Abstract
Introduction
The dominant location of electrical triggers for initiating atrial fibrillation (AF) originates from the muscle sleeves inside pulmonary veins (PVs). Currently, radiofrequency ablation (RFA) is performed outside of the PVs to isolate, rather than directly ablate these tissues, due to the risk of intraluminal PV stenosis.
Methods
In 4 chronic canine experiments, we performed direct PV muscle sleeve RFA ± post-ablation drug-coated balloon (DCB) treatment with paclitaxel/everolimus. Of the 4 PVs, 2 PVs were ablated and treated with DCB, 1 PV was ablated without DCB treatment (positive control), and 1 PV was left as a negative control. Local electrograms were assessed in PVs for near-field signals and were targeted for ablation. After 12-14 weeks survival, PVs were interrogated for absence of near-field PV potentials, and each PV was assessed for stenosis.
Results
All canines survived the study period without cardiorespiratory complications, and remained ambulatory. In all canines, PVs that were ablated and treated with DCB remained without any significant intraluminal stenosis. In contrast, PVs that were ablated and not treated with DCB showed near or complete intraluminal stenosis. At terminal study, PV potentials remained undetectable. A blinded, histologic analysis demonstrated that ablated PVs without DCB treatment had extensive thrombus, fibrin, mineralization, and elastin disruption.
Conclusion
Our chronic canine data suggest that direct PV tissue ablation without subsequent stenosis is feasible with the use of post-ablation DCBs.
Keywords: pulmonary vein stenosis, pulmonary vein isolation, catheter ablation, atrial fibrillation, anti-proliferative agent, neointimal hyperplasia
Introduction
Electrical activity originates within PV muscle sleeves, which are complex extensions of left atrial (LA) myocardium that permeates into the veins with a variable fiber thickness and orientation [1]. In a landmark study in 1998 by Haïssaguerre et al., PV muscle was shown to harbor electrical triggers that can spontaneously initiate AF [2]. These triggers were mapped inside the PV and subsequently targeted for direct RFA [2]. The mainstay of catheter-based treatment for AF has evolved to performance of circular ablation around the outside of the PV ostia to create electrical isolation to the LA [3], rather than a direct ablation of the triggers inside the PVs in order to minimize the potential for development of PV stenosis (Figure 1). Unfortunately, paroxysmal AF ablation is associated with a high rate of recurrence, and randomized controlled trials have demonstrated 66-86% efficacy at about 12 months post-ablation. [4]. Therefore, a critical unmet need exists to improve the efficacy of AF ablation, while maintaining procedural safety.
Figure 1. Illustration of pulmonary vein ablation versus isolation and potential for atrial fibrillation recurrence.
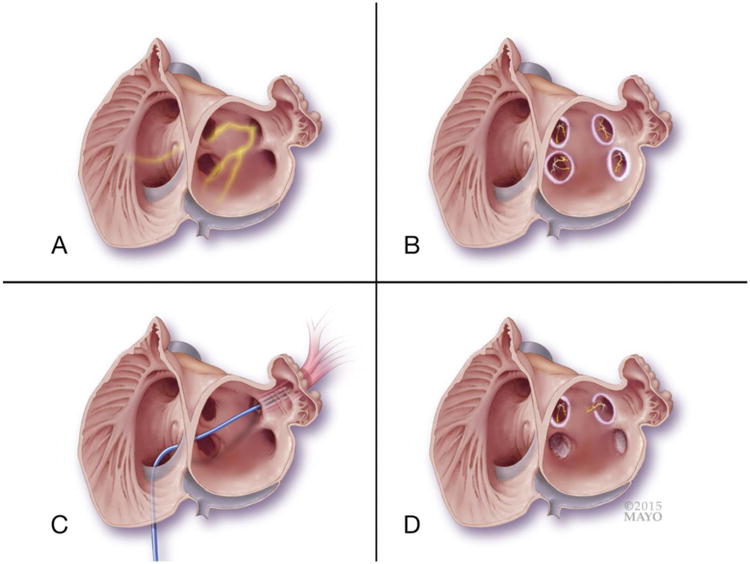
Panel A: a two chamber view of both right and left atria is shown, with en face view of the four ostia of the pulmonary veins (PVs). The left superior pulmonary vein is shown with the escape of electrical activity emerging from the PV myocardium, serving as a trigger for atrial fibrillation (AF). Panel B: illustrates that the electrical triggers within the PVs are present, but are now isolated from the rest of the atria, and thus preventing triggering of AF. The circular radiofrequency ablation lesions are outside the ostia of the PVs. Panel C: An example of direct PV myocardial tissue ablation is shown in the left superior pulmonary vein. The ablation catheter is shown ablating myocardium that exists within the lumen of the vein. Panel D: Comparison of post-ablation scenarios depending on the type of ablation method used. The right superior pulmonary vein has undergone PV isolation, and a complete circular lesion around the ostia persists. Note that the electrically active myocardium inside the PVs remains viable. The left superior pulmonary vein shows the concern with the PV isolation approach, as a gap in ablation will allow for escape electrical activity and the potential for AF recurrence. The right and left lower pulmonary veins are shown post direct PV myocardial ablation. Because of direct ablation of these muscle sleeves, the electrical trigger for AF is eliminated.
