Abstract
Background
Short-term targeted treatment can potentially prevent fall asthma exacerbations while limiting therapy exposure.
Objective
We sought to compare (1) omalizumab with placebo and (2) omalizumab with an inhaled corticosteroid (ICS) boost with regard to fall exacerbation rates when initiated 4 to 6 weeks before return to school.
Methods
A 3-arm, randomized, double-blind, double placebo-controlled, multicenter clinical trial was conducted among inner-city asthmatic children aged 6 to 17 years with 1 or more recent exacerbations (clincaltrials.gov #NCT01430403).
Guidelines-based therapy was continued over a 4- to 9-month run-in phase and a 4-month intervention phase. In a subset the effects of omalizumab on IFN-α responses to rhinovirus in PBMCs were examined.
Results
Before the falls of 2012 and 2013, 727 children were enrolled, 513 were randomized, and 478 were analyzed. The fall exacerbation rate was significantly lower in the omalizumab versus placebo arms (11.3% vs 21.0%; odds ratio [OR], 0.48; % CI, 0.25–.92), but there was no significant difference between omalizumab and ICS boost (8.4% vs 11.1%; OR, 0.73; 95% CI, 0.33–1.64). In a prespecified subgroup analysis, among participants with an exacerbation during the run-in phase, omalizumab was significantly more efficacious than both placebo (6.4% vs 36.3%; OR, 0.12; 95% CI, 0.02–0.64) and ICS boost (2.0% vs 27.8%; OR, 0.05; 95% CI, 0.002–0.98). Omalizumab improved IFN-α responses to rhinovirus, and within the omalizumab group, greater IFN-α increases were associated with fewer exacerbations (OR, 0.14; 95% CI, 0.01–0.88). Adverse events were rare and similar among arms.
Conclusions
Adding omalizumab before return to school to ongoing guidelines-based care among inner-city youth reduces fall asthma exacerbations, particularly among those with a recent exacerbation.
Keywords: Asthma, fall season, omalizumab, inhaled corticosteroid, asthma exacerbations, rhinovirus, IFN-α
Although implementation and adherence to asthma guidelines improves disease control,1 some children and adolescents continue to experience exacerbations despite treatment with doses of inhaled corticosteroids (ICSs) and long-acting β-agonists that reduce levels of impairment.2,3 The consequences of exacerbations are significant: greater morbidity, higher health care costs,4 and, possibly, disease progression.5 Furthermore, asthma exacerbations can occur at any time in any patient, but those with more severe disease, greater degrees of atopy, viral infection, and recent exacerbations appear most susceptible to recurrences, particularly during the fall after school resumes.6 These risks of exacerbations indicate that both innovative treatment approaches and a more specific targeting of treatment to mechanistic interactions between allergic sensitization and viral respiratory tract infections are necessary to reduce the frequency of these events.
Two prior Inner-City Asthma Consortium (ICAC) studies found the frequency of exacerbations was reduced with higher daily doses of ICSs2 or with omalizumab (anti-IgE mAb)3 when added to year-round guidelines-based treatment, with the effects of omalizumab being most striking during the fall season.3 Because continuous treatment with both therapeutic modalities can impart certain risks and increased costs and because the fall season is the peak period for exacerbations among the inner-city population,6 we designed the Preventative Omalizumab or Step-up Therapy for Fall Exacerbations (PROSE) study to determine whether a targeted strategy of beginning preventative therapy with omalizumab 4 to 6 weeks before the start of school and continuing it for the next 4 months would be more efficacious than (1) placebo or (2) an ICS boost in preventing fall asthma exacerbations among children already receiving guidelines-based therapy.
It is necessary to understand that viral respiratory tract infections, particularly rhinovirus infections, and underlying allergic sensitization are strong risk factors for fall asthma exacerbations to determine and possibly target the mechanisms of treatment in this setting.6–8 Consequently and based on findings that some patients with asthma have reduced antiviral type I and III interferon responses,9,10 a defect also noted with peripheral blood plasmacytoid dendritic cells isolated from asthmatic patients and associated with IgE concentration on the cell surface,11,12 we formulated the additional hypothesis that the beneficial effects of omalizumab on seasonal asthma exacerbations can be explained in part by an increased release of IFN-α from plasmacytoid dendritic cells on rhinovirus exposure.
METHODS
Study design
The PROSE study (clinicaltrials.gov #NCT01430403) was a 3-arm, randomized, double-blind, double placebo-controlled, multicenter clinical trial conducted among participants receiving ongoing guidelines-based asthma care (Expert Panel Report-3 [EPR3]).1 The study enrolled 2 cohorts at 8 urban clinical research sites before the fall seasons of 2012 and 2013. The primary study objectives were to compare (1) omalizumab with placebo and (2) omalizumab with a boost in ICS (with total daily dose not to exceed 1000 μg of fluticasone propionate equivalent) in preventing fall exacerbations. After enrollment (November-March for each cohort), participants completed a 4- to 9-month run-in phase during which guidelines-based care was delivered to achieve asthma control. Study intervention treatments were then added to ongoing guidelines-based treatment beginning 4 to 6 weeks before each participant’s school start date and ending 90 days after the school start date. The protocol (available in this article’s Online Repository at www.jacionline.org) was approved by all 8 institutional review boards. Written informed consent was obtained from each participant’s legal guardian. Participants provided assent according to local guidelines.
Participants
Eligibility criteria included age of 6 to 17 years, asthma diagnosis or symptoms for more than 1 year, 1 or more asthma exacerbations (requiring systemic corticosteroids) or hospitalization within the prior 19 months, a positive skin test response to 1 or more perennial allergens, body weight and total serum IgE levels suitable for omalizumab dosing based on the ICAC’s prior study (see Table E1, A, in this article’s Online Repository at www.jacionline.org),3,13 school attendance beginning the following August or September, residence in a low-income census tract in predefined inner-city areas, and insurance covering standard medications.
