Abstract
Embryos of the brine shrimp, Artemia franciscana, are genetically programmed to develop either ovoviparously or oviparously depending on environmental conditions. Shortly upon their release from the female, oviparous embryos enter diapause during which time they undergo major metabolic rate depression while simultaneously synthesize proteins that permit them to tolerate a wide range of stressful environmental events including prolonged periods of desiccation, freezing, and anoxia. Among the known stress-related proteins that accumulate in embryos entering diapause are the late embryogenesis abundant (LEA) proteins. This large group of intrinsically disordered proteins has been proposed to act as molecular shields or chaperones of macromolecules which are otherwise intolerant to harsh conditions associated with diapause. In this research, we used two model systems to study the potential function of the group 1 LEA proteins from Artemia. Expression of the Artemia group 1 gene (AfrLEA-1) in Escherichia coli inhibited growth in proportion to the number of 20-mer amino acid motifs expressed. As well, clones of E. coli, transformed with the AfrLEA-1 gene, expressed multiple bands of LEA proteins, either intrinsically or upon induction with isopropyl-β-thiogalactoside (IPTG), in a vector-specific manner. Expression of AfrLEA-1 in E. coli did not overcome the inhibitory effects of high concentrations of NaCl and KCl but modulated growth inhibition resulting from high concentrations of sorbitol in the growth medium. In contrast, expression of the AfrLEA-1 gene in Saccharomyces cerevisiae did not alter the growth kinetics or permit yeast to tolerate high concentrations of NaCl, KCl, or sorbitol. However, expression of AfrLEA-1 in yeast improved its tolerance to drying (desiccation) and freezing. Under our experimental conditions, both E. coli and S. cerevisiae appear to be potentially suitable hosts to study the function of Artemia group 1 LEA proteins under environmentally stressful conditions.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-015-0647-3) contains supplementary material, which is available to authorized users.
Keywords: Artemia, Late embryogenesis abundant, LEA proteins, E. coli, S. cerevisiae
Introduction
Important to survival of encysted embryos of the brine shrimp, Artemia franciscana, is their ability to tolerate various environmental stresses such as desiccation, freezing, high salinity, high osmotic pressures, and anoxia (Clegg and Trotman 2002; MacRae 2010; Warner and Clegg 2001; King and MacRae 2012; King et al. 2013; Toxopeus et al. 2014). Their stress tolerance is highest during diapause, a genetically programmed event in Artemia and many other invertebrates (Clegg and Conte 1980; MacRae 2003, 2010; Qiu and MacRae 2007; Tanguay et al. 2004; Liu et al. 2009; Wu et al. 2011). Preparation for diapause in Artemia is associated with the upregulation of at least 20 genes (Qiu and MacRae 2007; Liu et al. 2009) and downregulation of at least 4 genes estimated by subtractive hybridization of Artemia embryos that develop oviparously versus ovoviparously (Qiu and MacRae 2007). Two-dimensional sodium dodecyl sulfate polyacrylamide (SDS-PAGE) coupled with matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) of proteins isolated from Artemia cyst in diapause have revealed a similar number of highly regulated proteins (Wang et al. 2007). Upon termination of diapause, at least 133 genes are differentially expressed (DEG) during the first few hours in culture (Chen et al. 2009). The complement of DEG in early embryos of Artemia includes those associated with metabolic rate depression (Clegg et al. 1978; Clegg 1997), molecular chaperone activity (MacRae 2003; Sun et al. 2004; Warner et al. 2004; Clegg et al. 1995), small heat-shock proteins (Clegg et al. 1994; Chen et al. 2003; King and MacRae 2012; King et al. 2013; Qiu and MacRae 2008; Liang et al. 1997), and late embryogenesis abundant (LEA) proteins (Warner et al. 2010, 2012; Menze et al. 2009; Boswell et al. 2014). The molecular “trigger” needed to achieve such a major shift in metabolism is not known but probably involves one or more environmentally sensitive transcription factors (Qiu and MacRae 2007). Additional information about diapause in Artemia can be found in three excellent reviews on the subject (MacRae 2003, 2005, 2010).
Analyses of the Artemia proteome and EST databases have shown that at least six group 1 LEA genes are present in Artemia and expressed only during diapause in oviparous embryos (Warner et al. 2010; Chen et al. 2009; Toxopeus et al. 2014; Wu et al. 2011). As well, at least three group 3 LEA genes are active and possibly as many as twelve group 3 LEA proteins are present and functional, in Artemia embryos (Toxopeus et al. 2014; Warner et al. 2012; Boswell et al. 2014; Menze et al. 2009). The Artemia group 1 LEA proteins are localized mainly in the cytoplasm and mitochondria of dormant cysts, but small amounts may be present in nuclei and other organelles; they are not present in somatic tissues (Warner et al. 2010). At least one group 1 LEA protein appears in the nuclei of Artemia embryos under anoxia where it may bind to DNA and function in its regulation (Warner et al. 2010; Wu et al. 2013). So far, no group 2 LEA proteins (or their mRNAs) have been detected in the Artemia proteome or databases.
First identified by Dure, Greenway, and Galau in 1981, LEA proteins have been found in a wide array of plant seeds in several prokaryotes and notably in cysts of A. franciscana (Dure et al. 1981; Sharon et al. 2009; Warner et al. 2010; Chen et al. 2009; Toxopeus et al. 2014). In general, LEA proteins are intrinsically disordered proteins which fail to form stable structures, yet they recognize other proteins and nucleic acids forming a type of molecular shield against the deleterious effects of environmental stresses (Sun et al. 2013; Tompa 2002).
At least 12 classes of LEA proteins with multiple sub-groups have been proposed as reviewed previously (Warner et al. 2012). With respect to the group 1 LEA proteins, their presence in plant embryos and Artemia cysts which are desiccation-tolerant had led some researchers to suggest that these proteins may function in water retention to protect cells against desiccation and/or osmotic stresses (Swire-Clark and Marcotte 1999; Manfre et al. 2009; Gilles et al. 2007, and Campos et al. 2013). In desiccation-tolerant tissues, they are thought to achieve their functions by interacting with sugars (mainly trehalose) to form a highly viscous matrix or biological glasses (Buitink and Leprince 2008; Wolkers et al. 2001).
Among the several classes of LEA proteins identified in various organisms, those proteins belonging to groups 2 and 3 have been the most widely studied (Tunnacliffe and Wise 2007). Suggestions as to the function of the most commonly found LEA proteins include a role in formation of biological glasses (Buitink and Leprince 2008), ion sequestration (Tunnacliffe and Wise 2007), drying or desiccation tolerance (Manfre et al. 2009; Tunnacliffe and Wise 2007; Campos et al. 2006; Soulages et al. 2002; Hengherr et al. 2011; Li et al. 2012), osmotic resistance (Swire-Clark and Marcotte 1999), freeze tolerance (Goyal et al. 2005b), and molecular shielding (Chakrabortee et al. 2012; Goyal et al. 2005a).
Genes coding for LEA proteins are highly conserved among organism of all taxa and appear to be common in tissues and/or organisms which are desiccation-tolerant including cysts of Artemia (Tunnacliffe and Wise 2007; Warner et al. 2010). A recent study using RNA interference of group 1 LEA gene expression in the brine shrimp, A. franciscana, suggested that group 1 LEA proteins contribute to both desiccation and freeze tolerance of encysted embryos of Artemia (Toxopeus et al. 2014). Furthermore, at least one group 1 LEA gene appears to respond to hyper-saline stress during post-diapause development of encysted embryos of Artemia (Wu et al. 2011). Overall, group 1 LEA proteins are distinctly hydrophilic and characterized by a 20-mer amino acid signature motif which may be present in up to eight copies in tandem (Soulages et al. 2002; Tunnacliffe and Wise 2007). Finding suitable model systems to test for potential functions of the LEA proteins has proven to be a challenge.
Cysts of the brine shrimp, A. franciscana, demonstrated to be highly resistant to numerous environmental stresses, are an ideal organism to identify and study the full complement of LEA proteins during development. To study further the role of Artemia group 1 LEA proteins, we created recombinant plasmids containing various group 1 LEA genes, with two to eight characteristic motifs, in tandem, and used these vectors to transform strains of Escherichia coli and Saccharomyces cerevisiae. The main results of these experiments showed that the rate of growth of E. coli was inhibited upon expression of the Artemia LEA genes, and the extent of growth inhibition was dependent on the number of 20-mer amino acid motifs in the recombinant plasmid. The expression of the AfrLEA-1 gene in yeast (strain INVSc1) was modest and had little effect on growth inhibition by high osmoticums. However, S. cerevisiae transfected with the AfrLEA-1 gene showed improved tolerance to freezing and drying, suggesting that certain strains of yeast may be a good model to study the function of Artemia LEA proteins in vitro.
Materials and methods
Encysted embryos of the brine shrimp, A. franciscana, were obtained from the Great Salt Lake (Sanders Brine Shrimp Company, Ogden, UT, USA) and stored at −15 °C until needed. The Sepharose 12 and Mono Q columns and ECL reagents for immunoblotting were from Amersham Biosciences (Baie d’Urfe, QC, Canada). The C-18 reverse-phase column (Jupiter, 5 μm, 300 A) was from Phenomenex (Torrance, CA, USA). All gel electrophoresis materials were from Bio-Rad (Mississauga, ON, Canada), while the X-ray film and developer were Kodak products from Ultident (St. Laurent, QC, Canada). Plasmids pCR2.1, pYES2, and pYES3 and E. coli strains Top10, Top10F’, INVαF’, and S. cerevisiae, strain INVSc1, were from Invitrogen (Carlsbad, CA, USA). All restriction enzymes were from Fermentas (Burlington, ON, Canada), while Protease Arrest and BugBuster were from EMD Biosciences (San Diego, CA, USA). Bacteria and yeast growth media were from Sigma (Oakville, ON, Canada). All other chemicals were of reagent grade or better.
Preparation of the heat-soluble proteome from A. franciscana, E. coli, and S. cerevisiae
The heat-soluble proteins, including the LEA proteins, were obtained from Artemia cysts as described previously (Warner et al. 2012). To obtain the bacterial proteome, E. coli was grown in Luria-Bertani (LB) broth at 37 °C with constant shaking and then collected by centrifugation. The cells were washed with 20 mM NaCl containing 10 mM Tris-HCl, pH 8.0, then stored at −15 °C until needed. The bacterial proteins were obtained by suspending the frozen bacterial pellet in 10 volumes of HB (50 mM NaCl with 10 mM Tris-HCl, pH 8.0, and 1X Protease Arrest). The bacterial suspension was heated for 10 min at 80–85 °C, cooled on ice, and then centrifuged to collect the heat-soluble proteome.
Transfected yeast containing pYES2/cLEA constructs were grown at 30 °C with shaking in Synthetic Complete (SC) minimal medium, whereas doubly transfected yeast were grown in SC minimal medium lacking both uracil and tryptophan (−ura, −trp) (Ausubel et al. 1989). The protocols used in these studies were as previously described (Swire-Clark and Marcotte 1999). Non-inducible culture media contained either 2 % glucose or 2 % raffinose (min-raf) as the carbon source, while culture media designed to induce expression of the cloned LEA genes contained 1 % raffinose and 1 % galactose (min-gal). At the desired incubation times, yeast cells were collected by centrifugation at 6500×g, washed with sterile distilled water, and then either frozen at −15 °C or broken by vortexing with glass beads as described in the pYES2 user manual (Invitrogen). The cell suspension was vortexed five times for 30 s each, heated at 80–85 °C for 10 min, and then centrifuged to obtain the heat-soluble proteins. The heat-soluble proteins containing the LEA proteins (where present) were quantified as described below and elsewhere (Bradford 1976; Warner et al. 2012).
Detection of Artemia group 1 LEA proteins by gel electrophoresis and immunoblotting
Heat-soluble extracts from E. coli and S. cerevisiae were assayed for protein using the Bradford Reagent (Bio-Rad) and for group 1 LEA proteins on immunoblots (Westerns) as described previously (Warner et al. 2012). Total protein (7–10 μg per lane) was loaded on duplicate 10 % SDS-PAGE gels, and the proteins were separated over 1–2 h at 140 V. The proteins on one gel were stained using Coomassie Brilliant Blue (CBB), whereas the proteins from the second gel were transferred overnight to a nitrocellulose membrane at 100 mA. The LEA proteins were detected using a polyclonal antibody prepared in rabbits as described previously (Warner et al. 2012).
