Abstract
Objectives
To assess whether depression and anxiety increase the risk of mortality and major adverse cardiovascular events (MACE), among patients with and without coronary artery disease (CAD).
Design and setting, and patients
DECADE (Depression Effects on Coronary Artery Disease Events) is a prospective observational study of 2390 patients referred at the Montreal Heart Institute. Patients were followed for 8.8 years, between 1998 and 2009. Depression and anxiety were assessed using a psychiatric interview (Primary Care Evaluation of Mental Disorders, PRIME-MD). Outcomes data were obtained from Quebec provincial databases.
Main outcome measures
All-cause mortality and MACE.
Results
After adjustment for covariates, patients with depression were at increased risks of all-cause mortality (relative risk (RR)=2.84; 95% CI 1.25 to 6.49) compared with patients without depression. Anxiety was not associated with increased mortality risks (RR=0.86; 95% CI 0.31 to 2.36). When patients were stratified according to CAD status, depression increased the risk of mortality among patients with no CAD (RR=4.39; 95% CI 1.12 to 17.21), but not among patients with CAD (RR=2.32; 95% CI 0.78 to 6.88). Neither depression nor anxiety was associated with MACE among patients with or without CAD.
Conclusions and relevance
Depression, but not anxiety, was an independent risk factor for all-cause mortality in patients without CAD. The present study contributes to a better understanding of the relative and unique role of depression versus anxiety among patients with versus without CAD.
Keywords: EPIDEMIOLOGY, MENTAL HEALTH
Strengths and limitations of this study.
To our knowledge, it is the first study to examine the relative and independent effect of depressive and anxiety disorders on the prospective risk of major adverse cardiovascular events and mortality among patients with and without coronary artery disease.
The use of a valid structured clinical interview to assess depressive and anxiety disorders constitutes another important strength. The large sample size (n=2390), the inclusion of both men and women, the inclusion of several important covariates in the analyses, the relatively long follow-up period (8.8 years), and the use of objective administrative database data to assess outcomes are other important strengths.
Body mass index (BMI) had to be imputed for about half of the sample, which could have biased our results. However, we conducted the statistical models a second time without BMI as a covariate, and we obtained the same pattern of results, suggesting that our results have not been influenced by this limitation.
Despite a high sample size, the low prevalence of the individual psychiatric disorders (eg, major depression, generalised anxiety disorder) prevented us from assessing their individual effect on outcomes, which would have been interesting and informative. Also, the low mortality rate in our sample could have resulted in a lack of power to detect differences in our stratified analyses.
This was a primarily male (67%) sample, and may therefore not be representative of the general population. However, the proportions of men and women in this study are consistent with referral statistics to nuclear exercise stress tests.
Introduction
Depressive disorders are the most common psychiatric disorders among patients with coronary artery disease (CAD).1 2 Consequently, a large number of studies have assessed the relationship between depressive symptoms and disorders and CAD. A meta-analysis revealed a pooled ORs of 2.25 (95% CI 1.73 to 2.93) for all-cause mortality, 2.71 (95% CI 1.68 to 4.36) for cardiac mortality, and 1.59 (95% CI 1.37 to 1.85) for cardiac events, associated with depressive symptoms or disorders among patients postmyocardial infarction (MI).3 Recently, results of a review of meta-analyses further supported the recommendation that the American Heart Association elevate depression to the status of an official risk factor for adverse medical outcomes in patients with acute coronary syndrome.4 Studies have also found increased risks of morbidity and mortality associated with depression among initially healthy participants. A meta-analysis assessing its impact on the development of CAD revealed a relative risk (RR) of 1.64 (95% CI 1.29 to 2.08) associated with depression.5 Though there is an extensive literature linking depression to poorer cardiac outcomes in both patients with CAD and healthy cohorts, most studies have relied on self-report questionnaires (eg, Beck Depression Inventory, BDI) to assess depressive symptom levels,6 7 rather than psychiatric interviews to assess clinical depression.
Anxiety disorders are also very common among patients with CAD, but less evidence exists regarding their association with adverse outcomes in these patients with established CAD. For example, a review by Suls and Bunde8 identified 14 studies examining the relationship between anxious symptoms or disorders and cardiac morbidity and mortality among patients with CAD. Of these studies, five demonstrated positive associations, while eight found no association and one reported an inverse relationship suggesting a protective effect of anxiety. Differently, results appear more consistent across studies assessing the association between anxiety and the development of CAD. A meta-analysis of 20 studies including 249 846 initially CAD-free participants suggested that anxiety is associated with an increased risk of incident CAD (HR=1.26, 95% CI 1.15 to 1.38) and cardiac mortality (HR=1.48, 95% CI 1.14 to 1.92).9 The authors also reported a non-significant trend for an association between anxiety and non-fatal MI (HR=1.43, 95% CI 0.85 to 2.40). The overall anxiety data might reflect some inconsistencies in the anxiety-CAD relative to the depression-CAD literature.
