Abstract
Wolbachia is a vertically transmitted endosymbiont whose radiative success is mainly related to various host reproductive manipulations that led to consider this symbiont as a conflictual reproductive parasite. However, lately, some Wolbachia have been shown to act as beneficial symbionts by protecting hosts against a broad range of parasites. Still, this protection has been mostly demonstrated in artificial Wolbachia-host associations between partners that did not co-evolved together. Here, we tested in two terrestrial isopod species Armadillidium vulgare and Porcellio dilatatus whether resident Wolbachia (native or non-native) could confer protection during infections with Listeria ivanovii and Salmonella typhimurium and also during a transinfection with a Wolbachia strain that kills the recipient host (i.e., wVulC in P. dilatatus). Survival analyses showed that (i) A. vulgare lines hosting their native Wolbachia (wVulC) always exhibited higher survival than asymbiotic ones when infected with pathogenic bacteria (ii) P. dilatatus lines hosting their native wDil Wolbachia strain survived the S. typhimurium infection better, while lines hosting non-native wCon Wolbachia strain survived the L. ivanovii and also the transinfection with wVulC from A. vulgare better. By studying L. ivanovii and S. typhimurium loads in the hemolymph of the different host-Wolbachia systems, we showed that (i) the difference in survival between lines after L. ivanovii infections were not linked to the difference between their pathogenic bacterial loads, and (ii) the difference in survival after S. typhimurium infections corresponds to lower loads of pathogenic bacteria. Overall, our results demonstrate a beneficial effect of Wolbachia on survival of terrestrial isopods when infected with pathogenic intracellular bacteria. This protective effect may rely on different mechanisms depending on the resident symbiont and the invasive bacteria interacting together within the hosts.
Keywords: Wolbachia, infection, immunocompetence, Listeria, Salmonella, terrestrial isopods, Armadillidium vulgare, Porcellio dilatatus
Introduction
Symbioses, defined as intimate interactions between two or more species, can range from mutualism, where both partners benefit from the relationship, to parasitism where one of the partners exploits the other. In order to be maintained and become widespread in the host population, vertically transmitted symbionts adopt some strategies to manipulate their host’s reproduction and/or to provide their host with fitness benefits (Haine, 2008). The advantages of beneficial symbiotic relations can generally be categorized into two main functions: nutrition or protection (Douglas, 2011). In the case of vertically transmitted primary symbionts (i.e., via oocyte), this benefit is usually nutritional, through which the symbiont improves the specialized diet of the host, providing essential nutrients such as amino acids or vitamins (Gross et al., 2009). There is also clear evidence that symbionts can be involved in protection against predators and pathogens (Scarborough et al., 2005; Hedges and Johnson, 2008; Jaenike et al., 2010; Xie et al., 2010). This protection might be achieved with a direct interference with the pathogens or predators by the production of toxic compounds (Davidson et al., 2001; Jaenike and Perlman, 2002). Alternatively this protection can be caused indirectly by the competition between the symbiont and the pathogens for limited resources (Caragata et al., 2013) or by the modulation of host physiology including immune system (de Souza et al., 2009; Kaiser et al., 2010; Weiss et al., 2011). Although the underlying mechanisms of protective symbiosis are not yet unraveled (Douglas, 2011), the existence of protection against pathogens mediated by the presence of a vertically transmitted symbiont is supported by many studies (Haine, 2008; Brownlie and Johnson, 2009).
Even though symbiotic relationships are abundant in both vertebrates and invertebrates, an outstanding diversity of vertically transmitted symbionts has been shown only in the latter (Yen and Barr, 1971; Hurst et al., 1999; Stouthamer et al., 1999; Dunn and Smith, 2001; von der Schulenburg et al., 2001). Being one of the most common vertically transmitted symbiotic bacteria among Cuticulata, Wolbachia are found widespread in arthropods and may infect up to 50% of the arthropod species (Weinert et al., 2015). The success of their vertical transmission mainly relies on the manipulation of the reproduction of their hosts in different ways. For example, feminization induced by Wolbachia forces infected genetic males to develop in functional females, thus becoming able to transmit Wolbachia, resulting in a female bias in the population (Bouchon et al., 2008). Another example of a host reproduction manipulation strategy of Wolbachia is the cytoplasmic incompatibility (CI) that gives a fitness advantage to infected females by causing mortality of the embryos when uninfected females copulate with infected males (Serbus et al., 2008). In addition to the ability of the Wolbachia to manipulate their hosts reproduction, some interactions have been reported to be detrimental on various host life history traits, including body size (Hoffmann and Turelli, 1988), fecundity (Hoffmann et al., 1990; Fleury et al., 2000), survival (Fleury et al., 2000; Tagami et al., 2001), larval competitiveness (Huigens et al., 2004), mating choice (Rigaud and Moreau, 2004) as well as the hosts’ immunity (Fytrou et al., 2006; Braquart-Varnier et al., 2008; Sicard et al., 2010).
Recent studies showed that Wolbachia is not always conflictual and may also act as a mutualist with its hosts by being protective (i.e., increasing their host’s survival during a pathogenic challenge; Teixeira et al., 2008; Gross et al., 2009; Bian et al., 2010; Glaser and Meola, 2010; Zélé et al., 2012; Eleftherianos et al., 2013) or improving nutrition (Hosokawa et al., 2010). The first protective mutualistic effect of the presence of a native Wolbachia (wMel) against several viruses has been demonstrated in Drosophila melanogaster (Hedges et al., 2008; Teixeira et al., 2008). However, this protection seems limited to viruses (Rottschaefer and Lazzaro, 2012; Ye et al., 2013). On the other hand, in mosquitoes, in addition to the reports of a protective effect against viruses (i.e., Dengue, Chikugunya, in Aedes aegypti and Aedes albopictus; Moreira et al., 2009), protection against protozoans (Moreira et al., 2009), filarial nematodes (Kambris et al., 2009), as well as two bacteria species (i.e., Erwinia carotovora and Salmonella typhimurium; Kambris et al., 2009; Ye et al., 2013) have been linked to the presence of Wolbachia. However, all these observations were made using mosquitoes artificially transinfected with wMel and wMelpop from Drosophila (but see Zélé et al., 2012). Though interesting, these situations do not represent natural symbiotic systems where Wolbachia would have evolved toward being mutualistic by conferring protection to their host against pathogens.
The Wolbachia–isopod symbiotic systems constitute tractable experimental models for transinfection experiments and therefore to study the effects of native and non-native Wolbachia on the phenotype of their hosts (Moret et al., 2001; Le Clec’h et al., 2013). Besides, previous studies have been conducted to understand the effects of the Wolbachia on the hosts’ immune parameters. These studies have pointed out an immunodepressing effect of the presence of Wolbachia during ageing of the terrestrial isopod Armadillidium vulgare: 2-years-old females exhibited lower phenoloxidase (PO) activity (Sicard et al., 2010), lower hemocyte density and sometimes even bacteremia in their hemolymph (Braquart-Varnier et al., 2008). The reported presence, in some individuals, of bacteria from the environment suggests that those micro-organisms may constitute a threat in some cases, especially in older animals and/or when the isopod’s immune parameters are low (Braquart-Varnier et al., 2008). However, in younger individuals (i.e., 1-year-old animals) of two isopod species (A. vulgare and Porcellio dilatatus), no such negative effects of Wolbachia on immune parameters were observed suggesting that Wolbachia would only cause immunodepression in older animals (Sicard et al., 2010; Pigeault et al., 2014).
Considering the contrasted influence of Wolbachia on isopods’ (i) immune parameters (i.e., PO activity, hemocyte density, phagocytosis, proportion of hemocyte types; Braquart-Varnier et al., 2008; Sicard et al., 2010; Pigeault et al., 2014) and (ii) immune gene expression (Chevalier et al., 2012), the impact of the presence of Wolbachia on their host immunocompetence when facing a pathogen had to be directly assessed. As some environmental bacteria were detected, in some cases, in the isopod hemolymph, it appeared pertinent to study the impact of resident Wolbachia on the survival of terrestrial isopods against bacterial pathogens by injecting them directly into the hemolymph. Despite comprehensive investigations, no specific cultivable bacterial pathogens of terrestrial isopod have been described to date (Braquart-Varnier and Sicard, personal observation). Thus, we chose to investigate this question with two intracellular bacterial pathogens ecologically relevant and already demonstrated to be pathogenic toward arthropods: the Gram-positive Listeria ivanovii and the Gram-negative S. typhimurium, which were both detected in soil samples (Jacobsen and Bech, 2012; Sauders et al., 2012). In parallel with our investigation on ‘conventional’ bacterial pathogens, we also studied the impact of a resident vertically transmitted Wolbachia on the detrimental effect caused by the transinfection of the wVulC strain from A. vulgare to P. dilatatus (Le Clec’h et al., 2012). Indeed such transinfection has led to the death of all asymbiotic individuals (i.e., without Wolbachia; Le Clec’h et al., 2012). Resident Wolbachia (either native or non-native) might affect the host survival in case of such multiple infections (i.e., the resident Wolbachia strain and the invasive strain) in two main ways: (i) causing a decrease in wVulC induced mortality due to negative interference between the two Wolbachia strains, (ii) or causing an increase in wVulC induced mortality due to the additive costs that can result from multiple infections (López-Villavicencio et al., 2011). All the three “pathogens” used in this study are considered to share a similar intracellular niche with the resident Wolbachia and thus can potentially compete with the resident symbiont for resources (Caragata et al., 2013; Sicard et al., 2014b). Such competition could in return decrease the success of the pathogens (Loker et al., 2004). Wolbachia could also interfere with pathogenic bacteria directly via the production of toxic compounds or in a more indirect way, by modulating the host physiology (Kaiser et al., 2010), particularly the host immune system (Kambris et al., 2009).