Randomization and masking
By using a predefined EPR3-based treatment algorithm (see Table E1, B), clinicians determined each participant’s controller regimen (step level; see Table E1,C) based on symptoms, spirometric results, and exacerbation history (see Table E1, D), with assessments performed at 4 visits during the run-in phase. During the intervention period, step levels remained fixed. Randomization was done by using a blind, centralized, computer-based random allocation scheme. Participants had to require the equivalent of 200 μg/d or greater fluticasone propionate to be eligible for randomization. Because evidence suggests that using more than 1000 μg/d fluticasone propionate equivalent provides limited additional efficacy14 and increases the risk of side effects,15 participants requiring 500 μg of fluticasone or equivalent twice daily for control during the run-in phase (step 5) were not entered into the ICS boost arm. Instead, they were randomized at a ratio of 3:1 to omalizumab or injected placebo (Fig 1). The remaining participants (those receiving <500 μg of fluticasone propionate equivalence twice daily [steps 2–4]) were randomized at a ratio of 3:3:1 to omalizumab (with inhaled placebo), ICS boost (with injected placebo), or guidelines-based care with injected placebo and inhaled placebo. During the intervention, all participants (steps 2–5) had 2 inhalers, one obtained by prescription for ongoing guidelines-based care and one provided by blinded staff (a Diskus device [GlaxoSmithKline, Research Triangle Park, NC] containing either fluticasone propionate or placebo) for the ICS boost. The study Diskus provided to participants at step 5 always contained placebo.
FIG 1.
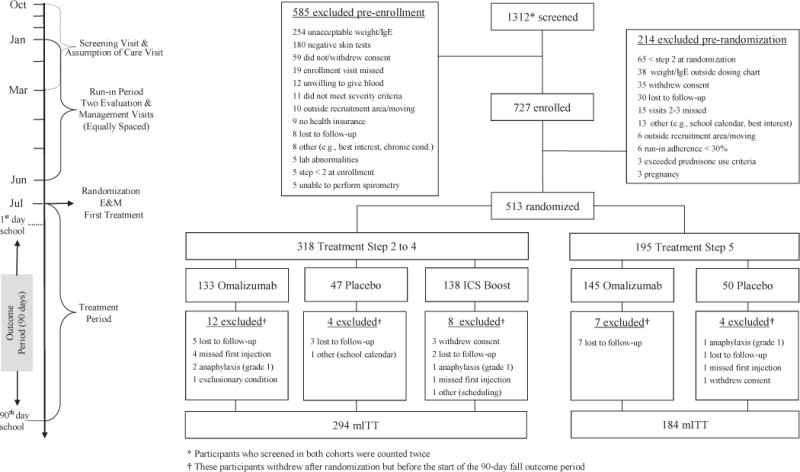
CONSORT diagram.
Omalizumab or its placebo was administered every 2 or 4 weeks by means of subcutaneous injection by unblinded nurses who had no other role in the trial, with dosing based on weight and serum IgE levels, as described previously (see Table E1, A).3,13 The ICS boost, fluticasone propionate inhalation powder (100 or 250 μg twice daily), effectively doubled the ICS dose of those patients at steps 2 to 4 at randomization (see Table E1, E).
Procedures
Clinical assessments before randomization included skin prick tests to 14 aeroallergen extracts (Greer Laboratories, Lenoir, NC), total and allergen-specific serum IgE measurement, and spirometry. Questionnaires (administered every 4–8 weeks during the run-in phase and every 2–4 weeks during the intervention phase) assessed asthma symptoms, respiratory illnesses, exacerbations, and adverse events (AEs). Adherence was measured by means of self-report for the inhaler used for ongoing care, counter for the ICS boost Diskus, and injection records for omalizumab/placebo.
During the intervention period, weekly nasal mucus samples were collected for viral detection (Respiratory Viral Panel [Luminex, Austin, Tex] and/or rhinovirus detection), as previously described.16 Also, eosinophilic cationic protein levels were measured in the same samples monthly.
PBMCs from a subset of subjects (n = 87) were incubated ex vivo with rhinovirus with or without IgE cross-linking to simulate allergen activation and IFN-α levels were measured in culture supernatants to determine whether omalizumab affected antiviral responses.11 Blood for these studies was obtained from subjects at 2 sites (UT Southwestern Medical Center, Dallas, Tex; National Jewish Health, Denver, Colo) before (prerandomization, at visit 3 or 4) and during (postrandomization, at visit 7 or 8) the intervention period. PBMCs were isolated by means of Ficoll-Paque (GE Healthcare, Fairfield, Conn) density gradient centrifugation and cultured at 0.5 × 106/0.2 mL in 96-well round-bottom plates in complete RPMI 1640 medium (supplemented with 10% heat-inactivated FBS, 1% penicillin-streptomycin, 1% sodium pyruvate, 1% HEPES buffer solution, 1% nonessential amino acids, 1% glutamate, 100 μmol/L β-mercaptoethanol, and 10 ng/mL IL-3). PBMCs were cultured for 18 hours in the presence or absence of an IgE cross-linking antibody (rabbit anti-human IgE, 1 μg/mL; Bethyl Laboratories, Montgomery, Tex) or control rabbit IgG antibody (1 μg/mL, Bethyl Laboratories). It is important to note that the IgE cross-linking antibody used in these in vitro assays differs from omalizumab because it binds and cross-links IgE on cell-surface FcɛRIα receptors (unlike omalizumab, which only binds to free IgE). After 18 hours, the PBMC conditions were stimulated with RV-A16 (106 plaque-forming units/mL; a gift from Wai-Ming Lee and Yury Bochkov, University of Wisconsin–Madison) for 24 hours. PBMC supernatants were stored at −80°C, and IFN-α concentrations were subsequently measured by means of ELISA (MabTech, Cincinnati, Ohio).
Outcomes
The primary outcome was an asthma exacerbation defined by a worsening of asthma control requiring systemic corticosteroids or hospitalization17 in the 90-day period beginning on the first day of each participant’s school year. The planned analysis also included 11 prespecified, nonmechanistic secondary outcomes (see the full protocol in this article’s Online Repository at www.jacionline.org). The protocol was monitored by a National Institute of Allergy and Infectious Diseases (NIAID) Data and Safety Monitoring Board and an NIAID Medical Monitor.
Statistical analysis
The primary outcome was analyzed as a dichotomous variable (occurrence or absence of exacerbations during the 90-day outcome period). Analysis was conducted by using a logistic regression model, adjusting for site, dosing schedule, and treatment step. The analysis of continuous secondary outcomes measured longitudinally was conducted by using a similarly adjusted linear mixed-effect model with random intercept (to account for the within-subject correlation). These analyses were performed on data from the modified intention-to-treat (mITT) population (ie, participants who were randomized, began study treatment, and had ≥1 study contact during the 90-day outcome period). Sensitivity analyses to assess the effect of missing data on the results are reported in Table E2 in this article’s Online Repository at www.jacionline.org. Eleven prespecified subgroup analyses were conducted to assess heterogeneity of treatment effects with a statistical test for interaction.18 All analyses were done with SAS (version 9.3; SAS Institute, Cary, NC) and R 3.2.0 (Vienna, Austria) software.
