Abstract
During physical activity, increased reactive oxygen species production occurs, which can lead to cell damage and in a decline of individual’s performance and health. The use of omega-3 polyunsaturated fatty acids as a supplement to protect the immune system has been increasing; however, their possible benefit to the anti-oxidant system is not well described. Thus, the aim of this study was to evaluate whether the omega-3 fatty acids (docosahexaenoic acid and eicosapentaenoic acid) can be beneficial to the anti-oxidant system in cultured skeletal muscle cells. C2C12 myocytes were differentiated and treated with either eicosapentaenoic acid or docosahexaenoic acid for 24 h. Superoxide content was quantified using the dihydroethidine oxidation method and superoxide dismutase, catalase, and glutathione peroxidase activity, and expression was quantified. We observed that the docosahexaenoic fatty acids caused an increase in superoxide production. Eicosapentaenoic acid induced catalase activity, while docosahexaenoic acid suppressed superoxide dismutase activity. In addition, we found an increased protein expression of the total manganese superoxide dismutase and catalase enzymes when cells were treated with eicosapentaenoic acid. Taken together, these data indicate that the use of eicosapentaenoic acid may present both acute and chronic benefits; however, the treatment with DHA may not be beneficial to muscle cells.
Keywords: Physical exercise, Skeletal muscle, Oxidative stress, Metabolism, Nutrition
Introduction
Oxidative compounds, such as reactive oxygen species (ROS), have been initially considered as deleterious species to skeletal muscle tissue (Barbieri and Sestili 2012). The predominant source of ROS in skeletal muscle cells is known to be mitochondria (Davies et al. 1982; Koren et al. 1983). Increased mitochondrial ROS generation occurs during various and different situations, such as in the contractile activity of muscle cells (Jackson et al. 2007). Early reports assumed that 2–5 % of the total oxygen consumed by mitochondria may undergo one-electron reduction with the generation of superoxide that can generate other reactive species, like hydrogen peroxide (H2O2) and hydroxyl radical (OH·), which can induce several cell damages (Halliwell and Gutteridge 2007; Loschen et al. 1974).
It is well known that the redox balance occurs in part to avoid oxidative damages and maintain cellular homeostasis and integrity, through a well-controlled balance between ROS production and its elimination by the cellular anti-oxidant systems (Nikolaidis et al. 2012). These anti-oxidant systems can be classified as nonenzymatic and enzymatic. Bilirubin, melatonin, coenzyme Q, uric acid, ascorbic acid, α-tocopherol, β-carotene, flavonoids, and glutathione are examples of nonenzymatic anti-oxidant systems. The enzymatic systems are mainly composed by the enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) (Brieger et al. 2012; Hernandez et al. 2012).
Omega-3 polyunsaturated fatty acids, mainly eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids, are commonly found in oils extracted from fishes from cold regions, such as salmon, tuna, sardines, trout, and mackerel. These fatty acids have been studied for nearly half a century for their beneficial effects on health, including the treatment of cardiovascular diseases and autoimmune diabetes (Martins et al. 2009; Woodman et al. 2003). It has been proposed that omega-3 polyunsaturated fatty acids can be incorporated to the cell membrane, affecting its fluidity, receptor function, enzymatic activity, and production of cytokines and eicosanoids (Nelson 2000). Oral supplementation with omega-3 from fish oil in healthy subjects can decrease the production of some pro-inflammatory cytokines in isolated monocytes, such as tumor necrosis factor, interleukin-1, and interleukin-2 (Martins et al. 2009; Russell and Burgin-Maunder 2012; Wu et al. 1996). The biological effects of these fatty acids are characterized by decreased levels of triglycerides, cholesterol, platelet adhesion, and improved membrane fluidity and vascular endothelium function, associated with the production of anti-inflammatory compounds (Karlsson 1997; Russell and Burgin-Maunder 2012).
Some authors have already suggested that omega-3 polyunsaturated fatty acids can be beneficial to increase the performance in aerobic activities due to its vasodilator property, which may improve O2 and nutrient flows to skeletal muscle tissue during activity (Bucci 1993). There are some indications that omega-3 polyunsaturated fatty acids can play an important role as an anti-oxidant that could lead to several benefits for skeletal muscle, increasing their performance during the exercise or even helping in the recovery period (Attaman et al. 2014; Mickleborough et al. 2008). However, it is important to emphasize that studies showing the possible anti-oxidant role of omega-3 fatty acids (modulating activity or expression of anti-oxidant enzymes) are limited and were not performed using isolated muscle cells (Kyrylenko et al. 2004; Pogozheva et al. 1994). Thus, the aim of this study was to evaluate the effects of the main omega-3 polyunsaturated fatty acids, EPA or DHA, on the anti-oxidant system of skeletal muscle cells.
