Abstract
AIM: To investigate the expression of mast cell tryptase and carboxypeptidase A in drug-related fatal anaphylaxis.
METHODS: The expression of mast cell tryptase and carboxypeptidase A in 15 autopsy cases of drug-related fatal anaphylaxis and 20 normal autopsy cases were detected. First, the expression of mast cell tryptase was determined in stomach, jejunum, lung, heart, and larynx by immunofluorescence. Different tissues were removed and fixed in paraformaldehyde solution, then paraffin sections were prepared for immunofluorescence. Using specific mast cell tryptase and carboxypeptidase A antibodies, the expression of tryptase and carboxypeptidase A in gastroenterology tract and other tissues were observed using fluorescent microscopy. The postmortem serum and pericardial fluid were collected from drug-related fatal anaphylaxis and normal autopsy cases. The level of mast cell tryptase and carboxypeptidase A in postmortem serum and pericardial fluid were measured using fluor enzyme linked immunosorbent assay (FEIA) and enzyme linked immunosorbent assay (ELISA) assay. The expression of mast cell tryptase and carboxypeptidase A was analyzed in drug-related fatal anaphylaxis cases and compared to normal autopsy cases.
RESULTS: The expression of carboxypeptidase A was less in the gastroenterology tract and other tissues from anaphylaxis-related death cadavers than normal controls. Immunofluorescence revealed that tryptase expression was significantly increased in multiple organs, especially the gastrointestinal tract, from anaphylaxis-related death cadavers compared to normal autopsy cases (46.67 ± 11.11 vs 4.88 ± 1.56 in stomach, 48.89 ± 11.02 vs 5.21 ± 1.34 in jejunum, 33.72 ± 5.76 vs 1.30 ± 1.02 in lung, 40.08 ± 7.56 vs 1.67 ± 1.03 in larynx, 7.11 ± 5.67 vs 1.10 ± 0.77 in heart, P < 0.05). Tryptase levels, as measured with FEIA, were significantly increased in both sera (43.50 ± 0.48 μg/L vs 5.40 ± 0.36 μg/L, P < 0.05) and pericardial fluid (28.64 ± 0.32 μg/L vs 4.60 ± 0.48 μg/L, P < 0.05) from the anaphylaxis group in comparison with the control group. As measured by ELISA, the concentration of carboxypeptidase A was also increased more than 2-fold in the anaphylaxis group compared to control (8.99 ± 3.91 ng/mL vs 3.25 ± 2.30 ng/mL in serum, 4.34 ± 2.41 ng/mL vs 1.43 ± 0.58 ng/mL in pericardial fluid, P < 0.05).
CONCLUSION: Detection of both mast cell tryptase and carboxypeptidase A could improve the forensic identification of drug-related fatal anaphylaxis.
Keywords: Gastrointestinal tract, Drug-related fatal anaphylaxis, Forensic Pathology, Mast cell carboxypeptidase A, Mast cell tryptase
Core tip: Drug-related fatal anaphylaxis is occasionally encountered in forensic pathology routine. However, markers in the identification of drug-related fatal anaphylaxis still need further exploration. This study identified two important markers in drug-related fatal anaphylaxis, tryptase and carboxypeptidase A, which may improve postmortem diagnosis of anaphylaxis in medicolegal expertise.
INTRODUCTION
Drug-induced anaphylaxis, also called allergic shock, is an immunologically mediated event that occurs after drug exposure in sensitized individuals and could lead to death[1-3]. However, postmortem diagnosis of anaphylaxis is difficult in medicolegal expertise. There was less different clinical symptom and pathological morphologic change between fatal anaphylaxis and general sudden death[4,5]. In current autopsy cases, general disease, intoxication, and violent death should be excluded first, and then exposure to allergen and clinical symptoms are evaluated in combination to identify anaphylaxis[2,6]. Therefore, exploration of novel, precise methods for anaphylaxis identification could be important in routine forensic pathology.
