident cases of probable AD identified from 1,869 individuals, the combination of low head circumference and APOE4 4 strongly predicted earlier onset of AD56.
Taken together, a few consistent and interesting findings emerge from the literature of early life body growth and dementia. There appears to be an association between early life markers of childhood body growth (height, arm length, leg length, head circumference) and clinical diagnosis of AD in some studies and the effect of body growth on AD risk likely follows a critical-window model. This association appears partly independent of education but may interact with gender, APOE status and handedness. Importantly, the relationship between early life body growth and pathologically defined AD has not been demonstrated and will be an important area of further research.
Childhood Adversity
Childhood adversity includes abuse and neglect, parental mental illness, substance abuse, criminal behavior, and domestic violence, as well as parental loss and divorce, childhood physical illness, and family economic adversity. Although the negative effect of childhood adversity on adult cognition and brain structure is well-established57, few studies have examined the relationship between exposure to childhood adversity and cognitive decline or dementia due to AD. Currently, only one study relates childhood adversity to clinical or pathological diagnosis of AD. In a population-based study of over four thousand older subjects in Utah, adults with who had suffered maternal death from eleven to seventeen years old were twice as likely to develop AD58. Though it did not study AD specifically, the Israel Heart Disease cohort study similarly demonstrated that the risk for incident dementia (of any type) was increased in individuals who reported experiencing parental death in childhood as compared to parental death in adulthood, particularly for individuals who experienced the event at a younger age in childhood59. In contrast, one study showed different relationships between childhood adversity and cognitive decline in African Americans and Caucasian, over a follow-up of 16 years60. The effects of childhood adversity on AD may follow a latent exposure model: initial sequelae of childhood trauma, such as reduced hippocampal size, would subsequently be exacerbated in the presence of late-life stressors and risk factors. Effects will likely depend upon the particular timing of the event during childhood.
Discussion
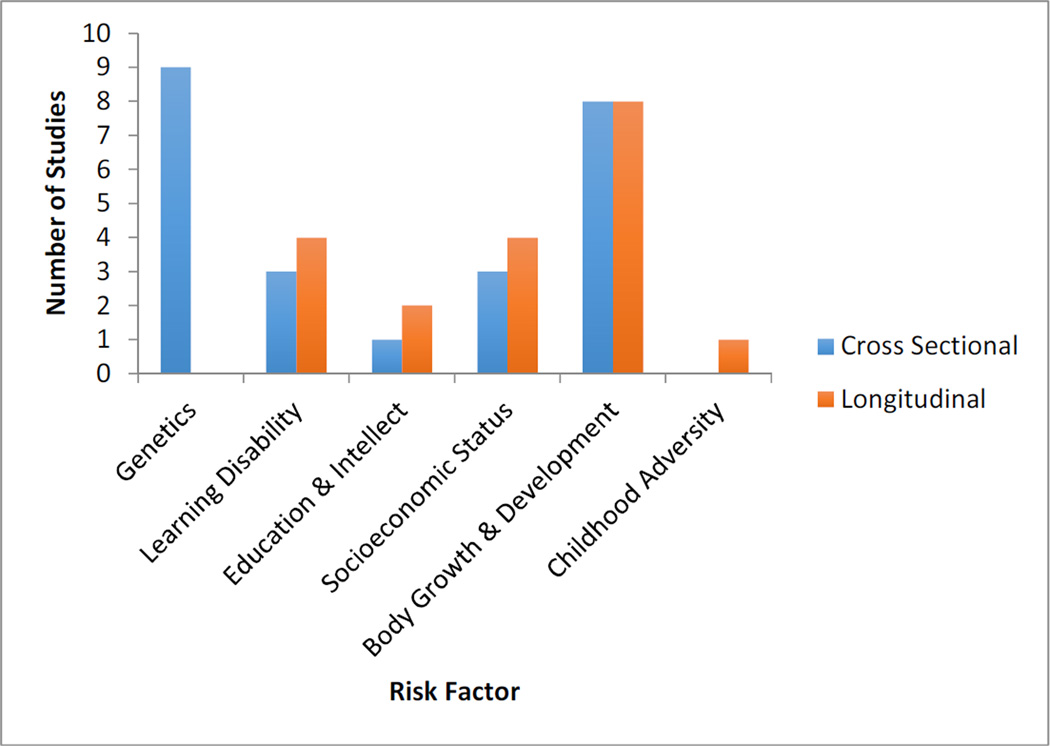
As shown in Figure 3, several early life factors have been associated with increased risk of clinical diagnosis of AD through their impact on adult brain structure. Table 1 shows the number of studies included in this review segregated by risk factor category. Below, we will briefly review the evidence and mechanistic model of risk of each of the early life exposures that are summarized in Table 2.