The present study aimed to test whether resident Wolbachia (either native or not) can modulate the survival of the terrestrial isopods when infected with the pathogenic intracellular bacteria L. ivanovii and S. typhimurium as well as after the transinfection with Wolbachia (wVulC). We showed in both A. vulgare and P. dilatatus that the presence of a native Wolbachia can increase their ability to survive pathogenic bacterial infections. We thus reveal a ‘mutualistic side’ of Wolbachia–isopod interactions, which were considered until now as quite conflictual ones.
Materials and Methods
Biological Material
Isopod Lines
We studied the impact of resident Wolbachia on their host survival when infected with intracellular pathogenic bacteria with lines of two terrestrial isopod host species. These two host species were chosen because each of them allowed to test the effect of different parameters: (i) the model A. vulgare allowed us to test the impact of the feminizing Wolbachia wVulC but also of their different host’s population of origin while (ii) the model P. dilatatus allowed us to test the effect of two CI-inducing Wolbachia genotypes (native versus non-native) on the same host genetic background. In A. vulgare lines, the impact of Wolbachia on the survival when injected with the pathogenic bacteria could not be tested in males as no males of this species were infected with Wolbachia due to the feminization process. However, for P. dilatatus, it was possible to test the effect of gender as they host CI-inducing strains, wDil and wCon, which infect both sexes (Sicard et al., 2014a).
In a previous study, Le Clec’h et al. (2013), had introduced by injection the native Wolbachia strain (wDil) isolated from a P. dilatatus symbiotic line (Sicard et al., 2014a), and a non-native CI Wolbachia strain (wCon) isolated from Cylisticus convexus (Moret et al., 2001) in recipient individuals from the asymbiotic P. dilatatus line (dilatatus A line; Table 1; Sicard et al., 2014a). Both Wolbachia are since then stably transmitted from injected mothers to further generations resulting in two independent lines maintained in the laboratory so called ‘dilatatus A-wCon’ and ‘dilatatus A-wDil’ (Table 1).
Table 1.
Isopod lines used in this study and their main characteristics: gender of studied animals, their origins, their symbiotic condition [asymbiotic, symbiotic (naturally or experimentally infected)], the Wolbachia strain that they harbor.
| Species | Line | Gender | Origin | Wolbachia status | Resident Wolbachia strain | Pathogen injections |
|---|---|---|---|---|---|---|
| Armadillidium vulgare | WXa |  |
Denmark | Asymbiotic | — | Salmonella typhimurium and Listeria ivanovii |
| WXw |  |
Denmark | Symbiotic | wVulCWX | S. typhimurium and L. ivanovii | |
| BF |  |
France | Asymbiotic | — | S. typhimurium and L. ivanovii | |
| BFwVulC |  |
BF line | Experimentally infected | wVulCZN | S. typhimurium and L. ivanovii | |
| ZN |  |
France | Symbiotic | wVulCZN | S. typhimurium and L. ivanovii | |
| Porcellio dilatatus | dilatatus A |
 and and 
|
France | Asymbiotic | — | wVulCWX, S. typhimurium and L. ivanovii |
| dilatatus A- wDil |
 and and 
|
dilatatus A line | Experimentally infected | wDil | wVulCWX, S. typhimurium and L. ivanovii | |
| dilatatus A- wCon |
 and and 
|
dilatatus A line | Experimentally infected | wCon | wVulCWX, S. typhimurium and L. ivanovii |
We used two A. vulgare asymbiotic lines (WXa from Helsingor and BF from Nice; Table 1) and two A. vulgare symbiotic lines infected with wVulC (ZN from Celles-Sur-Belles and WXw from Helsingor; Table 1). We also created the BFwVulC line to have BF individuals infected with Wolbachia wVulC with the same genetic background as BF asymbiotic population. This line was generated by injection of Wolbachia from ZN females in BF recipient females following the procedure described in Le Clec’h et al. (2013). Recipient females were then crossed with BF males to create this new line. Then, females and males from the BFwVulC line are crossed together at each generation to maintain the line. Animals from the BFwVulC line are infected with Wolbachia wVulC but have the ‘genetic background’ of BF asymbiotic line. The sex-ratio deviance due to Wolbachia infection in BFwVulC line was similar to the one observed in ZN line (data not shown). All the individuals from the different lines were grown at 20°C, in plastic breeding boxes, on humid soil and fed with dead lime-tree leaves. All animals used in this study were 1-year-old.
Pathogenic Intracellular Bacteria
Two phylogenetically distant intracellular pathogenic bacteria species, L. ivanovii (Phylum: Firmicutes) and S. typhimurium (Phylum: Proteobacteria), were injected into A. vulgare and P. dilatatus individuals from the different lines. L. ivanovii are Gram-positive intracellular bacteria, which are facultative anaerobe, non-spore forming rods (Vázquez-Boland et al., 2001). L. ivanovii has been shown to establish an intracellular infection causing moderate mortality in D. melanogaster (Rottschaefer and Lazzaro, 2012). The Gram-negative bacteria from the serotype Salmonella enterica serotype Typhimurium are facultative aerobe non-spore forming bacilli (Velge et al., 2012). S. typhimurium is considered as having a broad host range since they are able to infect the cells of many vertebrates (Velge et al., 2012) as well as invertebrates, such as D. melanogaster (Rottschaefer and Lazzaro, 2012). The strain used in this study expressed constitutively GFP.
Salmonella and Listeria Injections
Bacterial Cultures
For S. typhimurium and L. ivanovii injections, bacteria from glycerol stocks were cultured overnight at 37°C in liquid LB medium (Luria Bertani Broth Base, Invitrogen) or in BHI medium (Brain-Heart Infusion, BD) respectively. These cultures were then used to grow bacterial colonies on LB plates or BHI plates at 37°C (LB or BHI with 15 g/L agar) until colonies reached a 5 mm diameter. Before each injection experiment, one colony of either S. typhimurium or L. ivanovii from solid cultures was added to 5 mL of LB or BHI liquid medium and incubated overnight at 37°C. One hundred microliter of the overnight culture was added to 5 mL of LB or BHI and incubated at 37°C to reach a 0.7 optical density (OD) at 600 nm. One milliliter of the 0.7 OD culture was then centrifuged (at 13000 g, 4°C, 2 min). The supernatant was disposed and the pellet was resuspended in 1 mL of fresh LB or BHI medium. The resulting suspension contained around 105 bacteria/μL for S. typhimurium and 106 bacteria/μL for L. ivanovii. The S. typhimurium suspension was then diluted by 10 in order to obtain a 104 bacteria/μL suspension used for challenging both the A. vulgare and P. dilatatus individuals. Regarding the L. ivanovii, the initial 106 bacteria/μL suspension was used to challenge the A. vulgare individuals. Due to the higher susceptibility of P. dilatatus to L. ivanovii (data not shown), the L. ivanovii suspension was diluted by 10 to reach 105 bacteria/μL to challenge P. dilatatus.
For each independent replicate (for both survival and pathogen multiplication experiments), all the bacterial cultures were prepared separately. Additionally, to check the actual number of injected bacteria, serial dilutions were made to obtain around 1 bacteria/μL, and 100 μL of this dilution was streaked on solid LB plates or BHI plates depending on the bacteria.
Survival Assays
A Hamilton syringe with fine glass needle was used to inject 1 μL of the bacterial suspension (S. typhimurium or L. ivanovii) or 1 μL of the control solution (LB or BHI sterile liquid medium respectively) into the general cavity. All batches comprised six individuals of each line (WXa, WXw, BF, BFwVulC, ZN, dilatatus A, dilatatus A-wCon, and dilatatus A-wDil) and were independently replicated five times (n = 30). For each replicate, three individuals from each line received the control treatment (n = 15 per line). After the injections of L. ivanovii or S. typhimurium, the animals from the same replicate were kept in a plastic box with moist paper and checked every 4 h to record the mortality during 144 h for S. typhimurium challenged individuals or 200 h for L. ivanovii challenged individuals (as the mortality appeared later for L. ivanovii injected isopods).
Pathogen Multiplication in Asymbiotic versus Symbiotic Animals
In order to compare the L. ivanovii and S. typhimurium loads between symbiotic (BFwVulC, dilatatus A-wDil) and asymbiotic (BF, dilatatus A) lines, animals were injected in three independent replicates with S. typhimurium (BFwVulC n = 20, BF n = 20; dilatatus A n = 32, dilatatus A-wDil = 24) or with L. ivanovii (BFwVulC n = 30; BF n = 30; dilatatus A n = 15; dilatatus A-wDil n = 15). Five microliters of hemolymph of each injected animals were sampled 24 h post-injection (PI). Sampled hemolymph from S. typhimurium infected isopods was diluted by 106 in LB to reach a countable number of S. typhimurium colonies and 100 μL of these dilutions were streaked on two independent LB plates. All colonies grown on the plates were morphologically similar. However, to check whether the colonies on the plates were actually S. typhimurium, we spread them on a slide and checked under epifluorescence microscope (Olympus IX81) at λ = 411 nm for GFP expression. Sampled hemolymph from L. ivanovii infected isopods were added to 95 μL of BHI and directly streaked on BHI plates (no further dilution needed due to the low amount of L. ivanovii in hemolymph). The plates were then incubated overnight and the CFUs were counted the next day. To check whether the colonies showing the proper morphology all belonged to L. ivanovii, we sequenced randomly some of them on 16S rDNA.