Materials and methods
C2C12 skeletal muscle cell culture and treatment
Culture of mouse C2C12 skeletal muscle cells was performed according to the method described by Schmitz-Peiffer et al. (1999). C2C12 myocytes were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 20 % fetal bovine serum, 2 mM glutamine, 4.5 mg/mL glucose, and 500 U/mL penicillin-streptomycin, with 95 % O2 and 5 % CO2, at 37 C. After that, cells were placed on six-well plates (150,000 cells per well) and grown to reach 70–80 % of confluence. For differentiation of myocytes into skeletal muscle cells, cells were cultured in DMEM containing 2 % horse serum, 4.5 mg/mL glucose, and 500 U/mL penicillin-streptomycin for 5–7 days. The medium was changed every 2 days. After the complete differentiation, the cells were treated with 100 μM of DHA or EPA (Sigma-Aldrich) freshly prepared before each assay (using ethanol (1:100) as vehicle), for a period of 24 h. This concentration of DHA and EPA was used as previously described by Bryner et al. (2012). Moreover, this concentration did not reduce cells’ viability, as previously reported by Lee et al. (2013), where cells were treated with concentrations between 10 and 500 μM. These authors demonstrated that 100 μM of EPA or DHA treatment, for a period of 24 h, did not reduce cellular viability.
Evaluation of ROS production
To evaluate the effects of omega-3 polyunsaturated fatty acids on skeletal muscle cells, we determined superoxide production using the dihydroethidium (DHE) oxidation method (Fink et al. 2004; Zhao et al. 2003). After 24 h of treatment with 100 μM EPA or DHA freshly prepared before each assay (described above), cells were incubated with 1 μM DHE in DPBS containing 5 mM glucose, for 30 min, at 37 °C. Fluorescent microscopy was used to measure fluorescent signal which was quantified using ImageJ 1.46r (Wayne Rasband, NIH, USA) using two photos per well selected at random. Palmitic acid at 100 μM was used as positive control for superoxide production as shown in previous studies (Lambertucci et al. 2008, 2012).
Determination of the anti-oxidant enzyme activities
For the determination of each anti-oxidant enzyme activity (CAT, SOD, and GPx), after 24 h of incubation with 100 μM of omega-3 polyunsaturated fatty acids (DHA or EPA) freshly prepared before each assay, cells were homogenized in sodium phosphate buffer (10 mM, pH 7.5). After that, homogenates were centrifuged at 10,000g for 1 min (SPIN 1, Incibras, SP, Brazil) and the supernatant was used for the determination of the enzymatic activity by spectrophotometry, as previously described (Aebi 1984; Flohe and Otting 1984; Wendel 1981). Protein quantification (Bradford 1976) and enzyme analysis were always performed in duplicates.
Determination of total SOD activity (EC1.15.1.1)
Total SOD activity was monitored by overall reduction of cytochrome C (0.15 g/L) by superoxide radicals generated by the xanthine-xanthine oxidase system. The measurement was performed at 550 nm and 25 °C (Flohe and Otting 1984). Initially, the background was determined by finding the appropriate amount of buffer and xanthine oxidase to reach an absorbance nearest to 0.025. Then, it was added to each assay, 12 μL of sample, 3 μL of xanthine oxidase, and 185 μL of assay solution (cytochrome C, xanthine, and EDTA in 50 mM phosphate buffer).
Determination of CAT activity (EC1.11.1.6)
CAT activity was performed according to the method described by Aebi (1984). The enzyme activity was determined by the consumption of H2O2 at 230 nm, 30 °C, and pH 8.0. Each assay received 5 μL of sample, along with 15 μL of Tris base and 180 μL of 30 mM H2O2. CAT activity was calculated using the following equation: (Δ absorbance / 0.071) × dilution / mg protein.
Determination of GPx activity (EC1.11.1.9)
The activity of GPx was determined by the method described by Wendel (1981). Measurements were carried out indirectly by monitoring the consumption of NADPH at 340 nm. Each assay was performed with the addition of 10 μL of sample and 185 μL of stock buffer (143 mM sodium phosphate and 6.3 mM EDTA, pH 7.5), containing 0.25 mM NaN3, 0.25 U/mL glutathione reductase, 1 mM GSH, and 0.12 mM NADPH. After incubation at 37 C for approximately 30 s, 5 μL of t-butyl hydroperoxide (TBHP) was added and the reaction was monitored using stock buffer as a reference. Results were expressed as nanomole of TBHP reduced per minute per milligram protein, considering the extinction coefficient factor of NADPH (Σ NADPH = of 6.22 nM−1 × cm−1). Thus, the activity of GPX was obtained according to the equation: (Δ absorbance / 6.22) × factor of dilution / milligram protein.
Determination of the total protein content of anti-oxidant enzymes
After 24-h incubation with 100 μM of EPA or DHA (freshly prepared before each assay) at 37 °C, cells were homogenized in extraction buffer (100 mM Trizma pH 7.5, 10 mM EDTA, 100 mM NaF, 10 mM sodium phosphate, 10 mM sodium orthovanadate, 2 mM PMSF, 0.01 mg/mL aprotinin, at 4 °C). After that, Triton X-100 at 1 % was added and the samples were incubated for 30 min at 4 °C. Homogenates were then centrifuged at 13,000×g for 20 min at 4 °C. Aliquots of the supernatant (5 μL) were used for measurement of total protein as described by Bradford (1976). Equal amounts of protein from each sample (50 μg) were size-separated by electrophoresis. Western blotting was then performed according to the method described previously (Towbin et al. 1979). Briefly, proteins were transferred from the gel to a nitrocellulose membrane, at 120 V for 1 h. Nonspecific binding was blocked by incubating the membrane with a solution of 5 % bovine serum albumin (BSA) in basal solution (10 mM Trizma pH 7.5, 150 mM NaCl, and 0.05 % Tween 20) at room temperature for 2 h. The membranes were washed three times for 10 min each with basal solution and then incubated with specific antibodies in basal solution containing 3 % BSA at room temperature for 3 h. Antibodies against cytosolic superoxide dismutase (SOD1) (cat. # sc-11407), mitochondrial superoxide dismutase (SOD2) (cat. # sc-30080), catalase (CAT) (cat. # sc-50508), and glutathione peroxidase (GPx) (cat. # sc-30147) (all from Santa Cruz Biotechnology, Inc.) were used. The membranes were washed again (three times for 10 min each) and incubated with an anti-IgG antibody coupled with peroxidase in a solution containing 1 % BSA at room temperature for 1 h. After further washes, the membranes were incubated with a substrate for peroxidase (ECL, Western Blotting System Kit, GE Health Care, Little Chalfont, Buckinghamshire, England) for 1 min and immediately exposed to X-ray films. The films were then revealed and the bands quantified using the software ImageJ 1.46r (Wayne Rasband, NIH, USA). The final results were presented after normalization using a specific housekeeping protein (alpha-tubulin, Cell Signaling Technology, cat. #2148).