Construction of bacterial recombinant plasmid vectors
Since Artemia embryos in diapause contain LEA proteins in both the cytoplasm and mitochondria, vectors were constructed containing genes coding for both the cytoplasmic and mitochondrial group 1 LEA proteins. A collection of recombinant plasmids was constructed from polymerase chain reactions (PCR) products generated using an A. franciscana cDNA library as described previously (Sharon et al. 2009; Toxopeus et al. 2014) and commercially available vectors (Invitrogen, Carlsbad, CA, USA) using forward primer LEA-3F (5′-TTTAAACGAAGTTCAAGCGTTCTCCAT-3′) and reverse primer LEA-R1A (5′-AGCTTATTGCTGTCTTGCGAGACCTCCTTT-3′). The PCR reaction generated multiple bands on 1 % agarose gel, which were purified using gel extraction spin columns (QIAquick, Toronto, ON, Canada), and ligated to vector pCR2.1 with T overhangs according to the manufacturers’ protocol (Invitrogen). Competent E. coli (INVαF’, Top10, Top10F’) cells were transformed according to an established protocol (Invitrogen), spread on LB-agar plates containing ampicillin (75 μg/ml) and X-gal (32 μg/ml) for blue white screening. (Note: freshly prepared ampicillin and X-gal were added to the LB-agar plates immediately before use.) Several positive (white) clones were collected, grown in fresh LB media (with ampicillin), and their plasmids were isolated and purified using spin columns (BioBasic, Markam, ON, Canada). Several recombinant plasmids with the predicted size inserts were sequenced (Robarts Research, London, ON, Canada). Based on the above protocols and sequencing data, several bacterial clones were identified and amplified on LB/ampicillin media for future use. They contained plasmids with group 1 AfrLEA genes of different lengths with two, five, six, and eight 20-mer amino acid motifs in both normal and reversed orientations as summarized in Table 1. It should be noted that not all AfrLEA gene inserts in vector pCR2.1 were “in frame” with the lacZα promoter. However, all plasmids with the LEA gene in the correct orientation expressed the LEA proteins constitutively when transformed into either Top 10 or INVαF’ strains of E. coli.
Table 1.
Description of different bacterial and yeast plasmid constructs and clones used in this study
| Code/Clone | Descriptiona | Orientation | Tandem motifs |
|---|---|---|---|
| pCR2.1 | pCR2.1 vector only | Normal | 0 |
| B25 | pCR2.1/cLEA-1d | Reversed | 5-mer |
| E1A | pCR2.1/cLEA-1d | Normalb | 5-mer |
| E1B | pCR2.1/cLEA-1c | Normalb | 6-mer |
| E11 | pCR2.1/cLEA-1a | Reversed | 8-mer |
| E1C | pCR2.1/cLEA-1a | Normalb | 8-mer |
| E1C’ (2nd clone) | pCR2.1/cLEA-1a | Normalb | 8-mer |
| tE1C-2 | pCR2.1/cLEA-1a | Normal (in frame) | 8-mer |
| tE1C-3 | pCR2.1/cLEA-1a | Normal (in frame) | 8-mer |
| 4A1 | pCR2.1/mLEA-1a | Normalb | 8-mer |
| C5B | pCR2.1/cLEA-1g | Normalb | 2-mer |
| Yc | pYES-2/cLEA-1a | Normal | 8-mer |
| Ym | pYES-3/mLEA-1a | Normal | 8-mer |
| Y2,3 | pYES-2/pYES-3 only | Normal | 0 |
| Yc, m | pYES-2/cLEA-1a: | ||
| pYES-3/mLEA-1a | Normal | 8-mer |
All inserts were from PCR products confirmed by DNA sequencing
aThe letters after the LEA-1 designations refers to the group 1 LEA protein identified previously. Thus, LEA-1a refers to the largest LEA protein with eight 20-mer amino acid motifs, LEA-1b with seven 20-mer amino acid motifs, etc. (Warner et al. 2010). The small c or m indicates that the LEA protein is designated as cytoplasmic or mitochondrial
bNormal orientation but not in frame with the lacZ gene fragment
Additional recombinant plasmids were constructed using PCR to generate AfrLEA genes that would be in frame with the lacZα promoter when ligated into pCR2.1. Toward this objective, one forward PCR primer, LEA-3Ft (5′-TTTTAAACGAAGTTCAAGCGTTCTCCAT-3′), was paired with reverse primer LEA-R1A as described above to generate a full-length AfrLEA gene with eight 20-mer amino acid motifs (see supplemental figure S-1). The bold T was added to forward primers to place the PCR products in frame with the lacZα promoter. Transformation of E. coli Top10F’ with these plasmids resulted in clones tE1C-2 and tE1C-3 (see Table 1) which yielded LEA proteins when induced with isopropyl-β-thiogalactoside (IPTG).
Construction of recombinant yeast vectors
The shuttle vectors pYES2 and pYES3 carrying LEA gene constructs for both Artemia cytoplasmic and mitochondrial LEA proteins, respectively, were prepared using commercial kits (Invitrogen). Both vectors were inducible expression vectors for protein expression in yeast (S. cerevisiae) under control of the GAL1 promoter. pYES2 and pYES3 contain an ampicillin-resistant gene for selection on LB-agar plates supplemented with ampicillin at a final concentration of 75 μg/ml. They contain the CYC1 transcriptional terminator but lack an ATG initiation codon. They can be propagated in E. coli (Top10F’). The host cell of choice was S. cerevisiae (INVSc1), a diploid strain that is auxotrophic for uracil. Thus, yeast transfected with recombinant pYES2/LEA-1 grew on agar plates with minimal medium lacking uracil (SC(u)). Vector pYES3 is similar to pYES2 except that it is auxotrophic for tryptophan, allowing yeast transfected with pYES3 to grow in tryptophan-deficient media. Yeast transfected with both pYES2/cLEA-1 and pYES3/mLEA-1 sequentially (i.e., doubly transfected) grew in medium lacking both uracil and tryptophan.
Using the full-length Artemia group 1 LEA-1 gene [GenBank: EF656614] coding for Artemia cytoplasmic LEA protein of 182 amino acids, molecular mass 19,676 Da with eight 20-mer motifs as substrate, a PCR product was generated with forward primer Y-3Ft (5′-AGTGAATTCGGCTTTTAAACGAAGTTCCCACGT-3′) and reverse primer Y-R1A (5′-GCTATGCTCGAGAGCTTATTGCTGTCTTGCGAGACC-3′). The PCR product contained EcoRI and XhoI sites (underlined) for directional cloning in the pYES2 vector (see supplemental figure S-2). The PCR product was treated with EcoRI and XhoI then purified on a 1 % agarose gel. Next, it was inserted into pYES2 treated with EcoRI and XhoI following the suppliers protocol (Invitrogen). The recombinant vector was amplified in E. coli (Top10F’), and plasmids containing the predicted size were sent for DNA sequencing (Robarts, London, ON, Canada) to confirm the sequence of the Artemia group 1 AfrLEA-1 gene in pYES2. The recombinant plasmids were used to transfect S. cerevisiae (INVSc1) using the lithium-acetate method (Invitrogen). Clones with the correct plasmids were selected on agar plates prepared with SC minimal medium (−ura) and 2 % glucose. The yeast transfection was further confirmed by growing selected clones in media containing galactose and raffinose, while lacking glucose, and testing yeast protein extracts for LEA protein using immunoblotting.
Yeast vectors containing the Artemia gene (mRNA) coding for the full-length mitochondrial LEA protein [GenBank: GQ406334] were constructed as follows. Full-length Artemia group 1 AfrLEA gene coding for mitochondrial LEA protein was used as template in a PCR reaction with forward primer YF1A1 (5′-AGTGAATTCGGCTTAATGGAACTGTCGTCGAGT-3′) and reverse primer Y-R1A as described above. As for recombinant vector pYES2/LEA-1 described above, the PCR product generated an EcoRI and XhoI site for directional cloning. The PCR product was inserted into a DNA plasmid and amplified in E. coli (as for the pYES2 construct) followed by sequencing as described above. Plasmids with the desired sequence (pYES3/mLEA-1) were cloned in S. cerevisiae containing pYES2/cLEA-1 and selected by growth in medium containing glucose but lacking tryptophan. This resulted in doubly transfected S. cerevisiae with one vector (pYES2/AfrLEA-1) coding for cytoplasmic LEA-1 protein and one vector (pYES3/AfrLEA-1) coding for mitochondrial LEA-1 protein. The presence of both recombinant vectors in INVSc-1 was confirmed using PCR. The doubly transformed yeast produced 1.4 times the amount of LEA protein expressed in singly transformed yeast. An immunoblot showing the differences between singly and doubly transformed yeast is included as supplemental figure S-3. (Note that the cytoplasmic and mitochondrial LEA proteins are identical after processing of the 5′-leader sequence from the LEA protein designated for mitochondria.)
Prokaryotic expression
Recombinant plasmids were introduced into E. coli, strains Top10 or Top10F’, to create the following bacterial clones with different properties: pCR2.1/E11, pCR2.1/E1A, pCR2.1/E1B, pCR2.1/E1C, pCR2.1/E1C’, pCR2.1/4A1, pCR2.1/tE1C-2, pCR2.1/tE1C-3, pCR2.1/B25, pCR2.1/C5B, and empty plasmid (pCR2.1) (see Table 1). The transformed cells were grown at 37 °C and 225 rpm shaking in LB broth supplemented with ampicillin (75 μg/ml). In some cases, the expression of the LEA genes (clones tE1C-2, tE1C-3) were induced by adding 1 mM IPTG to the growth media. At the desired incubation times, cells were collected by centrifugation, washed with a dilute saline buffer (0.05 M NaCl, 10 mM Tris-HCl, pH 7.4) then heated to 80–85 °C for 10 min as described above. The heat-soluble proteins were analyzed on 10 % SDS-PAGE gels as described previously and on immunoblots to determine the pattern and/or presence of the LEA proteins produced under various treatments. Growth of the transformed bacteria was measured at either 600 or 650 nm.
Culture conditions of bacteria and yeast
Colonies from LB/agar plates containing E. coli transformed with various plasmid constructs were grown overnight in LB medium (with ampicillin) at 37 °C with shaking. The absorbance of the cultures was measured at 600 or 650 nm, and aliquots equal to 0.01 absorbance unit were added to fresh LB medium containing various concentrations of NaCl, KCl, or sorbitol plus ampicillin at a final concentration of 75 μg/ml as indicated in the figure legends. Where indicated, IPTG was added to cultures at a final concentration of 1 mM to induce expression of the AfrLEA gene in E. coli, strain Top 10 F’.
Colonies from agar plates containing S. cerevisiae transfected with various recombinant pYES plasmids were grown overnight in YPG with shaking at 30 °C (see the Invitrogen pYES manual for the composition of YPG). The growth was determined by absorbance at 600 nm at incubation times up to 30 h at 30 °C. The cell concentration was determined where 2 × 107 cells give an absorbance of 1.0 at 600 nm. Assays for the effect of Artemia LEA proteins on reactions containing NaCl, KCl, or sorbitol were exactly as described previously (Swire-Clark and Marcotte 1999). Assays for the effect of freezing and desiccation were as described previously (Swire-Clark and Marcotte 1999), except that appropriately diluted yeast cultures were collected on 0.45-μm sterile filters and then placed on agar/ampicillin plates supplemented with SC medium and 2 % glucose (see Table 2).
Table 2.
Drying- and freeze-tolerant S. cerevisiae transformed with the AfrLEA-1 gene coding for group 1 cytoplasmic or mitochondrial LEA proteins
| Drying (21 °C, air) | Freezing (−15 °C) | |||||
|---|---|---|---|---|---|---|
| Day | Control | Experimental | Control | Experimental | ||
| 0 | 100 % | 100 % | 100 % | 100 % | 100 % | 100 % |
| 1 | 32 % | 81 % | 25 % | – | 30 % | – |
| 2 | – | – | – | 6.7 % | – | 76 % |
| 3 | 14 % | 23 % | – | – | – | – |
| 4 | – | – | – | – | – | – |
| 5 | – | – | 6 % | 20 % | 21 % | 29 % |
| 6 | 2 % | 12 % | – | 4.8 % | – | 44 % |
Control yeast (containing empty vectors pYES2 and pYES3) and experimental yeast cultures, transfected with pYES2/cLEA-1/pYES3/mLEA-1, were grown to mid-log, then samples were either frozen at −16 °C or diluted with TE and aliquots containing 100 to 200 cells collected on 0.45 μm sterile filters and maintained at room temperature in a laminar flow hood for up to 6 days. At the days indicated, frozen samples were diluted to the desired concentration and collected on 0.45 μm filters. The filters were placed on plates containing SC(w) medium with 2 % glucose and 75 μg/ml ampicillin and incubated for 2 days at 30 °C. The cell count at day zero was considered to represent 100 % of viable cells for both air dried and frozen yeast. Each entry into the above table represents the average of two measurements for each sample. One experiment (two columns) was performed for drying, and two experiments (four columns) were carried out for freezing
The standard error of the mean was determined from the absorbance of three independent clones as shown in Figs. 4 and 7. Otherwise, the data represent the average of two absorbance measurements from duplicate samples taken at the times indicated.