Transinfection of P. dilatatus with wVulC from A. vulgare: Consequences on Survival and Mobility
Asymbiotic dilatatus A along with symbiotic dilatatus A-wCon and dilatatus A-wDil males (Table 1) were injected either with the wVulC suspension (obtained from crushed ovaries of infected females) or with the control suspension (obtained from crushed ovaries of uninfected females). To do so, a Hamilton syringe with a fine glass needle was used to inject 2 μL of filtered ovaries suspension from WXw or WXa females (Table 1) prepared as described in Le Clec’h et al. (2013) into the general cavity of the animals, through a small hole pierced at the posterior part of the animal. For each treatment, three independent replicates were conducted (n = 21 received wVulC injection for dilatatus A, dilatatus A-wCon, and dilatatus A-wDil while n = 27 received the control treatment).
After injection of wVulC into dilatatus A, dilatatus A-wCon, and dilatatus A-wDil individuals, injected animals from each replicate were kept at 20°C in a plastic rearing box on humid soil and fed with dried lime-tree leaves soil. The survival was recorded every 15 days starting at day 1 until day 112. In addition, a mobility test was performed on the survivors every 15 days, starting from day 60 until the day 105, by measuring the time the animals move in a Petri dish during a period of 180 s (Le Clec’h et al., 2012).
Detection and Density of Wolbachia
We quantified the density of Wolbachia by qPCR, both in the suspensions injected to P. dilatatus (i.e., when wVulC is injected as the invasive pathogen) and in the animals that received pathogenic bacterial injections (i.e., quantification of the resident Wolbachia: wCon, wDil in P. dilatatus and wVulC in A. vulgare) using either ovary or leg samples. The latter can be sampled without killing the animals allowing us to use the same animal both to measure the quantity of Wolbachia and to inject pathogens. Total DNA was extracted using the protocol described by Kocher et al. (1989) and the Nanodrop 1000 spectrophotometer was used to estimate the total DNA concentration and quality (ratios OD 260/280 nm). Reactions of qPCR were performed, as previously described in Le Clec’h et al. (2012), using Roche LightCycler 480 to measure the copy number of the Wolbachia surface protein (wsp) gene. In short, each 10 μL reaction contained 5 μL of SYBRGreen Master Mix (Roche), 0.5 μL of 10 μM specific primers wsp208f (5′-TGG-TGC-AGC-ATT-TAC-TCCAG-3′) and wsp413r (5′-TCG-CTT-GATAAG-CAA-AAC-CA-3′), 3 μL of sterile water and 1 μL of DNA (corresponding to a range of 5–50 ng). The thermal cycle starts with a 10 min initial denaturation at 95°C followed by 45 cycles of 10 s of denaturation at 95°C, 10 s of annealing at 60°C and 20 s of elongation at 72°C. The specificity of the PCR product was verified with a melting curve (65–97°C) that was recorded at the end of the each reaction. The wsp copy number was estimated with the help of the standard curve plotted using a dilution of the wsp purified PCR product (2.63 × 103 wsp.copies μL-1). The wsp copy number was then divided by the total DNA amount of the sample in ng in order to obtain normalized values for comparison between samples. For each sample two independent technical replicates were made.
Statistical Analyses
R 3.2.2 was used for all of the statistical analysis.
Wolbachia Titer
Shapiro-Wilk and Levene’s tests were conducted to check normality and homoscedasticity of the number of wsp copy/ng of total DNA. A t-test with Bonferroni correction for multiple testing was used when the data followed a normal distribution and variances of the samples were homogenous. A Wilcoxon-rank test was performed when the data was not normally distributed.
Isopod Survival After L. ivanovii and S. typhimurium Injections
A global mixed effects Cox proportional hazards model was fitted using the “coxme” R package. Bacterial injection (i.e., control/Salmonella or Listeria), Wolbachia status (presence/absence for A. vulgare; presence of different Wolbachia strains/absence of Wolbachia for P. dilatatus) as well as gender (for P. dilatatus) and population of origin (for A. vulgare) were included in the models as fixed effects. As the experiments were performed on different independent groups of individuals, a block effect was included as a random effect in the models. The latter analysis showed a low variance between independent groups (i.e., blocks) indicating the repeatability of the experiments. Each model was also fitted without control groups. This allowed us to compare the survival of the different asymbiotic and symbiotic lines when infected with the pathogenic bacteria. Survival of the different lines was compared pairwise to assess the difference between their survival times using the log-rank test.
Consequences of wVulC Transinfection on P. dilatatus Life History Traits
Models were fitted for the days 60, 75, and 105 PI in order to test differences in survival during the course of the infection of P. dilatatus by wVulC. Survival was modeled using a mixed effects Cox proportional hazards model as described above. For the analysis of the mobility, the ‘nlme’ package (version 3.1-120) was used to fit a mixed effect linear model with a random block effect. In both analyses, first a global model including all of the three lines (i.e., dilatatus A, dilatatus A wCon, and dilatatus A wDil) was performed. Then, further analysis was made to clarify the effect of the presence of each of the Wolbachia strains (i.e., wDil, wCon) on mobility and survival, when the host was infected with wVulC. Additionally, the log-rank test was used to compare the survival times of each lineage pairwise.
Results
Wolbachia Density in Symbiotic A. vulgare and P. dilatatus
Females of symbiotic A. vulgare lines were found infected with Wolbachia as expected (using leg samples; Figure 1A). The Wolbachia density did not differ significantly between the lines that are naturally (ZN, WXw) or experimentally (BFwVulC) infected with wVulC (t-test with Bonferroni correction; ZN and BFwVulC: t = 0.133, df = 17, p = 1.00; WXw and BFwVulC: t = 1.903, df = 17, p = 0.19; ZN and WXw: t = 2.228, df = 16, p = 0.16; Figure 1A).
FIGURE 1.
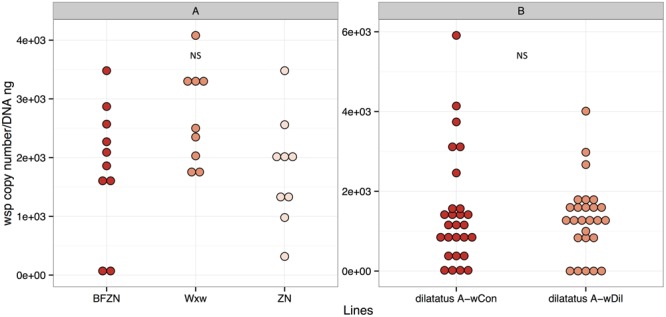
Wolbachia density in Armadillidium vulgare(A) and Porcellio dilatatus(B). The qPCR quantifications of wsp copy number/DNA ng in BFwVulC, ZN and WXw lineages and dilatatus A- wCon, dilatatus A- wDil lineages were made, using leg and ovary samples respectively. NS, not significant.
The symbiotic P. dilatatus individuals from the lines dilatatus A-wCon and dilatatus A-wDil were also proved to be all infected with Wolbachia using ovary samples (Figure 1B). The density of Wolbachia in both lines was similar as already reported in Pigeault et al. (2014) (Wilcoxon-rank sum test, W = 361, p = 0.866).
The number of wsp copies in the wVulC bacterial suspensions injected for transinfection of P. dilatatus was around of 1 × 106 wsp copy/μL.
The Impact of Wolbachia on the Survival of Terrestrial Isopods Infected with L. ivanovii and S. typhimurium
Global Survival Analysis
For both isopod species, the global survival models including all treatment groups (controls without injection of pathogens included), showed that L. ivanovii and S. typhimurium injections always significantly reduced the survival of terrestrial isopods (Table 2): both L. ivanovii and S. typhimurium were pathogenic for both isopod host species. The presence or absence of pathogenic bacteria in the injection explained an important part of the deviance between treatments. However, this was not the only significant explanatory factor. In the global model indeed, the symbiotic status (i.e., individual infected or not with Wolbachia) of the host also explained an important part of the deviance, with the exception of P. dilatatus individuals injected with L. ivanovii for which Wolbachia presence did not influence the survival (Table 2).
Table 2.
Survival analyses of the different A. vulgare and P. dilatatus lines when injected with S. typhimurium or L. ivanovii.
|
S. typhimurium injections |
L. ivanovii injections |
|||||
|---|---|---|---|---|---|---|
| df | Deviance | p | df | Deviance | p | |
| P. dilatatus | ||||||
| With control | ||||||
| Bacterial injection | 1 | 77.193 | <0.001 | 1 | 35.156 | <0.001 |
| Wolbachia status | 2 | 22.244 | <0.001 | 2 | 0.877 | 0.645 |
| Gender | 1 | 6.191 | <0.001 | 1 | 7.863 | 0.005 |
| Without control | ||||||
| Wolbachia status | 2 | 22.244 | <0.001 | 2 | 0.739 | 0.691 |
| Gender | 1 | 6.191 | 0.013 | 1 | 10.396 | 0.001 |
| A. vulgare | ||||||
| With control | ||||||
| Bacterial injection | 1 | 20.141 | <0.001 | 1 | 6.322 | 0.012 |
| Wolbachia status | 1 | 5.737 | 0.017 | 1 | 24.630 | <0.001 |
| Population of origin | 2 | 6.860 | 0.032 | 2 | 10.606 | 0.005 |
| Without control | ||||||
| Wolbachia status | 1 | 7.943 | 0.001 | 1 | 15.302 | <0.001 |
| Population of origin | 2 | 7.568 | 0.023 | 2 | 31.630 | <0.001 |
Isopods were injected either with S. typhimurium or L. ivanovii and their survival was recorded. A mixed effect Cox proportional hazards model was fitted with and without the controls using the survival data of the lineages to estimate the effects of the treatment, the presence of Wolbachia, the different Wolbachia strains, the gender (only in P. dilatatus), the population of origin (only in A. vulgare) on the survival and the replicate blocks as a random effect. In bold, statistically significant values (p < 0.05).
