Abstract
Background
Despite their energy density, walnuts can be included in the diet without adverse effects on weight or body composition. The effect of habitual walnut intake on total calorie intake is not well studied. Effects on overall diet quality have not been reported.
Methods
Randomized, controlled, modified Latin square parallel design study with 2 treatment arms. The 112 participants were randomly assigned to a diet with or without dietary counseling to adjust calorie intake. Within each treatment arm, participants were further randomized to 1 of the 2 possible sequence permutations to receive a walnut-included diet with 56 g (providing 366 kcal) of walnuts per day and a walnut-excluded diet. Participants were assessed for diet quality, body composition, and cardiac risk measures.
Results
When compared with a walnut-excluded diet, a walnut-included diet for 6 months, with or without dietary counseling to adjust caloric intake, significantly improved diet quality as measured by the Healthy Eating Index 2010 (9.14±17.71 vs 0.40±15.13; p=0.02 and 7.02±15.89 vs -5.92±21.84; p=0.001, respectively). Endothelial function, total and low-density lipoprotein (LDL) cholesterol improved significantly from baseline in the walnut-included diet. Body mass index, percent body fat, visceral fat, fasting glucose, glycated hemoglobin, and blood pressure did not change significantly.
Conclusions
The inclusion of walnuts in an ad libitum diet for 6 months, with or without dietary counseling to adjust calorie intake, significantly improved diet quality, endothelial function, total and LDL cholesterol, but had no effects on anthropometric measures, blood glucose level, and blood pressure.
Trial registration number:
Keywords: BMI, Body Fat Distribution, Body Composition
Key messages.
The inclusion of walnuts in habitual diet with or without dietary counseling to control calorie intake improved diet quality, endothelial function total and LDL cholesterol in adults at risk for diabetes.
Background
Prior research attests to the health benefits of consuming nuts or other foods high in polyunsaturated fats (PUFAs) for individuals at risk for diabetes and/or cardiovascular disease. The Nurses Health Study found an inverse association between the consumption of nuts and the risk of type 2 diabetes.1 Epidemiological and clinical trial evidence has consistently demonstrated the beneficial effects of nut consumption on risk factors associated with coronary heart disease.2 3 In addition, research has shown that diets rich in PUFAs can significantly reduce blood low-density lipoprotein (LDL) cholesterol levels, increase the total cholesterol/high-density lipoprotein (HDL) cholesterol ratio, and help achieve optimal fat consumption without adverse effects on total fat or energy intake.4 5
Among members of the nut family, walnuts have been found to be particularly promising in terms of health benefits. Compared with most other nuts, walnuts have a higher content of PUFAs, including α-linolenic acid (ALA), which may confer additional antiatherogenic influences.4 Epidemiological studies suggest that plant-derived ALA may confer particular cardiovascular benefits.6–8 According to a review of clinical trials, consumption of 2–3 servings of walnuts per day has been found to consistently decrease total cholesterol and LDL cholesterol.4 Consumption of walnuts has also been shown to improve endothelial function (EF) in individuals with hypercholesterolemia and type 2 diabetes.9–11 In addition, walnuts have been found to increase the insulin response during an oral glucose tolerance test, and to decrease levels of glycated hemoglobin (HbA1c), in individuals with polycystic ovary syndrome.12
Nuts are a rich source of nutrients (eg, vitamin E, magnesium, folate, essential fatty acids, fiber, and protein) and phytochemicals, which along with their proven health benefits have prompted recommendations to increase their consumption. However, due to their high energy density, they are also a theoretical contributor to positive energy balance and weight gain, which potentially raises questions about such recommendations.12 13
Despite this concern, walnuts and other nutrient-rich nuts have been found to contribute to satiety, which can help control appetite and total caloric intake.12–14 Numerous epidemiological and clinical studies thus far have shown that nuts are not associated with weight gain, likely due to their effects on satiety and possibly also due to inefficient absorption of caloric energy from nuts.11–13 15
The effects of walnuts on appetite, satiety, weight, and body composition in adults, including those at risk for diabetes, warrant further study. The relative effects of advice to consume walnuts on body composition, weight, diet quality, EF, and biomarkers for cardiovascular risk have been studied to a limited degree. The effects of advice to consume walnuts on these parameters, with and without regulation of total calorie intake, have not been compared.
Therefore, this study investigated the effects of walnut consumption in persons at risk for type 2 diabetes and assessing body composition in persons consuming with or without dietary counseling to adjust calorie intake. Specifically, this study investigated the inclusion of walnuts on diet quality, body composition, and markers of cardiac risk with or without calorie control, with greater improvements with calorie control.