Fig. 4.
Growth of E. coli transformed with different plasmid constructs. a Growth of E. coli Top10 after 22–23 h incubation at 37 °C in LB medium. pCR2.1 clone with the empty vector, B25 control clone with E1A sequence in reverse orientation, E1A clone with plasmid containing five 20-mer amino acid motifs in the correct orientation, E11 control clone with E1C sequence in reverse orientation, E1C plasmid with eight 20-mer amino acid motifs in the correct orientation. b SDS-PAGE analysis of transformed cells in (a). Lane 1 molecular weight marker, lane 2 clone with pCR2.1 empty plasmid, lane 3 clone B25 with five 20-mer motifs in the reverse orientation, lane 4 clone E1A with five motifs in the correct orientation, lane 5 clone E11 with eight motifs in the reverse orientation, lane 6 clone E1C with eight 20-mer amino acid motifs in the correct orientation. Each lane contained 9–10 μg protein. Asterisk indicates the position of a dominant protein missing or reduced in cells expressing the AfrLEA-1 gene. c Immunoblot of a duplicate gel to the one shown in (b). The light band of about 17 kDa in each lane, and in the previous figure, represents a background signal in the heat-soluble proteome of E. coli
Fig. 7.
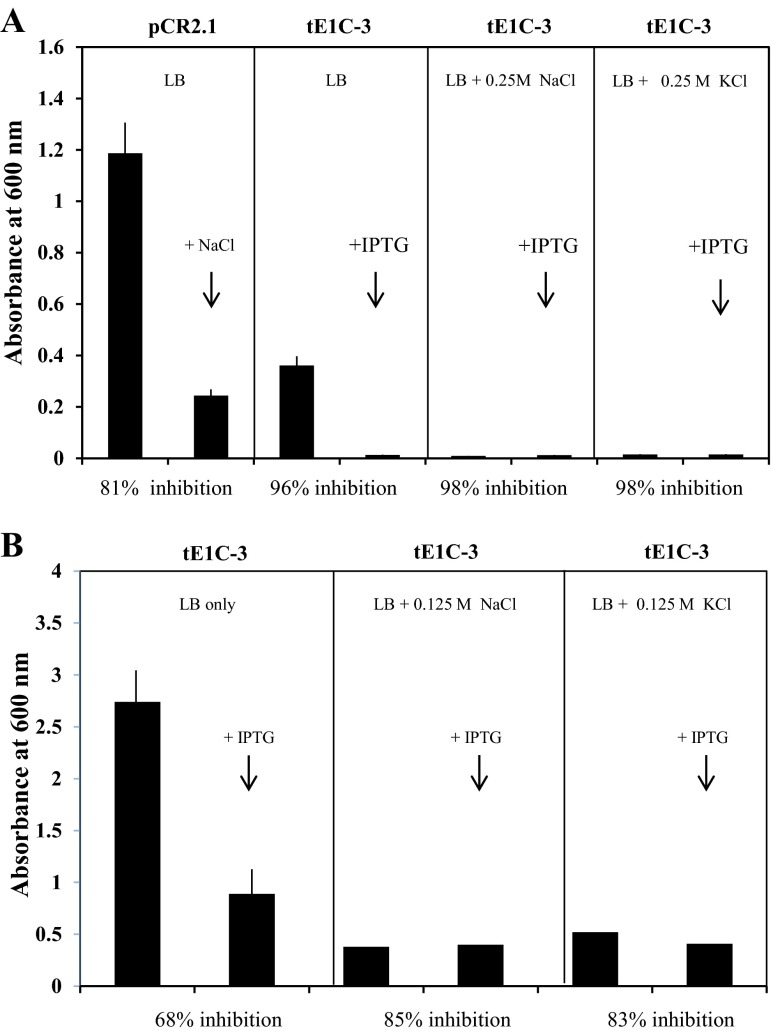
Effect of NaCl and KCl on growth of E. coli (Top10F’) transformed with Artemia LEA-1 gene (clone tE1C-3). a Growth after 18 h incubation at 37 °C in the presence of 0.25 M NaCl, KCl, and 1 mM IPTG. b Growth after 18 h incubation as in (a) in the presence of 0.125 M NaCl, KCl, and 1 mM IPTG
Results
Analysis of the heat-soluble proteome in E. coli transformed with the full-length AfrLEA-1 gene
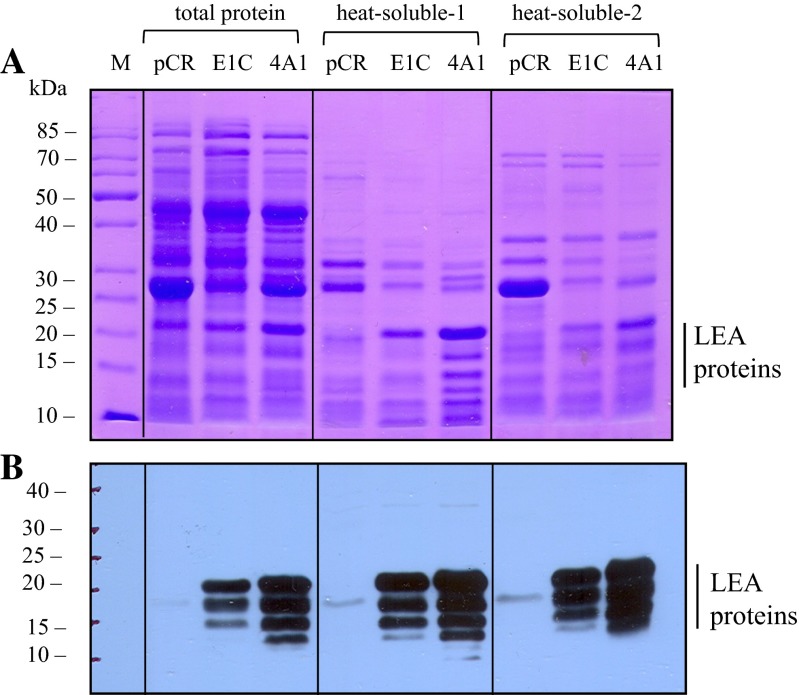
E. coli (Top10) transformed with the full-length cytoplasmic LEA-1 gene from A. franciscana (clone E1C; pCR2.1/cLEA-1, see Table 1) or the full-length mitochondrial LEA-1 gene from Artemia (clone 4A1; pCR2.1/mLEA-1, see Table 1) yielded a considerable amount of LEA proteins after 18 h of incubation without addition of an external inducer, despite being out of frame with the lacZ gene in the pCR2.1 vector (these sequences can be found in supplemental figures S-1 and S-2). Expression of the AfrLEA-1 gene (clones E1C or 4A1) yielded multiple LEA proteins ranging in size from about 21 to 13 kDa using either the direct heat-soluble protocol or the BugBuster reagent to solubilize the proteins as described under “Materials and methods.” These results are shown in Fig. 1. The reason for the multiple LEA proteins from a single cDNA is not clear. They could have arisen due to internal ribosome entry site (IRES) elements in the 5′ region of the gene (Huez et al. 1998; Colussi et al. 2015), by initiation at cryptic non-AUG sites in the gene (Touriol et al. 2003) or by proteolysis during the isolation procedure. Clearly, the translation mechanism functional here requires more study.
Fig. 1.
SDS-PAGE and immunoblot analysis of total and heat-soluble proteins in E. coli transformed with Artemia LEA-1 genes. a SDS-PAGE analysis. b Immunoblot analysis with anti-LEA proteins antibody. pCR lanes, E. coli (Top10 strain) transformed with empty vector; E1C lanes, E. coli transformed with Artemia cLEA-1 gene; 4A1 lanes, E. coli transformed with the full-length Artemia mLEA-1 gene. Total proteins (15 μg/lane) were isolated with the BugBuster reagent. Heat-soluble-1 proteins (10 μg/lane) were obtained by direct isolation (lysing) of E. coli cells, while heat-soluble-2 proteins (10 μg/lane) were obtained using first the BugBuster reagent and then heating to 80 °C as described under “Materials and methods”
Confirmation that the heat-soluble proteins in E. coli transformed with the AfrLEA-1 gene contained the Artemia group 1 LEA proteins
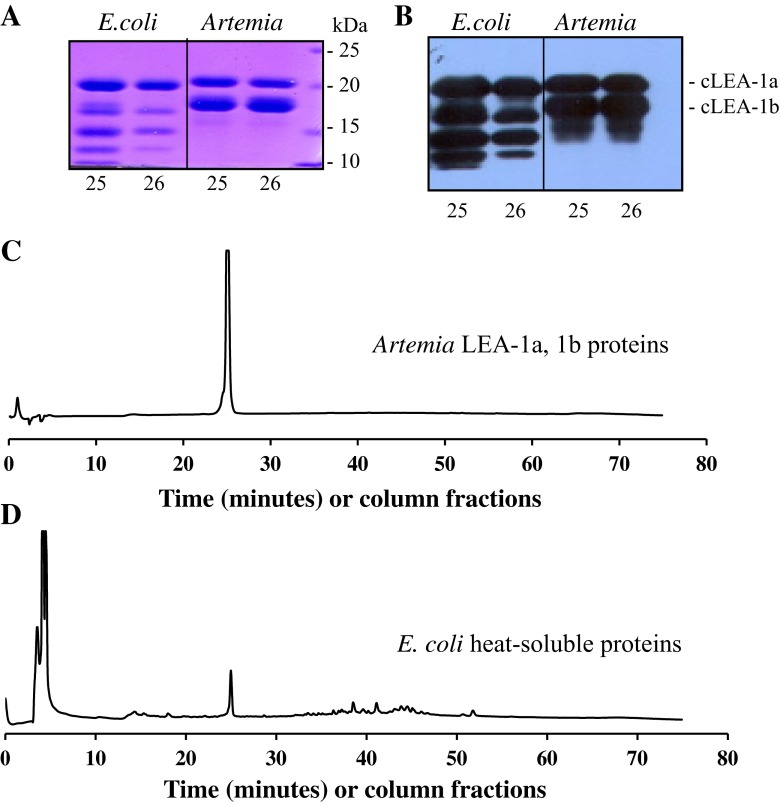
The heat-soluble proteins in E. coli transformed with the Artemia cLEA-1 gene were isolated, size-fractionated on Sepharose 12, and then purified on a Jupiter 5 μ C-18 column. The results in Fig. 2 show that the putative E. coli LEA proteins behave very similar to the major LEA proteins from Artemia on both SDS-PAGE and the reverse-phase column as described previously for the Artemia LEA proteins (Warner et al. 2010). No differences were observed in the largest band from Artemia and E. coli (panels A and B), while the additional smaller bands observed in E. coli are likely due to variable translation start sites in the transformed cells that are absent in Artemia.
Fig. 2.
