Abstract
AIM: To describe the application of complete robotic gastrectomy with transvaginal specimen extraction (TVSE) for gastric cancer patients.
METHODS: Between July and November 2014, eight female patients who were diagnosed with gastric adenocarcinoma underwent a TVSE following a full robot-sewn gastrectomy. According to the tumor location, the patients were allocated to two different groups; two patients received robotic total gastrectomy with TVSE and the other six received robotic distal gastrectomy with TVSE.
RESULTS: Surgical procedures were successfully performed in all eight cases without conversion. The mean age was 55.3 (range, 42-69) years, and the mean body mass index was 23.2 (range, 21.6-26.0) kg/m2. The mean total operative time and blood loss were 224 (range, 200-298) min and 62.5 (range, 50-150) mL, respectively. The mean postoperative hospital stay was 3.6 (range, 3-5) d. The mean number of lymph nodes resected was 23.6 (range, 17-27). None was readmitted within 30 d of postoperation. During the follow-up, no stricture developed nor was any anastomotic leakage detected.
CONCLUSION: It is possible to perform a TVSE following a full robot-sewn gastrectomy with standard D2 lymph node resection for female gastric cancer patients.
Keywords: Gastric cancer, Robotic surgery, Transvaginal, Natural orifice specimen extraction
Core tip: It is widely recognized that the natural orifice specimen extraction (NOSE) could reduce postoperative pain, length of stay, and morbidity. Although NOSE has been performed in different institutions, there has not been any report of transvaginal specimen extraction following full robot-sewn gastrectomy for female gastric cancer. This study describes the new application of complete robotic gastrectomy with transvaginal specimen extraction in eight patients with gastric cancer in Jinling Hospital. There were two different surgeries performed, robotic total gastrectomy and robotic distal gastrectomy.
INTRODUCTION
Gastric cancer is one of the most prevalent cancer in China[1]. Nearly a million new cases are diagnosed each year in the world, and it is the fourth most common type of cancer and the second leading cause of death worldwide[2]. For generations, surgeons have been contriving new surgical methods for inner organs, especially the stomach. These methods include open gastrectomy, laparoscopic gastrectomy, robotic gastrectomy, and natural orifice specimen extraction (NOSE)[3,4].
It is widely recognized that NOSE could reduce postoperative pain, length of stay, and morbidity. Although NOSE has been performed in a lot of institutions, there has not been any report of transvaginal specimen extraction (TVSE) following full robot-sewn gastrectomy for gastric cancer. This article aims to report robotic gastrectomy following a TVSE in eight patients with gastric cancer in Jinling Hospital. There were two different surgeries performed, robotic total gastrectomy (RTG) and robotic distal gastrectomy (RDG).
MATERIALS AND METHODS
Patients
Eight female patients with gastric cancer were enrolled in this study between July and November 2014. All patients were newly diagnosed with gastric cancer pathologically. The study was approved by the Research Ethics Committee of Nanjing University. All eight specimen extraction case series following full robot-sewn gastrectomy were performed in Jinling Hospital. Patients with a tumor that is too large or too difficult to resect were excluded in this study. Only multipara with no other underlying diseases was enrolled. Other organ (lung or kidney) dysfunction and abnormal clinical test results were not considered in this study. Patients who had taken chemotherapy or radiotherapy preoperatively were also excluded. According to the tumor location, the patients were primarily allocated to two different groups; two patients received RTG with TVSE and the other six received RDG with TVSE.
Surgical techniques
All eight robotic gastrectomies were carried out by the same surgeon (Jiang ZW) with full intracorporeal robot-sewn anastomosis[5]. The surgical procedure was carried out in four stages: (1) six of these patients underwent robotic distal gastrectomy followed by Billroth II reconstruction, and two underwent robotic total gastrectomy followed by Roux-en-Y reconstruction; (2) all the eight patients went through a standard D2 lymph node dissection (distal gastrectomy: 1, 3, 4Sb, 4d, 5, 6, 7, 8a, 9, 11p, 12a; total gastrectomy: 1, 2, 3, 4Sa, 4Sb, 4d, 5, 6, 7, 8a, 9, 10, 11, 12a )[6]; (3) partial omentectomy might be conducted if necessary; and (4) the TVSE and suture of wound were conducted.