Other explanatory factors were specific to each of the isopod species. In A. vulgare, the effect of the population of origin showed that this factor also significantly explained a part of the deviance (Table 2; Figures 2 and 5). In P. dilatatus, the global model showed that the survival depended significantly on the gender when injected with L. ivanovii or S. typhimurium: females survived better than males (Table 2; Figures 3 and 6).
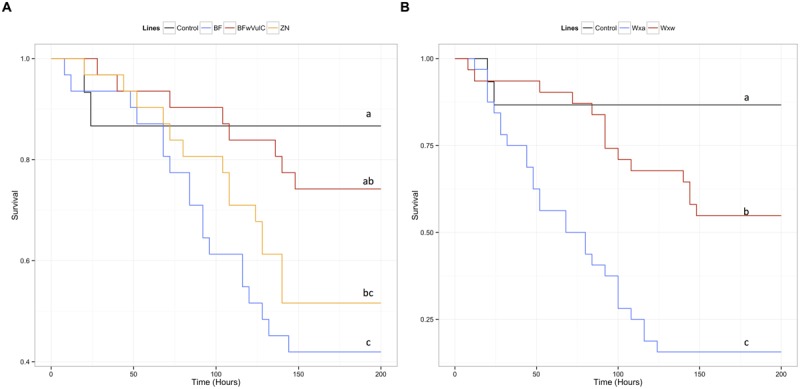
FIGURE 2.
Survival of the different A. vulgare lines when infected with S. typhimurium. A Cox proportional hazards model has been fitted using the survival data compared symbiotic and asymbiotic survival (A) BFwVulC and ZN/BF and (B) WXw/WXa A. vulgare lines after being injected with pathogenic bacteria S. typhimurium. Control groups were injected with liquid medium (LB). Different letters indicate significant differences between the survival curves of different lineages based on log-rank test p < 0.05.
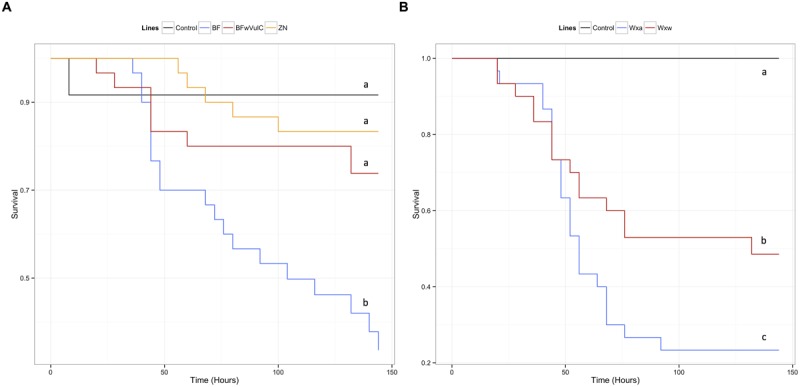
FIGURE 5.
Survival of the different A. vulgare lines when infected with L. ivanovii. A Cox proportional hazards model has been fitted using the survival data compared symbiotic and asymbiotic survival (A) BFwVulC and ZN/BF and (B) WXw/WXa. A. vulgare lines after being injected with pathogenic bacteria L. ivanovii. Control groups were injected with liquid medium (BHI). Different letters indicate significant differences between the survival curves of different lineages based on log-rank test p < 0.05.
FIGURE 3.
Survival of the different P. dilatatus lines when infected with S. typhimurium. A Cox proportional hazards model was fitted using the survival data of symbiotic (dilatatus A-wCon, dilatatus A-wDil) and asymbiotic (dilatatus A) P. dilatatus lines after being injected with pathogenic bacteria S. typhimurium. Control groups were injected with liquid medium (LB). Results for females (A) and for males (B) are presented separately. Different letters indicate significant differences between the survival curves of different lineages based on log-rank test p < 0.05.
FIGURE 6.
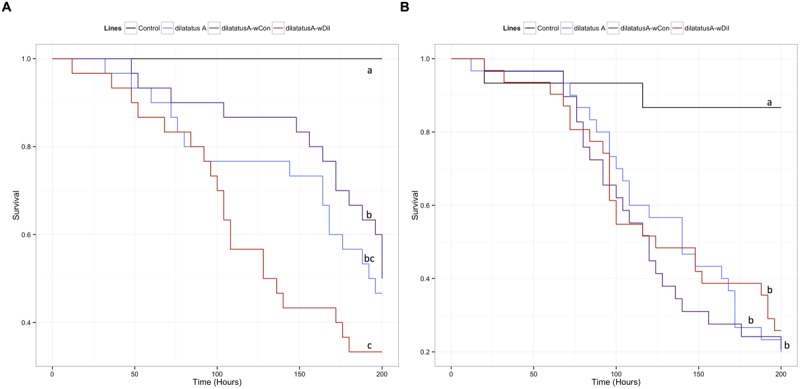
Survival of the different P. dilatatus lines when infected with L. ivanovii. A Cox proportional hazards model was fitted using the survival data of symbiotic (dilatatus A-wCon, dilatatus A-wDil) and asymbiotic (dilatatus A) individuals after being injected with pathogenic bacteria L. ivanovii. Results for females (A) and for males (B) are presented separately. Control groups were injected with liquid medium (BHI). Different letters indicate significant differences between the survival curves of different lineages based on log-rank test. p < 0.05.
Impact of Wolbachia on S. typhimurium Infections
Survival
A reduced submodel was fitted after excluding the control groups (i.e., injection of sterile culture media) to evaluate which parameter (gender, population of origin, Wolbachia status) still explained significantly the deviance in the survival of animals infected with S. typhimurium.
In A. vulgare, the presence of Wolbachia explained an important part of deviance in survival: symbiotic lines survived the S. typhimurium infection better than asymbiotic ones (Table 2). Originating from the same line and only differing in their symbiotic condition, BFwVulC individuals survived better than BF ones (log-rank test: χ2 = 7.2, df = 1, p = 0.007, Figure 2A). On the other hand, BFwVulC showed a very similar survival pattern to the other symbiotic lineage ZN (log-rank test: χ2 = 0.7, df = 1, p = 0.405, Figure 2A), even though they did not come from the same line but have the same Wolbachia strain. Similarly, the symbiotic lineage WXw survived better than the asymbiotic WXa originated from the same initial population (log-rank test: χ2 = 3.8, df = 1, p = 0.049, Figure 2B).
In P. dilatatus, the reduced submodel showed that both the Wolbachia status and at a lower extent the gender, explained significantly a part of the deviance (Table 2). The survival of P. dilatatus when injected with S. typhimurium differed not only between symbiotic and asymbiotic animals, but also depending on the resident Wolbachia strain present (i.e., native wDil and non-native wCon). Pairwise comparison of survival data with log-rank test showed that dilatatus A-wDil animals infected with native Wolbachia survived better than both dilatatus A-wCon and asymbiotic dilatatus A ones. This pattern was observed for females and males (for females, dilatatus A and dilatatus A-wDil: log-rank test, χ2 = 9.4, df = 1, p = 0.002; dilatatus A-wCon and dilatatus A-wDil: log-rank test, χ2 = 7.7, df = 1, p = 0.006, Figure 3A; for males, dilatatus A and dilatatus A-wDil: log-rank test, χ2 = 25.3, df = 1, p < 0.001, dilatatus A-wCon and dilatatus A-wDil: log-rank test, χ2 = 22.1, df = 1, p < 0.001, Figure 3B). However, the survival of asymbiotic dilatatus A and symbiotic dilatatus A-wCon was not significantly different (females: dilatatus A and dilatatus A-wCon: log-rank test, χ2 = 0.2, df = 1, p = 0.683, Figure 3A; males: dilatatus A and dilatatus A-wCon: log-rank test, χ2 = 0.6, df = 1, p = 0.435, Figure 3B). These results showed that only P. dilatatus individuals harboring the native Wolbachia strain wDil survived better than the other lines when infected with S. typhimurium.
Pathogen Load
Twenty-four hours PI, hemolymph samples were taken from animals injected with S. typhimurium to compare the pathogen load (i.e., CFUs) between asymbiotic (BF, dilatatus A) and symbiotic (BFwVulC, dilatatus A-wCon, dilatatus A-wDil) animals. In both A. vulgare and P. dilatatus, the pathogen load in the hemolymph was higher than the amount that was initially injected (at least 400 times higher). Moreover, in both A. vulgare and P. dilatatus, CFUs from S. typhimurium was significantly higher in asymbiotic animals compared to the symbiotic ones (BF/BFwVulC: W = 274, p = 0.043, Figure 4A; dilatatus A/dilatatus A-wDil W = 631.5, p = 0.006, Figure 4B).