Identification of LEA proteins in E. coli transformed with plasmid containing the full-length Artemia cLEA-1 cDNA. a SDS-PAGE analysis of CBB-stained proteins from C-18 RPC fractions 25 and 26 shown in (c) and (d). b Immunoblot of a duplicate gel to that shown in (a). c Chromatography on C-18 RPC of group 1 LEA proteins 1a and 1b from Artemia cysts. d Chromatography on a C-18 RPC of the heat-soluble proteins from E. coli transformed with pCR2.1 containing the full-length group 1 LEA-1 cDNA
Expression of the various Artemia LEA genes in E. coli
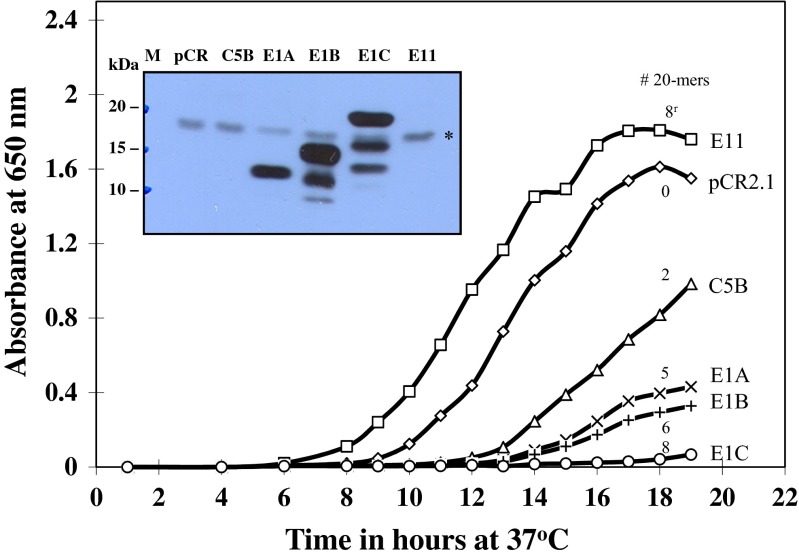
Growth of E. coli transformed with recombinant plasmids coding for Artemia group 1 LEA proteins containing from two to eight 20-mer motifs characteristic of group 1 LEA proteins from encysted embryos of Artemia is shown in Fig. 3. Clones E1A-, E1B-, and E1C-containing recombinant plasmids coding for LEA proteins with five, six, and eight 20-mer motifs, respectively, had the greatest inhibitory effect on growth, while clone C5B, encoding for two 20-mer motifs, showed the smallest inhibitory effect on growth. Clone E11 coded for eight 20-mer motifs; but in a reverse, non-functional orientation was inactive in LEA protein production as was clone pCR2.1 (empty vector). The latter two clones served as controls throughout this study. Overall, the extent of growth inhibition of E. coli transformed with different plasmid constructs appears to be dependent on the number of 20-mer motifs in the different (pCR2.1) plasmids. However, the total concentration of LEA proteins in the various clones may be important in determining the overall extent of growth inhibition in the transformed cells.
Fig. 3.
Growth of E. coli (INVαF’) transformed with plasmids containing different Artemia group 1 LEA gene constructs. The descriptors at the right have the same code as shown in Table 1. The numbers associated with each curve indicate the number of 20-mer amino acid repeats in each clone with one eight 20-mer amino acid repeat (E11), shown in reverse orientation to E1C (bottom curve). The insert is an immunoblot of the heat-soluble LEA proteins isolated from each clone after 19 h incubation. Each lane contained 4–5 μg protein. The symbol asterisk in the box shows the position of a weak antibody-reactive E. coli protein in each lane
Analysis of the heat-soluble proteins from the above clones by immunoblotting is shown in Fig. 3 (insert). Clones E1B and E1C containing recombinant plasmids coding for six and eight 20-mer motifs, respectively, yielded multiple LEA protein bands, while clone E1A containing plasmids coding for LEA proteins with five 20-mer motifs contained only one band of about 13 kDa as expected (Warner et al. 2010). E. coli transformed with a plasmid coding for two 20-mer motifs in tandem (lane 3, clone C5B) did not show a band on the immunoblot, as its size was either too small (about 5.5 kDa) to be detected with the anti-LEA protein antibodies or retained on the blotting membrane.
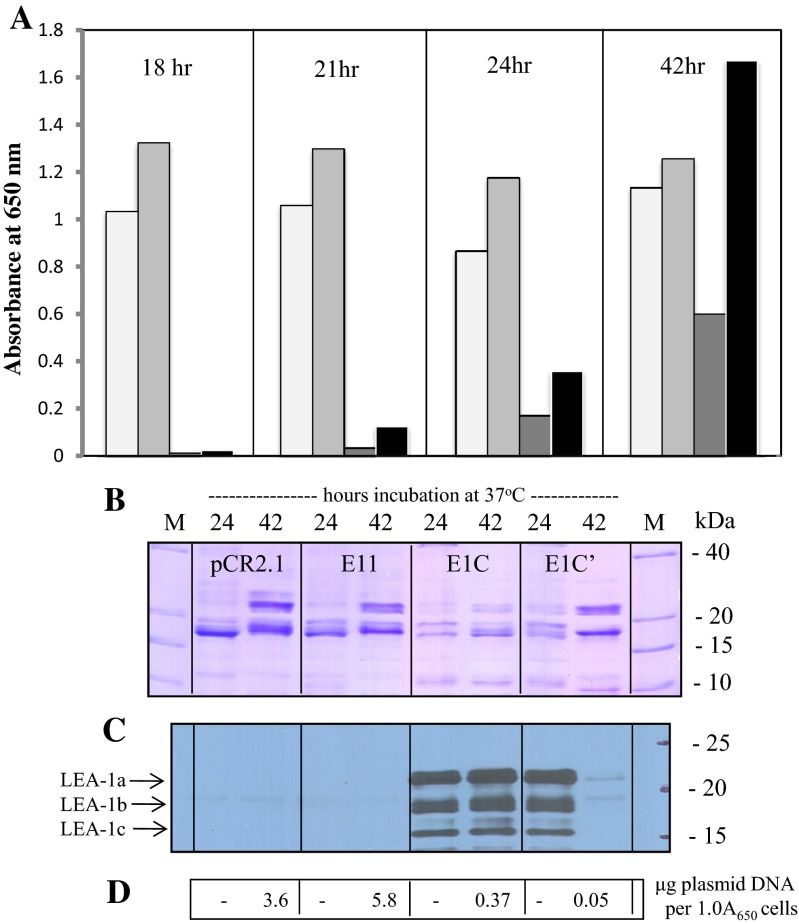
Further analysis of E. coli clones E1A and E1C (containing five and eight 20-mer motifs, respectively, in plasmid pCR2.1) resulted in 37 and 77 % inhibition of growth, compared with control clones after 22–23 h of incubation at 37 °C. These results are shown in Fig. 4. Not all clones of E. coli that transformed with plasmids containing the AfrLEA-1 gene showed the same growth kinetics. Some E1C and tE1C clones tended to lose their plasmids after multiple sub-culturing (see supplemental figure S-4). We have found this to be the case when INVαF’, Top10, and Top10F’ strains of E. coli were transformed with pCR2.1/AfrLEA-1. The results in Fig. 5 illustrate differences between two clones, E1C and E1C’, containing the full-length Artemia cDNA. The latter clone (E1C’) was derived from the former (E1C) after several sub-culturing on LB plates. When aliquots of 24 h cultures were transferred into fresh LB/ampicillin and incubated for an additional 18 h, clone E1C’ grew better than clone E1C from which it was derived. Prior to sub-culturing, the growth was apparent in both E1C and E1C’ cultures, albeit reduced considerably, in association with the presence of LEA proteins. However, after sub-culturing and 18 h of incubation (42 h total), clones E1C and E1C’ grew differently. After sub-culturing, the E1C’ culture had lost most of its ability to produce LEA proteins, compared with cultures containing E1C, which grew slower while producing LEA proteins (see Fig. 5c). The increase in growth rate appears to be due to the loss of plasmid (i.e., lower copy number) rather than loss of translational capacity of the transformed cells (see Fig. 5d). We have observed this phenomenon repeatedly over multiple sub-culturing (see supplemental figure S-4).
Fig. 5.
Growth of E. coli (strain Top 10) transformed with full-length Artemia LEA-1 cDNA. E. coli containing various plasmid constructs with AfrLEA-1 was incubated in LB medium with ampicillin (75 μg/ml). a Samples were taken (in duplicate) over 42 h incubation and the extent of growth determined by measuring the absorbance at 650 nm. Cultures of E. coli with empty pCR2.1 vector (unshaded bars), with full-length AfrLEA-1 in vector in the reverse orientation (E11, lightly shaded bars), and two clones with full-length AfrLEA-1 in vector pCR2.1 in the correct orientation (dark grey and black bars, E1C and E1C’, respectively). b SDS-PAGE analysis of the heat-soluble proteins (5 μg/lane) in various clones after 24 and 42 h incubation, stained with CBB. c Immunoblot analysis of a duplicate gel to that shown in (b) probed with group 1 anti-LEA protein antibodies. d Plasmid DNA content of clones shown in (b) after 42 h incubation
Inhibition of growth of E. coli when expression of the AfrLEA-1 gene is IPTG-dependent
When the AfrLEA-1 gene was cloned in frame with the lacZα gene in plasmid pCR2.1 and expressed in E. coli Top10F’, IPTG was required to induce the expression of the LEA-1 gene due to the large amount of the lacI gene product in the host cells. However, the growth characteristics of E. coli varied among clones transformed with pCR2.1/AfrLEA-1 compared with clones transformed with the control plasmid. For example, when two Top10F’ clones (tE1C-2 and tE1C-3), transformed with the same plasmid preparation (pCR2.1/AfrLEA-1) and in frame with the lacZα gene, were incubated in LB/ampicillin media, the growth rate was inhibited by about 30 %; while in the second clone (tE1C-3), the growth rate was inhibited by about 67 % after 18 h of incubation (see supplemental figure S-5). Thus, depending on the Top10F’ clone tested, we observed (overall) that the growth rate inhibition varied from about 24 to 80 % among sub-cultures derived from the same parental clone.
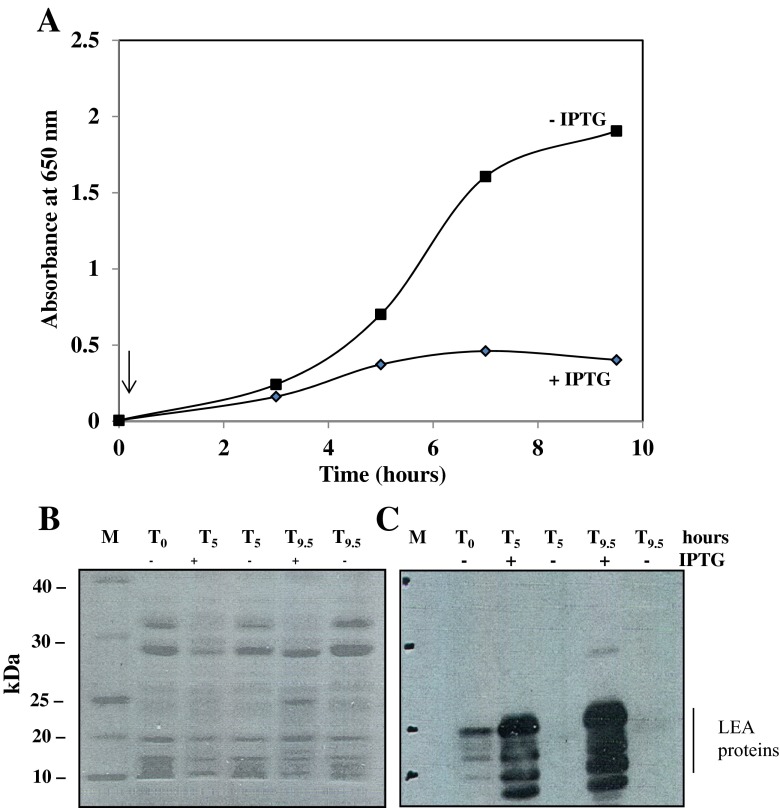
The kinetics of the IPTG effect on growth of E. coli Top10F’ transformed with pCR2.1/AfrLEA-1 (clone tE1C-3) are shown in Fig. 6. These results show that the induction of AfrLEA-1 gene expression is associated with inhibition of growth of Top10F’ E. coli, reaching 80 % after 9.5 h of incubation. Analysis of the heat-soluble proteome in these cells showed extensive induction of LEA proteins by 5 h of incubation (Fig. 6c). Occasionally, we observed some “leakiness” in overnight cultures grown in the absence of IPTG taken as starting cells (To, Fig. 6).
Fig. 6.
