Abstract
Sigma-1 receptor (Sig-1R) is an intracellular chaperone protein with many ligands, located at the endoplasmic reticulum. Binding of cocaine to Sig-1R has previously been found to modulate endothelial functions. In the present study, we show that cocaine dramatically inhibits store-operated Ca2+ entry (SOCE), a Ca2+ influx mechanism promoted by depletion of intracellular Ca2+ stores, in rat brain microvascular endothelial cells. Using either Sig-1R shRNA or pharmacological inhibition with the unrelated Sig-1R antagonists BD-1063 and NE-100, we show that cocaine-induced SOCE inhibition is dependent on Sig-1R. In addition to revealing new insight into fundamental mechanisms of cocaine-induced changes in endothelial function, these studies provide an unprecedented role for Sig-1R as a SOCE inhibitor.
Keywords: sigma-1 receptor, endothelial cell, calcium, calcium imaging, endoplasmic reticulum, store-operated calcium entry
Introduction
Sigma-1 receptors (Sig-1Rs) are chaperone proteins residing at the interface between endoplasmic reticulum (ER) and mitochondria [1, 2]; upon stimulation, they translocate to other cell regions [3, 4]. Sig-1Rs interact with various targets, including ion channels, G protein-coupled receptors, tyrosine kinase receptors, and kinases, to regulate cellular functions [3, 5], Stimulating factors include Sig-1R agonists and ER stress [2]. Moreover, activated Sig-1Rs typically associate with inositol 1,4,5-trisphosphate receptor type 3, favoring an increase in cytosolic Ca2+ concentration [2, 6].
Store-operated Ca2+ entry (SOCE) [7, 8] is an important mechanism for Ca2+ influx, described in a variety of cells, including the endothelium [8, 9]. Physiologically, SOCE is activated by depletion of intracellular Ca2+ pools following stimulation of plasma membrane receptors that couple to phosphoinositide hydrolysis and inositol 1,4,5-trisphosphate generation. These decreases in ER Ca2+ content are sensed by the ER Ca2+ sensor, stromal interaction molecule 1 (STIM1) [8, 10], resulting in activation of Ca2+ channels in the plasma membrane termed Orai1, also known as Ca2+ release-activated Ca2+ channels [8, 11].
Since cocaine is a Sig-1R agonist [12] and cocaine-induced activation of Sig-1Rs was reported to modulate the function of brain microvascular endothelium [13, 14], we examined the Sig-1R-mediated effect of cocaine on SOCE in rat brain microvascular endothelial cells (RBMVEC).
Experimental procedures
Chemicals
All chemicals were from Sigma Aldrich (St. Louis, MO), unless otherwise mentioned. Cocaine hydrochloride was generously supplied by NIDA; NE-100 hydrochloride was from Santa Cruz Biotechnology (Dallas, TX).
Cell Culture
RBMVEC from Cell Applications, Inc. (San Diego, CA, USA) were cultured in rat brain endothelial basal medium and rat brain endothelial growth supplement, according to the manufacturer's instructions (Cell Applications, Inc.). Cells were grown in T75 flasks coated with attachment factor (Cell Applications, Inc.) until 80% confluent. Cells were plated on round coverslips of 25 mm diameter coated with human fibronectin (Discovery Labware, Bedford, MA, USA) for measurements of cytosolic Ca2+ and membrane potential and on six-well plates for western blot analysis.
Cell transfections
RBMVEC were transfected 24 hours after plating with four unique 29mer shRNA constructs Oprs1 (Sig-1R) rat, with the following sequences: TF711128A (GCCATTCGGGACGATACTGGGCTGAGATT), TF711128B (ATCATCTCTGGCACTTTCCACCAGTGGAG), TF711128C (CCTGTTTCTGACTATTGTGGCGGTGCTGA), TF711128D (TTGCACGCCTCGCTGTCTGAGTACGTGCT), in pRFP-C-RS vector or scrambled negative control non-effective shRNA in pRFP-C-RS (OriGene Technologies Rockville, MD), using Cytofect endothelial cell transfection kit (Cell Applications, Inc.). Throughout the manuscript, the Sig-1R shRNA constructs were abbreviated Sig-1R shRNA (A), Sig-1R shRNA (B), Sig-1R shRNA (C) and Sig-1R shRNA (D).