IgE cross-linking effects on rhinovirus-induced IFN-α levels were determined by normalizing the rhinovirus-induced IFN-α produced in the presence of ex vivo IgE cross-linking to the rhinovirus-induced IFN-α produced in the absence of IgE cross-linking. The subgroups with high (above median) and low (below median) IFN-α level increases were then evaluated in relation to the rates of asthma exacerbation during the 90-day outcome period; odds ratios (ORs) for each subgroup were calculated.
Sample size calculation
We determined that enrollment of 453 participants (223 in the omalizumab arm, 155 in the ICS boost arm, and 75 in the placebo arm [52 in steps 2–4 and 23 in step 5]) would provide greater than 90% power to compare the omalizumab and placebo arms (11.8% vs 35.9% estimated effect) and 80% power to compare the omalizumab and ICS boost arms (12.9% vs 25.8% estimated effect).
Role of the funding source
This study was funded by the NIAID and an unrestricted grant from Novartis (Basel, Switzerland). Omalizumab and fluticasone propionate were administered in the intervention under a Food and Drug Administration Investigational New Drug Application (no. 100,210) sponsored by the NIAID. Omalizumab and matching placebo were donated by Novartis. The ICS boost and matching placebo were donated by GlaxoSmithKline. Both companies had the opportunity to comment on the study design, but they had no role in the trial’s performance, data analysis, manuscript preparation, or decision to submit the manuscript for publication. Epinephrine autoinjectors (EpiPens) were donated by Mylan (Canonsburg, Pa).
The ICAC Steering Committee designed the study. The individual study sites collected the data. Rho Federal Systems Division (Chapel Hill, NC) vouches for the data and their analysis. The corresponding author had final responsibility for the decision to submit for publication.
RESULTS
Participant enrollment characteristics
Before the fall seasons of 2012 and 2013, 345 and 382 participants, respectively, were enrolled in the 4- to 9-month run-in phase of our protocol. Of these 727 participants, 513 were subsequently randomized at the end of the run-in phase into the 3 treatment arms, and 478 were included in the mITT population (Fig 1). Characteristics of the mITT participants are provided in Table I and Table E3 in this article’s Online Repository at www.jacionline.org. Median adherence to asthma medications after randomization was high, as measured based on self-report for guidelines-directed care (92.1%; interquartile range [IQR], 82.2% to 97.9%), inhaler counter information for ICS boost (82.4%; IQR, 51.6% to 115.4%), and study records for injections (100%; IQR, 88.9% to 100%).
TABLE I.
Characteristics of the mITT participants at randomization*
| Characteristic | Overall (n = 478) | Treatment steps 2–5 | Treatment steps 2–4 | ||||
|---|---|---|---|---|---|---|---|
| Placebo (n = 89) | Omalizumab (n = 259) | P value | ICS boost (n = 130) | Omalizumab (n = 121) | P value | ||
| Study cohort, no. (%) | |||||||
| 2012 | 229 (47.9) | 43 (48.3) | 127 (49.0) | .99 | 59 (45.4) | 56 (46.3) | .99 |
| 2013 | 249 (52.1) | 46 (51.7) | 132 (51.0) | 71 (54.6) | 65 (53.7) | ||
| Demographics | |||||||
| Age (y) | 10.2 (2.93) | 10.1 (3.06) | 10.3 (2.99) | .60 | 9.84 (2.70) | 10.4 (3.11) | .13 |
| Male sex, no. (%) | 303 (63.4) | 59 (66.3) | 174 (67.2) | .98 | 70 (53.8) | 78 (64.5) | .11 |
| Clinical | |||||||
| Duration of asthma (y) | 7.40 (3.50) | 7.24 (3.56) | 7.72 (3.56) | .28 | 6.87 (3.28) | 7.62 (3.80) | .10 |
| Asthma control† | |||||||
| C-ACT score in previous month, age 4–11 y | 21.6 (3.63) | 21.3 (3.52) | 21.3 (3.70) | .91 | 22.4 (3.47) | 22.3 (3.84) | .91 |
| ACT score in previous month, age ≥12 y | 21.5 (3.18) | 21.2 (3.87) | 21.4 (3.05) | .84 | 22.0 (2.96) | 22.2 (2.62) | .80 |
| Asthma-related symptoms, days in prior 2 wk‡ | 2.34 (3.13) | 2.56 (2.95) | 2.51 (3.25) | .89 | 1.85 (2.97) | 1.83 (2.87) | .96 |
| Wheezing | 1.79 (2.65) | 1.98 (2.37) | 1.89 (2.70) | .77 | 1.46 (2.71) | 1.33 (2.35) | .68 |
| Interference with activity | 1.39 (2.61) | 1.72 (2.82) | 1.56 (2.84) | .65 | 0.82 (1.76) | 0.98 (2.02) | .48 |
| Nighttime sleep disruption | 0.77 (1.80) | 0.90 (1.98) | 0.88 (1.84) | .93 | 0.48 (1.54) | 0.52 (1.59) | .86 |
| Lung function | |||||||
| FEV1, (% predicted) | 90.1 (16.6) | 89.3 (21.2) | 88.7 (15.4) | .80 | 93.7 (15.0) | 91.3 (14.1) | .20 |
| FEV1/FVC × 100 | 77.8 (9.49) | 76.6 (10.9) | 77.2 (9.53) | .68 | 79.8 (8.05) | 79.0 (8.53) | .46 |
| Medication, no. (%)§ | |||||||
| Treatment steps 2–4 | 294 (61.5) | 43 (48.3) | 121 (46.7) | .89 | 130 (100) | 121 (100) | |
| Treatment step 5 | 184 (38.5) | 46 (51.7) | 138 (53.3) | ||||
| Asthma-related health care use during run-in phase, no. (%) | |||||||
| ≥1 Asthma exacerbation | 168 (35.1) | 35 (39.3) | 106 (40.9) | .89 | 27 (20.8) | 26 (21.5) | .99 |
Values are counts (percentages) or means (SDs).
Scores on the Childhood Asthma Control Test (C-ACT) and the Asthma Control Test (ACT) were measured on scales of 0 to 27 and 5 to 25, respectively. A score of 19 or less on either test indicates that asthma is not well controlled. The minimally important difference for ACT equals 3 points; for the C-ACT, a 3-point increase suggests a clinically relevant improvement in asthma control, whereas a 2-point decrease suggests a clinically relevant worsening.