Drug-induced anaphylaxis can be initiated by binding of foreign drugs to specific immunoglobulin E (IgE) on mast cells[7,8]. Subsequently, various kinds of mediators are secreted from the mast cells, thereby inducing anaphylaxis[7,9,10]. Tryptase is a serine protease mainly stored in the granules of mast cells that is released at the onset of anaphylaxis[11]. Several studies have reported that serum tryptase levels may be a reliable indicator of anaphylaxis because of its long serum half-life compared to other secreted mediators[11-13]. However, the normal value of tryptase varies in different countries. Thus, more a more precise standard should be determined. Another chemical mediator, mast cell carboxypeptidase A, has been the focus of postmortem diagnosis of anaphylaxis. Carboxypeptidase A is a secreted protease that may be released following activation of mast cells to mediate acute anaphylaxis[14,15].
Therefore, we determined whether the level of carboxypeptidase A or a combination of carboxypeptidase A and tryptase could be meaningful in the postmortem diagnosis of anaphylaxis. In this study, the expression of tryptase and carboxypeptidase A in multiple organs of cadavers was detected by immunofluorescence. Fluor enzyme linked immunosorbent assay (FEIA) and enzyme linked immunosorbent assay (ELISA) were used to measure the level of tryptase and carboxypeptidase A in postmortem serum and pericardial fluid, respectively.
MATERIALS AND METHODS
Immunofluorescence of tryptase in different tissues
During autopsy, the stomach, jejunum, lung, heart, and larynx were removed, fixed, and embedded in paraffin for preparation of sections. Immunofluorescence was performed as previously described with minor alterations[16]. Briefly, mouse anti-human mast cell tryptase, mouse anti-human carboxypeptidase A, and rabbit anti-mouse IgG-TRITC (Santa Cruz, Dallas, TX, United States) were used to detect tryptase in different organs. The sections were observed using fluorescence microscopy (BX61, Olympus, Tokyo, Japan). Ten random visual fields were imaged per section and the number of tryptase-positive cells was counted. All experiments were approved by the Ethics Committee of Shanxi Medical University.
Quantification of tryptase and carboxypeptidase A levels in serum and pericardial fluid
Blood was collected from the right cardiac cavity and centrifuged. The serum and pericardial fluid were stored at -80 °C until use. Samples from 35 autopsy cases were measured. The causes of death in the anaphylaxis group (15 cases, 10 male, five female) included three of penicillin, three of ceftriaxone, three of levofloxacin, five of lomefloxacin via intravenous drip, and one of ibuprofen via oral administration. Anaphylaxis was diagnosed by clinical features, where the anaphylaxis symptoms occurred in all cases within 30 min. All postmortem autopsies were performed within 72 h. For the control group, 20 cases without allergic reaction, craniocerebral injury, coronary heart disease, and recreational drug use were selected. The level of tryptase in serum and pericardial fluid was measured by a commercial FEIA kit (Pharmacia Diagnostics, Uppsala, Sweden). Carboxypeptidase A levels were determined using an ELISA kit (Huamei Bio, Wuhan, China) according to the manufacturer’s instructions.
Statistical analysis
Data were expressed as mean ± SE, and a student’t test was used to compare differences between groups. P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 17.0 software (Palo Alto, California, United States).
RESULTS
The expression of tryptase in different organs of anaphylaxis cadaver
Immunofluorescence was performed to detect the expression of carboxypeptidase A and tryptase in different organs. Less carboxypeptidase A was expressed in tissues from anaphylaxis cadaver than control (data not shown). Next, the expression of tryptase was detected in different organs. As shown in Figure 1, multiple tryptase-positive particles were observed in the mucous layer, with less in the muscular layer of the stomach and jejunum, from anaphylaxis cadaver. In contrast, the expression of tryptase was less in tissues from the control group. We also detected the expression of tryptase in some other tissues. Tryptase was observed in the bronchia wall and the small vessel wall in the lung, the small vessel wall in the submucosa of the larynx, and the periphery mesenchyme of the small vessels in the heart (Figure 1 and Table 1).
Figure 1.
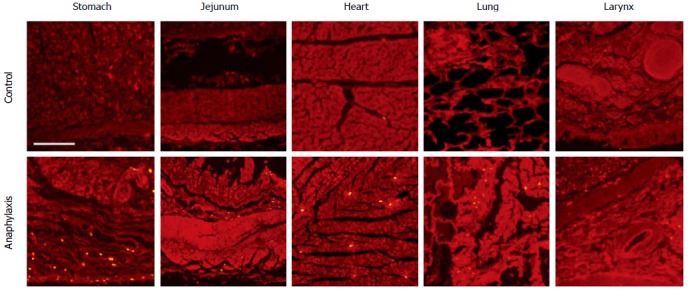
Immunofluorescence staining of tryptase in different organs. Scale bar = 200 μm.
