Abstract
Background
Inadequate medication adherence is a pervasive, under-recognized cause of poor health outcomes. Many intervention trials designed to improve medication adherence have targeted adults with adherence problems. No previous reviews have synthesized the effectiveness of medication adherence interventions focused on subjects with medication adherence difficulties.
Objective
This systematic review and meta-analysis synthesized findings from medication adherence intervention studies conducted among adults with medication adherence difficulties.
Methods
Primary research studies were eligible for inclusion if they tested an intervention designed to increase medication adherence among adults with documented adherence difficulties and reported medication adherence behavior outcomes. Comprehensive search strategies of 13 computerized databases, author and ancestry searches, and hand searches of 57 journals were used to locate eligible primary research. Participant demographics, intervention characteristics, and methodological features were reliably coded from reports along with medication adherence outcomes. Effect sizes for outcomes were calculated as standardized mean differences, and random effects models were used to estimate overall mean effects. Exploratory dichotomous and continuous variable moderator analyses were employed to examine potential associations between medication adherence effect size and sample, intervention, and methodological characteristics.
Results
Data were extracted from 53 reports of studies involving 8,243 individual primary study participants. The overall standardized mean difference effect size for treatment vs. control subjects was 0.301. For treatment pre- vs. post-intervention comparisons, the overall effect size was 0.533. Significantly larger effect sizes were associated with interventions incorporating prompts to take medications than interventions lacking medication prompts (0.497 vs. 0.234). Larger effect sizes were also found for interventions that linked medication taking with existing habits compared to interventions that did not (0.574 vs. 0.222).
Effect sizes were largest among studies that measured adherence by pill counts or electronic event monitoring systems. Analysis of study design features identified several potential risks of bias. Statistically significant publication bias was detected, but adherence effect sizes were not significantly associated with other risks of bias.
Conclusions
These findings document that interventions targeting individuals with medication adherence problems can have modest but significant effects on medication-taking behavior. The findings support the use of behavioral strategies such as prompts and linking medications to habits to increase medication adherence in adults with adherence challenges. Face-to-face interventions appear to be critical for patients who have experienced past problems with medication adherence.
Keywords: Medication adherence, intervention, meta-analysis, systematic review
Introduction
Medication adherence is an important component in the effective treatment of many acute and chronic diseases. Consequences of inadequate medication adherence include not only poor clinical outcomes with attendant increased morbidity and mortality but also diminished quality of life, decreased work and personal productivity, and increased health care costs.1–3 Poor medication adherence is a pervasive and long-standing problem; rates around 50% have been reported for decades.1,4–8 Inadequate medication adherence constitutes a global epidemic with estimated annual costs to the health care system of $100 billion in the US and €25 billion in the European Union.1,3,6,7
The problem of poor medication adherence (henceforth, adherence) has prompted many trials testing interventions to improve medication-taking behaviors.1,2 A number of these studies have intentionally recruited subjects who have difficulty with adherence.9–61 Targeting subjects who have adherence problems allows for potentially larger improvements in adherence scores than subjects who have good adherence at study entry.62 These larger increases in adherence may also result in concomitantly greater improvements in health outcomes.63
The present review and meta-analysis were conducted to assess the overall effectiveness of adherence interventions in subjects who have difficulties with medication taking. As such, this research fills a knowledge gap because no previously published meta-analyses have used subject baseline adherence level as a selection criterion.64–66 Focus in previous meta-analyses has been on specific types of medication adherence interventions,67–72 on populations with specific clinical conditions,69,72–76 or on specific demographic groups.77–79
The following questions were addressed in this report: 1) What is the overall average effect of interventions designed to increase adherence among subjects with adherence problems? 2) Do effects of interventions vary depending on sample and study characteristics? 3) Do the effects vary depending on intervention characteristics? 4) What risks of bias are present in studies, and what influence do they have on effect sizes?
Material and Methods
Widely accepted systematic review and meta-analysis methods, including PRISMA guidelines, were used.80–82 This study is part of a larger parent project consisting of a series of meta-analyses of medication adherence intervention trials. The protocol was not registered. This review emphasizes comparisons of adherence behavior outcomes between treatment and control subjects.
Eligibility Criteria
Eligible studies for the analysis were primary intervention studies designed to increase medication adherence in adult subjects recruited specifically because they had problems with adherence to prescription medications. For purposes of inclusion in the meta-analysis, adherence was defined as the extent to which medication consumption is consistent with professional health care provider recommendations.8 What constituted an adherence problem was defined by primary investigators. Small-sample studies with questionable statistical power were included because meta-analyses do not rely on p values to determine effect sizes.83,84 The project focused on treatment andcontrol group comparisons. Both randomized and nonrandomized studies were included in the meta-analysis. Subject allocation was examined as part of risk of bias assessment, which is described below.80,84–88 Pre-experimental studies were included in an analysis of single-group studies. These studies did not report control groups but instead compared post-intervention adherence to baseline values. All analyses were conducted separately for single-group and two-group comparisons. The single-group findings are reported only as ancillary information to the more valid two-group results.
To avoid bias, both unpublished and published studies were included because the most consistent difference between published and unpublished research is the statistical significance of the results.89–94 Although investigators used diverse methods to measure adherence, the method of measurement was not used as a selection criterion because in meta-analysis, primary study outcomes are converted to unitless indices.80
Only studies with adequate data to calculate effect sizes were included.95–97 When the data necessary for effect size calculation were lacking, author searches were conducted to locate other papers that might contain the necessary information. When the information could not be located in the literature, it was requested from corresponding authors. In the parent project, 2,897 potentially eligible primary studies were located that included a medication adherence intervention and mentioned medication adherence behavior outcomes. Adequate medication adherence behavior statistical information to calculate effect sizes was absent in 2,214 reports, so they were excluded from meta-analyses (see Figure 1).
Figure 1.
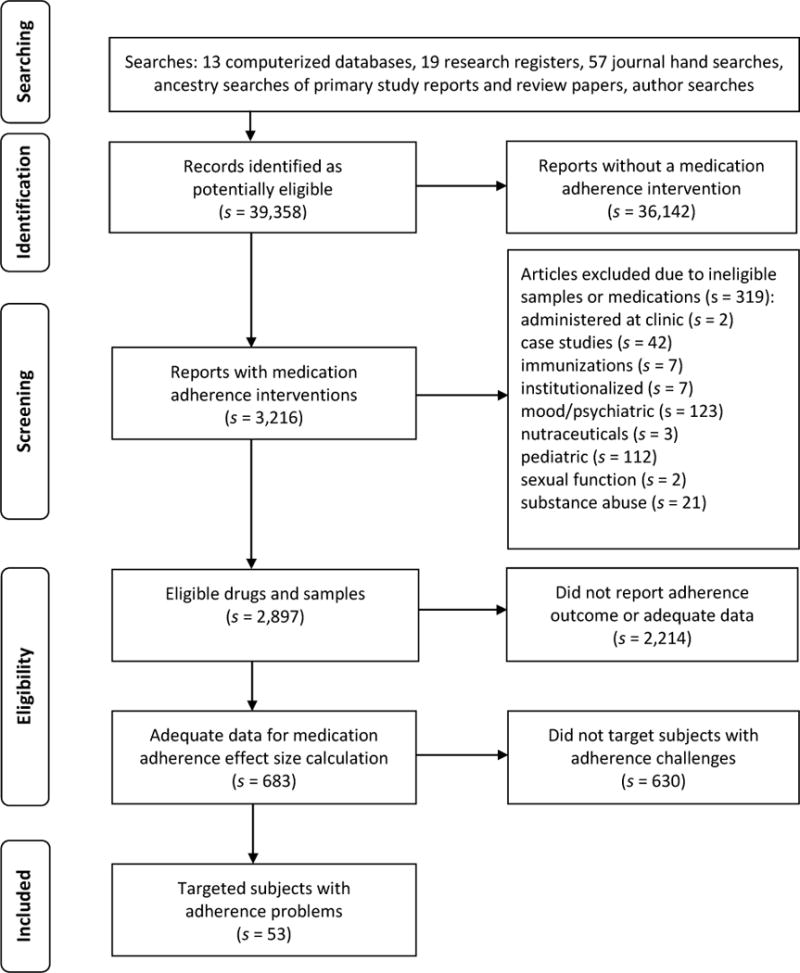
Flow of potentially eligible studies through review process
Note: s indicates the number of research reports
Strategies do exist for including studies that do not have adequate data for effect size determination, such as setting the effect size to 0, estimating possible effect sizes from other studies with significant or nonsignificant findings, or estimating effect size magnitude from similar studies reporting sample size and direction of effect information. However, none of these strategies were employed in the present study because they can distort estimates of heterogeneity and because imputing values requires assumptions that may not be justified.
This study focused on medications prescribed to prevent or treat acute or chronic physical disease. The project excluded primary research focused on subjects being treated for psychiatric conditions (e.g., schizophrenia) or substance abuse (e.g., alcohol) because decisions to skip or cease medications may be a consequence of patients’ impaired psychological status or addicted state.
Studies of patients who were prescribed contraceptive and sexual dysfunction medications were also excluded. These medications are most often prescribed for health promotion or as “lifestyle” medications, and patient decision-making about consuming such medications is expected. For example, contraceptives may be discontinued when a woman intends to become pregnant. Medications for sexual dysfunction are typically taken in an episodic, rather than a scheduled, regimen, which changes the conceptualization of adherence and adherence measurement for these medications. Different interventions would be needed for patients with major psychiatric diseases or for patients expected to modify consumption to meet personal goals. Thus, none of these medication classes fit the inclusion criteria for treating acute or chronic disease.
Studies of adherence to any of the following medications were also excluded: vitamins, supplements, or nutraceuticals not prescribed by a provider; medications administered by health care providers in clinical settings. Studies of institutionalized or incarcerated adults were not included in the meta-analysis sample because of institutional control over medication administration.
Information Sources and Search Strategies
Multiple search strategies were used to avoid potential bias resulting from narrow searches.93,98–104 An expert health sciences librarian conducted searches in PubMed, MEDLINE, PsycINFO, EBSCO, PDQT, ERIC, Cochrane Database of Systematic Reviews, Cochrane Central Trials Register, CINAHL, Communication and Mass Media, EBM Reviews, IndMED, and International Pharmaceutical Abstracts.100 The primary MeSH search terms were patient compliance for studies published before 2009 and medication adherence for studies published after 2008, the year medication adherence was introduced as a MeSH term. Other MeSH and text word search terms included compliant, compliance, adherent, adherence, noncompliant, noncompliance, nonadherent, nonadherence, prescription drugs, pharmaceutical preparations, drugs, dosage forms, or generic, prescription(s), prescribed, drug(s), medication(s), pill(s), tablet(s), regimen(s), improve, promote, enhance, encourage, foster, advocate, influence, incentive, ensure, remind, optimize, optimize, increase, impact, prevent, address, and decrease. Searches for the parent project were completed in 2013 to allow time for coding and analyses in 2014.
Several other methods were used to find additional potentially eligible studies. Authors having more than a single study in the parent project were contacted to solicit additional published or unpublished research.105,106 Abstracts from 48 conferences were examined. Searches were conducted in 19 research registers (e.g., Research Portfolio Online Reporting Tools), and investigators were contacted to obtain research reports of those studies.94,107,108 Hand searches were conducted in 57 journals where multiple eligible papers in the parent project were published.109,110
Study Selection
Potentially eligible studies were imported into bibliographic software and subsequently tracked with study-specific custom fields and terms. Studies were selected by extensively trained research specialists with graduate degrees and the principal investigator (VC). Each final eligibility decision was made by at least two research specialists. The 39,358 studies identified via comprehensive searching were examined using a multi-staged eligibility determination process. First, titles and abstracts were examined for visual heralds.111 Second, reports were examined for an intervention to increase adherence.112 Third, the sample and medications were examined for eligibility. Fourth, potentially eligible studies were assessed to determine whether adequate data were available for effect size calculation. If necessary, additional publication searches or author contacts were used to secure data for effect size calculations. To prevent sample overlap among coded studies and therefore ensure independence of data, author names of each potential study were checked against an author list of previously coded studies, and all potentially related studies were compared side by side.113 When necessary, corresponding authors were contacted to clarify the uniqueness of sample. Finally, primary studies were examined to determine if the sample was composed entirely of participants with adherence problems.