Statistical analysis
Data are presented as mean ± standard deviation of the mean. Samples were tested for normality (Kolmogorov-Smirnov), and then, a one-way ANOVA with Tukey’s (parametric data) or Dunn’s (nonparametric data) post hoc tests was used. ROUT test was performed to identify any outlier. Results were considered statistically different when p < 0.05. We used the GraphPad Prism 6 software (Graph Pad Software, Inc., San Diego, CA, USA).
Results
Evaluation of ROS production
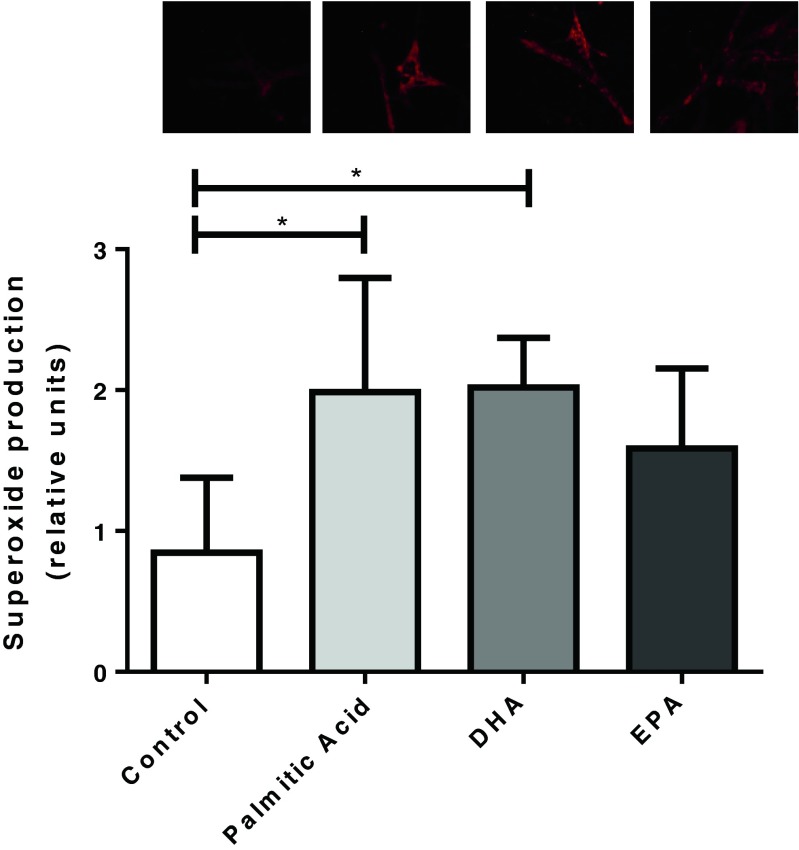
Superoxide production was evaluated according to the DHE oxidation method. Firstly, cells were treated with palmitic acid (a known inducer of superoxide production). As expected, we observed that palmitic acid induced an increased superoxide production when compared to the control group (0.84 ± 0.53 vs 1.97 ± 0.8, adjusted p = 0.0014). Cells were also treated with omega-3 polyunsaturated fatty acids (DHA and EPA). There was a higher superoxide production on cells treated with DHA only (0.84 ± 0.53 vs 2.01 ± 0.35, adjusted p = 0.001); however, EPA does not induced any alteration (0.84 ± 0.53 vs 1.58 ± 0.56 adjusted p = 0.054), as observed in Fig. 1.
Fig. 1.
Effect of palmitic, docosahexaenoic (DHA), and eicosapentaenoic (EPA) acids on superoxide anion production in skeletal muscle cells. Cells were incubated for 1 h in the absence or presence of 100 μM palmitic, DHA, or EPA acids in DPBS containing 5 mM glucose and 1 μM dihydroethidium. After this period, cells were observed in a fluorescence microscope with excitation and emission filters of 550 and 590 nm, respectively. Representative examples are shown above the graph. Results are presented as mean ± SD. *p < 0.05, n = 9
Anti-oxidant enzyme activities
The activities of the main skeletal muscle anti-oxidant enzymes (SOD, CAT, and GPx) were determined. For SOD activity, it was observed that DHA treatment presented lower values (68 %) (0.31 ± 0.2) when compared to control (0.97 ± 0.55, adjusted p = 0.01) or EPA (0.97 ± 0.6, adjusted p = 0.013) groups. However, EPA did not induce any alteration when compared to the control group (Fig. 2a). Furthermore, CAT activity showed to be higher in cells treated with EPA (98.66 %) when compared to cells treated with DHA (18.77 ± 8.42 vs 32.91 ± 4.42, adjusted p = 0.005) and control group (18.98 ± 2.3 vs 32.91 ± 4.42, adjusted p = 0.017) (Fig. 2b). Finally, neither DHA (59.77 ± 36.57 vs 80.54 ± 51.08, adjusted p = 0.48) nor EPA (59.77 ± 36.57 vs 58.83 ± 17.32, adjusted p = 0.99) induced any alteration on GPx activity when compared to the control group or between them (adjusted p = 0.44) (Fig. 2c).
