Abstract
Introduction
Current pharmacological therapies in patients with type 2 diabetes (T2D) are challenged by lack of sustainability and borderline firm evidence of real long-term health benefits. Accordingly, lifestyle intervention remains the corner stone in the management of T2D. However, there is a lack of knowledge regarding the optimal intervention programmes in T2D ensuring both compliance as well as long-term health outcomes. Our objective is to assess the effects of an intensive lifestyle intervention (the U-TURN intervention) on glycaemic control in patients with T2D. Our hypothesis is that intensive lifestyle changes are equally effective as standard diabetes care, including pharmacological treatment in maintaining glycaemic control (ie, glycated haemoglobin (HbA1c)) in patients with T2D. Furthermore, we expect that intensive lifestyle changes will decrease the need for antidiabetic medications.
Methods and analysis
The study is an assessor-blinded, parallel group and a 1-year randomised trial. The primary outcome is change in glycaemic control (HbA1c), with the key secondary outcome being reductions in antidiabetic medication. Participants will be patients with T2D (T2D duration <10 years) without complications who are randomised into an intensive lifestyle intervention (U-TURN) or a standard care intervention in a 2:1 fashion. Both groups will be exposed to the same standardised, blinded, target-driven pharmacological treatment and can thus maintain, increase, reduce or discontinue the pharmacological treatment. The decision is based on the standardised algorithm. The U-TURN intervention consists of increased training and basal physical activity level, and an antidiabetic diet including an intended weight loss. The standard care group as well as the U-TURN group is offered individual diabetes management counselling on top of the pharmacological treatment.
Ethics and dissemination
This study has been approved by the Scientific Ethical Committee at the Capital Region of Denmark (H-1–2014–114). Positive, negative or inconclusive findings will be disseminated in peer-reviewed journals, at national and international conferences.
Trial registration number
Keywords: Diabetes Mellitus, Type 2; Risk reduction behaviour; Exercise; Diet; Drug therapy
Introduction
The clinical care of type 2 diabetes (T2D) requires multifactorial intervention, including the pharmacological regulation of hyperglycaemia, hypertension and hyperlipidaemia to minimise T2D complications.1–3 Polypharmacy is often accompanied by an increased risk of adverse medication effects, a decreased quality of life and economical costs.4 5 Thus, the strategies of lifestyle interventions are equally efficient in maintaining glycaemic control as the pharmacological treatment is well warranted.
Lifestyle changes, such as healthy diet and increased physical activity, are established cornerstones in diabetes management.6–8 In addition to the beneficial effects on glycaemic control, improved physical activity and training are also likely to improve mental and physical well-being as well as reducing stress and distress in adults.9 Only one study has studied the effects of lifestyle-driven weight loss in patients with T2D (the Look AHEAD trial). These reports indicate that short and long-term reductions in antidiabetic, lipid lowering and antihypertensive medication can be achieved with a weight-loss intervention in patients with T2D.10 11 However, the weight loss was partially obtained by pharmacological treatment. Moreover, the pharmacological treatment was not standardised between the intervention and the control group, and the hypoglycaemic treatment was initially performed by the study physicians in the intervention group only.12 13 Accordingly, it is difficult to interpret to what extent healthy lifestyle changes (diet, aerobic and strength conditioning as well as decreased physical inactivity) per se can be used as a treatment for T2D as a substitute for the pharmacological treatment without compromising glycaemic control and metabolic health. Thus, studies of the effect of the U-TURN lifestyle intervention on the need for clinical T2D care alongside the effect on glycaemic control are needed in order to implement an intensive lifestyle treatment in clinical care.
A decade after the cessation of the predefined trial period in the UK Prospective Diabetes Study, significant reductions in the risk of myocardial infarction and improved mortality were observed in newly diagnosed patients with T2D allocated to early and aggressive glucose lowering treatment.14 It was then proposed that intensifying glycaemic control in patients with T2D with short T2D duration could be beneficial in reducing macrovascular and microvascular complications, whereas it had no effect or no adverse effects on patients with severe long-standing T2D.15 16 Thus, it could be speculated that an intensive lifestyle intervention could prove to be more efficient in patients with T2D with shorter disease duration.
Study objective and hypothesis
The objective of this study is to assess the clinical efficacy of the U-TURN lifestyle intervention in a sample of patients with short duration of T2D. We hypothesise that the U-TURN intervention would be comparable with the conventional multifactorial care in maintaining glycaemic control, while reducing the need for antidiabetic medications.
Methods and analysis
Trial design and study setting
The study is a parallel-arm, single-blinded, randomised clinical equivalence trial where the primary end point is glycated haemoglobin (HbA1c) monitored across 12 months. The participants are randomised in a 2:1 fashion to the lifestyle intervention (U-TURN) or standard care. The intervention is performed in a free-living environment with partial supervision of training and diet; all data collection will be performed at Copenhagen University Hospital at Rigshospitalet (primary trial sponsor, Blegdamsvej 9, 2100, Copenhagen, Denmark), and Glostrup (Denmark). Participants are recruited from the Capital Region of Denmark and the Region of Zealand, Denmark. The study has been registered at http://www.clinicaltrials.org (NCT02417012) on 14 April 2015. Amendments to the protocol are to be approved by the U-TURN steering committee and the Scientific Ethical Committee at the Capital Region of Denmark. Amendments are reported to http://www.clinicaltrials.org
Participants
Eligibility
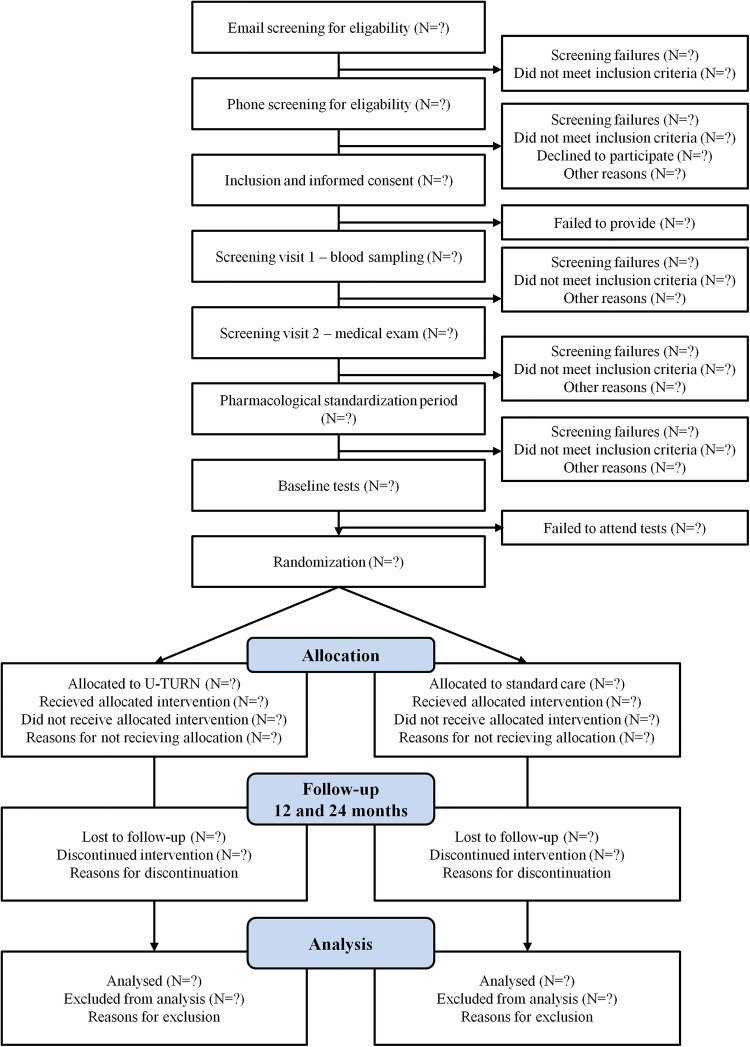
Flow of participants is described in figure 1. Initially inclusion and exclusion criteria are identified through a phone interview (preinclusion). If eligible after the phone interview, the participant will be included after providing informed oral and written consent before any additional study procedures are initiated. Next step includes a blood sample (postinclusion) and a thorough medical screening (postinclusion). The latter two screenings are included in the procedure to identify latent exclusion criteria. Inclusion and exclusion criteria are described in box 1.
Figure 1.
Flow of participants through the U-TURN study.
Box 1. Eligibility criteria.