Data Items and Collection
The coding frame was based on the research team’s previous experience conducting meta-analyses.114,115 Medication adherence-specific content was incorporated using suggestions from medication adherence and meta-analysis experts, examining adherence review articles, and by previewing 50 primary studies for the parent project.86,115,116 The coding frame included information about study source, study design and methods, participant characteristics, intervention features, plus outcome data and descriptive statistics.86,114,117 Type of study (e.g., journal article or dissertation), presence and type of funding, and year of distribution were coded as source attributes. Assignment to groups, allocation concealment, type of control group (i.e., attention control or true control), data collector masking, attrition, intention-to-treat analyses, and type of adherence measurement (e.g., electronic medication event monitoring, pharmacy refills, pill counts, or self-report) were coded as methodological features. Participant characteristics that were coded included the mean age and the gender and ethnic composition of the sample populations.
Intervention features coded included dose (i.e., number of sessions and duration of sessions), days over which the intervention was delivered, theoretical basis of intervention, delivery medium (e.g., face-to-face, telephone), and whether the intervention targeted adherence behavior alone or other health behaviors in addition to adherence (e.g., diet, exercise). Specific intervention content was coded including: prompts/cues to administer medications; self-monitoring of medication administration; self-monitoring of disease symptoms; written instructions; rewards for increased adherence; increased communication between providers and patients; providing feedback to participants about their adherence; goal setting about adherence; habit assessment/modification; and problem solving about adherence challenges.
Data coded for effect size determinations included sample sizes, means, measures of variability, and success rates. Whenever multiple reports were available about the same subjects, all were used to code study information. Two extensively trained research specialists independently coded all data from each study.114,115 Coders compared all data and discussed discrepancies to achieve 100% agreement.114 Data collected for effect size calculations were further verified by a doctorally prepared coder.
Summary Measures and Statistical Analysis
A unitless standardized mean difference effect size (d) was calculated for each treatment vs. control comparison.82,84,118,119 This effect size is the difference between treatment and control subjects divided by the pooled standard deviation. A better outcome for treatment than control participants is denoted by a positive effect size. Effect sizes were adjusted for bias, and each effect size was weighted by the inverse of its variance to give more weight to larger samples.84,120 Externally standardized residuals of effect sizes were examined to detect potential outliers, which were excluded from the calculation of the overall mean difference effect size.120 Although this review emphasizes treatment vs. control comparisons, effect sizes for treatment group pre-post comparisons and control group pre-post comparisons were additionally calculated.
Clinical and statistical effect size heterogeneity is common in behavior change research.121 To address heterogeneity in the sample, four strategies were employed. First, a random effects model was used to acknowledge that effect sizes vary due to both subject-level sampling error and study-level sources of error such as variations in methods and participant demographics.122–125 Second, the conventional heterogeneity statistic Q was calculated to test for the presence of heterogeneity,126 and the index of heterogeneity I2 was computed to determine the proportion of variation due to heterogeneity.82,126 Third, moderator analyses were used to explore potential sources of heterogeneity. Finally, findings were interpreted in the context of discovered heterogeneity.
To aid in interpretation, the overall standardized mean difference effect sizes for treatment vs. control comparisons was converted to an original adherence metric.84 To accomplish this, studies using identical adherence metrics were selected, and the individual reported baseline means and standard deviations were used to calculate a pooled mean and standard deviation of the baseline adherence. Adherence at outcome was calculated by multiplying the pooled baseline standard deviation by the effect size and adding this product to the pooled baseline mean.84
To explore whether adherence effect sizes were associated with specific intervention characteristics, exploratory moderator analyses were conducted for treatment vs. control comparisons. Dichotomous moderators were tested with between-group heterogeneity statistics (Qbetween) using a meta-analytic analogue of ANOVA. Continuous moderators were tested by analysis of unstandardized regression slopes using meta-regression.82
Risk of Bias Management and Assessment
Efforts were made to minimize the introduction of bias into effect size estimates. Comprehensive searching helped avoid bias related to using easy-to-locate primary research with larger effect sizes.89,90,96,105 Publication bias was addressed to the extent possible by including both unpublished and published studies.93 Small-sample studies, which may be underpowered, were included because meta-analyses do not utilize p values for determining effect sizes.82 Sample size variations were managed by statistically weighting effect sizes so more precise effect sizes from studies with larger sample sizes had proportionately more influence in the calculation of the overall effect size.82
To minimize bias related to preferential selection of outcome data when studies reported multiple methods of measuring medication adherence, decisions were made a priori regarding which outcomes to use for effect size calculations.127–129 To address design bias, effect sizes for treatment vs. control comparisons were analyzed separately from those for treatment group pre-post comparisons. Outliers detected by examination of externally standardized residuals of effect sizes were excluded from the calculation of overall effect sizes.
To determine if publication bias was present, funnel plots of study effect size vs. sampling variance were constructed.89,93,130 Plots were visually assessed for asymmetry suggestive of an association between effect size and variance.89 Begg’s test using Kendall’s method was conducted to determine whether associations between effect size and variance were greater than might be expected due to chance.89
To explore potential bias related to subjects’ mere participation in a trial, control group pre-post comparison effect sizes were calculated. To investigate risks of bias related to study design, moderator analyses of potential associations between methodological features and effect sizes were conducted as a form of sensitivity analysis.81,127 Indicators of methodological strength in treatment vs. control comparisons such as allocation concealment, random assignment of participants, control group management, data collector masking, and intention-to-treat analyses were analyzed as dichotomous moderators, whereas sample size and attrition were analyzed as continuous moderators.81 Risk of bias related to the technique used to measure adherence was also assessed by dichotomous moderator analysis.
Quality rating scales were not used to weight effect sizes because of problems with the scales.88,126,127,131–134 The scales have questionable validity, and they don’t adequately distinguish report from study design quality. Quality scales combine distinct aspects of quality and methodology into a single score that might obscure important differences among studies. Different aspects of quality may influence effect sizes in different ways. Finally, quality scales do not assess the measurement of medication adherence, which is an important methodological variation in this area of science.
Results
Study Selection and Characteristics
Comprehensive search strategies located 39,358 potentially eligible reports. The flow of these studies through the screening and selection process is depicted in Figure 1. From these citations, 53 reports of studies were identified that specifically targeted subjects with adherence problems.9–61 The eligible primary studies involved 8,423 individual participants. Forty of these reports, which involved 8,017 participants, were included in the meta-analytic sample for treatment vs. control comparisons. One study contributed two treatment groups compared to a single control group for a total of 41 comparisons in the meta-analytic sample. The pre-post treatment group meta-analytic sample consisted of 38 comparisons found in 37 reports involving 1,265 participants. The pre-post control sample consisted of 24 comparisons involving 842 participants.
The majority of studies were published since the year 2000. Forty-five reports were published in 2000 or later; five were published prior to 1990. Most reports were published journal articles (s = 43) (s indicates the number of reports; k denotes the number of comparisons). The sample included nine dissertations and one unpublished report.
Descriptive statistics for all the primary studies included in any meta-analysis are shown in Table 1. The median study sample size was 42 participants. The median of mean age of participants was 53 years. Women were well-represented in samples. In the studies reporting race/ethnicity of subjects, a median of 70% of participants were non-Caucasian. Median attrition rates were modest at 0.8%. All further results are about the treatment vs. control group comparisons, unless otherwise specified.
Table 1.
Descriptive statistics for primary studies Included in medication adherence meta-analyses
| Characteristic | k | Min | Q1 | Mdn | Q3 | Max |
|---|---|---|---|---|---|---|
| Total post – test sample size per study | 53 | 5 | 16 | 42 | 91 | 2,032 |
| Percentage attrition | 36 | 0 | 0 | 0.8 | 13.2 | 67.7 |
| Percentage female | 50 | 0 | 18.2 | 42.05 | 56.18 | 100 |
| Percentage non-Caucasian subjects | 38 | 5 | 40 | 70 | 92.5 | 100 |
| Mean age (years) | 49 | 26.3 | 44.08 | 52.9 | 60.5 | 77.76 |
| Percent adherence cutoff criterion for inclusion | 27 | <50 | <80 | <80 | <90 | <100 |
Note: Includes studies that contributed to primary analyses at least one effect size for any type of comparison.
k denotes number of comparisons providing data on characteristic. Q1=first quartile, Q3=third quartile.
Information about individual primary studies reporting treatment vs. control group outcomes is shown in Table 2. Thirty-two studies were conducted in the United States and three in Canada. The other studies were conducted in Australia, Hong Kong, Ireland, Netherlands, Switzerland, and the United Kingdom. Twenty comparisons reported specific criteria for determining subjects’ eligibility in relation to their adherence. The most common inclusion criterion was whether patients’ adherence fell below a threshold value of 80% (k = 9). Only eight studies reported the number of prescribed medications subjects were taking. The most common chronic diseases targeted by primary studies included HIV (k = 16) and hypertension (k = 10). Several studies possessed mixed samples of subjects having different diseases.
Table 2.
Individual primary study information for treatment vs. control comparisons
| Study & Location | Sample | Methods | Intervention | Effect Size |
|---|---|---|---|---|
| Alhalaiqa et al., 20129 Great Britain |
N: 136 Mean meds: 3.3 Health: 100% HTN % female: 53.7 % non-Caucasian: NA % MA criterion: NA |
Randomized: Yes Allocation concealed: Yes Blinded: Yes % Attrition: 0 ITT: Yes Comparison: True control Tx fidelity: NA MA Measure: Pill counts |
Theory: NA Interventionist: NA Content: Drug education, problem solving, thought restructuring Target: Medication adherence (MA) only Delivery: Face-to-face Dose: 7 sessions, 20 min each |
3.293 |
| Austin, 198611 United States |
N: 30 Mean meds: 3.1 Health: 100% HTN, 30% diabetes, 13% cardiac, 10% kidney disease, 3% gallbladder disease, 10% stroke, 17% other % female: 57 % non-Caucasian: 100 % MA criterion: 80 |
Randomized: Yes Allocation concealed: NA Blinded: No % Attrition: 0 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Self-report |
Theory: Social Cognitive Theory Interventionist: Registered nurse Content: Improve self-management skills, self-monitoring of MA and BP, feedback about MA and BP, habit linking, rewards, social support Target: MA and additional health behaviors Delivery: Face-to-face Dose: 3 sessions, 45 min each |
0.277 |
| Burrelle, 198613 United States |
N: 16 Mean meds: 5.94 Health: 100% HTN % female: 75 % non-Caucasian: 75 % MA criterion: NA |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: 0 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Pill counts |
Theory: NA Interventionist: Nurse, pharmacist, social worker Content: Medication-taking calendar, disease/drug education, pill boxes Target: MA only Delivery: Face-to-face, written materials Dose: 1 session |
2.303 |
| Cook et al., 201014 United States |
N: 10 Mean meds: NA Health: 100% glaucoma % female: 42 % non-Caucasian: 58.3 % MA criterion: 80 |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: 16.7 ITT: Yes Comparison: True control Tx fidelity: NA MA Measure: Electronic event monitoring device |
