Abstract
AIM: To investigate the effects of guggulsterone on the proliferation and apoptosis of human hepatoma HepG2 cells in vitro and relevant mechanisms.
METHODS: Human hepatocellular carcinoma HepG2 cells and normal human liver L-02 cells were treated with different concentrations of guggulsterone (5-100 μmol/L) for 24-72 h. Cell proliferation was tested by MTT assay. Cell cycle and apoptosis were investigated using flow cytometry (FACS). Bcl-2 and Bax mRNA and protein expression was detected by real-time PCR and Western blot, respectively. TGF-β1, TNF-α, and VEGF contents were determined by ELISA.
RESULTS: Guggulsterone significantly inhibited HepG2 cell proliferation in a dose- and time-dependent manner. FACS showed that guggulsterone arrested HepG2 cell cycle at G0/G1 phase. Guggulsterone induced apoptosis was also observed in HepG2 cells, with 24.91% ± 2.41% and 53.03% ± 2.28% of apoptotic cells in response to the treatment with 50 μmol/L and 75 μmol/L guggulsterone, respectively. Bax mRNA and protein expression was significantly increased and Bcl-2 mRNA and protein expression was decreased. ELISA analysis showed that the concentrations of TGF-β1 and VEGF were significantly decreased and TNF-α concentration was increased.
CONCLUSION: Guggulsterone exerts its anticancer effects by inhibiting cell proliferation and inducing apoptosis in HepG2 cells. Guggulsterone induces apoptosis by activation of the intrinsic mitochondrial pathway.
Keywords: Guggulsterone, Hepatocellular carcinoma cells, Apoptosis, Cell cycle, Mitochondrial pathway
Core tip: Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer and the second leading cause of tumor mortality worldwide. Guggulsterone (GS) is a phytosterol extracted from the gum resin of guggul plants, and its pro-apoptotic effect has been found to play a pivotal role in its anti-carcinogenic mechanisms. In this study, we investigated the anticancer effects of GS-induced apoptosis in human HCC cells and the underlying molecular mechanisms. Our results demonstrated that GS induced HepG2 cell apoptosis through regulating Bcl-2 and Bax expression levels.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer and the second leading cause of tumor mortality worldwide[1]. The number of new cases of HCC is increasing year by year and about 748000 new cases are being diagnosed annually, accounting for 9.2% of all new global tumor cases[2,3]. HCC has a low resectability rate, high recurrence rate, insidious onset, rapid progression, grave prognosis and high mortality[1], therefore development of anticancer drugs with explicit efficacy on HCC has now become a challenge worldwide.
Many phytochemicals derived from edible plants have shown cancer therapeutic potential[4,5]. Guggulsterone (GS) is a phytosterol extracted from the gum resin of guggul plants that shows strong antioxidant, anti-inflammatory, hypolipidemic and hypocholestremic properties[6-9], and has been used for treatment of several malignant diseases[10-13]. Pro-apoptotic effect of GS has been found to play a pivotal role in its anti-carcinogenic mechanisms[10,13].
Apoptosis is known as programmed cell death, accompanying specific morphological and biochemical changes such as cell shrinkage, nuclear and DNA fragmentation, and apoptotic body formation[14,15]. These changes are associated with a distinct set of signaling pathways including the intrinsic mitochondrial pathway and the extrinsic death receptor pathway[16]. A common step is the caspase activation in both pathways[17]. The mitochondrial pathway is activated by a variety of stimuli such as heat shock, DNA damage and reactive oxygen to increase permeability of mitochondrial outer membrane and release cytochrome C into the cytoplasm. In the presence of ATP, the cytochrome C is combined with apoptosis protease-activating factor (Apaf-1) that is combined with caspase recruitment domain and caspase-9 precursor to activate caspase-9, leading to activation of caspase-3 and caspase-7[14]. Bcl-2 family, which plays an important role in the mitochondrial pathway, contains anti-apoptotic proteins including Bcl-2, Bcl-XL and Mcl-1, and pro-apoptotic proteins such as Bax, Bad and Bek, in which Bcl-2 and Bax are more important proteins[16].
Previous studies have shown that GS can induce human colon cancer HT-29 cells apoptosis through decreasing expression of anti-apoptotic proteins including Bcl-2, cIAP-1, cIAP-2 and increasing the Bid protein expression[10]. Moreover, GS has been found to activate JNK signaling pathway in human prostate cancer cells, which subsequently upregulates the Bcl-2 family members including Bax and Bak, leading to the apoptosis of cancer cells[18,19]. Several studies have indicated that GS enhanced the sensitivity of HCC cell lines Hep3B and HepG2 to TRAIL-induced apoptosis via ROS-dependent ER stress induction[12,20,21]. However, it has not been determined whether GS has anti-HCC effects through other signaling pathways, such as the intrinsic mitochondrial pathway.
In this study, we investigated the anticancer effects of GS-induced apoptosis in human HCC cells and the underlying molecular mechanisms. Our results demonstrated that GS induced HepG2 cell apoptosis through regulating Bcl-2 and Bax expression levels.
