Abstract
Regulation of the endoplasmic reticulum (ER) stress-response pathway during the course of diabetes specifically in renal tubules is unclear. Since tubule cell dysfunction is critical to progression of diabetic nephropathy, this study analyzed markers of ER stress response and ER chaperones at different stages of diabetes and in different renal tubule subtypes of OVE26 type-1 diabetic mice. ER stress-response-induced chaperones GRP78, GRP94, and protein disulfide isomerase (PDI) were increased in isolated cortical tubules of older diabetic mice, while PDI was decreased in tubules of young diabetic mice. Immunofluorescence staining of kidneys from older mice showed GRP78 and PDI upregulation in all cortical tubule segments, with substantial induction of PDI in distal tubules. Protein kinase RNA-like endoplasmic reticulum kinase (PERK) phosphorylation was increased in cortical tubules of young diabetic mice, with no differences between older diabetic and control mice. Expression of ER stress-induced PERK inhibitor p58IPK was decreased and then increased in all tubule subtypes of young and older mice, respectively. Knockdown of PERK by small interfering RNA (siRNA) increased fibronectin secretion in cultured proximal tubule cells. Tubules of older diabetic mice had significantly more apoptotic cells, and ER stress-induced pro-apoptotic transcription factor C/EBP homologous protein (CHOP) was increased in proximal and distal tubules of diabetic mice and diabetic humans. CHOP induction in OVE26 mice was not altered by severity of proteinuria. Overexpression of CHOP in cultured proximal tubule cells increased expression of fibronectin. These findings demonstrate differential ER stress-response signaling in tubule subtypes of diabetic mice and implicate a role for PERK and CHOP in tubule cell matrix protein production.
Keywords: ER Chaperones, PERK, CHOP/GADD153, Apoptosis, P58IPK, Fibrosis
Introduction
Altered renal tubule function is critical to the development of diabetic nephropathy (DN), the leading cause of end-stage renal disease (Vallon 2011). Renal function and prognosis correlate well with tubule structural lesions and dysfunction and tubulointerstitial fibrosis. Early in diabetes, tubules undergo hypertrophy and expansion of the basement membrane and eventually atrophy and dilatation by the time DN is established (Hryciw et al. 2004; Magri and Fava 2009; Thomas et al. 2005). The presence of endoplasmic reticulum (ER) stress-response and ER stress-induced apoptotic markers has been shown in kidneys during diabetes (Brezniceanu et al. 2010; Zhuang and Forbes 2014; Lindenmeyer et al. 2008; Liu et al. 2008; Wu et al. 2010a, b). However, since the majority of the studies were conducted by analysis of markers in whole kidney homogenates, this stress response has not been clearly defined in different renal structures or tubule segments at different stages of diabetes. This stress response is an adaptive mechanism by the ER to couple increased unfolded protein load with increased protein folding capacity, to protect cells during stress. However, persistent stress stimuli ultimately lead to cell death in part by a process known as ER stress-induced apoptosis (Tabas and Ron 2011). Stress to the ER is sensed by the ER luminal chaperone, glucose-regulated protein 78 (GRP78). Accumulation of misfolded proteins in the ER lumen results in dissociation of GRP78 from, and activation of three ER transmembrane proteins responsible for transduction of stress signaling. These include protein kinase RNA-like endoplasmic reticulum kinase (PERK), activating transcription factor 6 (ATF6), and inositol requiring enzyme 1 (IRE-1) (Tabas and Ron 2011; Wang and Kaufman 2012). The sequence of events in the ER stress response results in arrest of global protein synthesis, induction of chaperone protein expression to increase protein folding capacity, and export of misfolded proteins from the ER for degradation or ER-associated degradation (ERAD).
Renal tubule cell apoptosis has been demonstrated in diabetic patients and animals models of DN and may be involved in tubular atrophy during diabetes (Brezniceanu et al. 2007; Sanchez-Niño et al. 2010; Verzola et al. 2007). Considering the critical role of tubule cell dysfunction in progression of DN, we analyzed ER-resident chaperones and stress-response signaling effectors specifically in renal tubules of a mouse model of type 1 diabetes mellitus (T1DM). We used the OVE26 transgenic mouse which develops early-onset type I diabetes. This mouse model displays characteristics of early and late-stage diabetic nephropathy in humans, including initial increase followed by decline in glomerular filtration rate, renal hypertrophy, mesangial expansion, and thickening of glomerular basement membrane, and they develop severe proteinuria, as well as tubulointerstitial fibrosis (Carlson et al. 1997; Powell et al. 2009; Zheng et al. 2004). To better correlate this stress response to characteristics associated with the pathogenesis of DN, diabetic OVE26 mice with mild to severe albuminuria were used.
Methods
Animal models
All studies on mice were approved by and conducted in accordance with the University of Louisville Institutional Animal Care and Use Committee (IACUC) guidelines. Transgenic female OVE26 (diabetic) and FVB (non-diabetic; background strain control) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Studies were conducted on young (2–3-month-old) and older (6–7-month-old) mice. OVE26-Nmt7 double transgenic mice were created and maintained by Dr. Paul Epstein at the University of Louisville. Animals were maintained on a 12-h light/dark cycle at 25 °C and given free access to water and food. Albuminuria was quantified as previously described by Zheng et al. (2004).
Immunohistochemistry and image analysis
Immunohistochemistry of paraffin-embedded kidneys sections from all mouse groups and human subjects was performed as previously described (Thongboonkerd et al. 2004). Sections from renal biopsies of diabetic patients (D-1 through D-5) and non-diabetic controls (ND-4 and ND-5) were kindly provided by Dr. Brad Rovin, Ohio State University. The non-diabetic control kidney tissues specimens provided by Dr. Rovin were obtained from normal living related kidney donors at the time of transplantation. These donors had been screened for hypertension and vascular disease and had normal creatinine clearances and no proteinuria. Additional fixed, paraffin-embedded renal biopsies from diabetic patients (D-6 through D-8) were provided by Dr. Susan Coventry, University of Louisville, and from non-diabetic subjects (ND-1 through ND-3) purchased from Bioserve (Beltsville, MD). Briefly, sections were cleared of paraffin with xylene, rehydrated in graded ethanols, and subject to antigen retrieval using a citrate based antigen unmasking buffer (Vector Labs, Burlingame, CA) at 95–99 °C for 20 min. Endogenous tissue peroxidase was quenched by incubation in 3 % hydrogen peroxide. Primary antibodies used were anti phospho-Thr 980 PERK (1:200 dilution) and anti-C/EBP homologous protein (CHOP; 1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). Sections were incubated with primary antibodies overnight at 4 °C. Negative controls were performed by treating additional sections with either isotype control antibodies or antibody dilution buffer (1 % bovine serum albumin (BSA), 0.05 % Triton X-100, 0.05 % Tween 20 in phosphate-buffered serum (PBS)) alone for the same duration. All sections including negative controls were then incubated with respective biotinylated secondary antibodies, followed by incubation in avidin/biotinylated enzyme complex (Vectastain Elite ABC kit, Vector Labs). Proteins were detected following color development using 3,3′-diaminobenzidine as substrate (Vector Labs). Digital Images were obtained with a Q Color 5 camera attached to an Olympus BX51 microscope using Image-Pro software. Similar regions in the kidney were used for comparative analysis of staining presented in the figures. Immunostaining was quantified with Image-Pro 6.2 software (Media Cybernetics, Silver Spring, MD). Five visual fields/cortex of kidneys were randomly selected and captured with a ×40 objective. Positive staining was defined in color range selection and the same profile applied to all images collected/sample. Values for total staining area were obtained and data represented as a ratio of staining in tubules from OVE26 diabetic mice to control FVB mice for each experimental date.
