Abstract
Objectives
The inhibitor of apoptosis (IAP) proteins are critical modulators of chemotherapeutic resistance in various cancers. To address the alarming emergence of chemotherapeutic resistance in pancreatic cancer, we investigated the efficacy of the turmeric derivative curcumin in reducing IAP protein and mRNA expression resulting in pancreatic cancer cell death.
Methods
The pancreatic adenocarcinoma cell line PANC-1 was used to assess curcumin’s effects in pancreatic cancer. Curcumin uptake was measured by spectral analysis and fluorescence microscopy. AlamarBlue and Trypan blue exclusion assays were used to determine PANC-1 cell viability following curcumin treatment. Visualization of PANC-1 cell death was performed using Hoffman Modulation Contrast microscopy. Western blot and PCR analyses were used to evaluate curcumin’s effects on IAP protein and mRNA expression.
Results
Curcumin enters PANC-1 cells and is ubiquitously present within the cell following treatment. Furthermore, curcumin reduces cell viability and induces morphological changes characteristic of cell death. Additionally, curcumin decreases IAP protein and mRNA expression in PANC-1 cells.
Conclusions
These data demonstrate that PANC-1 cells are sensitive to curcumin treatment. Furthermore, curcumin as a potential therapeutic tool for overcoming chemotherapeutic resistance mediated by IAPs, supports a role for curcumin as part of the therapeutic approach for pancreatic cancer.
Keywords: pancreatic cancer, curcumin, chemotherapy resistance, inhibitor of apoptosis
Introduction
Pancreatic cancer is an aggressive and devastating disease responsible for the highest mortality rates among cancer types with 94% of patients dying within five years of diagnosis. Pancreatic tumor resection remains the most efficacious treatment with 20 to 25% of the patients surviving 5 years post-surgery. However, early diagnosis, a prerequisite for the surgery, is made difficult by the absence of early signs or symptoms (1-3). As a result, most patients are ineligible for the surgery at the time of diagnosis and are offered chemotherapy (2-4). The gold standard chemotherapeutic for pancreatic cancer is Gemcitabine, which has shown significant clinical benefits, with survival rates ranging from six to fifteen months when resection is not an option and the patients exhibit either non-metastatic or metastatic disease (5-8). Unfortunately, emerging resistance to chemotherapy has hindered the efficacy of chemotherapeutics including Gemcitabine (9), highlighting the need for novel therapeutic approaches that address this rising resistance to therapy.
The inhibitor of apoptosis (IAP) proteins, including Survivin, cellular IAP 1 and 2 (cIAP1 and cIAP2), and X-chromosome linked IAP (XIAP), belong to a family of anti-apoptotic proteins known to confer resistance to treatment modalities such as radiation therapy and chemotherapy (10-13). Additionally, the overexpression of these IAP proteins has been positively correlated with the progression of a variety of cancer types, including pancreatic cancer, resulting in a decline in patient survival after chemotherapeutic treatment (14-21). Pre-clinical and clinical trials aimed at reducing IAP expression via antisense oligonucleotides and/or second mitochondria-derived activator of caspases (Smac) mimetics have yielded promising results in various cancers (14, 22-27); however, such studies have remained inconclusive in pancreatic cancer (28-29). This may be due to a compensatory effect of IAPs to targeted therapies. Further studies involving agents that cause simultaneous reduction in multiple IAPs are needed to investigate this notion.
Curcumin, a turmeric derivative, has been considered as a potential anti-cancer therapy due to its capacity to interrupt signaling pathways that are crucial for the initiation and progression of cancer (30). For instance, studies have demonstrated that curcumin inhibits the progression of various cancers by modulating the expression of anti-apoptotic factors (31-36). Furthermore, curcumin induces apoptosis in pancreatic cancer (37) and regulates IAP expression in a variety of other cancer types (38-39). Preclinical studies involving curcumin in pancreatic cancer have shown that curcumin enhances Gemcitabine sensitivity in vitro and in vivo (40-42). Moreover, Phase I and II clinical trials have yielded promising results on the use of curcumin as part of pancreatic cancer therapeutic strategies (43-48). This recent progress emphasizes the need for a better understanding of the mechanisms by which curcumin counteracts chemotherapeutic resistance. Therefore, the objective of this study was to determine curcumin’s impact alone on IAP expression in pancreatic cancer cells.
Materials and Methods
Cells and Culture Conditions
The pancreatic adenocarcinoma cell line PANC-1 was acquired from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in Dulbecco’s modified Eagle medium (DMEM; ATCC, Manassas, VA) supplemented with Normocin at a final concentration of 100 µg/mL (InvivoGen, San Diego, CA), 100 units of penicillin, 100 µg/mL of streptomycin, 300 µg/mL of L-glutamine and USDA-sourced 10% heat-inactivated fetal bovine serum (Mediatech, Manassas, VA). In all the experiments, cells were cultured at 37°C in a humidified atmosphere containing 5% CO2 to 70-80% confluency prior to use.
Preparation of Curcumin Solutions
Curcumin (Sigma-Aldrich, St. Louis, MO) stock solutions were prepared using DMSO and ethanol as solvents. Subsequent dilutions were made from this stock solution in DMEM. The final concentrations of DMSO and ethanol did not exceed 0.04 % and 0.6%, respectively, and cell viability was not affected at these concentrations (data not shown).
Cell Viability Assays
Cell viability following curcumin treatment was estimated by AlamarBlue and Trypan blue exclusion assays. For AlamarBlue assays, PANC-1 cells were cultured in 96-well plates at 1.0 × 104 cells per well and treated with 10, 50, and 100 μM curcumin for 24, 48 and 72 hours. Subsequently, the AlamarBlue reagent (Life Technologies, Grand Island, NY) was added to each sample (10% final concentration) and incubated at 37°C/5% CO2 for two hours. Viability was analyzed by detection of absorbance at 570 nm using 600 nm as a reference wavelength in a uQUANT spectrophotometer (Bio-Tek, Winooski, VT). For Trypan blue exclusion assays, cells were cultured in 6-well plates at 3.0 × 105 cells per well and treated with 10, 50, 100 μM curcumin for 24, 48 and 72 hours. Cells were then trypsinized and combined with the Trypan blue reagent (Life Technologies, Grand Island, NY) to calculate viability by counting the cells on a hemocytometer. The results presented are representative of three independent experiments. Data from curcumin-treated samples are normalized to the untreated control.