Using a PV isolation approach outside of the PV itself, the high frequency of AF recurrence is due to gaps within the ablation lesions, and electrical reconnection to the LA [4, 5]. A more effective ablation strategy would involve direct ablation of these triggers inside the PV. However, this approach is currently avoided because of the known complication of potentially life-threatening PV stenosis [6]. Although the exact mechanism for RF-induced PV stenosis remains undefined, canine studies have shown that histologic changes of neointimal hyperplasia, elastic lamina proliferation, thrombus, and collagen deposition occur [7]. In a recent porcine study, successful antagonism of the neointimal proliferation process by a paclitaxel-coated scoring balloon was demonstrated [8].
We hypothesized that post-ablation treatment with an anti-proliferative agent can prevent PV stenosis. We tested this hypothesis by performing RFA within canine PVs along with post-ablation delivery of drug via a coated balloon.
Methods
Animal study protocol, surgical preparation, and monitoring
Our experimental protocol was conducted according to the guidelines and regulations set forth by the Mayo Clinic Institutional Animal Care Use Committee (IACUC) (protocol #A48913). Five healthy, male mongrel dogs (30-40 kg) underwent experimental study of the four PVs draining into the LA (left superior pulmonary vein (LSPV), left inferior pulmonary vein (LIPV), right superior pulmonary vein (RSPV), and right inferior pulmonary vein (RIPV).
Canines were fasted 12 hours prior to each experimental procedure. Subcutaneous buprenorphine (0.15 mg/kg) and 50 mg/kg cefazolin were given intravenously (IV) 20 minutes prior to procedure for pain control and surgical prophylaxis. Canines were prepped in sterile fashion. Anesthetic induction for intubation was performed with the use of IV ketamine (10 mg/kg) and diazepam (0.5 mg/kg). Isoflurane (1-3%) was used for maintenance of anesthesia with volume-cycled ventilation. Continuous monitoring of arterial blood pressure, ECG, and level of anesthesia occurred throughout the procedure. Post-procedure, animals were monitored daily for pulmonary distress, hemoptysis, and bleeding. Aside from intra-procedural heparin, no antiplatelet or anticoagulation medications were used at any time during the study.
Vascular Access, Imaging, and Recording
A standard cut-down technique was utilized for access to the femoral arteries and veins, as well as the external jugular veins for sheath placement. An 18 gauge needle was placed in each vessel, a guidewire was introduced, and a sheath was advanced over the wire. A 12 Fr sheath was placed in the right external jugular vein for ICE imaging (Acuson Ultrasound SC2000) with a 10 Fr, 5.5-10 MHz steerable tip catheter. A 9 Fr sheath was placed in the right femoral artery for blood pressure recordings. Fluoroscopic imaging of the cardiac anatomy was performed using a CathLab Siemens ArtisZee X-ray machine (Siemens, Erlangen, Germany). All EP recordings were performed using the Prucka Cardiolab system (GE Healthcare Clinical Systems, Milwaukee, WI). Pacing was performed with the use of a Bloom DTU-215B EP stimulator (Fisher Medical Technologies Inc., Broomfield, CO). Bipolar signals were recorded with low and high pass filters set at 30Hz and 500 Hz, respectively, and a 60 Hz notch filter. Cardiac ablation was performed with the Maestro 3000 system (Boston Scientific, Marlborough, MA).
Experimental study
For each canine, an intervention strategy for each of the 4 PVs included: 1) one PV without ablation and without DCB (negative control), 2) one PV with RFA only (positive control), and 3) two remaining PVs served as treatment vessels for combined ablation and subsequent DCB delivery of an anti-proliferative agent (Figure 2). Each canine had an initial study performed that included fluoroscopic venography, intracardiac echocardiography (ICE) with Doppler imaging and measurements of the PVs, electrophysiologic (EP) assessment, and ablation/DCB intervention. Four canines completed the initial study and were survived an additional 12-14 weeks for a second procedure (a fifth canine exsanguinated prior to the experimental portion of the study; this was due to a defective transseptal sheath, which led to the transseptal needle piercing out the right side of the sheath and cannulating the aorta).
Figure 2. Schematic drawing of experimental approach to ablation and anti-stenosis drug application in canine studies.