Western blot analysis
Whole-cell lysates were separated on Mini-PROTEAN TGX 4–20% gels (Bio-Rad, Hercules, CA, USA) by sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by immunoblotting. Proteins were transferred to an Odyssey nitrocellulose membrane (Li-Cor Biosciences, Lincoln, NE, USA). After blocking with Odyssey blocking buffer, the membranes were incubated overnight with primary antibody against Sigma-1R (OPRS1, rabbit polyclonal, 1:1000, OriGene Technologies, Rockville, MD). An antibody against β-actin (mouse monoclonal, 1: 10,000; Sigma-Aldrich) was used to confirm equal protein loading. Membranes were washed with Tris-buffered saline-Tween 20 (TBST) and incubated with the secondary antibodies: IRDye 800CW conjugated goat anti-rabbit IgG, and IRDye 680 conjugated goat anti-mouse IgG (1 : 10,000, 1 h at 21°C). Membranes were then washed in TBST and scanned using an Li-Cor Odyssey Infrared Imager and analyzed using Odyssey software.
Cytosolic Ca2+ measurement
Intracellular Ca2+ levels were evaluated as previously described [15, 16]. Briefly, cells were incubated with 5 µM fura-2 AM (Molecular Probes, Eugene, OR, USA) in Hanks' balanced salt solution at 21 °C for 1 h and washed with dye-free Hanks' balanced salt solution. Coverslips were mounted in an open bath chamber (QR-40LP; Warner Instruments, Hamden, CT, USA) on the stage of an inverted microscope Nikon Eclipse TiE (Nikon Inc., Melville, NY, USA), equipped with a Perfect Focus System and a Photometrics CoolSnap HQ2 charge-coupled device camera (Photometrics, Tucson, AZ, USA). Ca2+ levels were determined ratiometrically using Fura-2 AM fluorescence (emission 510 nm), following alternate excitation at 340 and 380 nm. This was acquired at a frequency of 0.25 Hz, and the relative 340nm/380nm fluorescence ratio further plotted as a function of time. Images were acquired/analyzed using NIS-Elements AR software (Nikon, Inc.).
Measurement of membrane potential
The relative changes in membrane potential of single neurons were evaluated using bis-(l,3-dibutylbarbituric acid) trimethine oxonol, DiBAC4(3) (Invitrogen), a slow response voltage-sensitive dye, as previously described [15, 16]. Upon membrane hyperpolarization, the dye concentrates in the cell membrane, leading to a decrease in fluorescence intensity, while depolarization induces the sequestration of the dye into the cytosol, resulting in an increase of the fluorescence intensity. RBMVEC were incubated for 30 min in HBSS containing 0.5 mM DiBAC4(3) and the fluorescence monitored at 0.17 Hz, excitation/emission: 480 nm/540 nm. Calibration of DiBAC4(3) fluorescence following background subtraction was performed using the Na+-K+ ionophore gramicidin in Na+-free physiological solution and various concentrations of K+ (to alter membrane potential) and N-methylglucamine (to maintain osmolarity). Under these conditions, the membrane potential was approximately equal to the K+ equilibrium potential determined by the Nernst equation. The intracellular K+ and Na+ concentration were assumed to be 130 mM and 10 mM, respectively.
Statistical analysis
Data were expressed as mean ± standard error of mean (SEM). One-way ANOVA followed by post hoc analysis using Bonferroni and Tukey tests were used to evaluate significant differences between groups; P < 0.05 was considered statistically significant.