Methods
Study population
A cohort of 112 participants (31 men and 81 women) was recruited from the Lower Naugatuck Valley in Connecticut through flyers and newspaper advertisements. Interested participants (n=678) were prescreened over the telephone. This study included participants aged 25–75 years who were non-smokers and had a high risk for diabetes, which was defined as meeting at least one of the following criteria: overweight with increased waist circumference; prediabetes with fasting blood glucose >100 and <126 mg/dL or HbA1c 5.7–6.4%; metabolic syndrome, that is, meeting three out of five of the following criteria: (1) blood pressure ≥130/85 mm Hg or currently taking antihypertensive medication; (2) fasting plasma glucose >100 mg/dL (6.1 mmol/L); (3) serum triglycerides level >150 mg/dL (1.69 mmol/L); (4) HDL cholesterol <40 mg/dL (1.04 mmol/L) in men and<50 mg/dL (1.29 mmol/L) in women; (5) overweight (body mass index (BMI) ≥25 kg/m²) with waist circumference of more than 40 inches (102 cm) for men and more than 35 inches (88 cm) for women. Exclusion criteria included: allergy to walnuts or any other nuts; anticipated inability to complete study protocol for any reason; current eating disorder; restricted diets by choice (ie, vegetarian, vegan); receiving pharmacotherapy for obesity, including appetite suppressants; unstable use of lipid-lowering, antihypertensive medications or aspirin (ie, dose that had changed in the 3 months prior to enrollment) or unwilling to refrain from taking medication for 12 h prior to EF scanning; regular use of high doses of vitamin E (>400 IU/day) or vitamin C (>500 mg/day); intake of fish oil, flaxseed oil, ω-3 fatty acid, or fiber supplements unless welling to discontinue supplementation for the study duration; use of insulin, glucose-sensitizing medication, or vasoactive medications (including glucocorticoids, antineoplastic agents, psychoactive agents, or bronchodilators); diagnosed diabetes; diagnosed sleep apnea; established cardiovascular disease (including symptomatic coronary artery disease, myocardial infarction, peripheral vascular disease, congestive heart failure, or carotid stenosis); coagulopathy, known bleeding diathesis, or history of clinically significant hemorrhage; current use of warfarin; regular exercise defined as participation in moderate-intensity exercise ≥150 min/week; substance abuse (chronic alcoholism or other chemical dependency); any unstable medical condition (eg, cancer, AIDS, tuberculosis, psychotic disorder) that would limit the ability to participate fully in the trial; pregnant or lactating women; women receiving Depo-Provera shots; and/or women receiving hormone replacement therapy.
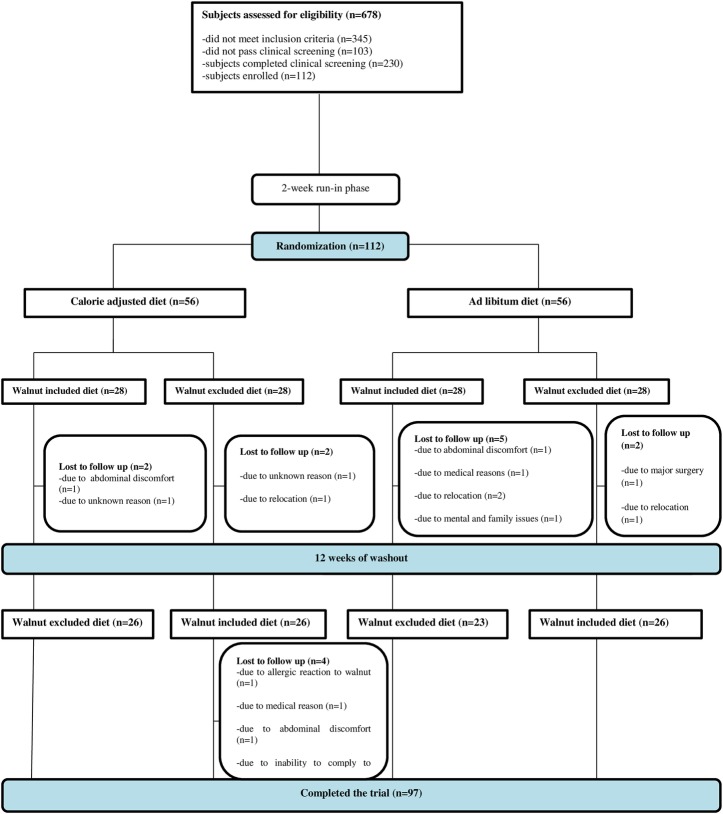
Participants who passed the telephone screening (n=333) underwent clinical screening examination consisting of the assessment of height, weight, BMI, and waist circumference; blood pressure measurements; and laboratory testing, including fasting serum lipids, fasting serum glucose, and HbA1c. The study protocol and consent form were approved by the Griffin Hospital (Derby, Connecticut, USA) Institutional Review Board. Signed informed consent was obtained from all study participants. All participants received monetary compensation for their participation. Participant participation and flow are shown in figure 1.