Immunofluorescence and confocal microscopy
Paraffin-embedded kidney sections were cleared of paraffin, rehydrated, and subject to antigen retrieval as described above for immunohistochemistry. Sections were blocked with a solution of 2 % goat serum, 1 % BSA, 0.05 % Tween 20, and 0.05 % Triton X-100 in PBS, for 1 h at room temperature. Primary antibodies used were anti p58IPK (1:500; Cell signaling, Danvers, MA), anti-GRP78 (1:500; Sigma, St. Louis, MO), anti PDI (1:200, Assay Designs, Farmingdale, NY), and anti-CHOP (1:100 dilution). All sections were incubated with primary or isotype control (negative control sections) antibodies overnight at 4 °C. Sections were then incubated with goat anti-rabbit rhodamine antibody then co-stained with fluorescein isothiocyanate (FITC)-Lotus Tetragonolobus Agglutinin (LTA; 1:1000; vector), washed ×3 in PBS, 4′,6-diamidino-2-phenylindole (DAPI)-stained, and mounted with vectashield hard-set aqueous mounting media (vector). Images were acquired using an Olympus Fluoview FV-1000 confocal coupled to an Olympus 1X81 inverted microscope, a PlanApoN ×60 objective, and FV-10 ASW 2.1 software. A multi-channel scanning configuration with sequential line scanning was set up for acquisition of rhodamine, FITC, and DAPI using HeNe 543 nm laser, 488 nm line of an Argon laser, and 405 diode, respectively. Optimal brightness setting for each channel was configured by determining the HV setting yielding maximal intensity without saturation. Each of the settings was tested against negative control sections to ensure exclusion of non-specific emission. Scanning was performed at a speed of 4 μs/pixel. Images presented are 1 μm single planes. Total sum of image intensity were used to quantify fluorescent staining intensity, using FV-10 ASW 2.1 software. Values for multiple images/sample were averaged.
Tubule isolation
Cortex of excised kidneys was dissected, minced with a razor blade, and subject to digestion with type IA collagenase (1 mg/ml) for 30 min, at 37 °C. The suspension was gently pressed through a 100-μm cell strainer, and the filtrate sequentially passed through an additional 100 μm and a 70-μm cell strainer. Glomeruli were retained on the 70-μm strainer while tubules passed through this strainer. The filtrate was spun at 120×g for 2 min, and purity of the pellet enriched with cortical tubule fragments was examined by visualization under a microscope.
Immunobloting
Equal amount of soluble proteins extracted from isolated tubules or cultured human proximal tubule cells were prepared for electrophoresis on 4–12 % Bis-Tris gels, transferred to nitrocellulose membranes and immunoblotted with anti-GRP78, anti-PDI, and anti-GRP94 (Assay Designs), p58IPK (Cell Signaling Technology), CHOP (Santa Cruz Biotechnology), phospho-PERK Thr 980 and PERK (Cell Signaling), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Millipore; Billerica, MA), or anti-actin (Santa Cruz Biotechnology). After incubation with respective secondary antibodies, proteins were visualized by incubation of membranes with enhanced chemiluminescent substrate (Pierce, Rockford, IL), or scanning on Odyssey Infrared Imager (Licor, Lincoln, NE), for phospho-PERK. Densitometric analysis of immunoblots was performed using Image J. For densitometric analysis, specific protein expression was normalized to densitometry values for β-actin or GAPDH in each lane. For analysis of fibronectin in HK-2 cell culture medium, equal volume of media from each sample was prepared for immunoblotting as above and fibronectin was detected using a monoclonal antibody (Santa Cruz Biotechnology).
TUNEL assay and analysis
In situ apoptosis detection in kidney sections was performed by terminal uridine deoxynucleotidyl transferase (TdT) nick end labeling (TUNEL) assay, using the ApopTag Plus Peroxidase Apoptosis detection Kit from Chemicon (Millipore). Kidney sections were analyzed for positively stained tubule cells by an observer blinded to the identity of the slides. Positively stained tubule cells on the outer edges of the sections and unattached cells in the tubule lumen were omitted from the analysis. Numerous, random visual fields were observed under a ×40 objective. To normalize values between samples, the number of positively stained tubule cells per section was divided by the number of visual fields observed for that section. Kidney sections from four different mice in each group were used for this analysis.
Tubule cell culture and transfections
HK-2 cells (human proximal tubule cell line) were obtained from American Type Culture Collection (Manassas, VA) and grown in keratinocyte serum-free media supplemented with 0.05 mg/ml bovine pituitary extract and 5 ng/ml recombinant epidermal growth factor. For small interfering RNA (siRNA) experiments, cells were plated to 65 % confluency in 12-well plates and subject to transfection with either 20 pmol control siRNA or PERK siRNA (Santa Cruz Biotechnology) for 48 h, using Lipofectamine 2000 (Invitrogen). HK-11 cells (human proximal tubule cell line) were obtained from Dr. Racusen (Racusen et al. 1997). Cells were cultured in Dulbecco’s modified Eagle’s medium/F-12 (Invitrogen) supplemented with 5 % fetal calf serum. For overexpression of CHOP, cells were grown to 85 % confluence and subject to transfection with 2 μg CHOP DNA (Origene, Rockville, MD) or vector control for 24 h, using Lipofectamine 2000.
Statistical analysis
Statistical analysis of TUNEL-positive cells and immunohistochemistry data between groups was performed using two-tailed, unpaired t test. P values <0.05 were considered significant. Statistical analysis of immunoblot data for all OVE26 and FVB groups and proximal tubule cells was performed using ANOVA, followed by post-hoc analysis to compare between two different experimental groups with two-tailed, unpaired t test, using GraphPad software and Microsoft Excel.
Results
General mouse characteristics
General characteristics of young (2 months) and older (6–7 months) non-diabetic and diabetic mice are presented in Table 1. As previously reported (Zheng et al. 2004), OVE26 diabetic mice used in this study have albuminuria by 2 months of age.
Table 1.