Hoffmann Modulation Contrast Microscopy
PANC-1 cells were cultured in T25 flasks at 7.0 × 105 cells per flask and treated with 10, 50, and 100 μM curcumin for 24 and 48 hours. Cells were then imaged using an Olympus IX70 microscope with Hoffmann modulation and an Insight Spot 2 Mega Sample camera and software. Three independent experiments were performed and within each experiment three images were captured in different sections of the T25 flasks to obtain a representative image.
Spectral Studies and Fluorescence Imaging
Spectral analysis of PANC-1 cell curcumin content post-treatment was performed as previously described (49-51). Briefly, cells were cultured in T75 flasks at 1.5 × 106 cells per flask and treated with 50 μM curcumin for 24 hours. Cells were then trypsinized, washed three times with 1X PBS, resuspended in 1 mL of 100% methanol and sonicated to disrupt the cell membrane integrity. The samples were then centrifuged at 10,000 rpm for 5 minutes at 4°C and the supernatant fraction collected for absorbance analysis at 420 nm using a uQUANT spectrophotometer and KC Junior software (Bio-Tek, Winooski, VT). Untreated cells were also lysed in methanol and subjected to spectral analysis to determine baseline auto-fluorescence of PANC-1 cells. Methanol-only, cell-free samples were used as blank controls. Data are representative of three independent experiments. For fluorescence microscopy of PANC-1 cells curcumin content post-treatment, PANC-1 cells were cultured in 6-well plates containing sterile cover slips at 3.0 × 105 cells per well and treated with 50 μM curcumin for 24 hours. Subsequently, cells were fixed with 4% paraformaldehyde and incubated at −20°C overnight. The following day, the samples were permeabilized using 0.1% Igepal in 1X PBS for 10 minutes at room temperature. The cover slips were then washed three times with 1X PBS and placed onto slides with the nuclear stain DAPI in mounting media for 5 minutes. Stained slides were imaged using a BZ-9000 BIOREVO fluorescence microscope (Keyence, Itasca, IL) with a 40X magnification objective. Results are representative of three independent experiments; within each experiment, three images were acquired from different portions of each slide to obtain a representative image.
Western Blot Analysis
PANC-1 cells were cultured in T25 flasks at 7.0 × 105 cells per flask and treated with 10, 50 and 100 μM curcumin for 24 and 48 hours. After treatment, lysates were prepared using a lysis buffer composed of 50mM Tris-HCl pH 7.5, 1% Triton-X, 0.25% DOC, 150mM sodium chloride, 1mM sodium orthovanadate, 20mM sodium fluoride, 0.2mM EGTA, 1mM EDTA, 1mM PMSF, 1X protease inhibitor cocktail (Roche Life Science, Indianapolis, IN) and sonicated. To remove cell debris, samples were centrifuged at 13,000 rpm for 20 minutes at 4°C. Protein concentration was determined using the Pierce BCA protein assay (Thermo Scientific, Waltham MA) according to the manufacturer’s protocol. Proteins (50 μg) were heated to 95°C for 5 minutes and fractionated using 10, 12 and 15% Bis-Tris polyacrylamide gels. Proteins were transferred onto nitrocellulose membranes (BioRad, Hercules, CA) and blocked for 1 hour at room temperature in 5% milk (w/v in 1X PBS-0.1% Tween). Subsequently, membranes were incubated overnight at 4°C in the following primary antibody solutions (final dilution 1:1000): rabbit polyclonal anti-Survivin (Novus, Littleton, CO), rabbit monoclonal anti-XIAP, rabbit monoclonal anti-cIAP1, rabbit monoclonal anti-cIAP2, and rabbit monoclonal anti-β-actin (Cell Signaling Technology, Beverly, MA). Membranes were washed with 1X PBST three times for 15 minutes each then probed for 1 hour at room temperature with goat anti-rabbit secondary antibodies labeled with IRDye 680 LT (LI-COR Biosciences, Lincoln, Nebraska), followed by three 15-minute washes in 1X PBST and imaging using the ODYSSEY infrared imaging system (LI-COR, Biosciences, Lincoln, Nebraska). β-actin was utilized as a loading control. Data are representative of 3-4 independent experiments. Densitometry analyses were performed using ImageJ software (http://imagej.nih.gov/ij/).
PCR Analysis
PANC-1 cells were cultured in T25 flasks at 7.0 × 105 cells per flask and treated with 10, 50 and 100 μM curcumin for 24 and 36 hours. Cells were then trypsinized and RNA isolation and purification was performed using the Tri-Reagent (Sigma-Aldrich, St. Louis, MO) following the manufacturer’s protocol. cDNA conversion was performed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Grand Island, NY). These cDNA products were used in combination with forward and reverse primers (IDT Technologies, San Diego, CA) designed for IAP mRNA (Table 1) and Platinum Taq DNA Polymerase (Life Technologies, Grand Island, NY) for PCR in a MasterCycler Gradient Thermo Cycler (Eppendorf, Hamburg, Germany). The PCR products were detected using a 1% agarose gel containing ethidium bromide and visualized using an Alpha Innotech imager (Protein Simple, Santa Clara, CA). Data are representative of three independent experiments. Densitometry analyses were performed using ImageJ software (http://imagej.nih.gov/ij/).
Table 1.
Primer sequences targeting IAPs
| Name | Primer Sequence |
|---|---|
| Survivin Fwd | 5’ – GCA TGG GTG CCC CGA CGT TG – 3’ |
| Survivin Rev | 5’ – GCT CCG GCC AGA GGC CTC AA – 3’ |
| cIAP1 Fwd | 5’ – ATT GTG TCA GCA CTT CTT AAT G – 3’ |
| cIAP1 Rev | 5’ – TTA AGA GAG AAA TGT ACG AAC AGT – 3’ |
| cIAP2 Fwd | 5’ – TGG AGA AGA CCA TTC AGA AGA T – 3’ |
| cIAP2 Rev | 5’ – TCA TGA AAG AAA TGT ACG AAC TGT – 3’ |
| XIAP Fwd | 5’ – ATG ACT TTT AAC AGT TTT GAA GGA – 3’ |
| XIAP Rev | 5’ – TTA AGA CAT AAA AAT TTT TTG CTT – 3’ |
| GAPDH Fwd | 5’ – ACG GAT TTG GTC GTA TTG GGC G – 3’ |
| GAPDH Rev | 5’ – CTC CTG GAA GAT GGT GAT GG – 3’ |
Primer sequences targeting IAPs. IAP = inhibitor of apoptosis, cIAP1 = cellular inhibitor of apoptosis 1, cIAP2 = cellular inhibitor of apoptosis 2, XIAP = X-linked inhibitor of apoptosis, GAPDH = glyceraldehyde 3-phosphate dehydrogenase, Fwd = forward primer, Rev = reverse primer.