Table 1.
Number of tryptase-positive particles in the anaphylaxis and control groups
| Control (× 100) | Anaphylaxis (× 100) | |
| Stomach | 4.88 ± 1.56 | 46.67 ± 11.111 |
| Jejunum | 5.21 ± 1.34 | 48.89 ± 11.021 |
| Lung | 1.30 ± 1.02 | 33.72 ± 5.761 |
| Larynx | 1.67 ± 1.03 | 40.08 ± 7.561 |
| Heart | 1.10 ± 0.77 | 7.11 ± 5.671 |
Denotes significant difference vs control, P < 0.05, n = 10.
Determination of tryptase and carboxypeptidase A in postmortem serum and pericardial fluid
We examined tryptase in the sera and pericardial fluid from 15 autopsy cases who died of anaphylaxis and 20 control cases. The levels of tryptase were significantly increased in both sera and pericardial fluid from the anaphylaxis group in comparison with control group (Table 2). The concentrations of carboxypeptidase A were increased more than 2-fold in the anaphylaxis group compared to the control group (Table 3). Taken together, our results suggested that both tryptase and carboxypeptidase A were increased in drug-related fatal anaphylaxis.
Table 2.
Expression of tryptase in serum and pericardial fluid
| n | Serum (μg/L) | Pericardial fluid (μg/L) | |
| Control | 20 | 5.40 ± 0.36 | 4.60 ± 0.48 |
| Anaphylaxis | 15 | 43.50 ± 0.481 | 28.64 ± 0.321 |
Denotes significant difference vs control, P < 0.05.
Table 3.
Expression of carboxypeptidase A in serum and pericardial fluid
| n | Serum (ng/mL) | Pericardial fluid (ng/mL) | |
| Control | 20 | 3.25 ± 2.30 | 1.43 ± 0.58 |
| Anaphylaxis | 15 | 8.99 ± 3.911 | 4.34 ± 2.411 |
Denotes significant difference vs control, P < 0.05.
DISCUSSION
Drug-induced fatal anaphylaxis is frequently encountered in medicolegal expertise. Some current indicators of anaphylaxis, including IgE and histamine, lack specify or stability[17-19]. Compared to other secreted mediators, tryptase and carboxypeptidase A have a long half-life in vivo, which led to the speculation that these two proteases may be superior indicators for the postmortem diagnosis of anaphylaxis[20,21]. In the present study, we measured the levels of mast cell tryptase and carboxypeptidase A in postmortem serum and pericardial fluid. Schwartz et al[22] had reported that the concentration of tryptase increased rapidly after allergic shock and that it could be detected up to 4 d in autopsy. Moreover, the severity of the allergic reaction was shown to be highly related to tryptase level[13,23,24]. Although the standard of serum tryptase is different in normal adults among countries, a tryptase value greater than 10 μg/L can be considered abnormal[25,26]. We found that the level of tryptase in the serum from the anaphylaxis group was 8-fold higher than control. Meanwhile, this value in the pericardial fluid was 6-fold greater in the anaphylaxis group than control. These results were consistent with previous findings suggesting that tryptase may be a specific marker in the postmortem diagnosis of anaphylaxis. However, it has also been reported that serum tryptase levels are increased in patients with coronary heart disease, mastocytosis patients, and some drug abusers[27-30]. Therefore, these causes of mortality should be excluded before making a diagnosis of anaphylaxis.
Another chemical mediator secreted from mast cells, carboxypeptidase A (also known as carboxypeptide A3, CPA3), was increased in allergic reactions, which was positively correlated to chymases[31,32]. As shown for tryptase, carboxypeptide was also highly expressed in the epithelium of asthma patients[33,34]. We confirmed that the level of carboxypeptidase A increased significantly in both postmortem serum and pericardial fluid from anaphylaxis cadavers compared with control. Although there was less in depth investigation of carboxypeptide levels in the postmortem serum from anaphylaxis cases, we speculate that the alteration of carboxypeptide was also meaningful. Measuring both carboxypeptide and tryptase could improve the postmortem diagnosis of anaphylaxis. Furthermore, determining levels of these mediators from the pericardial fluid in the closed serous cavity would help to avoid possible contamination after death.
