Abstract
Spinal cord injury (SCI) leads to increased anxiety and depression in as many as 60% of patients. Yet, despite extensive clinical research focused on understanding the variables influencing psychological well-being following SCI, risk factors that decrease it remain unclear. We hypothesized that excitation of the immune system, inherent to SCI, may contribute to the decrease in psychological well-being. To test this hypothesis, we used a battery of established behavioral tests to assess depression and anxiety in spinally contused rats. The behavioral tests, and subsequent statistical analyses, revealed three cohorts of subjects that displayed behavioral characteristics of 1) depression, 2) depression and anxiety, or 3) no signs of decreased psychological well-being. Subsequent molecular analyses demonstrated that the psychological cohorts differed not only in behavioral symptoms, but also in peripheral (serum) and central (hippocampi and spinal cord) levels of pro-inflammatory cytokines. Subjects exhibiting a purely depression-like profile showed higher levels of pro-inflammatory cytokines peripherally, whereas subjects exhibiting a depression- and anxiety-like profile showed higher levels of pro-inflammatory cytokines centrally (hippocampi and spinal cord). These changes in inflammation were not associated with injury severity; suggesting that the association between inflammation and the expression of behaviors characteristic of decreased psychological well-being was not confounded by differential impairments in motor ability. These data support the hypothesis that inflammatory changes are associated with decreased psychological well-being following SCI.
1. Introduction
In addition to its effects on physical function, spinal cord injury (SCI) significantly impacts quality of life and psychological well-being. As many as 60% of spinal cord injured patients suffer from depression (Shin, Goo et al. 2012), anxiety (Post and van Leeuwen 2012), and general decreased quality of life (Boakye, Leigh et al. 2012). Commensurate with these statistics, and the risks associated with depression (McCullumsmith, Kalpazian et al. 2015), suicide attempts and suicide ideation are estimated to be 3 or more times greater following SCI, than in the general population (DeVivo, Black et al. 1991, Soden, Walsh et al. 2000). Depression is also associated with long-term negative outcomes after SCI including an increased incidence of secondary complications (Malec and Neimeyer 1983, Herrick, Elliott et al. 1994, Elliott and Frank 1996) and lower functional independence (Gelis, Daures et al. 2011, Abdul-Sattar 2014). Given the high percentage of spinal cord injured patients experiencing psychological symptoms, and the significant impact of this mood disorder on rehabilitation outcomes and quality of life, it is imperative that we better understand the mechanisms underlying decreased psychological well-being following SCI.
There is compelling evidence to suggest that, in addition to psychosocial stressors, the activation of the immune system may be contributing to the manifestation of depression and anxiety in a subset of the clinical population. Both rodent and human studies support this hypothesis (Smith 1991, Maes 1999, van West and Maes 1999, Capuron, Ravaud et al. 2001, Capuron, Neurauter et al. 2003, Capuron, Ravaud et al. 2004, Miller, Haroon et al. 2013, Vogelzangs, Beekman et al. 2013). In the human clinical population, studies have found an association between elevated levels of pro-inflammatory cytokines and depression (Howren, Lamkin et al. 2009, Dowlati, Herrmann et al. 2010, Liu, Ho et al. 2012) as well as anxiety (Pace and Heim 2011, Miller, Haroon et al. 2013). In animal models, stressed rats were found to display increased spleen and brain (hippocampus, hypothalamus, and cortex) mRNA levels of pro-inflammatory cytokines relative to non-stressed rats (You, Luo et al. 2011). Further, in rodent models, central or systemic administration of pro-inflammatory cytokines produces sickness behavior characterized by behavioral and physiological changes resembling depression (Anisman, Merali et al. 2005), which can be alleviated with anti-depressants (Merali, Brennan et al. 2003). More recently, Wu et al. (2014) showed that brain microglia are activated in a mouse model of SCI, and that SCI increases cognitive dysfunction as well as depression-like symptoms. These data suggest that immune system activation may play a pivotal role in the development of depression and anxiety.
A role for the immune system in the development of depression and anxiety may be especially important after SCI. SCI is characterized by inflammation and activation of the immune system. Following injury, microglia, macrophages, and astrocytes are recruited to the site of trauma. Pro-inflammatory cytokine genes and other inflammation-related genes are up-regulated within hours of injury (Dumont, Okonkwo et al. 2001, Yip and Malaspina 2012), and some remain elevated for weeks following the injury (Malaspina, Jokic et al. 2008, Jokic, Yip et al. 2010). For example, in the hours following injury, the interleukin-6 gene (IL-6), the TNF gene, and the interleukin-1β gene (IL-1β) are up-regulated (Hayashi, Ueyama et al. 2000, Pan, Ni et al. 2002). In addition, as injury severity increases, pro-inflammatory cytokine levels increase (Yang, Jones et al. 2005). Immunocytochemical, RT-PCR and Western Blot assays on spinal cord tissue collected one to six hours post SCI indicated that IL-1β, IL-6 and TNF-α production is increased in neurons and microglia of the spinal cord following SCI, and that this increase in transcription is significantly greater following a severe compared to a mild injury (Yang, Jones et al. 2005). Therefore, immune system activation triggered by SCI may influence psychological well-being, and may do so incrementally as injury severity increases.
To test this hypothesis, the current experiment used an established behavioral ethogram (Luedtke, Maldonado-Bouchard et al. 2014) to phenotype spinally injured rodents based on psychological symptoms, and then examined the relationships between inflammation, depression and anxiety. We used pre-mixed multiplex assays to obtain an overall tableau of pro and anti-inflammatory cytokine expression following injury. We found that changes in cytokine expression are associated with the development of an anxiety- and depression-like profile in SCI rodents. Moreover changes in behavioral symptoms, of depression and anxiety, were not simply due to differences in the recovery of motor function. The incidence of depression, with or without co-morbid anxiety, did not differ across injury severity groups and locomotor recovery (BBB scale) levels were not significantly different across the psychological well-being cohorts. We posit that by activating the immune system, SCI may hinder psychological well-being, increasing the development of depression and anxiety-like signs post injury.
2. Methods
2.1 Subjects
Male Sprague-Dawley rats (Harlan Houston, TX), approximately 90–110 days old (300–350 g), were individually housed in Plexiglas bins [45.7 (length) x 23.5 (width) x 20.3 (height) cm] with food and water continuously available. The rats were maintained on a 12 hour light/dark cycle and all behavioral testing was conducted during the light cycle. Food consumption and subject weights were recorded daily. Following surgery, subjects’ bladders were manually expressed in the morning (8–9:30 a.m.) and in the evening (6–7:30 p.m.) until they regained full bladder control (which was operationally defined as three consecutive days with an empty bladder at the time of expression), and were checked daily for signs of autophagia and spasticity.
All of the experiments reported here were reviewed and approved by the Institutional Animal Care Committee at Texas A&M University and all NIH guidelines for the care and use of animal subjects were followed.
2.2 Procedures
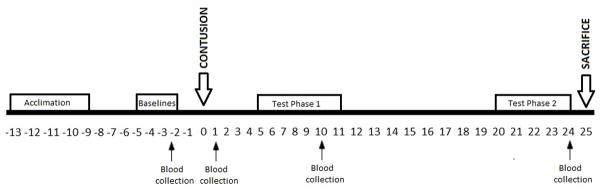
The timeline for experimental procedures is shown in Figure 1. Subjects (N=47) received a mild, moderate, or severe contusion or were intact controls (n=12/group). Psychological well-being was assessed at baseline (prior to injury) with a comprehensive battery of tests as well as during Test Phase 1 (days 5 and 10) and 2 (days 20–21) post injury. Intact subjects were tested at ages compatible with injured subjects.
Figure 1.
Experimental schedule.
2.3 Surgery
An Infinite Horizons (IH) impactor (Precision Systems and Instrumentation) fitted with a 2.5 mm impact probe was used to deliver a contusion injury to the T12 spinal cord. Subjects were anesthetized with 5% isoflurane gas, and once a stable level of anesthesia was reached, the concentration was lowered to a 2–3% maintenance level. An area extending approximately 2.5 cm above and below the injury site was then shaved and disinfected with iodine. A 3–4 cm incision was made in the skin along the midline of the animal, followed by 1.5 cm incisions on either side of the spinous processes at the level of injury. A laminectomy was performed removing the T12 vertebra and exposing the spinal cord. The animal was fixed in the IH impactor and the probe exerted a force onto the cord, remaining in contact with the cord for one second (dwell time). A force of 110 kDynes was used to produce a mild contusion injury, 150 kDynes a moderate injury, and 200 kDynes a severe injury. Immediately after the contusion, the incision was closed with Michel clips and subjects were treated with 100,000 units/kg Pfizerpen (penicillin G potassium), both following surgery and again two days later, to help prevent infection. To compensate for fluid loss, subjects received 3.0 ml of filtered saline at the time of surgery and again two days later. The rats were kept in a recovery room (maintained at 26.6°C) for 24 hours following the injury.
2.4 Assessment of psychological well-being
Anhedonia was assessed with the sucrose preference test (procedure described in detail in Luedtke et al., 2014). Briefly, the subjects were acclimated to the sucrose preference test in three sessions beginning 13 days prior to surgery. Baseline preferences, for sucrose-flavored or plain water, were collected five days prior to surgery. Sucrose preference was then measured on Days 10 (Test Phase 1) and 21 (Test Phase 2) post SCI. A decrease in sucrose preference is a sign of anhedonia, or lack of interest in rewarding stimuli.
Psychomotor activity and center field activity was assessed with the open field test (procedure described in detail in Luedtke et al., 2014). Subjects were acclimated to the open field environment [a black plywood box, 100 x 100 x 20 (height) cm] during three sessions beginning 13 days prior surgery. Baseline activity levels were collected five days prior to surgery, with post-injury measures occurring on Days 10 (Test Phase 1) and 21 (Test Phase 2). Open field activity was scored post hoc from video analyses. The floor of the open field box is partitioned into 100 squares delineated with silver marker, and the number of squares crossed in a 5 minute test session was recorded as a measure of activity. A decrease in the total number of squares crossed is interpreted as a sign of psychomotor retardation.
Center activity in an open field was used as a measure of anxiety-like behavior. The number of squares crossed in the center of the field (28 center squares) as well as time spent in the center squares were recorded, and center field activity was computed in the following manner: center squares crossed x center time. A decrease in center field activity is interpreted as a passive anxiety-like sign.
Social exploration was assessed in the same black plywood box used for open field activity (procedure described in detail in Luedtke et al., 2014). For this test, the interactions between the test subject and an unfamiliar juvenile rat are systematically scored. Subjects are videotaped for 5 minutes, and the time the focal subject spends performing social behaviors (i.e. moving toward, anogenital sniffing, and following the juvenile rat (Swain and Le 1998)) is determined post hoc. A decrease in time spent engaging in social behaviors is characteristic of depression. Baseline social exploration was assessed 5 days prior to surgery. Social exploration was assessed during Test Phase 1 and 2, on Days 10 and 21.
The forced swim test was used to assess learned helplessness (procedure described in detail in Luedtke et al., 2014). Briefly, subjects were placed in a cylinder [15 (diam.) x 40 (height) cm] filled with water (23 ± 1°C) from which they could not escape. They were video-recorded for a 10-minute test period, and time spent immobile was scored post hoc. Immobility is characterized as a lack of any movement except those required to keep the head above water (Porsolt, Bertin et al. 1977). Immobility is interpreted as a symptom of learned helplessness, and characteristic of depression. The forced swim test was conducted prior to injury (Baseline), and during Test Phase 2, on Day 23 post injury.
The shock probe burying task is widely used as a measure of active anxiety, and has been validated by several groups (Poling, Cleary et al. 1981, Treit, Pinel et al. 1981, De Boer and Koolhaas 2003). Rats were placed in a plastic box (56.51 cm L x 40.33 cm W x 33.35 cm H) with a small probe (5 cm) placed 2 cm above the bedding level at one far end of the box. The probe was built by wrapping two copper wires around a glass rod, and connecting the extremities to two dragon snaps. As soon as the subject came into contact with the probe (nose or forepaws touch), the electrical stimulation was turned off. The subjects were filmed for ten minutes after they contacted the probe. The time that the rats spent engaged in burying and freezing behavior was recorded from post hoc video analyses. Greater time spent burying is interpreted as a sign of active anxiety. The shock probe burying task was conducted during Test Phase 1 and 2, on Days 5 and 20 post injury. Given that performance on this test is sensitive to repeated testing, a baseline test of shock probe burying was not conducted (De Boer and Koolhaas 2003).
Food consumption was also collected daily as an index of appetite deviation, which is associated with depression in the clinical population. Food weight was recorded before and after injury, daily, at 9 AM. Food consumption was calculated by subtracting the current day’s food weight from the previous day’s food weight.
2.5 Assessment of recovery and pain reactivity
Locomotor function was assessed throughout the 21-day recovery period with the BBB scale (Basso, Beattie et al. 1995). Subjects were acclimated to the open enclosure (99 cm diameter and 23 cm deep) used for scoring for 5 min per day for 3 days prior to surgery. After injury, the locomotor capacity (BBB) of subjects was scored by trained observers on days 1–7, 9, 11, 13, 15, 18, and 21. Care was taken to ensure that all observers’ scoring behavior had high intra- and inter-observer reliability (all r’s > 0.90) and that they were blind to the subject’s experimental treatment. Locomotor scores were transformed to help assure that the data were amendable to parametric analyses (Ferguson, Hook et al. 2004).