Genetics may influence AD risk by altering the developmental trajectories and ultimate adult connectivity of functional brain networks. As discussed above, these networks show structural and functional abnormalities as early as childhood, suggesting that their pathological trajectories may influence cognitive health long before the appearance of dementia. In addition, epigenetic modifications may play a significant role in AD pathology; recent studies in humans have identified multiple genes methylated in AD that are linked to PTK2B, a regulator of hippocampal synaptic function91. Consistent with the concept of selective vulnerability, in which the developmental history of a brain region determines the course of its later degeneration, these modified functional networks will be particularly vulnerable to late-life neurodegeneration92.
Developmental learning disabilities and low native intellect may act as latent risks for different phenotypic variants of neurodegenerative disease. Dyslexia is present in 7% of the population87 and is thought to be caused by under-activity in the left temporal and occipital regions during reading and abnormal activation of the left inferior frontal gyrus88,89, 90. In this review, we described three studies linking dyslexia to atypical neurodegenerative disease and proposed that dyslexia anatomically predisposes the brain to an atypical variant of AD that presents as logopenic PPA. It remains to be seen whether other types of LD (dyscalculia, e.g.) influence the anatomical involvement and clinical phenotype of neurodegenerative disease and better imaging methods will be required to examine whether congenitally-affected brain regions in people with specific LDs are indeed the first to be affected in late-life degeneration.
Evidence for an association between early life SES and AD is equivocal, though multiple cross-sectional studies have shown significantly increased AD risk in groups with low childhood SES. Study of SES is particularly challenging because of its close relationship with educational opportunities. Early life SES affects adult cognitive outcomes partly by influencing adult SES and education levels61–66, while unfavorable SES is an independent risk factor for AD67. Biologically, early life SES alters telomere attrition rates and hippocampal volumes37, 68 and low childhood SES is a risk factor for stroke69. In addition, education increases cognitive and brain reserve; higher education has been associated with increased cortical thickness in the temporal lobe70, as well as lower CSF tau concentrations in patients with amnestic MCI71. Importantly, education is partially separable from other risk factors: though education may determine adult SES, low education is an independent risk factor for AD72, 73. The effects of childhood SES are consistent with a trajectory model as SES may determine the later educational, cognitive, and neuroanatomical path of the individual and ultimately lead to the development of AD.
The relationship between body measurements, physiologic variables like brain size and nutrition, and late-life AD are complex. Though this may be due to increased brain reserve81 (and cognitive reserve), other causal mechanisms are likely at work. Small head size and susceptibility to AD may share genetic influences, or small head size may reflect environmental exposures that increase risk of AD independently of brain size. Importantly, the critical window for nutrition is during pregnancy and the first two years of life82 and chronic undernutrition affects about 165 million children worldwide (primarily in Sub-Saharan Africa and South Asia)83. Anthropomorphic measurements of body growth are thus markers of early nutritional status. Neurobiologically, undernutrition can delay the rate of cell division; myelination is especially vulnerable to even moderate undernourishment and the resulting hypomyelination can permanently affect the brain even after nutritional restoration84. Metabolically, IGF-1 levels were recently related to incident AD85 and may be altered by suboptimal nutrition and subsequent insulin resistance86. Further research on the mechanisms and reliability of anthropomorphic measurements as proxies for critical developmental windows on brain reserve and AD risk is necessary.