During medicolegal expertise, the detection of indicators often occurs long after death, making it increasing difficult to obtain the serum or pericardial fluid samples. Therefore, determining the expression of chemical markers in different organs from the cadavers is important. Although carboxypeptidase A was expressed less in tissues from both normal and anaphylaxis cadaver, the expression of tryptase in stomach, jejunum, lung, heart, and larynx from the drug-induced anaphylaxis group was significantly greater than the control group.
In conclusion, the expression of mast cell tryptase and carboxypeptidase A in body fluid and postmortem organs, especially gastrointestinal tract, could be meaningful in the identification of drug-related fatal anaphylaxis. Taken together with immunofluorescent identification, measurement of serum mast cell-specific tryptase and carboxypeptidase A levels might be a novel precise method that could improve postmortem diagnosis of anaphylaxis in medicolegal expertise. In addition, the detection of tryptase level in postmortem organs could also be meaningful in cases where it is difficult to collect serum/pericardial fluid due to the advanced state of decay during medicolegal expertise.
COMMENTS
Background
Drug-related fatal anaphylaxis could be occasionally encountered in routine forensic pathology. However, additional markers for the identification of drug-related fatal anaphylaxis are still needed.
Research frontiers
The exploration of novel markers and methods for the identification of drug-related fatal anaphylaxis could be important in the forensic identification of anaphylaxis.
Innovations and breakthroughs
This article provides new evidence for the use of mast cell tryptase and carboxypeptidase A as biomarkers to identify drug-related fatal anaphylaxis. It is suggested that the expression of tryptase and carboxypeptidase A in the gastroenterology tract and other tissues might be important markers in the case that it is difficult to collect serum/pericardial fluid because of the advanced state of decay during medicolegal expertise.
Applications
Combination of mast cell tryptase and carboxypeptidase A detection could improve the forensic identification of drug-related fatal anaphylaxis.
Peer-review
This is an interesting study about drug-related fatal anaphylaxis. The expression of mast cell tryptase and carboxypeptidase A in drug-related fatal anaphylaxis are investigated. And the expression of mast cell tryptase and carboxypeptidase A in 15 autopsy cases of drug-related fatal anaphylaxis and 20 normal autopsy cases were detected. The authors concluded that combination of mast cell tryptase and carboxypeptidase A detection could improve the forensic identification of drug-related fatal anaphylaxis. And the detection of tryptase level in postmortem organs could also be meaningful in the case that hard to collect serum/pericardial fluid and advanced state of decay during medicolegal expertise.
Footnotes
Supported by the National Natural Science Foundation of China, No. 81172905; and Shanxi Province Science Foundation for Youths, No. 2012021032-2.
Institutional review board statement: All experiments were approved by the ethics committee of Shanxi Medical University.
Conflict-of-interest statement: The authors declare that there is no conflict of interest.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at 258187101@qq.com.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 25, 2015
First decision: June 25, 2015
Article in press: September 30, 2015
P- Reviewer: Ruffion A, Simpson ND S- Editor: Yu J L- Editor: Filipodia E- Editor: Liu XM
References