Physiologic parameters of mice
| Body weight (g) | Kidney weight (g) | Kidney weight/body weight | UAE (μg/24 h) | |
|---|---|---|---|---|
| 2 months | ||||
| FVB | 17.6 ± 0.9 | 0.133 ± 0.01 | 0.0076 ± 0.0005 | 103 ± 12.3 |
| OVE26 | 18.1 ± 0.9 | 0.21 ± 0.01* | 0.0117 ± 0.0005* | 255 ± 29.7* |
| 6 months | ||||
| FVB | 21.92 ± 1.8 | 0.191 ± 0.003‡ | 0.009 ± 0.0007 | 99 ± 6.6 |
| OVE26 | 20.5 ± 1.03 | 0.411 ± 0.03*, † | 0.020 ± 0.002*, † | 54,380 ± 12,661 *, † |
n = 8, 2-month-old and n = 6, 6-month-old mice used for kidney/body weight measurement; n = 7, FVB and n = 12 OVE26, 2-month old for UAE; n = 8 FVB and OVE26, 6-month-old for UAE
*P < 0.05 vs. FVB in the same age group; † P < 0.05 vs. 2-month-old OVE26; ‡ P < 0.05 vs. 2-month-old FVB
Differential induction of ER-resident chaperones in tubules of diabetic mice
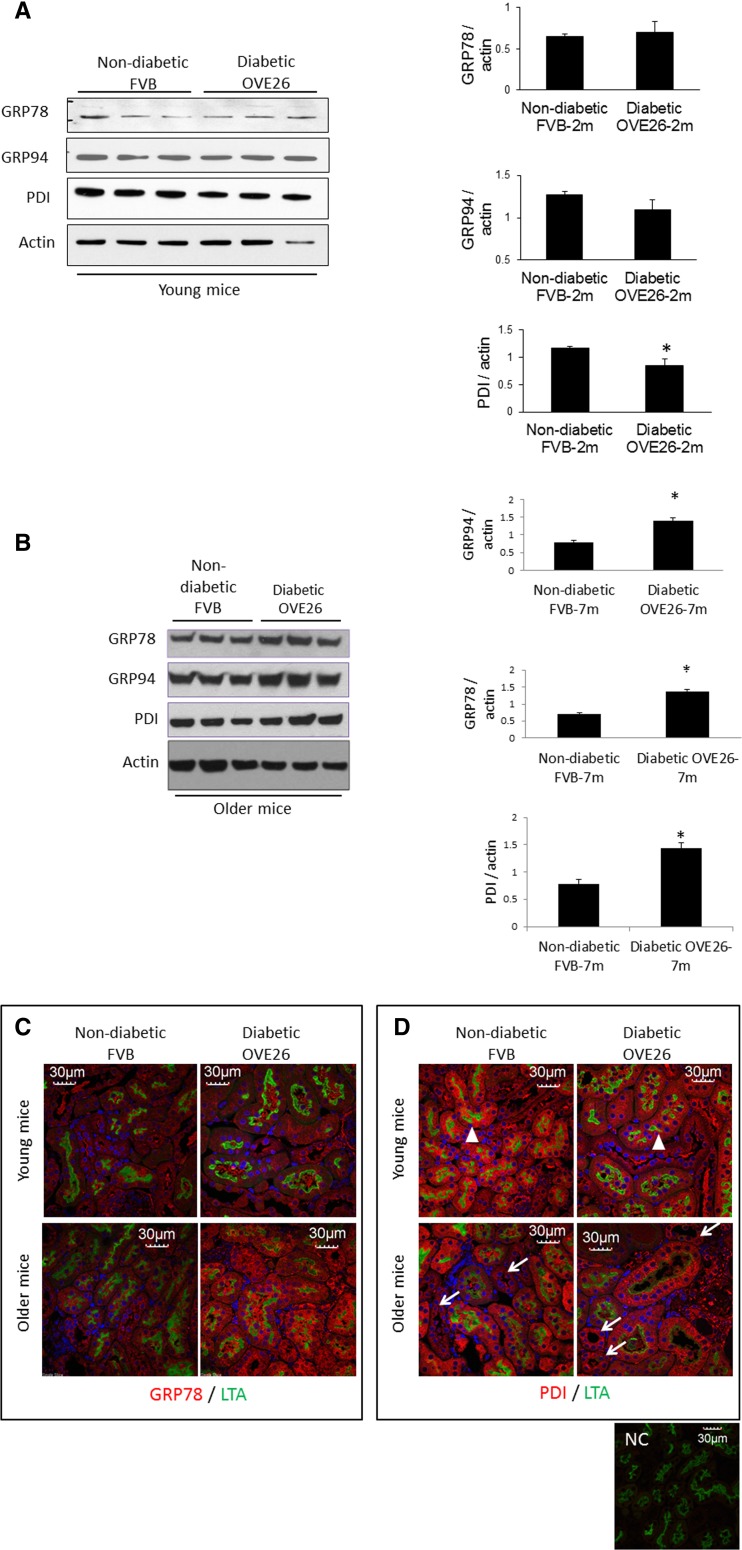
Upregulation of ER chaperone proteins in ER stress response supports folding of increased unfolded protein load to the ER. Expression of the ER stress sensor and chaperone GRP78 as well as GRP94 and, protein disulfide isomerase (PDI), was determined in isolated renal cortical tubules of young and older diabetic and non-diabetic mice. As shown in Fig. 1a, expression of GRP78 and GRP94 was unaltered while PDI was decreased in tubules of younger OVE26 diabetic mice, compared with age-matched FVB non-diabetic mice. Expression of all three ER chaperones was increased in tubules of older diabetic mice, compared with age-matched non-diabetic mice (Fig. 1b). To identify the specific tubule segments involved in altered chaperone protein expression, kidney sections from all mouse groups were immunofluorescence stained for GRP78 or PDI and stained with FITC-conjugated LTA to stain and identify proximal tubules (Barresi et al. 1988). As shown in Fig. 1c, GRP78 is upregulated in all tubule segments of older diabetic mice. Decreased PDI expression in tubules of young diabetic mice appears to occur mostly in LTA-positive proximal tubules (white arrowheads, Fig. 1d), while in older diabetic mice, PDI was increased more in distal, LTA-negative tubules (white arrows, Fig. 1d). Therefore, GRP78 and PDI are regulated differently in proximal and distal tubules during the course of diabetes.
Fig. 1.
Differential expression of ER-resident chaperones in tubules of diabetic mice. Expression of GRP78, GRP94, and PDI protein was analyzed in isolated cortical tubules from OVE26 diabetic and FVB non-diabetic mice. a, b Immunoblot of ER-resident chaperones in isolated tubules of young and older and 2- and 7-month-old mice, respectively. Bar graphs, densitometric quantitation of chaperone proteins normalized to actin (n = 6/group, 2- and 7-month old), average ± SE,*P < 0.05 OVE26 vs. FVB. c, d Immunofluorescence staining of kidney sections from young and older mice for GRP78 or PDI (red) and LTA staining (green). White arrows, non-LTA-stained tubules. White arrowheads, LTA-stained tubules to mark proximal tubules (images representative of six mice/group). NC negative control for staining
Early signaling of the endoplasmic reticulum stress response is induced in renal tubules of young diabetic mice
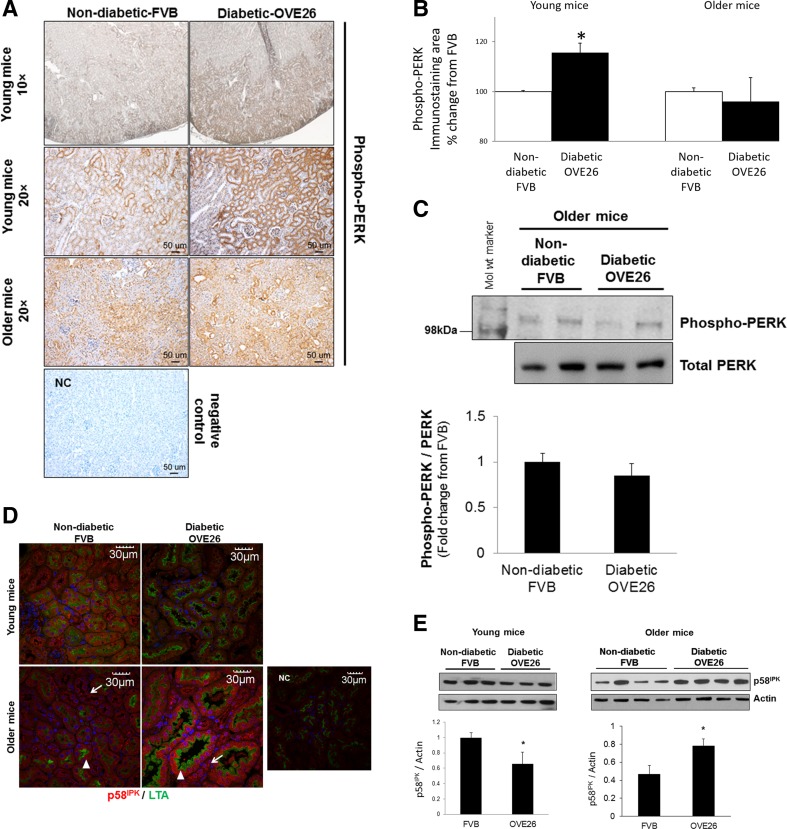
We next determined if signal transduction of the ER stress response is altered in renal tubules of young and older OVE26 diabetic mice, or in age groups with mild to high proteinuria. Stress-induced PERK autophosphorylation, the most proximal event in ER stress signal transduction which leads to global protein translation arrest, was analyzed by immunohistochemistry of kidneys from age-matched mice in both groups. During stress, GRP78 dissociates from PERK, allowing it to dimerize and autophosphorylate on Thr 980 residue (Wang and Kaufman 2012). As shown in Fig. 2a (top and second rows), PERK phosphorylation was higher in the renal cortex of young diabetic mice compared with age-matched non-diabetic mice. Figure 2b represents quantitative analysis of phospho-PERK staining in sections from young and older mice. Cortical phospho-PERK was not different between older diabetic animals and age-matched controls (Fig. 2a (3rd row), b). To further confirm immunohistochemistry findings in the older mouse groups, phospho-PERK was also analyzed by immunoblot of isolated cortical tubules. As shown in Fig. 2c, PERK phosphorylation was not different between tubules of older control and diabetic mice. These findings demonstrate activation of the earliest ER stress-response signaling event in tubules of young OVE26 diabetic mice with mild proteinuria.