Statistical Analysis
All statistical analyses in this study were performed using one-way ANOVA analysis and a probability of less than a 95% confidence limit (p < 0.05) was considered to be significant. Data are presented as mean ± SEM. Statistical analyses were performed using the Prism (Graphpad) software.
Results
Effect of curcumin on PANC-1 cell viability
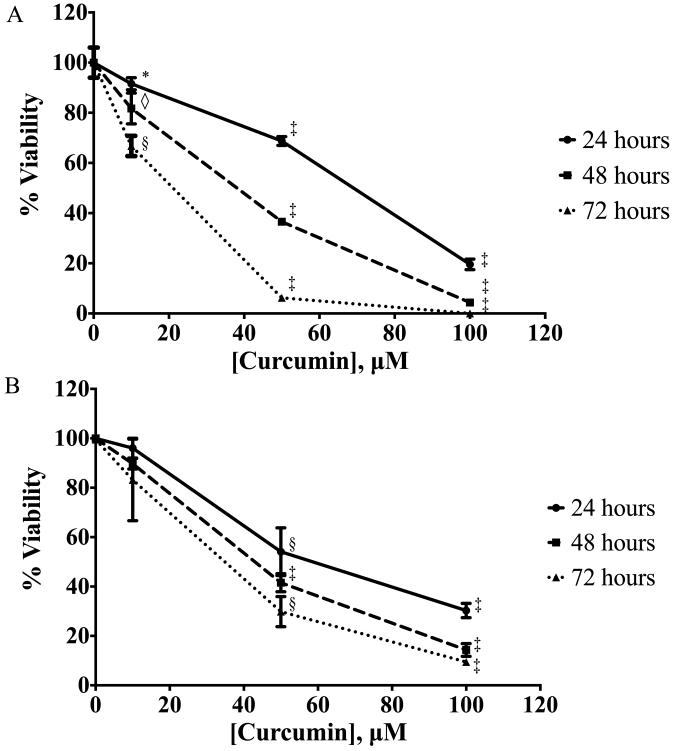
PANC-1 cells were cultured in increasing concentrations of curcumin for 24-72 hours and analyzed by AlamarBlue (Fig. 1A) and Trypan blue exclusion (Fig. 1B) viability assays. Curcumin demonstrated a significant dose- and time-dependent inhibitory effect on PANC-1 cell viability when compared with untreated controls. Curcumin concentrations (10uM and 50uM) flanking the IC50 were chosen for further experiments.
Figure 1.
Curcumin induces PANC-1 cell death, analyzed by AlamarBlue and Trypan blue exclusion assays. PANC-1 cells were cultured in medium supplemented with curcumin at increasing doses for 24, 48, and 72 hours followed by (A) AlamarBlue and (B) Trypan blue exclusion viability assays. Data are presented as mean (±SEM). * p ≤ 0.05, ◇ p ≤ 0.01, § p ≤ 0.001, ‡ p ≤ 0.0001, curcumin treatment versus untreated control.
Effect of curcumin on PANC-1 cell morphology
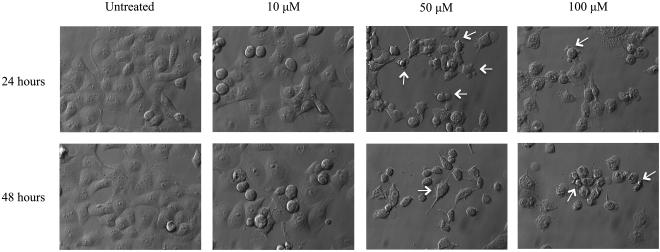
Apoptosis is a type of cell death with distinctive morphological features. To qualitatively evaluate the effects of curcumin treatment on PANC-1 cellular morphology, images were acquired using Hoffmann modulation contrast microscopy (Fig. 2). These images illustrate an increase in cell death in a dose- and time-dependent manner. The presence of membrane blebs and cell shrinkage, which are main morphological hallmarks of apoptotic cells, were observed following 50 and 100 μM curcumin treatment for 24 and 48 hours.
Figure 2.
Morphology of curcumin-treated PANC-1 cells, visualized by Hoffman modulation contrast microscopy. PANC-1 cells were cultured in medium supplemented with curcumin at increasing doses for 24 and 48 hours followed by imaging via Hoffman modulation contrast microscopy. White arrows indicate membrane blebs and cell shrinkage, morphological hallmarks of apoptosis. Results depicted in the figure are representative of findings from 3 independent experiments.
Curcumin detection within treated PANC-1 cells
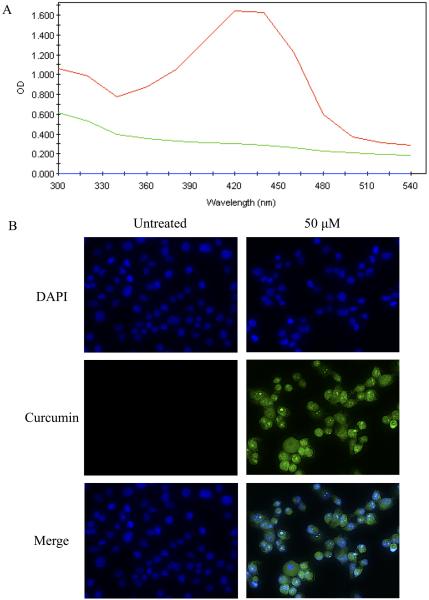
It has been reported previously that curcumin has excitation and emission spectra of 420 and 470 nm, respectively (65-67). These spectral properties of curcumin were used to detect its presence within PANC-1 cells using spectrophotometric studies and fluorescence microscopy. PANC-1 cells were cultured in the presence of 50uM curcumin for 24 hours and subsequently washed, trypsinized, and lysed in 100% methanol. The emission peak of curcumin-treated cell lysates at 420nm (red) was detected compared to cells not treated with curcumin (green) or blank/methanol-only samples (blue) (Fig. 3A). This peak is identical to the emission spectrum of 50uM curcumin in methanol (data not shown). The intrinsic fluorescence of curcumin was also exploited using fluorescence microscopy to visualize curcumin content in treated versus untreated PANC-1 cells (Fig. 3B). The DNA dye DAPI was used to visualize nuclei (blue). Curcumin demonstrates a pan-cellular staining pattern (green), with cytoplasmic, nuclear, and nucleolar pools visible by fluorescence microscopy. These results indicate that curcumin is capable of entering PANC-1 cells in vitro and is non-specific in its localization, suggesting multiple mechanisms of cytotoxicity.