MATERIALS AND METHODS
Reagents and antibodies
Z-guggulsterone (Z-GS) was purchased from ENZO (United States) and was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St Louis, MO) as a 16 mmol/L stock solution and stored at -20 °C. Various concentrations of Z-GS (0-100 μmol/L) were diluted in serum free RPMI1640 medium (HyClone, Utah, United States) with 0.5% (v/v) DMSO used as a vehicle control. 0.25% (w/v) trypsase was obtained from Hyclone (Utah, United States). The rabbit monoclonal antibodies against Bcl-2, Bax and β-actin were purchased from Santa Cruz Biotechnology (CA, United States). Horseradish peroxidase conjugated goat anti-rabbit and goat anti-mouse secondary antibodies were purchased from ABGENT Biotechnology (SD, United States).
Cell lines and cell culture
Human HCC cell line HepG2 and the normal human hepatic cell line L-02 were obtained from the Experimental Center of Xi’an Jiaotong University. Cells were cultured in RPMI1640 medium supplemented with 10% (v/v) fetal bovine serum (FBS, HyClone, Utah, United States), 100 U/mL penicillin (Sigma-Aldrich, St Louis, United States) and 100 μg/mL streptomycin (Sigma-Aldrich) in a humidified atmosphere of 95% (v/v) air and 5% (v/v) CO2 at 37 °C. Culture medium was changed every other day. When cells covered 80%-90% of the bottom of culture flasks, cell were washed twice with phosphate buffered saline (PBS, 137 mmol/L NaCl, 2.7 mmol/L KCl, 4.3 mmol/L Na2HPO4, 1.4 mmol/L KH2PO4, pH 7.4) and then were digested with 0.25% (w/v) trypsase. Cells were harvested using RPMI1640 medium followed by centrifugation at 1000 rpm for 10 min. Cells were re-suspended in RPMI1640 medium and were plated in appropriate plates at appropriate density and serum-starved for 24 h using serum free RPMI1640. Then the cells were treated with RPMI1640 medium containing various concentrations of Z-GS. After 24, 48 or 72 h of culture, cells were harvested as usual.
MTT assay
Cell viability was tested using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, St Louis, MO) assay as described previously[22]. Briefly, cells were plated in 96-well plates at a density of 6 × 103 cells/well followed by starvation for 24 h using serum free RPMI1640 culture medium. The culture medium was then replaced with RPMI1640 medium containing various concentrations of Z-GS (0-100 μmol/L). After 24, 48, and 72 h of culture, 20 μL of MTT solution (5 mg/mL) was added to each well and cells were continuously cultured for 4 h. Culture medium was then removed and 150 μL of DMSO was added to each well. After shaking the culture plates for 5 min, the solution was collected and the optical density (OD) was measured using a spectrophotometer (ND-1000, Thermo Fisher, United States) at a wavelength of 570 nm. The cell viability rate (%) was calculated as (ODtreated/ODcontrol) × 100%.
Cell cycle analysis
The logarithmic phase HepG2 cells and L-02 cells were plated in 6-well plates at a density of 6 × 105 cells/well and incubated in a humidified atmosphere of 95% (v/v) air and 5% (v/v) CO2 at 37 °C for 24 h. Cells were then treated with 50 μmol/L and 75 μmol/L Z-GS in RPMI1640 medium for 24 h. After washing with cold PBS twice, cells were fixed in ice-cold 70% (v/v) ethanol overnight at -20 °C. Cells were treated with Tris-HCl buffer (10 mmol/L Tris-HCl, pH 7.5) containing 1% (w/v) RNase A (Sigma-Aldrich) for 15 min, followed by incubation with propidium iodide (PI, Sigma-Aldrich) for 15 min. Cell cycles were then analyzed using a flow cytometer (CALIBUR, BD, United States), and the outputs were processed using ModFit LT2.0 software (Verity Software House, United States).
Apoptosis assay
Cell apoptosis was determined using the Annexin V-FITC and PI double staining (KaiJi, NanJing, China) as previously described[22]. Briefly, HepG2 cells and L-02 cells were plated in 6-well plates at 6 × 105 cells/well and treated with various concentrations of Z-GS (0 μmol/L, 50 μmol/L and 75 μmol/L) for 24 h. Cells were then harvested and stained with Annexin V-FITC and PI in the cold binding buffer (50 mmol/L HEPES, 700 mmol/L NaCl, 12.5 mmol/L CaCl2, pH 7.4) for 15 min at room temperature in the dark. After washing, cell apoptosis was analyzed using a flow cytometer (CALIBUR, BD, United States).
Real-time PCR analysis
Total RNA was extracted using Trizol reagent (Invitrogen, United States) as described previously[23]. RNA (1 μg) was then used to synthesize complementary DNA (cDNA) with AMV Reverse Transcriptase (RT) kit (TAKARA, Japan) according to the manufacturer’s protocols. The relative expression of Bcl-2 and Bax was analyzed by quantitative real-time PCR with SYBR Premix Ex Taq II kit (TAKARA, Japan) with GAPDH as an internal control. The reaction system contained 12.5 μL 2 × SYBR Ex Taq II, 2 μL RNA reaction, 1 μL each of forward and reverse primers and 8.5 μL sterile distilled water in a final volume of 25 μL. The reaction was performed at 95 °C for one cycle for 30 s, 95 °C for 5 s and 60 °C for 60 s for 40 cycles, and 72 °C for 60 s. Table 1 shows all the primer sequences. PCR products were run on 2% (w/v) agarose gels (Sigma-Aldrich) and stained with ethidium bromide (Sigma-Aldrich). RT-PCR outputs were detected using Bio-Rad iQ5 software (CA, United States).