Theory: Motivational interviewing Interventionist: Person other than HCP Content: Motivational interviewing, barriers management, problem solving education/counseling Target: MA only materials, drug Delivery: Face-to-face, telephone, written Dose: 3 in-person sessions, 30–45 min each, 3 telephone contacts, 5–10 min each |
1.295 |
| De Geest et al., 200615 Switzerland |
N: 13 Mean meds: NA Health: 100% kidney transplant % female: 21.4 % non-Caucasian: NA % MA criterion: 98 |
Randomized: Yes Allocation concealed: Yes Blinded: No % Attrition: 28 ITT: Yes Comparison: True control Tx fidelity: NA MA Measure: Electronic event monitoring device |
Theory: NA Interventionist: Nurse Content: Drug education, monitoring MA by device with feedback about MA, goal setting, problem solving, habit linking, patient empowerment, self-efficacy enhancement, self-management education Target: MA only Delivery: Face-to-face, telephone Dose: 4 sessions |
0.047 |
| Freedman, 200717 United States |
N: 16 Mean meds: NA Health: 100% HIV % female: 81.3 % non-Caucasian: 75 % MA criterion: NA |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: 0 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Self-report |
Theory: NA Interventionist: NA Content: Disease/drug education, improve patient communication with provider, goal setting, problem solving, stress management, investigator-formed support group Target: MA and additional health behaviors Delivery: Face-to-face, written materials Dose: 8 sessions, 90 min each |
0.386 |
| Gamble et al., 201118 Ireland |
N: 18 Mean meds: NA Health: 100% asthma % female: 85 % non-Caucasian: NA % MA criterion: 50 |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: 10 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Prescription refills |
Theory: Cognitive-behavioral Theory, motivational interviewing, Transtheoretical Stages of Change Model Interventionist: Nurse Content: Motivational interviewing, behavior modification, disease/drug education Target: MA only Delivery: Face-to-face Dose: 8 sessions |
1.431 |
| Glanz et al., 201219 United States |
N: 246 Mean meds: NA Health: 100% glaucoma % female: 37.5 % non-Caucasian: 90.7 % MA criterion: NA |
Randomized: No Allocation concealed: No Blinded: No % Attrition: 5 ITT: NA Comparison: True control Tx fidelity: NA MA Measure: Prescription refills |
Theory: NA Interventionist: Automated delivery Content: Disease/drug education, barriers management, problem solving Target: MA only Delivery: Telephone, mail Dose: 12 phone calls, 12 mailings |
−0.031 |
| Harper, 198421 United States |
N: 59 Mean meds: 5.25 Health: 100% HTN, 70% osteoarthritis, 55% cardiac disease, 45% diabetes % female: 100 % non-Caucasian: 100 % MA criterion: NA |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: 1.7 ITT: NA Comparison: True control Tx fidelity: NA MA Measure: Pill counts |
Theory: Orem’s Self-care Theory, General System Theory Interventionist: Nurse Content: Drug education, rewards, habit linking, cues/prompts, special labelling, side effects management Target: MA and additional health behaviors Delivery: Face-to-face Dose: 4 sessions |
1.047 |
| Haynes et al., 197622 Canada |
N: 38 Mean meds: NA Health: 100% HTN, mean 3.4 other chronic illnesses % female: 0 % non-Caucasian: NA % MA criterion: 80 |
Randomized: Yes Allocation concealed: No Blinded: Yes % Attrition: 2.6 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Pill count |
Theory: NA Interventionist: Person other than HCP Content: Drug reminder chart, habit linking, self-monitoring of medication taking, symptoms, cues/prompts Target: MA and additional health behaviors Delivery: Face-to-face Dose: 13 sessions, 30 min each |
0.569 |
| Kalichman et al., 201125 United States |
N: 39 Mean meds: NA Health: 100% HIV % female: 35.0 % non-Caucasian: 92.5 % MA criterion: 95 |
Randomized: Yes Allocation concealed: Yes Blinded: Yes % Attrition: 2.5 ITT: Yes Comparison: Attention control Tx fidelity: 99% MA Measure: Self-report |
Theory: Self-regulation Theory, Self-management Theory Interventionist: Person other than HCP Content: Improve self-management skills, barriers management, goal setting, problem solving, pill boxes, cues/prompts, habit linking, disease/drug education, drug counseling, diary to self-monitor MA, feedback about MA Target: MA only Delivery: Face-to-face, telephone Dose: 5 sessions |
0.460 |
| Kogos, 200426 United States |
N: 30 Mean meds: NA Health: various unspecified chronic illnesses % female: 0 % non-Caucasian: 27.0 % MA criterion: NA |
Randomized: No Allocation concealed: NA Blinded: NA % Attrition: 0 ITT: No Comparison: Attention control Tx fidelity: NA MA Measure: Pill counts |
Theory: Health Belief Model Interventionist: Person other than HCP Content: Barriers management, contracting, goal setting, problem solving, self-monitor medication taking, self-management education, intervention delivered in both group and individual contexts Target: MA and additional health behaviors Delivery: Face-to-face, written materials Dose: 5 sessions, 60 min each |
−0.312 |
| Levensky, 200629 United States |
N: 53 Mean meds: NA Health: 100% HIV % female: 15 % non-Caucasian: 22 % MA criterion: 90 |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: 1.9 ITT: Yes Comparison: True control Tx fidelity: NA MA Measure: Pill counts |
Theory: Motivational interviewing, Theory, Problem-solving Theory Social Cognitive Interventionist: Nurse, unspecified other HCP, person other than HCP Content: Motivational interviewing, behavior modification, self-efficacy enhancement, contracting/commitment to increased MA, barriers management, problem solving, goal setting, rewards, pill boxes, habit linking, diary o self-monitor MA, disease/drug education, drug counseling, simplifying medication regimen, side effects management, improve patient communication with health care provider, teach provider skills to improve communication with patient and promote MA; improve integration of health care Target: MA and additional health behaviors Delivery: Face-to-face, telephone, written materials Dose: 3–4 sessions depending on patient needs |
0.393 |
| Matteson, 201131 United States |
N: 5 Mean meds: NA Health: 100% inflammatory bowel disease % female: 42.1 % non-Caucasian: NA % MA criterion: 85 |
Randomized: Yes Allocation concealed: Yes Blinded: NA % Attrition: 0 ITT: No Comparison: Attention control Tx fidelity: NA MA Measure: Electronic event monitoring device |
Theory: Continuous Self-improvement Framework Interventionist: Advanced practice nurse Content: Behavior modification, personal system change, habit linking, feedback about MA, drug education Target: MA only Delivery: Face-to-face Dose: 1 session averaging 32.5 min |
1.821 |
| McPherson-Baker et al., 200032 United States |
N: 42 Mean meds: NA Health: 100% HIV % female: 0 % non-Caucasian: 88.1 % MA criterion: NA |
Randomized: No Allocation concealed: NA Blinded: NA % Attrition: 0 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Prescription refills |
Theory: Health BeliefModel Interventionist: Pharmacist Content: Disease/drug education, barriers management, problem solving, behavior rehearsal, modeling medication-taking behaviors, improve self-management skills Target: MA only Delivery: Face-to-face Dose: 5 sessions, 20–25 min each |
1.475 |
| Migneault et al., 201233 United States |
N: 337 Mean meds: 5.1 Health: 100% HTN, 37% diabetes, 7.7% history of stroke % female: 70.4 % non-Caucasian: 100 % MA criterion: NA |
Randomized: Yes Allocation concealed: Yes Blinded: No % Attrition: 0 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Self-report |
Theory: Motivational interviewing, Social Cognitive Theory, Transtheoretical Stages of Change Model Interventionist: Automated delivery Content: Motivational interviewing, drug education/counseling, self-monitoring of BP, feedback about MA Target: MA and additional health behaviors Delivery: Telephone Dose: 8 contacts |
0.233 |
| Mitchell, 199334 United States |
N: 109 Mean meds: NA Health: 100% HTN % female: 61 % non-Caucasian: 5 % MA criterion: NA |
Randomized: Yes Allocation concealed: Yes Blinded: NA % Attrition: NA ITT: No Comparison: True control Tx fidelity: NA MA Measure: self-report |
Theory: Health Promotion Model, Social Cognitive Theory Interventionist: Advanced practice nurse Content: Self-efficacy enhancement, value clarification, goal setting, problem solving, barriers management, monitoring MA by device with feedback about MA Target: MA and additional health behaviors Delivery: Face-to-face, telephone Dose: 4 sessions |
−0.315 |
| Moitra et al., 201135 United States |
N: 10 Mean meds: NA Health: 100% HIV % female: 7.7 % non-Caucasian: 84.6 % MA criterion: NA |
Randomized: yes Allocation concealed: NA Blinded: NA % Attrition: 67.7 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Self-report |
Theory: Acceptance and Commitment Theory Interventionist: Person other than HCP Content: Acceptance and commitment therapy, goal setting, self-re-evaluation, disease/drug education, drug counseling, investigator-formed support group Target: MA only Delivery: Face-to-face, written materials Dose: 4 sessions, 60 min each |
0 |
| Murphy et al., 200237 United States |
N: 33 Mean meds: NA Health: 100% HIV % female: 12.0 % non-Caucasian: 64 % MA criterion: NA |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: 36.5 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Self-report |
Theory: Cognitive-behavioral Theory, Social Cognitive Theory Interventionist: Nurse, person other than HCP Content: Behavior and cognitive modification, disease/drug education, cues/prompts, barriers management, goal setting, problem solving, relapse prevention, improve patient ability to communicate with provider, social support via experimenter-formed group Target: MA and exercise Delivery: Face-to-face, written materials Dose: 5 sessions |
0.795 |
| Murphy et al., 200738 United States |
N: 141 Mean meds: NA Health: 100% HIV % female: 17.6 % non-Caucasian: 70.4 % MA criterion: NA |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: NA ITT: Yes Comparison: True control Tx fidelity: NA MA Measure: Electronic event monitoring device |
Theory: Cognitive-behavioral Theory, Social Cognitive Theory Interventionist: Nurse, person other than HCP Content: Behavior and cognitive modification; disease education, cues/prompts, barriers management, problem solving, rewards, diary to self-monitor MA, improve patient ability to communicate with provider, social support via investigator-formed group Target: MA only Delivery: Face-to-face Dose: 5 sessions, 90 min + 4 sessions, 60 min |
0.249 |
| Nietert et al., 200939 United States |
N: 2,032 Mean meds: NA Health: 56.4% HTN or heart failure, 11.3% diabetes, 17.4% hyperlipidemia, 14.2% depression % female: NA % non-Caucasian: NA % MA criterion: NA |
Randomized: Yes Allocation concealed: NA Blinded: No % Attrition: 0 ITT: Yes Comparison: NA Tx fidelity: NA MA Measure: Prescription refills |
Theory: NA Interventionist: Pharmacist, person other than HCP Content: Drug education, telephone prompts to remind patient to refill prescriptions, barriers management, problem solving Target: MA only Delivery: Telephone Dose: NA |
−0.012 |
| Nietert et al., 200939 United States |
N: 2,030 Mean meds: NA Health: 56% HTN or heart failure, 11.3% diabetes, 17.3% hyperlipidemia, 14.9% depression % female: NA % non-Caucasian: NA % MA criterion: NA |
Randomized: Yes Allocation concealed: NA Blinded: No % Attrition: 0 ITT: Yes Comparison: NA Tx fidelity: NA MA Measure: Prescription refills |
Theory: NA Interventionist: None Content: Patient prescription refill information faxed to physician along with written prompts for physicians to encourage patients’ medication persistence Target: MA only Delivery: FAX sent to patients’ physicians Dose: NA |
−0.061 |
| Okeke et al., 200941 United States |
N: 66 Mean meds: NA Health: 100% glaucoma % female: 45.5 % non-Caucasian: 62.1 % MA criterion: 75 |
Randomized: Yes Allocation concealed: Yes Blinded: NA % Attrition: 0 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Electronic event monitoring device |
Theory: NA Interventionist: NA Content: Disease/drug education, barriers self-management, problem solving, cues/prompts, habit linking, drug reminder chart, diary to monitor MA, social support Target: MA only Delivery: Face-to-face, telephone, video Dose: 10 sessions |
0.844 |
| Oser, 200842 United States |
N: 22 Mean meds: NA Health: 100% HTN % female: 0 % non-Caucasian: 54.5 % MA criterion: 80 |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: 0 ITT: Yes Comparison: True control Tx fidelity: NA MA Measure: Pill counts |
Theory: Cognitive-behavioral Theory, motivational interviewing Interventionist: NA Content: Motivational interviewing, behavior modification, decisional balance activities, thought restructuring, self-re-evaluation, barriers management, problem solving, goal setting, habit linking, rewards, diary to self-monitor MA, relapse prevention, improve patient communication with provider, drug education, social support Target: MA and additional health behaviors Delivery: Written materials Dose: 1 contact |
0.405 |
| Ramirez Canada-Garcia & Cote, 201243 |
N: 44 Mean meds: NA Health: 100% HIV % female: 9.8 % non-Caucasian: NA % MA criterion: NA |
Randomized: Yes Allocation concealed: Yes Blinded: NA % Attrition: 13.7 ITT: Yes Comparison: True control Tx fidelity: NA MA Measure: Self-report |