Results and discussion
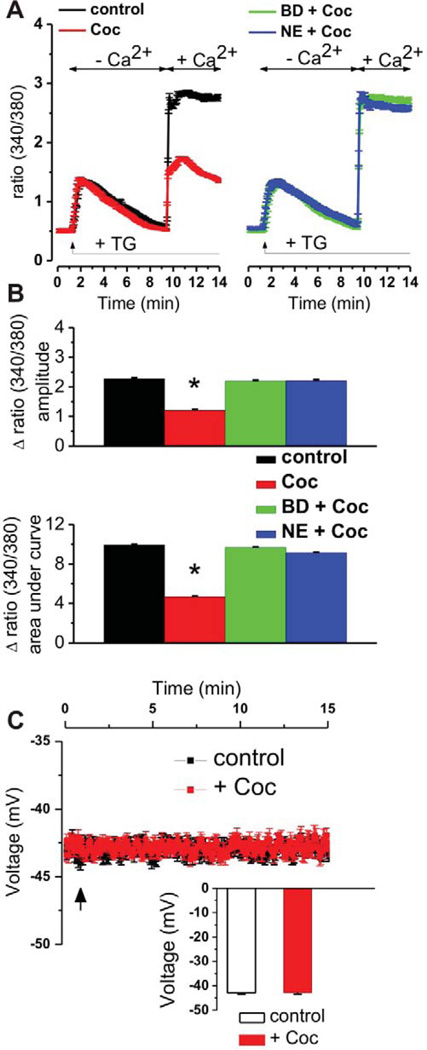
To determine the effect of cocaine on stored-operated Ca2+ entry, we used a classical protocol to induce SOCE. Briefly, ER Ca2+ stores were depleted in Fura-2-loaded cells using the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase inhibitor thapsigargin (1 µM) in the absence of extracellular Ca2+ (Fig 1A). No differences in the resultant rise in cytosolic Ca2+ were observed in the presence or absence of cocaine (3 µM, 15 min) indicating no effect of cocaine on ER Ca2+ content. SOCE was then stimulated by the addition of Ca2+ (1 mM). Surprisingly, a ~50% decrease in SOCE was observed in cocaine-pretreated cells (Fig 1A, B).
Figure 1. Cocaine inhibits SOCE in brain microvascular endothelial cells: pharmacological evidence of Sig-1R involvement.
A, Averaged traces of fura-2 fluorescence ratio (F340/F380) indicating the modulation of store-operated Ca2+ entry by pretreatment with cocaine (Coc, 3 µM, 15 min, n = 64 cells) alone, cocaine in the presence of Sig-R antagonist BD-1063 (BD, 10 µM, 20 min, n = 68 cells) or NE-100 (NE, 3 µM, 20 min, n = 57 cells) in RBMVEC cells. Cocaine reduced the SOCE amplitude as compared with control RBMVEC (n = 61 cells); the reduction was prevented by treatment with Sig-1R antagonists. B, Comparison of the amplitudes and areas under curve of SOCE elicited by cocaine in each of the conditions mentioned before. *P < 0.05 as compared with control. C, Cocaine does not change the resting membrane potential (RMP) of RBMVEC: averaged traces and comparison of medium RMP of cocaine-treated (n = 63 cells) and control, untreated cells (n = 47 cells); the arrow indicates the moment of cocaine application.
Since cocaine is a Sig-1R agonist [12], we assessed the possibility that Sig-1R mediates cocaine-induced SOCE inhibition. Pretreatment with the selective Sig-1R antagonists BD-1063 (10 µM, 20 min) [17] or NE-100 (3 µM, 20 min) [18, 19], completely eliminated cocaine-induced SOCE inhibition (Fig 1A, B).
To exclude any bias due to potential effects of cocaine on membrane potential, we examined the effect of cocaine on membrane potential in RBMVEC loaded with a voltage-sensitive dye, DiBAC4(3) [15]. We observed no changes in membrane potential in response to cocaine application (Fig. 1C).