Surprisingly, few studies have assessed the relative and independent effects of depressive and anxiety disorders on CAD morbidity and mortality. This is important as depressive and anxiety disorders are highly comorbid (both in the general population10 11 and in patients with CAD12 13). Moreover, though the two disorders share many clinical characteristics (eg, sleep disturbances, concentration difficulties11), they can also present with very different patterns of symptoms (eg, passivity and lack of motivation in depression vs compulsive and/or exaggerated proactive behaviours in anxiety).11 It is therefore important to assess their relative effects on CAD morbidity and mortality. To our knowledge, no studies to date have assessed these associations in patients with and without CAD within the same cohort. This makes it difficult to tease apart the importance of psychiatric morbidity at different stages of the CAD process.
In this study, we aimed to assess the relative and independent associations between depressive and anxiety disorders and all-cause mortality and major adverse cardiovascular events (MACE), in a cohort of stable patients with and without established CAD, referred for nuclear medicine-based exercise stress testing. We hypothesised that patients with a depressive or an anxiety disorder would be at increased risk of all-cause mortality and MACE, compared with patients with no depressive or anxiety disorder. Furthermore, we hypothesised that patients with and without CAD with a depressive or an anxiety disorder would be at increased risk of all-cause mortality and MACE, and that the relationship would be stronger among the CAD group compared with the non-CAD group.
Methods
Population
Patients referred for a single-photon emission CT (SPECT) exercise stress test between September 1998 and June 2002 in the Department of Nuclear Medicine of the Montreal Heart Institute (MHI) were approached to take part in this study. A total of 2460 patients agreed to participate in the study. Patients were eligible if they were between 18 and 75 years of age and could speak English or French. Patients were excluded if they had a medical condition that conferred a greater chance of morbidity or mortality than CAD (eg, cancer) or a documented cardiac event (eg, MI) within the past 6 weeks. Written informed consent was obtained from all participants.
Procedure
At baseline, patients were invited to participate in this study after completing their exercise testing. SPECT imaging was completed approximately 45 min after the end of the stress test. Immediately following SPECT imaging, patients were met by a research assistant who conducted a psychiatric, sociodemographic, and medical history interview.
For the follow-up measures, documented medical data covering the period between recruitment until 31 December 2008 were obtained from the Régie de l'assurance maladie du Québec (RAMQ), and included details and dates of all billable medical events occurring in the province. The RAMQ is the governmental institution responsible for the management of the Quebec public healthcare system and public medication insurance programme. Official mortality data over the same period was obtained from the Institut de la statistique du Québec, the governmental institution which is in charge of the recording and dissemination of Quebec's demographic and economic statistical data, including date and cause of death.
Baseline assessment of myocardial ischaemia
All participants underwent nuclear (SPECT) exercise stress testing (treadmill, Bruce protocol) according to standard procedures.14 As such, patients were on continuous ECG recording while exercising, using a standard 12-lead ECG configuration (Marquette Medical Systems Inc, Milwaukee, Wisconsin, USA), and a radioisotope (Technetium (99mTc) sestamibi) was injected when patients reached maximal exercise capacity. All patients exercised until exertion or until a technician terminated the test for clinical reasons (eg, significant ECG ischemia, arrhythmia). Patients underwent SPECT imaging 45 min after the completion of their treadmill test. A positive SPECT (Irix-3 model, Philips, Inc, Cleveland, Ohio, USA) diagnosis of myocardial ischaemia was defined as a two point change in the stress-rest differential score, independent of any underlying myocardial necrosis,14 using standard software (Autoquant, Media Cybernetics, Gale Group, Framingtion Hill, Michigan, USA). All diagnoses were made in the clinical setting by qualified nuclear medicine physicians.
Baseline assessment of risk factors
Data regarding medications, cigarette smoking, diabetes, hypertension, dyslipidaemia, body mass index (BMI) and history of CAD were collected through a sociodemographic and medical interview. Participants reporting current or history of cigarette smoking were categorised as smokers. History of CAD was determined by patients’ self-report of a previous MI, percutaneous coronary intervention or coronary artery bypass graft (CABG).
Baseline psychiatric assessment
Depressive and anxiety disorders were assessed at baseline using the Primary Care Evaluation of Mental Disorders (PRIME-MD).15 The PRIME-MD is a semistructured psychiatric interview which was developed to identify the most common Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) disorders seen in primary and tertiary care settings. Validation studies reported a κ value of 0.71, a specificity of 88% and a sensitivity of 83% for the depression module of the PRIME-MD, which is comparable to longer interviews such as the Structured Clinical Interview for DSM-IV (SCID).15 16
Follow-up outcomes
MACE were categorised according to the standard protocol published by the WHO,17 and included cardiac mortality, non-fatal MI, revascularisation procedures (percutaneous transluminal coronary angioplasty (PTCA) and CABG), and cerebrovascular events (ie, stroke). See online appendix 1 for a complete list of events (excluding cardiac mortality) and procedures coding classification according to International Classification of Diseases (ICD)-9 (before 1 April 2006), ICD-10 (from 1 April 2006) and the Manuel des médecins omnipraticiens et spécialistes (for intervention procedures). Cause of death was coded according to the ICD system. See online appendix 2 for the list of cardiac primary causes of death observed within the present sample (which were included in the definition of MACE).
