Abstract
Heatstroke is a serious illness in dogs characterized by core temperatures above 41 °C with central nervous system dysfunction. Experimental heatstroke models have tried to correlate biomarker levels with the severity of the syndrome. Serum heat shock protein (eHSP70) levels were recently evaluated as a biomarker of heat tolerance and acclimation, their role as a marker of heatstroke is inconclusive. Here, we monitored eHSP70 levels in correlation with systemic biomarkers in 30 naturally occurring canine heatstroke cases. Thirty dogs diagnosed with environmental (33 %) or exertional (66 %) heatstroke admitted to hospital (0–14 h post-injury) were tested for biomarkers of organ damage and coagulation parameters. eHSP70 levels were measured upon admission and 4, 12, and 24 h later (T1, T2, and T3, respectively). No differences were found between exertional and environmental heatstroke cases. The eHSP profile demonstrated an inverted bell shape, with the lowest levels at the 12 h time point. A positive correlation between eHSP70, lactate, and aPPT was also noted at T2 in all the dogs in the study. Twenty-four h after presentation, eHSP70 levels returned to those measured upon admission, this change was only significant in the survivors. The obtained results suggest that eHSP72 level profile may be predictive of survival.
Keywords: Extracellular heat shock protein 72, Canine, Serum, Hyperthermia, Chaperones
Introduction
Heatstroke is caused by the inability to dissipate accumulated heat (Bouchama and Knochel 2002; Bruchim et al. 2006; Epstein and Roberts 2011; Leon and Helwig 2010). In dogs, it is characterized by core temperatures above 41 °C (105.8 °F) with central nervous system (CNS) dysfunction. Heatstroke manifests clinically as hyperthermia, tachypnea, panting, collapse, shock, abnormal mentation, seizures, vomiting, diarrhea, petechia, and ecchymosis (Bruchim et al. 2006; Drobatz and Macintire 1996; Hausfater et al. 2008; Leon and Bouchama 2015).
The pathophysiology involves the activation of numerous inflammatory and hemostatic pathways causing systemic inflammatory response syndrome (SIRS) and multi-organ dysfunction syndrome (MODS) (Bouchama and Knochel 2002; Diehl et al. 2000; Leon and Helwig 2010; Roberts et al. 2008; Yang et al. 1998). Serious complications of heatstroke include rhabdomyolysis, acute kidney injury (AKI), acute respiratory distress syndrome (ARDS), and disseminated intravascular coagulation (DIC) (Bruchim et al. 2009; Hausfater et al. 2010; Yang et al. 1998). Consequently, despite appropriate cooling and other supportive treatments, mortality rates above 50 % have been reported in humans and canines suffering from heatstroke, because no specific treatment is available to stop the activated inflammatory and hemostatic pathways (Argaud et al. 2007; Aroch et al. 2009; Bruchim et al. 2006; Drobatz and Macintire 1996; Hausfater et al. 2010; Misset et al. 2006).
It seems that the main causes of death from heatstroke in canines are systemic hemodynamic deterioration and pulmonary lesions as determined from postmortem studies of 11 dogs that suffered from naturally occurring heatstroke (Bruchim et al. 2009). Similar histopathological findings of damage to the liver, kidney, and intestine were described in rats following experimentally induced heatstroke (King et al. 2015). However, in order to evaluate the damage as the disease progresses, serial monitoring of serum biomarkers is warranted.
Experimental models have attempted to link different biomarkers to the severity of the syndrome. Rodents with experimental heatstroke had significantly greater activity of serum creatine kinase (CK), alanine transaminase (ALT), and intestinal binding protein-2 than controls (King et al. 2015). Two human studies on naturally occurring exertional heatstroke showed that high mobility group box-1 protein (HMGB1) and procalcitonin were predictive serum biomarkers (Tong et al. 2012; Tong et al. 2011). Our previous studies on canines with naturally occurring heatstroke showed that various biomarkers and clinical signs may serve as prognostic markers, including the presence of: seizures at presentation, >50 % nucleated red blood cells (NRBC) in the peripheral blood, hypoglycemia and multiple coagulation abnormalities (prothrombin (PT), activated thromboplastin time (aPTT), protein C activity, and fibrinogen 12–24 h post presentation (PP)) (Aroch et al. 2009; Bruchim et al. 2006; Segev et al. 2015).
Serum heat shock protein (eHSP) was recently evaluated as biomarkers for heat tolerance and acclimation and during experimental heatstroke (Bruchim et al. 2014; Dehbi et al. 2010; Periard et al. 2012; Sandstrom et al. 2008). One study of induced heatstroke in six monkeys demonstrated that eHSP 72 serum levels correlated with the liver, myocardium, and skeletal muscle necrosis, and were lower in the survivors (Dehbi et al. 2010), therefore, eHSP72 levels may serve as a prognostic indicator of heatstroke (Dehbi et al. 2010). Ruell et al. observed human heatstroke patients immediately after a 14 km running event and noted that early neurological signs of heatstroke, (e.g., confusion), were associated with higher levels of eHSP 72, (Ruell et al. 2014). Another human study reported increases in eHSP serum levels after a single intense physical training session, and that the level correlated with the intensity and duration of the physical training and with rectal temperature (Tre) elevation (Walsh et al. 2001). Prior training induced an increase in basal eHSP levels, diminishing the need for further induction of this protein during exercise (Periard et al. 2012; Sandstrom et al. 2008). It is important to note that the findings may be species-specific, and those from animal models may not be consistent with naturally occurring disease.