Figure 3.
Curcumin in pancreatic cancer cells, analyzed by spectral analysis and fluorescent microscopy. (A) PANC-1 cells were cultured in medium supplemented with 50uM curcumin for 24 hours, trypsinized, lysed in 100% methanol and subjected to spectral analysis on a uQuant spectrophotometer at a 300-540nm wavelength range. Red = curcumin-treated cells; green = untreated cells; blue = methanol as a vehicle control. (B) PANC-1 cells were cultured in medium supplemented with 50uM curcumin (green) for 24 hours and stained with the DNA dye DAPI to show nuclei (blue) followed by imaging via fluorescence microscopy at 40X maginification. Results depicted in the figure are representative of findings from 3 independent experiments.
Curcumin decreases IAP protein and mRNA expression
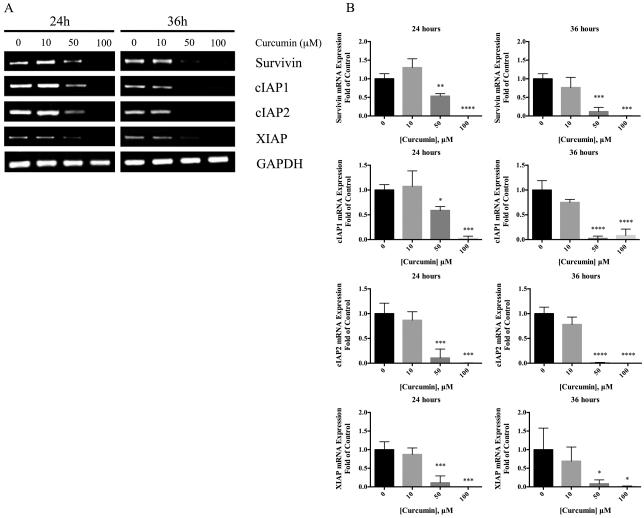
To determine the effects of curcumin on Survivin, cIAP1, cIAP2 and XIAP expression, Western blot (Fig. 4A) and reverse-transcription PCR (Fig. 5A) were performed following treatment with 10, 50 and 100uM curcumin. Protein expression of cIAP1, cIAP2 and XIAP were significantly decreased at 50 and 100uM curcumin treatment compared to untreated controls at 24 and 48 hours (Fig. 4B). In addition, Survivin protein expression was significantly decreased after 50uM curcumin treatment for 24 hours and after 50uM or 100uM curcumin treatment for 48 hours. Moreover, 10uM curcumin was sufficient to significantly reduce cIAP2 expression after 48 hours of treatment. Similarly, significant reduction in Survivin, cIAP1, cIAP2 and XIAP mRNA expression was observed at 50 and 100uM curcumin compared to untreated controls at 24 and 36 hours (Fig. 5B).
Figure 4.
Expression of IAP proteins in PANC-1 cells following curcumin treatment. (A) Cells were cultured in medium supplemented with 10-100uM curcumin for 24 and 48 hours. Whole-cell lysates were prepared and expression levels of Survivin, cIAP1, cIAP2, XIAP and actin proteins were analyzed by Western blot as described in the Materials and Methods. Data are representative of 3-4 independent experiments. (B) Protein levels of IAPs are presented as relative ratios to control cells without curcumin treatment after normalization to B-actin levels. Data are presented as mean (± SEM). * p ≤ 0.05, ◇ p ≤ 0.01, § p ≤ 0.001, ‡ p ≤ 0.0001, curcumin treatment versus untreated control.
Figure 5.
Expression of IAP mRNA following curcumin treatment in PANC-1 cells. (A) Cells were cultured in medium supplemented with 10-100uM curcumin for 24 and 36 hours. RNA was extracted using the Trizol-chloroform extraction method then converted to cDNA and probed using primers for Survivin, cIAP1, cIAP2, XIAP and GAPDH (Table 1). Data are representative of 3 independent experiments. (B) mRNA levels of IAPs are presented as relative ratios to control cells without curcumin treatment after normalization to GAPDH levels. Data are presented as mean (± SEM). * p ≤ 0.05, ◇ p ≤ 0.01, § p ≤ 0.001, ‡ p ≤ 0.0001, curcumin treatment versus untreated control.
Discussion
Pancreatic cancer is a deadly disease that causes higher mortality rates annually than other cancer types. Currently, surgical resection is one of the most effective therapeutic approaches for pancreatic cancer. However, pancreatic cancer does not exhibit notable signs or symptoms during early stages of development, making an early diagnosis difficult. For this reason, most patients with advanced non-metastatic or metastatic disease are ineligible for surgery and receive chemotherapy. Unfortunately, the efficacy of chemotherapeutic agents is limited by emerging drug resistance (1-4). Therefore, the pancreatic cancer field is moving toward investigating therapeutic approaches that target crucial mediators of chemoresistance. It has been well established that evasion of cell death is a required event in the development of resistance to chemotherapy (52-53) with this resistance linked to the up regulation of anti-apoptotic proteins such as the inhibitor of apoptosis (IAP) family members (54-55).
Apoptosis is a type of cell death highly dependent on the activation of caspases (cysteine- aspartic proteases) that cause endonuclease-mediated fragmentation of DNA and cellular demise. This series of events is a prerequisite for progressive cellular disassembly into apoptotic bodies that are subsequently consumed by phagocytic cells (56). The IAP family, particularly Survivin, cIAP1, cIAP2 and XIAP, are proteins that have substantial roles in modulating the inactivation of apoptosis (23). While the IAPs have been shown to bind caspases, only XIAP directly inhibits caspases (57). In addition to its role in cell cycle regulation, Survivin is thought to bind active caspases through cofactor proteins such as hepatitis B X-interacting protein (HBXIP) to prevent amplification by cleavage of other pro-caspase isoforms (58-59). cIAP1 and cIAP2, though capable of binding caspases, have been shown to inhibit apoptosis by interrupting caspase activation through their E3 ubiquitin ligase function (60-62).