Theory: Social Cognitive Theory, Persuasion Theory Interventionist: Nurse Content: Self-efficacy enhancement, thought restructuring, problem solving, goal setting, improve patient communication with provider, social support, drug education, side effects management Target: MA only Delivery: Face-to-face, written materials Dose: 4 sessions, 60 min each |
−0.411 |
| Remien et al., 200544 United States |
N: 115 Mean meds: NA Health: 100% HIV % female: 46 % non-Caucasian: 86 % MA criterion: 80 |
Randomized: Yes Allocation concealed: Yes Blinded: Yes % Attrition: 0 ITT: Yes Comparison: True control Tx fidelity: NA MA Measure: Electronic event monitoring device |
Theory: Ewart’s Social Action Theory Interventionist: Advanced practice nurse Content: Cognitive modification, behavior modification, disease/drug education, barriers management, problem solving, social support Target: MA and additional health behaviors Delivery: Face-to-face Dose: 4 sessions, 45–60 min each |
0.184 |
| Rosen et al., 200745 United States |
N: 56 Mean meds: NA Health: 100% HIV % female: 41 % non-Caucasian: 59 % MA criterion: 80 |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: 0 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Electronic event monitoring device |
Theory: NA Interventionist: Person other than HCP Content: Monitoring MA by device with feedback about MA, payment for taking medications, cues/prompts Target: MA and additional health behaviors Delivery: Face-to-face Dose: 16 sessions |
0.507 |
| Ruppar, 201046 United States |
N: 15 Mean meds: NA Health: 100% HTN % female: 73 % non-Caucasian: 33 % MA criterion: 85 |
Randomized: Yes Allocation concealed: Yes Blinded: No % Attrition: 0 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Electronic event monitoring device |
Theory: Self-Regulation Theory Interventionist: Advanced practice nurse Content: Disease/drug education, habit linking, BP self-monitoring, MA self-monitoring using electronic device, feedback about BP and MA, goal setting, pill boxes, special medication labels Target: MA only Delivery: Face-to-face Dose: 5 sessions |
1.039 |
| Russell et al., 201047 United States |
N: 13 Mean meds: NA Health: 100% kidney transplant % female: 53 % non-Caucasian: NA % MA criterion: 85 |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: 13.3 ITT: No Comparison: Attention control Tx fidelity: NA MA Measure: Electronic event monitoring device |
Theory: Continuous System Improvement Interventionist: Advanced practice nurse Content: Personal system change; habit linking, goal setting, problem solving, monitoring MA by device with feedback about MA Target: MA only Delivery: Face-to-face, written materials Dose: 6 sessions |
1.3682 |
| Safren et al., 200150 United States |
N: 53 Mean meds: NA Health: 100% HIV % female: 13 % non-Caucasian: 49 % MA criterion: 100 |
Randomized: No Allocation concealed: NA Blinded: NA % Attrition: 5.4 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Self-report |
Theory: Cognitive-behavioral Theory, motivational interviewing, Problem solving Theory Interventionist: Person other than HCP Content: Motivational interviewing, cognitive and behavior modification, disease/drug education, habit linking, cues/prompts, pill boxes improved patient communication and shared decision-making with health care provider, problem solving, thought restructuring, guided imagery, side effects management Target: MA only Delivery: Face-to-face, telephone, videotape Dose: 2 sessions |
0.060 |
| Safren et al., 200348 United States |
N: 44 Mean meds: NA Health: 100% HIV % female: 20 % non-Caucasian: 47 % MA criterion: 90 |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: NA ITT: No Comparison: True control Tx fidelity: NA MA Measure: Electronic event monitoring device |
Theory: NA Interventionist: NA Content: Pager system for medication taking reminders Target: MA only Delivery: Telephone Dose: NA |
0.470 |
| Sorensen et al., 200751 United States |
N: 66 Mean meds: NA Health: 100% HIV % female: 41 % non-Caucasian: 44 % MA criterion: 80 |
Randomized: Yes Allocation concealed: Yes Blinded: NA % Attrition: 0 ITT: Yes Comparison: True control Tx fidelity: NA MA Measure: Electronic event monitoring device |
Theory: Behavioral Modification Interventionist: Person other than HCP Content: Behavior modification, payment for taking medication Target: MA only Delivery: NA Dose: 24 contacts |
0.485 |
| Stewart et al., 200852 Australia |
N: 343 Mean meds: NA Health: 100% HTN, 73.5% cardiovascular disease, including stroke, cardiac disease, 35.5% diabetes, 34.5% depression % female: 48.9 % non-Caucasian: NA % MA criterion: NA |
Randomized: No Allocation concealed: Yes Blinded: NA % Attrition: 13.2 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Self-report |
Theory: Motivational interviewing Interventionist: Pharmacist Content: Motivational interviewing, medication review for appropriate prescription, self-monitor of symptoms/signs with feedback, drug education, pill boxes, cues/prompts to refill prescriptions, health care provider improved skills to enhance patient MA, health care integration Target: MA and additional health behaviors Delivery: Face-to-face, telephone, text messages, mail, written materials Dose: NA |
0.122 |
| Taylor, et al., 200353 United States |
N: 69 Mean meds: 6 Health: 76.8% HTN, 55% hyperlipidemia, 42% diabetes, 14.5% anti-coagulant therapy, 12% osteoarthritis % female: 68.1 % non-Caucasian: NA % MA criterion: NA |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: 14.8 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Self-report |
Theory: NA Interventionist: Pharmacist Content: Disease/drug education, medication review and reduction in number of prescriptions to increase MA, problem solving, pill boxes, teach skills related to administering medications and self-monitoring of signs Target: MA only Delivery: Face-to-face, written materials Dose: Number of sessions determined by frequency of clinic visits, 20 min each |
1.223 |
| Van Servellen et al., 200555 United States |
N: 69 Mean meds: NA Health: 100% HIV % female: 9.9 % non-Caucasian: 100 % MA criterion: NA |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: 18.8 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Self-report |
Theory: NA Interventionist: Advanced practice nurse, person other than HCP Content: Disease/drug education, increase health literacy, motivational interviewing, empower patients to improve communication with providers; problem solving, barriers management, stress management, social support Target: MA and additional health behaviors Delivery: Face-to-face, telephone, videotape, written materials Dose: NA |
0.074 |
| Vervloet et al., 201256 Netherlands |
N: 104 Mean meds: NA Health: 100% Type 2 diabetes % female: 45.2 % non-Caucasian: NA % MA criterion: 80 |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: 12.6 ITT: Yes Comparison: True control Tx fidelity: NA MA Measure: Electronic event monitoring device |
Theory: NA Interventionist: Automated delivery Content: Real-time electronic monitoring of medication-taking with transmission of short text-message reminders Target: MA only Delivery: Text messages Dose: NA |
0.544 |
| Villeneuve et al., 201057 Canada |
N: 225 Mean meds: NA Health: 100% hyperlipidemia, 64% HTN, 43% diabetes % female: 38 % non-Caucasian: NA % MA criterion: NA |
Randomized: No Allocation concealed: NA Blinded: NA % Attrition: 0 ITT: Yes Comparison: True control Tx fidelity: NA MA Measure: Prescription refills |
Theory: NA Interventionist: Physician, pharmacist Content: Organizational improvement; improved integration of health care, teach provider skills to improve communication with patient and promote MA, patient/provider concordance, drug education, feedback to patients on symptoms/signs, goal setting Target: MA and additional health behaviors Delivery: Face-to-face, written materials Dose: NA |
0.105 |
| Wall, et al., 199558 United States |
N: 25 Mean meds: 3.0 Health: 100% HIV % female: 48 % non-Caucasian: 68 % MA criterion: NA |
Randomized: Yes Allocation concealed: NA Blinded: NA % Attrition: 7.4 ITT: No Comparison: True control Tx fidelity: NA MA Measure: Electronic event monitoring device |
Theory: NA Interventionist: Registered nurse Content: Self-administration program with directly observed therapy, distance-to-pharmacy barriers management, charting MA as a clinical parameter, feedback about MA and clinical signs Target: MA only Delivery: Face-to-face Dose: 40 contacts |
0.175 |
| Watakakasol, 201059 United States |
N: 42 Mean meds: NA Health: 100% HIV % female: 40.5 % non-Caucasian: 7.1 % MA criterion: 95 |
Randomized: Yes Allocation concealed: Yes Blinded: Yes % Attrition: 0 ITT: Yes Comparison: True control Tx fidelity: NA MA Measure: Patient medication administration diary |
Theory: Motivational interviewing, Transtheoretical Stages of Change Model Interventionist: Person other than HCP Content: Motivational interviewing, decisional balance and decision-making activities related to MA, problem solving, rewards, intervention targeted to subject’s stage of change Target: MA only Delivery: Telephone Dose: 1 session, 60 min |
−0.199 |
| Wu et al., 200660 Hong Kong, China |
N: 442 Mean meds: 6 Health: various unspecified chronic illnesses % female: 51 % non-Caucasian: NA % MA criterion: 80 |
Randomized: Yes Allocation concealed: Yes Blinded: No % Attrition: 0 ITT: Yes Comparison: True control Tx fidelity: NA MA Measure: Self-report |
Theory: NA Interventionist: Pharmacist Content: Drug education, telephone conversations to encourage MA and self-monitoring of symptoms/side effects Target: MA and additional health behaviors Delivery: Telephone Dose: 7 contacts, 5–10 min each |
0.607 |
| Zuckerman et al., 200461 United States |
N: 1,675 Mean meds: NA Health: 100% coronary artery disease % female: NA % non-Caucasian: NA % MA criterion: NA |
Randomized: No Allocation concealed: NA Blinded: NA % Attrition: NA ITT: No Comparison: True control Tx fidelity: NA MA Measure: Prescription refills |
Theory: NA Interventionist: NA Content: Teach physicians skills to enhance patient MA Target: MA only Delivery: Written materials mailed to patients’ physicians Dose: NA |
0.134 |
Abbreviations and definitions: BP, blood pressure; HCP, health care provider; HTN, hypertension; MA, medication adherence; NA, not addressed; N, total number of subjects at outcome assessment ; % MA criterion, maximum adherence level for eligibility; Blinded, data collectors masked to group assignment; ITT, intention-to-treat analysis; Tx fidelity, treatment fidelity; Target, intervention focused on MA exclusively or MA plus other health behaviors; Effect size, standardized mean difference effect size.
Overall Effects of Interventions on Adherence Outcomes
Overall adherence effect sizes are presented in Table 3. For treatment vs. control comparisons, the overall standardized mean difference effect size for was 0.301. (Analysis of treatment vs. control effect size residuals revealed one outlier, and that effect size was excluded from estimation of the overall effect size. The overall mean effect size with the outlier included was 0.423). The effect size represents the degree of difference between treatment and control groups. The 0.301 effect size is consistent with mean adherence rates at outcome of 65% for treatment subjects and 57% for control subjects.
Table 3.
Random effects medication adherence estimates and tests
| k | Effect size | p (ES) | 95% Confidence interval | Standard error | I2 | Q | p(Q) | |
|---|---|---|---|---|---|---|---|---|
| Treatment vs. control comparisonsa | 40 | 0.301 | <.001 | 0.186, 0.415 | 0.058 | 68.472 | 123.701 | <.001 |
| Treatment subjects pre-post comparisonsb | 36 | 0.533 | <.001 | 0.404, 0.663 | 0.066 | 70.189 | 117.403 | <.001 |
| Control subjects pre-post comparisons | 24 | 0.011 | .861 | −0.115, 0.138 | 0.064 | 59.535 | 56.840 | <.001 |
k denotes number of comparisons. Q is a conventional heterogeneity statistic. I2 is the percentage of total variation among studies’ observed effect size due to heterogeneity.
One comparison excluded as an outlier. The overall effect size with inclusion of outlier was 0.423 (SE = 0.079, 95% CI: 0.277, 0.587).
Two comparisons excluded as outliers. The overall effect size with inclusion of outliers was 0.618 (SE = 0.084, 95% CI: 0.454, 0.783).
The overall mean effect size for treatment group pre- vs. post-intervention comparisons was 0.533 (mean effect size with two outliers included was 0.618). By contrast, the control group pre- vs. post-intervention adherence effect size was 0.011, which was not significantly different from zero. Chisquared tests of the heterogeneity statistic Q indicated significant between-studies variation for all three effect size estimates.
Moderator Analyses of Study and Sample Characteristics
Study attributes of publication status and fiscal support were investigated as dichotomous moderators (Table 4), and the year of dissemination was investigated as a continuous moderator (Table 5). Continuous moderator analysis was also conducted to examine the influence on effect size of participant age, percentage of women, and the proportion individuals belonging to underrepresented ethnic/racial groups (Table 5). Although published studies tended to have larger effect sizes than unpublished investigations (0.346 vs. 0.097), this difference did not achieve statistical significance. Effect size was also not related to whether studies received grant or other financial support. Studies that were conducted more recently had slightly smaller effect sizes than older studies (Table 5). No association was found between effect size and any of the three participant demographic variables analyzed (Table 5).
Table 4.