Fig. 2.
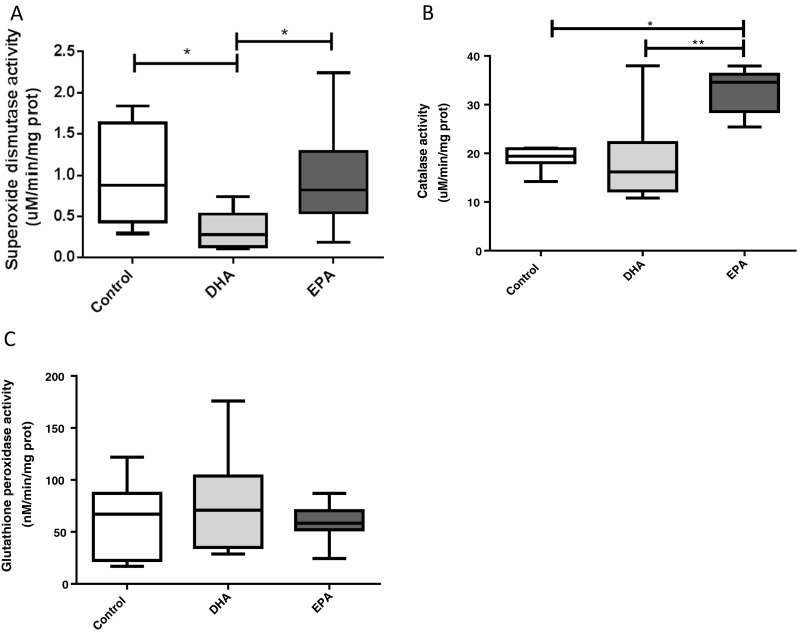
Effect of docosahexaenoic (DHA) and eicosapentaenoic (EPA) acids on skeletal muscle cells: a total superoxide dismutase (SOD) activity, b catalase (CAT) activity, and c glutathione peroxidase (GPx) activity. Cells were treated in the absence or presence of 100 μM of DHA or EPA. Results are presented as mean ± SD of two different experiments. *p < 0.05, n = 7 to 10
Anti-oxidant enzyme expression
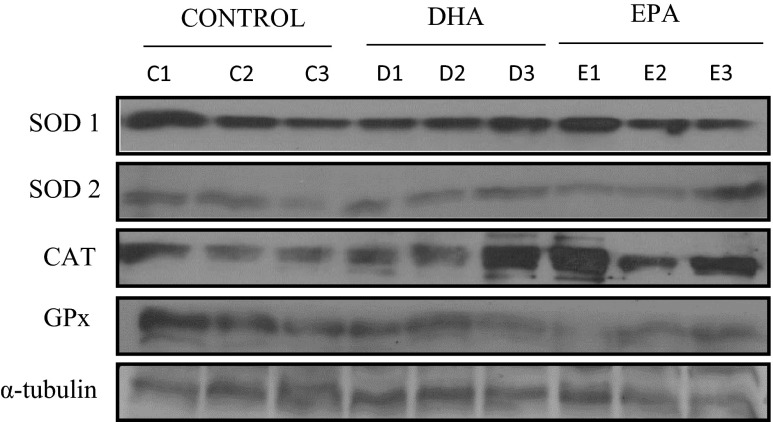
The total protein content of the skeletal muscle’s main anti-oxidant enzymes (SOD, CAT, and GPX) was determined by Western blotting. After incubation with DHA, no changes in Cu/Zn-SOD (SOD1) (1.12 ± 0.51 vs 0.79 ± 0.34, adjusted p = 0.42), CAT (0.96 ± 0.58 vs 0.81 ± 0.31, adjusted p = 0.99), and GPx (1.01 ± 0.24 vs 1.10 ± 0.38, adjusted p = 0.92) protein expression were observed when compared to control group. Similar response was observed after EPA treatment for SOD1 (0.97 ± 0.41 vs 0.79 ± 0.34, adjusted p = 0.75), CAT (2.09 ± 1.28 vs 0.81 vs 0.31, adjusted p = 0.09), and GPX (1.06 ± 0.44 vs 1.10 ± 0.38, adjusted p = 0.98) (Fig. 3a, c, d). However, Mn-SOD (SOD2) protein expression was 101.03 % higher in cells treated with EPA when compared to DHA treatment (0.78 ± 0.25 vs 1.9 ± 1.2, adjusted p = 0.03). When compared with the control group with EPA or DHA, it was not observed any alteration on SOD2 protein expression (0.97 ± 0.3 vs 1.9 ± 1.2, adjusted p = 0.64 and 0.78 ± 0.25 vs 0.97 ± 0.3, adjusted p = 0.64, respectively) (Fig. 3b). Representative blots of all anti-oxidant enzymes are presented in Fig. 4.