Growth kinetics of E. coli Top10F’ transformed with an inducible AfrLEA-1 gene in plasmid pCR2.1 (tE1C-3). a Growth kinetics over 9.5 h at 37 °C in the presence or absence of IPTG. The arrow indicates the time of IPTG addition. b SDS-PAGE gel stained with CBB of the heat-soluble proteins from E. coli collected at 0, 5, and 9.5 h as shown in (a). c Immunoblot of duplicate gel to that shown in (b) probed with the LEA protein antibody. To, starting cells from overnight culture showing some “leakyness” for expression of AfrLEA-1 gene in plasmid pCR2.1
Expression of AfrLEA-1 gene in E. coli does not overcome high-salt inhibition of growth
The growth rate of E. coli is generally strongly influenced by the salt content of the growth medium (Gowrishankar 1985). E. coli (Top10F’), clone tE1C-3, containing the full-length Artemia LEA gene in frame with the lacZα promoter, was grown overnight in LB (plus ampicillin), and aliquots were re-incubated with additional NaCl or KCl (0.125 or 0.25 M) and IPTG to induce expression of the AfrLEA-1 gene, giving the results shown in Fig. 7. Modest additions of NaCl or KCl to the growth medium (0.125 or 0.25 M, bringing the total salt concentration to 0.3 and 0.42 M, respectively) inhibited both control and transformed cells by 85–98 % after 18 h of incubation. The addition of IPTG (to induce LEA protein synthesis) did not reduce the level of growth inhibition by the salts.
Inhibition of growth of E. coli by sorbitol is modulated by expression of the AfrLEA-1 gene
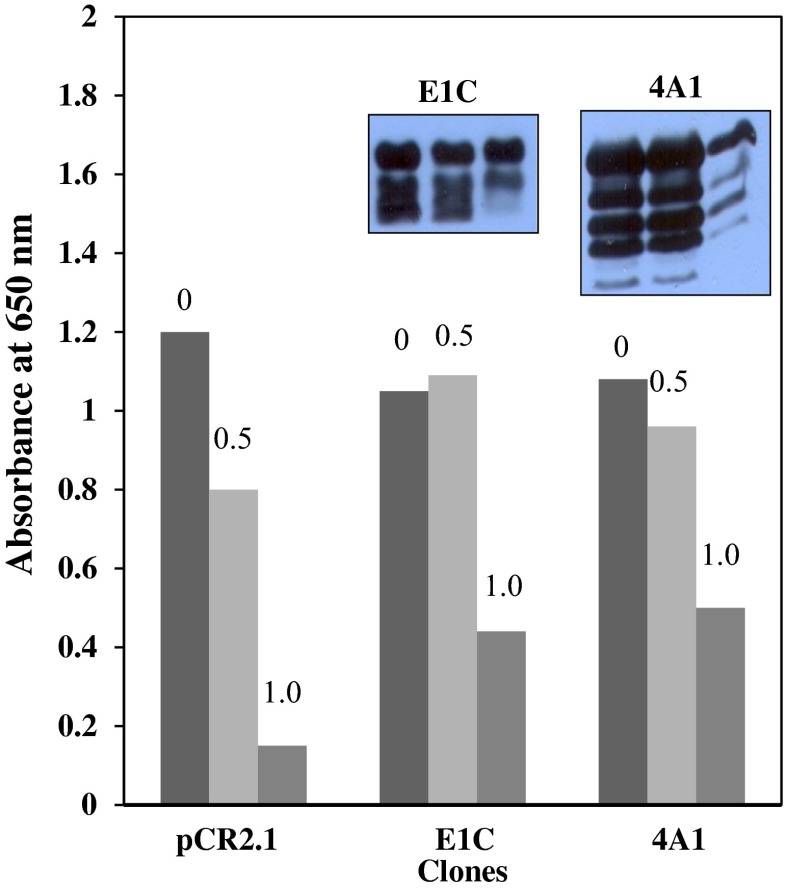
In general, group 1 LEA proteins from some plant sources have been shown to partially overcome growth inhibition in eukaryotic cells due to high concentrations of neutral osmoticums such as sorbitol in the culture medium (Swire-Clark and Marcotte 1999). However, our results with high concentrations of sorbitol were complex and difficult to interpret, depending on whether the AfrLEA-1 gene was cloned in frame with the lacZα promoter, requiring induction by IPTG, or cloned “out of frame” and translated by a non-AUG initiation mechanism. Growth of E. coli (Top10) transformed with plasmids containing the full-length AfrLEA-1 gene coding for both cytoplasmic (clone E1C), and mitochondrial LEA protein (clone 4A1) was not inhibited to any significant extent in the presence of 0.5 M sorbitol, compared to control clones transformed with the empty plasmid (pCR2.1). In fact, Top10 E. coli clones E1C and 4A1 grew more efficiently in the presence of 0.5 and 1.0 M sorbitol than control clones, probably due to the fact that E. coli has a transport system for sorbitol, which allows E. coli to utilize this sugar alcohol as a carbon source (Postma et al. 1993). These results are shown in Fig. 8. Immunoblot analysis showed that the levels of LEA proteins were not altered in clones E1C and 4A1 after 24 h of incubation in 0.5 M sorbitol but reduced in E. coli Top10 cells when incubated in the presence of 1.0 M sorbitol. The loss of LEA proteins correlates well with the enhanced growth rate of transformed cells compared with controls.
Fig. 8.
Effect of sorbitol on growth of E. coli Top10 transformed with LEA genes coding for cytoplasmic (clone E1C) and mitochondrial (clone 4A1) LEA proteins. Cells were grown for 24 h in LB medium, then equal amounts of cells were added to fresh LB medium containing 0, 0.5, or 1.0 M sorbitol. Starting cultures were transformed with empty plasmid (pCR2.1) or AfrLEA-1 genes (clones E1C and 4A1) containing LEA proteins due to the AUG-independent translation mechanism. Bars represent the average growth of duplicate cultures after 24 h, and inserts are immunoblots of the corresponding bars above clones E1C and 4A1
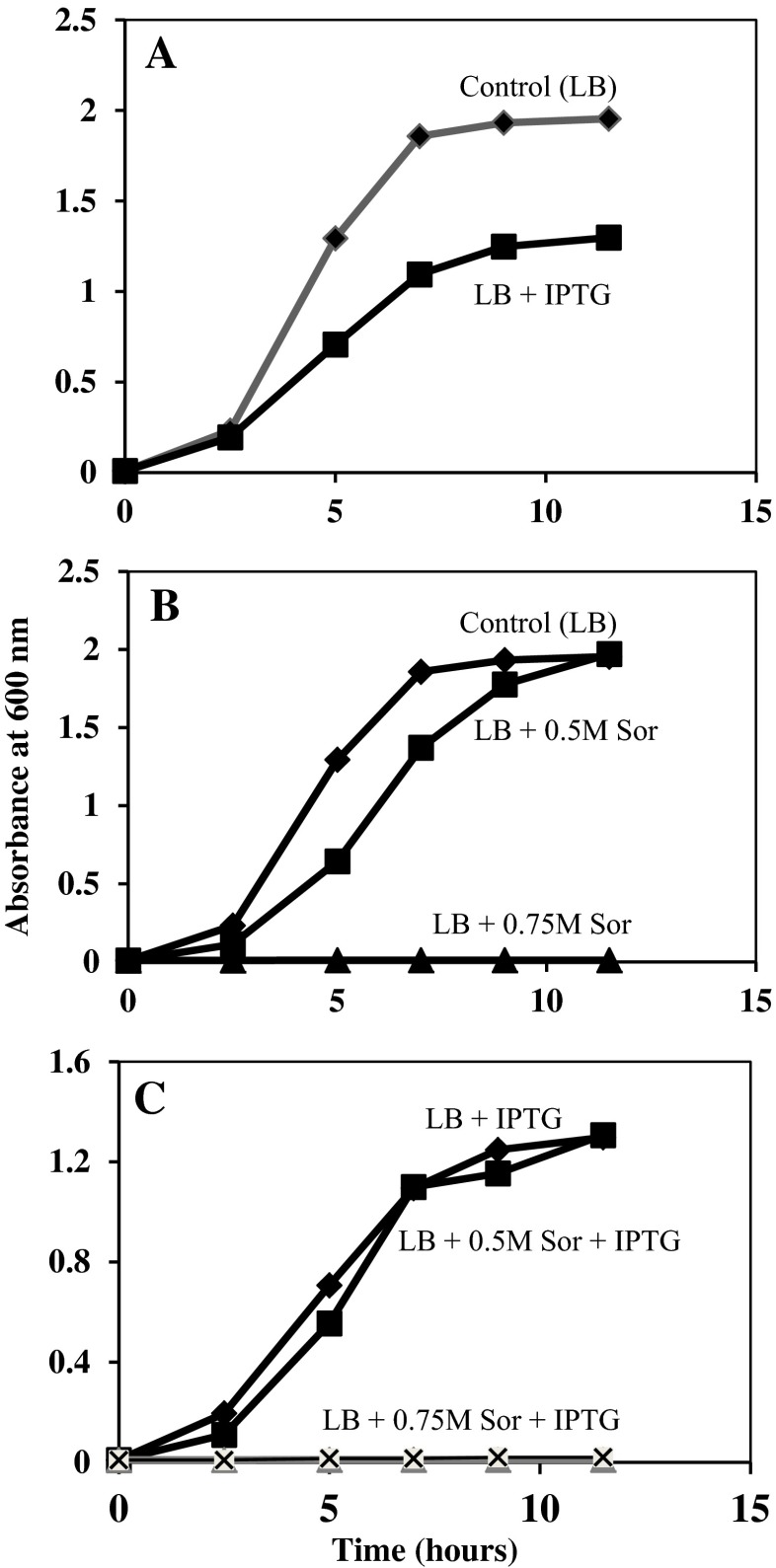
When E. coli Top10F’ cells were transformed with pCR2.1/tE1C-3 in frame with the lacZα promoter and induced with IPTG, growth inhibition was observed as expected (Fig. 9a). The inclusion of 0.5 M sorbitol alone had a more modest inhibitory effect on cell growth (Fig. 9b). However, when transformed cells were incubated in the presence of IPTG and 0.5 M sorbitol, this inhibitory effect was lost (Fig. 9c) and cell growth was indistinguishable from that of cells incubated with IPTG alone (Fig. 9c). Higher concentrations of sorbitol (0.75 M) in the culture medium completely inhibited the growth of E. coli Top10F’, even in the presence of LEA proteins. These findings contrast with those using E. coli Top10 cells containing endogenous LEA proteins synthesized by a non-AUG mechanism. The main difference in these results are that transformed Top 10 cultures were pre-loaded with LEA proteins, while transformed Top10F’ cultures contained no LEA proteins until induced by IPTG (compare Figs. 8 and 9).
Fig. 9.
Effect of sorbitol and IPTG on growth of E. coli Top10F’ transformed with pCR2.1/AfrLEA-1. Cells were grown overnight in LB medium (with ampicillin) and their concentration was determined. An equal number of cells (equivalent to 0.001 absorbance unit at 600 nm) was added to flasks containing LB as control and LB plus 0.5 M or 0.75 M sorbitol with or without IPTG (added at To hours) as indicated in (a–c). Cultures were maintained at 37 °C with shaking until the desired time at which samples were taken to determine their concentration by absorbance at 600 nm
Overall, these results (and others) show that Top10 and Top10F’ strains of E. coli are more growth tolerant to low concentrations of sorbitol when LEA proteins are present or induced. However, in the presence of high concentrations of sorbitol (0.50 to 1.0 M), LEA proteins were unable to overcome the growth inhibitory effect of sorbitol.