Inclusion criteria
Type 2 diabetes
Type 2 diabetes duration <10 years
Less than three antidiabetic medications
Age ≥18 years
Body mass index ≥25 but ≤40 kg/m2
Accept of medical regulation by the U-TURN endocrinologists
Accept of purchasing a fitness club membership through U-TURN collaborator
Exclusion criteria
Glycated haemoglobin > 9% (75 mmol/mol)
Insulin usage
-
Presence of one or more of the following microvascular and macrovascular complications of type 2 diabetes
Diabetic retinopathy (except mild non-proliferative retinopathy or early proliferative retinopathy)
Macro-albuminuria (urine albumin-creatinine ratio ≥ 300 mg/g) or nephropathy (plasma creatinine ≥130 µM)
Diabetic neuropathy (except mild affected vibratory testing (<50 V))
History or signs of ischaemic heart disease
History or signs of arterial insufficiency
Steroid treatment 3 months before the medical examination
Thyroid disease
Liver disease
Inability or contraindication to increased levels of physical activity17
Anaemia (haemoglobin <7.3 mmol/L (women) and 8.3 mmol/L (men))
History or signs of lung disease
History or signs of heart disease
Signs of kidney disease
Pregnancy
Interventions
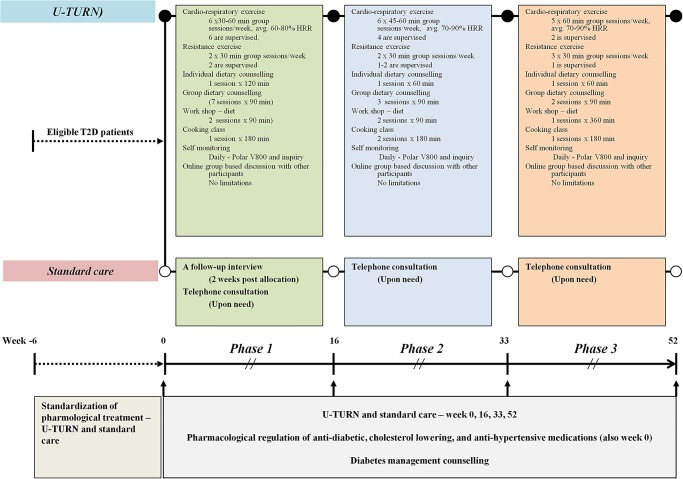
The intervention and standard care are summarised in figure 2. The U-TURN lifestyle intervention is a 1-year intervention consisting of two main components (1 and 2) with four online supplementary intervention components (3–6):
Increased levels of structured and supervised training
Antidiabetic diet
Increased levels of basal physical activity
Increased sleep duration
Self-monitoring of behaviours related to components 1–4 as well as perceived stress level, mood and motivation
Diabetes management education and networking.
Figure 2.
Description of the interventions and co-interventions. Participants are randomised either to intervention (U-TURN) (upper panel) or Standard care (Middle panel). Both groups receive pharmacological treatment and standard diabetes education (Lower panel—in grey). The intervention consists of three phases (1–3). The overall content in phases 1 through 3 is depicted in the green, light blue and light red boxes, respectively. HRR, heart rate reserve; Avg, average per training session.
The U-TURN intervention is delivered in three phases (see figure 2). The standard care intervention group receives the standard treatment according to the Danish clinical diabetes guidelines.3 The pharmacological treatment is delivered by the study's endocrinologist using the same titration and regulatory algorithms for both the groups. Both interventions and their rationale are described in detail below.
The U-TURN intervention
Intervention component 1: Increased levels of structured training
Current guidelines recommend that patients with T2D perform at least 150 min of moderate to vigorous intensity aerobic training for at least 3 days per week, with not more than two consecutive days between each training bout. In addition, resistance training is recommended three times per week at moderate to vigorous intensity.7 However, as evidence suggests, a greater reduction in HbA1c levels occurs with more than 150 min of structured training per week compared with 150 min of structured training per week or less.18 The training volume in the intervention will aim for 240–420 min training/week. During phase 1 and 2 (figure 2), the participants will complete four aerobic training sessions per week of 45–60 min duration. Additionally, two combined training sessions consisting of aerobic and resistance training are included. The aerobic part of the training session will have a time duration of 30–35 min and the resistance part will be of 30 min duration. In phase 3, the participants will complete two aerobic training sessions and three combined training sessions. The duration span of the aerobic training will be maintained in phase 3. Once a week the training session will take place outside whereas all the other training sessions will take place in a fitness centre. The training will be structured, with supervision across the entire project period as evidence supports the beneficial effect of structured training on improving glycaemic control in patients with T2D.19 The supervision is reduced across the intervention period (figure 2) and is supported with online supervision of the participants’ self-reported and objective training data.
All training is performed in groups of 4–8 participants. The groups are composed based on the geographical location of the participants’ home address. Each group will be assigned at least two certified coaches (minimum one physiotherapist) with one trainer being present at a supervised training session. Each week, a training programme is delivered from the intervention coordination centre to the coaches. An example of a weekly training programme sent to the coaches is provided in table 1. This will contain the overall distribution of aerobic and resistance training as well as the detailed programming. In the programme, the aerobic training is described, including the duration and intensity. Furthermore, the resistance training is described with muscle groups, sets and repetitions so that participants from across all groups follow the same training programme. The training modality within the aerobic training (eg, power walking and cycling) and resistance training (eg, machines and bodyweight) is the only factor that may vary between groups. The modality is decided by the trainers in order to prevent and minimise the frequency and severity of injuries. No running is permitted during phase 1. An example of a training programme is presented in table 1. Supervision is performed directly by the trainers during the training sessions, using heart rate measurements from the Polar V800 (Finland) and the online tool ‘Polar Flow for coach (Polar, Denmark). In phase 2 and 3, the heart rate of the participant and compliance to the unsupervised training session will be monitored online via the Polar V800 (Polar Inc, Denmark) and ‘Polar Flow for coach’.
Table 1.
Example of a weekly training programme—formed by the intervention coordination centre and administered by the coaches
| Week day | Aerobic training | Resistance training | Notes to the trainers |
|---|---|---|---|
| Monday | Duration: 40 min
|
Duration: 30 min
|
Make sure to inform the participant which muscle groups are activated and with time expand their “box” of different exercises. This will help participants to increase variation in their exercise programmes and thus increase their motivation. Furthermore, it will also help to minimize the risk of injuries. |
| Tuesday | Duration: 60 min
|
||
| Wednesday | Duration: 60 min
|
||
| Thursday | Duration: 30 min
|
Duration: 30 min
|
|
| Friday | Duration: 60 min
|
||
| Saturday | Rest day | ||
| Sunday | Duration: 60 min
|
Duration: 15 min
|
Intervention component 2: Antidiabetic diet, including an intended weight loss
The American Diabetes Association and the Canadian Diabetes Association support a macronutrient distribution within the range of 45–60E% carbohydrate, 15–20E% protein and 20–35E% fat (<7E% saturated fat).8 20 The U-TURN dietary intervention will be in line with these macronutrient distribution spans and will additionally focus on macronutrient quality: in particular, a diet with low glycaemic index (GI)/load (GL) as low GI or GL diets are related to a reduced HbA1c level, compared with high GI or GL diets, without inducing hypoglycaemia.21 22 As T2D is associated with comorbidities like cardiovascular disease and saturated fat intake is related to cardiovascular disease risk,23 the U-TURN intervention aims at reducing saturated fat intake to <7E% as proposed by ADA.8 As successful management of T2D is highly related to diets rich in whole grains, fruits, vegetables and nuts and legumes and low on refined grains, red or processed meat and sugar sweetened beverages,23 focus on these items will be central part of the meal plans.
A clinical dietician will prepare individual meal plans and the implementation is continuously discussed during group sessions (same groups as the training groups) and during individual counselling (figure 2). The meal plans will cover six daily meals (three main meals and three snack meals). Recipes will be changed continuously throughout the intervention. The principles of the meal plans by the dietician are described in table 2.
Table 2.