Table 1.
Primers used for real-time PCR analysis
| Name | Forward primer (5’-3’) | Reverse primer (5’-3’) |
| Bcl-2 (168 bp) | TGTGTGGAGAGCGTCAAC | GGAGAAATCAAACAGAGGC |
| Bax (164 bp) | ATGCGTCCACCAAGAAGC | CCAGTTGAAGTTGCCGTC |
| GAPDH (138 bp) | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA |
Western blot analysis
Bcl-2 and Bax protein expression was investigated using Western blot analysis as described previously[23]. Briefly, cells were harvested and re-suspended in RIPA lysis buffer [20 mmol/L Tris, 150 mmol/L NaCl, 1% (v/v) Triton X-100, 1% (w/v) digestive phosphatase inhibitors, 1% (w/v) protease inhibitors, 1% (w/v) phenylmethyl sulfonylfluoride (PMSF), pH 7.5] (Sigma-Aldrich). Protein concentration was determined using BCA assay (Kangweishiji, BeiJing, China) according to the manufacturer’s protocols. Equal amounts of protein (30 μg/lane) were separated in a 10% (w/v) sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel (Sigma-Aldrich), and were then electrotransferred onto polyvinylidenedifluoride (PVDF) membranes (Sigma-Aldrich). After blocking with 5% (w/v) bovine serum albumin (BSA, Sigma-Aldrich) in Tris-buffered saline (TBS, 0.1 mol/L, pH 7.4), membranes were incubated with primary antibodies (1:1000 dilution) overnight at 4 °C. After washing, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000 dilution) for 1 h at room temperature with agitation. All membranes were detected using the ECL Western Blotting Kit Reagent (Thermo Fisher, United States).
Cytokine detection
Cell supernatants were collected from Z-GS treated and untreated control cells and filtered through a 0.22 μm filter (Millipore, United States). The levels of TGF-β1, TNF-α and VEGF in the culture medium were determined using enzyme-linked immunosorbent assay kit (ELISA, eBioscience, United States) according to the manufacturer’s protocols.
Statistical analysis
Data are presented as mean ± SD, and were tested for normality and equal variance. Student’s t-test or one-way analysis of variance (ANOVA) plus Bonferroni’s post-test was carried out using SPSS 15.0 software (SPSS Inc., United States). P-values less than 0.05 were considered statistically significant.
RESULTS
GS inhibits the viability of human HCC cells
Compared with the control group, the viability of HepG2 and L-02 cells was significantly decreased in a dose- and time-dependent manner in response to 5, 10, 25, 50, 75 and 100 μmol/L of GS for 24 h (Figure 1A). Interestingly, 25, 50, 75 and 100 μmol/L of GS significantly reduced the HepG2 cell viability when compared with that in the L-02 cells after 24 h of treatment (Figure 1B), suggesting a less sensitivity to GS in L-02 cells relative to HepG2 cells. Similar results were revealed after 48 and 72 h of treatment (data not shown). The half maximal inhibitory concentration (IC50) of GS in HepG2 cells for 24 h was 75 μmol/L. Therefore, 50 μmol/L and 75 μmol/L GS was used for all subsequent experiments in HepG2 cells.
Figure 1.
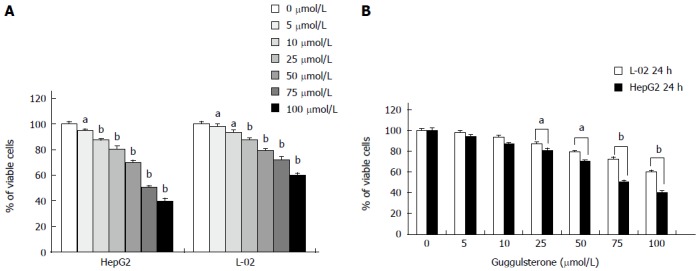
Human L-02 and HepG2 cells show different cell viabilities in response to guggulsterone treatment. Cell viability in L-02 and HepG2 cells was determined using MTT assay after incubation with 5, 10, 25, 50, 75 and 100 μmol/L guggulsterone, respectively, for 24 h. Guggulsterone significantly reduced cell viability in both L-02 and HepG2 cells in a dose- and time-dependent manner (A). Same concentration of guggulsterone differently reduced cytotoxicity between L-02 and HepG2 cells (B). aP < 0.05 or bP < 0.01, vs the control group.