We have shown that lymphocyte HSP72 protein levels and lymphocyte HSP72 mRNA are detectable and accurately measurable in working military dogs. In addition, in acclimated trained dogs, basal eHSP72 levels were higher than in naïve dogs, with significantly higher post-exercise levels in the acclimated dogs (Bruchim et al. 2014). To the best of our knowledge, eHSPs levels have never been assessed in naturally occurring heatstroke patients.
This study was designed to evaluate eHSP72 levels in relation to the standard systemic biomarkers used to assess disease severity in dogs suffering from naturally occurring heatstroke. We hypothesize that eHSP72 levels 24 h post-heat injury will be significantly different in survivors compared to non-survivors and can be used as a prognostic indicator.
The objectives of the study were three-fold: (1) to investigate whether eHSP72 is released into the circulation in dogs with naturally occurring heatstroke, (2) to follow changes in eHSP72 levels over time, and (3) to evaluate whether changes in eHSP72 levels over time correlate with organ or system damage and are associated with survival.
Materials and methods
Selection of dogs
This study was approved by the Institutional Ethics Committee of the Hebrew University Veterinary Teaching Hospital. Dogs were enrolled after a consent form was signed by their owners. Dogs diagnosed with naturally occurring environmental or exertional heatstroke were prospectively and consecutively enrolled. Heatstroke was diagnosed based on a history of strenuous exercise, exposure to a hot environment, or both, along with the development of appropriate clinical signs (i.e., acute collapse and acute CNS dysfunction, increased body temperature, tachypnea, or tachycardia). Dogs with pre-existing diseases, based on history and physical examination, were excluded. The dogs were physically examined at presentation and periodically throughout hospitalization. The duration of hospitalization, secondary hemostatic complications (i.e., DIC), and the outcome (died, euthanized, or discharged alive) were recorded.
Definition of complications
DIC was diagnosed if PT and aPTT were prolonged (>150 % reference interval (RI)) and thrombocytopenia (<150 × 109 cells/uL) was present along with clinical signs of spontaneous bleeding (e.g., petechia, hematochezia, hemoptysis). Dogs were diagnosed with AKI if their creatinine concentration was >2 mg/dl after 24 h of continuous IV fluid therapy, with the exclusion of pre- and post-renal causes of azotemia (Bruchim et al. 2006). Blood pressure was measured using an oscillometric device (Cardell, Midmark, Tampa, Florida, USA). Severity scoring system was described previously (Segev et al. 2015).
Serum HSP 72 analysis
Blood was collected in plain serum tubes upon admission, before any medical intervention or cooling were initiated, and at 4, 12, and 24 h PP (T0, T1, T2, and T3, respectively). Serum was separated immediately and stored at −80 °C pending analysis. Of all the samples, only <10 % were hemolytic mostly from the first sampling time. A commercially available kit HSP72 High-Sensitivity (Stress Xpress®, Bioscience, Victoria BC, Canada) was used to measure eHSP72 levels, according to the manufacturer's instructions. Briefly, each sample was divided into three aliquots, and analyses were done in triplicate. Samples were placed into the wells. The biotinylated antibody against HSP70 was added and the sealed plate was incubated for 2 h at room temperature. The wells were then washed with a Tris-buffered solution, leaving only plate-bound HSP70 and were then stained with a horseradish peroxide-catalyzed reaction for 30 min. The dyed solution was read by a spectrophotometer (Bio-Rad, i-MARK microplate reader, USA) at 450 nm. The amount of signal read by the spectrophotometer is directly proportional to the HSP70 concentration. The average optical density (OD) of each triplicate was calculated and used for statistical analysis. The normal range was determined to be 0.09–0.15 OD based on control dogs (Bruchim et al. 2014) and studies of eHSP72 in humans (Xiao et al. 2003) and monkeys (Dehbi et al. 2010).
Biomarkers for organ damage
To evaluate organ damage and the potential origin of eHSPs, several biochemical and hematological parameters were monitored. Serum activities of ALT, aspartate transaminase (AST), and alkaline phosphatase (ALP) were used to assess hepatobiliary damage. The kidney injury was assessed using serum creatinine concentration. Degree of rhabdomyolysis was assessed using serum activities of AST, CK, and ALT, and coagulation was assessed using PT and aPTT.
Hematological and biochemical tests
Blood samples for complete blood count (CBC) were collected in potassium-EDTA tubes and analyzed within 30 min of collection. Blood smears, stained with modified Wright’s stain solution was used to evaluate cell morphology, to exclude platelets clumping as a cause for spurious thrombocytopenia and as quality control for the automated platelet counter. Manual platelet count was performed by averaging platelet numbers in 10 randomly selected oil (×100) fields and multiplying the average by 20,000 cells/μL.