Fig. 2.
PERK phosphorylation and p58IPK expression in renal cortex and tubules of mice. a Immunohistochemistry of phospho-Thr 980 PERK in kidney sections from non-diabetic FVB and diabetic OVE26 mice. Top row, ×10 magnification, and all other images are ×20 magnification of cortex from young and older mice in each group. Images are representative of immunostained kidney sections in each group at each age. NC negative control for staining. b Immunostaining area analysis of phosphorylated PERK. *P < 0.05 compared with control group; n = 5. c Immunoblot of phospho-PERK Thr-980 in isolated tubules from older FVB and OVE26 mice. Far left lane, molecular weight marker. Data in bar graphs are densitometric quantitation of phospho-PERK normalized to PERK for each mouse and presented as fold change from FVB group, ±SEM, n = 5. d Immunofluorescence staining of kidney sections from young and older mice for p58IPK (red) and LTA staining (green). White arrows, non-LTA-stained tubules. White arrowheads, LTA-stained tubules to mark proximal tubules. Images representative of six mice/group. NC negative control for staining. e Immunoblot of p58IPK in isolated cortical tubules from young and older FVB non-diabetic and OVE26 diabetic mice. Bar graphs, densitometric quantitation of p58IPK normalized to actin. Data are average ± SE, *P < 0.05 (n = 5)
Since PERK phosphorylation was regulated with diabetes, we next analyzed expression of its inhibitor, p58IPK, an ER-resident chaperone induced by the ATF-6 arm of the ER stress response to reverse stress-induced translation repression (Yan et al. 2002). Kidney sections from young and older mice were immunostained for p58IPK and stained with FITC-LTA. As shown in Fig. 2d, p58IPK is downregulated in LTA-positive (proximal) and LTA-negative (distal and cortical collecting) tubules of young diabetic mice and uniformly increased across these tubule subtypes in older diabetic mice, compared with age-matched non-diabetic mice. Expression changes of p58IPK in tubules of young and older diabetic mice were also confirmed by immunoblot analysis of isolated cortical tubules (Fig. 2e). The data suggest activation of the ATF-6 arm of the ER stress response in older diabetic mice.
siRNA knockdown of PERK increases proximal tubule cell fibronectin secretion
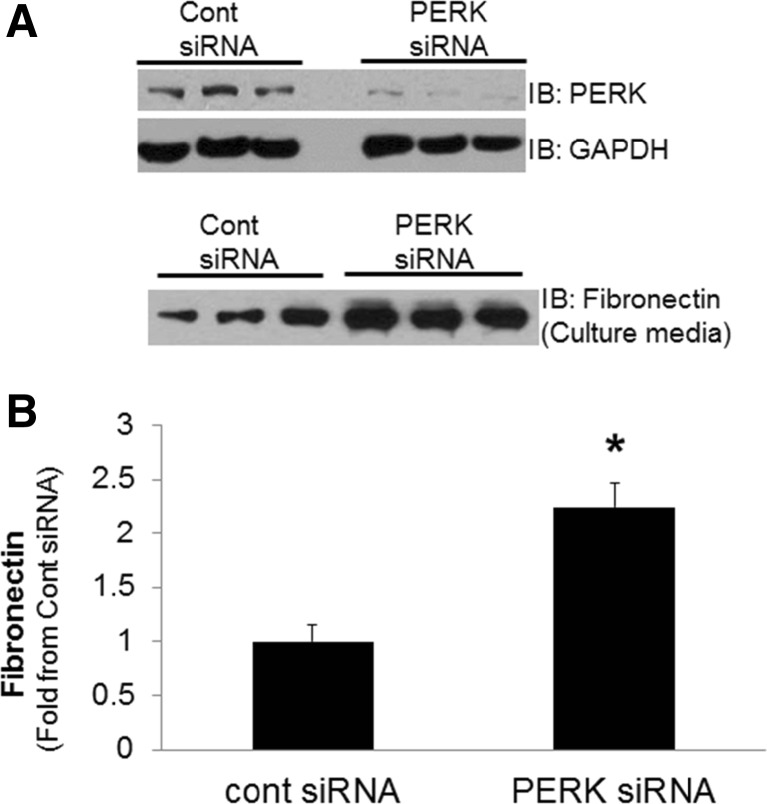
Induction of tubule PERK phosphorylation early in diabetes, suggests launch of protective mechanisms in tubule cells. To determine if PERK is protective to tubule cell responses relevant to diabetic nephropathy, siRNA knockdown of PERK in cultured proximal tubule cells was utilized. Since tubule cells synthesize and secrete more extracellular matrix proteins in diabetes, the following studies were aimed at determining the specific role of PERK on tubule cell fibronectin secretion. Human proximal tubule cells (HK-2 cells) were transfected with PERK siRNA or control siRNA for 48 h. Fibronectin in the cell culture media was determined by immunoblot analysis, representing secreted fibronectin. As shown in Fig. 3a, PERK siRNA significantly reduced PERK protein expression in HK-2 cells (Fig. 3a) and increased fibronectin secretion by proximal tubule cells (Fig. 3b).