Dichotomous moderator analysis of medication adherence effect size: Report and study design characteristics
| Moderator | k | Effect size | Standard error | Qbetween | p (Qbetween) |
|---|---|---|---|---|---|
| Report Moderators | |||||
|
| |||||
| Publication status | 3.244 | .072 | |||
| Published articles | 32 | 0.346 | 0.066 | ||
| Unpublished reports | 8 | 0.097 | 0.121 | ||
|
| |||||
| Presence of funding for research | 0.024 | .878 | |||
| Funded | 31 | 0.306 | 0.062 | ||
| Unfunded | 9 | 0.274 | 0.196 | ||
|
| |||||
| Design Moderators | |||||
|
| |||||
| Allocation to treatment groups | 2.621 | .105 | |||
| Randomization of individual subjects | 33 | 0.360 | 0.073 | ||
| Subjects not individually randomized | 7 | 0.156 | 0.103 | ||
|
| |||||
| Allocation concealment | 0.424 | .515 | |||
| Allocation concealed | 13 | 0.247 | 0.103 | ||
| Did not report allocation concealed | 27 | 0.329 | 0.072 | ||
|
| |||||
| Comparison group | 0.414 | .520 | |||
| True control group | 36 | 0.293 | 0.059 | ||
| Attention control group | 4 | 0.557 | 0.407 | ||
|
| |||||
| Data collector masking | 0.425 | .514 | |||
| Data collectors masked to group assignment | 4 | 0.218 | 0.131 | ||
| Did not report data collectors masked to group assignment | 36 | 0.312 | 0.063 | ||
|
| |||||
| Intention-to-treat approach | 2.847 | .092 | |||
| Reported intention-to-treat approach | 15 | 0.205 | 0.077 | ||
| Did not report intention-to-treat approach | 25 | 0.404 | 0.089 | ||
|
| |||||
| Adherence measured with electronic event monitoring system | 4.042 | .044 | |||
| Electronic event monitoring system data | 13 | 0.449 | 0.088 | ||
| Other measure of adherence | 27 | 0.227 | 0.066 | ||
|
| |||||
| Adherence measured with pharmacy refill information | 4.954 | .026 | |||
| Pharmacy refill data | 7 | 0.112 | 0.084 | ||
| Other measure of adherence | 33 | 0.358 | 0.071 | ||
|
| |||||
| Adherence measured with pill counts by research staff | 1.993 | .158 | |||
| Pill count data | 6 | 0.636 | 0.265 | ||
| Other measure of adherence | 34 | 0.253 | 0.057 | ||
|
| |||||
| Adherence measured with subjects’ self-report | 1.673 | .196 | |||
| Self-report data | 13 | 0.198 | 0.099 | ||
| Other measure of adherence | 27 | 0.357 | 0.073 | ||
k denotes number of comparisons. Effect size is standardized mean difference. Q is a conventional heterogeneity statistic.
Table 5.
Continuous moderator analysis of medication adherence effect size
| Moderator | k | Slope | Standard Error | τ2 | Qmodel | p (Slope) |
|---|---|---|---|---|---|---|
| Report Moderator | ||||||
| Year of publication | 40 | −0.017 | 0.005 | 0.055 | 10.773 | .001 |
| Sample Attribute Moderators | ||||||
| Age | 36 | −0.003 | 0.004 | 0.080 | 0.746 | .388 |
| Percent women | 37 | 0.002 | 0.002 | 0.088 | 1.379 | .240 |
| Percent non-Caucasian adults | 27 | −0.003 | 0.002 | 0.099 | 2.936 | .087 |
| Method Moderators | ||||||
| Sample size | 40 | 0 | 0 | 0.041 | 33.396 | <.001 |
| Attrition proportion | 36 | 0.712 | 0.462 | 0.080 | 2.373 | .123 |
k denotes number of comparisons. Q is a conventional heterogeneity statistic. τ2 is the between-studies variance.
Because certain interventions might be more effective for some disease conditions than others, such as due to the nature and complexity of the medication regimen, exploratory analysis was conducted to determine effect sizes for studies focusing on specific diseases. The two most frequent disease conditions in the meta-analysis sample were hypertension and HIV infection. Similar effect sizes were found for studies composed entirely of hypertensive subjects (d = 0.307, SE = 0.152, p = .044) and subjects with HIV (d = 0.303, SE = 0.093, p < .001). Other specific health problems were too infrequently reported to assess their potential as moderators of effect size.
An important potential moderator of effect size was the threshold adherence level used to determine subject eligibility. However, analysis of this moderator was precluded by a lack of variation in the sample; 14 of the 20 studies reporting an adherence inclusion criterion used <80–90% adherence for determination of eligibility.
Study Design Moderators and Risks of Bias
Several potential risks of bias related to study design attributes were identified in the meta-analysis sample: nonrandom assignment of subjects to treatment groups, nonconcealment of subject allocation, comparison group bias (attention control vs. true control), nonmasking of data collectors, on-treatment rather than intent-to-treat analysis, subject attrition, and small sample size. With the exception of study sample size, no evidence was found linking effect size to the design features analyzed. Treatment fidelity was not analyzed as a potential moderator because it was only reported by one primary study.
The method used to measure medication adherence was also investigated as a potential moderator of effect size (Table 4). Researchers used varied methods to measure adherence, including pill counts, electronic event monitoring systems, pharmacy refill data, and self-report questionnaires. The largest effect sizes were reported for studies using pill counts (0. 636) and electronic event monitoring systems (0.449). Effect sizes were significantly larger for studies with electronic monitoring systems than for studies employing other types of adherence measures (0.449 vs. 0.227, p = .044). Studies using pharmacy refills to measure adherence reported significantly smaller effect sizes than studies using other methods (0.112 vs. 0.358, p = .026). Effect sizes for studies using self-report instruments to assess adherence were smaller than for studies using other methods (0.198 vs. 0.357), but this difference was not statistically significant.
Funnel plot analysis of treatment vs. control comparisons revealed evidence of possible publication bias that was confirmed with Begg’s test (p = .02). Publication bias was also detected for treatment group pre-post comparisons and confirmed by a statistically significant Begg’s test (p = .03). The presence of publication bias suggested studies with small or negative effect sizes were not available for inclusion. In contrast, control group baseline vs. outcome effect sizes showed no evidence of publication bias.
Moderator Analyses of Intervention Characteristics
The results of moderator analyses to determine the impact of intervention characteristics on effect size are shown in Table 6. Interventions were significantly more effective if they were delivered face-to-face than if they were delivered through a medium such as telephone or email (0.411 vs. 0.182, p = .050). Effect size was not significantly impacted by whether medication adherence interventions were delivered alone or in conjunction with other health behaviors (0.318 vs. 0.282, p = 0.752).
Table 6.
Dichotomous moderator analysis of medication adherence effect size: Intervention characteristics
| Moderator | k | Effect size | Standard error | Qbetween | p (Qbetween) |
|---|---|---|---|---|---|
| Intervention delivery medium | 3.845 | .050 | |||
| Face to-face | 29 | 0.411 | 0.093 | ||
| Mediated delivery (e.g., telephone, mail) | 11 | 0.182 | 0.071 | ||
|
| |||||
| Behaviors targeted with intervention | 0.1 | .752 | |||
| Medication adherence exclusively targeted | 24 | 0.318 | 0.081 | ||
| Multiple behaviors including medication adherence | 16 | 0.282 | 0.091 | ||
|
| |||||
| Motivational interviewing theory/approach | 2.142 | .143 | |||
| Present | 9 | 0.186 | 0.074 | ||
| Absent | 31 | 0.336 | 0.070 | ||
|
| |||||
| Social Cognitive Theory | 3.590 | .058 | |||
| Present | 6 | 0.086 | 0.126 | ||
| Absent | 34 | 0.356 | 0.067 | ||
|
| |||||
| Subjects self-monitoring adherence behavior | 0.651 | .420 | |||
| Present | 8 | 0.382 | 0.105 | ||
| Absent | 32 | 0.283 | 0.065 | ||
|
| |||||
| Subjects self-monitoring signs of disease | 0.943 | .331 | |||
| Present | 5 | 0.213 | 0.073 | ||
| Absent | 35 | 0.309 | 0.066 | ||
|
| |||||
| Strategies to manage/reduce medication side effects | 0.026 | .873 | |||
| Present | 5 | 0.255 | 0.265 | ||
| Absent | 35 | 0.299 | 0.060 | ||
|
| |||||
| Prompts or cues to administer medications | 4.481 | .034 | |||
| Present | 10 | 0.497 | 0.107 | ||
| Absent | 30 | 0.234 | 0.063 | ||
|
| |||||
| Rewards or consequences for increased adherence | 0.189 | .663 | |||
| Present | 6 | 0.365 | 0.165 | ||
| Absent | 34 | 0.288 | 0.062 | ||
|
| |||||
| Habit assessment and linkage for adherence | 7.237 | .007 | |||
| Present | 12 | 0.574 | 0.116 | ||
| Absent | 28 | 0.222 | 0.061 | ||
|
| |||||
| Adherence goal setting | 3.024 | .082 | |||
| Present | 13 | 0.121 | 0.121 | ||
| Absent | 27 | 0.363 | 0.068 | ||
|
| |||||
| Improve communication between patients and providers | 0.943 | .332 | |||
| Present | 8 | 0.204 | 0.107 | ||
| Absent | 32 | 0.326 | 0.066 | ||
|
| |||||
| Feedback to patients about adherence levels | 0 | .992 | |||
| Present | 10 | 0.303 | 0.143 | ||
| Absent | 30 | 0.305 | 0.065 | ||
k denotes number of comparisons. Effect size is standardized mean difference. Q is a conventional heterogeneity statistic.
Whether theory-based interventions were more effective in increasing adherence could be assessed only for studies employing Motivational Interviewing and Social Cognitive Theory. No other theories were sufficiently represented in the sample to permit a moderator analysis. Effect sizes were lower for interventions using Motivational Interviewing theory/approaches compared to those that did not (0.186 vs. 0.336). Likewise, effect sizes were lower for interventions based on the Social Cognitive Theory than interventions that were not (0.086 vs. 0.356). Neither of these differences achieved statistical significance, owing in part to the small number of studies in the sample that used these theoretical approaches; only nine studies employed Motivational Interviewing and only six studies were grounded in Social Cognitive Theory.
With regard to intervention components that required patients to make specific changes in their behaviors, studies that employed prompts or cues for taking medications had larger effect sizes than studies that did not (0.497 vs. 0.234, p = .034). Typical prompts might include cell phone alarm reminders, locating medications in a particular location to cue medication taking such as on the kitchen table for medication to be consumed with meals, or placing reminders in strategic locations such as a note on the bathroom mirror. Habit-focused interventions in which participants’ daily habits were linked to taking medications were also effective in increasing medication adherence relative to interventions lacking this component (0.574 vs. 0.222, p = .007). Interventions directing participants to set medication adherence goals were not significantly more effective than those lacking a goal-setting component (0.121 vs. 0.363, p = .082).
Other intervention components that had no moderating influence on effect size included helping patients manage medication side effects, improving provider patient communication, providing rewards for adherence, and giving patients feedback on their adherence levels.
Discussion
This project was the first comprehensive review and meta-analysis of interventions specifically directed at individuals who have problems with medication adherence. For treatment vs. control comparisons, the statistically significant overall mean effect size of 0.301 documented that interventions do improve medication-taking behaviors in patients with adherence challenges. Although the magnitude of the effect was relatively modest, it was nevertheless comparable to the effects found in previous meta-analyses of medication adherence interventions conducted in general populations (d = 0.18–0.37).64,65 Effects were also comparable to those found in meta-analysis of interventions directed at targeted populations of underrepresented racial/ethnic groups (d = 0.211)135 and older adults (d = 0.33).79
Whether any given improvement in medication adherence translates into clinical improvements is difficult to assess because the level of adherence necessary to achieve therapeutic goals likely varies across diseases and among the medications used to treat any specific condition.136 In some situations, even a modest increase in adherence may be sufficient to realize a therapeutic effect, whereas in others, very high levels of adherence must be achieved.
Although participants with adherence problems may have more to gain in health benefits from improved adherence, it may be more difficult to induce behavior change in these individuals compared to those having fairly high adherence at baseline. It is plausible that individuals who have a history of adherence problems may lack confidence in their ability to become adherent. The overall modest effect size may reflect the inherent difficulty in changing adherence behaviors. Alternatively, it may indicate that, on average, the interventions were not adequately addressing the underlying reasons for participants’ nonadherence. A better understanding of patients’ past and current problems surrounding adherence could lead to increased intervention impact.
Analysis of Q statistics documented considerable heterogeneity among study effect sizes, indicating that some interventions were more effective than others or that intervention effectiveness might be related to sample characteristics. The exploratory moderator analyses of intervention characteristics provide direction for future research and interventions. Although mediated delivery of interventions may be attractive to increase the numbers of people who may be reached, the findings document that face-to-face delivery is more effective for people with adherence problems. The costs of face-to-face interventions may be less than the costs of nonadherence. The results also suggest that interventions can target multiple health behaviors without adversely affecting adherence outcomes. This is useful information given that clinical improvements can be achieved for some chronic conditions by changing additional health behaviors such as diet and physical activity.