Fig. 3.
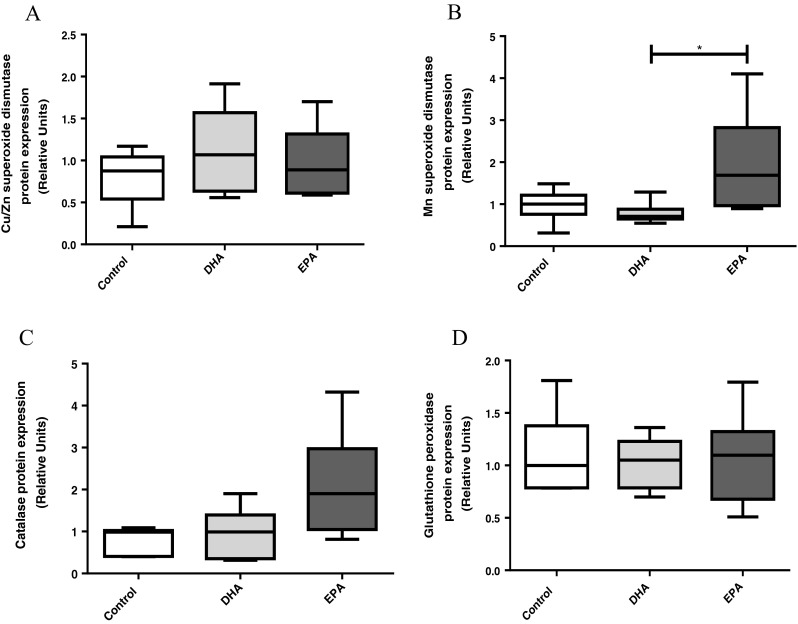
Effect of docosahexaenoic (DHA) and eicosapentaenoic (EPA) acids on skeletal muscle cells: a cytosolic superoxide dismutase (SOD1) protein content, b mitochondrial superoxide dismutase (SOD2) protein content, c catalase (CAT) protein content, and d glutathione peroxidase (GPx) protein content. Cells were treated in the absence or presence of 100 μM of DHA or EPA. Results are presented as mean ± SD of two different experiments. *p < 0.05, n = 6. All samples were normalized by its respective housekeeping values (relative units)
Fig. 4.
Representative blots of superoxide dismutase (SOD) (1 and 2), catalase (CAT), and glutathione peroxidase (GPx) protein expression evaluated by western blotting. Results were normalized using α-tubulin protein expression as reference. C control, D DHA, E EPA. The numbers 1, 2, and 3 related to each group represent a different sample
Discussion
The present study showed different responses for DHA and EPA muscle cells’ treatment. EPA results showed some indications linking its participation on muscle cell protection against oxidative stress, while DHA treatment was unable to induce the same.
We demonstrated that the DHA polyunsaturated fatty acids induced an increased production of superoxide anions, which may be associated with an augmented fatty acid oxidation; however, the same did not occur with EPA. Similar results were observed by our previous studies (Lambertucci et al. 2008) using primary cultures of skeletal muscle cells treated with palmitic acid. Rossary et al. (2007) also observed an increased ROS production using fibroblasts treated with 15 μM of DHA for 4 h (even using a shorter fatty acid concentration and incubation time). In addition, de Catalfo et al. (2013) showed similar results using different types of fatty acids using animal models. They observed that fatty acid (soybean oil and grape seed oil), incorporated in the diet for 60 days, caused an increase of oxidative stress markers in the liver of rats. The different responses induced by these fatty acids may be due to distinct metabolite formation and incorporation into plasma fractions and/or cell membrane, for example (Kelley and Adkins 2012; Sebaldt and Marignani 1997; Walker et al. 2014). According to Pisani et al. (2009), EPA and DHA may induce different ROS production, and this is a topic that still needs to be fully elucidated.
After evaluating ROS production, we also determined the activity of the main anti-oxidant enzymes found in skeletal muscle cells. We observed a lower activity of total SOD when cells were treated with DHA, but no difference was found when cells were treated with EPA. This response may be detrimental for cells because a decrease in this enzyme activity can induce an accumulation of superoxide, which may lead to cell damages. Similar results were obtained by Rossary et al. (2007), who found that SOD activity did not change when fibroblasts were treated with DHA or EPA omega-3 polyunsaturated fatty acid. On the other hand, Naqshbandi et al. (2012) showed an improvement in SOD activity in the intestine from rats treated with omega-3-enriched diet. The contradictory results of SOD activity may occur due to differences between experimental protocols, mainly related to the amount of omega 3 and/or the treatment duration.
SOD protein expression determination was carried out in the attempt to explain the results about its activity. No difference between the control and DHA groups was found. However, we observed a higher SOD2 protein expression when cells were treated with EPA, comparing to DHA group. Despite of this result, surprisingly, no alteration of SOD activity was noted. This effect may be related to the different response of the distinct SOD isoforms (increase activity for SOD2 and a decreased activity for SOD1, resulting in a balanced process). Even though there was no overall modulation of SOD activity after treating cells with EPA, an increased SOD2 expression can be important for cell health and survival, once this isoform is localized in mitochondria, which is known to be the main site of superoxide production in the muscle cell (Barja 2007).