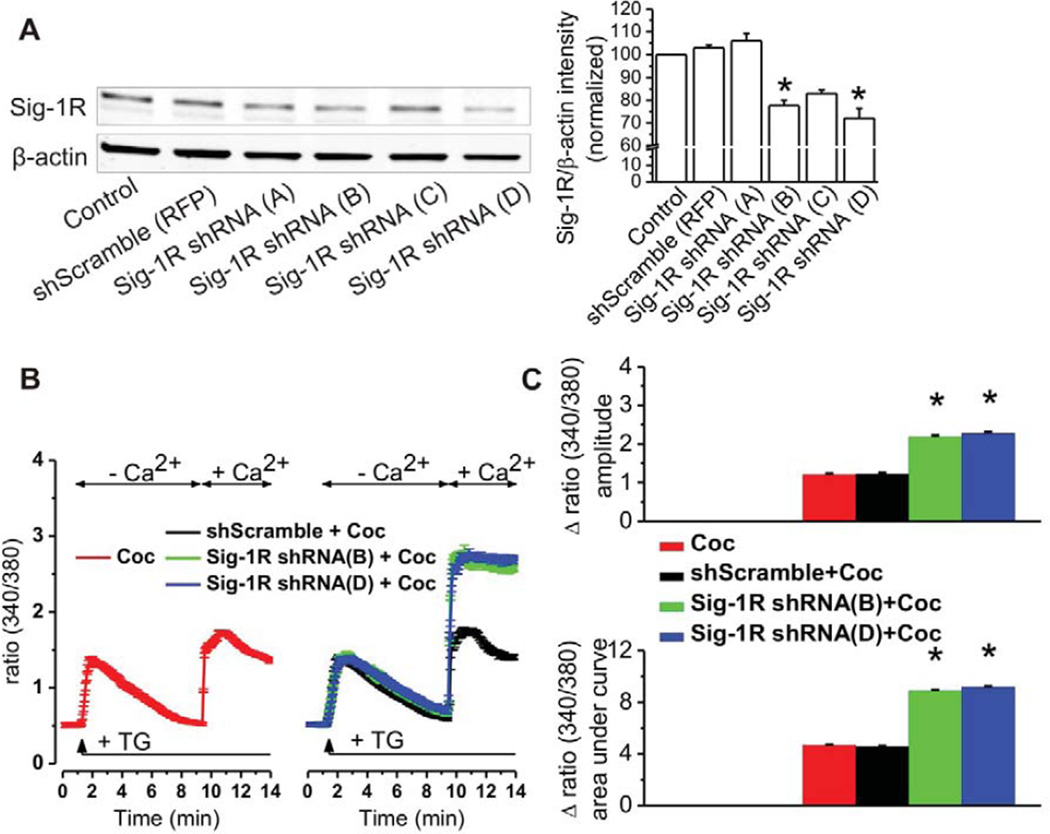
Finally, RBMVECs were transfected with four different Sig-1R shRNA constructs. Western analysis revealed that Sig-1R expression was decreased by 22.5 ± 2.5% (n = 3 independent experiments) after transfection with Sig-1R shRNA (B) (ATCATCTCTGGCACTTTCCACCAGTGGAG) and 28 ± 4.3% (n = 3) after transfection with Sig-1R shRNA (D) (TTGCACGCCTCGCTGTCTGAGTACGTGCT). Sig-1R shRNA (C) was less efficacious in knocking down Sig-1R, and (A) was ineffective; these constructs were not used in the subsequent functional studies. Fluorescence microscopic analysis of RFP expression revealed ~60% transfection efficiency. Since the 22.5 ± 2.5% and 28 ± 4.3% knockdowns observed by Western analysis were based on whole populations, the actual knockdowns in transfected cells (selectively measured in SOCE assay) were 45.6% for Sig-1R shRNA (B) and 56.8% for Sig-1R shRNA (D). As depicted in Fig 2B and 2C, under these conditions, cocaine again failed to inhibit SOCE. Considered collectively, these findings reveal that cocaine inhibits SOCE in cerebral microvascular endothelial cells via Sig-1R activation.
Figure 2. Knockdown of Sig-1Rs prevents cocaine-induced SOCE inhibition.
A, RBMVEC express Sig-1R protein; Sig-1R protein expression is significantly reduced by transfection with Sig-1R shRNA constructs B and D, but not altered by transfection with scrambled negative control non-effective shRNA-RFP (*P<0.05 as compared with control untransfected and with scrambled shRNA transfected conditions),. Immunoblot on the left is representative for three independent experiments; quantifications are indicated on the right. B, Averaged traces of fura-2 fluorescence ratio (F340/F380) revealing that SOCE inhibition by cocaine is reversed by Sig-1R knockdown using shRNA constructs B and D, but not by scrambled shRNA. C, Comparison of the amplitudes and areas under curve of SOCE elicited by cocaine in naive RBMVEC (n = 64 cells) or RBMVEC transfected with scrambled shRNA (n = 54 cells), Sig-1R shRNA (B) (n = 59 cells) or Sig-1R shRNA (D) (n = 62 cells); *P < 0.05 as compared with cocaine.