The moderator analyses identified some intriguing intervention characteristics that were strongly associated with improved adherence. Especially effective interventions were those employing prompts or cues for medication-taking, such as signs on refrigerator doors or placing medication containers where meals are eaten. Habit-based interventions that examined the participants’ daily routines and then linked medication administration to those routines were also particularly effective. Unlike many adherence interventions that attempt to change subjects’ knowledge, attitudes or beliefs79, these types of intervention focus on behaviors.
The typical health care provider focus on educating patients about medications may have limited effectiveness because lack of knowledge may not be an underlying reason for poor adherence. Educating patients about medications may still be a necessary component of interventions, but for reasons not directly related to increasing adherence. Patient education may be important for patient safety. Patient education may also help patients make informed decisions about their medications such as when to see their health care provider for potential medication side-effects. People with adherence challenges may not need to be persuaded of the importance of taking their medications but rather may require strategies to help them remember to take them. The relatively greater effectiveness of behavioral interventions compared to cognitive interventions has been demonstrated for other health behaviors.137–139
Another possible explanation for the strength of prompt- and habit-based interventions is their greater sustainability relative to education-based interventions. Interventions that focus on educating patients about medications occur once or over a limited period of time, so the impact on behavior may fade once the formal intervention period is completed. In contrast, setting up prompts or linking medication administration to existing habits provides an ongoing intervention.
The results provided no support for interventions that ask participants to self-monitor their disease symptoms or medication-taking behaviors. Interventions that included providing patients strategies to manage medication side effects, giving them feedback about their medication adherence, or having them set goals were also not effective methods for increasing medication adherence. Increased provider communication also was not an effective means of increasing medication adherence in patients with adherence problems. Future research should focus on other strategies and on novel ways of implementing effective strategies.
The findings from these studies did not support the use of Social Cognitive Theory or Motivational Interviewing to increase adherence among adults with adherence problems. This finding should be interpreted considering the implementation of theories in intervention research. Specifically, theories are often incompletely operationalized in interventions. The link between theory constructs and intervention content is in general poorly reported in primary research. Theory-linked medication adherence interventions often include multiple components, some of which may not be based on specific theories. Also, theory-linked intervention studies rarely measure mediating constructs, such as self-efficacy, that would provide information about change in theory constructs.
The moderator analyses revealed that intervention effectiveness was not related to sample age, sex, or racial/ethnic composition. Future research that tests interventions specifically designed for certain populations, such as adults from racially and ethnically underrepresented groups, may be useful to determine if these tailored interventions are more effective than standard interventions. Intervention effectiveness may be related to regimen complexity. Unfortunately, very few studies reported the number of medications patients were taking or other attributes of regimen complexity. Intervention effectiveness might also vary across health conditions. Future meta-analyses could compare intervention effectiveness across disease health conditions. This analysis found no evidence for a Hawthorne effect in which control subjects improve adherence by virtue of being enrolled in a trial, as has been reported in some primary investigations.15
Primary study investigators rarely reported whether participants’ lack of adherence was intentional or not. Very different kinds of interventions are necessary for patients who deliberately do not take medications.140 Future research should specify whether nonadherence was intentional or unintentional.
Risks of methodological bias were common in these studies.81 Although there was no significant association between most methodological attributes and effect sizes, the results nevertheless should be interpreted in the context of these methodological limitations. Some methodological difficulties, such as treatment integrity problems, were rarely addressed in primary studies.
Moderator analyses of the methods used to measure medication adherence suggested electronic event monitoring systems and pill counts may have better sensitivity to detect differences in adherence following interventions than other methods. Self-report measures are less expensive to administer but may lack accuracy and sensitivity. The most accurate methods for adherence measurement remain controversial.141,142
This study has limitations inherent to all meta-analysis research and specific to this project. Despite comprehensive searching, some eligible studies may have been missed. It is possible some potentially eligible studies were not included because the authors did not report that subjects were recruited because of adherence problems. The results should also be interpreted in the context of discovered publication bias.
The findings are limited to studies that targeted participants with adherence challenges. Other kinds of interventions may be more effective for patients without documented adherence challenges, such as patients starting new medication regimens. All of the moderator analyses should be considered exploratory and hypothesis generating. Those analyses performed with limited numbers of comparisons should be interpreted with particular caution.
Some potentially interesting variables were too infrequently reported to permit moderator analysis. For example, the number of medications patients were taking was rarely reported, so it was not possible to assess whether effectiveness of interventions was related to medication burden. Intervention effectiveness might be related to disease condition and should be explored in future research. Interventions may be differentially effective for subjects with intentional nonadherence compared to those with unintentional nonadherence. Likewise, specific intervention content is necessary for subjects with cognitive impairment, and the cognitive status of subjects is rarely reported in primary studies. Increased reporting of study details such as the number of medications prescribed to subjects would permit more complete analyses of moderators of intervention effectiveness.
Several gaps in knowledge were identified during this systematic review and meta-analysis. The relationship between effect size and regimen complexity needs to be further explored. More research is needed to test the effectiveness of newer delivery technologies such as text messaging and various social media platforms. Studies are necessary to compare the effectiveness of different intervention strategies among subjects who possess particular characteristics, such as similar health conditions. Another area that has been inadequately investigated is identifying which interventions are most effective when nonadherence is intentional vs. unintentional. Research directly comparing behavior-focused interventions to cognitively-focused interventions would clarify to what extent each component contributes to changing adherence behaviors.
Future investigations should fully implement theoretical frameworks and provide explicit information about intervention content linked with theories to allow for a robust assessment of the effectiveness of theory-linked interventions. Because subjects’ level of adherence at study entry can potentially influence effect size, more emphasis should be placed by authors on analyzing and reporting potential links between baseline adherence and intervention effectiveness. Finally, greater emphasis by investigators to design studies so as to minimize the risk of bias would enhance confidence in primary study findings.
Conclusion
Inadequate medication adherence contributes substantially to patient morbidity and mortality. Nonadherent patients have a significantly higher risk of hospitalization, often resulting in increased health care costs. This comprehensive systematic review and meta-analysis documented that interventions can result in modest but statistically significant improvements in medication-taking by patients with a history of adherence problems. The findings support face-to-face interventions for patients with adherence difficulties and behavioral interventions such as linking medication administration with existing habits or using cues for medication administration may be most effective in this population.
We meta-analyzed adherence interventions among adults with adherence problems.
The overall standardized mean difference effect size was 0.301.
Interventions that included prompts to take medications were effective.
Interventions that linked medication to daily habits were effective.
Acknowledgments
The project was supported by Award Number R01NR011990 (Conn-principal investigator) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agency had no role in study design; collection, analysis, and interpretation of data, in writing the report, or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
Contributor Information
Vicki S. Conn, Email: conn@missouri.edu.
Todd M. Ruppar, Email: ruppart@missouri.edu.
Maithe Enriquez, Email: enriquezm@missouri.edu.
Pam Cooper, Email: cooperps@missouri.edu.
References
- 1.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40:794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Christiansen AJ. Patient Adherence to Medical Treatment Regimens: Bridging the Gap Between Behavioral Science and Biomedicine. New York, NY: Yale University Press; 2004. [Google Scholar]
- 3.Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. Br Med J. 2006;333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosworth HB. Medication treatment adherence. In: Bosworth HB, Oddone EZ, Weinberger M, editors. Patient Treatment Adherence: Concepts, Interventions, and Measurement. New York, NY: Psychology Press; 2006. pp. 147–194. [Google Scholar]
- 5.Chaudhry HJ, McDermott B. Recognizing and improving patient nonadherence to statin therapy. Curr Atheroscler Rep. 2008;10:19–24. doi: 10.1007/s11883-008-0004-4. [DOI] [PubMed] [Google Scholar]
- 6.Friends of Europe. Just What the Doctor Ordered: An EU Response to Medication Nonadherence. Brussels, Belgium: Friends of Europe; Sep 28, 2010. [Google Scholar]
- 7.Starner T. The price of noncompliance. Human Resource Executive Online. 2006 http://www.hreonline.com/HRE/view/story.jhtml?id=5059249. Accessed April 15, 2015.
- 8.World Health Organization. Adherence to Long-term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 9.Alhalaiqa F, Deane KHO, Nawafleh AH, Clark A, Gray R. Adherence therapy for medication non-compliant patients with hypertension: a randomised controlled trial. J Hum Hypertens. 2012;26:117–126. doi: 10.1038/jhh.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali F, Laurin MY, Lariviere C, Tremblay D, Cloutier D. The effect of pharmacist intervention and patient education on lipid-lowering medication compliance and plasma cholesterol levels. Can J Clin Pharmacol. 2003;10:101–106. [PubMed] [Google Scholar]
- 11.Austin DL. Selected Nursing Interventions for Noncompliant Hypertensive Patients [dissertation] Denton, TX: Texas Women’s University; 1986. [Google Scholar]
- 12.Budde TR. Increasing Regimen Adherence in Young Adults with Type 1 Diabetes [dissertation] Milwaukee, WI: University of Wisconsin; 2009. [Google Scholar]
- 13.Burrelle TN. Evaluation of an interdisciplinary compliance service for elderly hypertensives. J Geriatr Drug Ther. 1986;1:23–51. [Google Scholar]
- 14.Cook PF, Bremer RW, Ayala AJ, Kahook MY. Feasibility of motivational interviewing delivered by a glaucoma educator to improve medication adherence. Clin Ophthalmol. 2010;4:1091–1101. doi: 10.2147/OPTH.S12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Geest S, Schafer-Keller P, Denhaerynck K, et al. Supporting medication adherence in renal transplantation (SMART): a pilot RCT to improve adherence to immunosuppressive regimens. Clin Transplant. 2006;20:359–368. doi: 10.1111/j.1399-0012.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 16.Desborough JA, Sach T, Bhattacharya D, Holland RC, Wright DJ. A cost-consequences analysis of an adherence focused pharmacistled medication review service. Int J Pharm Pract. 2012;20:41–49. doi: 10.1111/j.2042-7174.2011.00161.x. [DOI] [PubMed] [Google Scholar]