Principles of the U-TURN meal plan
| Principle | Additional comment |
|---|---|
| Homemade food | Recipes are included |
| Limit processed food items | |
| Include seasonal greens and fruits (minimum 600 g/day) | |
| Maximum two pieces of fruits per day | |
| Limit the amount of sodium | |
| Include fish (350 g/week) | 200 g should be ‘fat’ fish, for example, salmon or mackerel |
| Fibre rich food items (3 g/MJ) | |
| Hot meals should include fish once per week, one vegan meal per week | |
| Minced meat maximum twice per week | |
| Organic food items | Not a demand—but participants are encouraged to use organic food items |
| Hot meals should contain minimum 200 g vegetables per meal, maximum one-fourth of the plate should be meat, maximum one-fourth of the plate should be high glycaemic index/load food items | |
| Ad libitum intake of water and tea is allowed | |
| Maximum two cups of coffee/day | |
| No sugar sweetened beverages (including soda pops, juice or artificial sweetened beverages) | Juice is allowed in case of subjective signs of hypoglycaemia in relation to training (see below) |
| Alcohol is discouraged throughout the intervention period |
Energy requirement will be based on the age-adjusted Oxford equations.24 In a weight loss phase (phase 1, figure 2), the participants’ actual body weight is used for calculation of the energy requirement if the body mass index (BMI) <25 kg/m2. If BM I>25 kg/m2, the body weight in the equation is adjusted to equal a BMI=25 kg/m2. The weight loss phase is discontinued immediately for all participants if the BMI becomes lower than 25 kg/m2. At the individual counselling session primo phase 2 (figure 2), the clinical dietician will decide in collaboration with the participant whether to initiate another weight loss period. If BMI is >30 kg/m2 or waistline is > 94 cm for men and > 80 cm for women, the clinical dietician will recommend another weight loss period; otherwise, a maintenance period will be initiated. In the maintenance phase, the actual weight is applied in order to obtain energy balance. For all days, including structured training, 200 kcal/day will be added to the energy intake. In case of hypoglycaemic events, energy intake will be reassessed. In parallel to the training intervention, the clinical dietician will offer cooking classes and workshops on how to develop a meal plan and implement the plan. Participants are allowed to contact the clinical dietician by email once/week in case of any issues regarding implementation of or concerns about the meal plan.
To reduce the risk of hypoglycaemia, the participants are instructed to eat a snack meal just before (100–200 kcal) and after (200 kcal) a training session, and a main meal 2–3 h before a training session. In case of subjective signs of light hypoglycaemia (hunger, sweating, increased heart rate, feeling uncomfortable, dizziness and confusion), the participants are instructed to eat either one piece of fruit, drink a glass of juice in combination with a piece of rye bread or crisp bread.
Intervention component 3: Increased levels of basal physical activity
Physical inactivity and prolonged sedentary time comprises clinically important health risk factors.25 26 In a recent review it was concluded that minor increases in light intensity physical activity could improve glycaemic control in healthy persons and patients with T2D.27 As some evidence supports the beneficial effect of walking on glycaemic control in persons with T2D persons28 and as walking might prevent a deterioration in glycaemic control,29 U-TURN has adapted walking as a mode of physical activity to replace sedentary behaviour.
The aim is to reach an individual level of minimum 10 000 steps per day by gradually increasing the number of daily steps within the first month of intervention. Participants are encouraged to choose walking when possible and to incorporate light intensity physical activity breaks during prolonged sitting. Information to the participants about daily steps, the level of basal physical activity and sitting is provided using the Polar V800.
Intervention component 4: Increased sleep duration
It has been suggested that sleep duration is associated with improved glycaemic control in healthy persons and sleep deprivation reduces insulin sensitivity.30 Thus, in order to increase sleep duration, regular bedtimes and regular waking times are recommended throughout the week aiming at 7–8 h of sleep every night, with an additional requirement of 15–20 min in bed in order to fall asleep. All individuals will be recommended to shut down all electronic devices and dim the light at least 30 min before bedtime. Participants are requested to use the Polar V800 on a daily basis for monitoring sleep duration.
Intervention component 5: Self-monitoring of behaviours related to components 1–4
Self-regulation theory posits that self-monitoring is a pre-requisite to self-evaluation of progress made towards one's goal and self-reinforcement for the progress made.31 Thus, the process of changing habits may require well-developed self-regulatory skills. Self-monitoring is central to this process and includes paying deliberate attention to one's own actions as well as conditions under which these occur. In a review of 22 studies focusing on self-monitoring of diet, training or physical activity, Burke et al32 found that more frequent self-monitoring was significantly and consistently associated with larger weight loss.
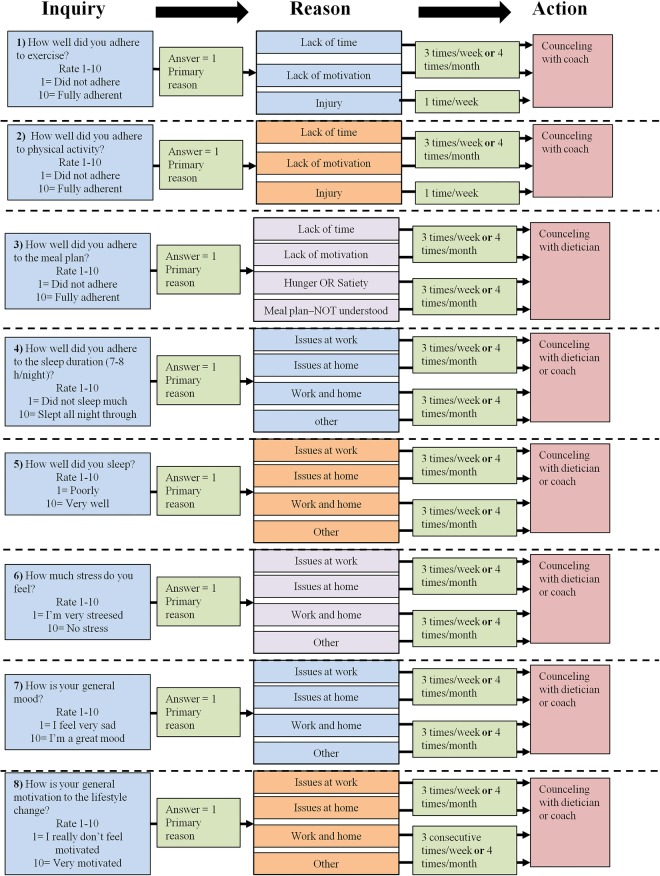
The U-TURN lifestyle intervention will entail a self-monitoring component, which is based on subjective evaluation on a daily basis. A simple questionnaire-containing eight inquiries regarding the intervention components and personal development is emailed to the participants every day. The participants rate the statements on a scale from 1 to 10 (1 is non-compliant and 10 is highly compliant). They rate the components of training (ability to follow training programme), daily physical activity (ability to comply with the physical activity goal—10 000 steps/day), diet (ability to follow the diet plan), sleep (sleep duration and sleep quality) and personal issues such as stress (perceived level), mood and general motivation. In case the participants’ score 1 (very low) in any of the items, the participants are asked to indicate the primary reasons for scoring so low. In case of a low score (figure 3) or when a participant repeatedly (three times/week) does not fill out the questionnaire, an email will be sent or a phone call will be made to the participant from the intervention coordination centre once a week. However, if the participant has contacted the coordination centre and informed them about circumstances which do not allow them to fill in the questionnaire (eg, vacation, work, etc) the follow-up procedure is not initiated. The dietician or training coach will follow-up with the participant directly based on the guidelines made by the intervention coordination centre. The self-monitoring serves two primary purposes: (1) to create a heightened awareness in each participant about their daily lifestyle choices in order to increase compliance with intervention guidelines, and (2) to prevent loss to follow-up. In addition, it offers the opportunity to adjust and individualise programmes within the overall framework for higher compliance and to prevent loss to follow-up.
Figure 3.
One electronic inquiry with eight (1–8 in figure) sub-inquiries is administrated to the participants’ intervention (U-TURN) and rated on a daily basis. The participants rate the inquiry from 1 (worst) to 10 (best). If rating is one, the participants are asked for the primary reason. Based on the frequency of the reasons action is taken. The answers are reviewed by the intervention coordination centre on a weekly basis. Based on ratings and frequency of reasons actions (red boxes) are taken.
Intervention component 6: Diabetes management education and networking
The literature supports individual33 and group-based diabetes counselling34 for improving glycaemic control, although the effect of group-based counselling may be more efficient.35 Thus, a group-based structure has been adapted. In addition, online encouragement and a participant platform for general experience sharing could potentially create a strong community feeling and enhance the face-to-face interaction.36
The U-TURN intervention participants are included in groups (see above). It also includes educational and informative elements, where the entire intervention group will participate in three 2 h lectures. The focus will be on disease pathology and diabetes management, diet and diet plans, training, sleep and motivational science. The participants will be assigned to a closed web-based group on http://www.facebook.com. It is essential to be aware of privacy settings; thus, U-TURN participants and health personnel will be requested to keep their engagement in the closed group and not ‘friend’ each other on Facebook. The intervention coordinators, diet and training councillors will post positive encouragements or encourage sharing success, fear, hope, etc. The diet and training councillors will monitor the discussions and add relevant inputs to the discussions.