Whole blood samples for hemostatic tests (PT, aPTT) were collected in 3.2 % trisodium-citrate tubes, according to the manufacturer’s instructions (trisodium-citrate: blood ratio of 1:9) at presentation and at 4, 12, and hrs PP. Blood samples were collected from a peripheral IV catheter upon its placement at presentation and from dedicated sampling peripheral IV catheters thereafter, using a standard method. Three syringes were prepared before sampling, two with 0.3 mL unfractionated heparin (1000 units/ml) and one plain syringe. One mL of blood was drawn from the catheter into a heparinized syringe, followed by 3 mL of blood into the plain syringe. The catheter was then flushed with the heparinized blood followed by 0.3 ml of heparinized saline to prevent intra-catheter clotting and thrombus formation. Samples were centrifuged within 30 min of collection. Harvested citrated plasma was either analyzed immediately or stored at −80 °C pending analysis, which was performed within 12 months, in two single runs. Routine hemostatic tests (PT, aPTT, and platelet count) were performed at the request of the attending clinician, and the results of these tests clearly influenced therapeutic decisions. However, the attending clinicians were blinded to the remaining hemostatic tests results. Automated coagulation tests were performed using coagulometric auto analyzers (ACL-9000, Instrumentation Laboratory, Milano, Italy) and included PT and aPTT.
Blood samples for serum biochemistry were centrifuged within 30 min of collection, and sera were either analyzed immediately or refrigerated (at 4 °C) pending analysis, that was performed within 12 h of collection or the samples were stored at −80 °C pending analysis using an auto analyzer (Cobas-Mira or Cobas Integra 400 Plus, Roche, Mannheim, Germany), at 37 °C. Urinary production was measured at 4, 12, and 24 h post presentation, after correction of pre-renal azotemia, from an indwelling urinary catheter connected to a monitoring system.
Treatment protocol
All dogs were treated according to the Hebrew University Veterinary Teaching Hospital (HUVTH) canine heatstroke protocol. Treatment included whole body irrigation with tap water and a fanning with a ventilator until reaching a core temperature of 39.5 °C in dogs admitted with hyperthermia. All dogs received a bolus of 50 ml/kg of ringer lactate solution followed by constant rate infusion of 7.5 ml/kg/h, broad spectrum antibiotics, H2 blockers, and an antiemetic (metoclopramide). All dogs but one (non-survivor) received fresh-frozen plasma (FFP). The number of FFP units per dog and the FFP dose (mL/kg) did not differ significantly between survivors and non-survivors (40.2 mL/kg vs. 36.5 mL/kg; P = 0.33, respectively). Urine output, neurological status, blood pressure, and ECG were continuously monitored for at least 24 h.
Statistical analysis
Data is presented as median and range. Non-parametric tests were used because of the small sample size and the non- normal distribution of the quantitative continuous variables (analyzed by Kolmogorov-Smirnov test). The comparison of continuous variables between the two groups was carried out using the non-parametric Mann-Whitney U test, which was also used for post-hoc (multiple pairwise comparisons) tests, with the Bonferroni correction when more than one group was compared. The chi-square and the Fisher's exact tests were applied for testing the association between two categorical variables. The non-parametric Spearman correlation coefficient was calculated to assess the correlation between two quantitative variables. All tests were two-tailed, and P ≤0.05 was considered significant. Statistical analyses were performed using a statistical software package (SPSS 22.0 for Windows, SPSS Inc, Chicago, IL, USA).
Results
Signalment and physical parameters
Thirty client-owned dogs with naturally occurring heatstroke were included in the study. The most common canine breeds included were brachycephalic breeds 11/30 (37 %), Golden and Labrador retrievers 6/30 (20 %), and Shepherd dogs 5/30 (16 %). There were significantly more males than females (26/30; 87 % vs. 4/30; 13 %, respectively, P = 0.001). The median age was 4 years (range 1–9 years) with survivors being significantly younger (3 years; range 1–8 years vs. 6.5 years; range 1.4–9 years, respectively, P = 0.029)(Table 1). Twenty (66 %) dogs were classified as having exertional heatstroke, while 10/30 (33 %) were classified as suffering from environmental heatstroke. Median elapsed time from heatstroke to admission was 3.75 h (range 0.5–14 h).
Table 1.