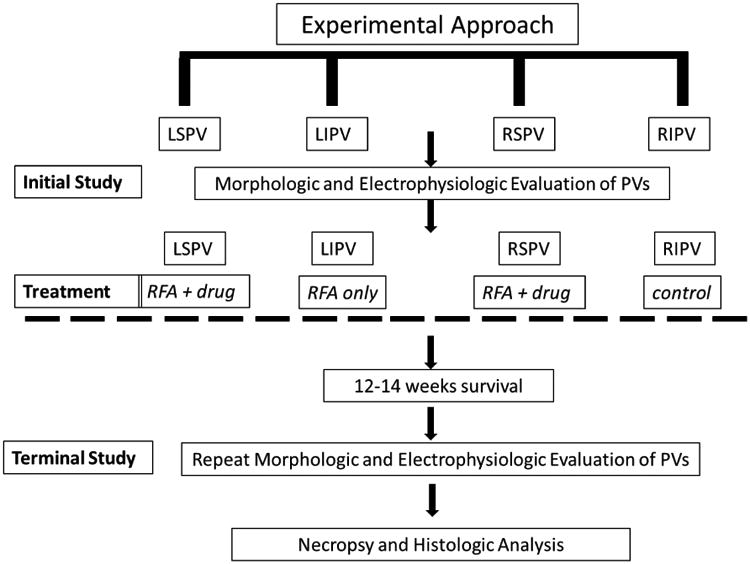
Timeline and treatment regimen for each of the canine pulmonary veins is shown. LSPV; left superior pulmonary vein, LIPV; left inferior pulmonary vein, RSPV; right superior pulmonary vein, RIPV; right inferior pulmonary vein. RFA; radiofrequency ablation. “Drug” denotes use of drug coated balloon (paclitaxel or everolimus) administered post-ablation.
Prior to transseptal puncture, unfractionated heparin was given intravenously at 100 U/kg as a bolus and followed by 30 U/kg/hr for the rest of the surgical procedure. The LA was then accessed with the use of a Brockenbrough needle through the atrial septum under ICE and fluoroscopic guidance, and the sheath was advanced into a stable position into the LA for easy PV access. Prior to ablation, PVs were imaged with ICE and LA contrast venography. PV ostial diameter and flow velocities were measured.
Using a combination of intracardiac ultrasound and angiography, the PV ostium and number was identified. A quadripolar, Blazer II ablation catheter (7Fr, 4 mm tip, 2.5 mm electrode spacing) (Boston Scientific, Marlborough, MA) was utilized for both mapping and ablating the electrical signals of the PV muscle sleeves. Ablation was performed in a point-to-point manner inside the PVs wherever near-field signals were found. These sites were treated with 120 sec applications of RF energy at 30 Watts/50°C. The endpoint of ablation at each site within the vein was the loss of the local PV near-field signal (Figure 3). The loss of signal was either from complete tissue obliteration, fragmentation, or only residual far-field signals. In doubtful cases, pacing from the ablation electrode was done to be sure that there was no capture of the PV myocardium.
Figure 3. Direct pulmonary vein myocardium ablation.
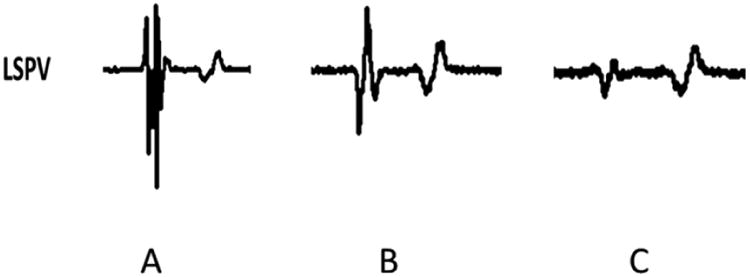
The local electrogram of the pulmonary vein myocardium of the left superior pulmonary vein of canine #4. Panel A shows a multi-component electrogram with near-field PV potential and a far-field left atrial signal pre-ablation with radiofrequency energy. Panel B from the same site shows decrease in the near-field PV signal during ablation. Panel C shows complete elimination of the near-field PV potential, and the remaining far-field left atrial signal.
After ablation was completed, 2 PVs underwent DCB treatment. This was performed with the use of an over-the-wire balloon catheter directed to the target vessel (provided by Boston Scientific, Marlborough, MA). The balloons were coated with either paclitaxel or everolimus (10 mm diameter; 30 mm in length) and covered the complete area that was ablated. Each balloon was expanded to contact the wall of PVs at 3-6 atmospheres (atm) of pressure for an application time of 60 seconds.
ICE with Doppler was performed to obtain ostial diameter and PV velocity (Figure 4). After 12-14 weeks survival, a second procedure was performed (terminal study). PV venography was repeated to evaluate for stenosis (Figure 5). All PVs were interrogated to ensure persistent elimination of PV potentials.