Cocaine is well defined as a modulator of endothelial function and has been shown to increase blood-brain barrier permeability [13, 20] and facilitate transmigration of inflammatory leukocytes into the brain [21–23]. Involvement of Sig-1R in this effect has also been established [13, 14]. What was not previously recognized, however, is the possibility that these effects of cocaine and Sig-1R on endothelial permeability and activation could be Ca2+-dependent. However, changes in Ca2+ concentration are well known to modulate a wide variety of endothelial functions [24] including barrier permeability [25], endothelial proliferation [9, 26] and expression of adhesion molecules responsible for inflammatory cell recruitment [27]. Hence, the current investigation provides a new role for SOCE modulation in cocaine- and Sig-1R-induced changes in endothelial function.
Based on the present findings, Sig-1Rs can be added to the growing list of endogenous molecules with the capacity to modulate SOCE [28]. Importantly, Sig-1Rs appear to mediate SOCE termination, the mechanisms of which have remained elusive.
Although we have not defined this mechanism in the current study, there are several possibilities ranging from interfering with STIM-Orai interaction to kinase modulation. Indeed, Sig-1R has been shown to modulate the functions of p38 MAPK [29], ERK1/2 [30] and calcineurin [31], all of which regulate STIM1 phosphorylation [32–34]. Moreover, both STIM1 [33, 35] and Orai1 [36, 37] are known to be negatively regulated by phosphorylation. Future investigations may provide further insight into the novel biochemical mechanism.
Summary statement.
We provide evidence that cocaine induces sigma-1 receptor-mediated inhibition of store-operated calcium entry (SOCE) in rat brain microvascular endothelial cells. Thus, we reveal sigma-1 receptors as SOCE blockers, adding novel insight regarding endothelial effects of cocaine and endogenous SOCE modulation.
Acknowledgments
Funding information: This work was supported by the NIH grants DA035926 (to MEA), P30DA013429 (to EMU).
Abbreviations
- ER
endoplasmic reticulum
- RBMVEC
rat brain microvascular endothelial cells
- RFP
red fluorescent protein
- Sig-1R
sigma-1 receptor
- SOCE
store-operated calcium entry
- STIM1
stromal interaction molecule 1
Footnotes
Declaration of interest: The authors declare no competing financial interest.
Author contributions: G.C.B., E.D., L.C.B., J.S. and E.B. performed experiments and analyzed data. G.C.B. and E.D. drafted the manuscript. M.E.A., E.M.U., J.S. analyzed data and revised the manuscript critically for important intellectual content. E.B. designed the study. All authors reviewed the results, read and approved the final version of the manuscript.
References
- 1.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 3.Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi T, Su TP. Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108-15 cells. J Pharmacol Exp Ther. 2003;306:726–733. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- 5.Kourrich S, Su TP, Fujimoto M, Bonci A. The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci. 2012;35:762–771. doi: 10.1016/j.tins.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai SY, Hayashi T, Mori T, Su TP. Sigma-1 receptor chaperones and diseases. Cent Nerv Syst Agents Med Chem. 2009;9:184–189. doi: 10.2174/1871524910909030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogan PG, Rao A. Store-operated calcium entry: Mechanisms and modulation. Biochem Biophys Res Commun. 2015;460:40–49. doi: 10.1016/j.bbrc.2015.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redondo PC, Rosado JA. Store-operated calcium entry: unveiling the calcium handling signalplex. Int Rev Cell Mol Biol. 2015;316:183–226. doi: 10.1016/bs.ircmb.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103:1289–1299. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smyth JT, Beg AM, Wu S, Putney JW, Jr, Rusan NM. Phosphoregulation of STIM1 leads to exclusion of the endoplasmic reticulum from the mitotic spindle. Curr Biol. 2012;22:1487–1493. doi: 10.1016/j.cub.2012.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharkey J, Glen KA, Wolfe S, Kuhar MJ. Cocaine binding at sigma receptors. Eur J Pharmacol. 1988;149:171–174. doi: 10.1016/0014-2999(88)90058-1. [DOI] [PubMed] [Google Scholar]