Signalment, physical parameters, type of heatstroke, exertion and exposure time and sum severity score, survivors vs. non-survivors in 30 dogs with naturally occurring heatstroke
| Parameter (median and range)/ Outcome | Age (years) median and range | Body weight (kg) Median and range |
Exertional heatstroke | Environmental heatstroke | Exposure time (min) | Exertion time (min) | Tre upon admission (celsius) | Heart rate BPM | Severity score | Systolic blood pressure (mmHg) | Duration of hospitalization (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Survivors (18 dogs) | 3.0 (1–8) | 33.7 (15.0–38.5) | 66 % (12/18) | 33 % (6/18) | 180 min (120–360) | 45 min (30–180) | 38.5 (35.0–41.6 0C) | 132 (75–180) | 33.4 (14.8–68.1) | 118 mmHg (90–175) | 3 days (1–12) |
| Non-survivors (12 dogs) | 6.5 (1.4–9) | 31.3 (12.0–60.0) | 66 % (8/12) | 33 % (4/12) | 150 min (60–240) | 60 min (10–120) | 39.6 0C (38.4–42.8) | 165 (80–200) | 49.7 (24.7–64.5) |
128.5 mmHg (63–172) | 3 days (1–9) |
| P value* | 0.029 | 0.772 | 0.552 | 0.553 | 0.853 | 0.528 | 0.046 | 0.028 | 0.047 | 0.940 | 0.368 |
*All tests were two-tailed, and P ≤0.05 was considered significant
The most common clinical signs upon admission included shock and collapse (25/30, 87 %), petechia (15/30, 50 %), seizures, and coma (9/30, 30 %). Median rectal temperature (Tre) upon arrival was 39.0 °C (range 35–42.8 °C). In 16/30 (53 %) dogs for which data were available, median Tre measured by the referring veterinarian prior to referral was 41.0 °C (range 39.4–43.0 °C). Most dogs (13/16, 81 %) presented to the referring veterinarian with Tre > 40 °C, while only 9/30 (30 %) had a Tre >40 °C upon admission to our emergency services. Median systolic and diastolic blood pressures were 127/74 mm/Hg, respectively. Only 3/30 and 6/30 dogs had systolic <100 mmHg and diastolic <60 mmHg pressures, respectively.
Serum biochemistry, hematology, complications, hospitalization time and outcome
Median CK upon admission was 13,667 U/l (range 195–111,802 U/l), median ALT activity was 2062 U/l (range 33–12,484 U/l), median AST activity was 297 U/l (range 44–3245 U/l), median ALP activity was 84 U/l (24–268 U/l), and median glucose was 80 mg/dl (range 19–254 mg/dl). Median PT and aPTT upon admission were 10 and 32 s, respectively (range 6.3–100 s and 11.8–100, respectively). Median platelet count was 121×109 cells/ul (range 26–534×109 cells/ul). DIC was diagnosed in 17/30 (57 %), and AKI was diagnosed in 14/30 (47 %). These biomarkers changed during the course of hospitalization, with a clear split between the survivors and non-survivors as shown in Table 2.
Table 2.
Serum heat shock protein 72, serum lactate, and enzyme activity and coagulation parameters, in 30 dogs with naturally occurring heatstroke
| Analyte (RI, units) |
Time PP1 (hours) |
Survivors (n = 18) | Non-survivors (n = 12) | P 3 value | ||
|---|---|---|---|---|---|---|
| n 2 | Median (range) | n 2 | Median (range) | |||
| Serum heat shock protein 72 (0.09–0.15 OD4) | 0 | 11 | 0.398 (0.055–1.74) | 7 | 0.202 (0.07–1.84) | 0.53 |
| 4 | 16 | 0.332 (0.08–2.25) | 10 | 0.111 (0.09–0.92) | 0.08 | |
| 12 | 15 | 0.164 (0.09–0.61) | 10 | 0.257 (0.075–1.63) | 0.25 | |
| 24 | 15 | 0.389 (0.08–2.34) | 11 | 0.211 (0.067–1.34) | 0.2 | |
| Lactate (0–14 mg/dl) | 0 | 15 | 27.4 (10.3–63.7) | 12 | 50.0 (24.2–62.3) | 0.04 |
| 12 | 16 | 13.6 (5.4–26.4) | 11 | 17.6 (11.0–75.4) | 0.025 | |