The number of days with symptoms was calculated as the largest of the following variables during the previous 2 weeks: number of days with wheezing, chest tightness, or cough; number of nights of sleep disturbance; and number of days when activities were affected. This symptom scale ranges from 0 to 14 days per 2-week period.
Six treatment steps were established, which is consistent with report 3 of the National Asthma Education and Prevention Program guidelines to standardize prescribing patterns according to levels of asthma severity; these steps are provided in full in Table E1, B, and are summarized here. Steps 1 and 2 apply to mild asthma, step 3 to moderate asthma, and steps 4 through 5 to severe asthma. At step 0, the recommendation is for no asthma control medication or albuterol as needed; at step 1, the recommendation is for 50 mg of fluticasone twice a day; at step 2, the recommendation is for 100 μg of fluticasone twice a day; at step 3, the recommendation is for 250 μg of fluticasone twice a day; at step 4, the recommendation is for 250 μg of fluticasone and 50 μg of salmeterol twice a day (Advair, GlaxoSmithKline); and at step 5, the recommendation is for 500 μg of fluticasone and 50 μg of salmeterol twice a day (Advair, GlaxoSmithKline).
Effect of run-in treatment on asthma control
The EPR3-based treatment algorithm used during the run-in phase significantly improved asthma control, as reflected by decreasing the mean number of symptom days reported over the 2 weeks before each assessment visit (4.5 [SD, 4.4] days at study entry to 2.3 [SD, 3.1] days before randomization, a difference of 2.1 days; 95% CI, 1.7–2.6 days; see Table E4 in this article’s Online Repository at www.jacionline.org). At randomization, 62% of the mITT participants were at treatment steps 2 to 4 and 38% were at step 5. Of those at steps 2 to 4 at randomization, 21.8% had experienced 1 or more asthma exacerbations during the run-in phase compared with 56.5% among those at step 5 (P <.001). After randomization, in the placebo arm the frequency of exacerbations continued to be significantly higher in step 5 participants versus that in step 2 to 4 participants (32.6% vs 12.7%, P = .03).
Effects of study interventions on the frequency of fall exacerbations
For the first of our primary hypotheses, the odds of participants at all step care levels (2–5) having at least 1 exacerbation during the 90-day outcome period were significantly lower in the omalizumab versus placebo arms (11.3% vs 21.0%; OR, 0.48; 95% CI, 0.25–0.92; Fig 2, A). Stratifying by treatment step, the effect of omalizumab was still found in those receiving step 5 care (Fig 2, B). For the second primary hypothesis, in which participants at steps 2 to 4 were used, we did not observe a difference between omalizumab and the ICS boost (8.4% vs 11.1%; OR, 0.73; 95% CI, 0.33–1.64; Fig 2, C).
FIG 2.
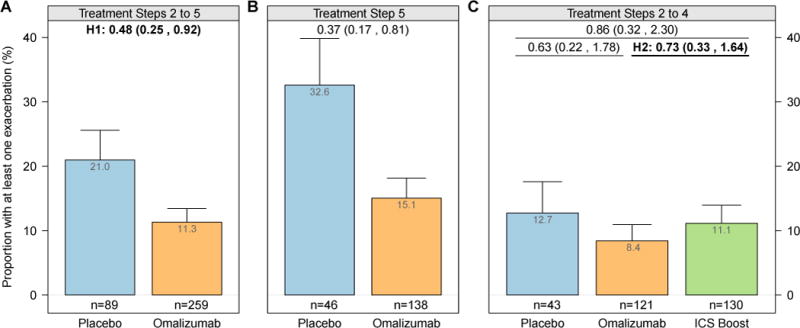
Proportion of participants by treatment arm with at least 1 exacerbation (bar) plus 1 SE (whisker) during the fall outcome period in the placebo and omalizumab arms randomized at steps 2 to 5 (A), in the placebo and omalizumab arms randomized at step 5 (B), and in the placebo, omalizumab, and ICS arms randomized at steps 2 to 4 (C). Values at the top of each panel are ORs (95% CIs). All values are adjusted for site, dosing group, and treatment step. H1, Primary hypothesis 1; H2, primary hypothesis 2.
Effects of an exacerbation during the run-in phase on responses to study treatments in the outcome period
As previously described,6 an exacerbation during the run-in phase significantly increased the odds of an exacerbation during the outcome period (23.2% in participants with ≥1 exacerbation vs 11.9% in those without an exacerbation; OR, 2.23; 95% CI, 1.36–3.67) in participants who received placebo. Comparison of the effect of our interventions in this subgroup was one of our predetermined subgroup analyses. At all treatment steps (steps 2–5), omalizumab was strikingly more efficacious than placebo in preventing exacerbations among participants who had experienced an exacerbation during the run-in phase (6.4% vs 36.3%; OR, 0.12; 95% CI, 0.02–0.64; Fig 3, A).Inaddition, at steps 2 to 4 and in this subgroup of participants, omalizumab was more efficacious than an ICS boost in preventing exacerbations during the outcome period (2.0% vs 27.8%; OR, 0.05; 95% CI, 0.003–0.98; Fig 3, B). In contrast, no effect of omalizumab compared with placebo was found in participants who had not experienced an asthma exacerbations during the run-in phase (steps 2–5: 12.2% vs 13.6%; OR, 0.88; 95% CI, 0.35–2.18; steps 2–4: 11.6% vs 8.9%; OR, 1.34; 95% CI, 0.56–3.25). Notably, even in the step 5 subjects, no effect of omalizumab was detected among participants who did not experience an exacerbation during the run-in phase. Among the other predetermined subgroups, no effects with similar consistency were detected (see Table E5 in this article’s Online Repository at www.jacionline.org).
FIG 3.