Fig. 3.
siRNA knockdown of PERK in proximal tubule cells. Human proximal tubule cells were transfected with control or PERK siRNA for 48 h. a Cell culture media subject to immunoblot analysis for expression of fibronectin and cell lysate for PERK and GAPDH to validate knockdown of PERK expression. b Densitometric quantitation of fibronectin immunoblots in (a) (n = 7–11/experimental condition), presented as fold change from control siRNA. Data are average ± SEM/condition, *P < 0.05 compared with control siRNA
Induction of CHOP in all tubules subtypes of diabetic mice
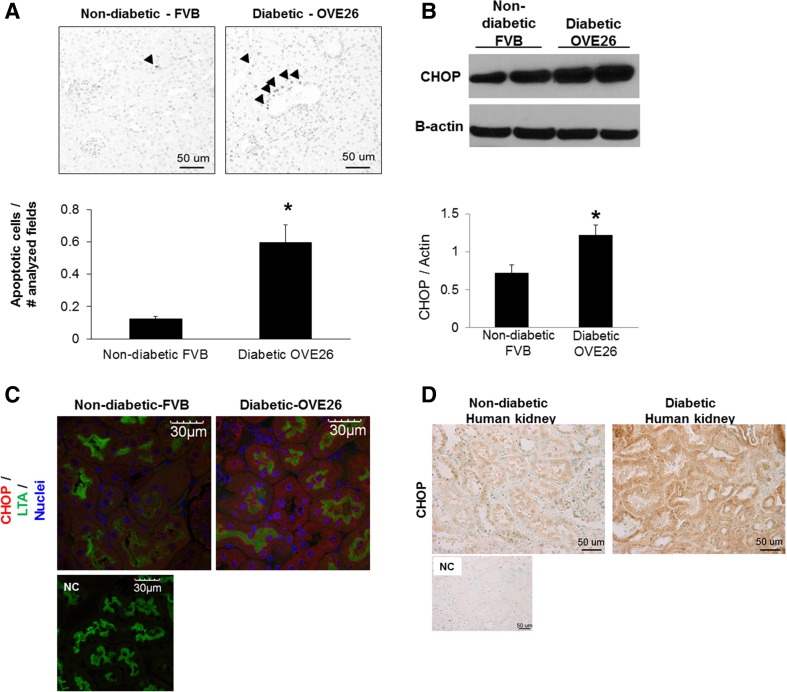
Death occurs in cells undergoing persistent ER stress (Xu et al. 2005) and is evident in renal tubule cells of patients with DN and diabetic animals (Brezniceanu et al. 2007; Verzola et al. 2007). To determine if ER stress response in tubules of OVE26 diabetic mice is associated with apoptosis, apoptosis in general was analyzed in kidneys of OVE26 diabetic mice by TUNEL assay. We consistently observed more TUNEL-positive tubule cells in kidneys of older diabetic mice compared with control (Fig. 4a). We next determined if expression of the pro-apoptotic transcription factor C/EBP homologous protein (CHOP) that is upregulated by activation of PERK/eIF2α, IRE-1, and ATF6 during ER stress, was regulated in tubules of the older diabetic mice. As shown in Fig. 4b, CHOP was upregulated in isolated cortical tubules from older OVE26 mice. Immunofluorescence staining of kidney sections for CHOP and staining with LTA shows CHOP expression is increased in tubules of nearly all portions of the nephron in these older diabetic mice (Fig. 4c). The presence of markers for or role for ER stress-induced apoptosis specifically in renal tubules of patients with DN remains unclear. Since CHOP protein is stabilized during persistent ER stress (Rutkowski et al. 2006), we next determined tubule CHOP protein expression in biopsies of diabetic patients and renal specimens from non-diabetic control subjects (Table 2), by immunohistochemistry. As shown in Fig. 4d, renal tissue from non-diabetic subjects shows ubiquitously low levels of CHOP protein while expression is increased in tubules of diabetic patients.
Fig. 4.
Renal tubule apoptosis analyzed by TUNEL assay and expression of CHOP in tubules. Representative TUNEL staining from older (6-month-old) non-diabetic FVB and diabetic OVE26 mouse kidney sections. Arrowheads indicate tubule cells with positive TUNEL staining. Magnification, ×40. Bar graph, quantitative analysis of apoptotic tubule cells by counting cells in random visual fields in the cortex under a ×40 objective. Values normalized between samples by dividing number of positively stained tubule cells by number of visual fields observed/section. Data are expressed as average ± SE, n = 6. *P < 0.05 vs. non-diabetic. b Immunoblot of CHOP protein in isolated cortical tubules from 7-month-old mice (n = 6). Densitometric quantitation of CHOP normalized to actin. Data are average ± SE, *P < 0.05 vs. non-diabetic FVB. c Immunofluorescence staining of kidney sections from older 7-month-old diabetic and control mice for CHOP (red) and staining with LTA (green), to mark proximal tubules (representative image, of n = 6). NC negative control for staining (see “Methods”). Scale bar, 30 μm. d Immunohistochemistry of CHOP in biopsy samples of patients with diabetic nephropathy and renal specimens from non-diabetic control subjects. Images are representative of biopsies from eight and five different diabetic and control patients, respectively. NC negative control for staining (see “Methods”)
Table 2.
Characteristics of donors for Human kidney biopsies
| Group | Gender | Age (year) | SCr (mg/dl) | HTN (Y/N) | Diabetes type | Retinopathy | Proteinuria (g) | Misc. |
|---|---|---|---|---|---|---|---|---|
| D-1 | M | 54 | na | Y | 2 | na | 2.7 | Obese |
| D-2 | M | 63 | 1.5 | Y | na | N | 1.5 | Obese |
| D-3 | F | 58 | 1.11 | Y | 2 | Y | na | Obese; ANCA1:640 |
| D-4 | M | 48 | 1.25 | Y | na | na | 6.5 | Solitary kidney |
| D-5 | M | 81 | 1 | Controlled | N | 3.3 | ||
| D-6 | M | 65 | na | Y | na | na | Nephrotic | ARF |
| D-7 | M | 15 | na | Y | 1 | na | na | Prot/Cr = 0.75 |
| D-8 | M | 18 | na | na | na | na | na | Prot/Cr = 2 |
| ND-1 | M | 54 | na | N | na | na | ||
| ND-2 | F | 59 | na | N | na | na | ||
| ND-3 | M | 30 | na | N | na | na | ||
| ND-4 | na | na | Normal | N | na | N | ||
| ND-5 | na | na | Normal | N | na | N |
D-1 through D-8 diabetic, ND-1 through ND-5 non-diabetic control, SCr serum creatinine, HTN hypertension, ARF acute renal failure, M male, F female, g grams; prot/Cr protein/creatinine, na not available, Y yes, N no
Severity of proteinuria does not augment CHOP induction in tubules of diabetic mice
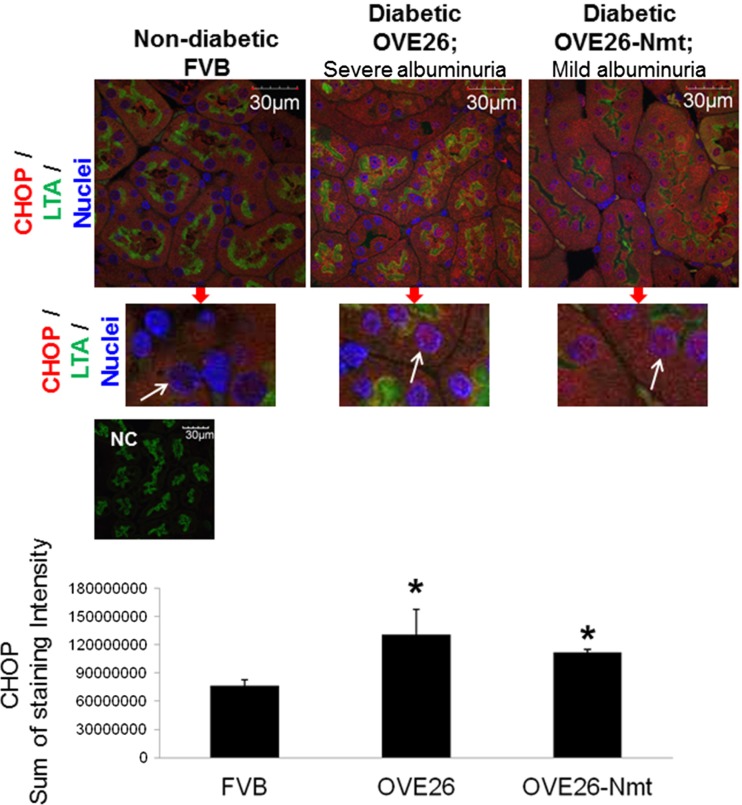
A previous study showed that high protein concentrations augment high glucose-induced GRP78 transcription in cultured proximal tubule cells (Lindenmeyer et al. 2008). In the current study, CHOP was upregulated in tubules of older diabetic mice with severe proteinuria. To determine whether excessive proteinuria plays a role in this induction, CHOP expression was analyzed in kidney sections from diabetic OVE26 and OVE26-Nmt7 mice. The OVE26-Nmt7 mice are a cross of OVE26 diabetic mice with Nmt transgenic mice which have podocyte-specific expression of metallothionein (Zheng et al. 2008). The OVE26-Nmt7 mice are similar to OVE26 diabetic mice in developing diabetes but exhibit much less podocyte and glomerular damage. For this set of studies, we used 4-month-old mice in each group since OVE26-Nmt7 diabetic mice have a fourfold reduction in albuminuria, compared with OVE26 mice at this age (Zheng et al. 2008). Average urinary albumin excretion of OVE-Nmt7 mice used for this study was 722.7 ± 192 μg/24 h, compared with 12,410.3 ± 4498 in 4-month-old OVE26 diabetic mice. As shown in Fig. 5, CHOP expression is higher and is distributed to both the cytoplasm and nuclei (white arrows) of tubules from both diabetic groups compared with tubules of non-diabetic mice, where CHOP is localized to the cytoplasm. Furthermore, CHOP expression is not different between severely proteinuric OVE26 and OVE26-Nmt mice with significantly less proteinuria (Fig. 5).