Among the main processes capable of controlling enzyme activities, some authors have proposed two important mechanisms: allosteric and covalent modulation (Anderssen et al. 2007; Ploug et al. 1990). According to Das and Plotkin (2013), SOD1, the cytosolic isoform, is a 32 kDa homo-dimeric enzyme, composed by 153 amino acids on each monomer, which binds two ions that regulate its activity and stability (one Cu and one Zn). They have showed that SOD1 can be modulated allosterically due to interference on metal-binding sites (decreasing its activity).
In a study done by Derogis et al. (2013), it was demonstrated that oxidation of DHA by free radicals can generate several by-products (ten positional isomers of HpDoHE (20-, 17-, 16-, 14-, 13-, 11-, 10-, 8-, 7-, and 4-HpDoHE)). Furthermore, there is evidence that these products may alter the action of SOD1 through the formation of protein aggregation (Kim et al. 2005). In our study, SOD1 activity was decreased after DHA treatment with no modification of its expression. We suggest that some products of DHA oxidation may induce either allosteric modulation or covalent modification of SOD1 enzyme, which may induce the observed decreased activity.
In opposition to the conflicting data about SOD activity and protein expression, the present study showed increased values of CAT activity when cells were treated with EPA compared to cells treated with DHA and control group. Currently, some studies have shown that EPA has the capability to directly and indirectly activate the nuclear factor kappa B (NF-κB) pathway (Kageyama et al. 2013; Zuniga et al. 2011), and this pathway is responsible for the control of the gene expression of several anti-oxidant enzymes, including CAT (Priyanka et al. 2013). Thus, the modulation of NF-κB pathway by EPA may modulate the CAT content, and consequently, its activity, furthermore, allosteric mechanisms, can modulate the enzyme activity as demonstrated above. However, we did not observe significant difference when we evaluated CAT protein expression. An allosteric mechanism appears to be a possible hypothesis to explain such surprising data.
In addition to CAT, the hydrogen peroxide can be also eliminated by GPx enzyme. Our study evaluated the activity and protein expression of this enzyme, and we did not observe significant difference when cells were treated with DHA or EPA. Other studies also found no difference in GPx activity using treatment with omega 3 fatty acids in different concentrations and cellular models (Filaire et al. 2010; Garrel et al. 2012; Kusunoki et al. 2013).
Taken together, according to the results present herein, EPA may act as an important anti-oxidant supplement when compared with DHA, helping cells to increase their enzymatic anti-oxidant system capacity, with a consequent prevention of cell oxidative damage. EPA was able of maintain the levels of superoxide associated to a higher activity of SOD and CAT enzymes when compared with DHA. An increased amount of SOD2 protein expression was observed as well. Similarly, a study performed by Gil (2002) also showed the anti-oxidant effect of EPA. Pal and Ghosh (2012) showed a beneficial effect of fatty acids for the cellular anti-oxidant system. They showed that linoleic acid improved the activity of SOD, CAT, and GPx enzymes in the liver and kidney from rats. Additionally, Khan et al. (2012) have shown the anti-oxidant effect of omega-3 polyunsaturated fatty acids in the kidney from rats. On the other hand, enhancement of anti-oxidant enzyme activity does not directly mean that cells are protected against oxidative damage. The present study did not measure oxidative damage parameters to conclude it. Moreover, the protective effects depend on the localization and type of ROS generation under specific conditions.
It is noteworthy that most studies used to discuss the results obtained in our study were performed using animal models or different cell types; however, the effect on the anti-oxidant system was similar in most of these studies.
Conclusion
In summary, the present study provides evidence about a possible beneficial effect of EPA for skeletal muscle cells regarding the anti-oxidant system, due to the improved anti-oxidant system capacity. However, the treatment with DHA may not be beneficial to muscle cells, since it induced an increase in superoxide production and a decrease of SOD activity, which may favor the occurrence of oxidative stress damage. Further studies have to be done to elucidate more precisely the mechanisms involved and the benefits of the EPA on the redox state for maintaining health and/or improved performance of exercise practitioners.
Acknowledgments
Financial support
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Conflict of interest
We declare that we have no conflict of interest.