| 24 | 16 | 11.7 (1.0–33.7) | 9 | 18.4 (2.1–38.9) | 0.59 | |
| Aspartate transaminase (14–80 U/L) | 0 | 18 | 1058 (100–8994) | 12 | 1250 (33–12,480) | 0.62 |
| 24 | 18 | 2450 (94–5186) | 12 | 2228 (198–17,177) | 0.61 | |
| Alanine aminotransferase (19–67U/L) | 0 | 18 | 240 (44–3245) | 12 | 326 (60–3244) | 0.92 |
| 24 | 18 | 601 (60–3684) | 12 | 1430 (44–17,675) | 0.055 | |
| Creatine Kinase (20–160U/L) | 0 | 18 | 14,653 (454–171,779) | 12 | 9368 (195–90,905) | 0.69 |
| 24 | 18 | 20,000 (387–87,135) | 12 | 18,840 (786–81,234) | 0.9 | |
| Alkaline phosphatase (13–190 U/L) | 0 | 18 | 67 (30–194) | 12 | 104 (24–268) | 0.09 |
| 24 | 18 | 86.5 (40–366) | 12 | 150 (27–382) | 0.1 | |
| Creatinine (0.5-1.2 mg/dl) | 0 | 18 | 1.42 (0.73–3.22) | 12 | 1.98 (0.5–2.33) | 0.88 |
| 24 | 18 | 1.02 (0.6–3.29) | 12 | 2.26 (0.96–4.31) | 0.027 | |
| Prothrombin time PT (6–8.4 s) | 0 | 18 | 10.0 (7.3–100) | 12 | 10.8 (6.3–22) | 0.39 |
| 12 | 18 | 11.4 (8–22.4) | 12 | 19.5 (7–100) | 0.03 | |
| 24 | 17 | 11.4 (7.4–23.2) | 10 | 17.4 (7–100) | 0.11 | |
| aPTT5 (11–17.4 s) | 0 | 18 | 30.6 (12–100) | 12 | 42 (15–100) | 0.59 |
| 12 | 18 | 30.8 (8.6–73) | 12 | 58.5 (12–100) | 0.02 | |
| 24 | 17 | 33.5 (16–48) | 10 | 45.9 (13–100) | 0.02 | |
| Urine production | 4 | 18 | 4.8 (0.1–13.8) | 12 | 3.58 (0.3–39.3) | 0.39 |
| 12 | 16 | 4.4 (0.03–10.9) | 12 | 2.2 (0.09–7.9) | 0.04 | |
| 24 | 15 | 7.0 (1.9–12.7) | 11 | 4.17 (0.6–11.5) | 0.14 | |
| Urine creatinine/protein ratio (<0.2) | 0 | 18 | 9.35 (0.4–46.1) | 12 | 4.17 (0.3–42.2) | 0.42 |
1, Time of sampling post presentation. 2, Number of dogs which data was available. 3. Kruskal-Wallis non-parametric test, all tests were two-tailed, and 4, Optical density. 5, Activated partial thromboplastin time
*P ≤0.05 was considered significant.
Serum heat shock protein 72 (eHSP 72)
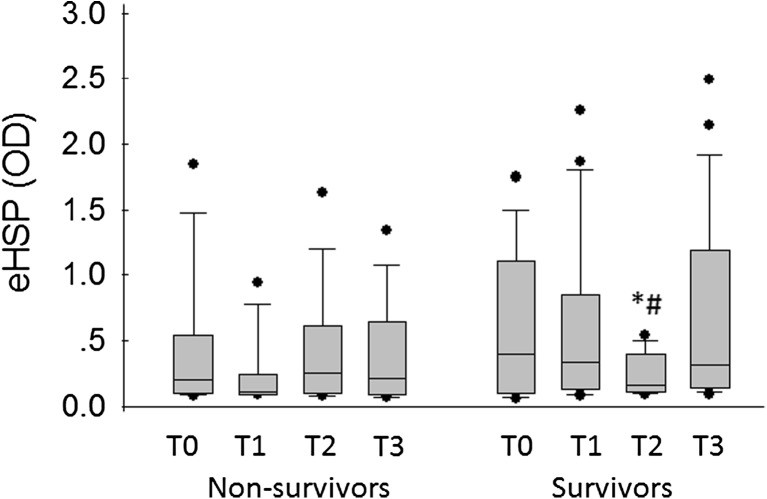
Median eHSPs 72 upon admission (T0), and at T1, T2, and T3 were 0.363 OD (range 0.055–1.847 OD), 0.235 OD (range 0.081–2.251), 0.186 OD (range 0.075–1.632), and 0.314 OD (range 0.068–2.488). Median values of T0, T1, and T3 were significantly higher than the reference interval in dogs (P = 0.009, P = 0.013, and P = 0.002, respectively). eHSP72 levels were above the reference interval, (0.09–0.15 OD) at presentation in 11/18 (61 %) of the dogs, at T1 in 15/26 (57 %) of the dogs, at T2 in 15/25 (60 %) of the dogs, and at T3 in 17/26 (65 %) of the dogs. Median eHSP72 levels were not significantly different between outcome groups at any of the time points (Table 2). Interestingly, however, the within group profile during hospitalization in the survivors group showed an inverted bell shape profile with the lowest value at T2. The median eHSP72 levels were significantly higher at T1 and T3 compared to T2 (0.332 vs. 0.164 OD, P = 0.04 and 0.389 vs. 0.164 OD, P = 0.02, respectively) (Fig. 1). There was no correlation between eHSP72 OD levels and admission Tre, hepatic enzyme activity (ALP, ALT, and AST), muscle enzyme activity (CK, AST, and ALT), PT, aPTT, and platelet count. At T2, a significant correlation was found between eHSP72 OD levels and aPTT (r = 0.65, P = 0.003), and between eHSP72 OD and lactate (r = 0.69, P = 0.015) in all the dogs in the study. No significant difference in eHSP72 levels was found between dogs with environmental and exertional heatstroke both in serum enzyme activity and in coagulation parameters at all-time points.
Fig. 1.