Expression of Artemia group 1 LEA-1 gene in S. cerevisiae
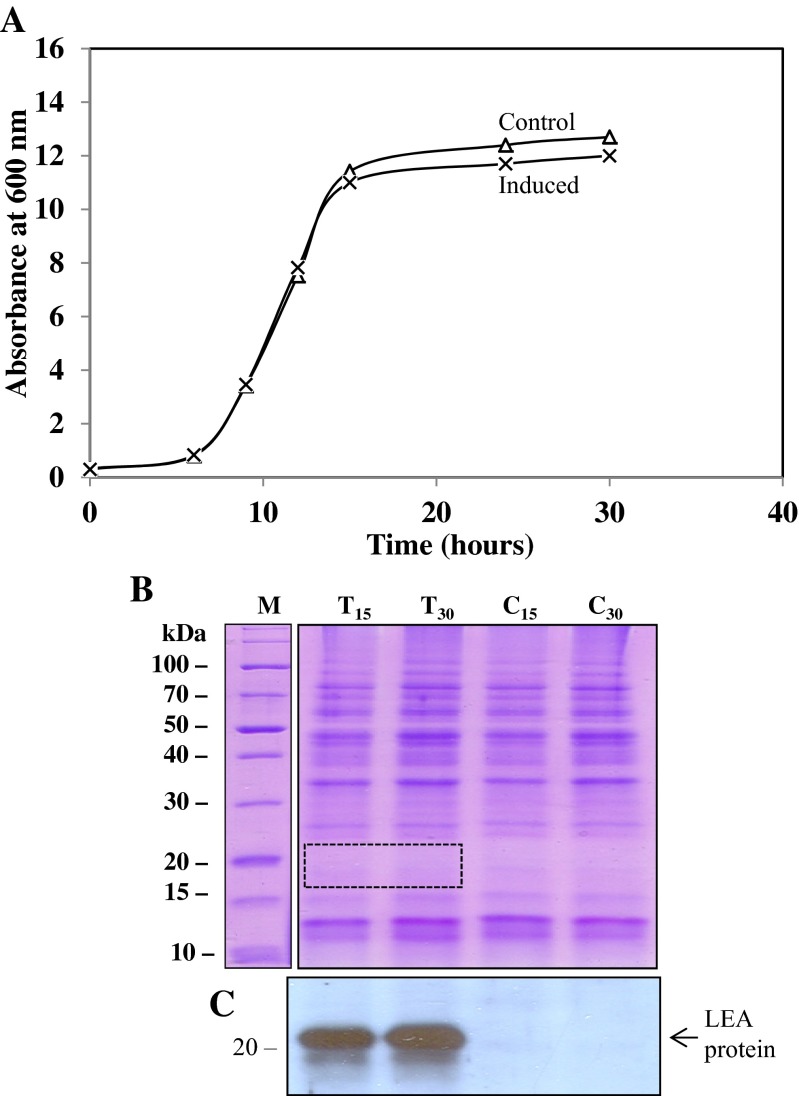
S. cerevisiae is one of the most commonly used cells as host for the expression of recombinant proteins (Ausubel et al. 1989; Calahan et al. 2011). Numerous stable cell lines can be generated with yeast and it has the molecular machinery to undertake post-translational modifications, where required, for studies of the functionality of cloned proteins in eukaryotes. In this study, we used the yeast strain INVSc1 to express Artemia group 1 LEA-1 gene to study their potential function in a heterologous cell system as described previously (Swire-Clark and Marcotte 1999). Doubly transfected yeast were grown overnight in SC(w) supplemented with 1 % raffinose and 1 % galactose to induce the cytoplasmic LEA-1 and mitochondrial LEA-1 proteins and used to inoculate fresh growth medium as described above. Results in Fig. 10 show that yeast transfected with the Artemia group 1 LEA-1 gene grew equally well compared with the control yeast clones, producing a single band of LEA proteins of about 21 kDa on an immunoblot. There was some evidence for proteolysis, but it was minimal given the time required to obtain good homogenates of each culture. The concentration of LEA proteins in transfected yeast was too low to be detected by CBB staining of an SDS-PAGE gel but not by immunoblotting (see Fig. 10) (Warner et al. 2010). At the low level of LEA proteins produced in yeast, no effect on growth was evident. These results contrast with LEA proteins expressed in E. coli which were detectable by both CBB staining of SDS-PAGE gels and immunoblot analysis (see Fig. 1).
Fig. 10.
Growth of S. cerevisiae doubly transfected with cytoplasmic and mitochondrial Artemia LEA-1 genes. Yeast were transfected with pYES2/Afr-cLEA-1 and pYES3/Afr-mLEA-1 or with pYES2 and pYES3 as controls and grown in SC(w) medium at 30 °C supplemented with 1 % raffinose and 1 % galactose. Starting cultures contained 5 × 105 cells from overnight cultures incubated in SC(w) medium containing 1 % raffinose and 1 % galactose to pre-induce cells with the LEA-1 genes. a Growth kinetics of control and induced yeast cells. b SDS-PAGE analysis of heat-soluble proteins (8 μg/lane) from control and transfected yeast after 15 and 30 h incubation stained with CBB. The dotted rectangle represents the area on the gel where the LEA proteins are expected, but too low in concentration to be detected with CBB. c Immunoblot of a duplicate gel to that in (b) showing the LEA protein antibody reactions
Expression of Artemia group 1 LEA-1 gene in S. cerevisiae does not protect yeast cells from high osmotic potentials
Previously, the wheat LEA protein Em was shown to protect yeast cells from the growth inhibitory effects of high concentrations of NaCl, KCl, and sorbitol (Swire-Clark and Marcotte 1999). Expression of the Em protein in yeast was shown to overcome, at least partially, any growth inhibition observed by various high osmoticums. In contrast to the wheat Em effect, expression of the Artemia group 1 LEA gene (AfrLEA-1) did not overcome growth inhibition by 1 M KCl, 1 M NaCl, or 1.5 M sorbitol as shown in Fig. 11a–c. Expression of AfrLEA-1 gene in yeast after 24 h incubation is also shown in the insert Fig. 11b. It should be noted that the addition of NaCl to the yeast cultures enhanced LEA gene expression in yeast, whether transfected singly (1.9-fold) or doubly (1.5-fold), with the AfrLEA-1 gene in the pYES vectors (see supplemental figure S-3).
Fig. 11.
Composite of three experiments to determine the effect of three osmoticums on growth of S. cerevisiae transfected with pYES2/pYES3 (control) or pYES2/Afr-cLEA-1 and pYES3/Afr-mLEA-1 (experimental). Control and transfected cells were grown overnight in SC(w) supplemented with 1 % raffinose and 1 % galactose and equal numbers of cells were added to culture flasks to start the incubation. a The effect of high concentration of KCl on growth of control yeast transfected with empty vectors compared with yeast doubly transfected with Artemia LEA genes. b The effect of 1 M NaCl on growth of yeast doubly transfected as in (a). The insert in (b) represents an immunoblot of control and experimental cells (i.e., LEA protein) collected after 24 h incubation. c The effect of 1.5 M sorbitol on growth of yeast doubly transfected as in (a). All osmoticums were added 6 h after the initial incubation period in SC-w/raf/gal medium
Effect of Artemia LEA-1 gene on freezing, desiccation, and heat tolerance in S. cerevisiae
Yeast transfected with Artemia LEA-1 genes coding for both cytoplasmic and mitochondrial LEA proteins displayed variable levels of protection during freezing (−15 °C) and drying (desiccation) at 22 °C when tested at daily intervals for up to 6 days, when the yeast were in log phase (see Table 2). A previous attempt to show a similar response to freezing of yeast transfected with the wheat Em protein was not successful (Swire-Clark and Marcotte 1999). The freezing and desiccation results with yeast transfected with the Artemia group 1 LEA-1 gene support recent results using RNA interference (RNAi) in Artemia embryos (Toxopeus et al. 2014). Finally, doubly transfected yeast with the Artemia LEA-1 gene was not tolerant to 15 min at 40 or 50 °C (data not shown).
Discussion
Early development in A. franciscana can occur along one of two pathways: ovoviparous and oviparous. In the former, development occurs primarily within the adult female to yield swimming nauplii, whereas in oviparous development, newly fertilized eggs become encysted in a thick shell before entering diapause, a genetically programmed event in Artemia and many other invertebrates (Clegg and Conte 1980; MacRae 2010; Wu et al. 2011).
Recently, RNA interference was used to demonstrate that group 1 LEA proteins are required for maximal tolerance of Artemia cysts in diapause to freezing and drying (Toxopeus et al. 2014). To explore further these properties of Artemia group 1 LEA proteins and potentially other stress-related functions, we tested the response of E. coli and S. cerevisiae, transfected with various Artemia group 1 LEA gene transcripts, to various stressful environmental conditions. Initially, we cloned the PCR product of Artemia LEA-1 gene into vector pCR2.1 with T overhangs at both ends of the plasmid which yielded numerous white colonies on LB/ampicillin plates. DNA sequence analysis of purified recombinant plasmids from selected white colonies gave the expected sequence of the 546 base pair AfrLEA-1 gene. However, the PCR product was often found to be cloned in the reverse, non-functional orientation within the lacZ gene of the pCR2.1 vector. Thus, most of the large white colonies of E. coli (strains Top10 and INVαF’) carried the recombinant AfrLEA-1 gene in the reverse orientation, while the small white colonies contained recombinant plasmids with the AfrLEA-1 gene in the correct (functional) orientation. As shown in Table 3, the small white colonies (E1C) with functional recombinant plasmids had a low plasmid copy number compared with the large white colonies (E11) with the AfrLEA-1 gene in the reverse, non-functional orientation along with clones transformed with the pCR2.1 vector only, which had a large copy number. Moreover, the small white colonies grown in LB, supplemented with 75 μg/ml ampicillin, yielded LEA proteins without induction, even though they were not in frame with the lacZα promoter. We had expected to see a single band of 21 kDa on immunoblots from E. coli transformed with the AfrLEA-1 gene, but multiple bands (about 4 to 5) were observed in all assays. As well, clones with the AfrLEA-1 gene coding for mitochondrial LEA protein (clone 4A1) usually contained additional bands on immunoblots (see Figs. 1, 2, and 8). Clones with spontaneously produced LEA-antibody-positive bands on immunoblots ranged in size from 21 kDa to about 13 kDa (see Fig. 1). A similar pattern of LEA protein production was observed when the PCR product of the AfrLEA-1 gene was inserted into pCR2.1 in frame with the lacZα gene, transformed into E. coli Top10F’ cells, and induced with IPTG (see Figs. 5 and 6). The LEA proteins appearing in E. coli (strains Top10 or Top10F’), transformed with the AfrLEA-1 gene coding for the largest Artemia LEA protein (ie., 21 kDa), behaved like those from cysts of Artemia on SDS-PAGE/immunoblots, although there were additional LEA proteins of different sizes (see Fig. 2). Given the fact that inclusion of various protease inhibitors in the breaking buffers used at 80–85 °C did not alter the pattern of LEA proteins appearing in E. coli, we suspect that the cloned sequence was translated in ways not common in most prokaryotes. Whether the multiple LEA protein bands, produced spontaneously in transformed E. coli or induced with IPTG, were the result of proteolysis during isolation of the bacterial heat-soluble proteome is not known. The translation mechanism in E. coli may use internal methionines or non-AUG codons to start translation, such as observed in several eukaryotic models, or use the IRES mechanism common among eukaryotes (Touriol et al. 2003; Colussi et al. 2015). If the multiple bands are due to partial amino acid sequences, they may have normal activity such as observed with clones E1A and E1B with only five or six amino acid motifs, respectively. We should note that site-directed mutagenesis of the first and second codons for methionine (to valine) in the AfrLEA-1 gene in the recombinant plasmid reduced the number of LEA proteins (i.e., bands on immunoblots) in the heat-soluble proteome of E. coli suggesting the use of internal methionines as start codons (data not shown).
Table 3.
Content of free plasmids in E. coli (Top 10) transformed with different constructs
| Source of free plasmids | Absorbance at 650 nm | Microgram of plasmid/A650 |
|---|---|---|
| pCR2.1 vector only | 0.75 to 1.5 | 4.80 ± 0.34 |
| E1C (8-mer, c.o.) | 1.40 to 1.7 | 1.66 ± 0.33 |
| E11 (8-mer, r.o.) | 0.60 to 1.4 | 4.42 ± 0.32 |
Cultures containing cells between 0.60 and 1.7 A650 densities were extracted of their plasmids using the BioBasic EZ-10 Spin columns and quantified at 260 nm. E. coli containing the pCR2.1 plasmid with group 1 LEA-1a cDNA insert in the correct orientation are designated as E1C, 8-mer, c.o., while cells containing the LEA-1 cDNA in the reverse orientation are designated as E11, 8-mer, r.o. (see Table 1). Cells with the empty vector are designated as pCR2.1
Generally, expression of LEA group 1 genes from plants has no effect on the growth kinetics of transformed E. coli cells. For example, overexpression of the group 1 gene (Em6) from Arabidopsis thaliana in E. coli did not alter the growth kinetics (Campos et al. 2006) nor did overexpression of the group 1 LEA gene (PM11; D-19) from soybean (Lan et al. 2005; Soulages et al. 2002). However, these earlier results contrast with our findings with E. coli transformed with the AfrLEA-1 gene which inhibited growth (see Figs. 3, 4, and 6). The reasons for the differences are not known, but all plant group 1 LEA proteins have an acidic pI, while the Artemia group 1 LEA proteins are basic with pIs above 7.9 (Tunnacliffe and Wise 2007; Warner et al. 2010). Also, the Artemia group 1 LEA proteins have two to eight of the characteristic 20-mer amino acid motifs in tandem, whereas the group 1 LEA proteins from A. thaliana (Em6) and Glycine max (PM-11 and D-19) contain only one 20-mer signature motif.