FIGURE 4.
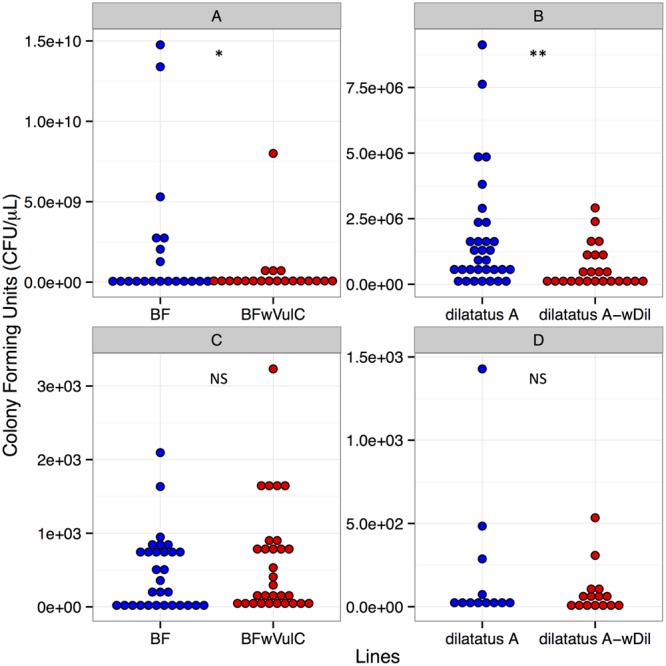
Salmonella typhimurium(A,B) and Listeria ivanovii(C,D) CFUs in A. vulgare and P. dilatatus. CFUs of S. typhimurium from A. vulgare hemolymph (A), from P. dilatatus hemolymph (B), CFUs of L. ivanovii from A. vulgare hemolymph (C) and CFUs of L. ivanovii from P. dilatatus hemolymph (D). ∗p < 0.05, ∗∗p < 0.01. NS, not significant.
Impact of Wolbachia on L. ivanovii Infections
Survival
In A. vulgare, similar to the global model, the reduced submodel showed that both the Wolbachia status and the origin of the population explained significantly a part of the deviance (Table 2). Pairwise comparison of survival data showed that symbiotic BFwVulC females survived significantly better than asymbiotic BF ones (log-rank test, χ2 = 4.7, df = 1, p = 0.029, Figure 5A) while survival of BFwVulC and ZN did not differ from each other (log-rank test, χ2 = 3.2, df = 1, p = 0.075, Figure 5A). In addition, symbiotic WXw females survived significantly better than asymbiotic WXa when injected with L. ivanovii (log-rank test, χ2 = 16.7, df = 1, p < 0,001, Figure 5B).
In P. dilatatus, a significant part of the deviance in survival was explained in the reduced submodel by the factor ‘gender’: females surviving better than males (Table 2). In this case, the Wolbachia status did not significantly affect the survival in the model (p = 0.691; Table 2). However, pairwise comparison of survival data showed a weak effect of Wolbachia status; there was actually no significant difference between the survival of asymbiotic and symbiotic females infected with L. ivanovii (dilatatus A and dilatatus A-wCon: log-rank test, χ2 = 0.3, df = 1, p = 0.579; dilatatus A and dilatatus A-wDil: log-rank test, χ2 = 1.8, df = 1, p = 0.180; Figure 6A). However, there was a significant difference in survival between females infected with different Wolbachia: dilatatus A-wCon females survived better than dilatatus A-wDil ones (log-rank test, χ2 = 4, df = 1, p = 0.046; Figure 6B). This pattern was not confirmed in males for which no difference due to the presence of any of the Wolbachia strains was detected (log-rank test: χ2 = 0.4, df = 2, p = 0.813; Figure 6B).
Pathogen Load
Twenty-four hours PI, hemolymph was sampled from L. ivanovii injected animals to compare the pathogen loads between asymbiotic (BF, dilatatus A) and symbiotic (BFwVulC, dilatatus A-wCon, dilatatus A-wDil) animals. The mean of the pathogen load in the hemolymph was more than 60 times lower than the initially injected L. ivanovii amount. No significant difference between asymbiotic and symbiotic animals was detected in any of the isopod models (for A. vulgare: W = 425.5, p = 0.723, Figure 4C; for P. dilatatus: W = 90, p = 1, Figure 4D).
Influence of Resident Wolbachia on Invasive Wolbachia
Survival
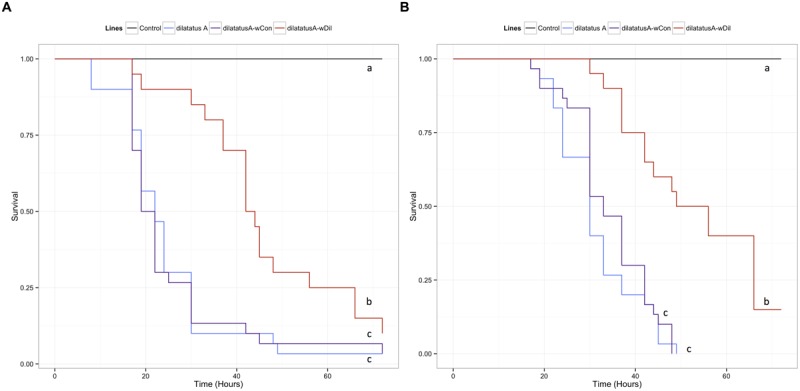
The negative effect of the wVulC infection on the survival of the animals was detected from day 90 PI (Figure 7; Table 3). Pairwise comparisons of survival data with log-rank test showed that the differential effect of the Wolbachia status (asymbiotic, dilatatus A-wDil, or dilatatus A-wCon) became apparent at day 105 PI (Table 3): globally, dilatatus A-wCon survived significantly longer than dilatatus A individuals and dilatatus A-wDil ones (log-rank test, dilatatus A-wDil and dilatatus A-wCon: χ2 = 12.2, df = 1, p < 0.001; dilatatus A and dilatatus A-wCon: χ2 = 5.8, df = 1, p = 0.015; Figure 7).
FIGURE 7.
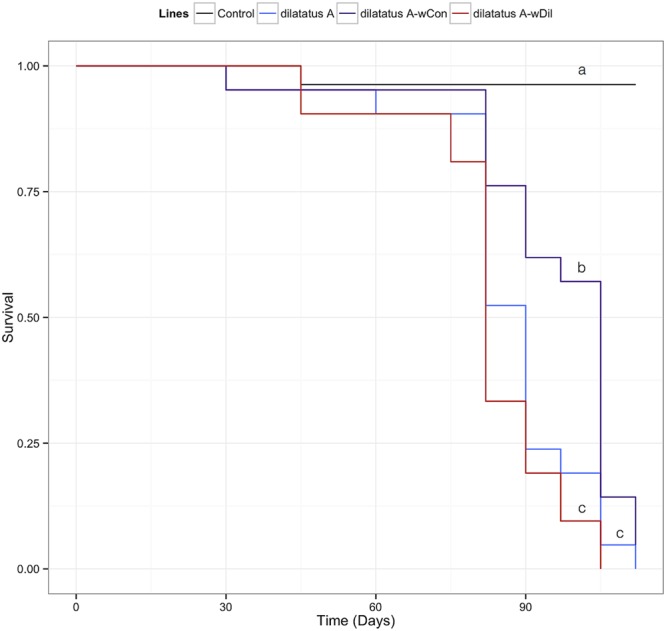
Influence of resident Wolbachia on the survival of P. dilatatus infected with wVulC. Porcellio dilatatus males were injected with invasive wVulC Wolbachia strain and their survival was recorded every 15 days. A Cox proportional hazards model was fitted. Different letters indicate significant differences between the survival curves of different lineages based on log-rank test. p < 0.05.
Table 3.
Influence of resident Wolbachia on the survival and mobility of P. dilatatus infected with wVulC.
| Day 60 PI |
Day 75 PI |
Day 105 PI |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| df | Deviance | p | df | Deviance | p | df | Deviance | p | |
| Comparisons between dilatatus A, dilatatus A-wCon, and dilatatus A-wDil | |||||||||
| Mobility | |||||||||
| Bacterial injection | 1 | 15.245 | <0.001 | 1 | 26.701 | <0.001 | 1 | 18.135 | <0.001 |
| Wolbachia status | 2 | 3.535 | 0.171 | 2 | 6.635 | 0.036 | 2 | 6.080 | 0.048 |
| Survival | |||||||||
| Bacterial injection | 1 | 0.604 | 0.436 | 1 | 1.285 | 0.257 | 1 | 42.591 | <0.001 |
| Wolbachia status | 2 | 0.993 | 0.608 | 2 | 1.576 | 0.448 | 2 | 9.218 | 0.01 |
| Comparisons between dilatatus A-wCon and dilatatus A-wDil | |||||||||
| Mobility | |||||||||
| Bacterial injection | 1 | 11.681 | 0.001 | 1 | 20.181 | <0.001 | 1 | 18.138 | <0.001 |
| Wolbachia status | 1 | 1.612 | 0.204 | 1 | 5.724 | 0.017 | 1 | 6.080 | 0.014 |
| Survival | |||||||||
| Bacterial injection | 1 | 0.214 | 0.643 | 1 | 3.413 | 0.064 | 1 | 32.185 | <0.001 |
| Wolbachia status | 1 | 2.078 | 0.149 | 1 | 0.149 | 0.245 | 1 | 8.475 | 0.004 |
Porcellio dilatatus males were injected with invasive wVulC Wolbachia strain and their survival as well as their mobility was recorded on the days 60, 75, and 105 PI. A mixed effects linear model with random block effect was used to analyze mobility data. A mixed effects Cox proportional hazards model was fitted using the survival data of the lineages to estimate the effects of the treatment and the Wolbachia status. For both models, first a global model with all of the lineages (symbiotic dilatatus A-wCon, dilatatus A-wDil and asymbiotic dilatatus A) was fitted, then further analysis was made to clarify the differences between symbiotic dilatatus A-wCon and dilatatus A-wDil lineages with help of a sub-model. In bold, statistically significant values (p < 0.05).