Figure 4. Intracardiac echocardiographic imaging with Doppler color flow demonstrates degree of vein stenosis and flow velocity within the pulmonary vein.
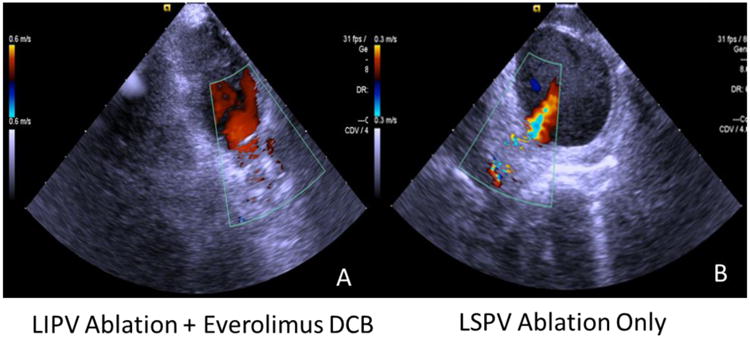
Panel A shows a color Doppler image of an LIPV that was ablated and treated with an everolimus-coated balloon (canine #5). In the same canine, Panel B shows a color Doppler of the LSPV (ablated only), showing severe stenosis along the vein, and high Doppler velocity evident from the noted aliasing. This aliasing correlated with an increasing velocity from 0.64 to 1.2 m/sec.
Figure 5. Venographic and anatomic demonstration of pulmonary veins post ablation and drug treatment.
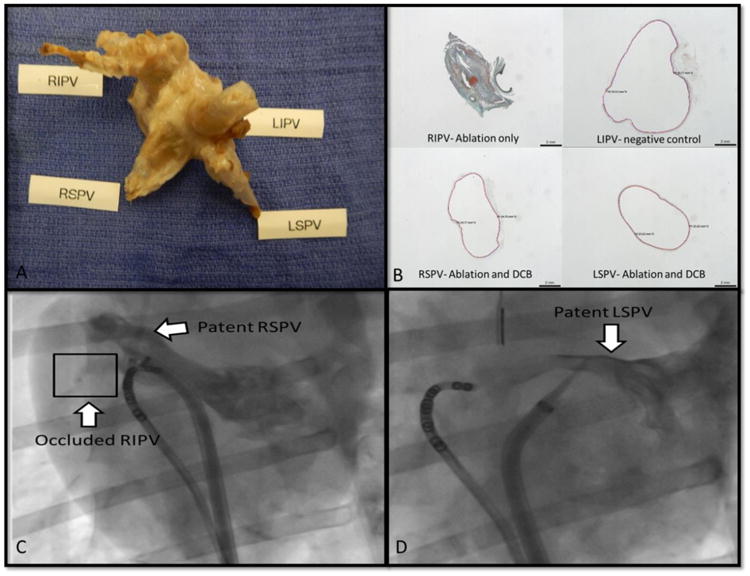
Panel A: Gross anatomic sample showing the stenotic RIPV (positive control; canine #1), while the rest of the PVs (LIPV – negative control; LSPV and RSPV- ablation + DCB) are grossly normal. Panel B: The intraluminal contents of the stenotic RIPV (positive control; canine #1) show thrombus, fibrin, collagen deposition, and mineralization. The remaining 3 PVs show almost no intraluminal contents or narrowing; these include the two PVs that were ablated and treated with DCB (RSPV and LSPV with paclitaxel) and the negative control (LIPV). Panel C shows a left atrial venogram that illustrates the outline of the canine RSPV. The occluded RIPV is not visualized past the ostium. Panel D depicts a selective venogram of a patent LSPV.
Post-mortem Analysis
After the terminal study, the animals were euthanized under anesthesia with direct current induction of ventricular fibrillation to the ventricle. Necropsy was performed to assess for gross damage to vital structures. Post-mortem en bloc heart and lungs were formalin fixed under pressure with 10% neutral buffered formalin at 25 mmHg for at least 2 hours. Gross anatomic evaluation of the PVs was performed (Figure 5). Tissue was stained with hematoxylin and eosin and Movat's pentachrome to assess for intraluminal stenosis (Figure 5) (American Preclinical Services, Bloomington, MN). The interpreting pathologist was blinded to PV treatment.
Statistical analysis
Statistical analysis was performed using SAS software (Cary, NC). Measurements of PV ostial diameter (cm) and flow velocity (m/sec) were performed in each PV prior to ablation and at terminal study. The percent change from baseline was calculated for each PV. For purposes of comparative statistical analysis, PVs were analyzed as independent variables, and averaged in groups according to intervention (negative control, positive control, or treatment veins). Generalized Estimating Equation models were used to compare the average percent change in ostial diameter (cm) and flow velocity (m/sec) between the groups. These models were used to account for multiple measures from each dog. The threshold for statistical significance was a two-tailed p value of <0.05. All data are shown as mean ± standard deviation unless.