Positioning and settlement of robotic arms
After general anesthesia, a 12-mm trocar was inserted into the abdomen through the sub-umbilical area, and a pneumoperitoneum was formed by insufflations of carbon dioxide. The intraperitoneal pressure was maintained at 11-13 mmHg. With patients placed in the reverse Trendelenburg position, distribution of trocars was as follows: a 12-mm camera port, three 8-mm robotic working ports, and one additional (12 mm) on-table assistant port (1-5; Figure 1). During the procedure, one ultrasonic scalpel, two Cadiere forceps, and one mega needle driver were used.
Figure 1.
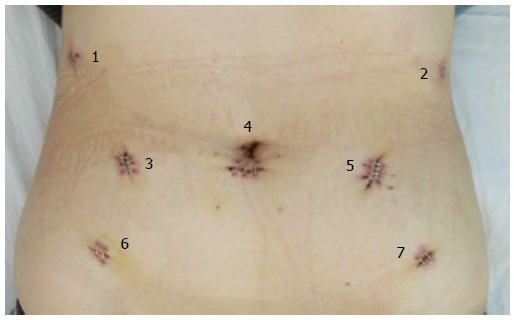
Placement of trocars.
Surgical procedure
The Da Vinci Robotic System was installed with its arms settled in position. Afterwards, the operator first inspected the abdominal cavity for any sign of tumor seeding or metastasis. The greater curvature of the stomach was mobilized by the ultrasonic scalpel. The gastrocolic ligament was cut with the ascending colon lifted by robotic arms. The left gastroepiploic artery was divided at its root after clipping. The dissection then proceeded towards the right gastroepiploic vessels, and then the left gastroepiploic vessels were ligated. The 4th set of lymph nodes along the greater curve of the stomach were dissected with the 6th set of the intrapyloric lymph nodes. Then the right gastric artery was dissected, clipped, cut and ligated, and the 12a lymph nodes were resected with the 5th set of suprapyloric lymph node. The duodenal stump was mobilized using an endo-GIA 60 (Covidien, Mansfield, MA, United States) to allow clipping at the root of the left gastric vessel. After the 7th lymph nodes were harvested, the 11p set of lymph nodes near the splenic artery were also resected with the 9th set of celiac trunk lymph nodes, and then, the 8th set of lymph nodes along the common hepatic artery. The 1st and 3rd sets of perigastric lymph nodes along the lesser curvature were dissected up to the site of the right paracardial region and the lesser omentum. The proximal stomach was divided using an endo-GIA 60 (Covidien, Mansfield, MA, United States). In RTG, additional lymph nodes should be dissected. The 2nd sets of lymph nodes along the left paracardial region, the 11d sets of lymph nodes over the spleen artery and the 10th sets of splenic hilar lymph nodes were dissected. Then, the division on the lower end of esophagus was made by an endo-GIA 60. A retrieval bag was introduced through the transabdominal trocar, and the specimen was transferred into it. The retrieval bag was then transferred into the pelvic cavity for transvaginal extraction (Figure 2).
Figure 2.
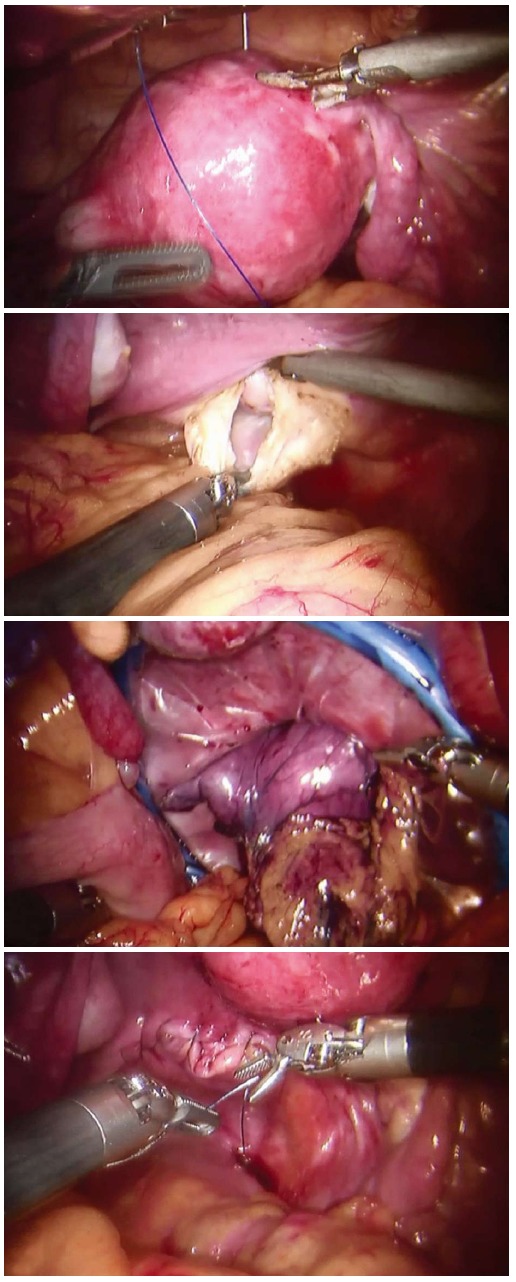
Procedure of robotic transvaginal specimen extraction.