Effects of childhood adversity on brain and behavior are remarkably timing-specific74, so a critical window model applies best. Exposure to childhood adversity is surprisingly common; 73% of respondents in one study reported exposure to at least one adversity75. One longitudinal study has shown a clear association between childhood adversity and AD, and others have linked adversity and all-cause dementia. The effects of adversity may be mediated by chronic stress, increased vulnerability to neurodegeneration, cellular senescence via shortened telomere length76, 77, or ineffective coping strategies such as tobacco use and alcoholism69, 78.. Exposure to childhood adversities also increases the risk of heart disease, stroke, and diabetes, all of which are risk factors for AD79, 80
Several methodological challenges preclude our ability to draw clear associations between early life factors and AD risk. Almost all studies relied on clinical diagnosis of AD using NINDCDS/NINDS criteria rather than neuropathological diagnosis. These criteria only detect 81% of pathologically confirmed cases of AD and are only 70% specific93 as AD dementia is associated with multiple underlying pathologies including amyloidopathy, cerebrovascular disease, synucleinopathy, and TDP-4394. Also, very few studies used an a priori approach to test mediating adult life mechanisms of early life factors and instead relied on self-report and indirect measures. Few studies were prospective and survival bias was prominent because early life factors may cause selective attrition of weaker individuals. Early life factors may explain the poorly understood phenomenon of phenotypic heterogeneity in AD. The typical, late-onset phenotype of AD begins with amnestic symptoms that correlate with neurofibrillary pathology in lateral entorhinal cortex. Atypical phenotypes of AD (posterior cortical atrophy and logopenic-type primary progressive aphasia), begin with non-amnestic symptoms and neurofibrillary pathology in non-hippocampal structures95. The varying susceptibility of different brain networks to AD pathology in different individuals may relate to the concept of selective vulnerability, which posits that the brain networks that developed least optimally during early life are among the first to degenerate during late life92. Intriguingly, AD preferentially affects the evolutionarily newest regions of the human brain (higher order association cortex)96 and the neurofibrillary neurodegeneration of AD seems to spread in reverse order to that of cortical myelination, beginning in the neurons which are the last to myelinate and the most poorly myelinated97. Because myelination is mostly finished by young adulthood, this may have implications for primary prevention.
There are myriad challenges challenges for future investigation of the relationship between early life factors and AD risk. The prevalence of childhood risk factors, particularly in children at genetic risk for AD, requires more accurate measurement and interactions between exposures and genetic risk for AD remain unclear. Though some mechanisms by which early life factors influence late life cognitive outcomes are partially understood and are discussed above, detailed molecular and anatomic pathophysiology has yet to be described. Timing effects, particularly during critical windows of brain development such as adolescence, and ongoing identification of gene candidates for learning disabilities represent particularly important opportunities for exploration. Finally, more epidemiological studies need to incorporate pathological endpoints as pathology can both confirm the clinical AD diagnosis and reveals much about the anatomical specificity of these myriad early life exposures.
Careful epidemiology, linking well-measured exposures to pathologically defined dementia outcomes, may provide clues as to how the brain systematically degenerates during AD and other dementias. Ideally, a better understanding of the lifelong time-course of AD may lead to preventative interventions delivered during critical life stages; the availability of biomarkers to detect Preclinical AD holds particular promise in this regard. Ultimately, identification of early life, causative risk factors for AD, especially in at-risk individuals such as those with family history of dementia, will help reduce the global burden of disability from dementia.
Figure 2.
Histogram showing number of included studies assessing early life risk factors.
Acknowledgements
Support included NIH/NIA grant 5T32NS007153 as well as the Leon Levy Foundation, the Taub Institute for Research on Alzheimer’s Disease and the Aging Brain, the Henry P. Panasci Fund, and the Charles and Ann Lee Brown Fellowship Fund.