Figure 1.
Participant flow diagram.
Study design
This study was a randomized, controlled, modified Latin square parallel design study with two treatment arms. The study participants were randomized using a SAS-generated random table. The eligible participants were randomized using a permuted design in a 1:1 ratio between a walnut intake arm with dietary counseling to adjust calorie intake or a walnut intake arm without dietary counseling to adjust calorie intake, and within each arm they were further assigned to one of two different sequence permutations: walnut-included diet/walnut-excluded diet or walnut-excluded diet/walnut-included diet. For the duration of the study, participants underwent an intervention, control, and washout phase. During the intervention phase, participants consumed 392 g of walnuts per week for 6 months, and during the control phase, they excluded walnuts from their diet for 6 months. A 3-month washout phase was incorporated between the two phases. Participants were evaluated on five occasions during the study: immediately prior to phase I, at the mid-point of phase I (3 months), at the end point of phase I (6 months), at the mid-point of phase II (12 months), and at the end point of phase II (15 months).
Intervention
Walnut intake without calorie regulation: Participants were provided 392 g of walnuts per week (56 g or 2 oz/day providing 366 kcal) to include in their diet. Their caloric intake was not monitored or regulated, and thus was allowed to float ad libitum.
Walnut intake with caloric regulation: The intervention group participants met with a registered dietitian and received instructions and recipes for inclusion of 392 g of walnuts per week (56 g or 2 oz/day providing 366 kcal) in their meal plan. Participants received instruction to preserve an isocaloric condition after the addition of walnuts. The study dietitian customized dietary adjustments to make room for walnuts in the diet, while accommodating the priorities of each study participant. The general approach emphasized general reduction in portion sizes; participants also received advice, based on baseline dietary intake analysis, of food eliminations that they might want to consider. While the isocaloric condition was encouraged and monitored by the dietitian, participants were provided latitude in determining how they make room for the walnut calories, to better approximate real-world conditions.
Control diet: During the control phase (ie, walnut-excluded phase), participants received instructions to consume an ad libitum diet while avoiding walnuts and specific walnut-containing products.
Outcome measures
Diet quality: Diet quality was the primary outcome measure for the study. It was assessed using the Healthy Eating Index 2010 (HEI-2010). Participants completed 24 h recalls using a web-based Automated Self-Administrated 24-Hour Recall.16 The participants completed one 24 h recall at each time point of assessment. The HEI-2010 is a tool used for assessing diet quality as specified by the 2010 Dietary Guidelines for Americans. The HEI-2010 is used to assess changes in diet quality over time and the efficacy of nutritional interventions. It is also used to better understand relationships between nutrients, foods, dietary patterns, and health-related outcomes. The basic steps we used to determine HEI-2010 scores include: identifying the set of foods under consideration; determining the amount of each relevant food group, subgroup, and nutrient in the set of foods; deriving the pertinent ratios; and scoring each component using the appropriate standard.17
EF assessment: The brachial artery reactivity studies’ methodology used was as described in the published ‘Guidelines for Ultrasound Assessment of Endothelial-dependent Flow-mediated Vasodilation of the Brachial Artery’.18 EF was measured as flow-mediated dilation (FMD), the percentage change of brachial artery diameter from before cuff inflation to 60 s after cuff release. In addition to brachial diameter at 60 s after cuff release, flow after cuff deflation within the first 15 s was used as an indicator of stimulus strength, hyperemic flow being the stimulus for endothelial reactivity. To account for potential variability in stimulus strength, FMD was divided by flow at 15 s after cuff deflation to create a stimulus-adjusted response measure.
Lipid profile, fasting blood glucose and HbA1c: The lipid profile was determined as follows: Total cholesterol (Tchol), triglycerides (TRIG) and HDL were obtained by direct measurements. Very-low-density lipoprotein (VLDL) and LDL were obtained by calculation: VLDL=TRIG/5; and LDL=Tchol−(VLDL+HDL). HDL:Tchol ratio. Fasting blood glucose and HbA1c were also measured at each visit.
Body composition: Body composition was measured using bioelectrical impedance analysis, which uses the resistance of electrical flow through the body to estimate body fat. The Tanita SC-240 Body Composition Analyzer was used to measure body composition. The SC-240 Body Composition Analyzer measured weight and calculated body fat percentage and total body water percentage in addition to BMI.
Anthropometric measures: Body weight was measured using a calibrated digital scale and height was measured by using a calibrated stadiometer. BMI was calculated as weight (kg) divided by height in meters (m) squared. Waist circumference was measured using the guidelines of the National Obesity Expert Panel Report.