Reconstruction
According to the location of tumor, reconstructions were performed differently. Six patients who underwent RDG received a Billroth II reconstruction while the other two patients who underwent RTG received a Roux-en-Y reconstruction. All lymph nodes dissection as well as the digestive restoration were performed fully by robotic arms intracorporeally as described in previous literature[5].
TVSE
The robotic system was moved from the headside to the footside with trocars reallocated; the trocar holes (1 and 2; Figure 1) left on the costal arch were sutured in case of air leakage. Additional trocars (6 and 7; Figure 1) were inserted. After the intraperitoneal pressure had been maintained back to 12 mmHg, the robotic arms were introduced into the abdominal cavity with the patients in lithotomy position. By purse-string suture, the uterus was lifted by a robotic arm. A 4-cm posterior colpotomy was performed with a robotic harmonic knife. Like others, we used a two-layer retrieval bag to deposit the specimen[7-9]. Then the retrieval bag containing the specimen was gently withdrawn until it reached the intra-abdominal tip of the transvaginal incision. The withdrawal of retrieval bag specimen was assisted by a tenaculum placed on the anterior lip of the cervix (3; Figure 2). After the removal of the transvaginal incision protector, the posterior incisions were closed with an self-anchoring barbed suture (Covidien, United States) suture (4; Figure 2). Then over 1000 mL of warm water was poured into the abdominal cavity through one of the trocar port to clean the cavity. It was carefully examined to ensure that no spot was left untreated. One drain was placed in the abdominal cavity through the ancillary port. Closure of the port incisions with subcuticular suture completed the operation.
RESULTS
Eight patients participated in the study. The mean age of the women was 55.3 (range, 42-69) years (See Table 1), and the mean body mass index (BMI) was 23.2 (range, 21.6-26.0) kg/m2. Histopathology confirmed poorly differentiated adenocarcinoma in all cases. The mean number of retrieved lymph nodes was 23.6 (range, 17-27). In case 1, 16 of 17 lymph nodes were found positive. Nevertheless, no metastatic lymph nodes were found in the other seven patients. Only the node-positive patients (case 1) underwent six cycles of postoperative chemotherapy.
Table 1.
Basic information of robotic transvaginal specimen extraction patients
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Mean | |
| Age/yr | 42 | 54 | 49 | 59 | 65 | 69 | 54 | 50 | 55.3 |
| BMI | 23.25 | 23.1 | 20.8 | 25.7 | 26 | 21.6 | 22.2 | 23.25 | 23.2 |
| Approach | RTG | RTG | RDG | RDG | RDG | RDG | RDG | RDG | |
| Reconstruction | Roux-en-Y | Roux-en-Y | Billroth II | Billroth II | Billroth II | Billroth II | Billroth II | Billroth II | |
| Number of retrieved LNs | 16/17 | 0/22 | 0/27 | 0/24 | 0/27 | 0/25 | 0/22 | 0/25 | 2/23.6 |
| WHO classification | PD | PD | PD | PD | PD | PD | PD | PD | |
| Lauren classification | Less curvature Antrum | Less curvature Antrum | Less curvature Body | Less curvature Body | Less curvature Body | Less curvature Body | Less curvature Body | Less curvature Body | |
| Histopathology | adenoma | adenoma | adenoma | adenoma | adenoma | adenoma | adenoma | adenoma | |
| History | Null | Null | Hemorrhoids 4 yr ago | Appendectomy 10 yr ago | Null | Null | Null | Caesarean section 22 yr ago | |
| Postoperative stay/d | 5 | 4 | 3 | 3 | 3 | 4 | 3 | 4 | 3.6 |
| Stage | |||||||||
| TNM | T4bN3M0 | T1N0M0 | T1N0M0 | T4bN0M0 | T1N0M0 | T1N0M0 | T1N0M0 | T1N0M0 | |
| Gross type | IIIC | IA | IA | IIIB | IA | IA | IA | IA | |
| During Operation | |||||||||
| Blood loss/mL | 50 | 50 | 100 | 100 | 50 | 50 | 50 | 50 | 62.5 |
| Blood transfusion/mL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Urine/mL | 600 | 400 | 500 | 300 | 300 | 400 | 300 | 450 | 406.3 |
| Operation time/min | 225 | 215 | 298 | 200 | 230 | 200 | 185 | 235 | 224 |
| Time to first flatus/h | 30 | 32 | 27 | 33 | 36 | 35 | 28 | 33 | 31.8 |
PD: Poorly differentiated; RTG: Robotic total gastrectomy; RDG: Robotic distal gastrectomy.