References
- 1.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:63–72. doi: 10.1097/01.wad.0000201854.62116.d7. [DOI] [PubMed] [Google Scholar]
- 3.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet neurology. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiner O, Shmueli A, Sapir T. Neuronal migration and neurodegeneration: 2 sides of the same coin. Cereb Cortex. 2009;19(Suppl 1):42–48. doi: 10.1093/cercor/bhp039. [DOI] [PubMed] [Google Scholar]
- 5.Miller DB, O’Callaghan JP. Do early-life insults contribute to the late-life development of Parkinson and Alzheimer diseases? Metabolism. 2008;57(Suppl 2):44–49. doi: 10.1016/j.metabol.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Zawia NH, Basha MR. Environmental risk factors and the developmental basis for Alzheimer’s disease. Rev Neurosci. 2005;16:325–337. doi: 10.1515/revneuro.2005.16.4.325. [DOI] [PubMed] [Google Scholar]
- 7.Lahiri DK, Zawia NH, Greig NH, Sambamurti K, Maloney B. Early-life events may trigger biochemical pathways for Alzheimer’s disease: the “LEARn” model. Biogerontology. 2008;9:375–379. doi: 10.1007/s10522-008-9162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whalley LJ, Dick FD, McNeill G. A life-course approach to the aetiology of late-onset dementias. Lancet neurology. 2006;5:87–96. doi: 10.1016/S1474-4422(05)70286-6. [DOI] [PubMed] [Google Scholar]
- 9.Dean DC, 3rd, Jerskey BA, Chen K, et al. Brain Differences in Infants at Differential Genetic Risk for Late-Onset Alzheimer Disease: A Cross-sectional Imaging Study. JAMA Neurol. 2013 doi: 10.1001/jamaneurol.2013.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felsky D, Szeszko P, Yu L, et al. The SORL1 gene and convergent neural risk for Alzheimer’s disease across the human lifespan. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller ZA, Mandelli ML, Rankin KP, et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain. 2013;136:3461–3473. doi: 10.1093/brain/awt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Jones RN, Glymour MM. Implications of Lifecourse Epidemiology for Research on Determinants of Adult Disease. Public Health Rev. 2010;32:489–511. doi: 10.1007/BF03391613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw P, Lerch JP, Pruessner JC, et al. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet neurology. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- 14.Lind J, Larsson A, Persson J, et al. Reduced hippocampal volume in non-demented carriers of the apolipoprotein E epsilon4: relation to chronological age and recognition memory. Neurosci Lett. 2006;396:23–27. doi: 10.1016/j.neulet.2005.11.070. [DOI] [PubMed] [Google Scholar]
- 15.Valla J, Yaari R, Wolf AB, et al. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer’s susceptibility gene. J Alzheimers Dis. 2010;22:307–313. doi: 10.3233/JAD-2010-100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloss CS, Delis DC, Salmon DP, Bondi MW. Decreased cognition in children with risk factors for Alzheimer’s disease. Biol Psychiatry. 2008;64:904–906. doi: 10.1016/j.biopsych.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen Y, Miyashita A, Kitamura N, et al. SORL1 is genetically associated with neuropathologically characterized late-onset Alzheimer’s disease. J Alzheimers Dis. 2013;35:387–394. doi: 10.3233/JAD-122395. [DOI] [PubMed] [Google Scholar]
- 18.Bralten J, Arias-Vasquez A, Makkinje R, et al. Association of the Alzheimer’s gene SORL1 with hippocampal volume in young, healthy adults. Am J Psychiatry. 2011;168:1083–1089. doi: 10.1176/appi.ajp.2011.10101509. [DOI] [PubMed] [Google Scholar]
- 19.Kim DH, Yeo SH, Park JM, et al. Genetic markers for diagnosis and pathogenesis of Alzheimer’s disease. Gene. 2014 doi: 10.1016/j.gene.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 20.Louvi A, Sisodia SS, Grove EA. Presenilin 1 in migration and morphogenesis in the central nervous system. Development. 2004;131:3093–3105. doi: 10.1242/dev.01191. [DOI] [PubMed] [Google Scholar]
- 21.Xu W, Tan L, Wang HF, et al. Education and Risk of Dementia: Dose-Response Meta-Analysis of Prospective Cohort Studies. Molecular neurobiology. 2015 doi: 10.1007/s12035-015-9211-5. [DOI] [PubMed] [Google Scholar]