Blood pressure: Blood pressure was measured using an automatic blood pressure monitor at each visit. Systolic and diastolic blood pressure was measured using an approved automated device. Blood pressure was measured (average of three measurements with 3 min between measurements) with the participants sitting in a quiet room.
Physical activity: Physical activity was measured using the International Physical Activity Questionnaire (IPAQ),19 a valid, comprehensive, and reliable tool used to assess physical activity in adults. The IPAQ was used to collect information on weekly involvement in household and yard-work activities, occupational activity, transport, leisure time physical activity, and sedentary behavior.
Statistical analysis
Descriptive and exploratory analyses of all measured outcomes were conducted before embarking on modeling or hypothesis testing procedures. Distributions of variables met the criteria for analysis with parametric statistics. Log transformation of data or non-parametric analytic techniques was employed. Linear mixed model regressions were used to analyze the design. Other factors were incorporated into the regression models in order to adjust for potential confounding factors (ie, covariate imbalance between the treatment groups), such as individual characteristics (ie, age, gender, caloric intake, fiber intake, monounsaturated fat (MUFA) intake, PUFA intake, ω-3 intake, and physical activity level). All analyses of end points were based on the intention-to-treat principle. A p value of <0.05 was considered statistically significant. SAS software for Windows V.9.3 (SAS Institute, Cary, North Carolina, USA) was used to carry out all statistical analyses. Results are expressed as means±SD in text and tables.
The sample size was estimated to allow for 25% attrition and non-compliance and to provide ≥80% power to detect a minimal difference of 7.5 in HEI score20 between treatment groups, with a SD of 10 points in the HEI scale and maximum allowable type I error of 5% adjusted for three pair-wise comparisons.
Results
Of the 112 participants (31 men and 81 women), 97 completed the study. Two participants withdrew from the study for medical reasons unrelated to walnut consumption, four due to relocation, six due to loss to follow-up, one due to an allergic reaction to walnuts, one due to an inability to comply with the study protocol, and one due to mental and family issues. The study participants randomized to receive walnuts with dietary counseling to adjust their caloric intake were comparable in terms of demographics, diet quality, body composition, and marker of cardiovascular risk to those randomized to receive walnuts without advice to control their caloric intake. Demographic characteristics and baseline information of the study participants are presented in tables 1 and 2.
Table 1.
Baseline characteristics of study participants
| Variable | Calorie-adjusted/walnuts (N=56) | No calorie-adjusted/walnuts (N=56) | p Value |
|---|---|---|---|
| Gender (female) | 69.6% | 75.0% | 0.53 |
| Age (years) | 56.5±11.7 | 53.3±11.1 | 0.15 |
| 2010 Healthy Eating Index score | 57.1±17.4 | 54.0±15.7 | 0.36 |
| FMD (%) | 8.9±3.1 | 8.6±2.1 | 0.54 |
| SARM | 0.1±0.1 | 0.1±0.1 | 0.21 |
| HbA1c (%) | 5.7±0.4 | 5.8±0.4 | 0.25 |
| Fasting blood glucose (mg/dL) | 93.3±8.2 | 95.4±10.3 | 0.23 |
| Total cholesterol (mg/dL) | 212.7±33.8 | 206.2±34.0 | 0.32 |
| Triglycerides (mg/dL) | 115.4±46.7 | 110.2±53.3 | 0.59 |
| HDL (mg/dL) | 60.8±16.7 | 58.5±14.9 | 0.46 |
| Low-density lipoprotein (mg/dL) | 129.2±31.2 | 126.1±31.1 | 0.60 |
| Total cholesterol/HDL | 3.7±1.2 | 3.7±1.1 | 0.92 |
| Systolic blood pressure (mm Hg) | 126.6±16.1 | 122.4±11.6 | 0.11 |
| Diastolic blood pressure (mm Hg) | 72.7±7.9 | 72.9±8.2 | 0.93 |
| Visceral fat | 11.5±4.0 | 10.7±3.5 | 0.27 |
| Waist circumference (cm) | 101.7±10.2 | 100.1±10.8 | 0.43 |
| Weight (lbs) | 180.4±31.7 | 184.2±31.1 | 0.52 |
| Body mass index (kg/m²) | 30.0±4.0 | 30.2±4.1 | 0.73 |
| Percent body fat (%) | 37.3±7.5 | 37.7±7.7 | 0.76 |
| Percent body water | 43.2±7.0 | 43.9±5.0 | 0.57 |
FMD, flow-mediated dilation; HDL, high-density lipoprotein; SARM, stimulus adjusted response measure.
Table 2.