Preventing discontinuation: The U-TURN Toolbox
Several rescue mechanisms are implemented to prevent loss to follow-up. The first line of action in case of problems with adherence relates to self-monitoring (see above). An extended toolbox contains procedures to modify the intervention components 1 (training) and 2 (diet). These procedures are described in table 3.
Table 3.
Extended tool box for prevention of loss to follow-up
| Intervention component 1 (training) | |
| If the participant contacts the therapist in person or by email and express concerns about participation in the training intervention | |
| Action 1 | The participant is offered a motivational interview with the coordination centre to get an overview over the possible challenges, that is, lack of time or worries. An adjusted plan is made and the trainers will follow-up at the supervised training. If the lacking compliance relates to injuries, pain or resistance to training modality, the training modality may be altered, whereas the training intensity will be maintained |
| Action 2 | If action 1 is insufficient, the participant is invited to a personal motivational interview with a motivational expert not involved with the daily training |
| Action 3 | If action 1 and 2 are insufficient, two training sessions per week are eliminated from the programme for 4 weeks. The training session will be gradually reintroduced |
| Intervention component 2 (diet) | |
| If the participant contacts the therapist in person or by email and express concerns about satiety, food preferences or food preparation techniques (by email to dietician or at group counselling) | |
| Action 1 | Participants are interviewed regarding compliance to the meal plan and provided with specific guidelines to practical changes in the plan by the clinical dieticians. For example, to increase adherence to food items increasing satiety or exchange some food items to match preferences |
| Action 2 | If action 1 is insufficient and the participant still experience lack of satiety, then the energy intake is increased in steps of 100 kcal/day until the level of satiety is acceptable by the participant. The process is performed via email with the dietician |
Conventional multicomponent care
Participants allocated to the standard care group will receive the standard Danish T2D treatment.3 37 Briefly, in Denmark patients with T2D are stratified based on the capability and severity of their condition determined by the patient's general practitioner (GP) to receive varying levels of rehabilitation and clinical care.37 The GP will cover the coordination with rehabilitation programmes, foot and eye specialists. However, the pharmacological treatment will be administered solely by the U-TURN endocrinologists (see description below). Furthermore, the participants receiving standard care will be interviewed 2 weeks postrandomisation about concerns regarding the allocation and will be provided with valid arguments to complete the project. The participants can contact a diabetes nurse by phone and/or email throughout the entire intervention period to discuss their concerns regarding the treatment.
Cointerventions (U-TURN and standard care)
Medical treatment in U-TURN
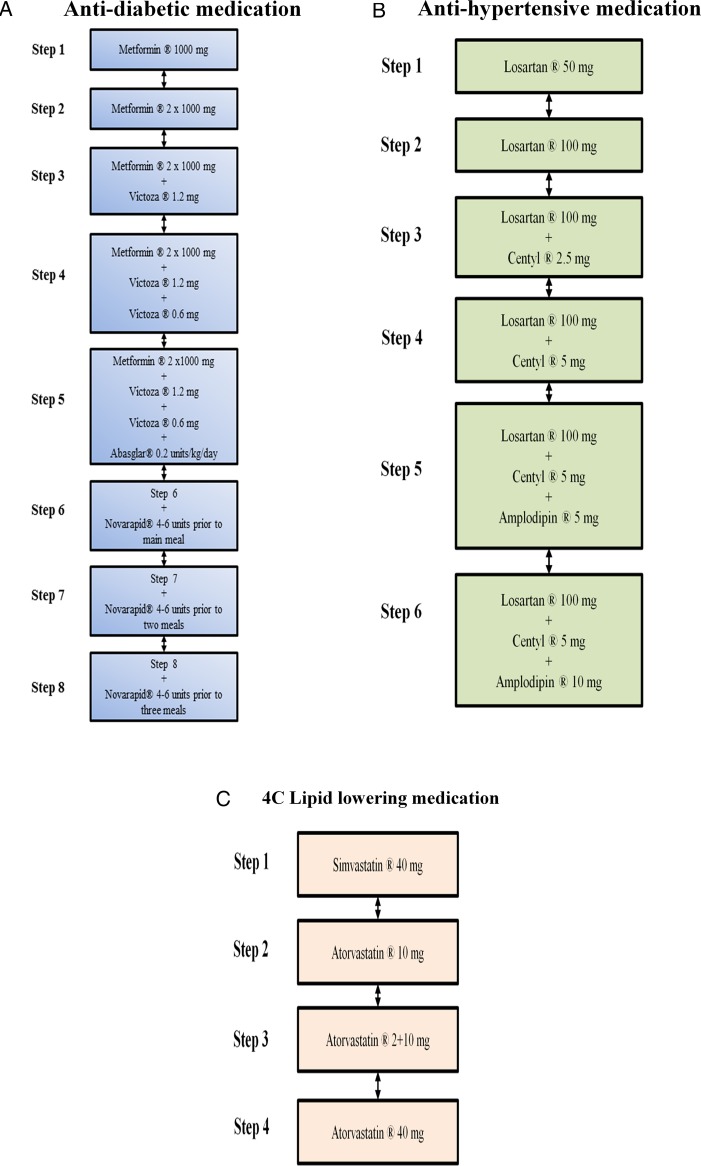
The medical regulation will be performed by two experienced endocrinologists. Owing to the blinding to group allocation, a study nurse is responsible for the main contact with the participants. Following the medical screening, but prior to the baseline measurements, all eligible participants will have their antidiabetic, lipid lowering and antihypertensive pharmacological treatment standardised using the predefined medications (figure 4A–C and table 4). The standardisation is employed in order to decrease the risk of reductions in medications not related directly to the intervention, but rather due to ongoing medical treatment at inclusion to the programme. The titration period is ≥6 weeks. Table 4 outlines treatment goals and intensification of treatment in U-TURN; while details of medication adjustments are outlined below and in figure 4A–C.
Figure 4.
(A) Illustration of antidiabetic treatment algorithm: Biguanid (tablet Metformin) is initiated at 500 mg once daily up to 1000 mg twice daily. If treatment goal is not reached, then a GLP-1 analogue (injection Victoza) is added at 1.2 mg increasing to 1.8 mg daily. In case of unacceptable adverse effects, a dipeptidyl peptidase inhibitor-4 inhibitor (tablet Januvia) is used at 100 mg daily instead of the GLP-1 analogue. If treatment goal is not reached, then basal insulin (injection Abasaglar) is added (0.2 units/kg once daily). If treatment goal is not reached then meal insulin is added (injection Novorapid titrated based on self-assessed pre-prandial blood glucose measurements in close cooperation with the study nurse). Detailed insulin adjustment is included in the online supplementary material. (B) Illustration of antihypertensive treatment algorithm: An angiotensin II receptor antagonist (tablet Losartan) is initiated at 50 mg daily up to 100 mg daily. If treatment goal is not reached, then a thiazide (tablet Centyl cum KCL) is added at 2.5 mg increasing to 5 mg daily. If treatment goal is not reached, then a calcium antagonist (tablet Amlodipine) is added at 5 mg increasing to 10 mg daily. In case of unacceptable adverse effects, a mineralocorticoid (tablet Spironolactone) is used at 25 mg increasing to 100 mg daily. (C) Illustration of lipid lowering treatment algorithm: A statin (tablet Simvastatin) is initiated at 40 mg daily. If treatment goal is not reached, treatment is replaced by another statin (tablet Atorvastatin) at 10 mg increasing to 40 mg daily.
Table 4.
Treatment goals for medical regulation
| Medication | Treatment goals | Intensification of treatment during standardisation | Intensification of treatment at follow-ups |
|---|---|---|---|
| Antidiabetics | HbA1c ≤48 mol/mol | HbA1c >64 mmol/L or 5 mmol/mol increment | HbA1c >58 mmol/L or 5 mmol/mol increment |
| Antihypertensive | BP ≤130/80 mm Hg | BP >150/95 mm Hg | BP >140/85 mm Hg |
| Antilipids | LDL ≤2.0 mmol/L TG ≤5.0 mmol/L |
LDL >2.0 mmol/L TG >5.0 mmol/L |
LDL >2.0 mmol/L TG >5.0 mmol/L |
The table shows treatment goals for the U-TURN intervention and intensification of treatment. If the treatment target is reached, the dose of the compound is halved at the following control time point (3 months later). In case of unchanged values or an additional drop, the compound is then discontinued.