- 22.Meng X, D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One. 2012;7:38268. doi: 10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta KM, Stewart AL, Langa KM, et al. “Below average” self-assessed school performance and Alzheimer’s disease in the Aging, Demographics, and Memory Study. Alzheimers Dement. 2009;5:380–387. doi: 10.1016/j.jalz.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snowdon DA, Greiner LH, Markesbery WR. Linguistic ability in early life, the neuropathology of Alzheimer’s disease, cerebrovascular disease Findings from the Nun Study. Ann N Y Acad Sci. 2000;903:34–38. doi: 10.1111/j.1749-6632.2000.tb06347.x. [DOI] [PubMed] [Google Scholar]
- 25.Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life, cognitive function Alzheimer’s disease in late life Findings from the Nun Study. Jama. 1996;275:528–532. [PubMed] [Google Scholar]
- 26.Rogalski E, Weintraub S, Mesulam MM. Are there susceptibility factors for primary progressive aphasia? Brain Lang. 2013;127:135–138. doi: 10.1016/j.bandl.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogalski E, Johnson N, Weintraub S, Mesulam M. Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives. Arch Neurol. 2008;65:244–248. doi: 10.1001/archneurol.2007.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sajjadi SA, Patterson K, Nestor PJ. Logopenic, mixed, or Alzheimer-related aphasia? Neurology. 2014;82:1127–1131. doi: 10.1212/WNL.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 29.Rogalski EJ, Rademaker A, Wieneke C, Bigio EH, Weintraub S, Mesulam MM. Association between the prevalence of learning disabilities and primary progressive aphasia. JAMA Neurol. 2014;71:1576–1577. doi: 10.1001/jamaneurol.2014.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seifan AAS, Huey ED, Mez J, Tsapanou A, Caccappolo E. Childhood Learning Disabilities and Atypical Dementia: A Retrospective Chart Review. PLoS One. 2015 doi: 10.1371/journal.pone.0129919. Accepted for Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Ann N Y Acad Sci. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- 32.Hall KS, Gao S, Unverzagt FW, Hendrie HC. Low education and childhood rural residence: risk for Alzheimer’s disease in African Americans. Neurology. 2000;54:95–99. doi: 10.1212/wnl.54.1.95. [DOI] [PubMed] [Google Scholar]
- 33.Moceri VM, Kukull WA, Emanual I, et al. Using census data and birth certificates to reconstruct the early-life socioeconomic environment and the relation to the development of Alzheimer’s disease. Epidemiology. 2001;12:383–389. doi: 10.1097/00001648-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Moceri VM, Kukull WA, Emanuel I, van Belle G, Larson EB. Early-life risk factors and the development of Alzheimer’s disease. Neurology. 2000;54:415–420. doi: 10.1212/wnl.54.2.415. [DOI] [PubMed] [Google Scholar]
- 35.Everson-Rose SA, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Early life conditions and cognitive functioning in later life. Am J Epidemiol. 2003;158:1083–1089. doi: 10.1093/aje/kwg263. [DOI] [PubMed] [Google Scholar]
- 36.Wilson RS, Scherr PA, Hoganson G, Bienias JL, Evans DA, Bennett DA. Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiology. 2005;25:8–14. doi: 10.1159/000085307. [DOI] [PubMed] [Google Scholar]
- 37.Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: childhood socioeconomic status influences adult hippocampal size. Ann Neurol. 2012;71:653–660. doi: 10.1002/ana.22631. [DOI] [PubMed] [Google Scholar]
- 38.Negash S, Wilson RS, Leurgans SE, et al. Resilient brain aging: characterization of discordance between Alzheimer’s disease pathology and cognition. Curr Alzheimer Res. 2013;10:844–851. doi: 10.2174/15672050113109990157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brewster PW, Melrose RJ, Marquine MJ, et al. Life experience and demographic influences on cognitive function in older adults. Neuropsychology. 2014;28:846–858. doi: 10.1037/neu0000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wadsworth ME, Hardy RJ, Paul AA, Marshall SF, Cole TJ. Leg and trunk length at 43 years in relation to childhood health, diet and family circumstances; evidence from the 1946 national birth cohort. Int J Epidemiol. 2002;31:383–390. [PubMed] [Google Scholar]