The nuclear factor-kappa B (NF-kB) transcription factor has been found to be constitutively activated in pancreatic cancer (63-64) and is known to regulate key mediators of cancer cell survival, proliferation, angiogenesis, and metastasis (65-68). NF-kB has been shown to regulate the production of certain IAPs, including Survivin, cIAP1, and XIAP (65-69). Furthermore, NF-kB activity and IAP expression have been implicated in resistance to Gemcitabine (69-70). Gemcitabine induces an increase in IAP expression in pancreatic cancer cells, particularly Survivin and XIAP, as well as cIAP1 in lung cancer cells (71-74).
Indeed, studies targeting NF-kB (70, 73, 75-76) or IAPs (73, 77-78) have demonstrated increased sensitivity to Gemcitabine. The increased sensitivity to Gemcitabine following IAP reduction is the rationale for the use of second mitochondria-derived activator of caspases (Smac) mimetics. Smac is an endogenous pro-apoptotic protein transcribed by the DIABLO gene. This protein promotes apoptosis by direct interaction and inhibition of XIAP and Survivin proteins. Several Smac mimetics are currently under investigation in clinical trials (14). While these Smac mimetics have shown promising results in preclinical trials in vitro and in vivo, both in the reduction of IAP expression and in re-sensitization to Gemcitabine (79), they have no known effects on NF-kB expression or activity. Recent studies have demonstrated that dual inhibition of NF-kB activity and IAP expression may have superior benefits than reducing IAP expression alone. Indeed, dual targeting NF-kB and XIAP was more effective in re-sensitizing pancreatic adenocarcinoma cells to Gemcitabine therapy than XIAP knockdown alone (73). Thus, the optimal “next step” in the development of a therapeutic strategy for pancreatic cancer involves compounds that target upstream mediators of IAP expression, such as NF-kB, as well as multiple IAPs simultaneously.
Curcumin, a turmeric derivative, is a candidate for such a therapeutic agent. It has been shown to inhibit pancreatic adenocarcinoma cell proliferation, survival, invasion and angiogenesis in vitro and in vivo (41, 80). In addition, studies by Kunukkamara et al. have demonstrated that curcumin attenuates NF-kB activation, resulting in decreased production of anti-apoptotic factors, including Survivin and cIAP1, as well as pro-angiogenic and metastatic factors, in MiaPaCa-2-derived xenograft tumors (69). Multiple studies have demonstrated synergistic activity between curcumin and Gemcitabine in pancreatic adenocarcinoma cells (40-42). Interestingly, while XIAP is considered to be the most potent regulator of apoptosis in humans, its levels following curcumin treatment remain to be elucidated. Furthermore, the effect of curcumin on mRNA expression of the IAPs remains to be investigated. This information is essential to understanding whether curcumin’s effects on IAP expression are due to transcriptional regulation or post-translational mechanisms. In this study, we explore curcumin’s effects on protein and mRNA expression of a panel of key IAPs, including Survivin, cIAP1, cIAP2 and XIAP in the pancreatic adenocarcinoma cell line PANC-1. Phase I and II clinical trials have been conducted to evaluate the safety and efficacy of curcumin, alone and in combination with standard Gemcitabine-based chemotherapy (45-48). The major challenge to curcumin’s clinical use is poor bioavailability. A recent Phase I clinical trial was conducted using a novel microparticle-based form of curcumin called Theracurmin in combination with standard Gemcitabine-based chemotherapy (48). This study reported promising results, increasing plasma levels over those reported in previous clinical trials, despite using approximately 5% of the dose of curcumin used in earlier studies (400mg vs. 8g/day) while inducing minimal toxicity in patients.
While some controversy exists as to the Gemcitabine-sensitivity of the pancreatic adenocarcinoma cell line MiaPaCa-2 (73, 75), PANC-1 cells are generally considered to be resistant to Gemcitabine. Therefore, we investigated the sensitivity of these cells to curcumin in vitro using AlamarBlue and Trypan blue exclusion viability assays. Our results are consistent with those published using other viability assays in PANC-1 cells (81-83), demonstrating dose- and time- dependent reduction in cell viability following curcumin treatment (Fig. 1). In addition, Hoffman modulation contrast microscopy illustrates the morphology of PANC-1 cells following curcumin treatment (Fig. 2). Cells treated with curcumin exhibit features characteristic of apoptotic cell death, including cell shrinkage and membrane blebbing.
To further elucidate the possible mechanisms of action of curcumin in PANC-1 cells, the spectral properties of curcumin (Fig. 3A) were used to determine the intracellular localization of the compound as analyzed by fluorescence microscopy (Fig. 3B). Consistent with the notion that curcumin exerts effects on multiple intracellular signaling pathways (84-87); our results demonstrate that curcumin displays a pan-cellular localization.
To determine the effects of curcumin on IAP protein and mRNA expression, we performed Western blot and RT-PCR analyses on curcumin-treated PANC-1 cells. Our data demonstrate that curcumin reduces protein and mRNA levels of Survivin, cIAP1 and cIAP2 and XIAP (Fig. 4 and 5), with the most marked effects on IAP expression demonstrated by cIAPs 1 and 2 and XIAP. Interestingly, while mRNA expression of Survivin diminishes with increasing curcumin concentrations and incubation times, Survivin’s protein level appears to be relatively stable at the highest dose (100uM) and time (48h) evaluated, remaining at approximately 50% of the level in untreated PANC-1 cells. These data suggest that mechanisms exist to stabilize Survivin protein under conditions of curcumin treatment, despite a reduction in Survivin mRNA production. This finding carries heavy implications for resistance to therapy, since Survivin itself has been found to bind and stabilize XIAP, enhancing the latter’s caspase-9 inhibiting activity (88). Thus, while curcumin exerts potent effects on upstream (NF-kB-mediated) signaling leading to reduced IAP expression, residual Survivin may represent a key mechanism for evasion of cell death in the context of this therapeutic strategy.
In summary, our data demonstrates for the first time that curcumin is effective in reducing expression of multiple IAPs critical to chemoresistance, both at the mRNA and protein level, resulting in increased cell death in vitro. The ability to modulate multiple members of the IAP family may prove to be a key factor in selecting compounds for further study as selection of individual IAP-specific targeting has been less than effective. Furthermore, we demonstrate that, of these IAPs, Survivin shows the least sensitivity to curcumin-mediated downregulation, suggesting a possible role in resistance to curcumin treatment, a phenomena that we are currently investigating in our laboratory.