BP, blood pressure; HbA1c, glycated haemoglobin; LDL, low-density lipoproteins; TG, triglycerides.
Procedures for regulation of medication (baseline to 12 months)
The study nurse presents the anonymised data to the blinded endocrinologists every third month and a decision on regulation of antidiabetic (baseline and every third month), cholesterol (baseline and every sixth month) and/or antihypertensive medication (baseline and every third month) is made (figure 4A–C). No information on group allocation is provided to the endocrinologists. The decisions will be based on HbA1c, cholesterols and home blood pressure measurements (18 home-based measurements over 3 days before each test round (Contour Next, Bayer, Copenhagen, Denmark)) using the algorithms described below. If insulin treatment is initiated, the antidiabetic pharmacological treatment is adjusted based on home glucose monitoring every 2–4 weeks (see below). Also, in case of glucagon-like peptide-1 analogue (GLP-1 analogue) or insulin treatment, an information meeting is arranged with the study nurse to educate the participant in glucose monitoring and insulin injection technique. If the treatment target is reached, the dose of the compound is halved at the following control time point (3 months later). In case of unchanged values or an additional drop in level, the compound is discontinued.
Regulation of medication (12–24 months)
All pharmacological treatment for the participants will be performed by their usual GP.
Medical regulation algorithms
Algorithms for regulation of antidiabetic medication
It is well recognised that tight glycaemic control in fragile diabetes patients (advanced age, known history of severe hypoglycaemia, overt cardiovascular disease) might be associated with higher incidence of cardiovascular mortality.16 Patients eligible for inclusion in the study are without known history of severe hypoglycaemia, advanced atherosclerosis, severe comorbidity or advanced age which allows for tighter glycaemic control in U-TURN. The goal is an HbA1c of 48 mmol/mol (6.5%) giving due consideration to the risk of hypoglycaemia in the individual patient.
Biguanid (tablet Metformin) is initiated as first-line treatment due to its long-standing evidence base for efficacy and safety; it is inexpensive, and may reduce the risk of cardiovascular events.38 If the patient exhibits unsatisfactory glycaemic control on metformin alone, GLP-1-analogue (Victoza) is added as second-line treatment. Despite an eventual less beneficial effect on glycaemic control in comparison with sulfonylurea, the GLP-1 analogues exhibit several other positive effects, including less frequent hypoglycaemia and weight loss.39 In case the patients experience unacceptable side effects on GLP-1 analogue treatment (eg, nausea), the treatment can be changed to metformin in combination with a dipeptidyl peptidase inhibitor (DPP-4 inhibitor, Januvia). However, with time supplementation with subcutaneous injections of insulin or insulin analogues is often necessary in order to compensate for insulin deficiency. As third-line treatment, one daily injection of insulin glargine biosimilar (Abasaglar initiating dose 0.2 U/kg/day) is, therefore, eventually added and titrated to an acceptable fasting blood glucose level. Further, analyses between these insulin doses have documented significant reductions in hypoglycaemia mainly at night with the insulin analogues, while no statistically significant differences for severe hypoglycaemia rates were shown in any of the trials.14 40 41 Most studies have shown that the combination of GLP-1 receptor agonists with basal insulin has an equal or slightly superior efficacy compared to the addition of prandial insulin with a subsequent weight loss and less hypoglycaemia.42 Eventually, if HbA1c is still above the target while fasting blood glucose is below 7 mmol/L, then meal insulin, fast acting Novorapid, is initiated.
Algorithms for regulation of lipid lowering and antihypertensive medication
Clinical studies in T2D have shown beneficial effects on cardiovascular outcomes from lowering of blood pressure to at least below 140 mm Hg systolic and 85 mm Hg diastolic.43 44 Therapeutic goal for blood pressure during the U-TURN study is 130/80 mm Hg.
An angiotensin receptor blocker (Losartan) is chosen as first-line antihypertensive treatment due to its additional protective effect on kidney function in patients with T2D.45 46 If the patients exhibit unsatisfactory blood pressure control from Losartan alone, a thiazide (Centyl) is added as second-line treatment based on its well validated and long-standing evidence as an efficient diuretic with an effect on cardiovascular outcomes. 47 48 As a third and eventually fourth antihypertensive drug in the treatment algorithm, a calcium channel blocker (amlodipine) and a mineralocorticoid antagonist (spiron) is chosen.
The increased prevalence of lipid abnormalities in patients with T2D and the associated risk of cardiovascular disease make lipid lowering treatment necessary. Therapeutic goals for lipids in the U-TURN study are low-density lipid cholesterol below 2.5 mmol/L and triglycerides below 5 mmol/L. Statin medication is preferred therapy as it helps to decrease all-cause and vascular mortality among diabetic patients.49 In the U-TURN study, two different kinds of statins are used, with atorvastatin replacing simvastatin in case of insufficient effect.
Safety criteria and adverse events
Participants will be informed about side effects as well as subjective signs of hypoglycaemia (hunger, sweating, increased heart rate, feeling uncomfortable, dizziness and confusion) and hyperglycaemia (thirst, polyuria, fatigue and confusion), and urged to contact the study nurse in case of any adverse symptoms. Severe hypoglycaemic events (see below) will be registered by the study nurse. The safety criteria employed include adverse events, health-related outcomes (for instance, episodes of angina or signs of atrial fibrillation) and subject-reported hypoglycaemic episodes (plasma glucose <4 mmol/L). Non-severe hypoglycaemic events are defined as those that can be self-treated; severe hypoglycaemic events are defined as plasma glucose <3 mmol/L or episodes requiring third-party assistance or medical intervention. In case of adverse effects, medication is changed according to the titration described. In case of hypoglycaemic episodes, antidiabetic medication is eventually adjusted. Severe hypoglycaemic periods are reported to the study nurse. The hypoglycaemic events are then presented to the endocrinologist and registered in a database. At any time, all necessary information, including information about intervention, medical history and adverse events, on the individual participant is available, but this is blinded to the endocrinologist in order to maintain group concealment. If considered necessary, the blinding will be repealed on a patient-to-patient basis and the participants will be contacted directly by the endocrinologist. This will be decided on a patient-to-patient basis and will be based on information provided by the study nurse. The participant's GP will be informed about the procedure and encouraged to contact the U-TURN project nurse in case of questions.
Injuries related to the intervention (acute and over-use) will be registered if reported. In case of reports of severe adverse events during the study period, the steering committee will be informed as will the Scientific Ethical Committee of the Capital Region of Denmark.
Diabetes education
All participants are invited to individual educational meetings and diabetes controls (30 min) with a trained diabetes nurse every third month (a total of four meetings). Home blood pressure and home glucose measurements are reviewed. At these meetings, challenges regarding the diabetes treatment, including compliance issues, are addressed. Moreover, general education regarding the importance of a healthy lifestyle is provided. Furthermore, all participants are invited to an introductory 2 h diabetes management course.
Treatment of obstructive sleep apnoea
As the prevalence of obstructive sleep apnoea is increased in patients with T2D and maybe casually linked to T2D,50 a screening of all participants is performed. Participants diagnosed with sleep apnoea (Apnoea-Hypopnoea Index >15 h) following baseline sleep testing with cardiorespiratory monitoring (see below) are offered sleep apnoea treatment (continuous positive airway pressure (CPAP)). To minimise the effect of CPAP treatment on retesting results, the participants with obstructive sleep apnoea are told to discontinue the CPAP treatment 1–2 days before retesting.
Retention
All participants will receive €300 (2250 Danish kroner) to cover lost earnings, transport and discomfort in relation to the testing procedures. To minimise loss to follow-up in the standard care group, participants are interviewed 2 weeks postallocation by the study nurse regarding their concerns about allocations to standard care. Furthermore, they are offered an interview about their progress after 1 year. All participants are allowed to contact the U-TURN study nurse by phone in case of study-related questions (eg, pharmacological treatment, sports injuries, etc).
Study end points and assessments
Table 5 describes the time points at which the outcomes are assessed during the intervention and follow-up period.
Table 5.