Tests of sensory function (tactile and thermal reactivity) were also conducted during Test Phase 1 (day 11) and Test Phase 2 (day 22). Thermal reactivity was assessed using radiant heat in the tail-flick test. Subjects were placed in the restraining tubes with their tail positioned in a 0.5 cm deep groove, cut into an aluminum block, and allowed to acclimate to the apparatus (IITC Inc., Life Science, CA) and testing room (maintained at 26.5°C) for 15 min. Thermal thresholds were then assessed. Thermal reactivity was tested using a halogen light that was focused onto the rat's tail. Prior to testing the temperature of the light, focused on the tail, was set to elicit a baseline tail-flick response in 3–4 sec (average). This pre-set temperature was maintained across all subjects. In testing, the latency to flick the tail away from the radiant heat source (light) was recorded. If a subject failed to respond, the test trial was automatically terminated after 8 s of heat exposure. Two tests occurred at 2-minute intervals, and the second test tail-flick latencies were recorded. To confirm that subjects did not respond in the absence of the heat stimulus, blank trials were also performed. A ‘false alarm’ was recorded if subjects made a motor or vocalization response during the blank tests. The blank trials were performed 1 min before or after each sensory test (in a counterbalanced fashion). No false alarms were recorded. After assessment of thermal reactivity, subjects were returned to their home cages for a minimum of 2 hours.
After 2 hours, subjects were placed back into restraint tubes and presented with von Frey stimulation (von Frey stimuli formed from nylon monofilaments; Semmes-Weinstein Anesthesiometer, Stoelting Co., Chicago, IL) to test tactile reactivity. Filaments of increasing strength were applied every 2s in sequence to the plantar surface of the paw. The stimuli were presented until subjects exhibited a paw withdrawal/motor and vocalization response. The intensity of the stimuli that produced the responses was reported using the formula provided by Semmes-Weinstein: Intensity=log10 (10,000 * g force). If one or both responses were not observed, testing was terminated at a force of 300g. Each subject was tested twice on each foot in a counterbalanced ABBA order.
2.6 Tissue collection
Blood was collected in each test phase (pre-injury, days 2, 10, and 24). Finally, at the end of the recovery assessment period, subjects received a lethal injection of beuthanasia (100 mg/kg, i.p.) and the injured spinal cord (1 cm section centered on the lesion) and brain tissue (hippocampus) were collected. The hippocampi were chosen for these brain analyses because pre-clinical and clinical studies of depression consistently find that depressed subjects show decreased hippocampal volume (Duman, Malberg et al. 1999, MacQueen, Campbell et al. 2003, Paradise, Naismith et al. 2012), increased microglial density (Stockmeier, Mahajan et al. 2004) and increased glucocorticoid levels (Bremner, Narayan et al. 2000). Thymus weight was also recorded as a physiological index of stress.
Serum
Twenty-four hours prior to blood collection, the rats’ hind limbs were shaved with an electric trimmer to expose the saphenous vein. The next day, topical lidocaine was applied to the shaven leg. After waiting for six minutes (to give lidocaine the time to act), each rat was inserted into a clean sock, with an opening for its nose, to breathe comfortably. A compression point was made at the base of the leg so that the saphenous vein would swell, and the vein was punctured using a 20G needle. Blood was collected in a blood collection tube with a clot activator (MCB 16440 with Serum/Clotting activator, Braintree Scientific). Then 2.5 ml (s.c.) of saline was administered to prevent dehydration. Care was taken to perform this procedure in two minutes or less. Blood was collected two hours prior to the beginning of the dark cycle on Day 1 and Day 24 post surgery. Saphenous veins punctured were alternated on each day of collection (i.e., left then right).
The collected blood was left at room temperature for 30 minutes to allow clotting. The samples were then centrifuged at 12 000 RPM for 15 minutes at 4 °C. The top clear layer (serum) of each sample was extracted with a pipette and stored at −20 °C.
Brain tissue
On Day 25 post-injury, subjects were anesthetized with beuthanasia (0.2 ml administered i.p.) and, after verification of a deep level of anesthesia (absence of pedal and corneal reflex), an incision was made above the skull. The nasal bone and the cranial bone were cut to extract the brain. The brain was placed immediately on a glass dish on ice, and the hippocampi were extracted and flash frozen in liquid nitrogen, in less than 3 minutes (Spijker 2011), for the subsequent extraction of total protein.
Spinal cord tissue and thymus
A 1 cm section of the spinal cord, centered over the injury site was removed and frozen in liquid nitrogen for the subsequent extraction of total protein. In addition, the thymus was extracted and weighed as an index of psychological stress. All tissue was collected on Day 25 post surgery.
Protein extraction
The lysis buffer was made as described in Bake, Selvamani, Cherry, & Sohrabji (2014) and Jezierski & Sohrabji (2001): 0.5M Tris (pH 7.4), 5M NaCL, 50% glycerol to stabilize protein, 100mM EGTA (pH 8) to inhibit metalloproteases and cysteine proteases, 10mM Na-orthovanadate (pH 10) as a phosphatase inhibitor, 1mM ZnCl2 as a phosphatase inhibitor, 0.5M NaF as a phosphatase inhibitor, reconstituted aprotinin as a phosphatase inhibitor, reconstituted leupeptin to inhibit serine and cysteine proteases, 10% triton X-100 to solubilize membranes and 200mM PMSF with DMSO added at the time of extraction to inhibit serine proteases.
Lysates were obtained by homogenizing the hippocampi and spinal cord, separately, in 600μL lysis buffer. The samples were next centrifuged at 18 000 rpm for 30 minutes at 4 °C, and the supernatants were collected and stored at −20 °C. A BCA Protein Assay Kit was used to assay the total protein concentration in the hippocampal and spinal cord supernatants (Pierce, Rockford, IL). Standards from 0 to 200 μg/mL were made from the albumin provided in the kit and MQ water.
Samples were prepared by mixing 5 μL of tissue (spinal or hippocampal) homogenate in lysis buffer and 45 μL of MQ water in 1.7 mL plastic tubes. The protein assay reagent was prepared in a 50:1 ratio for A:B, and 1mL was added to all standards and all prepared samples. All tubes (standards and samples) were incubated in a water bath for one hour at 37 °C. Then, 200 μL of each standard and sample were loaded in duplicate onto a 96-well microplate. Absorbance was measured at 562nm on a spectrophotometer (Biomate 3, Thermo Electron Corporation, Waltham, MA). Concentrations were obtained in μg protein/ μL.
2.7 Assays
Alpha-2 macroglobulin ELISA
Alpha-2 macroglobulin levels in the serum were assayed on Days 1 and 24 with ELISAs (Abcam 2013, kit ab157730). Dilution tests were run to determine the appropriate dilution rate for all serum samples. For intact subjects, the dilution rate was determined to be 300 x, whereas for all contused subject samples, the dilution rate was determined to be 3000 x. Assays were run following the manufacturer’s protocol. The best-fit equation was determined to be a 5-parameter logistic regression. The concentration readings were multiplied by the appropriate dilution factors (300 for intact subject samples, and 3000 for all contused subject samples).
Cytokine/Chemokine multiplex
The tissue collected included serum, hippocampi and spinal cords from subjects in the mild, moderate, or severe contusion conditions or in the intact control condition (n=12/group). Serum was collected on Days 1 and 24 post injury. The hippocampi and spinal cord were collected after euthanasia, on Day 25 post injury.
Serum and hippocampal levels of 27 cytokine/chemokines were assessed using a magnetic bead immunoassay (Millipore Corp. MA) following manufacturer’s instructions. Briefly, the assay plate was blocked with a buffer for 10 min and emptied. Standards and samples were added into appropriate wells, followed by addition of premixed beads and incubated for 2h at room temperature on a plate shaker. Wells were washed 2 times, 25 μl of detection antibody was added, incubated for 1h at room temperature (RT), and followed by 30 min of incubation with the addition of 25 μl of streptavidin-phycoerythrin per well. After 2 washes, beads were resuspended in 150 μl of sheath fluid and a minimum of 50 beads per analyte were analysed in a Bio-Plex suspension array system (Bio-Rad Laboratories,CA). Cytokine/chemokine levels were normalized to total protein content. The cytokines and chemokines assessed are listed in Tables 1, 2, and 3. The analytes are organized according to their most common roles in inflammation. Therefore, Table 1 displays the “pro-inflammatory” cytokines and chemokines, and Table 2 lists the “anti-inflammatory” cytokines and chemokines. Finally, we have added Table 3 to list the cytokines and chemokines for which there is no clearly defined pro versus anti-inflammatory role.
Table 1.
Pro-inflammatory cytokines and chemokines assessed with Multiplex assay; possible involvement in depression and anxiety.
| Analyte | Overall function and expression in anxiety/depression | References |
|---|---|---|
| Eotaxin | Activator and chemoattractor. Also referred to as CCL11. Found to be elevated in patients with mood disorder. | (Menzies-Gow, Ying et al. 2002; Magalhaes, Jansen et al. 2014) |
| GM-CSF (Granulocyte- macrophage colony- stimulating factor) |
Stimulates stem cells granulocyte production (neutrophils, eosinophils, and basophils) and monocytes. Found to be elevated in patients with major depressive disorder. Appears to play an important role in structural plasticity. | (Shi, Liu et al. 2006; Krieger, Both et al. 2012; Schmidt, Lichtblau et al. 2014) |
| IL-1α (interleukin-1α) |
Activates a set of immune system response processes shortly after infection. Up-regulation in the prefrontal cortex of patients with major depressive disorder. Mild depressive symptoms are associated with overexpression of monocyte-associated IL- 1α. | (Dinarello 2009; Suarez, Krishnan et al. 2003, Shelton, Claiborne et al. 2011) |
| MIP-1α (Macrophage inflammatory protein-1α) |
Involved in recruiting and activating leukocytes. Present in a subset of patients with moderate-severe depression. | (Cook 1996; Merendino, Di Pasquale et al. 2004) |
| IL-1β
(interleukin-1β) |
Activates microglia and immune cells (cytokines). Levels increase in the hippocampus and prefrontal cortex with stress. Can produce stress-induced learning impairments. | (Audet and Anisman 2013; Pugh, Fleshner et al. 2001) |
| IL-2 (interleukin-2) |
Involved in the differentiation of T cells. Increased in subjects with depression phenotype. | (Audet and Anisman 2013) |
| IL-6 (interleukin-6) |
Stimulates immune response. Circulating levels increased in depressed individuals. Also increased with psychosocial stress. | (Audet and Anisman 2013; Dowlati, Herrmann et al. 2010) |
| IL-12p70 (IL-12 phosphorylated 70 kDa) |
Involved in differentiating T cells to Th1 cells. Stimulates production of TNF- α and IFN-γ. | (Muller-Berghaus, Kern et al. 2004) |
| IFN-γ (interferon- γ) |
Activates microglia, natural killer cells and macrophages. Elevated in depressed individuals. Expression suppressed with stress. | (Audet and Anisman 2013; Curtin, Boyle et al. 2009, Dahl, Ormstad et al. 2014) |
| IL-5 (interleukin-5) |
Stimulates B cell growth. Involved in eosinophil activation. Elevated in depressed individuals. | (Kouro and Takatsu 2009; Dahl, Ormstad et al. 2014) |
| MCP-1 (monocyte chemotactic protein-1) |
Recruits monocytes, memory T cells and dendritic cells to inflammation site. Also referred to as chemokine C-C motif ligand 2 (CCL2). Elevated serum levels found in depressed patients. High expression in major depressive disorder patients is thought to modulate neuronal activity and neuroendocrine functions. | (Deshmane, Kremlev et al. 2009; Pae 2014, Young, Bruno et al. 2014) |
| IP-10 (interferon gamma- induced protein 10) |
Chemoattractant for activated T cells. Involved in recruiting T cells to sites of inflammation. Also referred to as C-X-C motif chemokine 10 (CXCL10). | (Dufour, Dziejman et al. 2002) |
| GRO-KC (growth-related oncogene/keratinocyte derived chemokine) |
Chemoattractant for neutrophils. Also referred to as CXCL1. | (Roy, Richard et al. 2012) |
| VEGF (Vascular endothelial growth factor) |
Critical in angiogenesis. Can increase IFN- γ and decrease IL-10 in T-cells, therefore increasing pro-inflammatory T-cell differentiation. | (Mor, Quintana et al. 2004) |
| LIX (lipopolysaccharide- induced CXC chemokine) |
Involved in cell migration and activation of neutrophils. Also referred to as CXCL5. | (Choong, Yong et al. 2004) |
| MIP-2 (macrophage inflammatory protein 2) |
Chemoattractant for neutrophils. Also referred to as CXCL2. | (Driscoll 1994) |
| TNF-α (Tumor necrosis factor- α) |
Activates microglia and immune cells. Circulating levels increased in depressed individuals. Can induce depression by upregulating the tryptofan-degrading enzyme indoleamine 2,3-dioxygenase (precursor for serotonin). | (Audet and Anisman 2013; Dowlati, Herrmann et al. 2010; Liu, Peng et al. 2015) |
| RANTES (regulated on activation, normal T cell expressed and secreted) |
Chemoattractant for T cells, eosinophils, and basophils. Also referred to as Chemokine (C-C motif) ligand 5 (CCL5). Can upregulate IL-10 expression, an anti- inflammatory cytokine. | (Kapp, Zeck-Kapp et al. 1994; Kim, Cha et al. 2015) |
| IL-17a (interleukin-17a) |
Involved in delayed-type reactions. Increases chemokine production for the recruitment of monocytes and neutrophils to inflammation site. Levels found to be positively correlated with anxiety. | (Jin and Dong 2013; Liu, Ho et al. 2012) |
| IL-18 (interleukin-18) |
Stimulates T cell and natural killer cell maturation, and stimulates IFN-γ production. Elevated in patients with moderate-severe depression. | (Audet and Anisman 2013; Merendino, Di Pasquale et al. 2004) |
Table 2.