Fig. 5.
Severe proteinuria does not augment CHOP expression in tubules of diabetic mice. Immunofluorescence of kidney sections from 4-month-old non-diabetic FVB and diabetic OVE26 and OVE26-Nmt for CHOP (red) and LTA (green) to mark proximal tubules. Nuclei stained with DAPI (blue). Portions of images cropped and enlarged with arrows pointing to nuclei to show CHOP localization (red) in nuclei of tubules from diabetic mice. CHOP is excluded from tubule nuclei of FVB control mice. Bar graph, quantitation of CHOP immunofluorescence staining. Data are average/group ± SEM. Sum of total intensity values/image was averaged for each mouse and then/group. *P < 0.05 compared with FVB non-diabetic mice. Scale bar, 30 μm. NC negative control for staining
Overexpression of CHOP in proximal tubule cells induces expression of fibronectin
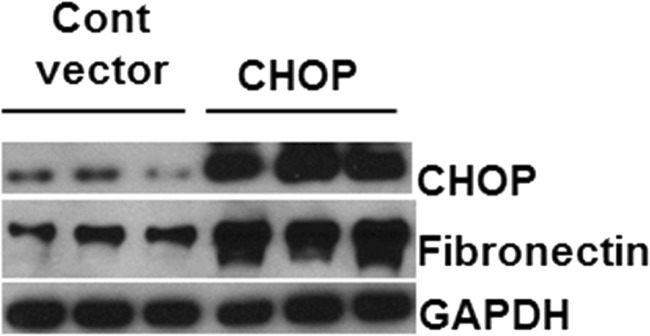
As shown in Figs. 4c and 5, expression of CHOP is increased by diabetes and localizes to the nucleus in the majority of tubule cells examined by immunofluorescence. However, while TUNEL analysis showed increased apoptosis in tubule cells of older diabetic mice, the majority of cells in each visual field were not apoptotic. Therefore, we next determined whether CHOP is involved in tubule cell responses associated with diabetes, other than apoptosis, such as induction of matrix proteins. CHOP was over-expressed in HK11 cells (human proximal tubule cells). Figure 6 shows marked overexpression of CHOP in cells transfected for 24 h with human CHOP DNA, compared with vector control. Overexpression of CHOP in tubule cells increased fibronectin expression (Fig. 6).
Fig. 6.
Overexpression of CHOP in proximal tubule cells. Human proximal tubule cells were transfected with control vector or vector containing human CHOP for 24 h. Cell lysate subject to immunoblot analysis for expression of CHOP to validate overexpression, fibronectin, and GAPDH
Discussion
This study shows temporal activation of ER stress-response transducer PERK early in the course of diabetes, and the first to show differential regulation of ER-resident chaperone proteins in different tubule subtypes during the course of diabetes in type I diabetic mice. Expression of CHOP protein was found to be ubiquitously increased in tubules of diabetic mice and humans and not altered by severity of proteinuria. Most importantly, this study identified novel roles for PERK and CHOP in regulation of tubule cell fibronectin production, implicating direct roles for an ER stress-response transducer and effector in matrix protein synthesis and secretion, a process significant to DN.
The results demonstrated differential regulation of ER chaperone proteins in different tubule subtypes. Expression of GRP78, GRP94, and PDI was increased in tubules of older diabetic mice, suggestive of a state of stress adaptation. For GRP78, the findings are similar to and supported by previous reports in tubules of 5-month-old db/db type II diabetic mice (Brezniceanu et al. 2010). Induction of GRP78 in cultured proximal tubule cells with pharmacologic ER stress inducers protects cells to additional toxic insults (Asmellash et al. 2005; Liu et al. 1997). In vivo, age-associated, and chronic protein overload-associated tubulointerstitial lesions are increased and accelerated, respectively, in a knock-in mouse model-expressing mutant, dysfunctional GRP78 (Kimura et al. 2008). The current study is the first report of PDI and GRP94 protein changes in tubules or kidneys during diabetes. The specific role for induction of these chaperone proteins to tubule cell function remains to be defined. In the ER, PDI is an oxidoreductase chaperone catalyzing formation, rearrangement, and breakdown of disulfide bonds (Laurindo et al. 2012). Potential role for increased PDI expression in distal tubule segments may be compensatory to diabetes-induced alterations in antioxidative mechanisms, specifically in these tubule segments (Advani et al. 2009), since cellular oxidative stress can alter ER oxidoreductase activity (Santos et al. 2009).
The cause for decreased expression of PDI in tubules of 2-month-old diabetic mice is not known; however, ER chaperones can be downregulated during ER stress. For example, ER chaperones can localize to the cell surface (Ni et al. 2011) or be subject to stress-induced degradation. The ER stress-inducer thapsigargin causes calpain-mediated GRP94 cleavage in LLC-PK1 proximal tubule cells (Muruganandan and Cribb 2006), and high glucose concentrations can increase calpain activity in these cells (Harwood et al. 2007). Calpain and cathepsin-mediated degradation of GRP78 occurs following deoxynivalenol-induced ER stress in macrophages (Shi et al. 2009). Thapsigargin also reduces GRP78 in INS-1E pancreatic beta cells by a combination of decreased synthesis and increased degradation (Rosengren et al. 2012). The effect of decreased tubule PDI expression in the early stages of diabetes on tubule cell dysfunction and/or loss in DN remains to be defined. Diabetes increases free sulfhydryl groups of PDI and decreases oxidoreductase activity in mouse liver cells (Nardai et al. 2005). Decreased PDI expression may decrease overall ER oxidoreductase activity and protein folding in tubules.