Serum heat shock protein in 30 dogs diagnosed with naturally occurring heatstroke at presentation and at 4, 12, and 24 h PP in both survivors and non-survivors (T0, T1, T2, and T3, respectively). Each box represents the interquartile range. The horizontal line within the box represents the median, and the whiskers represent the range. #Significant difference (P = 0.04) between eHSPs in sampling T1 and T2 in the survivors. *Significant difference (P = 0.02) between eHSPs in sampling time T2 and T3 in the survivors
Discussion
This study evaluates the dynamics of eHSP from the time of admission and during the first 24 h of hospitalization in 30 dogs with naturally occurring heatstroke. We show that eHSP72 levels were higher than the normal serum reference range at all measured time points. Survivor dogs demonstrated an inverted bell shape curve of the eHSP levels with the lowest amplitude 12 h post admission (Fig 1). The significant increase in serum eHSPs during heatstroke supports the notion that the heat stress response in dogs includes the release of eHSPs into circulation. A positive correlation between eHSP, aPTT, and lactate levels at T2 suggests a link between the consensus heatstroke markers of both exertional and passive heatstroke and the eHSP level. Unfortunately, the other parameters (serum enzyme activity) (Table 2) were not tested at T2, thus, the current data do not allow mechanistic explanation of these correlations.
The major difference between the current study and experimental heatstroke models is that monitoring was initiated at a different starting point and severity for each dog. Thus, the parameters showing significant differences over the duration of hospitalization between survivors and non-survivors are consensus markers of delayed/long-lasting effects, independent of the cause of the heatstroke (exertional vs. classical).
The dogs in the present study were admitted with acute collapse, abnormal mentation, hypoglycemia, and hyperemic mucus membranes, characteristic of distributive hypovolemic shock. Despite aggressive treatment individualized to each dog, a complication rate (DIC 57 %, AKI 47 %) and a high mortality rate (12/30, 40 %) were found, further confirming severe heatstroke. Nonetheless, the median systolic and diastolic blood pressures, except in one dog, were in the normal range (>90 mmHg and >60 mmHg, respectively, and HR of 160 BPM). This is in contrast to the hypotension documented in both experimental models and clinical reports (Dehbi et al. 2010; King et al. 2015; Li et al. 2001; Lin et al. 1997). The lack of hypotension implies that the dogs were in compensatory shock at the time of hospitalization (after they were cooled by their owners and/or by the referring veterinarian). The neural compensatory response of blood pressure is likely to be faster than the recovery/disruption of biochemical parameters.
In the current setup, median eHSP72 levels were significantly higher than the normal reference range observed in dogs at all-time points (Bruchim et al. 2014). The reference range used in this study was based on our previous study using military working dogs, before initiation of physical training and exposure to high environmental temperatures. The resting basal levels of dogs were found to be similar to the reported levels in humans (Periard et al. 2012), monkeys (Dehbi et al. 2010), but about 10–40 times lower than two other human studies in trained and in highly trained military working dogs (Bruchim et al. 2014; Sandstrom et al. 2008; Walsh et al. 2001). The conflicting data emphasizes that there is no consensus regarding the basal levels of eHSP72. The variability may be due to different methodologies or species or due to dissimilar acclimation states or physical performance abilities (Bruchim et al. 2014), diverse levels of heat exposure, and time elapsed from previous physical activity (Sandstrom et al. 2008). Therefore, eHSPs need to be monitored serially for each individual to enable evaluation of their dynamics over time.
In the present study, we followed 30 dogs with naturally occurring heatstroke and compared the levels of eHSP72 to other physiological parameters (e.g., Tre, pulse, and blood pressure), biochemical and coagulation parameters, for the first 24 h following hospital admission. This study demonstrates that eHSP levels change throughout the course of disease. The biological half-life of eHSP is 18 h (Gerner et al. 2002), therefore, the rapid changes we found cannot be attributed to the half-life of the protein. The changes may have been at least partially caused by the treatment that the dogs received, including high volumes of fluids with concurrent use of osmotic diuresis (i.e., mannitol), which can affect the renal clearance of these proteins. Dogs with heatstroke also sustain glomerular injury as reflected by high urine creatinine/protein (Table 2), which may also change protein elimination by the urinary system. Another possible factor that may influence eHSP level is lysis of the cells due to the heat injury and consequent DIC, and direct thermal damage to the erythrocytes seen in some of the samples. Nevertheless, only a small percent (<10 %) of the dogs suffered from hemolysis and usually it was only in the first sampling time upon admission.
The survivor dogs demonstrated an inverted bell shape curve of eHSP levels with the lowest value 12 h PP. At this time point, there was a positive correlation between eHSPs, lactate, and aPTT, two markers associated with hypoxic stress and inflammation/coagulation. In the surviving dogs, a recovery in the level of eHSPs was noted. In contrast, the non-survivors showed unchanged eHSP levels. The obtained results suggest that eHSP72 level profile may be predictive of survival. In the non-survivors, coagulation markers were further elevated at that time point and lactate level was significantly higher than in the survivor group (Table 2). The return to the initial high levels in the survivor group is surprising. It may be due to de novo cellular production and leakage to the serum along with decreased protein loss in the urine.