- 13.Yao H, Duan M, Buch S. Cocaine-mediated induction of platelet-derived growth factor: implication for increased vascular permeability. Blood. 2011;117:2538–2547. doi: 10.1182/blood-2010-10-313593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao H, Kim K, Duan M, Hayashi T, Guo M, Morgello S, Prat A, Wang J, Su TP, Buch S. Cocaine hijacks sigma1 receptor to initiate induction of activated leukocyte cell adhesion molecule: implication for increased monocyte adhesion and migration in the CNS. J Neurosci. 2011;31:5942–5955. doi: 10.1523/JNEUROSCI.5618-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altmann JB, Yan G, Meeks JF, Abood ME, Brailoiu E, Brailoiu GC. G protein-coupled estrogen receptor-mediated effects on cytosolic calcium and nanomechanics in brain microvascular endothelial cells. J Neurochem. 2015;133:629–639. doi: 10.1111/jnc.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barr JL, Deliu E, Brailoiu GC, Zhao P, Yan G, Abood ME, Unterwald EM, Brailoiu E. Mechanisms of activation of nucleus accumbens neurons by cocaine via sigma-1 receptor-inositol 1,4,5-trisphosphate-transient receptor potential canonical channel pathways. Cell Calcium. 2015 doi: 10.1016/j.ceca.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. Eur J Pharmacol. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- 18.Berardi F, Ferorelli S, Colabufo NA, Leopoldo M, Perrone R, Tortorella V. A multireceptorial binding reinvestigation on an extended class of sigma ligands: N-[omega-(indan-1-yl and tetralin-1-yl)alkyl] derivatives of 3,3-dimethylpiperidine reveal high affinities towards sigma1 and EBP sites. Bioorg Med Chem. 2001;9:1325–1335. doi: 10.1016/s0968-0896(01)00011-6. [DOI] [PubMed] [Google Scholar]
- 19.Chaki S, Okuyama S, Ogawa S, Tanaka M, Muramatsu M, Nakazato A, Tomisawa K. Solubilization and characterization of binding sites for [3H]NE-100, a novel and potent sigma 1 ligand, from guinea pig brain. Life Sci. 1996;59:1331–1340. doi: 10.1016/0024-3205(96)00458-4. [DOI] [PubMed] [Google Scholar]
- 20.Dhillon NK, Peng F, Bokhari S, Callen S, Shin SH, Zhu X, Kim KJ, Buch SJ. Cocaine-mediated alteration in tight junction protein expression and modulation of CCL2/CCR2 axis across the blood-brain barrier: implications for HIV-dementia. J Neuroimmune Pharmacol. 2008;3:52–56. doi: 10.1007/s11481-007-9091-1. [DOI] [PubMed] [Google Scholar]
- 21.Fiala M, Eshleman AJ, Cashman J, Lin J, Lossinsky AS, Suarez V, Yang W, Zhang J, Popik W, Singer E, Chiappelli F, Carro E, Weinand M, Witte M, Arthos J. Cocaine increases human immunodeficiency virus type 1 neuroinvasion through remodeling brain microvascular endothelial cells. J Neurovirol. 2005;11:281–291. doi: 10.1080/13550280590952835. [DOI] [PubMed] [Google Scholar]
- 22.Fiala M, Gan XH, Zhang L, House SD, Newton T, Graves MC, Shapshak P, Stins M, Kim KS, Witte M, Chang SL. Cocaine enhances monocyte migration across the blood-brain barrier. Cocaine's connection to AIDS dementia and vasculitis? Adv Exp Med Biol. 1998;437:199–205. doi: 10.1007/978-1-4615-5347-2_22. [DOI] [PubMed] [Google Scholar]
- 23.Gan X, Zhang L, Berger O, Stins MF, Way D, Taub DD, Chang SL, Kim KS, House SD, Weinand M, Witte M, Graves MC, Fiala M. Cocaine enhances brain endothelial adhesion molecules and leukocyte migration. Clin Immunol. 1999;91:68–76. doi: 10.1006/clim.1998.4683. [DOI] [PubMed] [Google Scholar]