Because the length of the incision was adapted to the size of the specimen and the tumor, with all tumors under 4 cm in diameter, the incision on the vagina was no larger than 4 cm. The mean operating time was 224 (range, 200-298) min, and the average blood loss was 62.5 (range, 50-150) mL and none of them needed blood transfusion. No intraoperative complications were noticed in any of the eight patients during the follow-up. The mean postoperative stay was 3.6 (range, 3-5) d. The first flatus of patients occurred at mean 28.5 (range, 24-33) h after surgery (Table 1).
There was no anastomotic leakage reported both within the hospital stay and 30 d after discharge from the hospital. During the follow-up, neither was there a sign of surgical site infection nor lung infection. No other complications were reported during the hospital stay. Thus, there was no one who needed re-admission in hospital. That is to say that both the mortality and morbidity were zero in 30 d (Table 2). The average VAS of six patients in the TVSE-RDG group decreased from 2.8 on postoperative day (POD) 1 to 1.7 on POD 2, and then 1.0 on POD 3 while the average VAS of the two patients in the TVSE-RTG group was 2, 1 and 1 on the POD 1, POD 2 and POD 3, respectively. The trend of postoperative mobility was also related to the VAS score.
Table 2.
Clinical features of robotic transvaginal specimen extraction patients
|
Adverse event |
Pain/ VAS |
activity/ step |
Sleep/h |
|||||||||
| Anastomotic leakage | 30 d re-admission | SSI or LI | POD 1 | POD 2 | POD 3 | POD 1 | POD 2 | POD 3 | POD 1 | POD 2 | POD 3 | |
| TVSE-RTG | 0/2 | 0/2 | 0/2 | 2 | 1 | 1 | 55 | 342 | 1100 | 7 | 6 | 6.5 |
| TVSE-RDG | 0/6 | 0/6 | 0/6 | 2.8 | 1.7 | 1 | 62 | 336 | 1078 | 7.1 | 6.8 | 7.9 |
SSI: Surgical site infection; LI: Lung infection; TVSE: Transvaginal specimen extraction.
All eight patients showed great progress in postoperative activity; their steps increased greatly for the TVSE-RDG group patients from 62 (range, 40-75) steps on POD 1, to 336 (range, 116-465) on POD 2, and then maybe with pain relief, the activity steps took a great leap to 1078 (range, 985-1200) steps on POD 3, nearing a normal daily level. The statistics from the TVSE-RTG group also showed the same trend (Table 2). There were 55 steps on POD 1, 342 on POD 2 and 1100 on POD 3 in the TVSE-RTG group. The sleep time measured by Fitbit Flex indicated an improvement on POD 2 to POD 3 by 0.5 h in the TVSE-RTG group and 1.1 h in the TVSE-RDG group.
During the perioperative course, the SpO2 showed a slight fluctuation (Table 3). The SpO2 decreased from preoperative 100% to 98.3% (range, 96%-99%) on POD 1, and then 99.3% (range, 98%-100%) on POD 3 in the TVSE-RDG group, while the SpO2 was 100, 98.5% (range, 98%-99%) and 99% (range, 98%-100%) on preoperative day, POD 1 and POD 3, respectively. Similarly, the heart rate (HR) also fluctuated from preoperative 66.2 (range, 58-69) to 77.5 (range, 62-102) on POD 1, and then down back to 72.5 (range, 60-85) on POD 3 in the TVSE-RDG group. However, we noticed that the grip strength strangely increased in 7 of the 8 patients, causing the average strength to increase from 25.6/25 kg to 27.8/26.3 kg in the TVSE-RTG group and 23.3/21.7 kg to 25.6/24.5 kg in the TVSE-RDG group. We also measured the body mass with a body machine (Inbody 230, South Korea). The body machine detected nearly no change in the bone (10.3%-10.2%), body fat (30.5%-30.2%), water (47.9%-48.1%) or muscle (32.0%-31.4%), which was also an indication for stability in keeping weight.