- 41.Kim JM, Stewart R, Shin IS, Yoon JS. Limb length and dementia in an older Korean population. J Neurol Neurosurg Psychiatry. 2003;74:427–432. doi: 10.1136/jnnp.74.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeong SK, Kim JM, Kweon SS, Shin MH, Seo MW, Kim YH. Does arm length indicate cognitive and functional reserve? Int J Geriatr Psychiatry. 2005;20:406–412. doi: 10.1002/gps.1295. [DOI] [PubMed] [Google Scholar]
- 43.Schofield PW, Mosesson RE, Stern Y, Mayeux R. The age at onset of Alzheimer’s disease, an intracranial area measurement A relationship. Arch Neurol. 1995;52:95–98. doi: 10.1001/archneur.1995.00540250103019. [DOI] [PubMed] [Google Scholar]
- 44.Schofield PW, Logroscino G, Andrews HF, Albert S, Stern Y. An association between head circumference and Alzheimer’s disease in a population-based study of aging and dementia. Neurology. 1997;49:30–37. doi: 10.1212/wnl.49.1.30. [DOI] [PubMed] [Google Scholar]
- 45.Vladeanu M, Giuffrida O, Bourne VJ. Prenatal sex hormone exposure and risk of Alzheimer disease: a pilot study using the 2D:4D digit length ratio. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology. 2014;27:102–106. doi: 10.1097/WNN.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 46.Luciano R, Barraco GM, Muraca M, et al. Biomarkers of Alzheimer disease, insulin resistance, and obesity in childhood. Pediatrics. 2015;135:1074–1081. doi: 10.1542/peds.2014-2391. [DOI] [PubMed] [Google Scholar]
- 47.Abbott RD, White LR, Ross GW, et al. Height as a marker of childhood development and late-life cognitive function: the Honolulu-Asia Aging Study. Pediatrics. 1998;102:602–609. doi: 10.1542/peds.102.3.602. [DOI] [PubMed] [Google Scholar]
- 48.Beeri MS, Davidson M, Silverman JM, Noy S, Schmeidler J, Goldbourt U. Relationship between body height and dementia. Am J Geriatr Psychiatry. 2005;13:116–123. doi: 10.1176/appi.ajgp.13.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang TL, Carlson MC, Fitzpatrick AL, Kuller LH, Fried LP, Zandi PP. Knee height and arm span: a reflection of early life environment and risk of dementia. Neurology. 2008;70:1818–1826. doi: 10.1212/01.wnl.0000311444.20490.98. [DOI] [PubMed] [Google Scholar]
- 50.Gale CR, Walton S, Martyn CN. Foetal and postnatal head growth and risk of cognitive decline in old age. Brain. 2003;126:2273–2278. doi: 10.1093/brain/awg225. [DOI] [PubMed] [Google Scholar]
- 51.Guo LH, Alexopoulos P, Wagenpfeil S, Kurz A, Perneczky R. Brain size and the compensation of Alzheimer’s disease symptoms: a longitudinal cohort study. Alzheimers Dement. 2013;9:580–586. doi: 10.1016/j.jalz.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Graves AB, Mortimer JA, Larson EB, Wenzlow A, Bowen JD, McCormick WC. Head circumference as a measure of cognitive reserve Association with severity of impairment in Alzheimer’s disease. Br J Psychiatry. 1996;169:86–92. doi: 10.1192/bjp.169.1.86. [DOI] [PubMed] [Google Scholar]
- 53.Jenkins R, Fox NC, Rossor AM, Harvey RJ, Rossor MN. Intracranial volume and Alzheimer disease: evidence against the cerebral reserve hypothesis. Arch Neurol. 2000;57:220–224. doi: 10.1001/archneur.57.2.220. [DOI] [PubMed] [Google Scholar]
- 54.Edland SD, Xu Y, Plevak M, et al. Total intracranial volume: normative values and lack of association with Alzheimer’s disease. Neurology. 2002;59:272–274. doi: 10.1212/wnl.59.2.272. [DOI] [PubMed] [Google Scholar]
- 55.Borenstein Graves A, Mortimer JA, Bowen JD, et al. Head circumference and incident Alzheimer’s disease: modification by apolipoprotein E. Neurology. 2001;57:1453–1460. doi: 10.1212/wnl.57.8.1453. [DOI] [PubMed] [Google Scholar]