Effect of GS on cell cycle in human HepG2 and L-02 cells
After treatment with 75 μmol/L of GS for 24 h, there was an increase in G0/G1 fraction (62.88% ± 2.67% vs 44.02% ± 1.07%) but decrease in G2/M fraction of HepG2 cells (13.33% ± 1.84% vs 28.33% ± 1.25%) in comparison with the untreated control cells (P < 0.05; Figure 2A). However, 50 μmol/L GS did not induce a significant difference in cell cycle fractions in HepG2 cells when compared with the control group (P > 0.05; Figure 2A). Differently, neither 75 μmol/L nor 50 μmol/L of GS induced a significant alteration in L-02 cell cycle (P > 0.05; Figure 2B).
Figure 2.
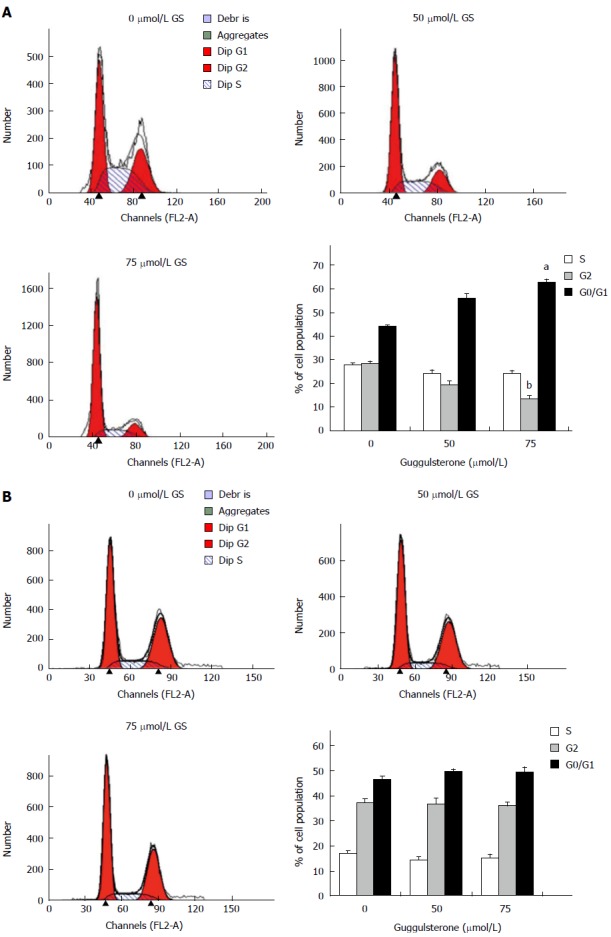
Guggulsterone alters cell cycle in human HepG2 and L-02 cells. Human HepG2 (A) and L-02 cells (B) were treated with 0 μmol/L, 50 μmol/L and 75 μmol/L of GS for 24 h and the cell cycle was analyzed using flow cytometry, respectively. The histogram showed mean % of cell population in each phase of cell cycle. aP < 0.05 or bP < 0.01, vs the untreated control group.
GS induces apoptosis of human HepG2 cells
After treatment with 50 μmol/L and 75 μmol/L GS for 24 h, the percentages of apoptotic cells in HepG2 cells were 24.91% ± 2.41% and 53.03% ± 2.28%, respectively, significantly higher than that in the untreated control cells (5.18% ± 1.74, P < 0.01) (Figure 3A). However there was no significant difference in L-02 cell apoptosis in response to 50 μmol/L and 75 μmol/L GS treatment (P > 0.05; Figure 3B).
Figure 3.

Guggulsterone induces apoptosis in human HepG2 and L-02 cells. Human HepG2 (A) and L-02 cells (B) were treated with 0 μmol/L, 50 μmol/L and 75 μmol/L of GS for 24 h and cell apoptosis was investigated using annexin V-FITC and PI. GS significantly increased HepG2 cell apoptosis in a dose-dependent manner. bP < 0.01, vs the control group.
GS induces apoptosis through activation of intrinsic mitochondrial signaling pathway
There was a significant increase in Bax mRNA and protein levels in HepG2 cells treated with 50 μmol/L and 75 μmol/L GS for 24 and 48 h, respectively (Figure 4A). Differently, Bcl-2 mRNA and protein contents in HepG2 cells were significantly reduced in response to 50 μmol/L and 75 μmol/L GS (Figure 4B). However, neither 50 μmol/L nor 75 μmol/L induced significant alterations in Bax (Figure 4C) or Bcl-2 expression (Figure 4D) in L-02 cells (P > 0.05).
Figure 4.