Summary of measures
| Measurement | Description | Baseline | 3 months | 6 months | 9 months | 12 months | 24 months |
|---|---|---|---|---|---|---|---|
| Blood sampling | Following an overnight fast (8 h) a blood sample is drawn. The plasma markers of metabolism (HbA1c*†‡, TC*, LDL*, HDL*, TG†, fasting insulin*† and glucose*†) will be analysed | Ѵ | Ѵ | Ѵ | Ѵ | Ѵ | – |
| Medications | Dose of antidiabetic*, lipid* and blood pressure lowering* medications | Ѵ | Ѵ | Ѵ | Ѵ | Ѵ | – |
| Adverse events | Major hypoglycaemic episodes*, cardiovascular events*, acute* and overuse* injuries related to the intervention component 1 | Ѵ | Ѵ | Ѵ | Ѵ | Ѵ | – |
| Glucose tolerance | Following an overnight fast (8 h) and after a 48 h medication and training pause, an antecubital intravenous line is placed and a standard 75 g oral glucose tolerance test will be performed. Blood will be drawn at the following time points: 0 (baseline), 15, 30, 60, 90 and 120 min. The plasma will be analysed for insulin*‡, C-peptide*‡ and glucose*‡. Area under the curve will be analysed*‡ | Ѵ | – | – | – | Ѵ | – |
| Physical fitness | Fitness*†‡ will be assessed by employing a progressive bicycle ergometer test protocol. Oxygen consumption will be assessed using continuous indirect calorimetric measurements (Cosmed, Italy) | Ѵ | – | – | – | Ѵ | – |
| Cognitive testing | Specific cognitive function areas (short-term memory, attention, executive functions, etc) will be tested using the CANTAB test package from Cambridge Cognition (Cambridge Cognition, UK) and BDNF-α is assessed from blood serum | Ѵ | – | – | – | Ѵ | – |
| Depression | Characteristics, attitudes and symptoms of depression will be assessed using the Beck Depression Inventory51 | Ѵ | – | – | – | Ѵ | – |
| Well-being, functional ability and motivation | Functional health and well-being will be assessed using the SF-36.52 Positive and negative effect on separate subscales will be assessed using the GMS. Dimensions of positive mental health, including emotional, psychological and social well-being, is measured using The Mental Health Continuum Short Form. To assess motivation for training, the BREQ-2 is employed | Ѵ | Ѵ | Ѵ | – | Ѵ | – |
| Dietary intake | A food frequency questionnaire is completed*53 | Ѵ | – | – | – | Ѵ | – |
| Body composition, anthropometry and blood pressure | Height*†‡§, weight*†‡§, waist and hip circumference will be measured by standard procedures. Dual X-ray absorptiometry (iDXA; Lunar, Madison, WI) and COREScan will be used to assess whole body composition*‡. Home-based diastolic* and systolic* blood pressure is assessed using an upper arm blood pressure monitor (Model BPM1C, Kinetik, UK) | Ѵ | Ѵ | Ѵ | Ѵ | Ѵ | Ѵ |
| Personal information | Age*†‡§, sex*†‡§, diabetes duration*†‡§ and educational level*†‡§ will be obtained using self-report | Ѵ | – | – | – | – | – |
| Physical activity | Information on the physical activity level*† will be obtained using questionnaire RPAQ | Ѵ | – | – | – | Ѵ | – |
| Personality type | Two questionnaires regarding personality, the NEO-Five Factor Inventory and a SES are incorporated¶54 55 | Ѵ | – | – | – | Ѵ | – |
| Sleep quality, disturbances, fatigue and sleepiness | Cardiorespiratory monitoring is used to measure the prevalence of obstructive sleep apnoea†. Participants complete sleep diaries for 2 weeks after each cardiorespiratory monitoring in order to record and describe potential changes in their sleep. The ESS† and the MFI† are employed to measure daytime sleepiness. Sleep quality is measured using the PSQI† | Ѵ | – | Ѵ | – | Ѵ | Ѵ |
| Arterial function¶ | An ultrasound system equipped with vascular software for two-dimensional imaging, colour and spectral Doppler is used to assess flow mediated dilation and shear stress in the femoral artery§ | Ѵ | – | – | – | Ѵ | – |
*Article 1: The effects of a head-to-head comparison of intensive life style intervention (U-TURN) versus standard glucose lowering medications in patients with type 2 diabetes mellitus: an assessor-blinded, parallel group, randomised trial.
†Article 2: The effects of the U-TURN lifestyle intervention on sleep quality and sleep apnoea in patients with type 2 diabetes.
‡Article 3: Predictors for improvements in glycaemic control after a comprehensive lifestyle intervention; A sub-study to the randomised trial study U-TURN.
§Article 4: Effect of lifestyle intervention on endothelial function in patients with type 2 diabetes assessed by flow mediated dilatation; A sub-study to the randomised trial U-TURN.
¶Measured in a subset of the sample (N=40).
–, not assessed; BREQ-2, Behavioral Regulation In Exercise Questionnaire 2; ESS, Epworth Sleepiness Scale; GMS, Global Mood Scale; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MFI, Multidimensional Fatigue Inventory; PSQI, Pittsburgh Sleep Quality Index; RPAQ, Recent physical activity questionnaire; SES, Sensation Seeking Scale; TC, total cholesterol; TG, triglyceride; Ѵ, assessed.
Primary outcome
The primary outcome measure is change in glycaemic control (HbA1c) from baseline to 12-month follow-up.
Secondary outcomes
The key secondary end point includes reductions in antidiabetic medications from baseline to 12-month follow-up. For exploratory purposes, the change in antidiabetic medication is also quantified according to dose from baseline to 12- month follow-up. Every available dose will be graded according to the titration algorithms described above and summed into a total dose score at 12-month follow-up. Every step in the titration (figure 4A) is awarded one point. A point is either added to the score when medication is added on (ie if increasing one step up on figure 4A) or subtracted upon discontinuation of medication (ie. if declining one step on figure 4A). A total of eight points can be accumulated if the participant is receiving full dose (ie, ends at step 8 figure 4A at 12-month follow-up) or 0 if no antiglycaemic medication is prescribed. Changes from the baseline score are reported. All secondary outcomes are assessed until 12-month follow-up except for changes in body composition, and personal and demographic variables which are also monitored until 24-month follow-up.
Sample size considerations
The sample size in this study was based on what was considered feasible, within the local context, enabling up to 120 participants to be enrolled in the trial period (29 April 2015–17 August 2017). The sample size is truncated at 120 participants or the N reached at the end of recruitment period—whichever is reached first. To increase the sensitivity to the U-TURN intervention it was decided to randomise the participants in a 2:1 fashion.
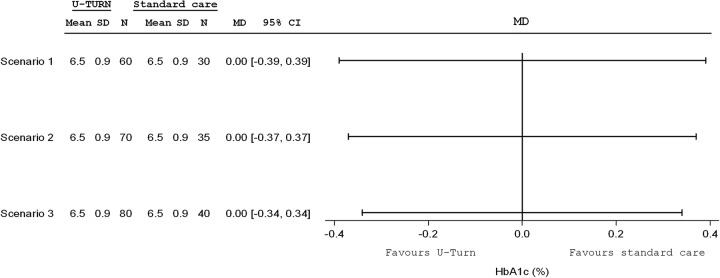
The study was not formally powered as an equivalence trial, but from the content experts it was decided that a reasonable equivalence margin would±0.4%-points for HbA1c for the between-group comparison. As presented in figure 5, assuming that the HbA1c is down to 6.5% in both groups, with an SD of 0.9%, we estimated that enrolling 120 participants in the intention-to-treat population (ITT; 80:40), testing a 2-tailed superiority hypothesis (based on 95% CIs56 would be reasonably precise to estimate within a reasonable equivalence margin; −0.34% to 0.34%). Further, according to the principle of sensitivity, our estimates support that even if we include only 90 (60:30) participants our confidence limits will be acceptable.
Figure 5.
Forrest plot depicting three scenarios of total sample size from a total of 90 participants (scenario 1), 105 (scenario 2) and 120 participants (scenario 3) with the respective with of 95% CIs. HbA1c, glycated haemoglobin N, number; MD, mean difference.
Randomisation, sequence generation and allocation concealment
Participants will be assigned randomly (2:1) in permuted blocks of three and six, according to computer-generated random numbers, to undergo either U-TURN or standard care after the baseline measurements. Participants will be stratified according to sex (male vs female). The sequence is generated centrally by a researcher not involved in the testing or allocation, and delivered to the data manager. The allocation information to the participants is given by a study nurse not involved in the testing, randomization or evaluation procedures. The study nurse receives the participant allocation directly from the data manager and the group allocation is delivered to the participants. The data will not be accessible until completion of the 12-month follow-up data collection.