References
- Aebi H (1984) Catalase in vitro. vol 105 [DOI] [PubMed]
- Anderssen SA, Carroll S, Urdal P, Holme I. Combined diet and exercise intervention reverses the metabolic syndrome in middle-aged males: results from the Oslo Diet and Exercise Study. Scand J Med Sci Sports. 2007;17:687–695. doi: 10.1111/j.1600-0838.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- Attaman JA, Stanic AK, Kim M, Lynch MP, Rueda BR, Styer AK. The anti-inflammatory impact of omega-3 polyunsaturated fatty acids during the establishment of endometriosis-like lesions. Am J Reprod Immunol. 2014 doi: 10.1111/aji.12276. [DOI] [PubMed] [Google Scholar]
- Barbieri E, Sestili P. Reactive oxygen species in skeletal muscle signaling. J Signal Transduct. 2012;2012:982794. doi: 10.1155/2012/982794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barja G. Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuvenation Res. 2007;10:215–224. doi: 10.1089/rej.2006.0516. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brieger K, Schiavone S, Miller FJ, Jr, Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly. 2012;142:w13659. doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- Bryner RW, Woodworth-Hobbs ME, Williamson DL, Alway SE. Docosahexaenoic acid protects muscle cells from palmitate-induced atrophy. ISRN Obes. 2012;2012:647348. doi: 10.5402/2012/647348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci LR. A functional analytical technique for monitoring nutrient status and repletion. Am Clin Lab. 1993;12(8):10. [PubMed] [Google Scholar]
- Das A, Plotkin SS. SOD1 exhibits allosteric frustration to facilitate metal binding affinity. Proc Natl Acad Sci U S A. 2013;110(10):3871–3876. doi: 10.1073/pnas.1216597110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/S0006-291X(82)80124-1. [DOI] [PubMed] [Google Scholar]
- de Catalfo GE, de Alaniz MJ, Marra CA. Dietary lipid-induced changes in enzymes of hepatic lipid metabolism. Nutrition. 2013;29:462–469. doi: 10.1016/j.nut.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Derogis PB, et al. The development of a specific and sensitive LC-MS-based method for the detection and quantification of hydroperoxy- and hydroxydocosahexaenoic acids as a tool for lipidomic analysis. PLoS One. 2013;8:e77561. doi: 10.1371/journal.pone.0077561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filaire E, et al. Effect of 6 weeks of n-3 fatty-acid supplementation on oxidative stress in Judo athletes. Int J Sport Nutr Exerc Metab. 2010;20:496–506. doi: 10.1123/ijsnem.20.6.496. [DOI] [PubMed] [Google Scholar]
- Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- Flohe L, Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/S0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- Garrel C, Alessandri JM, Guesnet P, Al-Gubory KH. Omega-3 fatty acids enhance mitochondrial superoxide dismutase activity in rat organs during post-natal development. Int J Biochem Cell Biol. 2012;44:123–131. doi: 10.1016/j.biocel.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Gil A. Polyunsaturated fatty acids and inflammatory diseases Biomedicine & pharmacotherapy. Biomed Pharmacother. 2002;56:388–396. doi: 10.1016/S0753-3322(02)00256-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J. Free radicals in biology and medicine. Oxford: Oxford Univ. Press; 2007. [Google Scholar]
- Hernandez A, Cheng A, Westerblad H. Antioxidants and skeletal muscle performance: "common knowledge" vs. experimental evidence. Front Physiol. 2012;3:46. doi: 10.3389/fphys.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MJ, Pye D, Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol. 2007;102:1664–1670. doi: 10.1152/japplphysiol.01102.2006. [DOI] [PubMed] [Google Scholar]
- Kageyama A, et al. Palmitic acid induces osteoblastic differentiation in vascular smooth muscle cells through ACSL3 and NF-kappaB, novel targets of eicosapentaenoic acid. PLoS One. 2013;8:e68197. doi: 10.1371/journal.pone.0068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J. Exercise, muscle metabolism and the antioxidant defense. World Rev Nutr Diet. 1997;82:81–100. doi: 10.1159/000059651. [DOI] [PubMed] [Google Scholar]
- Kelley DS, Adkins Y. Similarities and differences between the effects of EPA and DHA on markers of atherosclerosis in human subjects. Proc Nutr Soc. 2012;71:322–331. doi: 10.1017/S0029665112000080. [DOI] [PubMed] [Google Scholar]
- Khan MW, Priyamvada S, Khan SA, Khan S, Naqshbandi A, Yusufi AN. Protective effect of omega-3 polyunsaturated fatty acids (PUFAs) on sodium nitroprusside-induced nephrotoxicity and oxidative damage in rat kidney. Hum Exp Toxicol. 2012;31:1035–1049. doi: 10.1177/0960327112444475. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Nakatomi R, Akagi T, Hashikawa T, Takahashi R. Unsaturated fatty acids induce cytotoxic aggregate formation of amyotrophic lateral sclerosis-linked superoxide dismutase 1 mutants. J Biol Chem. 2005;280:21515–21521. doi: 10.1074/jbc.M502230200. [DOI] [PubMed] [Google Scholar]
- Koren A, Sauber C, Sentjurc M, Schara M. Free radicals in tetanic activity of isolated skeletal muscle. Comp Biochem Physiol B Comp Biochem. 1983;74:633–635. doi: 10.1016/0305-0491(83)90241-9. [DOI] [PubMed] [Google Scholar]
- Kusunoki C, et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/HO-1 pathway in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2013;430:225–230. doi: 10.1016/j.bbrc.2012.10.115. [DOI] [PubMed] [Google Scholar]
- Kyrylenko O, Kukoba TV, Moibenko OO, Nikula TD (2004) [Effect of omega-3 polyunsaturated fatty acids of animal origin on changes in regulation of lipid peroxidation in patients with ischemic heart disease]. Lik Sprava/Ministerstvo okhorony zdorov'ia Ukrainy 71–73 [PubMed]
- Lambertucci RH, Hirabara SM, Silveira Ldos R, Levada-Pires AC, Curi R, Pithon-Curi TC. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J Cell Physiol. 2008;216:796–804. doi: 10.1002/jcp.21463. [DOI] [PubMed] [Google Scholar]
- Lambertucci RH, et al. The effects of palmitic acid on nitric oxide production by rat skeletal muscle: mechanism via superoxide and iNOS activation. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2012;30:1169–1180. doi: 10.1159/000343307. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kim IH, Kim Y. Effects of eicosapentaenoic acid and docosahexaenoic acid on uncoupling protein 3 gene expression in C(2)C(12) muscle cells. Nutrients. 2013;5:1660–1671. doi: 10.3390/nu5051660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschen G, Azzi A, Richter C, Flohe L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS lett. 1974;42:68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- Martins C, Santos R, Gaya A, Twisk J, Ribeiro J, Mota J. Cardiorespiratory fitness predicts later body mass index, but not other cardiovascular risk factors from childhood to adolescence. Am J Human Biol Off J Human Biol Counc. 2009;21:121–123. doi: 10.1002/ajhb.20826. [DOI] [PubMed] [Google Scholar]
- Mickleborough TD, Lindley MR, Montgomery GS. Effect of fish oil-derived omega-3 polyunsaturated fatty acid supplementation on exercise-induced bronchoconstriction and immune function in athletes. Phys Sportsmed. 2008;36:11–17. doi: 10.3810/psm.2008.12.7. [DOI] [PubMed] [Google Scholar]
- Naqshbandi A, Rizwan S, Khan MW, Khan F. Dietary flaxseed oil supplementation ameliorates the effect of cisplatin on brush border membrane enzymes and antioxidant system in rat intestine. Hum Exp Toxicol. 2012 doi: 10.1177/0960327112438929. [DOI] [PubMed] [Google Scholar]
- Nelson DLC, M. M. (2000) Lehninger principles of biochemistry, vol 3. Worth Publishers, New York.