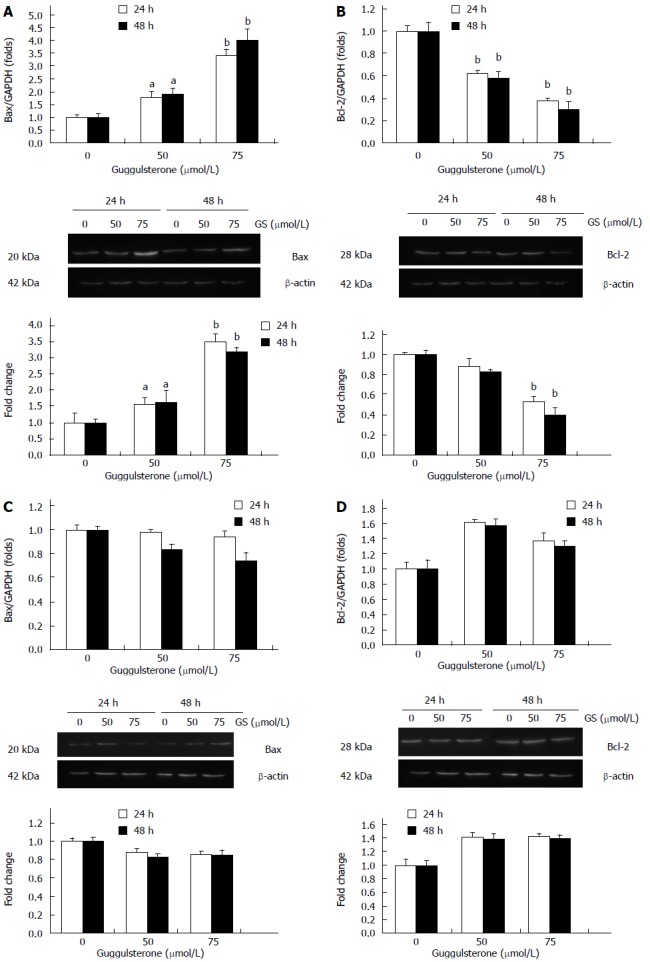
Guggulsterone differently alters Bax and Bcl-2 expression in HepG2 cell. HepG2 (A and B) and L-02 cells (C and D) were treated with 0 μmol/L, 50 μmol/L and 75 μmol/L of GS for 24 and 48 h, respectively. Bax and Bcl-2 mRNA and protein levels were investigated using real-time PCR and Western blot, respectively. aP < 0.05 or bP < 0.01, vs the control group.
Effect of GS on TGF-β1, TNF-α and VEGF cytokines
After treatment with 35, 50 and 75 μmol/L of GS for 24 h, TGF-β1 and VEGF levels in HepG2 cells were significantly decreased when compared with the control group (P < 0.01; Figure 5A and B). Differently, TNF-α contents in HepG2 cells were significantly increased in a dose-dependent manner in response to the treatment with 35, 50 and 75 μmol/L of GS when compared with the untreated cells (Figure 5C). Interestingly, there was no significant difference in TGF-β1 (Figure 5D), VEGF (Figure 5E) or TNF-α levels (Figure 5F) in L-02 cells after treatment with 35, 50 and 75 μmol/L of GS for 24 h.
Figure 5.
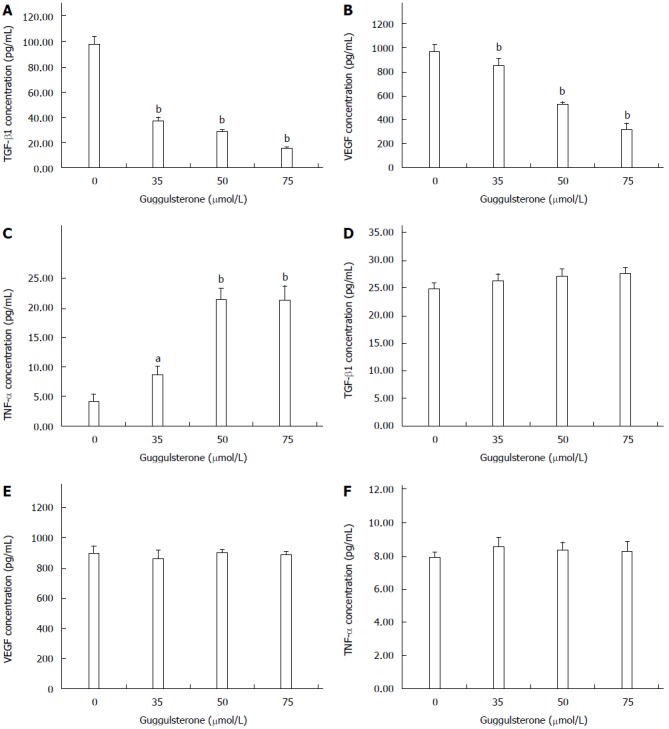
Guggulsterone alters TGF-β1, TNF-α and VEGF levels in HepG2 and L-02 cells. HepG2 (A, B, and C) and L-02 cells (D, E, and F) were treated with 0, 35, 50 or 75 μmol/L of GS for 24 h, respectively. TGF-β1, TNF-α and VEGF levels in the culture medium were investigated using ELISA. aP < 0.05 or bP < 0.01, vs the control group.
DISCUSSION
GS has been used as an anticancer agent due to its cell proliferation inhibition and pro-apoptosis effects in tumor cells[10,18]. However, whether GS can be used in HCC treatment is still largely unknown. In this study, the proliferation of HepG2 cells was significantly inhibited by GS in a dose- and time-dependent manner. More importantly, cell proliferation in normal hepatic cell line L-02 was less affected by GS when compared with HepG2 cells, suggesting that normal hepatocytes are significantly more resistant to growth inhibition by GS compared with HCC cells. Therefore, we investigated whether GS treatment has a selective activity to human HCC cells and normal liver cells by conducting experiments on the effects of GS on cell cycle and apoptosis of L-02, a normal human hepatic cell line.