Anti-inflammatory cytokines and chemokines assessed with Multiplex assay; possible involvement in depression and anxiety.
| Analyte | Overall function and expression in anxiety/depression | References |
|---|---|---|
| IL-4 (interleukin IL-4) |
Involved in a shift from Th1 to Th2. Decreases production of macrophages, IFN- gamma, and dendritic cell IL-12. IL-4 Knockout mice exhibit state anxiety-like signs. | (Audet & Anisman, 2013; Moon, Joesting et al. 2015) |
| EGF (epidermal growth factor) |
Stimulates cell growth, proliferation and differentiation. Single nucleotide polymorphisms within the EGF gene may be involved in the development of depression. Low levels of plasma EGF found in major depressive disorder patients. | (Menard et al., 2012; Tian, Zhang et al. 2012) |
| IL-13 (interleukin IL-13) |
Inhibits cytokine production by B cells and monocytes. Induces protein-degrading enzyme matric metalloproteinases (MPPs). | (Wynn, 2003) |
| IL-10 (interleukin IL-10) |
Involved in shift from Th1 to Th2. Reduces immune response activation. | (Audet & Anisman, 2013) |
Table 3.
Unclassified cytokines and chemokines assessed with Multiplex assay; possible involvement in depression and anxiety.
| Analyte | Overall function and expression in anxiety/depression | References |
|---|---|---|
| G-CSF (Granulocyte colony- stimulating factor) |
Neurogenesis, neuroplasticity. Counters apoptosis. Can have both pro- and anti- inflammatory effects. | (Boneberg & Hartung, 2002; Lawlor et al., 2004) |
| Leptin | Marker of inflammation. Regulates energy consumption. Related to appetite and hunger, metabolism, and behavior. Can have anxyolitic effects by facilitating fear extinction. | (Fantuzzi & Faggioni, 2000; Wang, Liu et al. 2015) |
| Fractalkine | Anti-apoptotic. Involved in chemotaxis and leukocyte adhesion, as well as the survival of various cells during inflammation. Also referred to as CX3CL1. High levels present in patients with moderate-severe depression. | (White & Greaves, 2012; Merendino, Di Pasquale et al. 2004) |
Spinal cord homogenate levels of 20 cytokine/chemokines were assessed using a magnetic bead panel Bio-Plex Pro Rat Cytokine 23-Plex Assay (L80-01V11S5; Bio-Rad Laboratories, Hercules, CA). This panel assessed the same analytes as the Milliplex MAP Kit, except that Eotaxin, Leptin, MIP-1α, EGF, IP-10, Fractalkine, and MIP-2 were not included. Both kits used the same reagents and followed equivalent protocols. The assays were run according to the manufacturer’s instructions, including for dilution recommendation.
The median fluorescent intensity of all 27 (or 20) analytes was obtained for all standards and samples, and the analyte concentrations in samples (in pg/mL) were calculated by deriving a 5-parameter logistic standard curve for each analyte separately. For serum samples, the calculated concentration for all analytes was multiplied by the dilution factor 2. For protein supernatant samples, the calculated concentration for all analytes was normalized to the total protein content, obtained from the BCA Protein Assay.
2.8 Statistical analyses
Principal components analysis
Subjects’ scores on each of the behavioral measures (sucrose preference, forced swim, open field psychomotor activity, center field activity, social exploration, and shock probe burying) were averaged across Test Phase 1 and 2 and then subjected to a principal components analysis using orthogonal Varimax rotation. The scores were averaged so that analyses would characterize behavioral signs that persisted into Test Phase 2 as well as have strong predictive value for Test Phase 1, or the early phase of SCI. Factors with loadings of ≥ 0.32 on more than one component (complex structure) were removed (Tabachnick and Fidell 2007) and the analysis was repeated until no complex structure factors remained.
Hierarchical cluster analysis
Hierarchical cluster analysis is a statistical procedure used to separate a sample into clusters that the experimenter can operationally define. A hierarchical cluster analysis was performed using the measures with moderate-strong loadings on the components retained in the PCA. Specifically, average scores (derived from both Test Phase 1 and 2), on each of the retained behavioral tests, were first standardized by z scores. Then a hierarchical cluster analysis was performed using Ward’s method and applying squared Euclidean distance as the distance measure. The number of appropriate clusters based on the behavioral measures was obtained by looking for a break in the agglomeration coefficient change and by examining the dendrogram, which illustrates the distance between linked clusters. It must be noted that the cluster sizes need not be even. Three clusters were identified, and the analysis was repeated using the same parameters but restricting it to a single solution of three clusters. A new variable, cluster membership, was generated for all subjects.
The three clusters’ performance on all measures retained by the principal component analysis was compared across the recovery period (Test Phase 1 and 2) using repeated measure analyses of variance (ANOVAs). Baseline scores were used as a covariate when significant (p<0.05). Based on the pattern of behaviors exhibited by each cluster, the subject cohorts were labeled as exhibiting “anxiety and depression-like signs,” “depression-like signs” or as being “healthy”.
Random Forest analyses
Principal component analyses and hierarchical cluster analyses allow visualization of clusters present in a given dataset, but do not allow any future predictions. That is, the variables retained by the principal component analysis, and the groups identified by the hierarchical cluster analysis can change with the addition of subjects. In contrast, random forest is versatile, and can be used for clustering of existing data, as well as to predict future data membership. Its goal is to derive a function from labeled training data which enables one to make predictions on future unseen un-labeled data (Hastie, Tibshirani et al. 2005, Mohri, Rostamizadeh et al. 2012.) Random forests are collection of thousands of trees. Each tree is grown using a bootstrap sample of the data. For each tree, 2/3 of the data is randomly selected for growing the tree and 1/3 of the data set is used for validation and testing. The clusters with the greatest number of validation through these cycles are retained.
We used random forest to create a learning algorithm that can generalize from the data of our original experiment to predict future subject psychological well-being membership. Consistent with previous analysis, principal component analysis was performed on average scores (derived from both Test Phase 1 and 2) and then the random forest model was built on the data obtained and classification accuracy of the model on test samples was analyzed.
3. Results
3.1 Injury severity groups differed on measures of physical and psychological recovery post SCI
Recovery of function
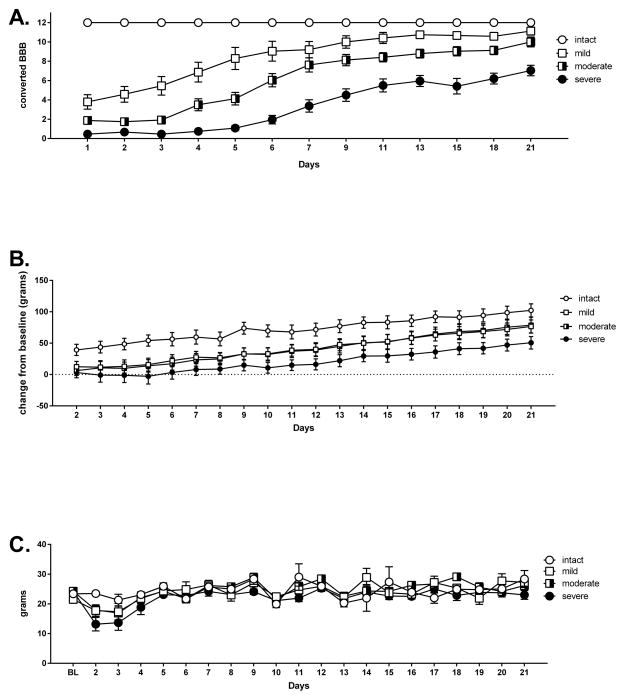
Repeated measure ANOVAS found main effects of injury severity on the recovery of locomotor function [F(3,35)=55.15 p<.05]. Post-hoc Sidak comparisons by injury severity confirmed that all four groups differed significantly from each other across time [ps ≤ .05, Figure 2A], with locomotor function decreasing as injury severity increased. Main effects of injury severity were also found for weight gain [F(3,35)=7.003, p<.05]. Post-hoc Sidak comparisons confirmed that across time, the severe injury group gained significantly less weight than all the other groups [ps ≤ .05, Figure 2B]. Finally, main effects of injury severity were found for food consumption [F(3,43)= 3.92, p<.05]. Post-hoc Sidak comparisons revealed that the severe injury group had significantly lower food consumption than the intact and moderate injury groups across time [ps ≤ .05, Figure 2C].
Figure 2.
Recovery as a function of injury severity. Commensurate with the severity of their injury, the groups showed significantly different converted BBB scores across the recovery period (A). Recovery of locomotor function decreased as injury severity increased. Similarly, the severe injury group showed significantly less weight gain than all other groups (B), and consumed less food than the moderate and intact groups (C). The mean (± SEM) performance of the intact, mild, moderate and severe injury groups is depicted.
Depression-like signs
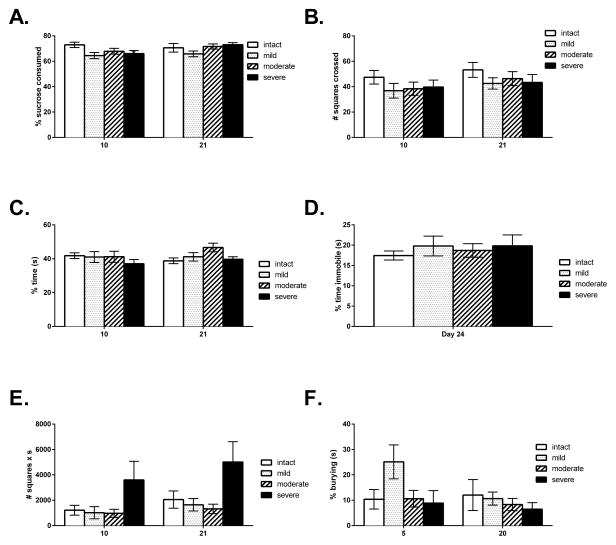
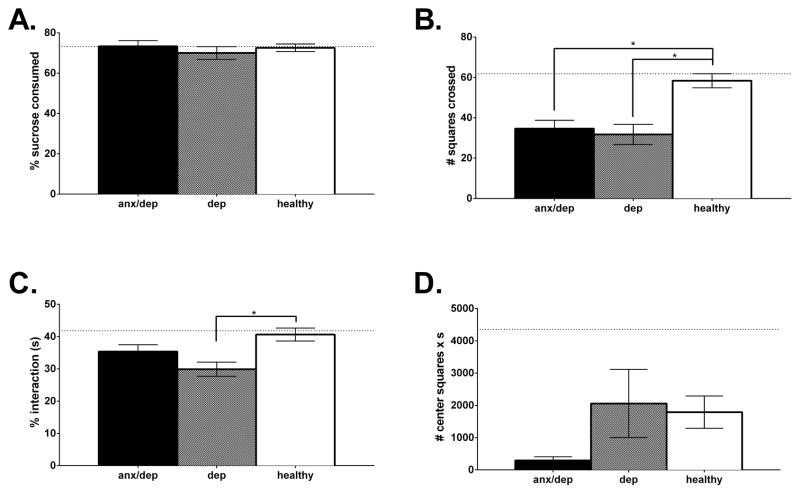
Despite significant differences in locomotor function, injury severity did not affect performance on the tests of depression. There was no effect of injury severity on sucrose preference [F(3,43)=1.67, p<.05], open field activity, [F(3,42)=2.12, p<.05], or social exploration [F(3,42)=1.55, p<.05]. There were also no effects of injury severity on immobility time in the forced swim test [F(3,41)=.18, p<.05, Figure 3A–3D].
Figure 3.
The panels of graphs depict the mean (± SEM) behavioral performance of the intact, mild, moderate and severe injury groups on the tests of depression and anxiety: Sucrose preference (A), Open field activity (B), Social exploration (C), Forced swim (D), Center activity (E) and Shock probe burying (F). No significant differences were found among injury severity groups on any of the measures.
Anxiety-like signs
A repeated measures ANOVA revealed a main effect of injury severity for center activity in the open field [F(3,43)=3.27, p<.05]. Subsequent ANOVAs, on Days 10 and 21 separately, confirmed a main effect on Day 21 [F(3,43)=3.08, p<.05]. Post-hoc Sidak comparisons indicated that on Day 21, the severe injury group showed a trend towards greater center activity than the moderate injury group and a tendency for greater center activity relative to the mild injury group [p=.05 and 0.10 respectively]. There was no effect of injury severity on shock probe burying [F(3,39)=1.37, p<.05, Figure 3E–3F].
Pain reactivity
Paw withdrawal thresholds on the tactile reactivity test were significantly different across groups [F(3,43)=8.55, p<.05]. Post-hoc Sidak comparisons confirmed that the intact group displayed significantly higher paw withdrawal thresholds than all other groups across the recovery period [ps • .05, graphs not shown]. No other injury severity effects on pain (vocalization responses or thermal) reactivity were observed.
As injury severity did not affect performance on the tests of depression, our subsequent statistical analyses focused on differences across psychological well-being groups and collapsed across injury severity conditions. Ideally, we would have had a 2x2 design allowing testing of injury severity x psychological well-being interactions. However, given that the psychological well-being groups were determined post injury, it was not possible to obtain equal numbers of subjects within each group, with a sufficient sample size per subgroup to allow analysis of interaction effects. This approach would have required running a much larger number of animals, to ensure a minimum of sample of 12 per subgroup (4 x 3). Thus, focusing solely on the differences across psychological well-being groups allowed us to run properly powered one-way analyses of variance.
3.2 Identification of three psychological well-being groups by principal component and hierarchical cluster analyses
The principal component analysis produced two components, which cumulatively explained 73.62% of the variance between subjects. The first component contained the sucrose preference test and the shock probe burying test. Open field activity and social exploration loaded on the second component (see Table 4). Both components had Eigenvalues greater than 1, and explained a significant proportion of the variance between subjects. They were retained for subsequent analyses.