Results
Assessment of pulmonary vein patency
In four survival canine experiments, we performed direct PV ablation in a total of 12 PVs (Table 1) with the remaining 4 serving as controls. All 4 canines survived the follow-up period (12-14 weeks) without any respiratory distress, hemoptysis, and remained ambulatory. Of the 12 PVs that underwent RFA, 8 were ablated and treated with a DCB (4 with paclitaxel; 4 with everolimus). These 8 PVs remained grossly patent and showed only minimal to no intraluminal narrowing. In contrast, the ablation-only PVs were severely stenosed or completely occluded. Figure 5 shows an example of two PVs in the same canine (canine #5) using ICE with Doppler imaging. The relatively patent LIPV (RFA + everolimus) shows laminar flow. This is in contrast to the severely stenosed LSPV (ablation only) that shows a high velocity jet with aliasing.
Table 1. Experimental treatment strategy used for canine pulmonary vein ablation and drug-coated balloon treatment.
| Canine | RSPV | RIPV | LSPV | LIPV | DCB | Survival | Complications |
|---|---|---|---|---|---|---|---|
| #1 | Ablation + DCB* | Ablation only | Ablation + DCB* | No treatment | Paclitaxel | 12 weeks | None |
| #2 | No treatment | Ablation + DCB | Ablation only | Ablation + DCB | Everolimus | 14 weeks | None |
| #3 | N/A | N/A | N/A | N/A | N/A | α Died | α Transseptal complication |
| #4 | Ablation + DCB | No treatment | Ablation only | Ablation + DCB | Paclitaxel | 14 weeks | None |
| #5 | Ablation + DCB | No treatment | Ablation only | Ablation + DCB | Everolimus | 12 weeks | None |
RSPV; right superior pulmonary vein; RIPV; right inferior pulmonary vein; LSPV; left superior pulmonary vein; LIPV; left inferior pulmonary vein. DCB; drug-coated balloon.
dog #3 died from a defect in the transseptal sheath causing the needle to exit prematurely and pierce the aorta.
Canine #1 had two DCB applications to each treated PV to ensure covering the ablation lesion.
We measured the ostial diameter (cm) and flow velocity (m/sec) in each PV prior to ablation and at the time of terminal study (Table 2). We compared the average change from baseline in ostial diameter and flow velocity among groups of PVs according to intervention (ablation only, ablation + DCB, and no intervention) (Table 3). In PVs that were ablated and treated with a DCB (n = 8), an average increase in ostial diameter of 4.7 ± 23.2% was seen. In contrast, PVs that underwent ablation only (n = 4) had an average reduction of 65.2 ± 26.7%. The difference between these groups was statistically significant (p= <0.001). In the negative control PVs (n= 4), an average increase in ostial diameter of 47.6 ± 33.4% was found, and this was significant when compared to the ablation + DCB group (p = <0.001). In evaluating the baseline to terminal study PV flow velocities, the ablation only group increased 12.1 ± 49.8%. There was no statistically significant difference when compared to the PV ablation +DCB group, where a decrease in velocity of 28.1 ± 33.3% was found (p value = 0.08). The negative control group had a decrease in flow velocity of 20.2 ± 38%, and no significant difference was found when compared to the ablation + DCB group (p value = 0.44).
Table 2. Canine pulmonary vein ostia diameter and flow velocities determined from intracardiac echocardiography and Doppler ultrasound.