- 17.Freedman D. The Effects of Group Mental Health Intervention on Adherence to Medication for People with HIV and AIDS [dissertation] New York, NY: New York University; 2007. [Google Scholar]
- 18.Gamble J, Stevenson M, Heaney LG. A study of a multi-level intervention to improve non-adherence in difficult to control asthma. Respir Med. 2011;105:1308–1315. doi: 10.1016/j.rmed.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Glanz K, Beck AD, Bundy L. Impact of a health communication intervention to improve glaucoma treatment adherence: results of the interactive study to increase glaucoma adherence to treatment trial. Arch Ophthalmol. 2012;130:1252–1258. doi: 10.1001/archophthalmol.2012.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths R, Johnson M, Piper M, Langdon R. A nursing intervention for the quality use of medicines by elderly community clients. Int J Nurs Pract. 2004;10:166–176. doi: 10.1111/j.1440-172X.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 21.Harper DC. Application of Orem’s theoretical constructs to self-care medication behaviors in the elderly. ANS Adv Nurs Sci. 1984;6:29–46. doi: 10.1097/00012272-198404000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Haynes RB, Sackett DL, Gibson ES, et al. Improvement of medication compliance in uncontrolled hypertension. Lancet. 1976;1:1265–1268. doi: 10.1016/s0140-6736(76)91737-2. [DOI] [PubMed] [Google Scholar]
- 23.Ingersoll KS, Farrell-Carnahan L, Cohen-Filipic J, et al. A pilot randomized clinical trial of two medication adherence and drug use interventions for HIV+ crack cocaine users. Drug Alcohol Depend. 2011;116:177–187. doi: 10.1016/j.drugalcdep.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalichman SC, Cherry J, Cain D. Nurse-delivered antiretroviral treatment adherence intervention for people with low literacy skills and living with HIV/AIDS. J Assoc Nurses AIDS Care. 2005;16:3–15. doi: 10.1016/j.jana.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Kalichman SC, Kalichman MO, Cherry C, et al. Brief behavioral self-regulation counseling for HIV treatment adherence delivered by cell phone: an initial test of concept trial. AIDS Patient Care STDS. 2011;25:303–310. doi: 10.1089/apc.2010.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kogos SC., Jr Support groups and treatment adherence in a geriatric outpatient clinic. J Clin Psychol Med Settings. 2004;11:275–282. [Google Scholar]
- 27.Lee VW, Leung PY. Glycemic control and medication compliance in diabetic patients in a pharmacist-managed clinic in Hong Kong. Am J Health Syst Pharm. 2003;60:2593–2596. doi: 10.1093/ajhp/60.24.2593. [DOI] [PubMed] [Google Scholar]
- 28.Leung LB, Busch AM, Nottage SL, et al. Approach to antihypertensive adherence: a feasibility study on the use of student health coaches for uninsured hypertensive adults. Behav Med. 2012;38:19–27. doi: 10.1080/08964289.2011.651174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levensky ER. Further Development and Evaluation of an Individualized Intervention for Increasing Adherence to HIV Medications [dissertation] Reno, NV: Unversity of Nevada; 2006. [Google Scholar]
- 30.Long JM, Kee CC, Graham MV, Saethang TB, Dames FD. Medication compliance and the older hemodialysis patient. ANNA J. 1998;25:43–49. [PubMed] [Google Scholar]
- 31.Matteson M. A Pilot Intervention to Improve Medication Adherence in Nonadherent Inflammatory Bowel Disease Patients [dissertation] Columbia, MO: University of Missouri; 2011. [Google Scholar]
- 32.McPherson-Baker S, Malow RM, Penedo F, Jones DL, Schneiderman N, Klimas NG. Enhancing adherence to combination antiretroviral therapy in non-adherent HIV-positive men. AIDS Care. 2000;12:399–404. doi: 10.1080/09540120050123792. [DOI] [PubMed] [Google Scholar]
- 33.Migneault J, Dedier J, Wright J, et al. A culturally adapted telecommunication system to improve physical activity, diet quality, and medication adherence among hypertensive African–Americans: a randomized controlled trial. Ann Behav Med. 2012;43:62–73. doi: 10.1007/s12160-011-9319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell ML. Effects of a Self-efficacy Intervention on Adherence to Antihypertensive Regimens [dissertation] Rochester, NY: University of Rochester; 1993. [Google Scholar]
- 35.Moitra E, Herbert JD, Forman EM. Acceptance-based behavior therapy to promote HIV medication adherence. AIDS Care. 2011;23:1660–1667. doi: 10.1080/09540121.2011.579945. [DOI] [PubMed] [Google Scholar]
- 36.Molassiotis A, Lopez-Nahas V, Chung WY, Lam SW. A pilot study of the effects of a behavioural intervention on treatment adherence in HIV-infected patients. AIDS Care. 2003;15:125–135. doi: 10.1080/0954012021000039833. [DOI] [PubMed] [Google Scholar]
- 37.Murphy DA, Lu MC, Martin D, Hoffman D, Marelich WD. Results of a pilot intervention trial to improve antiretroviral adherence among HIV-positive patients. J Assoc Nurses AIDS Care. 2002;13:57–69. doi: 10.1177/1055329002238026. [DOI] [PubMed] [Google Scholar]
- 38.Murphy DA, Marelich WD, Rappaport NB, Hoffman D, Farthing C. Results of an antiretroviral adherence intervention: STAR (Staying Healthy: Taking Antiretrovirals Regularly) J Int Assoc Physicians AIDS Care. 2007;6:113–124. doi: 10.1177/1545109707301243. [DOI] [PubMed] [Google Scholar]
- 39.Nietert PJ, Tilley BC, Zhao W, et al. Two pharmacy interventions to improve refill persistence for chronic disease medications: a randomized, controlled trial. Med Care. 2009;47:32–40. doi: 10.1097/MLR.0b013e3181808c17. [DOI] [PubMed] [Google Scholar]
- 40.Nochowitz B, Shapiro NL, Nutescu EA, Cavallari LH. Effect of a warfarin adherence aid on anticoagulation control in an inner-city anticoagulation clinic population. Ann Pharmacother. 2009;43:1165–1172. doi: 10.1345/aph.1L707. [DOI] [PubMed] [Google Scholar]
- 41.Okeke CO, Quigley HA, Jampel HD, et al. Interventions improve poor adherence with once daily glaucoma medications in electronically monitored patients. Ophthalmology. 2009;116:2286–2293. doi: 10.1016/j.ophtha.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oser M. Evaluation of a Bibliotherapy Intervention for Improving Patients’ Adherence to Antihypertensive Medications [dissertation] Reno, NV: University of Nevada; 2008. [Google Scholar]
- 43.Ramirez-Garcia P, Côté J. An individualized intervention to foster optimal antiretroviral treatment-taking behavior among persons living with HIV: a pilot randomized controlled trial. J Assoc Nurses AIDS Care. 2012;23:220–232. doi: 10.1016/j.jana.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Remien RH, Stirratt MJ, Dolezal C, et al. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. AIDS. 2005;19:807–814. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- 45.Rosen MI, Dieckhaus K, McMahon TJ, et al. Improved adherence with contingency management. AIDS Patient Care STDS. 2007;21:30–40. doi: 10.1089/apc.2006.0028. [DOI] [PubMed] [Google Scholar]
- 46.Ruppar TM. Randomized pilot study of a behavioral feedback intervention to improve medication adherence in older adults with hypertension. J Cardiovasc Nurs. 2010;25:470–479. doi: 10.1097/JCN.0b013e3181d5f9c5. [DOI] [PubMed] [Google Scholar]
- 47.Russell C, Conn V, Ashbaugh C, et al. Taking immunosuppressive medications effectively (TIMELink): a pilot randomized controlled trial in adult kidney transplant recipients. Clinical Transplant. 2011;25:864–870. doi: 10.1111/j.1399-0012.2010.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Safren SA, Hendriksen ES, Desousa N, Boswell SL, Mayer KH. Use of an on-line pager system to increase adherence to antiretroviral medications. AIDS Care. 2003;15:787–793. doi: 10.1080/09540120310001618630. [DOI] [PubMed] [Google Scholar]
- 49.Safren SA, Hendriksen ES, Mayer KH, Mimiaga MJ, Pickard R, Otto MW. Cognitive-behavioral therapy for HIV medication adherence and depression. Cogn Behav Pract. 2004;11:415–424. [Google Scholar]
- 50.Safren SA, Otto MW, Worth JL, et al. Two strategies to increase adherence to HIV antiretroviral medication: Life-Steps and medication monitoring. Behav Res Ther. 2001;39:1151–1162. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 51.Sorensen JL, Haug NA, Delucchi KL, et al. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: a randomized trial. Drug Alcohol Depend. 2007;88:54–63. doi: 10.1016/j.drugalcdep.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart K, George J, Jackson SL, et al. Increasing community pharmacy involvement in the prevention of cardiovascular disease. Pharmacy Guild of Australia; 2007. http://www.guild.org.au/docs/default-source/public-documents/services-and-programs/research-and-development/Fourth-Agreement-R-and-D/2007-08-10/full-final-report.pdf?sfvrsn=0. Project ID 2007/08-10. Accessed April 15, 2015. [Google Scholar]
- 53.Taylor CT, Byrd DC, Krueger K. Improving primary care in rural Alabama with a pharmacy initiative. Am J Health Syst Pharm. 2003;60:1123–1129. doi: 10.1093/ajhp/60.11.1123. [DOI] [PubMed] [Google Scholar]
- 54.Turner LC. Effects of a Behavioral Self-control Package on Drug Prescription Compliance Behavior of Chronic Arthritic Patients [dissertation] Winnepeg, MB, Canada: University of Manitoba; 1981. [Google Scholar]
- 55.van Servellen G, Nyamathi A, Carpio F, et al. Effects of a treatment adherence enhancement program on health literacy, patient-provider relationships, and adherence to HAART among low-income HIV-positive Spanish-speaking Latinos. AIDS Patient Care STDS. 2005;19:745–759. doi: 10.1089/apc.2005.19.745. [DOI] [PubMed] [Google Scholar]
- 56.Vervloet M, van Dijk L, Santen-Reestman J, et al. SMS reminders improve adherence to oral medication in type 2 diabetes patients who are real time electronically monitored. Int J Med Inform. 2012;81:594–604. doi: 10.1016/j.ijmedinf.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Villeneuve J, Genest J, Blais L, et al. A cluster randomized controlled trial to evaluate an ambulatory primary care management program for patients with dyslipidemia: the TEAM study. Can Med Assoc J. 2010;182:447–455. doi: 10.1503/cmaj.090533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wall TL, Sorensen JL, Batki SL, Delucchi KL, London JA, Chesney MA. Adherence to zidovudine (AZT) among HIV-infected methadone patients: a pilot study of supervised therapy and dispensing compared to usual care. Drug Alcohol Depend. 1995;37:261–269. doi: 10.1016/0376-8716(94)01080-5. [DOI] [PubMed] [Google Scholar]
- 59.Watakakosol R. Telephone-administered Intervention to Improve Medication Adherence in HIV-infected Rural Persons: A Pilot Randomized Clinical Trial [dissertation] Athens, OH: Ohio University; 2010. [Google Scholar]
- 60.Wu JYF, Leung WYS, Chang S, et al. Effectiveness of telephone counseling by a pharmacist in reducing mortality in patients receiving polypharmacy: randomised controlled trial. Br Med J. 2006;333:522–525. doi: 10.1136/bmj.38905.447118.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zuckerman IH, Weiss SR, McNally D, Layne B, Mullins CD, Wang J. Impact of an educational intervention for secondary prevention of myocardial infarction on Medicaid drug use and cost. Am J Manag Care. 2004;10:493–500. [PubMed] [Google Scholar]
- 62.Jones C. Medication adherence study looks at types of interventions. Manag Care. 2014;23:38–41. [PubMed] [Google Scholar]
- 63.Kravitz RL, Melnikow J. Medical adherence research: time for a change in direction? Med Care. 2004;42:197–199. doi: 10.1097/01.mlr.0000115957.44388.7c. [DOI] [PubMed] [Google Scholar]
- 64.Mullen PD, Green LW, Persinger GS. Clinical trials of patient education for chronic conditions: a comparative meta-analysis of intervention types. Prev Med. 1985;14:753–781. doi: 10.1016/0091-7435(85)90070-2. [DOI] [PubMed] [Google Scholar]
- 65.Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60:657–665. doi: 10.1093/ajhp/60.7.657. [DOI] [PubMed] [Google Scholar]
- 66.Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care. 1998;36:1138–1161. doi: 10.1097/00005650-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 67.Arbuthnott A, Sharpe D. The effect of physician-patient collaboration on patient adherence in non-psychiatric medicine. Patient Educ Couns. 2009;77:60–67. doi: 10.1016/j.pec.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 68.Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826–834. doi: 10.1097/MLR.0b013e31819a5acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iskedjian M, Einarson TR, MacKeigan LD, et al. Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: evidence from a meta-analysis. Clin Ther. 2002;24:302–316. doi: 10.1016/s0149-2918(02)85026-3. [DOI] [PubMed] [Google Scholar]
- 70.Mahtani KR, Heneghan CJ, Glasziou PP, Perera R. Reminder packaging for improving adherence to self-administered long-term medications. Cochrane Database Syst Rev. 2011;9:CD005025. doi: 10.1002/14651858.CD005025.pub3. [DOI] [PubMed] [Google Scholar]
- 71.Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120:713–719. doi: 10.1016/j.amjmed.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 72.Cutrona SL, Choudhry NK, Stedman M, et al. Physician effectiveness in interventions to improve cardiovascular medication adherence: a systematic review. J Gen Intern Med. 2010;25:1090–1096. doi: 10.1007/s11606-010-1387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chisholm-Burns MA, Spivey CA, Sredzinski E, Butler SL. Intervention toolbox to promote immunosuppressant therapy adherence in adult renal transplant recipients. J Am Pharm Assoc (2003) 2012;52:816–822. doi: 10.1331/JAPhA.2012.11083. [DOI] [PubMed] [Google Scholar]