Table 3.
SpO2 fluctuation during the perioperative course
|
SpO2/% |
HR/min |
Hand grip strength/(left/right) kg |
||||||
| Pre- | POD 1 | POD 3 | Pre- | POD 1 | POD 3 | Admission | Discharge | |
| TVSE-RTG | 100 | 98.5 | 99 | 60 | 62 | 60 | 25.6/25 | 27.8/26.3 |
| TVSE-RDG | 100 | 98.3 | 99.3 | 66.2 | 77.5 | 72.5 | 23.3/21.7 | 25.6/24.5 |
Pre-: Pre-operation; POD: Postoperative day; TVSE: Transvaginal specimen extraction; RTG: Robotic total gastrectomy; RDG: Robotic distal gastrectomy.
DISCUSSION
Thanks to the development of new technology and the enterprise of generations of surgeons in minimally invasive surgery, innovations and progresses have evolved to another level in the field of NOSE surgery.
There are four potential routes, namely, transesophageal, transrectal, transvaginal, and transurethral. The robotic surgery makes it possible for NOSE to become more and more feasible and thereby the optimal way of approaching gastric cancer.
Although NOSE is now relatively widely performed, it does not give any importance to gastric resection with specimen extraction through the transvaginal route for gastric cancer, especially robotic gastrectomy.
TVSE using a posterior colpotomy has extensively been reported during gynecologic procedures[10,11]. In 1993, Delvaux et al[12] performed the first transvaginal extraction. The gallbladder containing a stone was extracted through the transvaginal route following laparoscopic cholecystectomy in female patients. In 1996, Redwine et al[13] first described a segmental colectomy with transvaginal extraction for bowel endometriosis. Kim et al[14] in 1996 reported transvaginal extraction of the rectum in four patients following low anterior resection. In 2002, Gill et al[15] described vaginal extraction of the intact specimen following laparoscopic radical nephrectomy. Ghezzi et al[10] reported 60 cases of vaginal extraction of pelvic masses following operative laparoscopy in the same year. In 2007, a Chinese surgeon, Yuan et al[16] presented a 65-year-old female with invasive urothelial carcinoma of the urinary bladder and end-stage renal disease who underwent laparoscopic radical cystectomy combined with bilateral nephroureterectomy, where the specimen was extracted transvaginally. In 2008, Palanivelu et al[17] reported extraction of total colon and rectum following totally laparoscopic proctocolectomy. Also in 2008, a swine model for TVSE following total gastrectomy was successfully constructed by Nakajima, who later also succeeded in human the following year[18,19]. Despite the difficulty of transvaginal laparoscopic/endoscopic gastrectomy described by Lacy et al[20] in 2009, Jeong et al[21] succeeded in repeating the laparoscopic transvaginal extraction of gastric cancer in 2011.
Nevertheless, there have not been previous reports of transvaginal extraction following robotic gastrectomy, nor any transvaginal NOSE following gastrectomy with standard D2 lymph node resection for gastric cancer (Table 4). It was not until June 2014 that the first NOSE following gastrectomy was performed by Dostalik et al[22]. However, the natural orifice specimen was extracted through the oral-esophageal route. Even when carefully washed with water, the bad smell of blood mixed with stomach contents could not be disserved within a couple of weeks. Damage to the esophageal wall during extraction occurred in our previous experience. Moreover, in single port laparoscopic gastrectomy, lymph node resection was not as easy as the robotic surgery or the open abdomen surgery, nor was the specimen extraction. Colpotomy is considered safe and does not lead to surgical site infections or dyspareunia[23,24]. Transvaginal NOSE using a posterior colpotomy is considered a mature procedure used in gynecology. Not only is the robotic gastrectomy valued in the surgical safety but also in oncological performance as it is a safe way to perform the standard D2 lymph node harvest in gastric cancer[25,26].
Table 4.