Change in outcome measures from baseline to 6 months
| Variable | CAW | Calorie-adjusted/walnut-excluded | p Value | NCAW | No calorie-adjusted/walnut-excluded | p Value | CAW vs NCAW p Value |
|---|---|---|---|---|---|---|---|
| Healthy Eating Index score | 9.14±17.71** | 0.40±15.13 | 0.02 | 7.02±15.89¥ | −5.92±21.84¥ | 0.001 | 0.60 |
| FMD (%) | 1.94±3.76** | 1.54±4.31¥ | 0.62 | 2.21±4.01*** | 1.44±3.60* | 0.34 | 0.74 |
| SARM | 0.07±0.26¥ | 0.05±0.17 | 0.45 | −0.01±0.11 | −0.02±0.07 | 0.62 | 0.03 |
| HbA1c (%) | 0.05±0.14¥ | 0.06±0.14* | 0.64 | 0.10±0.21*** | 0.04±0.17 | 0.07 | 0.12 |
| Fasting blood glucose (mg/dL) | −1.75±7.29 | −0.33±5.42 | 0.35 | 0.02±9.67 | −1.08±7.27 | 0.47 | 0.25 |
| BMI (kg/m²) | −0.14±2.23 | −0.33±2.22 | 0.62 | 0.17±1.25 | −0.30±1.75 | 0.22 | 0.43 |
| Percent body fat (%) | 0.76±3.88 | 0.95±4.48 | 0.86 | 1.98±8.16¥ | 0.84±3.28 | 0.29 | 0.26 |
| Percent body water (%) | 0.72±7.19 | 0.54±6.96 | 0.87 | −0.39±2.84 | −0.60±2.19 | 0.84 | 0.31 |
| Visceral fat | 0.25±1.71 | 0.29±1.99 | 0.91 | 0.47±1.99¥ | 0.45±1.08 | 0.95 | 0.53 |
| Waist (cm) | −2.40±4.67** | −3.30±4.82*** | 0.34 | −1.28±4.84 | −1.89±4.11* | 0.52 | 0.24 |
| HDL (mg/dL) | −1.33±7.95 | −0.12±8.35 | 0.45 | −1.08±6.83 | −0.24±8.96 | 0.61 | 0.88 |
| LDL (mg/dL) | −14.52±24.11*** | −9.79±15.87** | 0.22 | −12.39±17.82*** | −11.84±19.10*** | 0.89 | 0.59 |
| Total cholesterol (mg/dL) | −16.04±27.34*** | −9.42±19.85* | 0.15 | −12.51±22.49*** | −11.14±21.78** | 0.77 | 0.45 |
| Triglycerides (mg/dL) | −1.15±34.34 | 2.44±39.60 | 0.69 | 4.53±53.69 | 4.57±48.89 | 0.99 | 0.53 |
| Total cholesterol/HDL | −0.29±0.74* | −0.15±0.64 | 0.33 | −0.19±0.67 | −0.22±0.82¥ | 0.82 | 0.49 |
| Diastolic blood pressure (mm Hg) | 0.46±6.42 | 0.60±7.36 | 0.93 | 0.82±7.77 | 1.80±8.41 | 0.52 | 0.82 |
| Systolic blood pressure (mm Hg) | −0.46±11.20 | 2.38±13.33 | 0.31 | 0.51±17.86 | 1.98±12.09 | 0.60 | 0.73 |
¥<0.05, *<0.01, **<0.001, ***<0.0001 indicates significant changes from baseline.
BMI, body mass index; CAW, calorie-adjusted/walnut-included; FMD, flow-mediated dilation; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NCAW, no calorie-adjusted/Walnut-included.
When compared with a walnut-excluded diet, a walnut-included diet for 6 months, with or without dietary counseling to adjust caloric intake, significantly improved diet quality as measured by the HEI-2010 (9.14±17.71 vs 0.40±15.13; p=0.02 and 7.02±15.89 vs −5.92±21.84; p=0.001, respectively).
EF, total and LDL cholesterol significantly improved from baseline after a walnut-included diet daily for 6 months with or without dietary counseling to adjust caloric intake. However, EF, total and LDL cholesterol did not significantly differ between the walnut-included diet for 6 months, with or without dietary counseling to adjust caloric intake compared with walnut-excluded diet. When compared with a walnut-excluded diet, a walnut-included diet for 6 months, with or without dietary counseling to adjust caloric intake, did not significantly improve (p>0.05) BMI, percent body fat, percent body water, or visceral fat. The walnut-included diet, when consumed without advice to adjust caloric intake, led to significant increases in percent body fat and visceral fat relative to baseline. Waist circumference significantly improved from baseline for the walnut-included diet for 6 months with dietary counseling to adjust caloric intake as well as in the walnut-excluded diet.