Blinding
Outcome assessors as well as the endocrinologists are blinded to the allocation at baseline and follow-up. The participants will be informed that they are not allowed to discuss their allocation during the follow-up measurements. Owing to the nature of the trial, participants, diabetes nurse and intervention coordinators cannot be blinded to the allocation.
Statistical methods
All data being collected longitudinally (including the primary outcome) will be analysed according to the ITT principle using repeated-measures analysis of covariance applied in mixed linear models. Patterns of missing data will be investigated. A priori, the less restrictive missing at random (MAR) assumption is considered more reasonable than the missing data being missing completely at random; MAR assumes that drop-out may depend on observed outcomes or covariates, but does not depend on unobserved data. The prespecified efficacy analyses are based on the full-analysis set, which include all participants who are randomised and have had an outcome assessment at baseline. Under the MAR assumption, likelihood-based approaches, such as mixed effects models, will produce valid inferences. Thus, based on mixed linear models, our primary model is based on analysis of covariance for continuous end points. The model will include group and sex as fixed effects, with the baseline value of the relevant variable as a covariate. Categorical data for dichotomous end points will be analysed with the use of logistic regression with the same fixed effects and covariates as the respective analysis of covariance.
Assuming that the data on potential drop-outs are MAR, both linear mixed models and multiple imputation procedures would be applicable to handle missing data.
While the null hypothesis in a superiority trial is that treatment effects are identical (with HA implying that these are not), the null hypothesis for the primary outcome in this trial is defined with reference to an acceptable clinical difference in treatment effects (ie, equivalence margin).57 As mentioned above, we define the threshold for not ‘too different’ or not ‘unacceptably worse’ as a potential difference (95% CI) in HbA1c of±0.4% points. Sensitivity analyses will be performed to assess the robustness of the primary analyses, including repeated measures with baseline observation carried forward, and multiple-imputation techniques (with the latter using model based approaches to ‘replace’ missing data).
Exploratory analyses of the treatment effects will be performed on the secondary outcomes. The analysis plan will be developed prior to data analysis, and will be performed in parallel independently by two blinded researchers. Group allocation is not disclosed before consensus about the interpretation of the data is reached. In essence, the information about treatment and the N is concealed until consensus is reached. Discrepancies in the analysis outcome between the researchers will be resolved using a blinded third party statistician.
Ethics and dissemination
The U-TURN study is expected to provide evidence that lifestyle change (without use of weight loss enhancing pharmacological additives as a part of the treatment) is equally effective in treating T2D as the recommended multicomponent care. Furthermore, the study will provide valuable insights into motivational mediators and moderators for adherence to intensive lifestyle treatment. Although intensive, the intervention contains components which can be directly included in the rehabilitation of the patients with T2D and thus, it might have a broad appeal. If the intervention is effective in maintaining glycaemic control and reduces the need for antidiabetic pharmacological care, it will provide an alternative to the current standard care and thus decrease some of the side effects induced by the medications used in standard clinical care.
The study is unique as the methodology is stringent and systematic, thus providing scientifically viable data. A major strength of the study is the blinding of assessors and participants at the baseline assessment, and the assessor and endocrinologist blinding at all follow-up assessments thereby limiting the risk of bias. It is not possible to blind the participants during follow-up in this type of trial, which could introduce a bias. The inclusion of predefined medications used in the pharmacological care (ie, not all combinations and medications are considered) limits the interpretation to patients receiving these combinations. As the choice of medications in the study is in line with the recommended first line-treatments,3 58 the observations made in this study will still apply to a large number of patients. Furthermore, the inclusion of self-reported physical activity and adherence pose a potential information bias, limiting the interpretation of the effects of the single intervention components on HbA1c.
The U-TURN study is initiated with a 6-week prerandomisation open label run-in period for all participants, in which the medical treatment is adjusted according to the treatment algorithm used in the study. This is done in order to adjust for differences in prestudy medication due to either non-compliance or insufficient prescriptions (too little or too much medication) and to have a uniform status of medication at baseline. A titration period of 6 weeks is chosen to ensure steady state of new prescribed medication before allocation. In order to dissect the combined effect of medication and intervention in contrast to medication alone, we aim for homogenous pharmacological treatment and reliable estimates of adherence. This is done by a clear pharmaceutical treatment algorithm and administering of an interview every third month focusing on adherence to medication. The study is designed as a treat-to-target study aiming at specific goals for glycaemic control, blood pressure and lipids. This implies intensification or decrease in medical treatment according to clinical data (HbA1c, glucose measurements, blood pressure and lipids). We are aware that regulation of medication prior to allocation and during the study period may have a ‘carry-over effect’ affecting the following measurements. Hence, a 3-month period between follow-ups is chosen as this should be sufficient time to obtain sustained effect of medical regulation. Also tapering of medication is done gradually to ensure that any improvement in clinical outcome is sustained even after withdrawal of medication.
The study will be conducted according to the principles of the Helsinki Declaration II.59 Prior to inclusion, all participants received written and oral information about the study and provided written as well as oral informed consent. The participants can discontinue participation in the full study or part of the study at all times with no obligation to provide a reason. This will not have any consequences for their future treatment. If discontinued, the participants will receive the standard T2D care according to the Danish clinical guidelines; however, this will be delivered by their own GP.3 Medical regulation will be performed by experienced endocrinologists throughout the study in accordance with the predefined algorithms and strict safety criteria, and registration of adverse outcomes is employed. Thus, the study is expected to result in limited risks, adverse effects and discomfort to the participants. The results are to be reported according to the CONSORT guidelines.60 Negative, positive and inconclusive results will be disseminated in international peer-reviewed scientific journals at national and international conferences. Access to data can be granted on approval for a formal request to the steering committee.
All participants will be provided with their results. Postrandomisation, all participants are ascribed a unique personal identifier to anonymise the data. Thus, no participant identifying information is stored alongside study data. A transfer key (ID to personal information) is available, but is encrypted and stored separately from the trial data. After trial cession all data are anonymised and stored on the server of Copenhagen University Hospital. Additional biological materials are stored in a research bio-bank for up to 20 years.
As far as we are aware, the U-TURN study is the first study to investigate if lifestyle intervention is effective in maintaining glycaemic control while aiming at discontinuing pharmacological care in patients with T2D. The results from this trial are of great importance for clinical care of T2D and can provide important knowledge on how to implement and conduct treatments using the lifestyle changes.
Footnotes
Contributors: MR-L, RC, KBH and KK drafted the manuscript. All authors made substantial contributions to conception and design, and revised the manuscript critically for important intellectual content. All authors have given their final approval for the manuscript to be published.
Funding: The work is supported by the Tryg foundation, Denmark. Professor Christensen (The Parker Institute) is supported by unrestricted grants from the Oak Foundation. Mathias Ried-Larsen is supported by a post-doctoral grant from the Danish Diabetes Academy supported by the Novo Nordisk Foundation.
Competing interests: The Contour Next monitors were provided by Bayer A/S, Copenhagen, Denmark.
Ethics approval: The Scientific Ethical Committee at the Capital Region of Denmark (H-1-2014-114).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Remaining study material not designated to planned articles (specified in the protocol) is available by contacting the corresponding author. A proposal is to be submitted to the U-TURN steering committee. If approved, data are available.