G0/G1 fractions of cell cycles were increased but G2/M fractions were decreased in HepG2 cells in response to treatment with GS, indicating that GS arrested HepG2 cell cycle in G0/G1 phase, and thereby inhibited HCC cell proliferation. The precise mechanism is not clear, however, previous studies performed in other tumor cell types have illustrated that GS regulates cell cycle via downregulated expression level of cell cycle regulatory protein cyclin D1 and induced expression of cyclin dependent kinase inhibitor P21WAF1/CIP1 and P27[24,25].
Furthermore, we found that GS induced HepG2 cell apoptosis in a dose-dependent way, which is also consistent with previous studies[10,24,26]. Our further investigation indicated that GS increased Bax but decreased Bcl-2 gene and protein expression in HepG2 cells, illustrating that the intrinsic mitochondrial pathway was involved in the pro-apoptosis effect of GS. A variety of stress stimuli including growth factor withdrawal, heat shock, and oxidative damage have been shown to activate the apoptosis intrinsic or mitochondrial pathway[27-29]. TGF-β is a multifunctional cytokine involved in the regulation of apoptosis of many cell types and is implicated in the pathogenesis of human diseases, including carcinogenesis. The VEGF family plays a pivotal role in tumor angiogenesis and is responsible for solid tumor growth and metastasis[29-31]. We therefore investigated the effects of GS on the levels of TGF and VEGF which have been confirmed to be involved in the HCC occurrence and development. As expected, TGF-β1 and VEGF concentrations were significantly decreased in response to the treatment with GS in a dose-dependent manner, indicating that these two growth factors may be negatively involved in the HCC cell apoptosis. The underlying mechanism is still unknown, however, previous studies have shown that the low expression of their receptors on the cell surface induced by GS may be involved[32,33].
Accumulating evidence has suggested that GS has chemopreventive and chemotherapeutic potential for cancer treatment. Although the underlying mechanisms are not fully understood, these studies including ours clearly indicated that anticancer activity of GS is associated with apoptosis induction. Specifically, GS treatment of cancer cells induces the alteration and regulation of Bcl-2 gene family proteins, NF-κB signaling, MAPK pathways, farnesoid X receptor, EGFR-STAT3 signaling, etc. However, these results are largely from other cancer cell types. It is still largely unknown whether GS has similar effects on HCC cells. In the present study, for the first time, we showed that GS treatment significantly inhibited cell growth, induced cell cycle arrest and caused apoptotic cell death in human HCC cells. Our results revealed a novel anti-HCC mechanism of GS, in which the mitochondrial pathway is involved in the GS induced apoptosis in human hepatocarcinoma.
Interestingly, GS treatment did not induce a significant cell cycle arrest and apoptosis in L-02 cells, which may explain the reason why a normal human hepatocyte cell line is more resistant to the growth inhibition by GS as described above. Unsurprisingly, our further investigation showed less alterations in mitochondrial pathway protein bax and Bcl-2, as well as growth factors/cytokines including TGF-β1, VEGF and TNF-α levels in L-02 cells in response to the treatment with GS. These results indicated that normal hepatocytes are more resistant to the growth inhibition by GS when compared with the HCC cells, which is consistent to previous results in other cancer cell types[34]. The mechanism is largely unknown, however, uncharacterized constituent(s) of GS may interact additively or synergistically to inhibit the viability of the cancer cells.
In conclusion, we demonstrated that GS inhibited the viability of human HCC cells by regulated cell cycle, induced tumor cell apoptosis by activation of the mitochondrial pathway, and decreased TGF-β1 and VEGF but increased TNF-α levels. These results suggest that GS influences HCC phenotypes by inhibiting cell proliferation, promoting cell death and regulating carcinogenesis-related growth factors, and therefore is a potential anticancer agent for the treatment of human HCC.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer and the second leading cause of tumor mortality worldwide. Guggulsterone (GS) is a phytosterol extracted from the gum resin of guggul plants that shows strong antioxidant, anti-inflammatory, hypolipidemic and hypocholestremic properties, and has been used for treatment of several malignant diseases. Apoptosis is known as programmed cell death, which is associated with a distinct set of signaling pathways including the intrinsic mitochondrial pathway and the extrinsic death receptor pathway. Previous studies have shown that GS can induce cancer cell apoptosis through activation of the extrinsic death receptor pathway. However, it has not been determined whether GS has anti-HCC effects through other signaling pathways, such as the intrinsic mitochondrial pathway.
Research frontiers
Due to the strong antioxidant, anti-inflammatory, hypolipidemic and hypocholestremic properties of GS, it has been used for treatment of several malignant diseases including HCC. Pro-apoptotic effect of GS has been found to play a pivotal role in its anti-carcinogenic mechanisms, however, its precise mechanism is not fully understood. Therefore, the relationship between GS and cell apoptosis becomes an important research area in this field.