Phosphorylation of PERK was higher in renal cortical tubules of younger diabetic mice suggesting adaptive mechanisms by tubule cells to ER stress-inducing stimuli, such as high glucose concentrations (Lindenmeyer et al. 2008). Unaltered PERK phosphorylation in tubules of older (6–7-month-old) diabetic mice correlated with increased p58IPK and suggests induction of negative feedback mechanisms and activation of the ATF-6 arm of ER stress response. Activation of the ATF-6 pathway is significant to stress adaptation and maintenance of ER functional capacity in the setting of chronic stress, as ATF-6 null cells are more sensitive to repeated or long-term stress (Wu et al. 2007). In the ER lumen, p58IPK interacts with PERK to inhibit its activity and functions as a co-chaperone for folding of newly translated proteins (Rutkowski et al. 2007). By regulating PERK, its induction is significant to recovery from ER stress and resumption of global protein synthesis. Haploinsufficiency for p58IPK exacerbates renal injury in mice transgenic for folding mutants of carbonic anhydrase IV (Datta et al. 2010), suggesting promotion of cell injury with decreased p58IPK expression. Whether decreased p58IPK in tubules of younger diabetic mice is part of stress-response PERK activation or has other functional consequences is not known.
Tubulointerstitial fibrosis resulting from accumulation of extracellular matrix proteins is significant to progression of renal injury in DN (Brosius 2008). Increased fibronectin secretion by tubule cells following siRNA knockdown of PERK is a novel finding and suggests that signaling downstream of PERK activation may regulate extracellular matrix protein secretion. Furthermore, this may simply reflect one of several roles that PERK can play in tubule cell stress response. The in vivo relevance of PERK knockdown on tubule function in diabetes remains to be determined. Since induction of the ER stress response is shown to be protective in tubule cells (Asmellash et al. 2005; Liu et al. 1997), the in vitro results suggest that increased PERK activation early in diabetes may counteract pro-fibrotic signaling associated with diabetes, whereas decreased or unaltered PERK activation later in diabetes may contribute to increased fibronectin expression in tubulointerstitium of OVE26 mice ≥6 months of age, as previously reported by our group (Powell et al. 2009).
The significance of CHOP to renal injury is shown by CHOP knockout mice having lower albuminuria during STZ diabetes (Wu et al. 2010a, b) and less renal toxicity and tubule apoptosis following treatment with tunicamycin (Marciniak et al. 2004; Zinszner et al. 1998). Compared with other reports of CHOP in kidneys during diabetes (Brezniceanu et al. 2010; Liu et al. 2008), this is the first report of increased CHOP protein specifically in proximal and distal tubule subtypes of a mouse model of type I DM. Lack of apoptosis in numerous tubule cells of diabetic mice expressing CHOP suggests that continued induction of ER chaperones in these cells likely allows stress adaptive pathways to alleviate some stress in the chronic setting of diabetes and suppress pro-apoptotic pathways and stress-induced apoptosis (Rutkowski et al. 2006; Rutkowski and Kaufman 2007; Wu et al. 2007). Previously, no change in CHOP transcript levels was found in tubulointerstitial tissue from kidney biopsies of patients with established DN (Lindenmeyer et al. 2008). Results of the current study suggest the possibility for increased CHOP protein stabilization, which occurs during stress (Rutkowski et al. 2006). In addition, transcript and protein levels of CHOP may be differentially regulated by the degree of nephropathy, such that more advanced nephropathy may result in loss of cells that have already undergone ER stress-induced apoptosis. In the current study, tubule CHOP protein was not different between age-matched severely proteinuric OVE26 and mildly proteinuric OVE26-Nmt diabetic mice. High glucose, humoral and hormonal factors, growth factors, and cytokines, even prior to the onset of proteinuria during diabetes, could cause maximal induction of CHOP. These data do not suggest CHOP induction is independent of albuminuria, since previous reports clearly show the ability of albumin to induce ER stress and CHOP in cultured tubule cells and animal models of albumin overload and proteinuric nephropathy (Brezniceanu et al. 2010; Lindenmeyer et al. 2008; Ohse et al. 2006; Wu et al. 2010a, b). Alternatively, the mild proteinuria in OVE26-Nmt diabetic mice may be sufficient for maximal stress-response induction. The ability of CHOP to induce fibronectin expression in tubule cells implicates a novel role for CHOP in tubule cell biology and promotion of tubulointerstitial fibrosis in diabetic nephropathy, and may explain why CHOP protein expression remains high in non-apoptotic tubule cells during diabetes.
In conclusion, this study demonstrates that ER-resident chaperones are regulated differently in proximal and distal tubule subtypes during diabetes. Furthermore, novel roles for the ER stress-response transducer PERK and a downstream effector of its activation, CHOP, in tubule cell production of fibronectin has been identified. Together, these studies provide a more direct role for the ER stress-response effectors in progression of tubulointerstitial fibrosis and development of DN.
Acknowledgment
B.D.K. current affiliation: Indiana University Purdue University Indianapolis School of Informatics.
Grants
This work was supported by National Institutes of Health (NIH) grant K01-DK080951 (M.T.B.). J.B.K. was supported by a Department of Energy grant and the Kentucky Research Challenge Trust. M.J.R. was supported by NIH R01-075212, D.W.P. was supported by NIH R21-AI103980, and L.C. was supported by NIH 1R01DK091338.
Footnotes
Madhavi J. Rane and Jon B. Klein are equal contributing senior authors.