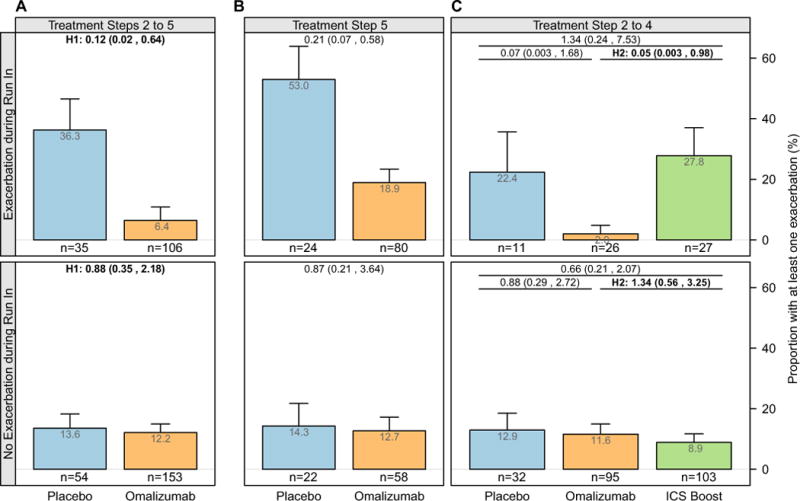
Proportion of participants by treatment arm with at least 1 exacerbation (bar) plus 1 SE (whisker) during the fall outcome period stratified by exacerbation status during the run-in phase among participants in the placebo and omalizumab arms randomized at steps 2 to 5 (A), in the placebo and omalizumab arms randomized at step 5 (B), and in the placebo, omalizumab, and ICS arms randomized at steps 2 to 4 (C). Values at the top of each panel are ORs (95% CIs). All values are adjusted for site, dosing group, and treatment step. H1, Primary hypothesis 1; H2, primary hypothesis 2. For H1 and H2, there was a significant interaction between subgroups (P < .05).
Participants who experienced an exacerbation during the run-in phase compared with those without an exacerbation had evidence of higher levels of allergic inflammation. At the time of randomization, those with an exacerbation during the run-in phase had higher peripheral blood eosinophil counts of 350 cells/μL (IQR, 210–520 cells/μL) and fraction of exhaled nitric oxide (FENO) levels of 29.0 ppb (IQR, 15.5–51.2 ppb) versus respective values in those without an exacerbation during the run-in phase: 280 cells/μL (IQR, 170–430 cells/μL; P < .01) and 19.0 ppb (IQR, 11.4–39.0 ppb; P < .01), respectively.
Effect of treatment interventions on asthma symptoms
Compared with placebo, omalizumab significantly decreased mean days with symptoms reported by participants in the 2 weeks before each assessment. Compared with ICS boost at steps 2 to 4, omalizumab did not significantly decrease symptom days (see Table E6 in this article’s Online Repository at www.jacionline.org).
Respiratory tract virus detection in association with exacerbations
Of the 86 recorded exacerbations, 75 had 1 or more nasal samples obtained within 7 days of the exacerbation. Of these 75 exacerbations, 67 (89%) had a respiratory tract virus detected, most commonly rhinovirus (61/75 [81%]). When all nasal samples were considered, rhinoviruses were found more often in samples temporally related to exacerbations (97/171 [57%]) than in samples not related to exacerbations (2150/5959 [36%]; OR, 2.32; 95% CI, 1.71–3.16). Compared with placebo, omalizumab treatment was associated with a trend toward a lower odds of a respiratory virus–associated exacerbation (OR, 0.52; 95% CI, 0.24–1.13); this effect was statistically significant for step 5 participants (OR, 0.35; 95% CI, 0.15–0.85; see Fig E1 in this article’s Online Repository at www.jacionline.org). There were too few exacerbations (n = 8) not associated with a virus to determine the effect of treatment on this group.
Effect of omalizumab treatment on PBMC generation of IFN-α on rhinovirus challenge and frequency of exacerbations
Before randomization, IgE receptor cross-linking in PBMCs significantly suppressed rhinovirus-induced IFN-α responses in both the placebo- and omalizumab-treated groups (Fig 4, A, left panel; P < .001). When the same assessments were repeated during the intervention phase, IFN-α responses to rhinovirus were significantly increased in the omalizumab-treated group (Fig 4, A, right panel; P =.03). Notably, among the omalizumab-treated group, participants with increases in ex vivo IFN-α responses to rhinovirus to greater than the median value had a significantly lower rate of exacerbations during the outcome period (Fig 4, B; OR, 0.14; 95% CI, 0.01–0.88).
FIG 4.
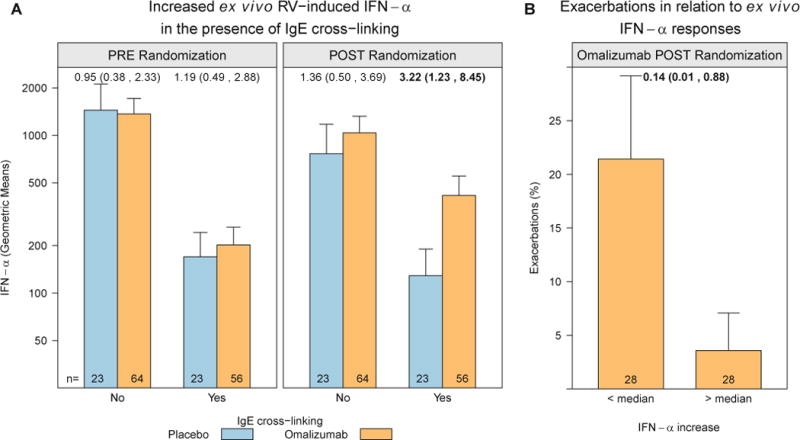
Enhanced ex vivo IFN-α responses to rhinovirus (RV) in the omalizumab group and relationship to exacerbation rates. PBMCs were incubated ex vivo with rhinovirus in the presence or absence of an IgE cross-linking antibody, and IFN-α levels were measured in culture supernatants. Rhinovirus-induced IFN-α was significantly reduced by IgE cross-linking; the IFN-α response was significantly increased in the omalizumab group during the intervention phase of the study. A, A 3.22-fold increase in omalizumab versus placebo in the postrandomization phase (P = .03). B, Among participants treated with omalizumab, those with the greatest increase in ex vivo IFN-α responses in the presence of IgE cross-linking were less likely to have an asthma exacerbation during the outcome period. Values at the top of each panel are ORs (95% CIs).
AEs
One or more AEs were reported by 54.5% of participants in the omalizumab arm and 54.8% of participants in the placebo arm (P > .99, steps 2–5) during the intervention phase. One or more AEs were reported by 43.5% of participants in the ICS boost arm and 53.3% of participants in the placebo arm (P =.30, steps 2–4; Table II). Three cases of grade 1 anaphylaxis occurred in the ICS boost, 2 in the placebo, and 3 in the omalizumab arms. Two serious AEs occurred during the intervention period, one each in the placebo (seventh nerve palsy) and ICS boost (anaphylaxis) arms. There were no deaths and no non–asthma-related hospitalizations during the intervention phase.