A walnut-included diet for 6 months, with or without dietary counseling to adjust caloric intake, did not improve (p>0.05) blood pressure and fasting blood glucose level in this sample of adults at risk for diabetes. HbA1c significantly increased from baseline after daily consumption of walnuts for 6 months with or without dietary counseling to adjust caloric intake. HbA1c level significantly increased from baseline also in the walnut-excluded diet.
Dietary counseling to adjust caloric intake to keep it constant with the addition of walnuts to the diet did not (p>0.05) enhance the beneficial effects of walnut consumption on diet quality, body composition, and vascular function.
Our findings persisted after controlling for age, gender, caloric intake, fiber intake, MUFA intake, PUFA intake, ω-3 intake, and physical activity level in regression models.
Discussion
Our data suggest that inclusion of walnuts in the diet, with or without dietary counseling to adjust caloric intake, improved diet quality and may also improve EF, reduce total and LDL cholesterol in this sample of adults at risk for diabetes. Inclusion of walnuts in the diet, with or without dietary counseling to adjust caloric intake, did not affect anthropometric measures, insulin response, and blood pressure in these participants at risk for diabetes. No differential treatment effects were observed in our outcome measures, whether or not the participants received dietary counseling to adjust caloric intake to compensate for the inclusion of walnuts in their diet.
The inclusion of 56 g walnuts/day in the diet, with or without dietary counseling to adjust caloric intake, significantly improved diet quality as measured by the 2010 HEI in this sample of adults at risk for type 2 diabetic. Improving diet quality has been associated with a reduction of cardiometabolic risk and chronic diseases in general and also reduces the risk of mortality due to chronic diseases,1 21–24 The Dietary Guideline for Americans recommends the Mediterranean dietary pattern to promote health and prevent chronic diseases.25 The Mediterranean dietary pattern, which is rich in nuts in general and walnuts in particular, has been associated with low incidence of cardiovascular disease and reduction of mortality due to chronic diseases.21 26 27 Walnuts are rich in heart-healthy MUFA, protein, and vitamin E, and have a very low ω-6/ω-3 fatty acid ratio relative to other nuts. ω-3 fatty acids are thought to slow down the growth of plaques in the arteries.
Consumption of walnuts has also been shown to improve EF in individuals with hypercholesterolemia, overweight adults with visceral obesity and type 2 diabetes.9–11 15 The EF of our study participants improved significantly from baseline with the inclusion of walnuts in the diet, with or without dietary counseling to adjust caloric intake. Interestingly, EF also improved in the walnut-excluded diet phase, probably due to variability inherent in the diet. The improvement in EF associated with consumption of walnuts is probably due to the low ω-6 to ω-3 ratio in walnuts, and to the high content of fiber, magnesium, folate, and antioxidants.
We observed a significant reduction of total and LDL cholesterol from baseline with inclusion of walnuts in the diet. However, when compared with the walnut-excluded phase, the walnut-included diet showed no significant improvement, probably due to the placebo effect. In a pooled analysis of 25 studies conducted by Sabaté et al,28 walnut consumption reduced cholesterol levels in lean individuals. In a meta-analysis by Banel and Hu,29 walnut consumption reduced total and LDL cholesterol. Some studies have shown no significant improvement in cholesterol level with the consumption of walnuts.15 30 The inconsistency of the results of the effects of walnuts on cholesterol may be due to the diversity of the population studied, and/or to different doses and duration of the studies. Walnut consumption has been found to increase the insulin response during an oral glucose tolerance test, and to decrease levels of HbA1c in individuals with polycystic ovary syndrome, a condition commonly associated with insulin resistance.31 The Nurses Health Study found an inverse association between the consumption of nuts and the risk of type 2 diabetes.1 However, we did not find an increase in insulin response and/or a decrease in HbA1c in this study and our previous studies.11 15 This may relate to variations in study populations, study duration, or the treatment dose, among other potential explanations.
Body weight of our study participants remained constant with no increase in visceral and percent body fat with the inclusion of walnuts in the diet, with or without dietary counseling to adjust caloric intake. These results are consistent with our prior research, where daily consumption of 56 g of walnuts for 8 weeks as part of an ad libitum diet did not lead to a significant change in anthropometric measures in overweight adults, despite an increase in self-reported caloric intake.15Numerous epidemiological and clinical studies thus far have shown that nut intake is not associated with weight gain.11 29 31 32 This is likely due to the fact that nuts exert their effects on satiety and also possibly due to inefficient absorption of caloric energy from nuts.13
Including walnuts in the diet, with or without dietary counseling to adjust caloric intake, did not improve blood pressure for these participants at risk for diabetes. Studies assessing the effects of walnuts on blood pressure have shown mixed results. In our previous studies, consumption of walnuts did not improve blood pressure in overweight adults or in patients with diabetes.11 15 West et al33 showed significant improvement in blood pressure in hypercholesterolemic participants with walnut consumption. These conflicting results of walnuts on blood pressure may be due to different amount of walnuts, study duration, and population characteristics at baseline. The beneficial effects of walnuts on blood pressure that have been seen in some studies could be due to the high content of ω-3, fiber, and magnesium in walnuts.