Acknowledgments
Sources of Financial Support
This work would have been impossible if not for a generous grant from the Hirshberg Foundation for Pancreatic Cancer Research and the friendship, inspiration and mentoring of Agi Hirshberg. This work was also supported by grants from the National Institutes of Health (NIH) National Center on Minority Health and Health Disparities (NIH-NCMHD 5P20MD001632, 1P20MD006988 and 2R25GM060507). Funding was also obtained from a National Merit Test Bed (NMTB) award sponsored by the Department of the Army under Cooperative Agreement Number DAMD17-97-2-7016 (NRW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Address for Reprints
Same as corresponding author.
References
- 1.American Cancer Society . Cancer Facts & Figures 2013. American Cancer Society; Atlanta: 2013. [Google Scholar]
- 2.Huang ZQ, Saluja AK, Dudeja V, et al. Molecular targeted approaches for treatment of pancreatic cancer. Curr Pharm Des. 2011;17(21):2221–2238. doi: 10.2174/138161211796957427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller MW, Friess H, Koninger J, et al. Factors influencing survival after bypass procedures in patients with advanced pancreatic adenocarcinomas. Am J Surg. 2008;195(2):221–228. doi: 10.1016/j.amjsurg.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Alexakis N, Halloran C, Raraty M, et al. Current standards of surgery for pancreatic cancer. Br J Surg. 2004;91(11):1410–1427. doi: 10.1002/bjs.4794. [DOI] [PubMed] [Google Scholar]
- 5.Kim R. FOLFIRINOX: a new standard treatment for advanced pancreatic cancer? Lancet Oncol. 2011;12(1):8–9. doi: 10.1016/S1470-2045(10)70237-0. [DOI] [PubMed] [Google Scholar]
- 6.Furuse J. Current status and future directions of chemotherapy for pancreatic cancer. Nihon Shokakibyo Gakkai Zasshi = The Japanese Journal of Gastroenterology. 2013;110(12):2060–2065. [PubMed] [Google Scholar]
- 7.Li J, Wientjes MG, Au JL. Pancreatic cancer: pathobiology, treatment options, and drug delivery. AAPS J. 2010;12(2):223–232. doi: 10.1208/s12248-010-9181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueno H, Kiyosawa K, Kaniwa N. Pharmacogenomics of gemcitabine: can genetic studies lead to tailor-made therapy? Br J Cancer. 2007;97(2):145–151. doi: 10.1038/sj.bjc.6603860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long J, Zhang Y, Yu X, et al. Overcoming drug resistance in pancreatic cancer. Expert Opin Ther Targets. 2011;15(7):817–828. doi: 10.1517/14728222.2011.566216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai Y, Lawrence TS, Xu L. Overcoming cancer therapy resistance by targeting inhibitors of apoptosis proteins and nuclear factor-kappa B. Am J Transl Res. 2009;1(1):1–15. [PMC free article] [PubMed] [Google Scholar]
- 11.Mita AC, Mita MM, Nawrocki ST, et al. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14(16):5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 12.Kami K, Doi R, Koizumi M, et al. Downregulation of survivin by siRNA diminishes radioresistance of pancreatic cancer cells. Surgery. 2005;138(2):299–305. doi: 10.1016/j.surg.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Shen X, Zheng JY, Shi H, et al. Survivin knockdown enhances gastric cancer cell sensitivity to radiation and chemotherapy in vitro and in nude mice. Am J Med Sci. 2012;344(1):52–58. doi: 10.1097/MAJ.0b013e318239c4ee. [DOI] [PubMed] [Google Scholar]
- 14.Dubrez L, Berthelet J, Glorian V. IAP proteins as targets for drug development in oncology. Onco Targets Ther. 2013;9:1285–1304. doi: 10.2147/OTT.S33375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanimoto T, Tsuda H, Imazeki N, et al. Nuclear expression of cIAP-1, an apoptosis inhibiting protein, predicts lymph node metastasis and poor patient prognosis in head and neck squamous cell carcinomas. Cancer Lett. 2005;224(1):141–151. doi: 10.1016/j.canlet.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 16.Che X, Yang D, Zong H, et al. Nuclear cIAP1 overexpression is a tumor stage- and grade-independent predictor of poor prognosis in human bladder cancer patients. Urol Oncol. 2012;30(4):450–456. doi: 10.1016/j.urolonc.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Zhu J, Tang Y, et al. X-linked inhibitor of apoptosis positive nuclear labeling: a new independent prognostic biomarker of breast invasive ductal carcinoma. Diagnostic Pathology. 2011;6:49. doi: 10.1186/1746-1596-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seligson DB, Hongo F, Huerta-Yepez S, et al. Expression of X-linked inhibitor of apoptosis protein is a strong predictor of human prostate cancer recurrence. Clin Cancer Res. 2007;13(20):6056–6063. doi: 10.1158/1078-0432.CCR-07-0960. [DOI] [PubMed] [Google Scholar]
- 19.Krajewska M, Krajewski S, Banares S, et al. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res. 2003;9(13):4914–4925. [PubMed] [Google Scholar]
- 20.Ferreira CG, van der Valk P, Span SW, et al. Expression of X-linked inhibitor of apoptosis as a novel prognostic marker in radically resected non-small cell lung cancer patients. Clin Cancer Res. 2001;7(8):2468–2474. [PubMed] [Google Scholar]
- 21.Ferreira CG, van der Valk P, Span SW, et al. Assessment of IAP (inhibitor of apoptosis) proteins as predictors of response to chemotherapy in advanced non-small-cell lung cancer patients. Ann Oncol. 2001;12(6):799–805. doi: 10.1023/a:1011167113067. [DOI] [PubMed] [Google Scholar]
- 22.LaCasse EC, Mahoney DJ, Cheung HH, et al. IAP-targeted therapies for cancer. Oncogene. 2008;27(48):6252–6275. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 23.Saleem M, Qadir MI, Perveen N, et al. Inhibitors of apoptotic proteins: new targets for anticancer therapy. Chem Biol Drug Des. 2013;82(3):243–251. doi: 10.1111/cbdd.12176. [DOI] [PubMed] [Google Scholar]
- 24.Oberoi-Khanuja TK, Murali A, Rajalingam K. IAPs on the move: role of inhibitors of apoptosis proteins in cell migration. Cell Death Dis. 2013;4:e784. doi: 10.1038/cddis.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu JL, Wang Y, Jiang J, et al. Inhibition of survivin expression and mechanisms of reversing drug-resistance of human lung adenocarcinoma cells by siRNA. Chin Med J (Engl) 2010;123(20):2901–2907. [PubMed] [Google Scholar]