Mobility
Leg tremors and seizures caused by the wVulC infection were observed during the course of experiment for all lines. The mobility of the animals injected with wVulC decreased compared to the control group, starting from the day 60 PI (Table 3). This decrease continued until the last days of the infection monitoring in all lines. However, the decrease of mobility due to wVulC was lower for symbiotic dilatatus A-wCon individuals compared to the other symbiotic lineage dilatatus A-wDil at both days 75 and 105 PI (Table 3; Figure 8).
FIGURE 8.
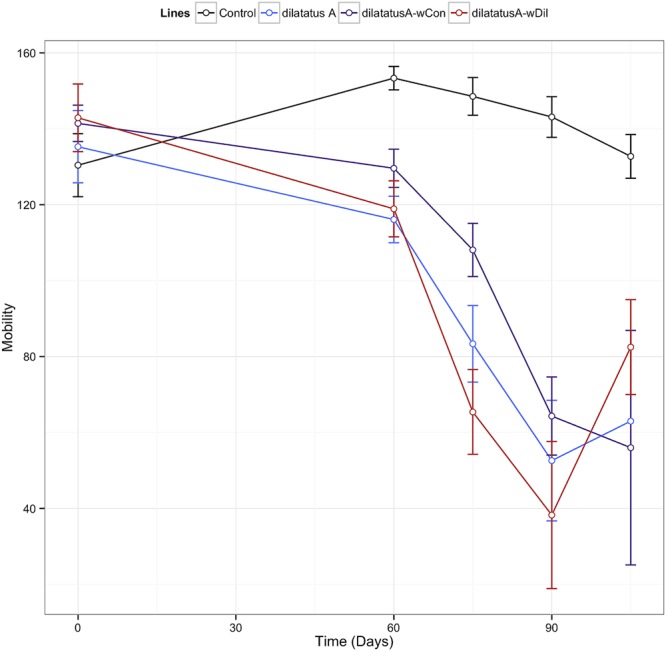
Influence of resident Wolbachia on the mobility of P. dilatatus infected with wVulC. P. dilatatus males were injected with pathogenic wVulC Wolbachia strain and their mobility was recorded every 15 days. The mobility test was performed measuring the time the animals move in a Petri dish during a period of 180 s. Data points indicate the mean (±SEM).
Discussion
The potential mutualistic nature of Wolbachia as a protective symbiont was revealed few years ago by the interactions between Wolbachia and RNA viruses in Drosophila (Hedges et al., 2008; Teixeira et al., 2008). Since then, the protection conferred by the presence of Wolbachia has been reported against various natural enemies. However, this has mainly been observed in artificially established host-Wolbachia associations either when a naturally naive host was transinfected with Wolbachia (Kambris et al., 2009; Ye et al., 2013), or when a non-naive host (i.e., host species for which some individuals are infected by another Wolbachia strain) was transinfected with a new Wolbachia strain (Blagrove et al., 2012). The aim of our study was to find out whether a resident Wolbachia has a protective effect against intracellular bacterial pathogens in terrestrial isopods.
In A. vulgare, we showed that lines harboring the feminizing Wolbachia wVulC (BFwVulC, ZN, WXw) survived both L. ivanovii and S. typhimurium injections better than their asymbiotic counterparts (BF, WXa). This demonstrates that wVulC behaves as a protective symbiont for A. vulgare. Moreover, even if our analyses of the different lines coming from different populations of origins showed an influence of ‘host-background’ in the protection phenotype, the major source of variation in survival to pathogenic bacteria is clearly the absence or presence of Wolbachia wVulC. A prevailing effect of Wolbachia on A. vulgare physiology was already demonstrated by studying several immune parameters (Braquart-Varnier et al., 2008; Sicard et al., 2010). In 1-year-old A. vulgare, the same age as the animals used in the present study, the presence of wVulC influences both PO activities (Sicard et al., 2010) and hemocyte type proportions (i.e., increase in hyaline and semi-granular hemocyte, and decrease in granular hemocyte percentage; Chevalier et al., 2011). Besides native wVulC Wolbachia presence leads to a down regulation of some immune genes in the whole body of A. vulgare, including genes involved in stress response, detoxification, autophagy, AMP synthesis (including two Gram-positive AMPs: armadillidin and crustin), pathogen recognition and proteolytic cascades in ovaries (Chevalier et al., 2012), while most of these genes tend, on the contrary, to be up-regulated in the immune tissues of Wolbachia infected animals (Chevalier et al., 2012). The previously described immunodepressive effects of Wolbachia presence might be somehow compensated by the general up-regulation of immune genes specifically in the immune tissues hence causing the observed protective effect. However, the global picture of Wolbachia influence on A. vulgare immune system is not yet unraveled and the protection against bacterial pathogens demonstrated here cannot be linked to any previously demonstrated effect of Wolbachia presence. Besides any immune system stimulation, such protection effect of the presence of Wolbachia could also be the result of negative interferences between resident symbiont and invasive bacteria in diverse possible ways.
In P. dilatatus, the two CI-inducing Wolbachia strains (non-native wCon and native wDil) both conferred protection of both females and males against pathogenic bacteria. However, each resident Wolbachia increased survival against different invasive pathogenic bacteria: the native wDil conferred better resistance to S. typhimurium infection while wCon conferred a slightly higher resistance against L. ivanovii. Previous assessments of the influence of both Wolbachia strains on some P. dilatatus immune parameters (PO, hemocyte load and phagocytosis rate) indicated that the two Wolbachia strains influence the immune system differently: the non-native wCon is overall more immuno-stimulating than the native wDil (Pigeault et al., 2014). wCon as a non-native Wolbachia strain of P. dilatatus might trigger more general immune pathways than the native wDil strain does, suggesting an immune stimulation due to the presence of the foreign Wolbachia. However, an alternative hypothesis not implying mediation via immunity may also explain such patterns of protection: wDil could interact harder with Salmonella and counteracts its multiplication while wCon would interact harder with Listeria. For P. dilatatus, we also demonstrate an effect of gender on survival: females survived better than males when challenged with pathogenic bacteria. This suggests that isopod females invest more in immunity than males, as already reported by measuring immune parameters in Pigeault et al. (2014).
Previous transinfection experiments with P. dilatatus as a recipient host demonstrated that wVulC coming from A. vulgare resulted in strong pathogenicity when interacting with this new host (Le Clec’h et al., 2012, 2013, 2014). In the present work, the wVulC strain was injected into asymbiotic (dilatatus A) but also for the first time in symbiotic animals (i.e., dilatatus A-wCon or dilatatus A-wDil). Our results confirmed the pathogenicity of wVulC on P. dilatatus and we observed the previously reported symptoms (reduced mobility, leg tremors, seizures) suggesting a neurologic pathology (Le Clec’h et al., 2012, 2013). Nonetheless, dilatatus A-wCon survived longer and some symptoms such as reduced mobility were postponed compared to the other lines. The difference in survival rates following wVulC injections was not related to the load of resident Wolbachia. Therefore the reason for this difference could result from difference in the interactions between wVulC and the resident strain within the intracellular niche.
Our results demonstrate for the first time in any model a protection conferred by native Wolbachia strains against pathogenic intracellular bacteria: an important proportion (up to 70%) of the symbiotic animals survived the infection (even several weeks after infection; personal observation) while all the asymbiotic animals died. Such a strong benefit due to the presence of Wolbachia has only been previously reported for infection of extracellular E. carotovora in the naive host A. aegypti transinfected with non-native Wolbachia strains from Drosophila (Kambris et al., 2009; Ye et al., 2013). Other investigations on bacterial protection conferred by Wolbachia conducted on D. melanogaster naturally infected with native Wolbachia showed that symbiotic animals did not survive better than their asymbiotic counterparts when infected with pathogenic bacteria such as L. ivanovii and S. typhimurium as well as Burkholderia cepacia, E. carotovora, and Mycobacterium marinum (Rottschaefer and Lazzaro, 2012; Ye et al., 2013). Similarly, Drosophila simulans naturally infected with Wolbachia did not show any difference in terms of mortality compared to the flies without Wolbachia, when infected with E. carotovora, Pseudomonas aeruginosa, and Serratia marcescens (Wong et al., 2011). Based on these studies, it was suggested that the mechanism responsible for the protective effect against pathogenic bacteria, only observed in artificial associations between Wolbachia and mosquitoes, could result from an immune stimulation triggered only by non-native transinfected Wolbachia (Moreira et al., 2009; Bian et al., 2010; Ye et al., 2013). As such immune priming would be unlikely to be triggered in long-term associations between Wolbachia and their arthropod hosts, several authors concluded that a protective effect of Wolbachia against intracellular bacteria in natural symbiotic systems was not likely to be found (Rottschaefer and Lazzaro, 2012; Ye et al., 2013). However, in isopod models, we showed a protection against pathogenic bacteria in situations for which immune stimulation caused by the introduction of non-native Wolbachia in a new host cannot explain the protection phenotype. Even though in the BFwVulC and dilatatus-wDil lines, the Wolbachia have been artificially introduced, the Wolbachia strain wVulC is widely distributed all over the world in A. vulgare populations (Cordaux et al., 2004) while wDil is widely distributed in P. dilatatus (Grève, personal communication). Therefore, there are co-evolutionary histories between these symbiotic partners.