TABLE II.
Number of AEs (safety population)*
| System organ class | Treatment steps 2–5 | Treatment steps 2–4 | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 93) | Omalizumab (n = 268) | P value† | Placebo (n = 45) | ICS boost (n = 131) | P value† | |
| Blood and lymphatic system disorders | 1 (1) | 1 (1) | .45 | 0 (0) | 1 (1) | 1.00 |
| Ear and labyrinth disorders | 1 (1) | 4 (4) | 1.00 | 0 (0) | 4 (3) | .57 |
| Eye disorders | 0 (0) | 2 (2) | 1.00 | 0 (0) | 7 (6) | .34 |
| Gastrointestinal disorders | 10 (7) | 19 (17) | .64 | 5 (4) | 3 (3) | .07 |
| General disorders and administration-site conditions | 10 (6) | 63 (41) | .03 | 6 (3) | 18 (11) | 1.00 |
| Immune system disorders | 2 (2) | 5 (5) | 1.00 | 0 (0) | 3 (3) | .57 |
| Infections and infestations | 22 (17) | 63 (50) | 1.00 | 13 (10) | 26 (23) | .51 |
| Injury, poisoning, and procedural complications | 7 (6) | 32 (28) | .31 | 4 (4) | 10 (9) | .74 |
| Investigations | 2 (2) | 1 (1) | .16 | 0 (0) | 2 (2) | 1.00 |
| Musculoskeletal and connective tissue disorders | 1 (1) | 8 (8) | .46 | 0 (0) | 1 (1) | 1.00 |
| Neoplasms: benign, malignant, and unspecified | 0 (0) | 1 (1) | 1.00 | 0 (0) | 0 (0) | |
| Nervous system disorders | 9 (8) | 21 (20) | .82 | 4 (4) | 7 (6) | .28 |
| Pregnancy, puerperium, and perinatal conditions | 0 (0) | 1 (1) | 1.00 | 0 (0) | 0 (0) | |
| Psychiatric disorders | 2 (2) | 7 (6) | 1.00 | 0 (0) | 1 (1) | 1.00 |
| Reproductive system and breast disorders | 1 (1) | 1 (1) | .45 | 1 (1) | 2 (2) | 1.00 |
| Respiratory, thoracic, and mediastinal disorders | 11 (8) | 29 (24) | 1.00 | 5 (4) | 13 (11) | 1.00 |
| Skin and subcutaneous tissue disorders | 18 (14) | 41 (36) | .73 | 10 (8) | 20 (19) | .63 |
| Surgical and medical procedures | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1.00 | |
| Vascular disorders | 0 (0) | 1 (1) | 1.00 | 0 (0) | 0 (0) | |
| Total | 97 (51) | 300 (146) | 1.00 | 48 (24) | 119 (57) | .30 |
In each case the reported data are the total number of combined serious and nonserious AEs. Numbers in parentheses are numbers of unique patients reporting such events. Only AEs during the treatment period are considered; these include AEs that occurred between randomization and 30 days after the end of the fall outcome period in participants who received at least 1 dose of study medication. Two serious AEs occurred after initiation of the intervention: Bell palsy in a participant receiving placebo and grade 1 anaphylaxis in a participant receiving an ICS boost.
The Fisher exact test was used to compare the number of participants with at least 1 AE between groups.
DISCUSSION
In the PROSE study we showed, in the context of our first primary objective, that a 4-month targeted treatment strategy with the addition of omalizumab beginning 4 to 6 weeks before the start of a school year to ongoing guidelines-based management significantly reduced asthma exacerbations during the fall season among at-risk inner-city youth (Fig 2, A). This seasonal approach in treatment adjustment represents a first-time report of this novel strategy aiming to more effectively prevent exacerbations during what is referred to as the September epidemic of asthma.7 Our seasonal strategy in the PROSE study was as effective in reducing fall exacerbations as year-round treatment with omalizumab3 and also indicates that the protective onset of omalizumab begins well within 4 to 6 weeks of initiating this intervention. Moreover, through predetermined subgroup analysis, we found that children with exacerbations during the run-in phase were highly benefited by a seasonal addition of omalizumab, with a greater than 80% protection from fall exacerbations; in the absence of a run-in exacerbation, omalizumab was not beneficial (Fig 3).
Our second primary objective was to compare the addition of omalizumab with an ICS boost in preventing fall exacerbations. Per the study design, this comparison was only available in participants randomized at steps 2 to 4 (<1000 μg of fluticasone propionate equivalent daily). We limited the ICS boost to not exceed 1000 μg/d fluticasone propionate equivalence because larger doses over an extended time period provide minimal benefit in preventing exacerbations14 and increase the risk for systemic adverse effects.15 We found no significant difference in exacerbation rates between omalizumab and ICS boost (Fig 2, C) or between any of these 2 interventions and placebo, which might relate to the overall low rate of fall exacerbations in patients at steps 2 to 4.
In contrast, among participants in the step 2 to 4 group who experienced an exacerbation during the run-in phase, the addition of omalizumab was more efficacious than the ICS boost in preventing fall exacerbations (Fig 3, B, upper panel). In those who experienced an exacerbation during the run-in phase, peripheral blood eosinophil counts and FENO values were greater than in those who did not experience an exacerbation. This might reflect higher levels of inflammation despite guidelines-directed treatment, which could have resulted from the recent exacerbation together with an incomplete response to the standard use of systemic corticosteroids. These greater levels of inflammation might have enhanced the susceptibility for a subsequent exacerbation at the time of a respiratory tract infection.
From our results in the PROSE study, 2 asthma groups or phenotypes emerge as candidates who will benefit from the seasonal addition of omalizumab. First, patients with severe asthma, as reflected by the need for step 5 care, benefited from omalizumab over the 4-month treatment period (Fig 2, A). In our previous study, the Inner-City Anti-IgE Therapy for Asthma Study (ICATA),3 patients with asthma of all severities had a reduced frequency of exacerbations with omalizumab treatment administered over a 1-year period. This difference with the study reflects the shorter double-blind period (90 days) in the PROSE study, which resulted in a lower number of exacerbations among participants receiving step 2 to 4, thus reducing the statistical power to find a difference. In addition, a novel observation in the PROSE study was the striking benefit of seasonal omalizumab in patients with a recent exacerbation; in this setting the preventative efficacy of omalizumab was noted across all levels of disease severity (Fig 3). Without this additional prespecified analysis and based only on data from Fig 2, it might have been concluded that a seasonal benefit of omalizumab is restricted to only those with the most severe disease. This conclusion would lead to both seasonal undertreatment and overtreatment with omalizumab to prevent fall exacerbations. Undertreatment would occur in patients with low severity and a recent exacerbation, whereas overtreatment would occur among patients with high severity without a recent exacerbations.