References
- 1.Davidson MB. Triple therapy: definitions, application, and treating to target. Diabetes care 2004;27:1834–5. 10.2337/diacare.27.7.1834 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl 1):S11–63. 10.2337/dc12-s011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snorgaard OD, Drivsholm TO, Breum L et al. . Farmakologisk behandling af type 2 diabetes—mål og algoritmer—2014. In. Copenhagen; 2014. [Google Scholar]
- 4.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care 2004;27:1218–24. 10.2337/diacare.27.5.1218 [DOI] [PubMed] [Google Scholar]
- 5.Huang ES, Brown SE, Ewigman BG et al. . Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care 2007;30:2478–83. 10.2337/dc07-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl 1):S11–61. 10.2337/dc11-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colberg SR, Sigal RJ, Fernhall B et al. . Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010;33:e147–167. 10.2337/dc10-9990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evert AB, Boucher JL, Cypress M et al. . Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2013;36:3821–42. 10.2337/dc13-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eime RM, Young JA, Harvey JT et al. . A systematic review of the psychological and social benefits of participation in sport for adults: informing development of a conceptual model of health through sport. Int J Behav Nutr Phys Act 2013;10:135 10.1186/1479-5868-10-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redmon JB, Bertoni AG, Connelly S et al. . Effect of the look AHEAD study intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care 2010;33:1153–8. 10.2337/dc09-2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espeland MA, Glick HA, Bertoni A et al. . Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care 2014;37:2548–56. 10.2337/dc14-0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan DH, Espeland MA, Foster GD et al. . Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–28. 10.1016/S0197-2456(03)00064-3 [DOI] [PubMed] [Google Scholar]
- 13.Wadden TA, West DS, Delahanty L et al. . The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–52. 10.1038/oby.2006.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holman RR, Paul SK, Bethel MA et al. . 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 15.Chalmers J, Cooper ME. UKPDS and the legacy effect. N Engl J Med 2008;359:1618–20. 10.1056/NEJMe0807625 [DOI] [PubMed] [Google Scholar]
- 16.Bianchi C, Del Prato S. Metabolic memory and individual treatment aims in type 2 diabetes--outcome-lessons learned from large clinical trials. Rev Diabet Stud 2011;8:432–40. 10.1900/RDS.2011.8.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 2006;16(Suppl 1):3–63. 10.1111/j.1600-0838.2006.00520.x [DOI] [PubMed] [Google Scholar]
- 18.Umpierre D, Ribeiro PA, Schaan BD et al. . Volume of supervised exercise training impacts glycaemic control in patients with type 2 diabetes: a systematic review with meta-regression analysis. Diabetologia 2013;56:242–51. 10.1007/s00125-012-2774-z [DOI] [PubMed] [Google Scholar]
- 19.Umpierre D, Ribeiro PA, Kramer CK et al. . Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011;305:1790–9. 10.1001/jama.2011.576 [DOI] [PubMed] [Google Scholar]
- 20.Sievenpiper JL, Dworatzek PD. Food and dietary pattern-based recommendations: an emerging approach to clinical practice guidelines for nutrition therapy in diabetes. Can J Diabetes 2013;37:51–7. 10.1016/j.jcjd.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 21.Thomas D, Elliott EJ. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev 2009;(1):CD006296 10.1002/14651858.CD006296.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br J Nutr 2010;104:797–802. 10.1017/S0007114510001534 [DOI] [PubMed] [Google Scholar]
- 23.Ley SH, Hamdy O, Mohan V et al. . Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 2014;383:1999–2007. 10.1016/S0140-6736(14)60613-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry CJ. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr 2005;8:1133–52. 10.1079/PHN2005801 [DOI] [PubMed] [Google Scholar]
- 25.Lee IM, Shiroma EJ, Lobelo F et al. . Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219–29. 10.1016/S0140-6736(12)61031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas A, Oh PI, Faulkner GE et al. . Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med 2015;162:123–32. 10.7326/M14-1651 [DOI] [PubMed] [Google Scholar]
- 27.Benatti FB, Ried-Larsen M. The effects of breaking up prolonged sitting: a review of experimental studies. Med Sci Sports Exerc 2015;47:2053–61. 10.1249/MSS.0000000000000654 [DOI] [PubMed] [Google Scholar]
- 28.Funk M, Taylor EL. Pedometer-based walking interventions for free-living adults with type 2 diabetes: a systematic review. Curr Diabetes Rev 2013;9:462–71. 10.2174/15733998113096660084 [DOI] [PubMed] [Google Scholar]
- 29.Karstoft K, Winding K, Knudsen SH et al. . The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2013;36:228–36. 10.2337/dc12-0658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci 2014;1311:151–73. 10.1111/nyas.12355 [DOI] [PubMed] [Google Scholar]
- 31.Kanfer FH, Lisa. Helping people change: a textbook of methods vol. Vol 52 Elmsford, NY, US: Pergamon Press, 1991. [Google Scholar]
- 32.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc 2011;111:92–102. 10.1016/j.jada.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duke SA, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2009;(1):CD005268 10.1002/14651858.CD005268.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deakin T, McShane CE, Cade JE et al. . Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;(2):CD003417 10.1002/14651858.CD003417.pub2 [DOI] [PubMed] [Google Scholar]
- 35.Trento M, Passera P, Tomalino M et al. . Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care 2001;24:995–1000. 10.2337/diacare.24.6.995 [DOI] [PubMed] [Google Scholar]
- 36.George DR, Rovniak LS, Kraschnewski JL. Dangers and opportunities for social media in medicine. Clin Obstet Gynecol 2013;56:453–62. 10.1097/GRF.0b013e318297dc38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drivsholm TB, Snorgaard O. [Organization of treatment and control of type 2 diabetic patients]. Ugeskr Laeger 2012;174:2159–62. [PubMed] [Google Scholar]
- 38.Alberti A, Pirino S, Pintore F et al. . Ovis aries Papillomavirus 3: a prototype of a novel genus in the family Papillomaviridae associated with ovine squamous cell carcinoma. Virology 2010;407:352–9. 10.1016/j.virol.2010.08.034 [DOI] [PubMed] [Google Scholar]
- 39.Monami M, Marchionni N, Mannucci E. Glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized clinical trials. Eur J Endocrinol 2009;160:909–17. 10.1530/EJE-09-0101 [DOI] [PubMed] [Google Scholar]
- 40.Home PD, Fritsche A, Schinzel S et al. . Meta-analysis of individual patient data to assess the risk of hypoglycaemia in people with type 2 diabetes using NPH insulin or insulin glargine. Diabetes Obes Metab 2010;12:772–9. 10.1111/j.1463-1326.2010.01232.x [DOI] [PubMed] [Google Scholar]
- 41.Horvath K, Jeitler K, Berghold A et al. . Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007;(2):CD005613 10.1002/14651858.CD005613.pub3 [DOI] [PubMed] [Google Scholar]
- 42.Eng C, Kramer CK, Zinman B et al. . Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet 2014;384:2228–34. 10.1016/S0140-6736(14)61335-0 [DOI] [PubMed] [Google Scholar]
- 43.No authors listed]. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998;317:703–13. 10.1136/bmj.317.7160.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holman RR, Paul SK, Bethel MA et al. . Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med 2008;359:1565–76. 10.1056/NEJMoa0806359 [DOI] [PubMed] [Google Scholar]
- 45.Brenner BM, Cooper ME, de Zeeuw D et al. . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–9. 10.1056/NEJMoa011161 [DOI] [PubMed] [Google Scholar]
- 46.Lewis EJ, Hunsicker LG, Clarke WR et al. . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–60. 10.1056/NEJMoa011303 [DOI] [PubMed] [Google Scholar]
- 47.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981–97. 10.1001/jama.288.23.2981 [DOI] [PubMed] [Google Scholar]
- 48.Chobanian AV, Bakris GL, Black HR et al. . The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72. 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 49.Kearney PM, Blackwell L, Collins R et al. . Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–25. 10.1016/S0140-6736(08)60104-X [DOI] [PubMed] [Google Scholar]
- 50.Shaw JE, Punjabi NM, Wilding JP et al. . Sleep-disordered breathing and type 2 diabetes: a report from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Diabetes Res Clin Pract 2008;81:2–12. 10.1016/j.diabres.2008.04.025 [DOI] [PubMed] [Google Scholar]
- 51.Beck AT, Ward CH, Mendelson M et al. . An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–71. 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 52.McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med care 1993;31:247–63. 10.1097/00005650-199303000-00006 [DOI] [PubMed] [Google Scholar]
- 53.Eriksen L, Gronbaek M, Helge JW et al. . The Danish Health Examination Survey 2007–2008 (DANHES 2007–2008). Scand J Public Health 2011;39:203–11. 10.1177/1403494810393557 [DOI] [PubMed] [Google Scholar]
- 54.Mortensen EL, Flensborg-Madsen T, Molbo D et al. . Personality in late midlife: associations with demographic factors and cognitive ability. J Aging Health 2014;26:21–36. 10.1177/0898264313519317 [DOI] [PubMed] [Google Scholar]
- 55.Kolin EA, Price L, Zoob I. Development of a sensation-seeking scale. J Consult Psychol 1964;28:477–82. 10.1037/h0040995 [DOI] [PubMed] [Google Scholar]
- 56.Bland JM. The tyranny of power: is there a better way to calculate sample size? BMJ 2009;339:b3985 10.1136/bmj.b3985 [DOI] [PubMed] [Google Scholar]
- 57.Piaggio G, Elbourne DR, Pocock SJ et al. . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308:2594–604. 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- 58.Inzucchi SE, Bergenstal RM, Buse JB et al. . Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–96. 10.1007/s00125-012-2534-0 [DOI] [PubMed] [Google Scholar]
- 59.World Medical Association. World medical association declaration of helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 60.Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]