- 1.Aun MV, Blanca M, Garro LS, Ribeiro MR, Kalil J, Motta AA, Castells M, Giavina-Bianchi P. Nonsteroidal anti-inflammatory drugs are major causes of drug-induced anaphylaxis. J Allergy Clin Immunol Pract. 2014;2:414–420. doi: 10.1016/j.jaip.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Da Broi U, Moreschi C. Post-mortem diagnosis of anaphylaxis: A difficult task in forensic medicine. Forensic Sci Int. 2011;204:1–5. doi: 10.1016/j.forsciint.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 3.Kuruvilla M, Khan DA. Anaphylaxis to drugs. Immunol Allergy Clin North Am. 2015;35:303–319. doi: 10.1016/j.iac.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Dworzynski K, Ardern-Jones M, Nasser S. Diagnosis and management of drug allergy in adults, children and young people: summary of NICE guidance. BMJ. 2014;349:g4852. doi: 10.1136/bmj.g4852. [DOI] [PubMed] [Google Scholar]
- 5.Jerschow E, Lin RY, Scaperotti MM, McGinn AP. Fatal anaphylaxis in the United States, 1999-2010: temporal patterns and demographic associations. J Allergy Clin Immunol. 2014;134:1318–1328.e7. doi: 10.1016/j.jaci.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kannan JA, Bernstein JA. Perioperative anaphylaxis: diagnosis, evaluation, and management. Immunol Allergy Clin North Am. 2015;35:321–334. doi: 10.1016/j.iac.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Drain KL, Volcheck GW. Preventing and managing drug-induced anaphylaxis. Drug Saf. 2001;24:843–853. doi: 10.2165/00002018-200124110-00005. [DOI] [PubMed] [Google Scholar]
- 8.Burton OT, Noval Rivas M, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, Roers A, Houshyar H, Crackower MA, Chatila TA, et al. Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity. 2014;41:141–151. doi: 10.1016/j.immuni.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruchalla RS. Clinical assessment of drug-induced disease. Lancet. 2000;356:1505–1511. doi: 10.1016/S0140-6736(00)02885-3. [DOI] [PubMed] [Google Scholar]
- 10.Akin C. Mast cell activation syndromes presenting as anaphylaxis. Immunol Allergy Clin North Am. 2015;35:277–285. doi: 10.1016/j.iac.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Matsson P, Enander I, Andersson AS, Nystrand J, Schwartz L, Watkins J. Evaluation of mast cell activation (tryptase) in two patients suffering from drug-induced hypotensoid reactions. Agents Actions. 1991;33:218–220. doi: 10.1007/BF01993172. [DOI] [PubMed] [Google Scholar]
- 12.Cianferoni A, Novembre E, Mugnaini L, Lombardi E, Bernardini R, Pucci N, Vierucci A. Clinical features of acute anaphylaxis in patients admitted to a university hospital: an 11-year retrospective review (1985-1996) Ann Allergy Asthma Immunol. 2001;87:27–32. doi: 10.1016/S1081-1206(10)62318-6. [DOI] [PubMed] [Google Scholar]
- 13.Sprung J, Weingarten TN, Schwartz LB. Presence or absence of elevated acute total serum tryptase by itself is not a definitive marker for an allergic reaction. Anesthesiology. 2015;122:713–714. doi: 10.1097/ALN.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 14.Lützelschwab C, Pejler G, Aveskogh M, Hellman L. Secretory granule proteases in rat mast cells. Cloning of 10 different serine proteases and a carboxypeptidase A from various rat mast cell populations. J Exp Med. 1997;185:13–29. doi: 10.1084/jem.185.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing D, Zhang R, Li S, Huang P, Luo C, Hei Z, Xia Z, Gan X. Pivotal role of mast cell carboxypeptidase A in mediating protection against small intestinal ischemia-reperfusion injury in rats after ischemic preconditioning. J Surg Res. 2014;192:177–186. doi: 10.1016/j.jss.2014.05.050. [DOI] [PubMed] [Google Scholar]
- 16.Chen M, Sun P, Liu XY, Dong D, Du J, Gu L, Ge YB. α-fetoprotein involvement during glucocorticoid-induced precocious maturation in rat colon. World J Gastroenterol. 2011;17:2933–2940. doi: 10.3748/wjg.v17.i24.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malo JL, Cartier A. Occupational reactions in the seafood industry. Clin Rev Allergy. 1993;11:223–240. doi: 10.1007/BF02914472. [DOI] [PubMed] [Google Scholar]
- 18.Tyler D. Disability and medical management of natural latex sensitivity claims. J Allergy Clin Immunol. 2002;110:S129–S136. doi: 10.1067/mai.2002.125259. [DOI] [PubMed] [Google Scholar]