| Canine #1 | Measurement | Pre-intervention | End Study | Treatment |
|---|---|---|---|---|
| LSPV | ostial diameter | 0.9 cm | 0.65 cm | Ablation + DCB |
| LSPV | flow velocity | 0.52 m/sec | 0.61 m/sec | Ablation + DCB |
| LIPV | ostial diameter | 0.53 cm | 0.83 cm | No ablation |
| LIPV | flow velocity | 0.41 m/sec | 0.48 m/sec | No ablation |
| RSPV | ostial diameter | 0.92 cm | 0.97 cm | Ablation + DCB |
| RSPV | flow velocity | 0.68 m/sec | 0.34 m/sec | Ablation + DCB |
| RIPV | ostial diameter | 1.14 cm | 0 cm | Ablation only |
| RIPV | flow velocity | 0.61 m/sec | N/A | Ablation only |
| Canine #2 | ||||
| LSPV | ostial diameter | 0.9 cm | 0.57 cm | Ablation only |
| LSPV | flow velocity | 0.64 m/sec | 0.43 m/sec | Ablation only |
| LIPV | ostial diameter | 0.75 cm | 0.77 cm | Ablation + DCB |
| LIPV | flow velocity | 0.79 m/sec | 0.28 m/sec | Ablation + DCB |
| RSPV | ostial diameter | 1.02 cm | 1.77 cm | No ablation |
| RSPV | flow velocity | 0.55 m/sec | 0.34 m/sec | No ablation |
| RIPV | ostial diameter | 0.90 cm | 1.28 cm | Ablation + DCB |
| RIPV | flow velocity | 0.6 m/sec | 0.35 m/sec | Ablation + DCB |
| Canine #3 | ||||
| LSPV | ostial diameter | 0.78 cm | 0.35 cm | Ablation only |
| LSPV | flow velocity | 0.60 m/sec | 0.62 m/sec | Ablation only |
| LIPV | ostial diameter | 0.98 cm | 0.98 cm | Ablation + DCB |
| LIPV | flow velocity | 0.47 m/sec | 0.40 m/sec | Ablation + DCB |
| RSPV | ostial diameter | 1.35 cm | 1.03 cm | Ablation + DCB |
| RSPV | flow velocity | 0.67 m/sec | 0.22 m/sec | Ablation + DCB |
| RIPV | ostial diameter | 1.46 cm | 1.44 cm | No ablation |
| RIPV | flow velocity | 0.82 m/sec | 0.29 m/sec | No ablation |
| Canine #4 | ||||
| LSPV | ostial diameter | 0.96 cm | 0.30 cm | Ablation only |
| LSPV | flow velocity | 0.35 m/sec | 0.58 m/sec | Ablation only |
| LIPV | ostial diameter | 0.74 cm | 0.85 cm | Ablation + DCB |
| LIPV | flow velocity | 0.38 m/sec | 0.44 m/sec | Ablation + DCB |
| RSPV | ostial diameter | 1.36 cm | 1.68 cm | Ablation + DCB |
| RSPV | flow velocity | 0.30 m/sec | 0.24 m/sec | Ablation + DCB |
| RIPV | ostial diameter | 0.99 cm | 1.60 cm | No ablation |
| RIPV | flow velocity | 0.40 m/sec | 0.42 m/sec | No ablation |
PV; pulmonary vein. RSPV; right superior pulmonary vein; RIPV; right inferior pulmonary vein; LSPV; left superior pulmonary vein; LIPV; left inferior pulmonary vein.
Table 3. Analysis of pulmonary vein ostial diameter and pulmonary vein flow velocity changes taken prior to ablation and at terminal study.
| Intervention Group | Average % change in ostial diameter | SD | P value | Average % change in flow velocity | SD | P value |
|---|---|---|---|---|---|---|
| Ablated only | -65.2 | 26.7 | <0.001 | 12.1 | 49.8 | 0.08 |
| No intervention | 47.6 | 33.4 | <0.001 | -20.2 | 38 | 0.44 |
| Ablated + DCB | 4.7 | 23.2 | ------ | -28.1 | 33.3 | ------ |
The average percent change of ostial diameter and pulmonary vein flow velocity are shown. Measurements were taken at each PV prior to ablation and at the time of terminal study. The average percent change was calculated according to intervention, and compared across groups. Student's t test and p value <0.05. Comparisons were made between Ablated + DCB group to the negative (no intervention) and positive (ablation only) control groups using a Student's t test. DCB; drug-coated balloon.
Acute and chronic assessment of direct pulmonary vein ablation efficacy
In all 4 chronic canines, all 16 veins (LSPV, LIPV, RSPV, and RIPV in each canine) showed electrically active muscle tissue upon PV interrogation. In each PV targeted for ablation, 100% (12/12) had complete PV potential elimination and conduction block. After 12-14 weeks of survival, each PV that was ablated (12/12) remained without PV potential and without electrical reconnection, with persistent conduction block (canine #1 RIPV was completed occluded and electrical assessment was not performed at terminal study).
Gross anatomic and histologic findings
At necropsy, no gross signs of vital organ damage were seen. All canine PVs were examined after disconnection from the lungs (canine #1- Figure 5). A histologic analysis was performed between canine #1 (paclitaxel DCB) and #2 (everolimus DCB). The sectioned PVs showed similar histologic findings that reflected minor fibrous tissue replacement. In contrast, the ablation only PVs were found to have intraluminal fibrin, elastin disruption, thrombus, and mineralization. An example of 4 PVs sectioned distal to the os is shown in Figure 5.
Discussion
The main finding of our study is that direct RFA inside PV muscle sleeves can be performed while maintaining patency in canines survived at least 3 months. In addition, direct PV ablation is effective at eliminating electrical triggers for at least 3 months. Furthermore, this proof-of-concept approach held independent of the type of DCB used. To the best of our knowledge, this is the first reported experiment where a DCB was used post-RFA to prevent stenosis in PVs.