By measuring the concentration of pathogenic bacteria in the hemolymph of the injected animals, we investigated whether the observed protection would be due to a true resistance phenomenon which would result in reduced pathogen load, or tolerance which would result in a similar pathogen load but increased survival (Schneider and Ayres, 2008). In S. typhimurium infection, symbiotic BFwVulC (A. vulgare) and dilatatus A-wDil (P. dilatatus) lines showed lower pathogen load in their hemolymph than asymbiotic BF (A. vulgare) and dilatatus A (P. dilatatus) lines respectively. Therefore, the better survival of symbiotic animals would correspond to a decrease in bacterial load in the hemolymph (i.e., higher resistance). On the other hand, we did not find any difference in L. ivanovii load in the hemolymph of the isopods 24 h PI (Figure 4) or even 48 h PI (personal observation). These results suggest that the difference in survival between lines after Listeria injection was not linked to difference in pathogenic bacterial loads. However, further investigations would be required to have a firm conclusion on the latter, since in our experiments Listeria was clearly pathogenic while we were not able to detect a proper infection (i.e., multiplication) in the hemolymph.
Maternally inherited Wolbachia strains induce either feminization or CI in terrestrial isopods. As these reproductive manipulations and in general Wolbachia infections can have severe costs on host fitness, they are considered as conflictual interactions. Indeed various Wolbachia strains are linked to some detrimental effects on their host’s fitness, such as wVulC strain in A. vulgare causing reduced progeny and survival (Braquart-Varnier et al., 2008; Sicard et al., 2010). Given that Wolbachia are quite widespread in host populations despite their apparent fitness costs, we hypothesize that the observed protection effect could compensate for the costs, and that Wolbachia can become a mutualist, especially if infections by environmental bacteria constitute an important threat for terrestrial isopods. Moreover in P. dilatatus, the native wDil strain has recently been shown to increase the reproduction of its host (Pigeault et al., 2014). If antibacterial protection conferred by the same Wolbachia is paired with a reproductive benefit (Pigeault et al., 2014), reproductive parasitism might continue to manipulate host reproduction while being a mutualist at the same time, resulting in a so called ‘Jekyll and Hyde’ infection, where beneficial and conflictual traits co-exist together in the same symbiotic system (Zug and Hammerstein, 2014).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Winka Le Clec’h for her valuable comments on the project. We thank Julien Dauphin, Carine Delaunay, Gaetan Mappa, and Maryline Raimond for their technical assistance. We also thank all the technical staff of the UMR EBI CNRS 7267.
Footnotes
Funding. This work was supported by the Ministère de l’Enseignement Supérieur et de la Recherche and the Agence Nationale de la Recherche (ADaWOL ANR-09-JCJC-0109-01 coordinated by MS and ImmunSymbArt ANR-10-BLAN-1701 coordinated by DB). MA was financially supported by the European Commission through the program Erasmus Mundus Master Course – International Master in Applied Ecology (EMMC-IMAE; FPA 532524-1-FR-2012-ERA MUNDUS-EMMC).
References
- Bian G., Xu Y., Lu P., Xie Y., Xi Z. (2010). The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 6:e1000833 10.1371/journal.ppat.1000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagrove M. S. C., Arias-Goeta C., Failloux A.-B., Sinkins S. P. (2012). Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc. Natl. Acad. Sci. U.S.A. 109 255–260. 10.1073/pnas.1112021108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchon D., Cordaux R., Grève P. (2008). “Feminizing Wolbachia and the evolution of sex determination in isopods,” in Insect Symbiosis, Vol. 3 Contemporary Topics in Entomology eds Bourtzis K., Miller T. A. (Boca Raton, FL: CRC Press: ) 273–294. 10.1201/9781420064117.ch12 [DOI] [Google Scholar]
- Braquart-Varnier C., Lachat M., Herbinière J., Johnson M., Caubet Y., Bouchon D., et al. (2008). Wolbachia mediate variation of host immunocompetence. PLoS ONE 3:e3286 10.1371/journal.pone.0003286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie J. C., Johnson K. N. (2009). Symbiont-mediated protection in insect hosts. Trends Microbiol. 17 348–354. 10.1016/j.tim.2009.05.005 [DOI] [PubMed] [Google Scholar]
- Caragata E. P., Rancès E., Hedges L. M., Gofton A. W., Johnson K. N., O’Neill S. L., et al. (2013). Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 9:e1003459 10.1371/journal.ppat.1003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier F., Herbinière-Gaboreau J., Bertaux J., Raimond M., Morel F., Bouchon D., et al. (2011). The immune cellular effectors of terrestrial isopod Armadillidium vulgare: meeting with their invaders, Wolbachia. PLoS ONE 6:e18531 10.1371/journal.pone.0018531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier F., Herbinière-Gaboreau J., Charif D., Mitta G., Gavory F., Wincker P., et al. (2012). Feminizing Wolbachia: a transcriptomics approach with insights on the immune response genes in Armadillidium vulgare. BMC Microbiol. 12:S1 10.1186/1471-2180-12-S1-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaux R., Michel-Salzat A., Frelon-Raimond M., Rigaud T., Bouchon D. (2004). Evidence for a new feminizing Wolbachia strain in the isopod Armadillidium vulgare: evolutionary implications. Heredity (Edinb.) 93 78–84. 10.1038/sj.hdy.6800482 [DOI] [PubMed] [Google Scholar]
- Davidson S. K., Allen S. W., Lim G. E., Anderson C. M., Haygood M. G. (2001). Evidence for the biosynthesis of bryostatins by the bacterial symbiont “candidatus endobugula sertula” of the bryozoan Bugula neritina. Appl. Environ. Microbiol. 67 4531–4537. 10.1128/AEM.67.10.4531-4537.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza D. J., Bézier A., Depoix D., Drezen J.-M., Lenoir A. (2009). Blochmannia endosymbionts improve colony growth and immune defence in the ant Camponotus fellah. BMC Microbiol. 9:29 10.1186/1471-2180-9-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas A. E. (2011). Lessons from studying insect symbioses. Cell Host Microbe 10 359–367. 10.1016/j.chom.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A. M., Smith J. E. (2001). Microsporidian life cycles and diversity: the relationship between virulence and transmission. Microbes Infect. 3 381–388. 10.1016/S1286-4579(01)01394-6 [DOI] [PubMed] [Google Scholar]
- Eleftherianos L., Atri J., Accetta J., Castillo J. C. (2013). Endosymbiotic bacteria in insects: guardians of the immune system? Front. Physiol. 4:46 10.3389/fphys.2013.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury F., Vavre F., Ris N., Fouillet P., Boulétreau M. (2000). Physiological cost induced by the maternally-transmitted endosymbiont Wolbachia in the Drosophila parasitoid Leptopilina heterotoma. Parasitol. 121(Pt 5) 493–500. 10.1017/S0031182099006599 [DOI] [PubMed] [Google Scholar]
- Fytrou A., Schofield P. G., Kraaijeveld A. R., Hubbard S. F. (2006). Wolbachia infection suppresses both host defence and parasitoid counter-defence. Proc. Biol. Sci. 273 791–796. 10.1098/rspb.2005.3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R. L., Meola M. A. (2010). The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to west nile virus infection. PLoS ONE 5:e11977 10.1371/journal.pone.0011977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R., Vavre F., Heddi A., Hurst G. D. D., Zchori-Fein E., Bourtzis K. (2009). Immunity and symbiosis. Mol. Microbiol. 73 751–759. 10.1111/j.1365-2958.2009.06820.x [DOI] [PubMed] [Google Scholar]
- Haine E. R. (2008). Symbiont-mediated protection. Proc. Biol. Sci. 275 353–361. 10.1098/rspb.2007.1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges L. M., Brownlie J. C., O’Neill S. L., Johnson K. N. (2008). Wolbachia and virus protection in insects. Science 322:702 10.1126/science.1162418 [DOI] [PubMed] [Google Scholar]
- Hedges L. M., Johnson K. N. (2008). Induction of host defence responses by Drosophila C virus. J. Gen. Virol. 89 1497–1501. 10.1099/vir.0.83684-0 [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A., Turelli M. (1988). Unidirectional incompatibility in Drosophila simulans: inheritance, geographic variation and fitness effects. Genetics 119 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A. A., Turelli M., Harshman L. G. (1990). Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics 126 933–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T., Koga R., Kikuchi Y., Meng X.-Y., Fukatsu T. (2010). Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl. Acad. Sci. U.S.A. 107 769–774. 10.1073/pnas.0911476107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huigens M. E., Hohmann C. L., Luck R. F., Gort G., Stouthamer R. (2004). Reduced competitive ability due to Wolbachia infection in the parasitoid wasp Trichogramma kaykai. Entomol. Exp. Appl. 110 115–123. 10.1111/j.0013-8703.2004.00126.x [DOI] [Google Scholar]