The rationale for our design of comparing omalizumab with an ICS boost was based on our previous work in which we found greater suppression of seasonal exacerbations in children and adolescents who received higher ICS doses as year-round treatment.2 The addition of the ICS boost arm in the PROSE study was also meant to contribute to addressing the question of whether the risk for an asthma exacerbation might represent undertreatment with ICS.2,3 Other approaches to reduce asthma exacerbations, especially during the fall, have had little success, including adding montelukast to existing treatment at the start of school,19 increasing ICSs at the beginning of an exacerbation,20 or prescribing clarithromycin in adults21 and azithromycin in children.22
The mechanisms underlying fall asthma exacerbations are complex23 and include viral respiratory tract infections (predominantly with rhinoviruses),8 allergic sensitization,24 allergen exposure,25 and diminished generation of type I and III interferons.9 It is likely that IgE plays an important role in the promotion of rhinovirus infections progressing to exacerbations by generating an inflammatory milieu. Omalizumab removes free circulating IgE, resulting in less IgE bound to mast cells and basophils, as well as suppression of allergic reactions and type 2 inflammation.26 In our study evidence for this effect is provided by the reduction in eosinophilic cationic protein levels in nasal secretions (see Table E6). Most interestingly, in vitro IgE receptor activation decreases the generation of IFN-α from plasmacytoid dendritic cells11,12; the possibility that this mechanism occurs in vivo has not been previously tested. Our data provide support to this hypothesis and raise the possibility that restoration of virus-induced IFN-α responses might be a mechanism for the preventative effects of omalizumab on respiratory virus–associated exacerbations (Fig 4).
A challenge to the use of biologic agents in asthmatic patients is to select the patients most likely to respond to these treatments. Our findings in the PROSE study might help clarify patient selection for omalizumab. We recently published observations related to factors associated with seasonal asthma exacerbations.6 In particular, we noted that patients’ characteristics associated with an increased risk for a fall exacerbation include an exacerbation during the prior season. This finding was replicated in the PROSE study, in which we also found that an exacerbation in the run-in phase was a major predictor of efficacy for seasonal administration of omalizumab (ie, >80% reduction in fall exacerbations). Not only did the results in the PROSE study confirm that recent exacerbations are a risk factor for a subsequent event but also our findings point to possible explanations for a recurrent exacerbation in the outcome period. The increased peripheral blood eosinophil counts and FENO values found at randomization in the participants who had an exacerbation during the preceding 4 to 9 months of the run-in phase might reflect increased airway inflammation, perhaps resulting from the recent exacerbation with incomplete resolution despite systemic corticosteroids, which in turn led to greater susceptibility to a subsequent exacerbation. These findings parallel observations by Hanania et al,27 who found increases in these 2 biomarkers predicting greater future risk for an exacerbation and greater likelihood to benefit with omalizumab. Furthermore, the persistence of increased peripheral blood eosinophil counts and FENO values, despite a higher treatment dose of ICS (ie, step 5 care), parallels findings with mepolizumab and might represent biomarkers earmarking a risk for exacerbations, which cannot be prevented by conventional therapy but might require alternative treatment approaches, such as type 2–directed biologic agents.28,29
Interpretation of the PROSE study requires several considerations. First, our results are derived from a largely minority low-income population. However, year-round omalizumab reduces exacerbations to a similar degree in other groups.3,30,31
Second, our study population was limited to allergen-sensitized participants eligible for omalizumab therapy. Consequently, 36.0% of our screened patients were ineligible for enrollment because of negative skin test results or unsuitable weight/IgE measurements (Fig 1).13
Finally, the cost of omalizumab is a limitation, but our findings help identify populations most likely to respond to preseasonal treatment. For those patients, the reduced cost of treatment for only the fall season to prevent an exacerbation compared with a full year of treatment might be justifiable.
Our findings that an effective preventative strategy for fall exacerbations can be achieved with targeted seasonal treatment suggest a paradigm shift for managing high-risk patients, although further research is needed to refine the subgroups of asthmatic patients most likely to benefit from seasonal treatment with omalizumab. In addition, we need to identify more effective treatment strategies for those patients who have exacerbations despite the addition of omalizumab therapy. An early step to personalized or precision medicine is recognition of the correct phenotype to guide treatment selection. The results of the PROSE study are a step toward an effective and time-limited treatment approach for a specific exacerbation-prone asthmatic phenotype.
Supplementary Material
Clinical implications.
Inner-city children might benefit from the addition of omalizumab to ongoing guidelines-based therapy before the fall season to prevent exacerbations if they have severe disease and particularly if they have a history of a recent exacerbation, irrespective of disease severity.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contracts HHSN272200900052C and HHSN272201000052I. Additional support was provided by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, under grants NCRR/NIH UL1TR000451, 1UL1RR025780, UL1TR000075 and NCATS/NIH UL1 TR000154, UL1 TR000077-04, NCATS/NIH UL1TR000040, UL1TR000150, and UL1TR001105, NIH NIAID 5R01AI098077, and UM1AI109565. The following were donated: omalizumab and matching placebo by Novartis and fluticasone and matching placebo by GlaxoSmithKline under a clinical trial agreement with the University of Wisconsin–Madison; EpiPens by Mylan; and Ayr nasal rinse by B.F. Ascher & Company.