Interpretation of our results
In addition to gross evaluation of the PVs, we assessed the degree of stenosis/narrowing by comparing the change at terminal study to that at baseline by measuring PV ostial diameter (cm) and flow velocity (m/sec). As expected, RFA within the PVs led to severe narrowing or even complete occlusion. In comparing the ablation only PV group, to the ablation + DCB group, we found > 10-fold reduction in ostial diameter (60% vs 5%).
We found a difference in PV flow velocity when comparing the ablation only group (+12%) with the ablation + DCB group (-28%), although this was not significant (p = 0.14). This may be due to being underpowered to detect a difference, or because of error associated with small variations in technique of reproducibly lining up the pulse wave Doppler in these small canine PVs. In future studies, we wish to improve the fidelity of our quantification methods, but also to pursue a clinical correlation to PV stenosis. Human studies using radionuclide imaging have suggested that a luminal narrowing of 80% or an associated 5 mmHg LA-PV pressure gradient was found to be associated with a lung perfusion defect [9].
We found an increase in PV ostial diameter of 48% in our negative control group of PVs. Consistent with the increase in ostial size, there was a concomitant decrease in PV flow velocity (m/sec) of 20% from baseline, measured at the terminal study. The most likely explanation for this finding is that in ablating PV myocardium, the vein may become aneurysmal. However, this is not typically seen because this is usually offset by endothelial proliferation. Thus, if the endothelial proliferation is prevented, such as in the case of our experiments with the use of anti-proliferative agents such as paclitaxel or everolimus, then it is likely that we are observing the unopposed dilatation.
Potential mechanism of pulmonary vein stenosis and action of anti-proliferative agents
The mechanism of PV stenosis remains undefined. This has been previously examined in animal and human studies. The acute thermal effects of RFA on canine PVs were studied by Kok et al., and suggested that acute PV stenosis may be due to collagen denaturation and vessel contraction [10]. However, the investigators had removed the muscular sleeves from the PVs prior to testing, and thus the thermal effect on the natural PV could not be addressed. The authors note this effect is in contrast to that in chronic PV stenosis, which is suggested to result from a rigorous inflammatory response at the site of ablation [7, 10]. Taylor et al., performed RFA within PVs to evaluate histologic changes over time [7]. At 1-2 months follow-up they showed intimal thickening, thrombus formation, altered smooth muscle/collagen matrix, and elastic laminae proliferation occurred [7]. Histologic analysis on patients with congenital PV stenosis has been performed. At post-mortem evaluation, a stenosed PV was noted to have prominent intimal proliferation, along with collagen, and a disarray of elastic fibers [11].
Paclitaxel-coated balloons have been shown to prevent neointimal hyperproliferation, and are currently being used to prevent coronary and peripheral arterial restenosis [12]. The activity of paclitaxel is based on blocking microtubule assembly, cell division and migration, and disrupting overall cellular proliferation [12]. Everolimus works by inhibiting smooth muscle proliferation and migration by halting the cell cycle in the G1 phase, and inhibiting the mammalian target of rapamycin (mTOR) [13, 14]. In keeping with our histologic analysis of stenosed PVs, we found the presence of fibrin, thrombus, calcification, elastic laminae changes, and neointimal proliferation. It is tempting to speculate that the local uptake of either drug at the site of tissue ablation interrupts the inflammatory response, especially tissue hyperproliferation, which in concert with fibrin and thrombus can lead towards intraluminal stenosis. However, further study is needed before this hypothesis can be supported.
Conclusions
Our data suggest that RFA of muscle sleeves inside PVs while maintaining luminal patency is feasible, and is effective in maintaining conduction block in canines survived at least 3 months.
Clinical implications
These preliminary findings may form the basis for a combined PV isolation and ablation approach to increase the efficacy and safety of AF ablation without the complication of stenosis.
Limitations
Our testing was performed in healthy canines. Although not the aim of this early, proof-of-concept experiment, the creation of sustained PV exit block without stenosis remains to be tested in an animal model of AF. We did not specify a randomized treatment approach to PVs a priori. In addition to randomization of treatment approach, these early findings should be confirmed in a larger number of canines, and over a longer survival period. While we hypothesized that the effect of paclitaxel/everolimus is to block neointimal tissue proliferation, we have no evidence to demonstrate that this is the mechanism by which PV stenosis is prevented. Further study to delineate the histologic changes post-ablation is needed. Furthermore, we have no data on the uptake of these drugs into PV tissue, nor do we have any data if there is a dose-dependency of this effect.