Table 4.
Components of principal component analyses. Component loadings are shown for the behavioral tests retained. To be retained, behavioral tests had to load with >.03 on one component only.
| Behavioral test | Components |
|
|---|---|---|
| 1 | 2 | |
|
| ||
| Sucrose preference | .178 | .817 |
| Social exploration | .872 | −.150 |
| Open field activity | .872 | .214 |
| Shock probe burying | .120 | −.802 |
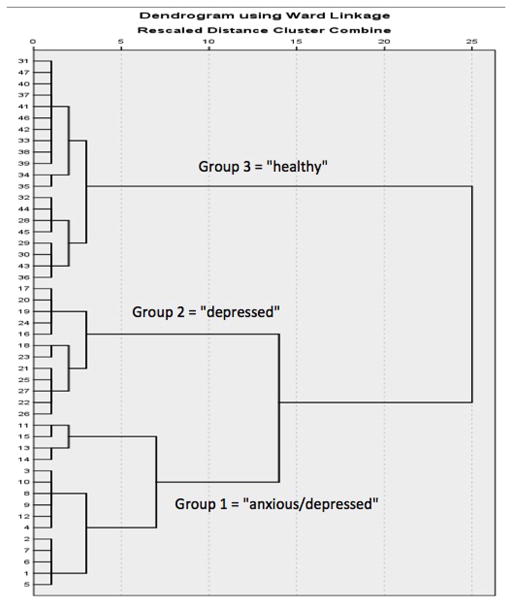
Average scores (derived from both Test Phases 1 and 2), for each of the behavioral tests retained in the principal component analysis (sucrose preference, shock probe burying, open field and social exploration), were used in the hierarchical cluster analysis. The dendrogram produced by this analysis showed that subjects separated into three psychological well-being clusters, with 15 subjects in Hierarchical Cluster (HC) Group 1, 12 subjects in HC group 2, and 20 subjects in HC group 3 (see Figure 4).
Figure 4.
Hierarchical cluster dendrogram. The dendrogram illustrates the results of the hierarchical cluster analysis, which separated the sample into three main cohorts (Group 1 (n=15), Group 2 (n=12), Group 3 (n=20)), based on the subjects’ average performance on the behavioral tests retained by the principal component analyses. The numbers on the y-axis represent the individual subjects.
To identify the psychological well-being characteristics of each cluster, the three groups were compared on each of the behavioral measures retained in the principal component analysis (See section 3.3), across Test Phase 1 and 2. Based on their performance on these measures, subjects in HC group 1 were labeled as exhibiting anxiety and depression-like signs (ANX/DEP), subjects in HC group 2 as exhibiting depression-like signs (DEP) and subjects in HC group 3 as being healthy (HEALTHY).
To determine whether pre-injury behavior was predictive of post-injury behavior, and the clusters derived, we also compared baseline measures across the psychological well-being groups. Baseline differences were only significant for the social exploration and the open field activity test [F(2,44)=5.82, p<.05 and F(2,44)=12.88, p<.05, respectively]. Post-hoc analyses revealed that at baseline, the groups labeled as DEP and ANX/DEP on Day 10 showed lower activity in an open field relative to subjects identified as HEALTHY on Day 10 [p<.05]. Similarly, we found that the group subsequently identified as DEP on Day 10 exhibited less social exploration than the HEALTHY group [p<.05, (Figure 5)].
Figure 5.
Comparison of baseline measures of anxiety and depression (Mean ± SEM), across the psychological well-being groups. There were no significant baseline differences across groups for sucrose preference (A). However, the DEP and ANX/DEP showed lower activity in an open field relative to HEALTHY subjects at baseline (B), and the DEP group exhibited less social exploration than the HEALTHY group (C). Baseline differences were not found for the measure of passive anxiety, center time in the open field (D). * p <.05.
3.3 Hierarchical clusters differed on psychological well-being measures retained by PCA
Repeated measure ANOVAs revealed a main effect of psychological well-being (HC group) for the sucrose preference test [F(2,44)=8.39, p<.05], the open field activity test [F(2,43)=20.95, p<.05], the social exploration test [F(2,43)=7.67, p<.05], and the shock probe burying test [F(2,40)=41.32, p<.05].
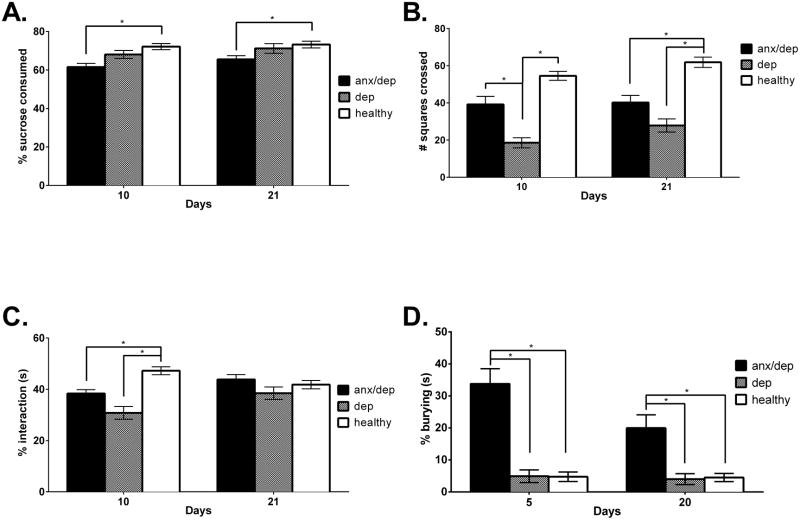
For sucrose preference, subsequent ANOVAs (on Days 10 and 21 separately) confirmed that the HC groups differed at both time points [F(2,44)=8.67, p<.05, F(2,44)=3.89, p<.05, for Day 10 and 21 respectively]. Post hoc analyses revealed that on both Day 10 and 21, the ANX/DEP group showed significantly lower sucrose preferences than the HEALTHY group [p<.05, Figure 6A].
Figure 6.
Post hierarchical cluster differences: The panels of graphs depict the mean (± SEM) behavioral performance of the ANX/DEP, DEP, and HEALTHY groups on the tests of depression and anxiety: Sucrose preference (A), Open field activity (B), Social exploration (C), and Shock probe burying (D). ANX/DEP subjects showed decreased sucrose preference relative to the HEALTHY group on Days 10 and 21, as well as increased shock probe burying relative to the DEP and HEALTHY groups on Days 5 and 20. The DEP group showed decreased open field activity relative to the ANX/DEP and HEALTHY group on Day 10 and relative to the HEALTHY group on Day 21. The DEP group also showed decreased social exploration on Day 10 relative to the HEALTHY group. * p <.05.
For open field activity, separate ANOVAs on Days 10 and 21 also confirmed main effects of psychological well-being at both time points [F(2,43)=14.93, p<.05, F(2,43)=11.65, p<.05.] Post-hoc analyses revealed that on Day 10, the DEP group showed decreased activity in an open field relative to the ANX/DEP and HEALTHY group [ps < .05] and at Day 21, both the ANX/DEP and the DEP group showed decreased activity relative to the HEALTHY group [ps ≤ .05, Figure 6B].
On the social exploration test, subsequent ANOVAs revealed that the HC groups differed significantly on Day 10 [F(2,43)=14.27, p <.05]. Post-hoc analyses revealed that on Day 10, both the ANX/DEP and the DEP group showed significantly lower social exploration time than the HEALTHY group [ps ≤ .05, Figure 6C].
Finally, on the shock probe burying test, separate ANOVAs for Days 5 and 20 confirmed that the HC groups differed significantly on shock probe burying at both time points [F(2,43)=28.66, p<.05, F(2,44)=11.12, p<.05]. Post-hoc analyses revealed that the ANX/DEP group spent a significantly higher percent time burying, than both the DEP and HEALTHY groups, on both Days 5 and 20 [ps ≤ .05, Figure 6D].
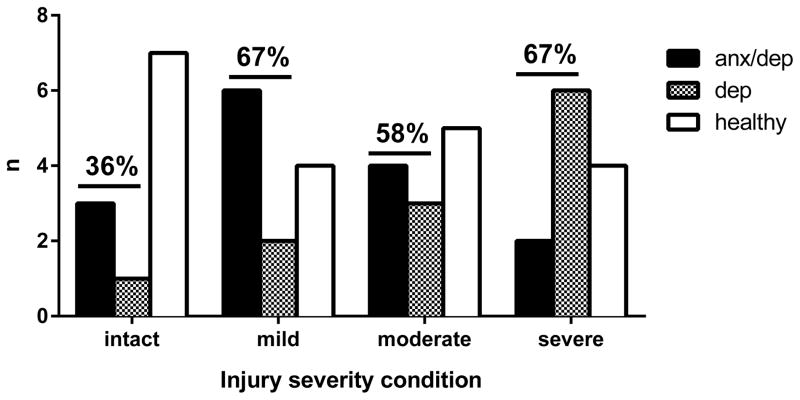
As shown in Figure 7, examining group membership by psychological well-being and injury severity, we found that most intact subjects were classified as HEALTHY, whereas most contused subjects were classified either as DEP or ANX/DEP. Although it did appear that the number of DEP subjects increased with injury severity and that the number of ANX/DEP subjects decreased with injury severity, no significant trend emerged indicating a relationship between increased injury severity and the number of subjects classified as either DEP or ANX/DEP.
Figure 7.
Psychological well-being membership by injury severity. This figure shows group membership by injury severity, and indicates the percentage of subjects identified as either anxious/depressed or depressed for each injury severity level.
Finally, group membership was confirmed by random forest analyses. Consistent with the previous analysis, we performed PCA on average scores (derived from both Test Phase 1 and 2), and then grew one hundred thousand trees. The classification accuracy of the model on the test data out of bag samples) was recorded. The model achieved 90.48% correct classification accuracy on test data. In addition to confirming the groups obtained from PCA and HC analysis, the Random Forest algorithm generated a model that can be used for future data point prediction (code can be used at this link: https://sites.google.com/site/druseffaghihi/research-interests/spinal-cord-injury). Random Forest was implemented in R using Random Forest library (Liaw and Wiener 2002).
3.4 Clusters differed on additional measures of anxiety as well as recovery of function, pain and physiological markers following spinal cord injury
Next, we compared the psychological well-being cohorts on measures other than those used for hierarchical clustering including center activity, recovery of function, pain, and physiological markers. Pearson-product moment correlations were also conducted to further investigate associations between pain reactivity (tactile and tail-flick tests), behavioral measures (open field activity, social exploration, sucrose preference, forced swim, shock probe burying, and center activity), recovery of function (BBB scores) and peripheral inflammation (serum alpha-2 macroglobulin levels). Significant correlations are reported.
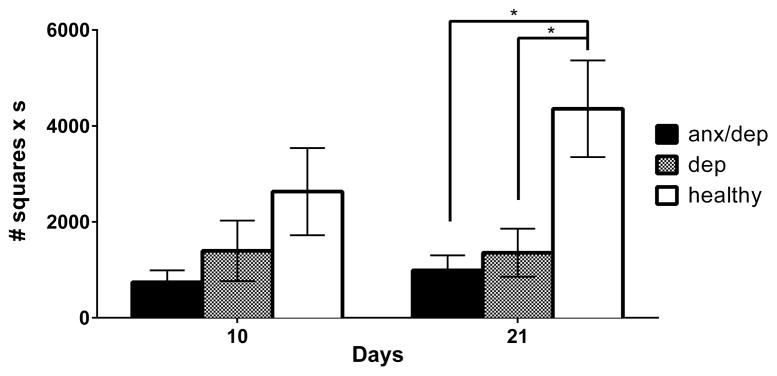
Center activity
A repeated measure ANOVA by psychological well-being revealed a main effect of psychological well-being for center activity [F(2,44)=4.19, p<.05]. Subsequent separate ANOVAs, for Days 10 and 21, confirmed a main effect of psychological well-being on Day 21 [F(2,43)=5.63, p<.05]. Post-hoc Sidak comparisons indicated that on Day 21, both the ANX/DEP and DEP groups showed reduced center activity relative to the HEALTHY group [ps ≤ .05, Figure 8].
Figure 8.
Center activity as measure of passive anxiety. The mean (± SEM) performance of the ANX/DEP, DEP, and HEALTHY for center activity in an open field is depicted for Days 10 and 21. On Day 21, the ANX/DEP and DEP groups showed decreased center activity relative to the HEALTHY group. * p <.05.