- 56.Borenstein AR, Wu Y, Mortimer JA, et al. Developmental and vascular risk factors for Alzheimer’s disease. Neurobiol Aging. 2005;26:325–334. doi: 10.1016/j.neurobiolaging.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Norton MC, Smith KR, Ostbye T, et al. Early parental death and remarriage of widowed parents as risk factors for Alzheimer disease: the Cache County study. Am J Geriatr Psychiatry. 2011;19:814–824. doi: 10.1097/JGP.0b013e3182011b38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ravona-Springer R, Beeri MS, Goldbourt U. Younger age at crisis following parental death in male children and adolescents is associated with higher risk for dementia at old age. Alzheimer Dis Assoc Disord. 2012;26:68–73. doi: 10.1097/WAD.0b013e3182191f86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnes LL, Wilson RS, Everson-Rose SA, Hayward MD, Evans DA, Mendes de Leon CF. Effects of early-life adversity on cognitive decline in older African Americans and whites. Neurology. 2012;79:2321–2327. doi: 10.1212/WNL.0b013e318278b607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fors S, Lennartsson C, Lundberg O. Childhood living conditions, socioeconomic position in adulthood, and cognition in later life: exploring the associations. J Gerontol B Psychol Sci Soc Sci. 2009;64:750–757. doi: 10.1093/geronb/gbp029. [DOI] [PubMed] [Google Scholar]
- 62.Kobrosly RW, van Wijngaarden E, Galea S, et al. Socioeconomic position and cognitive function in the Seychelles: a life course analysis. Neuroepidemiology. 2011;36:162–168. doi: 10.1159/000325779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh-Manoux A, Richards M, Marmot M. Socioeconomic position across the lifecourse: how does it relate to cognitive function in mid-life? Ann Epidemiol. 2005;15:572–578. doi: 10.1016/j.annepidem.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 64.Scazufca M, Menezes PR, Araya R, et al. Risk factors across the life course and dementia in a Brazilian population: results from the Sao Paulo Ageing & Health Study (SPAH) Int J Epidemiol. 2008;37:879–890. doi: 10.1093/ije/dyn125. [DOI] [PubMed] [Google Scholar]
- 65.Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemiol. 2001;30:256–263. doi: 10.1093/ije/30.2.256. [DOI] [PubMed] [Google Scholar]
- 66.Luo Y, Waite LJ. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. J Gerontol B Psychol Sci Soc Sci. 2005;60:S93–S101. doi: 10.1093/geronb/60.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sattler C, Toro P, Schonknecht P, Schroder J. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Res. 2012;196:90–95. doi: 10.1016/j.psychres.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 68.Cherkas LF, Aviv A, Valdes AM, et al. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 69.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 70.Liu Y, Julkunen V, Paajanen T, et al. Education increases reserve against Alzheimer’s disease--evidence from structural MRI analysis. Neuroradiology. 2012;54:929–938. doi: 10.1007/s00234-012-1005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rolstad S, Nordlund A, Eckerstrom C, et al. High education may offer protection against tauopathy in patients with mild cognitive impairment. J Alzheimers Dis. 2010;21:221–228. doi: 10.3233/JAD-2010-091012. [DOI] [PubMed] [Google Scholar]
- 72.Evans DA, Hebert LE, Beckett LA, et al. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol. 1997;54:1399–1405. doi: 10.1001/archneur.1997.00550230066019. [DOI] [PubMed] [Google Scholar]
- 73.Karp A, Kareholt I, Qiu C, Bellander T, Winblad B, Fratiglioni L. Relation of education and occupation-based socioeconomic status to incident Alzheimer’s disease. Am J Epidemiol. 2004;159:175–183. doi: 10.1093/aje/kwh018. [DOI] [PubMed] [Google Scholar]
- 74.Maercker A, Michael T, Fehm L, Becker ES, Margraf J. Age of traumatisation as a predictor of post-traumatic stress disorder or major depression in young women. Br J Psychiatry. 2004;184:482–487. doi: 10.1192/bjp.184.6.482. [DOI] [PubMed] [Google Scholar]
- 75.McGurn B, Deary IJ, Starr JM. Childhood cognitive ability and risk of late-onset Alzheimer and vascular dementia. Neurology. 2008;71:1051–1056. doi: 10.1212/01.wnl.0000319692.20283.10. [DOI] [PubMed] [Google Scholar]
- 76.Kume K, Kikukawa M, Hanyu H, et al. Telomere length shortening in patients with dementia with Lewy bodies. Eur J Neurol. 2012;19:905–910. doi: 10.1111/j.1468-1331.2011.03655.x. [DOI] [PubMed] [Google Scholar]