References
- Advani A, Gilbert RE, Thai K, Gow RM, Langham RG, Cox AJ, Connelly KA, Zhang Y, Herzenberg AM, Christensen PK, Pollock CA, Qi W, Tan SM, Parving HH, Kelly DJ. Expression, localization, and function of the thioredoxin system in diabetic nephropathy. J Am Soc Nephrol. 2009;20:730–741. doi: 10.1681/ASN.2008020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmellash S, Stevens JL, Ichimura T. Modulating the endoplasmic reticulum stress response with trans-4,5-dihydroxy-1,2-dithiane prevents chemically induced renal injury in vivo. Toxicol Sci. 2005;88:576–584. doi: 10.1093/toxsci/kfi303. [DOI] [PubMed] [Google Scholar]
- Barresi G, Tuccari G, Arena F. Peanut and Lotus tetragonolobus binding sites in human kidney from congenital nephrotic syndrome of Finnish type. Histochemistry. 1988;89:117–120. doi: 10.1007/BF00489914. [DOI] [PubMed] [Google Scholar]
- Brezniceanu ML, Liu F, Wei CC, Tran S, Sachetelli S, Zhang SL, Guo DF, Filep JG, Ingelfinger JR, Chan JS. Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int. 2007;71:912–923. doi: 10.1038/sj.ki.5002188. [DOI] [PubMed] [Google Scholar]
- Brezniceanu ML, Lau CJ, Godin N, Chénier I, Duclos A, Ethier J, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy. J Am Soc Nephrol. 2010;21:943–954. doi: 10.1681/ASN.2009030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius FC., 3rd New insights into the mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev Endocr Metab Disord. 2008;9:245–254. doi: 10.1007/s11154-008-9100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson EC, Audette JL, Klevay LM, Nguyen H, Epstein PN. Ultrastructural and functional analyses of nephropathy in calmodulin-induced diabetic transgenic mice. Anat Rec. 1997;247:9–19. doi: 10.1002/(SICI)1097-0185(199701)247:1<9::AID-AR2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Datta R, Shah GN, Rubbelke TS, Waheed A, Rauchman M, Goodman AG, Katze MG, Sly WS. Progressive renal injury from transgenic expression of human carbonic anhydrase IV folding mutants is enhanced by deficiency of p58IPK. Proc Natl Acad Sci U S A. 2010;107:6448–6452. doi: 10.1073/pnas.1001905107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood SM, Allen DA, Raftery MJ, Yaqoob MM. High glucose initiates calpain-induced necrosis before apoptosis in LLC-PK1 cells. Kidney Int. 2007;71:655–663. doi: 10.1038/sj.ki.5002106. [DOI] [PubMed] [Google Scholar]
- Hryciw DH, Lee EM, Pollock CA, Poronnik P. Molecular changes in proximal tubule function in diabetes mellitus. Clin Exp Pharmacol Physiol. 2004;31:372–379. doi: 10.1111/j.1440-1681.2004.04001.x. [DOI] [PubMed] [Google Scholar]
- Kimura K, Jin H, Ogawa M, Aoe T. Dysfunction of the ER chaperone BiP accelerates the renal tubular injury. Biochem Biophys Res Commun. 2008;366:1048–1053. doi: 10.1016/j.bbrc.2007.12.098. [DOI] [PubMed] [Google Scholar]
- Laurindo FR, Pescatore LA, Fernandes Dde C. Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radic Biol Med. 2012;52:1954–1969. doi: 10.1016/j.freeradbiomed.2012.02.037. [DOI] [PubMed] [Google Scholar]
- Lindenmeyer MT, Rastaldi MP, Ikehata M, Neusser MA, Kretzler M, Cohen CD, Schlöndorff D. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J Am Soc Nephrol. 2008;19:2225–2236. doi: 10.1681/ASN.2007121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Bowes RC, 3rd, van de Water B, Sillence C, Nagelkerke JF, Stevens JL. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J Biol Chem. 1997;272:21751–21759. doi: 10.1074/jbc.272.35.21751. [DOI] [PubMed] [Google Scholar]
- Liu G, Sun Y, Li Z, Song T, Wang H, Zhang Y, Ge Z. Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem Biophys Res Commun. 2008;370:651–656. doi: 10.1016/j.bbrc.2008.04.031. [DOI] [PubMed] [Google Scholar]
- Magri CJ, Fava S. The role of tubular injury in diabetic nephropathy. Eur J Intern Med. 2009;20:551–555. doi: 10.1016/j.ejim.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruganandan S, Cribb AE. Calpain-induced endoplasmic reticulum stress and cell death following cytotoxic damage to renal cells. Toxicol Sci. 2006;94:118–128. doi: 10.1093/toxsci/kfl084. [DOI] [PubMed] [Google Scholar]
- Nardai G, Stadler K, Papp E, Korcsmáros T, Jakus J, Csermely P. Diabetic changes in the redox status of the microsomal protein folding machinery. Biochem Biophys Res Commun. 2005;334:787–795. doi: 10.1016/j.bbrc.2005.06.172. [DOI] [PubMed] [Google Scholar]
- Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011;434:181–188. doi: 10.1042/BJ20101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohse T, Inagi R, Tanaka T, Ota T, Miyata T, Kojima I, Ingelfinger JR, Ogawa S, Fujita T, Nangaku M. Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int. 2006;70:1447–1455. doi: 10.1038/sj.ki.5001704. [DOI] [PubMed] [Google Scholar]
- Powell DW, Bertram CC, Cummins TD, Barati MT, Zheng S, Epstein PN, Klein JB. Renal tubulointerstitial fibrosis in OVE26 type 1 diabetic mice. Nephron Exp Nephrol. 2009;111:e11–e19. doi: 10.1159/000178763. [DOI] [PubMed] [Google Scholar]
- Racusen LC, Monteil C, Sgrignoli A, Lucskay M, Marouillat S, Rhim JG, Morin JP. J Lab Clin Med. 1997;129:318–329. doi: 10.1016/S0022-2143(97)90180-3. [DOI] [PubMed] [Google Scholar]
- Rosengren V, Johansson H, Lehtiö J, Fransson L, Sjöholm A, Ortsäter H. Thapsigargin down-regulates protein levels of GRP78/BiP in INS-1E cells. J Cell Biochem. 2012;113:1635–1644. doi: 10.1002/jcb.24032. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007;32:469–476. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Kang SW, Goodman AG, Garrison JL, Taunton J, Katze MG, Kaufman RJ, Hegde RS. The role of p58IPK in protecting the stressed endoplasmic reticulum. Mol Biol Cell. 2007;18:3681–3691. doi: 10.1091/mbc.E07-03-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Niño MD, Benito-Martin A, Ortiz A. New paradigms in cell death in human diabetic nephropathy. Kidney Int. 2010;78:737–744. doi: 10.1038/ki.2010.270. [DOI] [PubMed] [Google Scholar]
- Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- Shi Y, Porter K, Parameswaran N, Bae HK, Pestka JJ. Role of GRP78/BiP degradation and ER stress in deoxynivalenol-induced interleukin-6 upregulation in the macrophage. Toxicol Sci. 2009;109:247–255. doi: 10.1093/toxsci/kfp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MC, Burns WC, Cooper ME. Tubular changes in early diabetic nephropathy. Adv Chronic Kidney Dis. 2005;12:177–186. doi: 10.1053/j.ackd.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Thongboonkerd V, Barati MT, McLeish KR, Benarafa C, Remold-O’Donnell E, Zheng S, Rovin BH, Pierce WM, Epstein PN, Klein JB. Alterations in the renal elastin-elastase system in type 1 diabetic nephropathy identified by proteomic analysis. J Am Soc Nephrol. 2004;15:650–662. doi: 10.1097/01.ASN.0000115334.65095.9B. [DOI] [PubMed] [Google Scholar]
- Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1009–R1022. doi: 10.1152/ajpregu.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzola D, Gandolfo MT, Ferrario F, Rastaldi MP, Villaggio B, Gianiorio F, Giannoni M, Rimoldi L, Lauria F, Miji M, Deferrari G, Garibotto G. Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int. 2007;72:1262–1272. doi: 10.1038/sj.ki.5002531. [DOI] [PubMed] [Google Scholar]
- Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhang R, Torreggiani M, Ting A, Xiong H, Striker GE, Vlassara H, Zheng F. Induction of diabetes in aged C57B6 mice results in severe nephropathy: an association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am J Pathol. 2010;176:2163–2176. doi: 10.2353/ajpath.2010.090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, He Y, Jing Y, Li K, Zhang J. Albumin overload induces apoptosis in renal tubular epithelial cells through a CHOP-dependent pathway. OMICS. 2010;14:61–73. doi: 10.1089/omi.2009.0073. [DOI] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Frank CL, Korth MJ, Sopher BL, Novoa I, Ron D, Katze MG. Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci U S A. 2002;99:15920–15925. doi: 10.1073/pnas.252341799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Noonan WT, Metreveli NS, Coventry S, Kralik PM, Carlson EC, Epstein PN. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes. 2004;53:3248–3257. doi: 10.2337/diabetes.53.12.3248. [DOI] [PubMed] [Google Scholar]
- Zheng S, Carlson EC, Yang L, Kralik PM, Huang Y, Epstein PN. Podocyte-specific overexpression of the antioxidant metallothionein reduces diabetic nephropathy. J Am Soc Nephrol. 2008;19:2077–2085. doi: 10.1681/ASN.2007080967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang A, Forbes JM. Stress in the kidney is the road to pERdition: is endoplasmic reticulum stress a pathogenic mediator of diabetic nephropathy? J Endocrinol. 2014;222:R97–R111. doi: 10.1530/JOE-13-0517. [DOI] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]