Innovations and breakthroughs
In this study, the authors demonstrated that GS inhibited the viability of human HCC cells by regulated cell cycle, induced tumor cell apoptosis by activation of the mitochondrial pathway, and decreased TGF-β1 and VEGF but increased TNF-α levels. These results suggest that GS influences HCC phenotypes by inhibiting cell proliferation, promoting cell death and regulating carcinogenesis-related growth factors, and therefore is a potential anticancer agent for the treatment of human HCC. This is the first systematical study demonstrating that GS induces HCC cell apoptosis through activation of the mitochondrial pathway, and evidence shown here expands our knowledge of the precise mechanism how GS inhibits HCC development.
Applications
The study results suggest that GS induces HCC apoptosis and could be a potential treatment drug for this commonly prevalent tumor in humans in the future.
Peer-review
Other reports have shown that GS is pro-apoptotic and modulates various genes. These authors show that GS arrested HepG2 cycle at G0/G1 phase. Bax mRNA was increased together with other changes, suggesting that the intrinsic mitochondrial pathway is involved in GS effects. The study indicates that GS is a potential anticancer therapy for HCC.
Footnotes
Supported by Science and Technology Foundation of Shaanxi Province, China, No. 2007K16-07(9).
Institutional review board statement: No human or animal subjects in the study.
Institutional animal care and use committee statement: Animals were not used in the study.
Conflict-of-interest statement: To the best of our knowledge, no conflict of interest exists.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 8, 2015
First decision: July 19, 2015
Article in press: October 20, 2015
P- Reviewer: Shukla SD S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Kew MC. Hepatocellular carcinoma: epidemiology and risk factors. Available from: http://www.dovepress.com/hepatocellular-carcinoma-epidemiology-and-risk-factors-peer-reviewed-article-JHC#. [DOI] [PMC free article] [PubMed]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Takada Y, Oommen OV. From chemoprevention to chemotherapy: common targets and common goals. Expert Opin Investig Drugs. 2004;13:1327–1338. doi: 10.1517/13543784.13.10.1327. [DOI] [PubMed] [Google Scholar]
- 6.Lee JY, Lee KT, Lee JK, Lee KH, Jang KT, Heo JS, Choi SH, Kim Y, Rhee JC. Farnesoid X receptor, overexpressed in pancreatic cancer with lymph node metastasis promotes cell migration and invasion. Br J Cancer. 2011;104:1027–1037. doi: 10.1038/bjc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niranjan R, Kamat PK, Nath C, Shukla R. Evaluation of guggulipid and nimesulide on production of inflammatory mediators and GFAP expression in LPS stimulated rat astrocytoma, cell line (C6) J Ethnopharmacol. 2010;127:625–630. doi: 10.1016/j.jep.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Yang D, Yang J, Shi D, Xiao D, Chen YT, Black C, Deng R, Yan B. Hypolipidemic agent Z-guggulsterone: metabolism interplays with induction of carboxylesterase and bile salt export pump. J Lipid Res. 2012;53:529–539. doi: 10.1194/jlr.M014688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almazari I, Park JM, Park SA, Suh JY, Na HK, Cha YN, Surh YJ. Guggulsterone induces heme oxygenase-1 expression through activation of Nrf2 in human mammary epithelial cells: PTEN as a putative target. Carcinogenesis. 2012;33:368–376. doi: 10.1093/carcin/bgr259. [DOI] [PubMed] [Google Scholar]
- 10.An MJ, Cheon JH, Kim SW, Kim ES, Kim TI, Kim WH. Guggulsterone induces apoptosis in colon cancer cells and inhibits tumor growth in murine colorectal cancer xenografts. Cancer Lett. 2009;279:93–100. doi: 10.1016/j.canlet.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Yamada T, Osawa S, Hamaya Y, Furuta T, Hishida A, Kajimura M, Ikuma M. Guggulsterone suppresses bile acid-induced and constitutive caudal-related homeobox 2 expression in gut-derived adenocarcinoma cells. Anticancer Res. 2010;30:1953–1960. [PubMed] [Google Scholar]
- 12.Chen KF, Chen HL, Liu CY, Tai WT, Ichikawa K, Chen PJ, Cheng AL. Dovitinib sensitizes hepatocellular carcinoma cells to TRAIL and tigatuzumab, a novel anti-DR5 antibody, through SHP-1-dependent inhibition of STAT3. Biochem Pharmacol. 2012;83:769–777. doi: 10.1016/j.bcp.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 13.Shishodia S, Sethi G, Ahn KS, Aggarwal BB. Guggulsterone inhibits tumor cell proliferation, induces S-phase arrest, and promotes apoptosis through activation of c-Jun N-terminal kinase, suppression of Akt pathway, and downregulation of antiapoptotic gene products. Biochem Pharmacol. 2007;74:118–130. doi: 10.1016/j.bcp.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debatin KM. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother. 