- 77.Honig LS, Kang MS, Schupf N, Lee JH, Mayeux R. Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch Neurol. 2012;69:1332–1339. doi: 10.1001/archneurol.2012.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kananen L, Surakka I, Pirkola S, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One. 2010;5:10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scott KM, Von Korff M, Angermeyer MC, et al. Association of childhood adversities and early-onset mental disorders with adult-onset chronic physical conditions. Arch Gen Psychiatry. 2011;68:838–844. doi: 10.1001/archgenpsychiatry.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med. 2004;34:509–520. doi: 10.1017/s003329170300134x. [DOI] [PubMed] [Google Scholar]
- 81.Rushton JP, Ankney CD. Brain size and cognitive ability: Correlations with age, sex, social class, and race. Psychon Bull Rev. 1996;3:21–36. doi: 10.3758/BF03210739. [DOI] [PubMed] [Google Scholar]
- 82.Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125:473–480. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- 83.UNICEF. Improving child nutrition: the achievable imperative for global progress. 2013 [Google Scholar]
- 84.Royland J, Klinkhachorn P, Konat G, Wiggins RC. How much undernourishment is required to retard brain myelin development. Neurochem Int. 1992;21:269–274. doi: 10.1016/0197-0186(92)90157-m. [DOI] [PubMed] [Google Scholar]
- 85.Westwood AJ, Beiser A, Decarli C, et al. Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology. 2014;82:1613–1619. doi: 10.1212/WNL.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clark GM, Zamenhof S, Van Mathens E, Grauel L, Kruger L. The effect of prenatal malnutrition on dimensions of cerebral cortex. Brain Res. 1973;54:397–402. doi: 10.1016/0006-8993(73)90068-1. [DOI] [PubMed] [Google Scholar]
- 87.Peterson RL, Pennington BF. Developmental dyslexia. Lancet. 2012;379:1997–2007. doi: 10.1016/S0140-6736(12)60198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paulesu E, Demonet JF, Fazio F, et al. Dyslexia: cultural diversity and biological unity. Science. 2001;291:2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- 89.Guttorm TK, Leppanen PH, Tolvanen A, Lyytinen H. Event-related potentials in newborns with and without familial risk for dyslexia: principal component analysis reveals differences between the groups. J Neural Transm. 2003;110:1059–1074. doi: 10.1007/s00702-003-0014-x. [DOI] [PubMed] [Google Scholar]
- 90.Raschle NM, Chang M, Gaab N. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage. 2011;57:742–749. doi: 10.1016/j.neuroimage.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lord J, Cruchaga C. The epigenetic landscape of Alzheimer’s disease. Nature neuroscience. 2014;17:1138–1140. doi: 10.1038/nn.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mesulam MM. Primary progressive aphasia, the language network: the 2013 HHouston Merritt Lecture. Neurology. 2013;81:456–462. doi: 10.1212/WNL.0b013e31829d87df. [DOI] [PubMed] [Google Scholar]
- 93.Blacker D, Albert MS, Bassett SS, Go RC, Harrell LE, Folstein MF. Reliability validity of NINCDS-ADRDA criteria for Alzheimer’s disease The National Institute of Mental Health Genetics Initiative. Arch Neurol. 1994;51:1198–1204. doi: 10.1001/archneur.1994.00540240042014. [DOI] [PubMed] [Google Scholar]
- 94.Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol. 2014 doi: 10.1007/s00401-014-1269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Janocko NJ, Brodersen KA, Soto-Ortolaza AI, et al. Neuropathologically defined subtypes of Alzheimer’s disease differ significantly from neurofibrillary tangle-predominant dementia. Acta Neuropathol. 2012;124:681–692. doi: 10.1007/s00401-012-1044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Braak H, Del Tredici K. Evolutional aspects of Alzheimer’s disease pathogenesis. J Alzheimers Dis. 2013;33(Suppl 1):155–161. doi: 10.3233/JAD-2012-129029. [DOI] [PubMed] [Google Scholar]
- 97.Braak H, Rub U, Schultz C, Del Tredici K. Vulnerability of cortical neurons to Alzheimer’s and Parkinson’s diseases. J Alzheimers Dis. 2006;9:35–44. doi: 10.3233/jad-2006-9s305. [DOI] [PubMed] [Google Scholar]
- 98.Luciano R, Barraco GM, Muraca M, et al. Biomarkers of Alzheimer Disease, Insulin Resistance, and Obesity in Childhood. Pediatrics. 2015 doi: 10.1542/peds.2014-2391. [DOI] [PubMed] [Google Scholar]