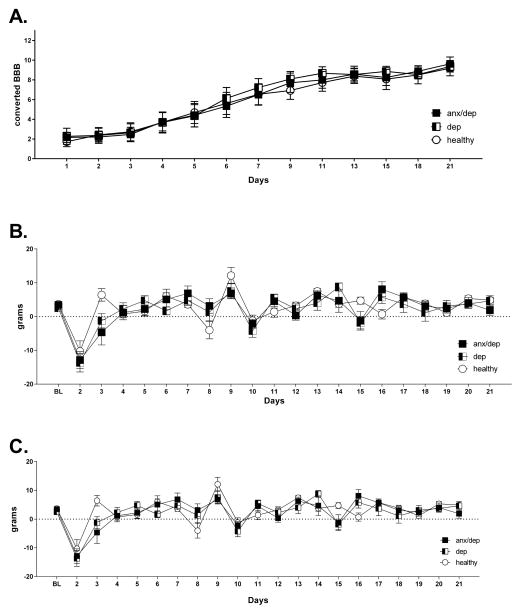
Recovery of function
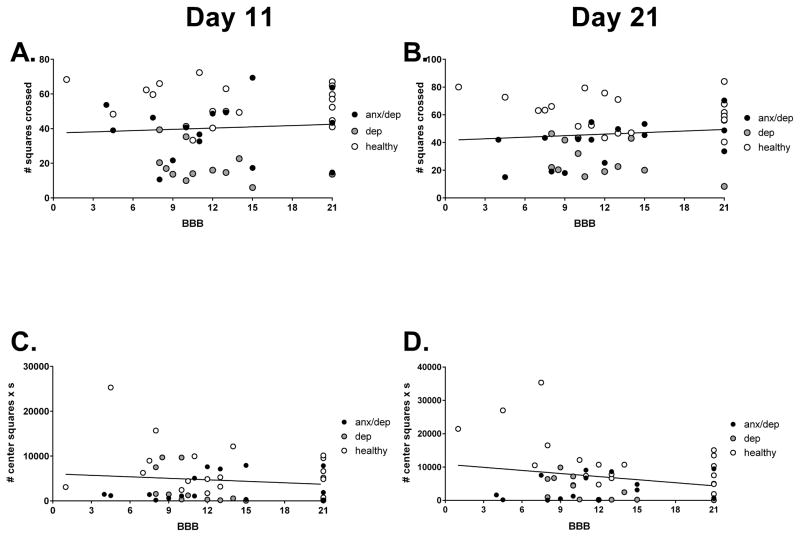
Repeated measures ANOVAs did not reveal any significant differences among psychological well-being groups for locomotor recovery, weight gain, or food consumption (see Figure 9). There was also no correlation between BBB scores and performances on the behavioral tests of depression and anxiety. Figure 10 shows the absence of correlation between BBB scores and open field activity and center activity.
Figure 9.
Recovery measures by psychological well-being. No differences were found among ANX/DEP, DEP and HEALTHY groups for converted BBB scores (A), weight gain (B), or food consumption (C).
Figure 10.
No correlations were found between BBB scores and performances on behavioral tests of depression [open field (A) and (B)] and anxiety [center activity in an open field (C) and (D)].
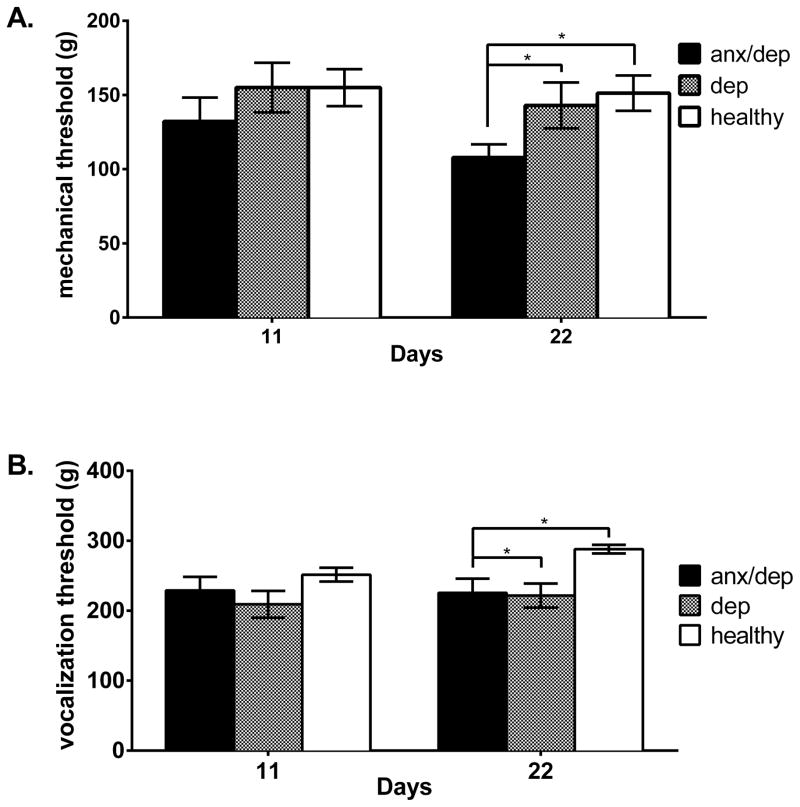
Pain reactivity
While there was no effect of psychological well-being on thermal reactivity, a repeated measures ANOVA revealed a trend for differences among psychological well-being groups for paw withdrawal thresholds on the tactile test [F(2,44)=2.758, p >.05]. Subsequent ANOVAs on Days 11 and 22 separately confirmed a main effect of psychological well-being on Day 22 [F(2,44)=3.451, p <.05]. Post-hoc Sidak comparisons indicated that on Day 22, the ANX/DEP group showed significantly lower paw withdrawal thresholds than the HEALTHY group [p <.05, Figure 11A]. Similarly, there was a main effect of psychological well-being for the vocalization threshold on the tactile test [F(2,44)=8.75, p <.05]. Subsequent ANOVAs, comparing vocalization thresholds on Days 11 and 22 separately, confirmed a main effect of psychological well-being on test Day 22 [F(2,44)=7.024, p <.05], but not on Day 11. Post-hoc Sidak comparisons by psychological well-being indicated that on Day 22, the DEP group and the ANX/DEP group showed a lower vocalization threshold than the HEALTHY group [ps <.05, Figure 11B]. Vocalization thresholds on Day 22 were negatively correlated with percent time spent burying on the shock probe burying test on Day 20 [r = −.30, p < .05, data not depicted].
Figure 11.
Tactile withdrawal (A) and vocalization thresholds (B) measures by psychological well-being, on Days 11 and 22. The mean (± SEM) performance of the ANX/DEP, DEP, and HEALTHY groups are shown on the tactile tests. The ANX/DEP group showed a lower paw withdrawal threshold than the HEALTHY group on Day 22. Similarly, both the ANX/DEP and the DEP group showed a lower vocalization threshold than the HEALTHY group. * p <.05.
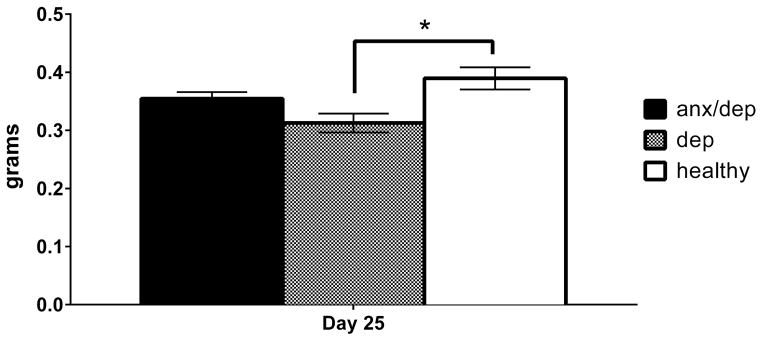
Thymus
An ANOVA revealed a main effect of psychological well-being on thymus weight, with or without intact subjects included in the analysis [F(2,44)= 5.09, p <.05, F(2,33)= 5.16, p <.05, respectively]. Post-hoc Sidak comparisons confirmed that thymus weight was significantly decreased in the DEP group relative to the HEALTHY group [p < .05, Figure 12].
Figure 12.
Thymus weight by psychological well-being groups (intact subjects excluded). The mean (± SEM) thymus weight of the DEP group was significantly lower than that of the HEALTHY group. * p <.05.
Alpha-2 macroglobulin
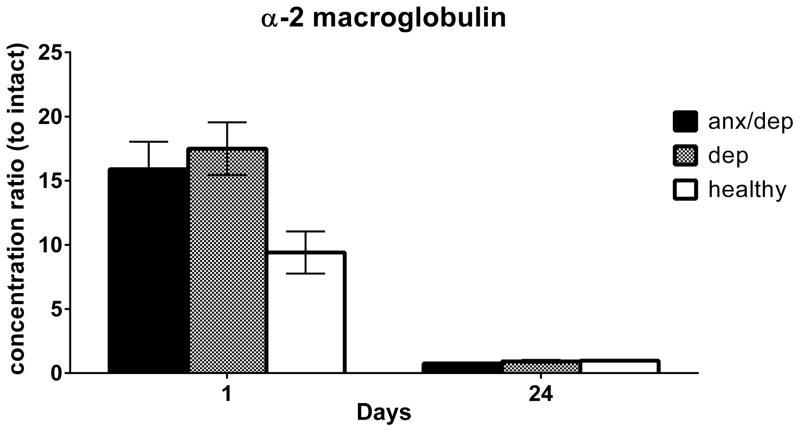
It was expected that levels of alpha-2 macroglobulin would be extremely low in intact subjects. To obtain alpha-2 macroglobulin values relative to controls (intact subjects), intact subjects were excluded from analyses, and all alpha-2 macroglobulin levels were normalized to intact animals by dividing each data point for a given day of collection by the intact subjects’ average level of alpha-2 macroglobulin for that same day. Furthermore, because alpha-2 macroglobulin is an acute phase protein, we expected group differences to be evident shortly after the spinal cord injury only. Therefore, we conducted ANOVAs on days 1 and 24 separately. There was no main effect of injury severity for alpha-2 macroglobulin on either day, but there was a trend towards a main effect of psychological well-being on Day 1 post injury [F(2,31)= 3.01, p >.05]. Post-hoc analyses revealed a trend for both the ANX/DEP and DEP groups to show increased levels of serum alpha-2 macroglobulin on Day 1 relative to the HEALTHY group [p ≥.05, Figure 13].
Figure 13.
Alpha-2 macroglobulin levels in serum on Days 1 and 24 by psychological well-being. The mean (± SEM) alpha-2 macroglobulin levels, normalized to intact subjects, are shown by psychological well-being. A trend for both the ANX/DEP and DEP groups to show increased levels of serum alpha-2 macroglobulin on Day 1 relative to the HEALTHY group was found, p = .16 and .09 respectively.
3.5 The immune response differs by psychological well-being subtype
One-way ANOVAs were conducted to examine the effects of psychological well-being on cytokine and chemokine levels in the serum, hippocampi, and spinal cord. For these analyses samples from only half of the subjects were run, due to plate space limitations and costs. The subsets of subjects analyzed were randomly selected and were representative of the behavioral characteristics associated with their psychological well-being cohort. Serum IL-6 median fluorescent intensities were out of range for all subjects; therefore, pg/mL concentrations were not obtained.
Serum levels of pro-inflammatory cytokines are increased in depressed and anxiety/depressed clusters relative to the healthy cluster
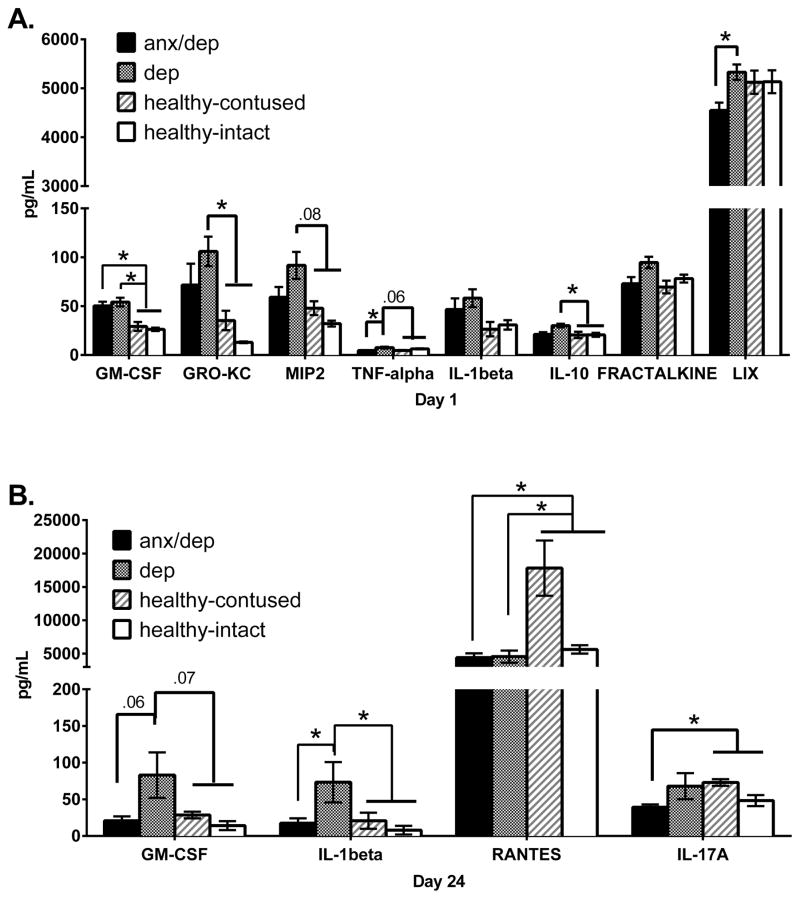
We assessed the levels of 27 cytokine/chemokines in serum on Day 1 and Day 24 post SCI. On Day 1 post SCI, one-way ANOVAs only found a main effect of injury severity for MCP-1 [F(3,26)=7.11, p<.05]. Post-hoc Sidak comparisons indicated that the intact group exhibited a significantly lower level of MCP-1 than the moderate and severe injury groups [ps ≤ .05]. Collapsing across injury conditions, one-way ANOVAs revealed a main effect of psychological well-being for GM-CSF [F(2,27)=11.923, p <.05], GRO-KC [F(2,27)=5.832, p <.05], MIP-2 [F(2,27)=5.66, p <.05], TNF-α [F(2,27)=4.889, p <.05], IL-10 [F(2,27)=4.098, p <.05], Fractalkine [F(2,27)=3.537, p <.05], and LIX [F(2,27)=3.731, p <.05]. Post-hoc Sidak comparisons revealed that the DEP and ANX/DEP groups showed higher levels of GM-CSF than the HEALTHY group [ps ≤ .05]. The DEP group also exhibited levels of GRO-KC and MIP-2 that were higher than those of the HEALTHY group [ps ≤ .05]. Moreover, the DEP group showed levels of TNF-α and LIX that were higher than those of the ANX/DEP group [ps ≤ .05]. Finally, the DEP group showed higher levels of IL-10 than the HEALTHY group [p <.05]. Levels of other analytes did not differ by psychological well-being group (Figure 14A).