- Nikolaidis MG, Kyparos A, Spanou C, Paschalis V, Theodorou AA, Vrabas IS. Redox biology of exercise: an integrative and comparative consideration of some overlooked issues. J Exp Biol. 2012;215:1615–1625. doi: 10.1242/jeb.067470. [DOI] [PubMed] [Google Scholar]
- Pal M, Ghosh M. Studies on comparative efficacy of alpha-linolenic acid and alpha-eleostearic acid on prevention of organic mercury-induced oxidative stress in kidney and liver of rat. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2012;50:1066–1072. doi: 10.1016/j.fct.2011.12.042. [DOI] [PubMed] [Google Scholar]
- Pisani LF, Lecchi C, Invernizzi G, Sartorelli P, Savoini G, Ceciliani F. In vitro modulatory effect of omega-3 polyunsaturated fatty acid (EPA and DHA) on phagocytosis and ROS production of goat neutrophils. Vet Immunol Immunopathol. 2009;131:79–85. doi: 10.1016/j.vetimm.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Ploug T, Stallknecht BM, Pedersen O, Kahn BB, Ohkuwa T, Vinten J, Galbo H. Effect of endurance training on glucose transport capacity and glucose transporter expression in rat skeletal muscle. Am J Physiol. 1990;259:E778–E786. doi: 10.1152/ajpendo.1990.259.6.E778. [DOI] [PubMed] [Google Scholar]
- Pogozheva AV, Martynova EA, Samsonov MA, Egorova NI, Ksenofontova EA, Gorozhanskaia EG (1994) [Dietary effects of PUFA omega-3 on lipid peroxidation and antioxidant system in patients with IHD, hyperlipoproteinemia and hypertension] Vopr Pitan 40–42 [PubMed]
- Priyanka HP, Bala P, Ankisettipalle S, ThyagaRajan S. Bacopa monnieri and L-deprenyl differentially enhance the activities of antioxidant enzymes and the expression of tyrosine hydroxylase and nerve growth factor via ERK 1/2 and NF-kappaB pathways in the spleen of female wistar rats. Neurochem Res. 2013;38:141–152. doi: 10.1007/s11064-012-0902-2. [DOI] [PubMed] [Google Scholar]
- Rossary A, Arab K, Steghens JP. Polyunsaturated fatty acids modulate NOX 4 anion superoxide production in human fibroblasts. Biochem J. 2007;406:77–83. doi: 10.1042/BJ20061009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell FD, Burgin-Maunder CS. Distinguishing health benefits of eicosapentaenoic and docosahexaenoic acids. Mar Drugs. 2012;10:2535–2559. doi: 10.3390/md10112535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- Sebaldt RJ, Marignani P. Diradylglycerol formation is altered by n-3 highly unsaturated fatty acids, with differences between eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids. Adv Exp Med Biol. 1997;400B:937–945. [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CG, Browning LM, Mander AP, Madden J, West AL, Calder PC, Jebb SA. Age and sex differences in the incorporation of EPA and DHA into plasma fractions, cells and adipose tissue in humans. Br J Nutr. 2014;111:679–689. doi: 10.1017/S0007114513002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel A. Glutathione peroxidase. Methods Enzymol. 1981;77:325–333. doi: 10.1016/S0076-6879(81)77046-0. [DOI] [PubMed] [Google Scholar]
- Woodman RJ, Mori TA, Burke V, Puddey IB, Barden A, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis. 2003;166:85–93. doi: 10.1016/S0021-9150(02)00307-6. [DOI] [PubMed] [Google Scholar]
- Wu D, Meydani SN, Meydani M, Hayek MG, Huth P, Nicolosi RJ. Immunologic effects of marine- and plant-derived n-3 polyunsaturated fatty acids in nonhuman primates. Am J Clin Nutr. 1996;63:273–280. doi: 10.1093/ajcn/63.2.273. [DOI] [PubMed] [Google Scholar]
- Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/S0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- Zuniga J, et al. N-3 PUFA supplementation triggers PPAR-alpha activation and PPAR-alpha/NF-kappaB interaction: anti-inflammatory implications in liver ischemia-reperfusion injury. PLoS One. 2011;6:e28502. doi: 10.1371/journal.pone.0028502. [DOI] [PMC free article] [PubMed] [Google Scholar]