- 26.He SQ, Rehman H, Gong MG, et al. Inhibiting survivin expression enhances TRAIL-induced tumoricidal activity in human hepatocellular carcinoma via cell cycle arrest. Cancer Biol Ther. 2007;6(8):1247–1257. doi: 10.4161/cbt.6.8.4444. [DOI] [PubMed] [Google Scholar]
- 27.Rodel F, Frey B, Leitmann W, et al. Survivin antisense oligonucleotides effectively radiosensitize colorectal cancer cells in both tissue culture and murine xenograft models. Int J Radiat Oncol Biol Phys. 2008;71(1):247–255. doi: 10.1016/j.ijrobp.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Ma WW, Zhang H, Hylander B, et al. TL32711, a novel Smac mimetic, exerts significant antitumor efficacy in primary pancreatic adenocarcinoma model. Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research; Chicago, Illinois. Philadelphia (PA). 2012. Mar 31-Apr 4. AACR; 2012. Abstract nr1939. [Google Scholar]
- 29.Mahadevan D, Chalasani P, Rensvold D, et al. Phase I trial of AEG35156 an antisense oligonucleotide to XIAP plus gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma. Am J Clin Oncol. 2013;36(3):239–243. doi: 10.1097/COC.0b013e3182467a13. [DOI] [PubMed] [Google Scholar]
- 30.Shehzad A, Lee J, Lee YS. Curcumin in various cancers. BioFactors (Oxford, England) 2013;39(1):56–68. doi: 10.1002/biof.1068. [DOI] [PubMed] [Google Scholar]
- 31.Woo JH, Kim YH, Choi YJ, et al. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24(7):1199–1208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 32.Park S, Cho DH, Andera L, et al. Curcumin enhances TRAIL-induced apoptosis of breast cancer cells by regulating apoptosis-related proteins. Mol Cell Biochem. 2013;383(1-2):39–48. doi: 10.1007/s11010-013-1752-1. [DOI] [PubMed] [Google Scholar]
- 33.Reuter S, Eifes S, Dicato M, et al. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem Pharmacol. 2008;76(11):1340–1351. doi: 10.1016/j.bcp.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 34.Wang JB, Qi LL, Zheng SD, et al. Curcumin induces apoptosis through the mitochondria-mediated apoptotic pathway in HT-29 cells. J Zhejiang Univ Sci B. 2009;10(2):93–102. doi: 10.1631/jzus.B0820238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shankar S, Chen Q, Sarva K, et al. Curcumin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells: molecular mechanisms of apoptosis, migration and angiogenesis. J Mol Signal. 2007;2:10. doi: 10.1186/1750-2187-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009;11(3):495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedman L, Lin L, Ball S, et al. Curcumin analogues exhibit enhanced growth suppressive activity in human pancreatic cancer cells. Anticancer Drugs. 2009;20(6):444–449. doi: 10.1097/CAD.0b013e32832afc04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aggarwal S, Ichikawa H, Takada Y, et al. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69(1):195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 39.Notarbartolo M, Poma P, Perri D, et al. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005;224(1):53–65. doi: 10.1016/j.canlet.2004.10.051. [DOI] [PubMed] [Google Scholar]
- 40.Lev-Ari S, Vexler A, Starr A, et al. Curcumin augments gemcitabine cytotoxic effect on pancreatic adenocarcinoma cell lines. Cancer Invest. 2007;25(6):411–418. doi: 10.1080/07357900701359577. [DOI] [PubMed] [Google Scholar]
- 41.Bimonte S, Barbieri A, Palma G, et al. Curcumin inhibits tumor growth and angiogenesis in an orthotopic mouse model of human pancreatic cancer. Biomed Res Int. 2013;2013:810423. doi: 10.1155/2013/810423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramachandran C, Resek AP, Escalon E, et al. Potentiation of gemcitabine by Turmeric Force in pancreatic cancer cell lines. Oncol Rep. 2010;23(6):1529–1535. doi: 10.3892/or_00000792. [DOI] [PubMed] [Google Scholar]
- 43.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Archiv der Pharmazie. 2010;343(9):489–499. doi: 10.1002/ardp.200900319. [DOI] [PubMed] [Google Scholar]
- 45.Dhillon N, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14(14):4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 46.Epelbaum R, Schaffer M, Vizel B, et al. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62(8):1137–1141. doi: 10.1080/01635581.2010.513802. [DOI] [PubMed] [Google Scholar]
- 47.Kanai M, Yoshimura K, Asada M, et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. 2011;68(1):157–164. doi: 10.1007/s00280-010-1470-2. [DOI] [PubMed] [Google Scholar]
- 48.Kanai M, Otsuka Y, Otsuka K, et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother Pharmacol. 2013;71(6):1521–1530. doi: 10.1007/s00280-013-2151-8. [DOI] [PubMed] [Google Scholar]
- 49.Kunwar A, Barik A, Pandey R, et al. Transport of liposomal and albumin loaded curcumin to living cells: an absorption and fluorescence spectroscopic study. Biochim Biophys Acta. 2006;1760(10):1513–1520. doi: 10.1016/j.bbagen.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 50.Kunwar A, Barik A, Mishra B, et al. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim Biophys Acta. 2008;1780(4):673–679. doi: 10.1016/j.bbagen.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 51.Nardo L, Andreoni A, Masson M, et al. Studies on curcumin and curcuminoids. XXXIX. Photophysical properties of bisdemethoxycurcumin. J Fluoresc. 2011;21(2):627–635. doi: 10.1007/s10895-010-0750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 53.Rebucci M, Michiels C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol. 2013;85(9):1219–1226. doi: 10.1016/j.bcp.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 54.W Owens T. Inhibitor of Apoptosis Proteins: Promising Targets for Cancer Therapy. J Carcinog Mutagen. 2013:S14. doi: 10.4172/2157-2518.S14-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fulda S. Targeting apoptosis signaling in pancreatic cancer. Cancers. 2011;3(1):241–251. doi: 10.3390/cancers3010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ouyang L, Shi Z, Zhao S, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–498. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7(10):988–994. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamm I, Wang Y, Sausville E, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58(23):5315–5320. [PubMed] [Google Scholar]