- 24.Tran QK, Watanabe H. Calcium signalling in the endothelium. Handb Exp Pharmacol. 2006:145–187. doi: 10.1007/3-540-32967-6_5. [DOI] [PubMed] [Google Scholar]
- 25.Wu S, Cioffi EA, Alvarez D, Sayner SL, Chen H, Cioffi DL, King J, Creighton JR, Townsley M, Goodman SR, Stevens T. Essential role of a Ca2+-selective, store-operated current (ISOC) in endothelial cell permeability: determinants of the vascular leak site. Circ Res. 2005;96:856–863. doi: 10.1161/01.RES.0000163632.67282.1f. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Cubbon RM, Wilson LA, Amer MS, McKeown L, Hou B, Majeed Y, Tumova S, Seymour VA, Taylor H, Stacey M, O'Regan D, Foster R, Porter KE, Kearney MT, Beech DJ. Orai1 and CRAC channel dependence of VEGF-activated Ca2+ entry and endothelial tube formation. Circ Res. 2011;108:1190–1198. doi: 10.1161/CIRCRESAHA.111.243352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H, Razmpour R, Yang XF, Houser SR, Chen J, Koch WJ, Wang H, Soboloff J, Gill DL, Madesh M. Blockade of N0X2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest. 2013;123:887–902. doi: 10.1172/JCI65647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon JY, Roh DH, Yoon SY, Kang SY, Choi SR, Kwon SG, Choi HS, Han HJ, Beitz AJ, Lee JH. Sigma-1 receptor-mediated increase in spinal p38 MAPK phosphorylation leads to the induction of mechanical allodynia in mice and neuropathic rats. Exp Neurol. 2013;247:383–391. doi: 10.1016/j.expneurol.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Mueller BH, 2nd, Park Y, Ma HY, Dibas A, Ellis DZ, Clark AF, Yorio T. Sigma-1 receptor stimulation protects retinal ganglion cells from ischemia-like insult through the activation of extracellular-signal-regulated kinases 1/2. Exp Eye Res. 2014;128:156–169. doi: 10.1016/j.exer.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Mari Y, Katnik C, Cuevas J. sigma-1 Receptor Inhibition of ASIC1a Channels is Dependent on a Pertussis Toxin-Sensitive G-Protein and an AKAP150/Calcineurin Complex. Neurochem Res. 2014 doi: 10.1007/s11064-014-1324-0. [DOI] [PubMed] [Google Scholar]
- 32.Pozo-Guisado E, Casas-Rua V, Tomas-Martin P, Lopez-Guerrero AM, Alvarez-Barrientos A, Martin-Romero FJ. Phosphorylation of STIM1 at ERK1/2 target sites regulates interaction with the microtubule plus-end binding protein EB1. J Cell Sci. 2013;126:3170–3180. doi: 10.1242/jcs.125054. [DOI] [PubMed] [Google Scholar]
- 33.Sundivakkam PC, Natarajan V, Malik AB, Tiruppathi C. Store-operated Ca2+ entry (SOCE) induced by protease-activated receptor-1 mediates STIM1 protein phosphorylation to inhibit SOCE in endothelial cells through AMP-activated protein kinase and p38beta mitogen-activated protein kinase. J Biol Chem. 2013;288:17030–17041. doi: 10.1074/jbc.M112.411272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasauskas AA, Chen H, Wu S, Cioffi DL. The senne-threonine phosphatase calcineurin is a regulator of endothelial store-operated calcium entry. Pulm Circ. 2014;4:116–127. doi: 10.1086/675641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smyth JT, Petranka JG, Boyles RR, DeHaven WI, Fukushima M, Johnson KL, Williams JG, Putney JW., Jr Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat Cell Biol. 2009;11:1465–1472. doi: 10.1038/ncb1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawasaki T, Ueyama T, Lange I, Feske S, Saito N. Protein kinase C-induced phosphorylation of Orai1 regulates the intracellular Ca2+ level via the store-operated Ca2+ channel. J Biol Chem. 2010;285:25720–25730. doi: 10.1074/jbc.M109.022996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hooper R, Zhang X, Webster M, Go C, Kedra J, Marchbank K, Gill DL, Weeraratna AT, Trebak M, Soboloff J. Novel PKC-mediated Control of Orai1 function in Invasive Melanoma. Mol Cell Biol. 2015 doi: 10.1128/MCB.01500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]