- 74.Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: translating research findings to the real world clinic. Current HIV/AIDS Reports. 2010;7:44–51. doi: 10.1007/s11904-009-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takiya LN, Peterson AM, Finley RS. Meta-analysis of interventions for medication adherence to antihypertensives. Ann Pharmacother. 2004;38:1617–1624. doi: 10.1345/aph.1D268. [DOI] [PubMed] [Google Scholar]
- 76.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41:285–297. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 77.Bailey EJ, Cates CJ, Kruske SG, Morris PS, Brown N, Chang AB. Culture-specific programs for children and adults from minority groups who have asthma. Cochrane Database of Syst Rev. 2009;2:CD006580. [Google Scholar]
- 78.Manias E, Williams A. Medication adherence in people of culturally and linguistically diverse backgrounds: a meta-analysis. Ann Pharmacother. 2010;44:964–982. doi: 10.1345/aph.1M572. [DOI] [PubMed] [Google Scholar]
- 79.Conn VS, Hafdahl AR, Cooper PS, Ruppar TM, Mehr DR, Russell CL. Interventions to improve medication adherence among older adults: meta-analysis of adherence outcomes among randomized controlled trials. Gerontologist. 2009;49:447–462. doi: 10.1093/geront/gnp037. [DOI] [PubMed] [Google Scholar]
- 80.Cooper H, Hedges LV, Valentine JC. The Handbook of Research Synthesis and Meta-analysis. 2nd. New York, NY: Russell Sage Foundation; 2009. [Google Scholar]
- 81.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 82.Borenstein M, Hedges L, Higgins JPT, Rothstein H. Introduction to Meta-analysis. West Sussex, England: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 83.Cooper H. Synthesizing Research. 3rd. Thousand Oaks, CA: Sage; 1998. [Google Scholar]
- 84.Lipsey M, Wilson D. Practical Meta-analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 85.Lipsey MW. Those confounded moderators in meta-analysis: good, bad, and ugly. Ann Am Acad Pol Soc Sci. 2003;587:69–81. [Google Scholar]
- 86.Lipsey M. Identifying interesting variables and analysis opportunities. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd. New York, NY: Russell Sage Foundation; 2009. pp. 147–158. [Google Scholar]
- 87.Lohr KN, Carey TS. Assessing “best evidence”: issues in grading the quality of studies for systematic reviews. Jt Comm J Qual Improv. 1999;25:470–479. doi: 10.1016/s1070-3241(16)30461-8. [DOI] [PubMed] [Google Scholar]
- 88.Moher D, Olkin I. Meta-analysis of randomized controlled trials: a concern for standards. JAMA. 1995;274:1962–1964. [PubMed] [Google Scholar]
- 89.Sutton AJ. Publication bias. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-analysis. 2nd. New York, NY: Russell Sage Foundation; 2009. pp. 435–452. [Google Scholar]
- 90.Conn VS, Valentine JC, Cooper HM, Rantz MJ. Grey literature in meta-analyses. Nurs Res. 2003;52:256–261. doi: 10.1097/00006199-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 91.Jadad AR, Moher D, Klassen TP. Guides for reading and interpreting systematic reviews: II. How did the authors find the studies and assess their quality? Arch Pediatr Adolesc Med. 1998;152:812–817. doi: 10.1001/archpedi.152.8.812. [DOI] [PubMed] [Google Scholar]
- 92.McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet. 2000;356:1228–1231. doi: 10.1016/S0140-6736(00)02786-0. [DOI] [PubMed] [Google Scholar]
- 93.Rothstein HR, Hopewell S. Grey literature. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-analysis. 2nd. New York, NY: Russell Sage Foundation; 2009. pp. 103–125. [Google Scholar]
- 94.Stern JM, Simes RJ. Publication bias: evidence of delayed publication in a cohort study of clinical research projects. BMJ. 1997;315:640–645. doi: 10.1136/bmj.315.7109.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matt G, Cook T. Threats to the validity of research synthesis. In: Cooper H, Hedges L, editors. The handbook of research synthesis. New York, NY: Russell Sage Foundation; 1994. pp. 503–519. [Google Scholar]
- 96.Nony P, Cucherat M, Haugh MC, Boissel JP. Critical reading of the meta-analysis of clinical trials. Therapie. 1995;50:339–351. [PubMed] [Google Scholar]
- 97.Pigott T. Handling missing data. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd. New York, NY: Russell Sage Foundation; 2009. pp. 399–416. [Google Scholar]
- 98.White H. Scientific communication and literature retrieval. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-analysis. 2nd. New York, NY: Russell Sage Foundation; 2009. pp. 51–71. [Google Scholar]
- 99.Dickersin K, Scherer R, Suci ES, Gil-Montero M. Problems with indexing and citation of articles with group authorship. JAMA. 2002;287:2772–2774. doi: 10.1001/jama.287.21.2772. [DOI] [PubMed] [Google Scholar]
- 100.Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ. 1994;309:1286–1291. doi: 10.1136/bmj.309.6964.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fergusson D, Laupacis A, Salmi LR, McAlister FA, Huet C. What should be included in meta-analyses? An exploration of methodological issues using the ISPOT meta-analyses. Int J Technol Assess Health Care. 2000;16:1109–1119. doi: 10.1017/s0266462300103150. [DOI] [PubMed] [Google Scholar]
- 102.Reed J, Baxter P. Using reference databases. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd. New York, NY: Russell Sage Foundation; 2009. pp. 73–101. [Google Scholar]
- 103.Scoville CL, Johnson ED, McConnell AL. When A. Rose is not A. Rose: the vagaries of author searching. Med Ref Serv Q. 2003;22:1–11. doi: 10.1300/J115v22n04_01. [DOI] [PubMed] [Google Scholar]
- 104.Dickersin K, Olson CM, Rennie D, et al. Association between time interval to publication and statistical significance. JAMA. 2002;287:2829–2831. doi: 10.1001/jama.287.21.2829. [DOI] [PubMed] [Google Scholar]
- 105.Helmer D, Savoie I, Green C, Kazanjian A. Evidence-based practice: extending the search to find material for the systematic review. Bull Med Libr Assoc. 2001;89:346–352. [PMC free article] [PubMed] [Google Scholar]
- 106.Sindhu F, Dickson R. The complexity of searching the literature. Int J Nurs Pract. 1997;3:211–217. doi: 10.1111/j.1440-172x.1997.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 107.Easterbrook PJ. Directory of registries of clinical trials. Stat Med. 1992;11:363–423. [PubMed] [Google Scholar]
- 108.Lefebvre C, Lusher A, Dickersin K, Manheimer E. Literature searches. Lancet. 2002;359:896. doi: 10.1016/S0140-6736(02)07942-4. [DOI] [PubMed] [Google Scholar]
- 109.Hek G, Langton H, Blunden G. Systematically searching and reviewing literature. Nurse Res. 2000;7:40–57. [Google Scholar]
- 110.Langham J, Thompson E, Rowen K. Identification of randomized controlled trials from the emergency medicine literature: comparison of hand searching versus MEDLINE searching. Ann Emerg Med. 1999;34:25–34. doi: 10.1016/s0196-0644(99)70268-4. [DOI] [PubMed] [Google Scholar]
- 111.Smith JT, Jr, Smith MC, Stullenbarger E. Decision points in the integrative research review process: a flow-chart approach. Med Ref Serv Q. 1991;10:47–72. doi: 10.1300/J115v10n02_04. [DOI] [PubMed] [Google Scholar]
- 112.Cooper H, Ribble R. Influences on the outcomes of literature searches for integrative research reviews. Knowledge. 1989;10:179–201. [Google Scholar]
- 113.Wood JA. Methodology for dealing with duplicate study effects in a meta-analysis. Orgn Res Methods. 2008;11:79–95. [Google Scholar]
- 114.Devine E. Issues and challenges in coding interventions for meta-analysis of prevention research. In: Bukoski W, editor. Meta-analysis of Drug Abuse Prevention Programs. Rockville, MD: National Institute on Drug Abuse; 1997. pp. 130–146. (NIDA research monograph; 170). http://archives.drugabuse.gov/pdf/monographs/monograph170/monograph170.pdf. Accessed May 18, 2015. [PubMed] [Google Scholar]
- 115.Orwin R, Vevea J. Evaluating coding decisions. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd. New York, NY: Russell Sage Foundation; 2009. pp. 177–203. [Google Scholar]
- 116.Wilson D. Systematic coding. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd. New York, NY: Russell Sage Foundation; 2009. pp. 159–176. [Google Scholar]
- 117.Lipsey MW, Wilson DB. The way in which intervention studies have “personality” and why it is important to meta-analysis. Eval Health Prof. 2001;24:236–254. doi: 10.1177/016327870102400302. [DOI] [PubMed] [Google Scholar]
- 118.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2d. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 119.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 120.Hedges L, Olkin I. Statistical Methods for Meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- 121.Conn VS, Hafdahl AR, Mehr DR, LeMaster JW, Brown SA, Nielsen PJ. Metabolic effects of interventions to increase exercise in adults with type 2 diabetes. Diabetologia. 2007;50:913–921. doi: 10.1007/s00125-007-0625-0. [DOI] [PubMed] [Google Scholar]
- 122.Raudenbush S. Random effects models. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-analysis. 2nd. New York, NY: Russell Sage Foundation; 2009. pp. 295–315. [Google Scholar]
- 123.Hedges L, Vevea J. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3:486–504. [Google Scholar]
- 124.Andes AD, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25:646–654. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 125.Kisamore JL, Brannick MT. An illustration of the consequences of meta-analysis model choice. Organizational Research Methods. 2008;11:35–53. [Google Scholar]
- 126.Shadish W, Haddock C. Combining estimates of effect size. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd. New York, NY: Russell Sage Foundation; 2009. pp. 257–277. [Google Scholar]
- 127.Conn VS, Rantz MJ. Research methods: managing primary study quality in meta-analyses. Res Nurs Health. 2003;26:322–333. doi: 10.1002/nur.10092. [DOI] [PubMed] [Google Scholar]
- 128.Phillips CV. Publication bias in situ. BMC Med Res Methodol. 2004;4:20. doi: 10.1186/1471-2288-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Williamson PR, Gamble C, Altman DG, Hutton JL. Outcome selection bias in meta-analysis. Stat Methods Med Res. 2005;14:515–524. doi: 10.1191/0962280205sm415oa. [DOI] [PubMed] [Google Scholar]
- 130.Mahid SS, Qadan M, Hornung CA, Galandiuk S. Assessment of publication bias for the surgeon scientist. Br J Surg. 2008;95:943–949. doi: 10.1002/bjs.6302. [DOI] [PubMed] [Google Scholar]
- 131.de Vet HC, de Bie RA, van der Heijden GJ, Verhagen AP, Sijpkes P, Kipschild P. Systematic review on the basis of methodological criteria. Physiotherapy. 1997;1997:284–289. [Google Scholar]
- 132.Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 133.Moher D, Cook DJ, Jadad AR, et al. Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses. Health Technol Assess. 1999;3:i–iv. 1–98. [PubMed] [Google Scholar]
- 134.Valentine J. Judging the quality of primary research. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd. New York, NY: Russell Sage Foundation; 2009. pp. 129–146. [Google Scholar]
- 135.Conn V, Enriquez M, Ruppar TM, Chan KC. Cultural relevance in medication adherence interventions with underrepresented adults: systematic review and meta-analysis of outcomes. Prev Med. 2014;69:239–247. doi: 10.1016/j.ypmed.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peeters B, Van Tongelen I, Boussery K, Mehuys E, Remon JP, Willems S. Factors associated with medication adherence to oral hypoglycaemic agents in different ethnic groups suffering from type 2 diabetes: a systematic literature review and suggestions for further research. Diabet Med. 2011;28:262–275. doi: 10.1111/j.1464-5491.2010.03133.x. [DOI] [PubMed] [Google Scholar]
- 137.Conn VS, Hafdahl AR, Brown SA, Brown LM. Meta-analysis of patient education interventions to increase physical activity among chronically ill adults. Patient Educ Couns. 2008;70:157–172. doi: 10.1016/j.pec.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Conn VS, Hafdahl AR, Mehr DR. Interventions to increase physical activity among healthy adults: meta-analysis of outcomes. Am J Public Health. 2011;101:751–758. doi: 10.2105/AJPH.2010.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Conn VS, Valentine JC, Cooper HM. Interventions to increase physical activity among aging adults: a meta-analysis. Ann Behav Med. 2002;24:190–200. doi: 10.1207/S15324796ABM2403_04. [DOI] [PubMed] [Google Scholar]
- 140.Rivers PH. Compliance aids—do they work? Drugs Aging. 1992;2:103–111. doi: 10.2165/00002512-199202020-00004. [DOI] [PubMed] [Google Scholar]
- 141.Cook P, Schmiege S, McClean M, Aagaard L, Kahook M. Practical and analytic issues in the electronic assessment of adherence. West J Nurs Res. 2012;34:598–620. doi: 10.1177/0193945911427153. [DOI] [PubMed] [Google Scholar]
- 142.Dunbar-Jacob J, Sereika SM, Houze M, Luyster FS, Callan JA. Accuracy of measures of medication adherence in a cholesterol-lowering regimen. West J Nurs Res. 2012;34:578–597. doi: 10.1177/0193945912439251. [DOI] [PMC free article] [PubMed] [Google Scholar]