Laboratory features of robotic transvaginal specimen extraction patients
|
CRP (mg/L) |
HB (g/L) |
ALB (g/L) |
PAB (g/L) |
TFN (g/L) |
|||||||||||
| Pre- | POD 1 | POD 3 | Pre- | POD 1 | POD 3 | Pre- | POD 1 | POD 3 | Pre- | POD 1 | POD 3 | Pre- | POD 1 | POD 3 | |
| TVSE-RTG | 0.4 | 1.8 | 32.2 | 132 | 131 | 123 | 41.6 | 34.8 | 28.4 | 321 | 227 | 200 | 2.7 | 2.6 | 2.2 |
| TVSE-RDG | 0.5 | 19.4 | 35 | 133 | 124 | 116.3 | 41.4 | 33.5 | 29.6 | 273.7 | 236.8 | 205.8 | 2.8 | 2.5 | 2.3 |
POD: Postoperative day; CRP: C-reactive protein; HB: Haemoglobin; ALB: Albumin; PAB: Prealbumin; TFN: Transferrin; HR: Heart rate; TVSE: Transvaginal specimen extraction; RTG: Robotic total gastrectomy; RDG: Robotic distal gastrectomy.
In 2013, we have succeeded in publishing an investigation on robotic procedure which provides us a safe and feasible approach to robotic gastrectomy[5]. This new procedure for gastrectomy is performed daily in our hospital with the anastomosis sewn fully by robot intracorporeally. This daily training provides us a possibility to perform the TVSE following full robot-sewn gastrectomy.
We are glad to see a decrease in the morbidity and mortality in patients with TVSE following full intracorporeal robot-sewn gastrectomy. This study showed the safety of eight cases of TVSE following gastrectomy, indicating that it could be a more feasible way of approaching gastric cancer.
In comparison with transrectal extraction, the transvaginal extraction is feasible and carries a low risk of infection and postoperative leakage[27]. Despite the potential advantages, there are still potential risks in transvaginal NOSE, for example, infertility or dyspareunia to some extent. The complicated surgical procedure requires more technique and consumes more time to perform the transvaginal NOSE. The mean time of TVSE following robotic gastrectomy was 224 min in the eight patients. The mean operative times were 229 min and 212 min for Awad and McKenzie, even though they are laparoscopic colectomy which seems easier than those in gastrectomy[28,29]. However, there were no significant side effects clinically detected in the post-vaginal-resection patients[30,31].
Robotic TVSE in gastric cancer might be a feasible and alternative operative procedure for patients with gastric cancer. It showed minimal postoperative pain with small incisions; minimal invasiveness as well as less severe postoperative complications such as anastomotic fistulae, stenosis, and bleeding.
This innovation with robotic surgery provides a new approach to gastric cancer with pure NOSE approach. However, we must realize that this TVSE following standard D2 gastric surgery study with eight patients is small. The follow-up period of six months is a little too short for the judgement of oncological safety. Extensive studies with larger sample size of patients focusing on the oncologic safety with long-term surveillance are needed for adequate confirmation.
COMMENTS
Background
Despite the advantages of robotic gastrectomy, the need for an incision in the abdominal wall to remove the surgical specimen is a morbid factor.
Research frontiers
The development of natural orifice translumenal robotic surgery and natural orifice specimen extraction (NOSE) appears to be the next major frontier in robotic surgery.
Innovations and breakthroughs
Present research showed that robotic gastrectomy with transvaginal extraction is a safe and effective procedure. This technique is feasible and simple to perform, and avoids the abdominal wall incision and its potential complications.
Applications
NOSE may provide both an attractive way to reduce abdominal wall morbidity and a bridge to pure natural orifice transluminal endoscopic surgery for robotic gastrectomy.
Terminology
NOSE in robotic gastrectomy prevents the need for an enlarged port site or minilaparotomy to extract the surgical specimen. The current trend to develop less invasive techniques by reducing the number and size of abdominal incisions has spurred new interest in practice.
Peer-review
Authors conducted the case series analysis about the transvaginal specimen extraction following robotic total or partial gastrectomy for gastric cancer. Authors concluded that this surgical procedure is safe and feasible for patients with gastric cancer. This paper is very interesting and well written.
Footnotes
Institutional review board statement: This clinical study was approved by the Ethic Committee of Jinling Hospital, Nanjing University.
Informed consent statement: All patients involved in this study gave their written informed consent authorizing use and disclosure of their protected health information.