S. J. Teach has received grants from Novartis, PCORI, the Fight for Children Foundation, the Stewart Foundation, EJF Philanthropies, the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID), and the NIH/National Heart, Lung, and Blood Institute (NHLBI). M. Gill has received grants from the NIH/NIAID Inner City Asthma Consortium II and the NIH/NIAID R01. C. A. Sorkness has received grants from the NIH/NIAID, the NIH/NHLBI, and Novartis. S. J. Arbes has a contract with the NIH/NIAID. A. Calatroni, J. J. Wildfire, and K. A. Grindle have received grants from the NIH/NIAID. J. A. Pongracic received the study drug for this study from Genentech, has a subcontract for the NIAID-sponsored Inner City Asthma Consortium from the University of Wisconsin and has received the study drug for a food allergy clinical trial from Genentech. C. M. Kercsmar has received a grant from the NIH and has received personal fees from GlaxoSmithKline. G. K. Khurana Hershey and E. M. Zoratti have received grants from the NIH. R. S. Gruchalla has served as a special government employee for the Center for Biologics Evaluation and Research and has consulted for the Massachusetts Medical Society. A. H. Liu has served as a member of a data monitoring committee for GlaxoSmithKline and has received payment for lectures from Merck. M. Kattan has received a grant from the NIH/NIAID and is on the advisory board for Novartis Pharma. J. E. Gern has received grants from the NIH, GlaxoSmithKline, and Merck; has consultant arrangements with GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Genentech, Amgen, and Novartis; and has stock/stock options in 3V BioSciences. W. W. Busse has received grants from the NIH/NIAID; has received partial study funding, drug, and placebo from Novartis; is on Data Safety Monitoring Boards for Boston Scientific and Circassia; is on the Study Oversight Committee for ICON; and has consultant arrangements with Novartis, GlaxoSmithKline, Genentech, Roche, Pfizer, Merck, Boehringer Ingelheim, Sanofi, AstraZeneca, Gilead, Teva, Tekeda, and Aerocrine. S. J. Szefler has received grants from the NIAID-sponsored Inner City Asthma Consortium and GlaxoSmithKline; has consultant arrangements with Merck, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Aerocrine, Novartis, and AstraZeneca; and has received payment for lectures from Merck.
Abbreviations
- AE
Adverse event
- EPR3
Expert Panel Report-3
- FENO
Fraction of exhaled nitric oxide
- ICAC
Inner-City Asthma Consortium
- ICATA
Inner-City Anti-IgE Therapy for Asthma
- ICS
Inhaled corticosteroid
- mITT
Modified intention-to-treat
- NIAID
National Institute of Allergy and Infectious Diseases
- OR
Odds ratio
- PROSE
Preventative Omalizumab or Step-up Therapy for Fall Exacerbations
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma–summary report 2007. J Allergy Clin Immunol. 2007;120(suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O’Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065–72. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:10050–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129:1229–35. doi: 10.1016/j.jaci.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 5.Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J. 2007;30:452–6. doi: 10.1183/09031936.00165106. [DOI] [PubMed] [Google Scholar]
- 6.Teach SJ, Gergen PJ, Szefler SJ, Mitchell HE, Calatroni A, Wildfire J, et al. Seasonal risk factors for asthma exacerbations among inner-city children. J Allergy Clin Immunol. 2015;135:1465–73.e5. doi: 10.1016/j.jaci.2014.12.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston NW, Johnston SL, Duncan JM, Greene JM, Kebadze T, Keith PK, et al. The September epidemic of asthma exacerbations in children: a search for etiology. J Allergy Clin Immunol. 2005;115:132–8. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–34. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baraldo S, Contoli M, Bazzan E, Turato G, Padovani A, Marku B, et al. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. J Allergy Clin Immunol. 2012;130:1307–14. doi: 10.1016/j.jaci.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–47. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, et al. Counterregulation between the FcɛRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durrani SR, Montville DJ, Pratt AS, Sahu S, DeVries MK, Rajamanickam V, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130:489–95. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorkness CA, Wildfire JJ, Calatroni A, Mitchell HE, Busse WW, O’Connor GT, et al. Reassessment of omalizumab-dosing strategies and pharmacodynamics in inner-city children and adolescents. J Allergy Clin Immunology Pract. 2013;1:163–71. doi: 10.1016/j.jaip.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masoli M, Weatherall M, Holt S, Beasley R. Systematic review of the dose-response relation of inhaled fluticasone propionate. Arch Dis Child. 2004;89:902–7. doi: 10.1136/adc.2003.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly HW, Nelson HS. Potential adverse effects of the inhaled corticosteroids. J Allergy Clin Immunol. 2003;112:469–79. [PubMed] [Google Scholar]
- 16.Bochkov YA, Grindle K, Vang F, Evans MD, Gern JE. Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol. 2014;52:2461–71. doi: 10.1128/JCM.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA, Jr, Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol. 2012;129(suppl):S34–48. doi: 10.1016/j.jaci.2011.12.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–94. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 19.Weiss KB, Gern JE, Johnston NW, Sears MR, Jones CA, Jia G, et al. The Back to School asthma study: the effect of montelukast on asthma burden when initiated prophylactically at the start of the school year. Ann Allergy Asthma Immunol. 2010;105:174–81. doi: 10.1016/j.anai.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Oborne J, Mortimer K, Hubbard RB, Tattersfield AE, Harrison TW. Quadrupling the dose of inhaled corticosteroid to prevent asthma exacerbations: a randomized, double-blind, placebo-controlled, parallel-group clinical trial. Am J Respir Crit Care Med. 2009;180:598–602. doi: 10.1164/rccm.200904-0616OC. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland ER, King TS, Icitovic N, Ameredes BT, Bleecker E, Boushey HA, et al. A trial of clarithromycin for the treatment of suboptimally controlled asthma. J Allergy Clin Immunol. 2010;126:747–53. doi: 10.1016/j.jaci.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strunk RC, Bacharier LB, Phillips BR, Szefler SJ, Zeiger RS, Chinchilli VM, et al. Azithromycin or montelukast as inhaled corticosteroid-sparing agents in moderate-to-severe childhood asthma study. J Allergy Clin Immunol. 2008;122:1138–44.e4. doi: 10.1016/j.jaci.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011;128:1165–74. doi: 10.1016/j.jaci.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soto-Quiros M, Avila L, Platts-Mills TA, Hunt JF, Erdman DD, Carper H, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol. 2012;129:1499–505.e5. doi: 10.1016/j.jaci.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray CS, Poletti G, Kebadze T, Morris J, Woodcock A, Johnston SL, et al. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax. 2006;61:376–82. doi: 10.1136/thx.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170:583–93. doi: 10.1164/rccm.200312-1651OC. [DOI] [PubMed] [Google Scholar]
- 27.Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804–11. doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 28.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–9. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 30.Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154:573–82. doi: 10.7326/0003-4819-154-9-201105030-00002. [DOI] [PubMed] [Google Scholar]
- 31.Humbert M, Boulet LP, Niven RM, Panahloo Z, Blogg M, Ayre G. Omalizumab therapy: patients who achieve greatest benefit for their asthma experience greatest benefit for rhinitis. Allergy. 2009;64:81–4. doi: 10.1111/j.1398-9995.2008.01846.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