Limitations
This study has several limitations. The study sample was predominantly comprised of white women, which limits our ability to generalize these findings. Another limitation is that our sample size calculations for this study were powered based only on our primary outcome measure, diet quality. Thus, this study may be underpowered for our secondary outcome measures. This study relied on self-report by the participants for dietary intake, which can introduce measurement and recall biases. However, surveys used to capture these data have been validated and tested for reliability, which therefore limits such measurement errors. Another limitation was that the participants were not administered a restricted diet and their dietary intake was not monitored on a daily basis. However, this can also be viewed as strength of the study because it provides a more realistic scenario and potentially improves external validity.
Conclusions
Our data suggest that a walnut-included diet, when consumed daily for 6 months, with or without dietary counseling to adjust caloric intake, improved diet quality as measured by the HEI-2010. The HEI inversely correlates with chronic disease outcome. EF, total and LDL cholesterol improved from baseline regardless of treatment assignments in this sample of adults at risk for diabetes. Daily consumption of walnuts for 6 months, with or without dietary counseling to adjust caloric intake in this sample of adults at risk for diabetes, did not improve fasting blood glucose, HDL cholesterol, blood pressure, body composition, or anthropometric measures. Controlling caloric intake to keep it constant with the addition of walnuts to the diet did not seem to enhance the beneficial effects of walnut ingestion on diet quality, vascular function, insulin response, body composition, and anthropometric measures. Further investigation is warranted in a more diverse population to replicate these findings.
Acknowledgments
The authors wish to acknowledge the technical assistance of Mrs Susan Acheychek RDMS for assessing the clinical outcomes.
Footnotes
Contributors: VYN was involved in study design, project oversight, data analysis, data interpretation, developed manuscript draft, final approval. RA is project coordinator. PP conducted statistical analysis and data interpretation. JAT is study dietitian and was involved in critical review. DLK was involved in study design, project oversight, data interpretation, critical review of paper, final approval.
Funding: Funding for this study has been provided by the California Walnut Commission (#5U48DP001945-02).
Competing interests: DLK has been compensated for public speaking by the California Walnut Commission.
Ethics approval: Griffin Hospital Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Jiang R, Manson JE, Stampfer MJ et al. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 2002;288:2554–60. 10.1001/jama.288.20.2554 [DOI] [PubMed] [Google Scholar]
- 2.Albert CM, Gaziano JM, Willett WC et al. Nut consumption and decreased risk of sudden cardiac death in the Physicians’ Health Study. Arch Intern Med 2002;162:1382–7. 10.1001/archinte.162.12.1382 [DOI] [PubMed] [Google Scholar]
- 3.Kris-Etherton PM, Zhao G, Binkoski AE et al. The effects of nuts on coronary heart disease risk. Nutr Rev 2001;59:103–11. 10.1111/j.1753-4887.2001.tb06996.x [DOI] [PubMed] [Google Scholar]
- 4.Feldman EB. The scientific evidence for a beneficial health relationship between walnuts and coronary heart disease. J Nutr 2002;132:1062S–101S. [DOI] [PubMed] [Google Scholar]
- 5.Tapsell LC, Gillen LJ, Patch CS et al. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care 2004;27:2777–83. 10.2337/diacare.27.12.2777 [DOI] [PubMed] [Google Scholar]
- 6.Egert S, Stehle P. Impact of n-3 fatty acids on endothelial function: results from human interventions studies. Curr Opin Clin Nutr Metab Care 2011;14:121–31. 10.1097/MCO.0b013e3283439622 [DOI] [PubMed] [Google Scholar]
- 7.Holy EW, Forestier M, Richter EK et al. Dietary α-linolenic acid inhibits arterial thrombus formation, tissue factor expression, and platelet activation. Arterioscler Thromb Vasc Biol 2011;31:1772–80. 10.1161/ATVBAHA.111.226118 [DOI] [PubMed] [Google Scholar]
- 8.Damasceno NR, Pérez-Heras A, Serra M et al. Crossover study of diets enriched with virgin olive oil, walnuts or almonds. Effects on lipids and other cardiovascular risk markers. Nutr Metab Cardiovasc Dis 2011;21:S14–20. 10.1016/j.numecd.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 9.Ros E, Núñez I, Pérez-Heras A et al. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation 2004;109:1609–14. 10.1161/01.CIR.0000124477.91474.FF [DOI] [PubMed] [Google Scholar]
- 10.Cortes B, Núñez I, Cofán M et al. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J Am Coll Cardiol 2006;48:1666–71. 10.1016/j.jacc.2006.06.057 [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Njike VY, Millet J et al. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabetes Care 2010;33:227–32. 10.2337/dc09-1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan AM, Sweeney LL, Liu X et al. Walnut consumption increases satiation but has no effect on insulin resistance or the metabolic profile over a 4-day period. Obesity (Silver Spring) 2010;18:1176–82. 10.1038/oby.2009.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattes RD, Dreher ML. Nuts and healthy body weight maintenance mechanisms. Asia Pac J Clin Nutr 2010;19:137–41. [PubMed] [Google Scholar]
- 14.Department of Health and Human Services. Healthy People. 2010. http://www.cdc.gov/nchs/healthy_people/hp2010.htm. Accessed 10/8/2015.