Among all clones tested, Artemia recombinant clone C5B with two 20-mer repeats in tandem had the least inhibitory effect on growth rate, whereas recombinant clone E1C (or tE1C-3) with 8 repeats had the greatest inhibitory effect. Clone E1A with five repeats in tandem had an intermediate growth inhibitory effect (see Figs. 3 and 4). Thus, the extent of growth inhibition in E. coli appears to be dependent on the number of 20-mer motifs coded for by different recombinant plasmids; however, an earlier report suggested that the N-terminal helical domain, and not the 20-mer characteristic amino acid motif of group 1 LEA protein, is required for enzyme protection against drying (Gilles et al. 2007). Clearly, further work is required to resolve these different results.
It is noteworthy to mention that the group 1 LEA protein content in Artemia is maximal (∼1 % of the heat-soluble proteins) during diapause when DNA synthesis and cell division are quiescent (Warner et al. 2010, 2012). We should also note that the plasmid copy number (i.e., DNA content) is highly reduced in E. coli transformed with AfrLEA-1 gene in the correct orientation, suggesting that the presence of LEA proteins inhibits plasmid replication in these cells. Whether the full-length LEA proteins from Artemia are active in the regulation of DNA synthesis, as suggested from our results and computational analysis of group 1 LEA protein of maize, remains to be determined (Wu et al. 2013).
Using E. coli as the host cell for overexpression of the Artemia group 1 LEA gene, we noted that the transformed cells contained a different complement of heat-soluble proteins than control cells. Inspection of CBB-stained SDS-PAGE gels showed that in addition to the appearance of the LEA proteins, at least four heat-stable proteins were downregulated when the AfrLEA-1 gene was expressed (see Figs. 1, 4, 5, and 6). We analyzed these heat-soluble E. coli proteins by MALDI-TOF-MS and identified them to be as follows: d-galactose binding periplasmic protein (35.7 kDa), d-ribose binding protein (30.9 kDa), inorganic pyrophosphatase (19.7 kDa), and cold-shock protein E (10.5 kDa). These results suggest that expression of the Artemia group 1 LEA genes in E. coli affected several transcription units, but the level of control is not known.
Overexpression of group 1 LEA genes from plants in E. coli has been shown to provide increased tolerance to the harmful effects of high salinity environments (Lan et al. 2005). In our studies, E. coli transformed with the AfrLEA-1 gene was not tolerant to moderately high concentrations of NaCl and KCl (see Fig. 7). These observations are consistent with recent studies on Artemia, which suggest that the cyst shell is primarily responsible for the embryos’ protection against the deleterious effects of high-salt concentrations (Dai et al. 2011; Wu et al. 2011). Clearly, there appears to be considerable functional diversity among the various group 1 LEA proteins, especially between plant and animal sources, when tested in a bacterial host.
Previous studies designed to test the potential role of LEA proteins in osmo-regulation have used E. coli as a host cell to test the expression of LEA genes without consideration of the fact that E. coli has a membrane-associated phosphotransferase systems to facilitate the uptake of osmoticants such as sorbitol. In the cell, sorbitol is converted to the glycolytic intermediate fructose-6-phosphate which serves as a carbon source for growth (Liu and Zheng 2005; Manfre et al. 2009; Postma et al. 1993). In our experiments, the response of E. coli transformed with the AfrLEA-1 gene to high concentrations of sorbitol is complex and dependent on whether the LEA gene is expressed constitutively or only after addition of an inducer such as IPTG. Data in Fig. 8 show that high concentrations of sorbitol (0.5 to 1.0 M) inhibit growth in control cells but not nearly to the same extent when LEA proteins are present. When the LEA proteins are induced in the presence of 0.5 M sorbitol, the growth inhibitory effects of the LEA proteins and sorbitol appear to neutralize one another (see Fig. 9b). The presence of LEA proteins is not able to overcome the growth inhibition produced by higher concentrations (0.75 M) of sorbitol (see Fig. 9b, c).
Since Artemia embryos in diapause contain LEA proteins in both the cytoplasm and mitochondria, we transfected S. cerevisiae with vectors containing genes coding for both the cytoplasmic (pYES2/AfrLEA-1) and mitochondrial group 1 LEA proteins (pYES3/AfrLEA-1m). In our experiments, doubly transfected S. cerevisiae showed the same growth kinetics as control yeast in standard growth media (see Figs. 10 and 11). This finding was similar to that observed by other researchers after transfecting the same strain of yeast (INVSc1) with the wheat Em gene in the pYES2 vector, even though the host cells were not transfected with an LEA gene coding for LEA proteins targeted to mitochondria (Swire-Clark and Marcotte 1999). Also, expression of the group 1 LEA gene of A. thaliana (Em6) in yeast did not alter the growth pattern (Dang et al. 2014) nor did transgenic Oryza sativa (rice) containing the wheat Em gene (Cheng et al. 2002). The lack of LEA proteins targeted to mitochondria in yeast, as described previously, may account for the inability of yeast to withstand freezing, compared to our results (Swire-Clark and Marcotte 1999).
Unlike the role of the Em protein from wheat in promoting salt tolerance in yeast, expression of the Artemia LEA gene in yeast did not improve the tolerance of yeast to high salts and sorbitol in the growth medium (see Fig. 11). Media with high osmolarity (1.0–1.5 M) are known to inhibit growth in yeast (Gaxiola et al. 1992). As was the case with wheat Em (a group 1 LEA protein), Artemia group 1 LEA proteins, induced in yeast by galactose, were undetectable on SDS-PAGE gels by CBB staining of the heat-soluble yeast proteome (see boxed area in Fig. 10). However, a distinct band of 21 kDa was detected on immunoblots from transfected yeast using Artemia anti-LEA antibodies. The low level of LEA proteins produced in yeast, regulated by the GAL1 promoter, was estimated to represent 0.1 % of the heat-soluble proteins. It should be noted that the wheat Em and Artemia group 1 LEA proteins are only 31 % identical, including the 20-mer motif characteristic of group 1 LEA proteins. Such a low concentration and weak similarity for a non-enzymatic LEA protein may account for the lack of effect of the Artemia group 1 LEA protein on the cell cycle in yeast. By comparison, expression of the AfrLEA-1 gene in E. coli yielded LEA proteins detectable by CBB staining on SDS-PAGE gels. Yet the high level of LEA proteins in transformed E. coli did not improve tolerance to high-salt concentrations in the growth medium, but they inhibited growth (see Figs. 3 and 4). As well, the low concentration of Artemia LEA protein (induced) in yeast may be insufficient to improve the tolerance of yeast to high concentrations of NaCl or KCl and/or alter the cell cycle. However, and despite the low level of wheat Em protein expressed in yeast, the Em protein was able to modulate the growth inhibitory effects of high osmoticums in yeast (Swire-Clark and Marcotte 1999).
In a limited study, we attempted to test the role of the AfrLEA protein in two mammalian cells, HEK 293T and HeLa, transiently transfected with a Flag-tagged group 1 AfrLEA-1 gene, compared with control cells transfected with Flag-empty vectors, similar to a recent study using Kc167 Drosophila melanogaster transgenetic cells (Marunde et al. 2013). Despite evidence for the localization of the Artemia LEA protein in the transfected mammalian cells, we were not able to increase cellular viability after air drying and osmotic stress (data not shown).
The group 1 LEA proteins in cysts of Artemia are non-enzymatic proteins representing 1–2 % of the heat-soluble proteome in Artemia (Sharon et al. 2009). The cysts can survive for years in the desiccated, frozen state due to the combined effect of the shell, abundance of trehalose, molecular chaperones, and complement of stress-related proteins (MacRae 2003, 2010; Clegg 2011; Liu et al. 2009). The results of our studies overall, together with data from RNAi treatment of embryos developing along the oviparous pathway, suggest that Artemia group 1 LEA proteins are important in desiccation and freeze tolerance but have little, if any, effect as osmo-protectants of encysted embryos in diapause or during early post-diapause development. The osmotic stability of encysted embryos of Artemia appears to reside mainly with the chitinous shell that surrounds the embryo (Liu et al. 2009).
Electronic supplementary material
(DOC 24 kb)
(DOC 24 kb)
(PPT 461 kb)
(DOC 1349 kb)
(PPT 196 kb)
(PPT 215 kb)
Acknowledgments
The authors wish to thank P. O. Vacratsis and A. Steevensz of the Department of Chemistry and Biochemistry for their help with the mass spectrometry analysis of selected proteins in transformed E. coli and to C. Jhajj and H. Anandan for their contribution to preliminary studies leading to this work. Funding for this work was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) through a Discovery Grant 2909 to AHW and NSERC Discovery Grant 05947 to JWH.
References
- Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K. Yeast vectors and assays for expression of cloned genes. Curr Protoc Mol Biol. 1989;2:13.16.11. [Google Scholar]
- Boswell L, Moore D, Hand S. Quantification of cellular protein expression and molecular features of group 3 LEA proteins from embryos of Artemia franciscana. Cell Stress Chaperones. 2014;19:329–341. doi: 10.1007/s12192-013-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buitink J, Leprince O. Intracellular glasses and seed survival in the dry state. C R Biol. 2008;331:788–795. doi: 10.1016/j.crvi.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Calahan D, Dunham M, Desevo C, Koshland D. Genetic analysis of desiccation tolerance in Saccharomyces cerevisiae. Genetics. 2011;189:507–519. doi: 10.1534/genetics.111.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos F, Zamudio F, Covarrubias AA. Two different late embryogenesis abundant proteins from Arabidopsis thaliana contain specific domains that inhibit Escherichia coli growth. Biochem Biophys Res Commun. 2006;342:406–413. doi: 10.1016/j.bbrc.2006.01.151. [DOI] [PubMed] [Google Scholar]
- Campos F, Cuevas-Velazquez D, Fares MA, Reyes JL, Covarrubias AA. Group 1 LEA proteins, an ancestral plant protein group, are also present in other eukaryotes, and in the archeae and bacteria domains. Mol Gen Genomics. 2013;288:503–517. doi: 10.1007/s00438-013-0768-2. [DOI] [PubMed] [Google Scholar]
- Chakrabortee S, Tripathi R, Watson M, Schierle GS, Kurniawan DP, Kaminski CF, Wise MJ, Tunnacliffe A. Intrinsically disordered proteins as molecular shields. Mol BioSyst. 2012;8:210–219. doi: 10.1039/C1MB05263B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Amons R, Clegg JS, Warner AH, Macrae TH. Molecular characterization of artemin and ferritin from Artemia franciscana. Eur J Biochem. 2003;270:137–145. doi: 10.1046/j.1432-1033.2003.03373.x. [DOI] [PubMed] [Google Scholar]
- Chen WH, Ge X, Wang W, Yu J, Hu S. A gene catalogue for post-diapause development of an anhydrobiotic arthropod Artemia franciscana. BMC Genomics. 2009;10:52. doi: 10.1186/1471-2164-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Targolli J, Huang X, Wu R. Wheat LEA genes, PMA80 and PMA1959, enhance dehydration tolerance of transgenic rice (Oryza sativa L.) Mol Breed. 2002;10:71–82. doi: 10.1023/A:1020329401191. [DOI] [Google Scholar]
- Clegg JS. Embryos of Artemia franciscana survive four years of continuous anoxia: the case for complete metabolic rate depression. J Exp Biol. 1997;200:467–475. doi: 10.1242/jeb.200.3.467. [DOI] [PubMed] [Google Scholar]
- Clegg JS. Stress-related proteins compared in diapause and in activated, anoxic encysted embryos of the animal extremophile, Artemia franciscana. J Insect Physiol. 2011;57:660–664. doi: 10.1016/j.jinsphys.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Clegg J, Conte FP. A review of the cellular and developmental biology of Artemia. In: Persoone P, Sorgeloos P, Roels O, Jaspers E, editors. The brine shrimp Artemia. Wetteren: Universa; 1980. pp. 11–54. [Google Scholar]