2004;53:153–159. doi: 10.1007/s00262-003-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 16.Sprick MR, Walczak H. The interplay between the Bcl-2 family and death receptor-mediated apoptosis. Biochim Biophys Acta. 2004;1644:125–132. doi: 10.1016/j.bbamcr.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Ricci MS, Zong WX. Chemotherapeutic approaches for targeting cell death pathways. Oncologist. 2006;11:342–357. doi: 10.1634/theoncologist.11-4-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh SV, Choi S, Zeng Y, Hahm ER, Xiao D. Guggulsterone-induced apoptosis in human prostate cancer cells is caused by reactive oxygen intermediate dependent activation of c-Jun NH2-terminal kinase. Cancer Res. 2007;67:7439–7449. doi: 10.1158/0008-5472.CAN-07-0120. [DOI] [PubMed] [Google Scholar]
- 19.Singh SV, Zeng Y, Xiao D, Vogel VG, Nelson JB, Dhir R, Tripathi YB. Caspase-dependent apoptosis induction by guggulsterone, a constituent of Ayurvedic medicinal plant Commiphora mukul, in PC-3 human prostate cancer cells is mediated by Bax and Bak. Mol Cancer Ther. 2005;4:1747–1754. doi: 10.1158/1535-7163.MCT-05-0223. [DOI] [PubMed] [Google Scholar]
- 20.Anan A, Gores GJ. A new TRAIL to therapy of hepatocellular carcinoma: blocking the proteasome. Hepatology. 2005;42:527–529. doi: 10.1002/hep.20869. [DOI] [PubMed] [Google Scholar]
- 21.Moon DO, Park SY, Choi YH, Ahn JS, Kim GY. Guggulsterone sensitizes hepatoma cells to TRAIL-induced apoptosis through the induction of CHOP-dependent DR5: involvement of ROS-dependent ER-stress. Biochem Pharmacol. 2011;82:1641–1650. doi: 10.1016/j.bcp.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Sun MZ, Dang SS, Wang WJ, Jia XL, Zhai S, Zhang X, Li M, Li YP, Xun M. Cytokeratin 8 is increased in hepatitis C virus cells and its ectopic expression induces apoptosis of SMMC7721 cells. World J Gastroenterol. 2013;19:6178–6187. doi: 10.3748/wjg.v19.i37.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dang SS, Sun MZ, Yang E, Xun M, Ma L, Jia ZS, Wang WJ, Jia XL. Prohibitin is overexpressed in Huh-7-HCV and Huh-7.5-HCV cells harboring in vitro transcribed full-length hepatitis C virus RNA. Virol J. 2011;8:424. doi: 10.1186/1743-422X-8-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macha MA, Matta A, Chauhan S, Siu KM, Ralhan R. 14-3-3 zeta is a molecular target in guggulsterone induced apoptosis in head and neck cancer cells. BMC Cancer. 2010;10:655. doi: 10.1186/1471-2407-10-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leeman-Neill RJ, Wheeler SE, Singh SV, Thomas SM, Seethala RR, Neill DB, Panahandeh MC, Hahm ER, Joyce SC, Sen M, et al. Guggulsterone enhances head and neck cancer therapies via inhibition of signal transducer and activator of transcription-3. Carcinogenesis. 2009;30:1848–1856. doi: 10.1093/carcin/bgp211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel MP, Masood A, Patel PS, Chanan-Khan AA. Targeting the Bcl-2. Curr Opin Oncol. 2009;21:516–523. doi: 10.1097/CCO.0b013e328331a7a4. [DOI] [PubMed] [Google Scholar]
- 27.Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87:1157–1180. doi: 10.1007/s00204-013-1034-4. [DOI] [PubMed] [Google Scholar]
- 28.Schulz R, Moll UM. Targeting the heat shock protein 90: a rational way to inhibit macrophage migration inhibitory factor function in cancer. Curr Opin Oncol. 2014;26:108–113. doi: 10.1097/CCO.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 29.Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L, Pasche B, Lee C, Grippo PJ. TGF-β: duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst. 2014;106:djt369. doi: 10.1093/jnci/djt369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakowlew SB. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev. 2006;25:435–457. doi: 10.1007/s10555-006-9006-2. [DOI] [PubMed] [Google Scholar]
- 31.Shahneh FZ, Baradaran B, Zamani F, Aghebati-Maleki L. Tumor angiogenesis and anti-angiogenic therapies. Hum Antibodies. 2013;22:15–19. doi: 10.3233/HAB-130267. [DOI] [PubMed] [Google Scholar]
- 32.Kim ES, Hong SY, Lee HK, Kim SW, An MJ, Kim TI, Lee KR, Kim WH, Cheon JH. Guggulsterone inhibits angiogenesis by blocking STAT3 and VEGF expression in colon cancer cells. Oncol Rep. 2008;20:1321–1327. [PubMed] [Google Scholar]
- 33.Xiao D, Singh SV. z-Guggulsterone, a constituent of Ayurvedic medicinal plant Commiphora mukul, inhibits angiogenesis in vitro and in vivo. Mol Cancer Ther. 2008;7:171–180. doi: 10.1158/1535-7163.MCT-07-0491. [DOI] [PubMed] [Google Scholar]
- 34.Jiang G, Xiao X, Zeng Y, Nagabhushanam K, Majeed M, Xiao D. Targeting beta-catenin signaling to induce apoptosis in human breast cancer cells by z-guggulsterone and Gugulipid extract of Ayurvedic medicine plant Commiphora mukul. BMC Complement Altern Med. 2013;13:203. doi: 10.1186/1472-6882-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]