- 15.Katz DL, Davidhi A, Ma Y et al. Effects of walnuts on endothelial function in overweight adults with signs of metabolic syndrome: a randomized, controlled, cross-over trial. J Am Coll Nutr 2012;31:415–23. 10.1080/07315724.2012.10720468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute, N.C. http://riskfactor.cancer.gov/tools/instruments/asa24/.
- 17.Reedy J, Krebs-Smith SM, Bosire C. Evaluating the food environment: application of the Healthy Eating Index-2005. Am J Prev Med 2010;38:465–47. 10.1016/j.amepre.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 18.Corretti MC, Anderson TJ, Benjamin EJ et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery. A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;16:257–65. 10.1016/S0735-1097(01)01746-6 [DOI] [PubMed] [Google Scholar]
- 19.Craig CL, Marshall AL, Sjöström M et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 20.McCullough ML, Feskanich D, Stampfer MJ et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261–71. [DOI] [PubMed] [Google Scholar]
- 21.Estruch R, Ros E, Salas-Salvadó J et al. , PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. 10.1056/NEJMoa1200303 [DOI] [PubMed] [Google Scholar]
- 22.Akbaraly TN, Ferrie JE, Berr C et al. Alternative Healthy Eating Index and mortality over 18 y of follow-up: results from the Whitehall II cohort. Am J Clin Nutr 2011;94:247–53. 10.3945/ajcn.111.013128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atkins JL, Whincup PH, Morris RW et al. High diet quality is associated with a lower risk of cardiovascular disease and all-cause mortality in older men. J Nutr 2014;144:673–80. 10.3945/jn.113.186486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reedy J, Krebs-Smith SM, Miller PE et al. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr 2014;144:881–9. 10.3945/jn.113.189407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Services, U.S.D.o.H.a.H. Dietary Guidelines for Americans 2010. 2010. cited 2014 12/23/2014 http://health.gov/dietaryguidelines/dga2010/dietaryguidelines2010.pdf. Access 10/8/2015.
- 26.George SM, Ballard-Barbash R, Manson JE et al. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women's Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol 2014;180:616–25. 10.1093/aje/kwu173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Garcia E, Rodriguez-Artalejo F, Li TY et al. The Mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. Am J Clin Nutr 2014;99:172–80. 10.3945/ajcn.113.068106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med 2010;170:821–7. 10.1001/archinternmed.2010.79 [DOI] [PubMed] [Google Scholar]
- 29.Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr 2009;90:56–63. 10.3945/ajcn.2009.27457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan JM, Horton K, Reese D et al. Effects of walnut consumption as part of a low-fat, low-cholesterol diet on serum cardiovascular risk factors. Int J Vitam Nutr Res 2002;72:341–7. 10.1024/0300-9831.72.5.341 [DOI] [PubMed] [Google Scholar]
- 31.Kalgaonkar S, Almario RU, Gurusinghe D et al. Differential effects of walnuts vs almonds on improving metabolic and endocrine parameters in PCOS. Eur J Clin Nutr 2011;65:386–93. 10.1038/ejcn.2010.266 [DOI] [PubMed] [Google Scholar]
- 32.Fitschen PJ, Rolfhus KR, Winfrey MR et al. Cardiovascular effects of consumption of black versus English walnuts. J Med Food 2011;14:890–8. 10.1089/jmf.2010.0169 [DOI] [PubMed] [Google Scholar]
- 33.West SG, Krick AL, Klein LC et al. Effects of diets high in walnuts and flax oil on hemodynamic responses to stress and vascular endothelial function. J Am Coll Nutr 2010;29:595–603. 10.1080/07315724.2010.10719898 [DOI] [PMC free article] [PubMed] [Google Scholar]