- Clegg JS, Trotman CAN. Physiological and biochemical aspects of Artemia ecology. Dordrecht: Kluwer Academic; 2002. [Google Scholar]
- Clegg JS, Zettlemoyer AC, Hsing HH. On the residual water content of dried but viable cells. Experientia. 1978;34:734–735. doi: 10.1007/BF01947290. [DOI] [PubMed] [Google Scholar]
- Clegg JS, Jackson SA, Warner AH. Extensive intracellular translocations of a major protein accompany anoxia in embryos of Artemia franciscana. Exp Cell Res. 1994;212:77–83. doi: 10.1006/excr.1994.1120. [DOI] [PubMed] [Google Scholar]
- Clegg JS, Jackson SA, Liang P, Macrae TH. Nuclear-cytoplasmic translocations of protein p26 during aerobic-anoxic transitions in embryos of Artemia franciscana. Exp Cell Res. 1995;219:1–7. doi: 10.1006/excr.1995.1197. [DOI] [PubMed] [Google Scholar]
- Colussi T, Costantino D, Zhu J, Donohue J, Korostelev A, Jaafar Z, Plank T, Noller H, Kieft J. Initiation of translation in bacteria by a structured eukaryotic IRES RNA. Nature. 2015;519:110–113. doi: 10.1038/nature14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Chen D-F, Liu Y-L, Zhao Y, Yang F, Yang J-S, Yang W-J. Extracellular matrix peptides of Artemia cyst shell participate in protecting encysted embryos from extreme environments. PLoS One. 2011;6:e20187. doi: 10.1371/journal.pone.0020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang N, Popova A, Hundertmark M, Hincha D. Functional characterization of selected LEA proteins from Arabidopsis thaliana in yeast and in vitro. Planta. 2014;240:325–336. doi: 10.1007/s00425-014-2089-z. [DOI] [PubMed] [Google Scholar]
- Dure L, 3rd, Greenway SC, Galau GA. Developmental biochemistry of cottonseed embryogenesis and germination: changing messenger ribonucleic acid populations as shown by in vitro and in vivo protein synthesis. Biochemistry. 1981;20:4162–4168. doi: 10.1021/bi00517a033. [DOI] [PubMed] [Google Scholar]
- Gaxiola R, Delarrinoa I, Villalba J, Serrano R. A novel and conserved salt-induced protein is an important determinant of salt tolerance in yeast. EMBO J. 1992;11:3157–3164. doi: 10.1002/j.1460-2075.1992.tb05392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles GJ, Hines KM, Manfre AJ, Marcotte WR. A predicted N-terminal helical domain of a group 1 LEA protein is required for protection of enzyme activity from drying. Plant Physiol Biochem. 2007;45:3890399. doi: 10.1016/j.plaphy.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Gowrishankar J. Identification of osmoresponsive genes in Escherichia coli: evidence for participation of potassium and proline transport systems in osmoregulation. J Bacteriol. 1985;164:434–445. doi: 10.1128/jb.164.1.434-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal K, Pinelli C, Maslen SL, Rastogi RK, Stephens E, Tunnacliffe A. Dehydration-regulated processing of late embryogenesis abundant protein in a desiccation-tolerant nematode. FEBS Lett. 2005;579:4093–4098. doi: 10.1016/j.febslet.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Goyal K, Walton LJ, Tunnacliffe A. LEA proteins prevent protein aggregation due to water stress. Biochem J. 2005;388:151–157. doi: 10.1042/BJ20041931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengherr S, Schill RO, Clegg JS. Mechanisms associated with cellular desiccation tolerance in the animal extremophile Artemia. Physiol Biochem Zool. 2011;84:249–257. doi: 10.1086/659314. [DOI] [PubMed] [Google Scholar]
- Huez I, Creancier L, Audigier S, Gensac MC, Prats AC, Prats H (1998) Two independent internal ribosome entry sites are involved in translation initiation of vascular endothelial growth factor mRNA. Mol Cell Biol 18:6178–6190 [DOI] [PMC free article] [PubMed]
- King AM, Macrae TH. The small heat shock protein p26 aids development of encysting Artemia embryos, prevents spontaneous diapause termination and protects against stress. PLoS One. 2012;7:e43723. doi: 10.1371/journal.pone.0043723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AM, Toxopus J, Macrae TH. Functional differentiation of small heat shock proteins in diapause-destined Artemia embryos. FEBS J. 2013;280:4761–4772. doi: 10.1111/febs.12442. [DOI] [PubMed] [Google Scholar]
- Lan Y, Cai D, Zheng Y-Z. Expression in Escherichia coli of three different soybean late embryogenesis abundant (LEA) genes to investigate enhanced stress tolerance. J Integr Plant Biol. 2005;47:613–621. doi: 10.1111/j.1744-7909.2005.00025.x. [DOI] [Google Scholar]
- Li S, Chakraborty N, Borcar A, Menze M, Toner M, Hand S. Late embryogenesis abundant proteins protect human hepatoma cells during acute desiccation. Proc Natl Acad Sci U S A. 2012;109:20859–20864. doi: 10.1073/pnas.1214893109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, Amons R, Macrae TH, Clegg JS. Purification, structure and in vitro molecular-chaperone activity of Artemia p26, a small heat-shock/alpha-crystallin protein. Eur J Biochem. 1997;243:225–232. doi: 10.1111/j.1432-1033.1997.0225a.x. [DOI] [PubMed] [Google Scholar]
- Liu Y-L, Zheng Y-Z. PM2, a group 3 LEA protein from soybean, and its 22-mer repeating region confer salt tolerance in Escherichia coli. Biochem Biophys Res Commun. 2005;331:325–332. doi: 10.1016/j.bbrc.2005.03.165. [DOI] [PubMed] [Google Scholar]
- Liu YL, Zhao Y, Dai ZM, Chen HM, Yang WJ. Formation of diapause cyst shell in brine shrimp, Artemia parthenogenetica, and its resistance role in environmental stresses. J Biol Chem. 2009;284:16931–16938. doi: 10.1074/jbc.M109.004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae TH. Molecular chaperones, stress resistance and development in Artemia franciscana. Semin Cell Dev Biol. 2003;14:251–258. doi: 10.1016/j.semcdb.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Macrae TH. Diapause: diverse states of developmental and metabolic arrest. J Biol Res. 2005;3:3–14. [Google Scholar]
- Macrae TH. Gene expression, metabolic regulation and stress tolerance during diapause. Cell Mol Life Sci. 2010 doi: 10.1007/s00018-010-0311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfre AJ, Lahatte GA, Climer CR, Marcotte WR. Seed dehydration and the establishment of desiccation tolerance during seed maturation is altered in the Arabidopsis thaliana mutant atem6-1. Plant Cell Physiol. 2009;50:243–253. doi: 10.1093/pcp/pcn185. [DOI] [PubMed] [Google Scholar]
- Marunde MR, Samarajeewa DA, Anderson J, Li S, Hand SC. Improved tolerance to salt and water stress in Drosophila melanogaster cells conferred by late embryogenesis abundant protein. J Insect Physiol. 2013;59:377–386. doi: 10.1016/j.jinsphys.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Menze MA, Boswell L, Toner M, Hand SC. Occurrence of mitochondria-targeted Late Embryogenesis Abundant (LEA) gene in animals increases organelle resistance to water stress. J Biol Chem. 2009;284:10714–10719. doi: 10.1074/jbc.C900001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P, Lengeler J, Jacobson G. Phosphopenolpyruvate: carbohydrate phosphotransferase systems in bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Macrae TH. Developmentally regulated synthesis of p8, a stress-associated transcription cofactor, in diapause-destined embryos of Artemia franciscana. Cell Stress Chaperones. 2007;12:255–264. doi: 10.1379/CSC-275.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Macrae TH. ArHsp22, a developmentally regulated small heat shock protein produced in diapause-destined Artemia embryos, is stress inducible in adults. FEBS J. 2008;275:3556–3566. doi: 10.1111/j.1742-4658.2008.06501.x. [DOI] [PubMed] [Google Scholar]
- Sharon MA, Kozarova A, Clegg JS, Vacratsis PO, Warner AH. Characterization of a group 1 late embryogenesis abundant protein in encysted embryos of the brine shrimp Artemia franciscana. Biochem Cell Biol. 2009;87:415–430. doi: 10.1139/O09-001. [DOI] [PubMed] [Google Scholar]
- Soulages JL, Kim K, Walters C, Cushman JC. Temperature-induced extended helix/random coil transitions in a group 1 late embryogenesis-abundant protein from soybean. Plant Physiol. 2002;128:822–832. doi: 10.1104/pp.010521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Mansour M, Crack JA, Gass GL, Macrae TH. Oligomerization, chaperone activity, and nuclear localization of p26, a small heat shock protein from Artemia franciscana. J Biol Chem. 2004;279:39999–40006. doi: 10.1074/jbc.M406999200. [DOI] [PubMed] [Google Scholar]
- Sun X, Rikkerink EHA, Jones WT, Uversky VN. Multifarious roles of intrinsic disorder in proteins illustrate its broad impact on plant biology. Plant Cell. 2013;25:38–55. doi: 10.1105/tpc.112.106062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swire-Clark GA, Marcotte WR. The wheat LEA protein Em functions as an osmoprotective molecule in Saccharomyces cerevisiae. Plant Mol Biol. 1999;39:117–128. doi: 10.1023/A:1006106906345. [DOI] [PubMed] [Google Scholar]
- Tanguay JA, Reyes RC, Clegg JS. Habitat diversity and adaptation to environmental stress in encysted embryos of the crustacean Artemia. J Biosci. 2004;29:489–501. doi: 10.1007/BF02712121. [DOI] [PubMed] [Google Scholar]
- Tompa P. Intrinsically unstructured proteins. Trends Biochem Sci. 2002;27:527–533. doi: 10.1016/S0968-0004(02)02169-2. [DOI] [PubMed] [Google Scholar]
- Touriol C, Bornes S, Bonnal S, Audigier S, Prats H, Prats A-C, Vagner S. Generation of protein diversity by alternate initiation of translation at non-AUG codons. Biol Cell. 2003;95:169–178. doi: 10.1016/S0248-4900(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Toxopeus J, Warner AH, Macrae TH. Group 1 LEA proteins contribute to the desiccation and freeze tolerance of Artemia franciscana embryos. Cell Stress Chaperones. 2014;19:939–948. doi: 10.1007/s12192-014-0518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnacliffe A, Wise MJ. The continuing conundrum of the LEA proteins. Naturwissenschaften. 2007;94:791–812. doi: 10.1007/s00114-007-0254-y. [DOI] [PubMed] [Google Scholar]
- Wang W, Meng B, Chen W, Ge X, Liu S, Yu J. A proteomic study on postdiapaused embryonic development of brine shrimp (Artemia franciscana) Proteomics. 2007;7:3580–3591. doi: 10.1002/pmic.200700259. [DOI] [PubMed] [Google Scholar]
- Warner AH, Clegg JS. Diguanosine nucleotide metabolism and the survival of artemia embryos during years of continuous anoxia. Eur J Biochem. 2001;268:1568–1576. doi: 10.1046/j.1432-1327.2001.01993.x. [DOI] [PubMed] [Google Scholar]
- Warner AH, Brunet RT, Macrae TH, Clegg JS. Artemin is an RNA-binding protein with high thermal stability and potential RNA chaperone activity. Arch Biochem Biophys. 2004;424:189–200. doi: 10.1016/j.abb.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Warner AH, Miroshnychenko O, Kozarova A, Vacratsis PO, Macrae TH, Kim J, Clegg JS. Evidence for multiple group 1 late embryogenesis abundant proteins in encysted embryos of Artemia and their organelles. J Biochem. 2010;148:581–592. doi: 10.1093/jb/mvq091. [DOI] [PubMed] [Google Scholar]
- Warner AH, Chakrabortee S, Tunnacliffe A, Clegg JS. Complexity of the heat-soluble LEA proteome in Artemia species. Comp Biochem Physiol Part D Genomics Proteomics. 2012;7:260–267. doi: 10.1016/j.cbd.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Wolkers WF, Mccready S, Brandt WF, Lindsey GG, Hoekstra FA. Isolation and characterization of a D-7 LEA protein from pollen that stabilizes glasses in vitro. Biochim Biophys Acta. 2001;1544:196–206. doi: 10.1016/S0167-4838(00)00220-X. [DOI] [PubMed] [Google Scholar]
- Wu G, Zhang H, Sun J, Liu F, Ge X, Chen W-H, Yu J, Wang W. Diverse LEA (late embryogenesis abundant) and LEA-like genes and their responses to hypersaline stress in post-diapause embryonic development of Artemia franciscana. Comp Biochem Physiol B Biochem Mol Biol. 2011;160:32–39. doi: 10.1016/j.cbpb.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Wu X, Gong F, Wang W. Functional assignment to maize group 1 LEA protein EM B564 within the cell nucleus using computational analysis. Bioinformation. 2013;9:276–280. doi: 10.6026/97320630009276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 24 kb)
(DOC 24 kb)
(PPT 461 kb)
(DOC 1349 kb)
(PPT 196 kb)
(PPT 215 kb)