Conflict-of-interest statement: We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “Robotic Gastrectomy with Transvaginal Specimen Extraction for Female Gastric Cancer Patients”.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 23, 2015
First decision: June 19, 2015
Article in press: September 14, 2015
P- Reviewer: Murata A S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Guo P, Huang ZL, Yu P, Li K. Trends in cancer mortality in China: an update. Ann Oncol. 2012;23:2755–2762. doi: 10.1093/annonc/mds069. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Franklin ME, Ramos R, Rosenthal D, Schuessler W. Laparoscopic colonic procedures. World J Surg. 1993;17:51–56. doi: 10.1007/BF01655705. [DOI] [PubMed] [Google Scholar]
- 4.Darzi A, Super P, Guillou PJ, Monson JR. Laparoscopic sigmoid colectomy: total laparoscopic approach. Dis Colon Rectum. 1994;37:268–271. doi: 10.1007/BF02048165. [DOI] [PubMed] [Google Scholar]
- 5.Liu XX, Jiang ZW, Chen P, Zhao Y, Pan HF, Li JS. Full robot-assisted gastrectomy with intracorporeal robot-sewn anastomosis produces satisfying outcomes. World J Gastroenterol. 2013;19:6427–6437. doi: 10.3748/wjg.v19.i38.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 7.Boni L, Tenconi S, Beretta P, Cromi A, Dionigi G, Rovera F, Dionigi R, Ghezzi F. Laparoscopic colorectal resections with transvaginal specimen extraction for severe endometriosis. Surg Oncol. 2007;16 Suppl 1:S157–S160. doi: 10.1016/j.suronc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Tarantino I, Linke GR, Lange J, Siercks I, Warschkow R, Zerz A. Transvaginal rigid-hybrid natural orifice transluminal endoscopic surgery technique for anterior resection treatment of diverticulitis: a feasibility study. Surg Endosc. 2011;25:3034–3042. doi: 10.1007/s00464-011-1666-5. [DOI] [PubMed] [Google Scholar]
- 9.Torres RA, Orban RD, Tocaimaza L, Vallejos Pereira G, Arévalo JR. Transvaginal specimen extraction after laparoscopic colectomy. World J Surg. 2012;36:1699–1702. doi: 10.1007/s00268-012-1528-x. [DOI] [PubMed] [Google Scholar]
- 10.Ghezzi F, Raio L, Mueller MD, Gyr T, Buttarelli M, Franchi M. Vaginal extraction of pelvic masses following operative laparoscopy. Surg Endosc. 2002;16:1691–1696. doi: 10.1007/s00464-002-9043-z. [DOI] [PubMed] [Google Scholar]
- 11.Diana M, Perretta S, Wall J, Costantino FA, Leroy J, Demartines N, Marescaux J. Transvaginal specimen extraction in colorectal surgery: current state of the art. Colorectal Dis. 2011;13:e104–e111. doi: 10.1111/j.1463-1318.2011.02599.x. [DOI] [PubMed] [Google Scholar]
- 12.Delvaux G, Devroey P, De Waele B, Willems G. Transvaginal removal of gallbladders with large stones after laparoscopic cholecystectomy. Surg Laparosc Endosc. 1993;3:307–309. [PubMed] [Google Scholar]
- 13.Redwine DB, Koning M, Sharpe DR. Laparoscopically assisted transvaginal segmental resection of the rectosigmoid colon for endometriosis. Fertil Steril. 1996;65:193–197. doi: 10.1016/s0015-0282(16)58051-0. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Shim M, Kwun K. Laparoscopic-assisted transvaginal resection of the rectum. Dis Colon Rectum. 1996;39:582–583. doi: 10.1007/BF02058716. [DOI] [PubMed] [Google Scholar]
- 15.Gill IS, Cherullo EE, Meraney AM, Borsuk F, Murphy DP, Falcone T. Vaginal extraction of the intact specimen following laparoscopic radical nephrectomy. J Urol. 2002;167:238–241. [PubMed] [Google Scholar]
- 16.Yuan LH, Chung HJ, Chen KK. Laparoscopic radical cystectomy combined with bilateral nephroureterectomy and specimen extraction through the vagina. J Chin Med Assoc. 2007;70:260–261. doi: 10.1016/S1726-4901(09)70371-5. [DOI] [PubMed] [Google Scholar]