A correlation between eHSP levels and an attenuated inflammatory response has been reported by several investigators (Campisi and Fleshner 2003). In contrast to their intracellular anti-inflammatory action, extracellular HSPs (eHSPs) exert immune stimulatory effects by interacting with pattern recognition receptors such as toll-like receptor, thereby activating the immune and inflammatory systems (Asea 2008, 2000, 2002, Asea et al. 2000; 2002). Indeed, Dehbi et al. (2010) have recently reported the important cytoprotective role of the TOL4 pathway in recovery from heatstroke. We hypothesize that eHSPs are not only useful as biomarkers of stress but also act as a help signal, and when sufficient levels have accumulated, eHSPs accelerate spontaneous recovery.
In summary, the current study shows that in contrast to experimental models of heatstroke, where disease severity is followed from the onset of the heat event, in the natural occurring heatstroke victims only parameters reflecting delayed compensatory responses are valid. Data from our protocol shows that serum levels at the 12-hour time point post-hospitalization seem to be critical (e.g., shorter coagulation time in the survivors). The elevation of eHSP in the 24-hour sample may be associated with enhanced immunological activity and protection.
Compliance with ethical standards
This study was approved by the Institutional Ethics Committee of the Hebrew University Veterinary Teaching Hospital.
References
- Argaud L, Ferry T, Le QH, Marfisi A, Ciorba D, Achache P, Robert D. Short- and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch Intern Med. 2007;167(20):2177–2183. doi: 10.1001/archinte.167.20.ioi70147. [DOI] [PubMed] [Google Scholar]
- Aroch I, Segev G, Loeb E, Bruchim Y. Peripheral nucleated red blood cells as a prognostic indicator in heatstroke in dogs. J Vet Intern Med. 2009;23(3):544–551. doi: 10.1111/j.1939-1676.2009.0305.x. [DOI] [PubMed] [Google Scholar]
- Asea A (2008) Heat shock proteins and toll-like receptors. Handb Exp Pharmacol(183):111-127. doi: 10.1007/978-3-540-72167-3_6 [DOI] [PubMed]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277(17):15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- Bruchim Y, Aroch I, Eliav A, Abbas A, Frank I, Kelmer E, Horowitz M. Two years of combined high-intensity physical training and heat acclimatization affect lymphocyte and serum HSP70 in purebred military working dogs. J Appl Physiol (1985) 2014;117(2):112–118. doi: 10.1152/japplphysiol.00090.2014. [DOI] [PubMed] [Google Scholar]
- Bruchim Y, Klement E, Saragusty J, Finkeilstein E, Kass P, Aroch I. Heat stroke in dogs: a retrospective study of 54 cases (1999–2004) and analysis of risk factors for death. J Vet Intern Med. 2006;20(1):38–46. doi: 10.1892/0891-6640(2006)20[38:hsidar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bruchim Y, Loeb E, Saragusty J, Aroch I. Pathological findings in dogs with fatal heatstroke. J Comp Pathol. 2009;140(2-3):97–104. doi: 10.1016/j.jcpa.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Campisi J, Fleshner M. Role of extracellular HSP72 in acute stress-induced potentiation of innate immunity in active rats. J Appl Physiol (1985) 2003;94(1):43–52. doi: 10.1152/japplphysiol.00681.2002. [DOI] [PubMed] [Google Scholar]
- Dehbi M, Baturcam E, Eldali A, Ahmed M, Kwaasi A, Chishti MA, Bouchama A. Hsp-72, a candidate prognostic indicator of heatstroke. Cell Stress Chaperones. 2010;15(5):593–603. doi: 10.1007/s12192-010-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl KA, Crawford E, Shinko PD, Tallman RD, Jr, Oglesbee MJ. Alterations in hemostasis associated with hyperthermia in a canine model. Am J Hematol. 2000;64(4):262–270. doi: 10.1002/1096-8652(200008)64:4<262::AID-AJH5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Drobatz KJ, Macintire DK. Heat-induced illness in dogs: 42 cases (1976-1993) J Am Vet Med Assoc. 1996;209(11):1894–1899. [PubMed] [Google Scholar]
- Epstein Y, Roberts WO. The pathophysiology of heat stroke: an integrative view of the final common pathway. Scand J Med Sci Sports. 2011;21(6):742–748. doi: 10.1111/j.1600-0838.2011.01333.x. [DOI] [PubMed] [Google Scholar]
- Gerner C, Vejda S, Gelbmann D, Bayer E, Gotzmann J, Schulte-Hermann R, Mikulits W. Concomitant determination of absolute values of cellular protein amounts, synthesis rates, and turnover rates by quantitative proteome profiling. Mol Cell Proteomics. 2002;1(7):528–537. doi: 10.1074/mcp.M200026-MCP200. [DOI] [PubMed] [Google Scholar]