Figure 14.
The serum expression levels of pro- and anti-inflammatory cytokines in the serum on Days 1 (A) and 24 (B). The mean (± SEM) concentration of analytes that differed significantly across psychological well-being groups are shown. The graph depicts the original data, with the HEALTHY group separated into healthy contused subjects and healthy intact subjects to denote possible HEALTHY group differences due to intact subjects. The statistical differences reported here are based on post-hoc Sidak comparisons between the ANX/DEP, DEP and HEALTHY groups, with intacts removed. The ANX/DEP and DEP groups showed increases in both pro-inflammatory cytokines and chemokines on both days 1 and 24. * p <.05.
On Day 24 post SCI, no main effects of injury severity were found. However, one-way ANOVAs revealed a main effect of psychological well-being for GM-CSF [F(2,33)=5.372, p <.05], IL-1β [F(2,33)=4.561, p<.05], Rantes [F(2,33)=4.042, p <.05], and IL-17A [F(2,32)=3.321, p <.05]. Post-hoc Sidak comparisons revealed that the DEP group showed higher levels of GM-CSF and IL-1β than the ANX/DEP and the HEALTHY group [ps ≤ .05]. Both the DEP group and the ANX/DEP group also showed lower levels of Rantes than the HEALTHY group [ps ≤ .05]. Finally, the ANX/DEP group showed lower levels of IL-17A than the HEALTHY group [p <.05]. Although no significant differences were found in anti-inflammatory cytokines, a trend is observed for IL-13, with the DEP group showing increased levels relative to both the ANX/DEP and the HEALTHY group (Figure 14B).
Increase in hippocampal levels of pro-inflammatory cytokines is observed in anxiety/depressed subgroup only
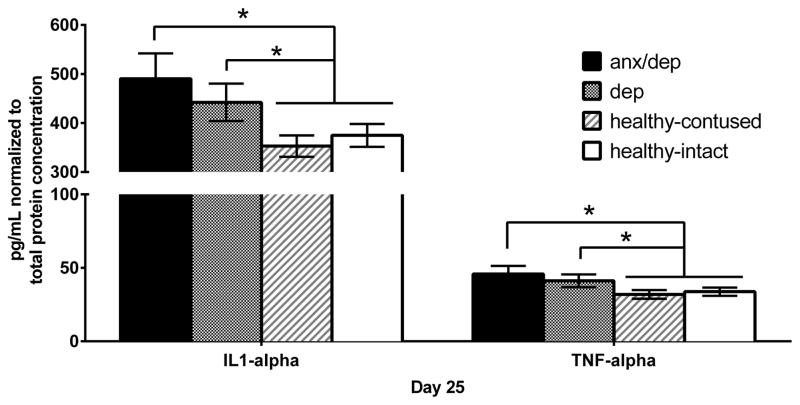
We assessed the levels of 27 cytokine/chemokines in the hippocampi protein supernatant (left and right combined). No main effects of injury severity were found. However, one-way ANOVAs revealed a main effect of psychological well-being for two pro-inflammatory cytokines, IL-1α [F(2,32)=4.463, p <.05] and TNF-α [F(2,32)=3.537, p <.05]. Post-hoc Sidak comparisons revealed that the ANX/DEP group showed higher levels of IL-1α and TNF-α than the HEALTHY group [ps ≤ .05]. No significant differences were found for anti-inflammatory cytokines and chemokines (Figure 15).
Figure 15.
The mean (± SEM) concentration of analytes in lysate (normalized to total protein concentration) found to differ by psychological well-being are shown. The graph depicts the original data, with the HEALTHY group separated into healthy contused subjects and healthy intact subjects to identify possible HEALTHY group differences driven by intact subjects (rather than psychological well-being). The statistical differences reported here are based on post-hoc Sidak comparisons between the ANX/DEP, DEP and HEALTHY groups, with intacts removed. As the graphs indicate, even with intacts removed, the ANX/DEP group shows increases in both pro-inflammatory cytokines and chemokines.*p<.05.
Increase in spinal cord levels of pro-inflammatory cytokines is observed in anxiety/depressed subgroup only
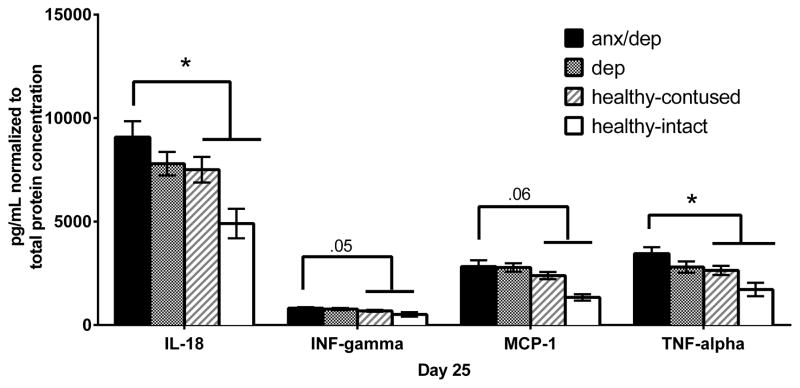
We assessed the levels of 20 cytokine/chemokines in the spinal cord protein supernatant. One-way ANOVAs by psychological well-being revealed a main effect for IL-18 [F(2,34)=3.426, p <.05], IFN-γ [F(2,34)=3.516, p <.05], MCP-1 [F(2,34)=3.849, p <.05], and TNF-α [F(2,34)=4.496, p <.05]. Post-hoc Sidak comparisons revealed that the ANX/DEP group showed higher levels of IL-18, MCP-1, TNF-α, and IFN-γthan the HEALTHY group [ps ≤ .05 for all except MCP-1, p >.05; Figure 16].
Figure 16.
The mean (± SEM) concentration of analytes in lysate (normalized to total protein concentration) found to differ by psychological well-being are shown. The graph depicts the original data, with the HEALTHY group separated into healthy contused subjects and healthy intact subjects to denote possible HEALTHY group differences due to intact subjects alone. The statistical differences reported here are based on post-hoc Sidak comparisons between the ANX/DEP, DEP and HEALTHY groups, with intacts removed. As the graphs indicate, even with intacts removed, the ANX/DEP group shows increases in both pro-inflammatory cytokines and chemokines. * p <.05.
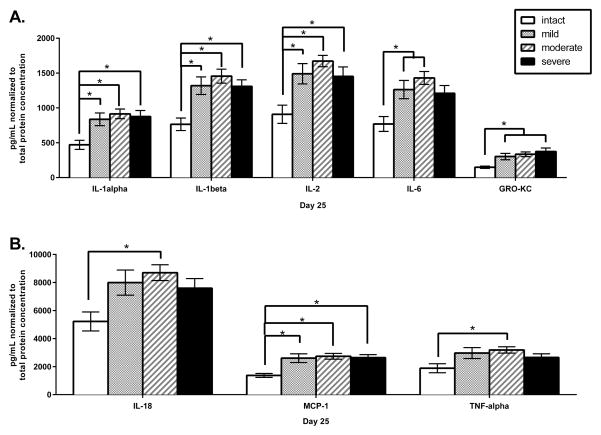
One-way ANOVAs by injury severity revealed a main effect for IL-1α [F(3,32)=4.803, p <.05], IL-1β [F(3,33)=6.361, p <.05], IL-2 [F(3,33)=5.456, p <.05], IL-6 [F(3,33)=4.931, p <.05], IL-18 [F(3,33)=3.349, p <.05], GRO-KC [F(3,33)=4.452, p <.05], MCP-1 [F(3,33)=5.636, p <.05], and TNF-α [F(3,33)=2.838, p <.05]. Post-hoc Sidak comparisons revealed that the mild, moderate, and severe injury groups had higher levels of IL-1α, IL-1β, IL-2, and MCP-1 than the intact group [ps ≤ .05, Figure 17]. The mild and moderate injury groups showed higher levels of IL-6 than the intact group [ps ≤ .05]. The moderate and severe injury groups showed higher levels of GRO-KC than the intact group [ps ≤ .05]. Finally, the moderate injury group showed higher levels of IL-18 and TNF-α than the intact group [ps ≤ .05]. No main effects of injury severity were observed for the anti-inflammatory or the unclassified cytokines/chemokines.
Figure 17.
The mean (± SEM) concentrations of pro-inflammatory cytokines and chemokines in lysate (normalized to total protein concentration) are shown for the intact, mild, moderate and severe injury groups. Analytes are separated into graphs A and B to clearly show differences for cytokines/ chemokines with higher or lower concentrations. * p <.05.
4. Discussion
Consistent with the incidence of depression and anxiety in the clinical population, we found that 63.8% of contused rodents displayed depression or anxiety-like signs following SCI. Specifically, one subgroup of contused rodents exhibited a purely depression-like profile (30.5% of contused subjects), while a second exhibited a profile characterized by both depression and anxiety-like signs combined (33.3%). The random forest analysis confirmed the hierarchical cluster and principal component analyses, with 90% concordance for subject placement in the anxiety and depression subgroups. Behaviorally, the depressed subgroup showed symptoms thought to be analogous to depression in humans, such as psychomotor retardation, decreased interest in social interactions, and passive anxiety. Similarly the anxious/depressed subgroup showed depression-like symptoms, such as anhedonia, psychomotor retardation and decreased interest in social interactions, as well as active and passive anxiety.
Importantly, the behavioral differences observed, between psychological well-being subgroups, were not simply due to differences in motor ability. There was no relationship between psychological well-being and locomotor recovery post SCI. Moreover, performance on the behavioral tests of depression and anxiety did not decrease as a function of injury severity. In fact, the severe injury group displayed greater movement in some tests, such as the open field activity test. These data concur with our previous studies (Luedtke et al. 2014). Luedtke et al. (2014) also found that locomotor recovery did not differ between depressed and not depressed SCI subjects, and found no correlation between white matter sparing in the contused spinal cord and behavioral performances on the tests of depression (i.e. open field activity). The behaviors observed, therefore, appear to be valid measures of psychomotor retardation and reduced motivation, characteristics of depression and anxiety, in the rodent model of SCI.
While locomotor function was not related to psychological well-being, there was a relationship between pain and anxiety. We found vocalization thresholds on the tactile test to be negatively correlated with anxiety-like signs. This supports the clinical observations of comorbidity between pain and anxiety (Yalcin and Barrot 2014). Because this effect was subtle, however, it is unlikely that it explains the manifestation of depression-like behavior. Rather, we propose that it may be a second outcome of a common underlying cause, such as increased inflammation. Interestingly, there were also some differences in pre-injury behavior among the three psychological well-being groups. The group later identified as “anxious/depressed” and “depressed” displayed lower psychomotor activity prior to injury than the group later identified as “healthy.” These data suggest that pre-injury behavioral characteristics may help predict psychological well-being following SCI and that, not surprisingly, pre-dispositions may be exacerbated by injury. The extent to which potentially latent individual predispositions for depression and/or anxiety affects psychological and physical recovery (i.e., pain reactivity), as well as molecular responses to injury, needs to be further explored (Maldonado and Hook, 2014).
4.1 Spinal cord injury results in increased inflammation
While individual differences may predict post-injury well-being, our data suggest that psychological well-being is also related to differences in levels of pro and anti-inflammatory cytokines following SCI. As predicted, we found that SCI results in increased inflammation both peripherally and centrally. We observed significant increases in serum alpha-2 macroglobulin, an acute phase protein, in contused subjects one day post SCI. Further, focusing on injury alone (without psychological well-being), we found that the cytokine and chemokine levels of the contused subjects were only significantly increased in the spinal cord. Also, despite significant increases in spinal cytokine expression with SCI, we did not find that cytokine or chemokine levels increased as a function of injury severity. This contrasts with previous studies showing an increase in inflammation in the spinal tissue as injury severity increases, but was likely affected by the time of tissue collection (Yang, Jones et al. 2005). Our results suggest that injury severity dependent changes in inflammation may only be present transiently, at the level of the spinal cord, and may not transfer to an injury severity dependent change in inflammation in the periphery, or a long-term change in inflammation in the central nervous system.
While injury severity did not affect cytokine levels in the spinal tissue, we found that serum, hippocampal, and spinal cord cytokine and chemokine levels were elevated in subsets of SCI rodents even 25 days post SCI. These subjects exhibited distinct behavioral profiles associated with depression and anxiety. These data are reminiscent of the increased serum levels of pro-inflammatory cytokines reported more than one year after the injury in a subpopulation of spinal cord injured patients (Hayes, Hull et al. 2002).
4.2 Site of inflammation varies by depression sub-type
We found both depression and anxiety to be associated with higher levels of an acute phase protein, alpha-2 macroglobulin on Day 1 post SCI. Similarly, human studies have found depression and anxiety to be associated with higher levels of circulating C-reactive protein, an acute phase protein equivalent to alpha-2 macroglobulin in rats (Howren, Lamkin et al. 2009, Copeland, Shanahan et al. 2012). However, when we examined the inflammation changes post SCI by psychological well-being sub-group more closely, we found two interesting and distinctive patterns: the purely depressed sub-group showed increased inflammation in the periphery, whereas the anxious/depressed group showed increased inflammation in the central nervous system.