- 59.Marusawa H, Matsuzawa S, Welsh K, et al. HBXIP functions as a cofactor of survivin in apoptosis suppression. EMBO J. 2003;22(11):2729–2740. doi: 10.1093/emboj/cdg263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi YE, Butterworth M, Malladi S, et al. The E3 ubiquitin ligase cIAP1 binds and ubiquitinates caspase-3 and -7 via unique mechanisms at distinct steps in their processing. J Biol Chem. 2009;284(19):12772–12782. doi: 10.1074/jbc.M807550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang H, Joazeiro CA, Bonfoco E, et al. The inhibitor of apoptosis, cIAP2, functions as a ubiquitin-protein ligase and promotes in vitro monoubiquitination of caspases 3 and 7. J Biol Chem. 2000;275(35):26661–26664. doi: 10.1074/jbc.C000199200. [DOI] [PubMed] [Google Scholar]
- 62.Vandenabeele P, Galluzzi L, Vanden Berghe T, et al. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11(10):700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 63.Wang W, Abbruzzese JL, Evans DB, et al. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5(1):119–127. [PubMed] [Google Scholar]
- 64.Liptay S, Weber CK, Ludwig L, et al. Mitogenic and antiapoptotic role of constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J Cancer. 2003;105(6):735–746. doi: 10.1002/ijc.11081. [DOI] [PubMed] [Google Scholar]
- 65.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6(3):203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 66.Fujioka S, Sclabas GM, Schmidt C, et al. Function of nuclear factor kappaB in pancreatic cancer metastasis. Clin Cancer Res. 2003;9(1):346–354. [PubMed] [Google Scholar]
- 67.Xiong HQ, Abbruzzese JL, Lin E, et al. NF-kappaB activity blockade impairs the angiogenic potential of human pancreatic cancer cells. Int J Cancer. 2004;108(2):181–188. doi: 10.1002/ijc.11562. [DOI] [PubMed] [Google Scholar]
- 68.Greten FR, Weber CK, Greten TF, et al. Stat3 and NF-kappaB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterol. 2002;123(6):2052–2063. doi: 10.1053/gast.2002.37075. [DOI] [PubMed] [Google Scholar]
- 69.Kunnumakkara AB, Guha S, Krishnan S, et al. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67(8):3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 70.Arlt A, Gehrz A, Muerkoster S, et al. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22(21):3243–3251. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 71.Galloway NR, Aspe JR, Sellers C, et al. Enhanced antitumor effect of combined gemcitabine and proton radiation in the treatment of pancreatic cancer. Pancreas. 2009;38(7):782–790. doi: 10.1097/MPA.0b013e3181a85999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo HC, Bu HQ, Luo J, et al. Emodin potentiates the antitumor effects of gemcitabine in PANC-1 pancreatic cancer xenograft model in vivo via inhibition of inhibitors of apoptosis. Int J Oncol. 2012;40(6):1849–1857. doi: 10.3892/ijo.2012.1389. [DOI] [PubMed] [Google Scholar]
- 73.Cao LP, Song JL, Yi XP, et al. Double inhibition of NF-kappaB and XIAP via RNAi enhances the sensitivity of pancreatic cancer cells to gemcitabine. Oncology Rep. 2013;29(4):1659–1665. doi: 10.3892/or.2013.2246. [DOI] [PubMed] [Google Scholar]
- 74.Bandala E, Espinosa M, Maldonado V, et al. Inhibitor of apoptosis-1 (IAP-1) expression and apoptosis in non-small-cell lung cancer cells exposed to gemcitabine. Biochem Pharmacol. 2001;62(1):13–19. doi: 10.1016/s0006-2952(01)00632-3. [DOI] [PubMed] [Google Scholar]
- 75.Pan X, Arumugam T, Yamamoto T, et al. Nuclear factor-kappaB p65/relA silencing induces apoptosis and increases gemcitabine effectiveness in a subset of pancreatic cancer cells. Clin Cancer Res. 2008;14(24):8143–8151. doi: 10.1158/1078-0432.CCR-08-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kong R, Sun B, Jiang H, et al. Downregulation of nuclear factor-kappaB p65 subunit by small interfering RNA synergizes with gemcitabine to inhibit the growth of pancreatic cancer. Cancer Lett. 2010;291(1):90–98. doi: 10.1016/j.canlet.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 77.Shrikhande SV, Kleeff J, Kayed H, et al. Silencing of X-linked inhibitor of apoptosis (XIAP) decreases gemcitabine resistance of pancreatic cancer cells. Anticancer Res. 2006;26(5a):3265–3273. [PubMed] [Google Scholar]
- 78.Liu WS, Yan HJ, Qin RY, et al. siRNA directed against survivin enhances pancreatic cancer cell gemcitabine chemosensitivity. Dig Dis Sci. 2009;54(1):89–96. doi: 10.1007/s10620-008-0329-4. [DOI] [PubMed] [Google Scholar]
- 79.Dineen SP, Roland CL, Greer R, et al. Smac mimetic increases chemotherapy response and improves survival in mice with pancreatic cancer. Cancer Res. 2010;70(7):2852–2861. doi: 10.1158/0008-5472.CAN-09-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Youns M, Fathy GM. Upregulation of extrinsic apoptotic pathway in curcumin-mediated antiproliferative effect on human pancreatic carcinogenesis. J Cell Biochem. 2013;114(12):2654–2665. doi: 10.1002/jcb.24612. [DOI] [PubMed] [Google Scholar]
- 81.Wang Z, Zhang Y, Banerjee S, et al. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106(11):2503–2513. doi: 10.1002/cncr.21904. [DOI] [PubMed] [Google Scholar]
- 82.Lev-Ari S, Zinger H, Kazanov D, et al. Curcumin synergistically potentiates the growth inhibitory and pro-apoptotic effects of celecoxib in pancreatic adenocarcinoma cells. Biomed Pharmacother. 2005;59(Suppl 2):S276–280. doi: 10.1016/s0753-3322(05)80045-9. [DOI] [PubMed] [Google Scholar]
- 83.Lev-Ari S, Starr A, Vexler A, et al. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006;26(6b):4423–4430. [PubMed] [Google Scholar]
- 84.Prakobwong S, Gupta SC, Kim JH, et al. Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis. 2011;32(9):1372–1380. doi: 10.1093/carcin/bgr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gupta SC, Prasad S, Kim JH, et al. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011;28(12):1937–1955. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maheshwari RK, Singh AK, Gaddipati J, et al. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78(18):2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 87.Shehzad A, Lee YS. Molecular mechanisms of curcumin action: signal transduction. BioFactors (Oxford, England) 2013;39(1):27–36. doi: 10.1002/biof.1065. [DOI] [PubMed] [Google Scholar]
- 88.Dohi T, Okada K, Xia F, et al. An IAP-IAP complex inhibits apoptosis. J Biol Chem. 2004;279(33):34087–34090. doi: 10.1074/jbc.C400236200. [DOI] [PubMed] [Google Scholar]