- 19.Sakatani A, Doi Y, Matsuda T, Sasai Y, Nishida N, Sakamoto M, Uenoyama N, Matsumoto Y, Kinoshita K. Protracted anaphylaxis developed after peginterferon α-2a administration for chronic hepatitis C. World J Gastroenterol. 2015;21:2826–2829. doi: 10.3748/wjg.v21.i9.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishio H, Takai S, Miyazaki M, Horiuchi H, Osawa M, Uemura K, Yoshida K, Mukaida M, Ueno Y, Suzuki K. Usefulness of serum mast cell-specific chymase levels for postmortem diagnosis of anaphylaxis. Int J Legal Med. 2005;119:331–334. doi: 10.1007/s00414-005-0524-1. [DOI] [PubMed] [Google Scholar]
- 21.Ciccarelli A, Calabrò C, Imperatore C, Scala G. Prick by prick induced anaphylaxis in a patient with peanuts and lupine allergy: awareness of risks and role of component resolved diagnosis. Case Rep Med. 2014;2014:892394. doi: 10.1155/2014/892394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz LB, Yunginger JW, Miller J, Bokhari R, Dull D. Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. J Clin Invest. 1989;83:1551–1555. doi: 10.1172/JCI114051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haeberli G, Brönnimann M, Hunziker T, Müller U. Elevated basal serum tryptase and hymenoptera venom allergy: relation to severity of sting reactions and to safety and efficacy of venom immunotherapy. Clin Exp Allergy. 2003;33:1216–1220. doi: 10.1046/j.1365-2222.2003.01755.x. [DOI] [PubMed] [Google Scholar]
- 24.Sprung J, Larson KJ, Divekar RD, Butterfield JH, Schwartz LB, Weingarten TN. Refractory intraoperative hypotension with elevated serum tryptase. Asia Pac Allergy. 2015;5:47–50. doi: 10.5415/apallergy.2015.5.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vadas P, Perelman B, Liss G. Platelet-activating factor, histamine, and tryptase levels in human anaphylaxis. J Allergy Clin Immunol. 2013;131:144–149. doi: 10.1016/j.jaci.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Edston E, van Hage-Hamsten M. Mast cell tryptase and hemolysis after trauma. Forensic Sci Int. 2003;131:8–13. doi: 10.1016/s0379-0738(02)00383-3. [DOI] [PubMed] [Google Scholar]
- 27.Deliargyris EN, Upadhya B, Sane DC, Dehmer GJ, Pye J, Smith SC, Boucher WS, Theoharides TC. Mast cell tryptase: a new biomarker in patients with stable coronary artery disease. Atherosclerosis. 2005;178:381–386. doi: 10.1016/j.atherosclerosis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Sperr WR, Jordan JH, Fiegl M, Escribano L, Bellas C, Dirnhofer S, Semper H, Simonitsch-Klupp I, Horny HP, Valent P. Serum tryptase levels in patients with mastocytosis: correlation with mast cell burden and implication for defining the category of disease. Int Arch Allergy Immunol. 2002;128:136–141. doi: 10.1159/000059404. [DOI] [PubMed] [Google Scholar]
- 29.Edston E, van Hage-Hamsten M. Anaphylactoid shock--a common cause of death in heroin addicts? Allergy. 1997;52:950–954. doi: 10.1111/j.1398-9995.1997.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 30.Maurer U, Kager C, Fellinger C, Loader D, Pollesböck A, Spitzer B, Jarisch R. Risk of anaphylaxis in opioid dependent persons: effects of heroin versus substitution substance. Subst Abuse Treat Prev Policy. 2014;9:12. doi: 10.1186/1747-597X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayorga C, Sanz ML, Gamboa PM, García BE, Caballero MT, García JM, Labrador M, Lahoz C, Longo Areso N, López Hoyos M, et al. In vitro diagnosis of immediate allergic reactions to drugs: an update. J Investig Allergol Clin Immunol. 2010;20:103–109. [PubMed] [Google Scholar]
- 32.Arias Á, Lucendo AJ, Martínez-Fernández P, González-Castro AM, Fortea M, González-Cervera J, Yagüe-Compadre JL, Mota-Huertas T, Vicario M. Dietary Treatment Modulates Mast Cell Phenotype, Density, and Activity in Adult Eosinophilic Esophagitis. Clin Exp Allergy. 2015:Epub ahead of print. doi: 10.1111/cea.12504. [DOI] [PubMed] [Google Scholar]
- 33.Christ-Crain M, Müller B. Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 2007;30:556–573. doi: 10.1183/09031936.00166106. [DOI] [PubMed] [Google Scholar]
- 34.Kemona-Chetnik I, Kowal K, Kucharewicz I, Pampuch A, Bodzenta-Lukaszyk A. [Thrombin activatable fibrinolysis inhibitor (TAFI) in allergic asthma patients] Przegl Lek. 2006;63:1281–1285. [PubMed] [Google Scholar]