The depressed subjects had increased serum levels of several pro-inflammatory cytokines and chemokines in both the acute and chronic phase of injury. Specifically, depressed subjects had significant increases in IL-1β, GM-CSF, GRO-KC, MIP-2, LIX and TNF-α levels, relative to anxious/depressed and/or healthy subjects. As summarized in Table 1, these cytokines have been implicated in depression in the human clinical population, and in other animal models. In fact, there is a growing body of data implicating pro-inflammatory cytokines as causal factors in depression (Maes, Bosmans et al. 1997, Maes 1999, van West and Maes 1999, Dantzer, O'Connor et al. 2008, Audet and Anisman 2013, Walker, Kavelaars et al. 2014), particularly after a nervous system injury or illness (Norman et al. 2010; Juengst, Kumar et al. 2014). According to the cytokine hypothesis, pro-inflammatory cytokines may lead to the development of depression in several ways. First, cytokines such as TNF-α and interleukin-6 (IL-6) are known to increase the enzymatic activity of indolamine 2,3-dioxygenase (IDO) (Maes, Leonard, Myint, Kubera, & Verkerk, 2011; Roman, Kreiner, & Nalepa, 2013), which catalyzes the conversion of tryptophan into kynurenine, thereby decreasing the substrate availability for serotonin production (Capuron et al., 2003; Capuron et al., 2001). Alternatively, cytokines such as IL-6 and TNF-α, assist in the breakdown of 5-hydroxytryptophan (5-HT) into 5-hydroxyindoleacetic acid (5-HIAA). Thus, cytokines not only reduce the amount of serotonin produced, but can also increase the amount of serotonin degraded, thereby contributing to the development of depression.
Whereas the cytokine hypothesis supposes a direct relationship between cytokines and serotonin levels, the HPA axis hypothesis suggests that increased levels of cytokines such as interleukin-1 (IL-1), IL-6, TNF-α and IFN-γ contribute to the development of depression and anxiety via activation of the HPA axis and the alteration of glucocorticoid levels (Holsboer, 2000; Pariante & Miller, 2001). In our study, it is possible that elevated glucocorticoid levels might have contributed to increase inflammation in the hippocampus of anxious/depressed subjects. It would be important to measure levels of glucocorticoid levels in future studies. Indeed, it is likely that inflammation contributes to the development of depression in multiple ways; engaging discrete pathways with distinct pro-inflammatory cytokines. Moreover, as suggested by Anisman and Merali (1999), the symptoms of depression that are manifest may be modulated by the specific cytokine that precipitates the molecular changes. For example, IL-1 receptor knockout mice show decreased anxiety in an open field relative to wild type mice (Murray, Obiang et al. 2013). IL-1α was also significantly increased in the hippocampi of the subjects with signs of anxiety, relative to healthy controls, in the current study. IL-1β and TNF-α, pro-inflammatory cytokines elevated in the serum and hippocampi of the depression and anxiety groups respectively in the current study, have also been shown to induce anhedonia (Brebner et al. 2000) and reduce social interaction (Bluthe et al. 1994) in other models. Makino et al. (2000) also demonstrated that learned helplessness (immobility in the forced swim test) is increased by systemic IFN-α administration, which reduces 5-hydroxytryptamine (5-HT) mediated neuronal activity and synthesis (Morikawa et al. 1998; Menkes and MacDonald, 2000; Capuron et al. 2002). Although we did not measure serotonin levels in the current study, we found no changes for IFN-α (or immobility on the forced swim test) in the depressed subjects in the current study. While the precise mechanisms mediating the development of depression after SCI remain to be elucidated our data suggests that, as found for humans, pro-inflammatory cytokines may play a pivotal role. By developing animal models of depression and anxiety, and by identifying the associated cytokine profiles, we will be able to develop more efficacious, targeted therapeutic strategies for the treatment of clinical depression.
While depression was associated with changes in serum levels of pro-inflammatory cytokines, we did not find changes in the spinal cord or hippocampi of the depressed subjects, relative to healthy controls. We propose that depression per se may be associated with changes in brain areas other than the hippocampi. In fact, hippocampal changes are mostly observed in human depressed patients with comorbid anxiety, and in animal models in which depression is induced with chronic stress exposure (Price and Drevets 2010, Bora, Fornito et al. 2012, Marsden 2013). Similarly, in the current study, while depression alone was not associated with hippocampal changes, we did observe higher levels of TNF-α in the hippocampus of anxious/depressed subjects. For the anxious/depressed group, pro-inflammatory cytokines were also modulated in the spinal cord. IL-18, MCP-1, TNF-α, and IFN-γ were increased relative to healthy controls. These data concur with other studies showing that, for example, intracerebroventricular administration of TNF-α induces depression and anxiety behavior in rats and mice (Connor, Song et al. 1998, Kaster, Gadotti et al. 2012, Neis, Manosso et al. 2014), as well as reports demonstrating that anxiety in the open field test is highly suppressed in IL-18 knockout mice, compared to wild type mice (Yaguchi, Nagata et al. 2010). In future studies, it will be important to examine other brain regions, such as the frontal cortex, for depression-induced inflammation.
3. Conclusion
Overall, our results provide a first line of evidence for a relationship between increased peripheral cytokine levels and decreased psychological well-being following SCI. Human studies of SCI commonly observe depression post SCI, but are unable to identify clear predicting factors, for they are faced with a multitude of psychosocial variables such as financial difficulties and loss of independence. Here, with the use of an animal model, we avoided the confounding psychosocial factors. We were therefore able to demonstrate that even in a context devoid of psychosocial factors, SCI results in depression in a sub-group of injured subjects.
Importantly, we do not claim that psychosocial factors do not contribute to the decreased psychological well-being experienced by some patients after SCI. In fact, social stressors may interact with SCI to increase the immune response after injury. Indeed in the current study, single housing may have potentiated depression and/or anxiety-like signs for some subjects. We routinely house SCI subjects individually, particularly after surgery, to prevent inadvertent infections with wound opening when the animals interact with one another (i.e. grooming). However, social isolation is known to induce depression and anxiety in both humans and animal subjects (Liu, Wu et al. 2013, Krugel, Fischer et al. 2014). Given that all subjects were single housed (irrespective of condition), this variable alone cannot account for the differences observed across groups in the current study, but it may have potentiated individual predispositions. Future studies assessing the effects of social and environmental variables that might influence depression-like behavior, at both the molecular and behavioral level, will be important for understanding the significance of this variable on psychological well-being after SCI.
In sum, however, these data suggest that the depression and anxiety patients experience following SCI is not due solely to psychosocial factors, but may also result from increased immune activation inherent to the injury per se. Spinal cord injured patients with chronic increases in peripheral and central inflammation may be susceptible to the development of depression and anxiety. For these patients, medications that reduce the immune response may be more effective than traditional anti-depressants, targeting monoamine or HPA axis dysregulation. Further elucidation of the molecular mechanisms underlying depression and anxiety, such as those engaged by pro-inflammatory cytokines will significantly improve our understanding of mental health following SCI, and improve quality of life for spinal cord injured patients.
HIGHLIGHTS.
Three groups emerged post SCI: 1) depression, 2) depression/anxiety, 3) neither.
Inflammation post SCI was associated with decreased psychological well-being.
Depression-like profiles were associated with peripheral inflammation.
Anxiety/depression-like profiles were associated with brain/spinal inflammation.
Acknowledgments
These experiments were supported by DA 031197 (to M.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Sattar AB. Predictors of functional outcome in patients with traumatic spinal cord injury after inpatient rehabilitation: in Saudi Arabia. NeuroRehabilitation. 2014;35(2):341–347. doi: 10.3233/NRE-141111. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11(8):963–972. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- Audet MC, Anisman H. Interplay between pro-inflammatory cytokines and growth factors in depressive illnesses. Front Cell Neurosci. 2013;7:68. doi: 10.3389/fncel.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake S, Selvamani A, Cherry J, Sohrabji F. Blood brain barrier and neuroinflammation are critical targets of IGF-1-mediated neuroprotection in stroke for middle-aged female rats. PLoS One. 2014;9(3):e91427. doi: 10.1371/journal.pone.0091427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. Journal of Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Boakye M, Leigh BC, Skelly AC. Quality of life in persons with spinal cord injury: comparisons with other populations. JNS: Spine Special Supplements. 2012;17(1):29–37. doi: 10.3171/2012.6.AOSPINE1252. [DOI] [PubMed] [Google Scholar]
- Boneberg EM, Hartung T. Molecular aspects of anti-inflammatory action of G-CSF. Inflamm Res. 2002;513:119–128. doi: 10.1007/pl00000283. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138(1–2):9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. Interferon-alpha–induced changes in tryptophan metabolism. Biological psychiatry. 2003;54(9):906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Gualde N, Bosmans E, Dantzer R, Maes M, Neveu PJ. Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology. 2001;26(8):797–808. doi: 10.1016/s0306-4530(01)00030-0. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun. 2004;18(3):205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Choong ML, Yong YP, Tan AC, Luo B, Lodish HF. LIX: a chemokine with a role in hematopoietic stem cells maintenance. Cytokine. 2004;25(6):239–245. doi: 10.1016/j.cyto.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Christensen MD, Hulsebosch CE. Chronic central pain after spinal cord injury. J Neurotrauma. 1997;14(8):517–537. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Song C, Leonard BE, Merali Z, Anisman H. An assessment of the effects of central interleukin-1beta, -2, -6, and tumor necrosis factor-alpha administration on some behavioural, neurochemical, endocrine and immune parameters in the rat. Neuroscience. 1998;84(3):923–933. doi: 10.1016/s0306-4522(97)00533-2. [DOI] [PubMed] [Google Scholar]
- Cook DN. The role of MIP-1 alpha in inflammation and hematopoiesis. J Leukoc Biol. 1996;59(1):61–66. doi: 10.1002/jlb.59.1.61. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Generalized anxiety and C-reactive protein levels: a prospective, longitudinal analysis. Psychol Med. 2012;42(12):2641–2650. doi: 10.1017/S0033291712000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin NM, Boyle NT, Mills KH, Connor TJ. Psychological stress suppresses innate IFN-gamma production via glucocorticoid receptor activation: reversal by the anxiolytic chlordiazepoxide. Brain Behav Immun. 2009;23(4):535–547. doi: 10.1016/j.bbi.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Dahl J, Ormstad H, Aass HC, Malt UF, Bendz LT, Sandvik L, Brundin L, Andreassen OA. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. 2014;45:77–86. doi: 10.1016/j.psyneuen.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. European Journal of Pharmacology. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVivo MJ, Black KJ, Richards JS, Stover SL. Suicide following spinal cord injury. Paraplegia. 1991;29(9):620–627. doi: 10.1038/sc.1991.91. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Driscoll KE. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res. 1994;20(6):473–490. doi: 10.3109/01902149409031733. [DOI] [PubMed] [Google Scholar]
- Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168(7):3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46(9):1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- Dumont RJ, Okonkwo DO, Verma S, Hurlbert JR, Boulos PT, Ellegala DB, Dumont AS. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clinical Neuropharmacology. 2001;24(5):254–264. doi: 10.1097/00002826-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Elliott TR, Frank RG. Depression following spinal cord injury. Arch Phys Med Rehabil. 1996;77(8):816–823. doi: 10.1016/s0003-9993(96)90263-4. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68(4):437–446. [PubMed] [Google Scholar]
- Ferguson AR, Hook MA, Garcia G, Bresnahan JC, Beattie MS, Grau JW. A simple post hoc transformation that improves the metric properties of the BBB scale for rats with moderate to severe spinal cord injury. J Neurotrauma. 2004;21(11):1601–1613. doi: 10.1089/neu.2004.21.1601. [DOI] [PubMed] [Google Scholar]
- Gelis A, Daures JP, Benaim C, Kennedy P, Albert T, Colin D, Joseph PA, Pelissier J, Fattal C. Evaluating self-reported pressure ulcer prevention measures in persons with spinal cord injury using the revised Skin Management Needs Assessment Checklist: reliability study. Spinal Cord. 2011;49(5):653–658. doi: 10.1038/sc.2010.177. [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R, Friedman J, Franklin J. The elements of statistical learning: data mining, inference and prediction. The Mathematical Intelligencer. 2005;27(2):83–85. [Google Scholar]
- Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E. Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. Journal of Neurotrauma. 2000;17(3):203–218. doi: 10.1089/neu.2000.17.203. [DOI] [PubMed] [Google Scholar]
- Hayes KC, Hull TC, Delaney GA, Potter PJ, Sequeira KA, Campbell K, Popovich PG. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma. 2002;19(6):753–761. doi: 10.1089/08977150260139129. [DOI] [PubMed] [Google Scholar]