As with all large animal studies, we cannot extrapolate these findings to ablation in human PVs, as the inflammatory response and uptake of these drugs may behave in a different manner. Finally, in order to translate this approach to improve AF therapies, we will most likely need to employ a combined approach of PV ablation along with isolation to ablate the trigger as well as the antrum-ostial interface. While we presume that direct pulmonary vein ablation, as with direct ablation of arrhythmia substrate anywhere else in the heart, is more permanent than isolation approach, there may be additional benefits with antral ablation including autonomic modulation, etc, which may not be present with direct pulmonary vein ablation. Furthermore, subsequent prototypes will allow the flexibility of ablating both or either and the antrum and within the pulmonary vein itself
List of Abbreviations
- PV
Pulmonary vein
- LSPV
left superior pulmonary vein
- LIPV
left inferior pulmonary vein
- RSPV
right superior pulmonary vein
- RIPV
right inferior pulmonary vein DCB, drug-coated balloon
- DCB
drug-coated balloon
- RFA
radiofrequency ablation ICE, intracardiac echocardiography
- ICE
intracardiac echocardiography
Footnotes
Mayo Clinic Ventures has filed a patent application around the concept disclosed in this manuscript.
Drug coated balloons were supplied in-kind from Boston Scientific.
Author disclosures: None
References
- 1.Ho SY, Cabrera JA, Tran VH, Farré J, Anderson RH, Sánchez-Quintana D. Architecture of the pulmonary veins: relevance to radiofrequency ablation. Heart. 2001;86(3):265–270. doi: 10.1136/heart.86.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. New England Journal of Medicine. 1998;339(10):659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 3.Dewire J, Calkins H. Update on atrial fibrillation catheter ablation technologies and techniques. Nat Rev Cardiol. 2013;10(10):599–612. doi: 10.1038/nrcardio.2013.121. [DOI] [PubMed] [Google Scholar]
- 4.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJG, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Patient Selection, Procedural Techniques, Patient Management and Follow-up, Definitions, Endpoints, and Research Trial Design: A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9(4):632–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Nanthakumar K, Plumb VJ, Epstein AE, Veenhuyzen GD, Link D, Kay GN. Resumption of Electrical Conduction in Previously Isolated Pulmonary Veins:Rationale for a Different Strategy? Circulation. 2004;109(10):1226–1229. doi: 10.1161/01.CIR.0000121423.78120.49. [DOI] [PubMed] [Google Scholar]
- 6.Robbins IM, Colvin EV, Doyle TP, Kemp WE, Loyd JE, McMahon WS, Kay GN. Pulmonary Vein Stenosis After Catheter Ablation of Atrial Fibrillation. Circulation. 1998;98(17):1769–1775. doi: 10.1161/01.cir.98.17.1769. [DOI] [PubMed] [Google Scholar]
- 7.Taylor GW, Kay GN, Zheng X, Bishop S, Ideker RE. Pathological Effects of Extensive Radiofrequency Energy Applications in the Pulmonary Veins in Dogs. Circulation. 2000;101(14):1736–1742. doi: 10.1161/01.cir.101.14.1736. [DOI] [PubMed] [Google Scholar]
- 8.Cremers B, Schmitmeier S, Clever YP, Gershony G, Speck U, Scheller B. Inhibition of neo-intimal hyperplasia in porcine coronary arteries utilizing a novel paclitaxel-coated scoring balloon catheter. Catheterization and Cardiovascular Interventions. 2014;84(7):1089–1098. doi: 10.1002/ccd.25296. [DOI] [PubMed] [Google Scholar]
- 9.Nanthakumar K, Mountz JM, Plumb VJ, Epstein AE, Kay GN. Functional assessment of pulmonary vein stenosis using radionuclide ventilation/perfusion imaging*. Chest. 2004;126(2):645–651. doi: 10.1378/chest.126.2.645. [DOI] [PubMed] [Google Scholar]
- 10.Kok LC, Everett TH, Akar JG, Haines DE. Effect of Heating on Pulmonary Veins. Journal of Cardiovascular Electrophysiology. 2003;14(3):250–254. doi: 10.1046/j.1540-8167.2003.02490.x. [DOI] [PubMed] [Google Scholar]
- 11.Lock JE, Bass JL, Castaneda-Zuniga W, Fuhrman BP, Rashkind WJ, Lucas RV. Dilation angioplasty of congenital or operative narrowings of venous channels. Circulation. 1984;70(3):457–64. doi: 10.1161/01.cir.70.3.457. [DOI] [PubMed] [Google Scholar]
- 12.Wöhrle J. Drug-Coated Balloons for Coronary and Peripheral Interventional Procedures. Current Cardiology Reports. 2012;14(5):635–641. doi: 10.1007/s11886-012-0290-x. [DOI] [PubMed] [Google Scholar]
- 13.Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-Stent Restenosis in the Drug-Eluting Stent Era. Journal of the American College of Cardiology. 2010;56(23):1897–1907. doi: 10.1016/j.jacc.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Costa MA, Simon DI. Molecular Basis of Restenosis and Drug-Eluting Stents. Circulation. 2005;111(17):2257–2273. doi: 10.1161/01.CIR.0000163587.36485.A7. [DOI] [PubMed] [Google Scholar]