- Hurst G. D. D., Bandi C., Sacchi L., Cochrane A. G., Bertrand D., Karaca I., et al. (1999). Adonia variegata (Coleoptera: Coccinellidae) bears maternally inherited Flavobacteria that kill males only. Parasitology 118 125–134. 10.1017/S0031182098003655 [DOI] [PubMed] [Google Scholar]
- Jacobsen C. S., Bech T. B. (2012). Soil survival of Salmonella and transfer to freshwater and fresh produce. Food Res. Int. 45 557–566. 10.1016/j.foodres.2011.07.026 [DOI] [Google Scholar]
- Jaenike J., Perlman S. J. (2002). Ecology and evolution of host-parasite associations: mycophagous Drosophila and their parasitic nematodes. Am. Nat. 160(Suppl.) S23–S39. 10.1086/342137 [DOI] [PubMed] [Google Scholar]
- Jaenike J., Unckless R., Cockburn S. N., Boelio L. M., Perlman S. J. (2010). Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329 212–215. 10.1126/science.1188235 [DOI] [PubMed] [Google Scholar]
- Kaiser W., Huguet E., Casas J., Commin C., Giron D. (2010). Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts. Proc. R. Soc. B Biol. Sci. 277 2311–2319. 10.1098/rspb.2010.0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambris Z., Cook P. E., Phuc H. K., Sinkins S. P. (2009). Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326 134–136. 10.1126/science.1177531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher T. D., Thomas W. K., Meyer A., Edwards S. V., Pääbo S., Villablanca F. X., et al. (1989). Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. U.S.A. 86 6196–6200. 10.1073/pnas.86.16.6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clec’h W., Braquart-Varnier C., Raimond M., Ferdy J. B., Bouchon D., Sicard M. (2012). High virulence of Wolbachia after host switching: when autophagy hurts. PLoS Pathog. 8:e1002844 10.1371/journal.ppat.1002844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clec’h W., Raimond M., Bouchon D., Sicard M. (2014). Strength of the pathogenicity caused by feminizing Wolbachia after transfer in a new host: strain or dose effect? J. Invertebr. Pathol. 116 18–26. 10.1016/j.jip.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Le Clec’h W., Raimond M., Guillot S., Bouchon D., Sicard M. (2013). Horizontal transfers of feminizing versus non-feminizing Wolbachia strains: from harmless passengers to pathogens. Environ. Microbiol. 15 2922–2936. 10.1111/1462-2920.12172 [DOI] [PubMed] [Google Scholar]
- Loker E., Loker E., Adema C., Adema C., Zhang S., Zhang S., et al. (2004). Invertebrate immune systems-not homogonous, not simple, not well understood. Immunol. Rev. 198 10–24. 10.1111/j.0105-2896.2004.0117.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Villavicencio M., Courjol F., Gibson A. K., Hood M. E., Jonot O., Shykoff J. A., et al. (2011). Competition, cooperation among kin, and virulence in multiple infections. Evolution (N. Y.) 65 1357–1366. 10.1111/j.1558-5646.2010.01207.x [DOI] [PubMed] [Google Scholar]
- Moreira L. A., Iturbe-Ormaetxe I., Jeffery J. A., Lu G., Pyke A. T., Hedges L. M., et al. (2009). A Wolbachia Symbiont in Aedes aegypti limits infection with dengue, chikungunya, and plasmodium. Cell 139 1268–1278. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- Moret Y., Juchault P., Rigaud T. (2001). Wolbachia endosymbiont responsible for cytoplasmic incompatibility in a terrestrial crustacean: effects in natural and foreign hosts. Heredity (Edinb.) 86 325–332. 10.1046/j.1365-2540.2001.00831.x [DOI] [PubMed] [Google Scholar]
- Pigeault R., Braquart-Varnier C., Marcadé I., Mappa G., Mottin E., Sicard M. (2014). Modulation of host immunity and reproduction by horizontally acquired Wolbachia. J. Insect Physiol. 70 125–133. 10.1016/j.jinsphys.2014.07.005 [DOI] [PubMed] [Google Scholar]
- Rigaud T., Moreau J. (2004). A cost of Wolbachia-induced sex reversal and female-biased sex ratios: decrease in female fertility after sperm depletion in a terrestrial isopod. Proc. Biol. Sci. 271 1941–1946. 10.1098/rspb.2004.2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschaefer S. M., Lazzaro B. P. (2012). No effect of Wolbachia on resistance to intracellular infection by pathogenic bacteria in Drosophila melanogaster. PLoS ONE 7:e40500 10.1371/journal.pone.0040500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauders B. D., Overdevest J., Fortes E., Windham K., Schukken Y., Lembo A., et al. (2012). Diversity of Listeria species in urban and natural environments. Appl. Environ. Microbiol. 78 4420–4433. 10.1128/AEM.00282-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough C. L., Ferrari J., Godfray H. C. J. (2005). Aphid protected from pathogen by endosymbiont. Science 310:1781 10.1126/science.1120180 [DOI] [PubMed] [Google Scholar]
- Schneider D. S., Ayres J. S. (2008). Us about treating infectious diseases. Nat. Rev. Immunol. 8 889–895. 10.1038/nri2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbus L. R., Casper-Lindley C., Landmann F., Sullivan W. (2008). The genetics and cell biology of Wolbachia-host interactions. Annu. Rev. Genet. 42 683–707. 10.1146/annurev.genet.41.110306.130354 [DOI] [PubMed] [Google Scholar]
- Sicard M., Bouchon D., Ceyrac L., Raimond R., Thierry M., Le Clec’h W., et al. (2014a). Bidirectional cytoplasmic incompatibility caused by Wolbachia in the terrestrial isopod Porcellio dilatatus. J. Invertebr. Pathol. 121 28–36. 10.1016/j.jip.2014.06.007 [DOI] [PubMed] [Google Scholar]
- Sicard M., Dittmer J., Grève P., Bouchon D., Braquart-Varnier C. (2014b). A host as an ecosystem: Wolbachia coping with environmental constraints. Environ. Microbiol. 16 3583–3607. 10.1111/1462-2920.12573 [DOI] [PubMed] [Google Scholar]
- Sicard M., Chevalier F., De Vlechouver M., Bouchon D., Grève P., Braquart-Varnier C. (2010). Variations of immune parameters in terrestrial isopods: a matter of gender, aging and Wolbachia. Naturwissenschaften 97 819–826. 10.1007/s00114-010-0699-2 [DOI] [PubMed] [Google Scholar]
- Stouthamer R., Breeuwer J. A., Hurst G. D. (1999). Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 53 71–102. 10.1146/annurev.micro.53.1.71 [DOI] [PubMed] [Google Scholar]
- Tagami Y., Miura K., Stouthamer R. (2001). How does infection with parthenogenesis-inducing Wolbachia reduce the fitness of Trichogramma? J. Invertebr. Pathol. 78 267–271. 10.1006/jipa.2002.5080 [DOI] [PubMed] [Google Scholar]
- Teixeira L., Ferreira Á, Ashburner M. (2008). The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6:e2 10.1371/journal.pbio.1000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Boland J. A., Kuhn M., Berche P., Chakraborty T., Domi G., González-zorn B., et al. (2001). Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14 584–640. 10.1128/CMR.14.3.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velge P., Wiedemann A., Rosselin M., Abed N., Boumart Z., Chaussé A. M., et al. (2012). Multiplicity of Salmonella entry mechanisms, a new paradigm for Salmonella pathogenesis. Microbiologyopen 1 243–258. 10.1002/mbo3.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Schulenburg J. H. G., Habig M., Sloggett J. J., Webberley K. M., Bertrand D., Hurst G. D. D., et al. (2001). Incidence of male killing Rickettsia spp. (alpha proteobacteria) in the ten spot ladybird beetle Adalia decempunctata L. (Coleoptera: Coccinellidae). Appl. Environ. Microbiol. 67 270–277. 10.1128/AEM.67.1.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert L. A., Araujo-Jnr E. V., Ahmed M. Z., Welch J. J. (2015). The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. R. Soc. Lond. B Biol. Sci. 282 10.1098/rspb.2015.0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. L., Wang J., Aksoy S. (2011). Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 9:e1000619 10.1371/journal.pbio.1000619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Z. S., Hedges L. M., Brownlie J. C., Johnson K. N. (2011). Wolbachia-mediated antibacterial protection and immune gene regulation in Drosophila. PLoS ONE 6:e1000619 10.1371/journal.pone.0025430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Vilchez I., Mateos M. (2010). Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLoS ONE 5:e12149 10.1371/journal.pone.0012149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y. H., Woolfit M., Rancès E., O’Neill S. L., McGraw E. A. (2013). Wolbachia-associated bacterial protection in the mosquito Aedes aegypti. PLoS Negl. Trop. Dis. 7:e2362 10.1371/journal.pntd.0002362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J. H., Barr A. R. (1971). New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature 232 657–658. 10.1038/232657a0 [DOI] [PubMed] [Google Scholar]
- Zélé F., Nicot A., Duron O., Rivero A. (2012). Infection with Wolbachia protects mosquitoes against Plasmodium-induced mortality in a natural system. J. Evol. Biol. 25 1243–1252. 10.1111/j.1420-9101.2012.02519.x [DOI] [PubMed] [Google Scholar]
- Zug R., Hammerstein P. (2014). Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. 90 89–111. 10.1111/brv.12098 [DOI] [PubMed] [Google Scholar]