- Herrick S, Elliott TR, Crow F. Self-appraised problem-solving skills and the prediction of secondary complications among persons with spinal cord injuries. J Clin Psychol Med Settings. 1994;1(3):269–283. doi: 10.1007/BF01989628. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Jezierski MK, Sohrabji F. Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol Aging. 2001;22(2):309–319. doi: 10.1016/s0197-4580(00)00230-x. [DOI] [PubMed] [Google Scholar]
- Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect. 2013;2:e60. doi: 10.1038/emi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokic N, Yip P, Michael-Titus A, Priestley J, Malaspina A. The human G93A-SOD1 mutation in a pre-symptomatic rat model of amyotrophic lateral sclerosis increases the vulnerability to a mild spinal cord compression. BMC Genomics. 2010;11(1):633. doi: 10.1186/1471-2164-11-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juengst SB, Kumar RG, Failla MD, Goyal A, Wagner AK. Acute Inflammatory Biomarker Profiles Predict Depression Risk Following Moderate to Severe Traumatic Brain Injury. J Head Trauma Rehabil. 2014 doi: 10.1097/HTR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- Kapp A, Zeck-Kapp G, Czech W, Schopf E. The chemokine RANTES is more than a chemoattractant: characterization of its effect on human eosinophil oxidative metabolism and morphology in comparison with IL-5 and GM-CSF. J Invest Dermatol. 1994;102(6):906–914. doi: 10.1111/1523-1747.ep12383399. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Gadotti VM, Calixto JB, Santos AR, Rodrigues AL. Depressive-like behavior induced by tumor necrosis factor-alpha in mice. Neuropharmacology. 2012;62(1):419–426. doi: 10.1016/j.neuropharm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Kim HY, Cha HJ, Kim HS. CCL5 upregulates IL-10 expression and partially mediates the antihypertensive effects of IL-10 in the vascular smooth muscle cells of spontaneously hypertensive rats. Hypertens Res. 2015 doi: 10.1038/hr.2015.62. [DOI] [PubMed] [Google Scholar]
- Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21(12):1303–1309. doi: 10.1093/intimm/dxp102. [DOI] [PubMed] [Google Scholar]
- Krieger M, Both M, Kranig SA, Pitzer C, Klugmann M, Vogt G, Draguhn A, Schneider A. The hematopoietic cytokine granulocyte-macrophage colony stimulating factor is important for cognitive functions. Sci Rep. 2012;2:697. doi: 10.1038/srep00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugel U, Fischer J, Bauer K, Sack U, Himmerich H. The impact of social isolation on immunological parameters in rats. Arch Toxicol. 2014;88(3):853–855. doi: 10.1007/s00204-014-1203-0. [DOI] [PubMed] [Google Scholar]
- Liaw A, Wiener M. Classification and regression by randomForest. R news. 2002;2(3):18–22. [Google Scholar]
- Liu YN, Peng YL, Liu L, Wu TY, Zhang Y, Lian YJ, Yang YY, Kelley KW, Jiang CL, Wang YX. TNFalpha mediates stress-induced depression by upregulating indoleamine 2,3-dioxygenase in a mouse model of unpredictable chronic mild stress. Eur Cytokine Netw. 2015;26(1):15–25. doi: 10.1684/ecn.2015.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wu R, Tai F, Ma L, Wei B, Yang X, Zhang X, Jia R. Effects of group housing on stress induced emotional and neuroendocrine alterations. Brain Res. 2013;1502:71–80. doi: 10.1016/j.brainres.2013.01.044. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139(3):230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ho RC, Mak A. The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int J Rheum Dis. 2012;15(2):183–187. doi: 10.1111/j.1756-185X.2011.01673.x. [DOI] [PubMed] [Google Scholar]
- Luedtke K, Maldonado-Bouchard S, Woller SA, Funk MK, Aceves M, Hook MA. Assessment of Depression in a Rodent Model of Spinal Cord Injury. J Neurotrauma. 2014 doi: 10.1089/neu.2013.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100(3):1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9(11):853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- Magalhaes PV, Jansen K, Stertz L, Ferrari P, Pinheiro RT, da Silva RA, Kapczinski F. Peripheral eotaxin-1 (CCL11) levels and mood disorder diagnosis in a population-based sample of young adults. J Psychiatr Res. 2014;48(1):13–15. doi: 10.1016/j.jpsychires.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Malaspina A, Jokic N, Huang W, Priestley J. Comparative analysis of the time-dependent functional and molecular changes in spinal cord degeneration induced by the G93A SOD1 gene mutation and by mechanical compression. BMC Genomics. 2008;9(1):500. doi: 10.1186/1471-2164-9-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malec J, Neimeyer R. Psychologic prediction of duration of inpatient spinal cord injury rehabilitation and performance of self-care. Arch Phys Med Rehabil. 1983;64(8):359–363. [PubMed] [Google Scholar]
- Marsden WN. Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:168–184. doi: 10.1016/j.pnpbp.2012.12.012. [DOI] [PubMed] [Google Scholar]
- McCullumsmith CB, Kalpazian C, Richards JS, Forchheimer M, Heinemann A, Richardson E, Wilson C, Barber J, Temkin N, Fann JR, Bombardier CH, Investigators P. Novel risk factors associated with current suicidal ideation and lifetime suicide attempt in individuals with spinal cord injury. Arch Phys Med Rehabil. 2015 doi: 10.1016/j.apmr.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Menard D, Tremblay E, Ferretti E, Babakissa C, Perron N, Seidman EG, Beaulieu JF. Anti-inflammatory effects of epidermal growth factor on the immature human intestine. Physiol Genomics. 2012;44(4):268–280. doi: 10.1152/physiolgenomics.00101.2011. [DOI] [PubMed] [Google Scholar]
- Menzies-Gow A, Ying S, Sabroe I, Stubbs VL, Soler D, Williams TJ, Kay AB. Eotaxin (CCL11) and eotaxin-2 (CCL24) induce recruitment of eosinophils, basophils, neutrophils, and macrophages as well as features of early- and late-phase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J Immunol. 2002;169(5):2712–2718. doi: 10.4049/jimmunol.169.5.2712. [DOI] [PubMed] [Google Scholar]
- Merali Z, Brennan K, Brau P, Anisman H. Dissociating anorexia and anhedonia elicited by interleukin-1beta: antidepressant and gender effects on responding for “free chow” and “earned” sucrose intake. Psychopharmacology (Berl) 2003;165(4):413–418. doi: 10.1007/s00213-002-1273-1. [DOI] [PubMed] [Google Scholar]
- Merendino RA, Di Pasquale G, De Luca F, Di Pasquale L, Ferlazzo E, Martino G, Palumbo MC, Morabito F, Gangemi S. Involvement of fractalkine and macrophage inflammatory protein-1 alpha in moderate-severe depression. Mediators Inflamm. 2004;13(3):205–207. doi: 10.1080/09511920410001713484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depression and Anxiety. 2013;30(4):297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri M, Rostamizadeh A, Talwalkar A. Foundations of machine learning. MIT press; 2012. [Google Scholar]
- Moon ML, Joesting JJ, Blevins NA, Lawson MA, Gainey SJ, Towers AE, McNeil LK, Freund GG. IL-4 Knock Out Mice Display Anxiety-Like Behavior. Behav Genet. 2015;45(4):451–460. doi: 10.1007/s10519-015-9714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol. 2004;172(7):4618–4623. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- Muller-Berghaus J, Kern K, Paschen A, Nguyen XD, Kluter H, Morahan G, Schadendorf D. Deficient IL-12p70 secretion by dendritic cells based on IL12B promoter genotype. Genes Immun. 2004;5(5):431–434. doi: 10.1038/sj.gene.6364102. [DOI] [PubMed] [Google Scholar]
- Murray CL, Obiang P, Bannerman D, Cunningham C. Endogenous IL-1 in cognitive function and anxiety: a study in IL-1RI−/− mice. PLoS One. 2013;8(10):e78385. doi: 10.1371/journal.pone.0078385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neis VB, Manosso LM, Moretti M, Freitas AE, Daufenbach J, Rodrigues AL. Depressive-like behavior induced by tumor necrosis factor-alpha is abolished by agmatine administration. Behav Brain Res. 2014;261:336–344. doi: 10.1016/j.bbr.2013.12.038. [DOI] [PubMed] [Google Scholar]
- Pace TW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun. 2011;25(1):6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Pae CU. The potential role of monocyte chemoattractant protein-1 for major depressive disorder. Psychiatry Investig. 2014;11(3):217–222. doi: 10.4306/pi.2014.11.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JZ, Ni L, Sodhi A, Aguanno A, Young W, Hart RP. Cytokine activity contributes to induction of inflammatory cytokine mRNAs in spinal cord following contusion. Journal of Neuroscience Research. 2002;68(3):315–322. doi: 10.1002/jnr.10215. [DOI] [PubMed] [Google Scholar]
- Paradise MB, Naismith SL, Norrie LM, Graeber MB, Hickie IB. The role of glia in late-life depression. Int Psychogeriatr. 2012;24(12):1878–1890. doi: 10.1017/S1041610212000828. [DOI] [PubMed] [Google Scholar]
- Poling A, Cleary J, Monaghan M. Burying by rats in response to aversive and nonaversive stimuli. Journal of the experimental analysis of behavior. 1981;35(1):31–44. doi: 10.1901/jeab.1981.35-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–336. [PubMed] [Google Scholar]
- Post MWM, van Leeuwen CMC. Psychosocial issues in spinal cord injury: a review. Spinal Cord. 2012;50(5):382–389. doi: 10.1038/sc.2011.182. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh RC, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neurosci Biobehav Rev. 2001;25(1):29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Rivat C, Becker C, Blugeot A, Zeau B, Mauborgne A, Pohl M, Benoliel JJ. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain. 2010;150(2):358–368. doi: 10.1016/j.pain.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Roy M, Richard J-F, Dumas A, Vallieres L. CXCL1 can be regulated by IL-6 and promotes granulocyte adhesion to brain capillaries during bacterial toxin exposure and encephalomyelitis. Journal of Neuroinflammation. 2012;9(1):18. doi: 10.1186/1742-2094-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FM, Lichtblau N, Minkwitz J, Chittka T, Thormann J, Kirkby KC, Sander C, Mergl R, Fasshauer M, Stumvoll M, Holdt LM, Teupser D, Hegerl U, Himmerich H. Cytokine levels in depressed and non-depressed subjects, and masking effects of obesity. J Psychiatr Res. 2014;55:29–34. doi: 10.1016/j.jpsychires.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, Mirnics K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. 2011;16(7):751–762. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, Zhang Y, Zhang L, Yuan ZR, Tan HS, Das G, Devadas S. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don't know. Cell Res. 2006;16(2):126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- Shin JC, Goo HR, Yu SJ, Kim DH, Yoon SY. Depression and quality of life in patients within the first 6 months after the spinal cord injury. Annals of Rehabilitation Medicine. 2012;36(1):119–125. doi: 10.5535/arm.2012.36.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Zhang J, Zhang K, Yang H, Sun Y, Shen Y, Xu Q. A study of the functional significance of epidermal growth factor in major depressive disorder. Psychiatr Genet. 2012;22(4):161–167. doi: 10.1097/YPG.0b013e3283539550. [DOI] [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35(4):298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Soden RJ, Walsh J, Middleton JW, Craven ML, Rutkowski SB, Yeo JD. Causes of death after spinal cord injury. Spinal Cord. 2000;38(10):604–610. doi: 10.1038/sj.sc.3101080. [DOI] [PubMed] [Google Scholar]
- Spijker S. Dissection of rodent brain regions. In: Li KW, editor. Neuroproteomics. Vol. 57. Springer, Humana Press; 2011. pp. 13–26. [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56(9):640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez EC, Krishnan RR, Lewis JG. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom Med. 2003;65(3):362–368. doi: 10.1097/01.psy.0000035719.79068.2b. [DOI] [PubMed] [Google Scholar]
- Swain MG, Le T. Chronic cholestasis in rats induces anhedonia and a loss of social interest. Hepatology. 1998;28(1):6–10. doi: 10.1002/hep.510280102. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. Boston: Pearson Education Inc; 2007. Principal Components and Factor Analysis; p. 649. [Google Scholar]
- Treit D, Pinel JPJ, Fibiger HC. Conditioned defensive burying: A new paradigm for the study of anxiolytic agents. Pharmacology Biochemistry and Behavior. 1981;15(4):619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- van West D, Maes M. Activation of the inflammatory response system: A new look at the etiopathogenesis of major depression. Neuro Endocrinol Lett. 1999;20(1–2):11–17. [PubMed] [Google Scholar]
- Vogelzangs N, Beekman ATF, de Jonge P, Penninx BWJH. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Kavelaars A, Heijnen CJ, Dantzer R. Neuroinflammation and Comorbidity of Pain and Depression. Pharmacological Reviews. 2014;66(1):80–101. doi: 10.1124/pr.113.008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White GE, Greaves DR. Fractalkine: a survivor's guide: chemokines as antiapoptotic mediators. Arterioscler Thromb Vasc Biol. 2012;32(3):589–594. doi: 10.1161/ATVBAHA.111.237412. [DOI] [PubMed] [Google Scholar]
- Wilson CB, McLaughlin LD, Nair A, Ebenezer PJ, Dange R, Francis J. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS One. 2013;8(10):e76146. doi: 10.1371/journal.pone.0076146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33(34):13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Zhao Z, Sabirzhanov B, Stoica BA, Kumar A, Luo T, Skovira J, Faden AI. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J Neurosci. 2014;34(33):10989–11006. doi: 10.1523/JNEUROSCI.5110-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- Yaguchi T, Nagata T, Yang D, Nishizaki T. Interleukin-18 regulates motor activity, anxiety and spatial learning without affecting synaptic plasticity. Behav Brain Res. 2010;206(1):47–51. doi: 10.1016/j.bbr.2009.08.033. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Barrot M. The anxiodepressive comorbidity in chronic pain. Curr Opin Anaesthesiol. 2014;27(5):520–527. doi: 10.1097/ACO.0000000000000116. [DOI] [PubMed] [Google Scholar]
- Yang L, Jones NR, Blumbergs PC, Van Den Heuvel C, Moore EJ, Manavis J, Sarvestani GT, Ghabriel MN. Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J Clin Neurosci. 2005;12(3):276–284. doi: 10.1016/j.jocn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Yip PK, Malaspina A. Spinal cord trauma and the molecular point of no return. Molecular Neurodegeneration. 2012;7(6) doi: 10.1186/1750-1326-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z, Luo C, Zhang W, Chen Y, He J, Zhao Q, Zuo R, Wu Y. Pro- and anti-inflammatory cytokines expression in rat's brain and spleen exposed to chronic mild stress: involvement in depression. Behav Brain Res. 2011;225(1):135–141. doi: 10.1016/j.bbr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Young JJ, Bruno D, Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J Affect Disord. 2014;169:15–20. doi: 10.1016/j.jad.2014.07.032. [DOI] [PubMed] [Google Scholar]