- Hausfater P, Hurtado M, Pease S, Juillien G, Lvovschi VE, Salehabadi S, Riou B. Is procalcitonin a marker of critical illness in heatstroke? Intensive Care Med. 2008;34(8):1377–1383. doi: 10.1007/s00134-008-1083-y. [DOI] [PubMed] [Google Scholar]
- Hausfater P, Megarbane B, Dautheville S, Patzak A, Andronikof M, Santin A, Riou B. Prognostic factors in non-exertional heatstroke. Intensive Care Med. 2010;36(2):272–280. doi: 10.1007/s00134-009-1694-y. [DOI] [PubMed] [Google Scholar]
- King MA, Leon LR, Mustico DL, Haines JM, Clanton TL. Biomarkers of multiorgan injury in a preclinical model of exertional heat stroke. J Appl Physiol (1985) 2015;118(10):1207–1220. doi: 10.1152/japplphysiol.01051.2014. [DOI] [PubMed] [Google Scholar]
- Leon LR, Bouchama A. Heat stroke. Compr Physiol. 2015;5(2):611–647. doi: 10.1002/cphy.c140017. [DOI] [PubMed] [Google Scholar]
- Leon LR, Helwig BG. Heat stroke: role of the systemic inflammatory response. J Appl Physiol. 2010;109(6):1980–1988. doi: 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- Li PL, Chao YM, Chan SH, Chan JY. Potentiation of baroreceptor reflex response by heat shock protein 70 in nucleus tractus solitarii confers cardiovascular protection during heatstroke. Circulation. 2001;103(16):2114–2119. doi: 10.1161/01.CIR.103.16.2114. [DOI] [PubMed] [Google Scholar]
- Lin MT, Liu HH, Yang YL. Involvement of interleukin-1 receptor mechanisms in development of arterial hypotension in rat heatstroke. Am J Physiol. 1997;273(4 Pt 2):H2072–2077. doi: 10.1152/ajpheart.1997.273.4.H2072. [DOI] [PubMed] [Google Scholar]
- Misset B, De Jonghe B, Bastuji-Garin S, Gattolliat O, Boughrara E, Annane D, Carlet J. Mortality of patients with heatstroke admitted to intensive care units during the 2003 heat wave in France: a national multiple-center risk-factor study. Crit Care Med. 2006;34(4):1087–1092. doi: 10.1097/01.CCM.0000206469.33615.02. [DOI] [PubMed] [Google Scholar]
- Periard JD, Ruell P, Caillaud C, Thompson MW. Plasma Hsp72 (HSPA1A) and Hsp27 (HSPB1) expression under heat stress: influence of exercise intensity. Cell Stress Chaperones. 2012;17(3):375–383. doi: 10.1007/s12192-011-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GT, Ghebeh H, Chishti MA, Al-Mohanna F, El-Sayed R, Bouchama A. Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke: a study in baboon model. Arterioscler Thromb Vasc Biol. 2008;28(6):1130–1136. doi: 10.1161/ATVBAHA.107.158709. [DOI] [PubMed] [Google Scholar]
- Ruell PA, Simar D, Periard JD, Best S, Caillaud C, Thompson MW. Plasma and lymphocyte Hsp72 responses to exercise in athletes with prior exertional heat illness. Amino Acids. 2014;46(6):1491–1499. doi: 10.1007/s00726-014-1721-3. [DOI] [PubMed] [Google Scholar]
- Sandstrom ME, Siegler JC, Lovell RJ, Madden LA, McNaughton L. The effect of 15 consecutive days of heat-exercise acclimation on heat shock protein 70. Cell Stress Chaperones. 2008;13(2):169–175. doi: 10.1007/s12192-008-0022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev G, Aroch I, Savoray M, Kass PH, Bruchim Y. A novel severity scoring system for dogs with heatstroke. J Vet Emerg Crit Care (San Antonio) 2015;25(2):240–247. doi: 10.1111/vec.12284. [DOI] [PubMed] [Google Scholar]
- Tong HS, Liu YS, Wen Q, Tang YQ, Yuan FF, Su L. Serum procalcitonin predicting mortality in exertional heatstroke. Emerg Med J. 2012;29(2):113–117. doi: 10.1136/emj.2010.107680. [DOI] [PubMed] [Google Scholar]
- Tong HS, Tang YQ, Chen Y, Qiu JM, Wen Q, Su L. Early elevated HMGB1 level predicting the outcome in exertional heatstroke. J Trauma. 2011;71(4):808–814. doi: 10.1097/TA.0b013e318220b957. [DOI] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6(4):386–393. doi: 10.1379/1466-1268(2001)006<0386:EISHIH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Wu T, Ren A, Pan Q, Chen S, Wu F, Tanguay RM (2003, Spring). Basal and inducible levels of Hsp70 in patients with acute heat illness induced during training. Cell Stress Chaperones. Retrieved 1, 8, from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12820658 [DOI] [PMC free article] [PubMed]
- Yang YL, Lu KT, Tsay HJ, Lin CH, Lin MT. Heat shock protein expression protects against death following exposure to heatstroke in rats. Neurosci Lett. 1998;252(1):9–12. doi: 10.1016/S0304-3940(98)00508-4. [DOI] [PubMed] [Google Scholar]