- 17.Palanivelu C, Rangarajan M, Jategaonkar PA, Anand NV. An innovative technique for colorectal specimen retrieval: a new era of „natural orifice specimen extraction“ (N.O.S.E) Dis Colon Rectum. 2008;51:1120–1124. doi: 10.1007/s10350-008-9316-2. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima K, Takahashi T, Souma Y, Shinzaki S, Yamada T, Yoshio T, Nishida T. Transvaginal endoscopic partial gastrectomy in porcine models: the role of an extra endoscope for gastric control. Surg Endosc. 2008;22:2733–2736. doi: 10.1007/s00464-008-0122-7. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima K, Nishida T, Takahashi T, Souma Y, Hara J, Yamada T, Yoshio T, Tsutsui T, Yokoi T, Mori M, et al. Partial gastrectomy using natural orifice translumenal endoscopic surgery (NOTES) for gastric submucosal tumors: early experience in humans. Surg Endosc. 2009;23:2650–2655. doi: 10.1007/s00464-009-0474-7. [DOI] [PubMed] [Google Scholar]
- 20.Lacy AM, Delgado S, Rojas OA, Ibarzabal A, Fernandez-Esparrach G, Taura P. Hybrid vaginal MA-NOS sleeve gastrectomy: technical note on the procedure in a patient. Surg Endosc. 2009;23:1130–1137. doi: 10.1007/s00464-008-0292-3. [DOI] [PubMed] [Google Scholar]
- 21.Jeong SH, Lee YJ, Choi WJ, Paik WY, Jeong CY, Park ST, Choi SK, Hong SC, Jung EJ, Joo YT, et al. Trans-vaginal specimen extraction following totally laparoscopic subtotal gastrectomy in early gastric cancer. Gastric Cancer. 2011;14:91–96. doi: 10.1007/s10120-011-0006-8. [DOI] [PubMed] [Google Scholar]
- 22.Dostalik J, Gunkova P, Gunka I, Mazur M, Mrazek T. Laparoscopic gastric resection with natural orifice specimen extraction for postulcer pyloric stenosis. Wideochir Inne Tech Maloinwazyjne. 2014;9:282–285. doi: 10.5114/wiitm.2014.41622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lomanto D, Chua HC, Myat MM, So J, Shabbir A, Ho L. Microbiological contamination during transgastric and transvaginal endoscopic techniques. J Laparoendosc Adv Surg Tech A. 2009;19:465–469. doi: 10.1089/lap.2009.0007. [DOI] [PubMed] [Google Scholar]
- 24.Wood SG, Panait L, Duffy AJ, Bell RL, Roberts KE. Complications of transvaginal natural orifice transluminal endoscopic surgery: a series of 102 patients. Ann Surg. 2014;259:744–749. doi: 10.1097/SLA.0b013e3182916138. [DOI] [PubMed] [Google Scholar]
- 25.Patriti A, Ceccarelli G, Bellochi R, Bartoli A, Spaziani A, Di Zitti L, Casciola L. Robot-assisted laparoscopic total and partial gastric resection with D2 lymph node dissection for adenocarcinoma. Surg Endosc. 2008;22:2753–2760. doi: 10.1007/s00464-008-0129-0. [DOI] [PubMed] [Google Scholar]
- 26.Liao G, Chen J, Ren C, Li R, Du S, Xie G, Deng H, Yang K, Yuan Y. Robotic versus open gastrectomy for gastric cancer: a meta-analysis. PLoS One. 2013;8:e81946. doi: 10.1371/journal.pone.0081946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolthuis AM, de Buck van Overstraeten A, D’Hoore A. Laparoscopic natural orifice specimen extraction-colectomy: a systematic review. World J Gastroenterol. 2014;20:12981–12992. doi: 10.3748/wjg.v20.i36.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awad ZT, Qureshi I, Seibel B, Sharma S, Dobbertien MA. Laparoscopic right hemicolectomy with transvaginal colon extraction using a laparoscopic posterior colpotomy: a 2-year series from a single institution. Surg Laparosc Endosc Percutan Tech. 2011;21:403–408. doi: 10.1097/SLE.0b013e31823945ac. [DOI] [PubMed] [Google Scholar]
- 29.McKenzie S, Baek JH, Wakabayashi M, Garcia-Aguilar J, Pigazzi A. Totally laparoscopic right colectomy with transvaginal specimen extraction: the authors’ initial institutional experience. Surg Endosc. 2010;24:2048–2052. doi: 10.1007/s00464-009-0870-z. [DOI] [PubMed] [Google Scholar]
- 30.Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762–1771. doi: 10.1016/j.ajog.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Roovers JP, van der Bom JG, van der Vaart CH, van Leeuwen JH, Scholten PC, Heintz AP. A randomized comparison of post-operative pain, quality of life, and physical performance during the first 6 weeks after abdominal or vaginal surgical correction of descensus uteri. Neurourol Urodyn. 2005;24:334–340. doi: 10.1002/nau.20104. [DOI] [PubMed] [Google Scholar]