Data reduction and statistical analyses
All data were analysed using SAS V.9.2 (SAS Institute, Cary, North Carolina, USA). Of the 2460 participants initially recruited, 70 were lost to follow-up due to missing data in the matching process (eg, absence of social insurance number or birth date) with the RAMQ databases, resulting in a final sample of 2390 patients. One-way general linear models and χ2 analyses were used to compare baseline sociodemographic and clinical characteristics of patients with and without depressive and anxiety disorders, and with and without CAD. Since height and weight were collected among the second half of the sample only, BMI could not be calculated for 66% of the sample. As data were missing for some variables (see table 1 for details), multiple imputation procedures18 were performed to estimate missing values. This procedure creates five data sets for each variable containing missing data. More specifically, it uses all other available data to calculate plausible values representing the uncertainty about the right data to impute. Each of these imputed data sets is then analysed using standard procedures for complete data, and the final results come from the combination of these five analyses, which is obtained through the SAS PROC MIANALYZE statement.
Table 1.
Baseline characteristics as a function of psychiatric groups and CAD status
| Per cent (n) | Depressive disorder n=572 |
No depressive disorder n=1818 |
Anxiety disorder n=476 |
No anxiety disorder n=1914 |
CAD n=917 |
No CAD n=1295 |
Missing data (n) |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age, years (mean±SD) | 57±8.52 | 56±7.86 | 55±8.98 | 54±8.06 | 56±8.52 | 54±8.41 | 8* |
| Female | 41 (235)† | 30 (545) | 43 (88)‡ | 30 (574) | 16 (147)§ | 45 (583) | 9* |
| Caucasian | 99 (566) | 99 (1800) | 99 (226) | 99 (1895) | 99 (908) | 99 (1282) | 183 |
| Postsecondary education | 57 (326) | 59 (1073) | 52 (115)‡ | 60 (1148) | 56 (514) | 60 (777) | 1340 |
| Cohabitating | 64 (366)† | 73 (1327) | 67 (66) | 72 (1378) | 72 (660) | 70 (907) | 1340 |
| Medical history | |||||||
| Smoking, past or current | 71 (406) | 70 (1273) | 72 (160) | 70 (1340) | 81 (743)§ | 62 (803) | 335* |
| BMI (mean±SD) | 27±4.83 | 27±4.31 | 28±4.46 | 27±4.67 | 28±4.61 | 27±4.89 | 1577* |
| Hypertension | 44 (252) | 43 (113) | 43 (782) | 44 (842) | 49 (449)§ | 40 (518) | 185* |
| Dyslipidaemia | 64 (366)† | 58 (157) | 58 (1054) | 59 (1129) | 79 (724)§ | 45 (583) | 184* |
| Diabetes | 11 (63) | 11 (33) | 9 (164) | 11 (211) | 14 (128)§ | 8 (104) | 185* |
| History of CAD | 41 (235) | 41 (124) | 36 (654)‡ | 43 (823) | NA | NA | 178* |
| Exercise-induced ischaemia | 40 (229) | 41 (124) | 38 (691) | 41 (785) | 59 (541)§ | 29 (376) | 65* |
| Medications | |||||||
| ACE inhibitors | 15 (86) | 17 (41) | 15 (1273) | 17 (325) | 24 (220)§ | 12 (155) | 182 |
| Vasodilators | 32 (183) | 31 (88) | 34 (618) | 30 (574) | 47 (431)§ | 19 (246) | 182 |
| β-blockers | 35 (200) | 36 (110) | 34 (618) | 36 (689) | 53 (486)§ | 24 (311) | 182 |
| Diuretics | 6 (34) | 8 (14) | 7 (127) | 8 (153) | 8 (73) | 7 (91) | 182 |
| Any anti-BP medication | 54 (309) | 58 (154) | 54 (982) | 56 (1072) | 74 (679)§ | 42 (544) | 182 |
| Ca-channel blockers | 20 (114) | 18 (55) | 18 (327) | 18 (345) | 25 (229)§ | 14 (181) | 181 |
| Any lipid-lowering medications | 52 (297) | 48 (149) | 44 (800)‡ | 50 (957) | 75 (688)§ | 31 (401) | 181 |
| Any anti-ischaemic | 57 (326) | 55 (160) | 57 (1036) | 55 (1053) | 77 (706)§ | 40 (518) | 185 |
| Aspirin | 48 (275)† | 54 (149) | 48 (873)‡ | 54 (1034) | 80 (734)§ | 34 (440) | 185 |
| Antidiabetic | 8 (46) | 8 (25) | 7 (127) | 8 (153) | 11 (101)§ | 6 (78) | 184 |
*Variables included in the multiple imputation procedure.
†Significantly different from the ‘no depressive disorder’ group.
‡Significantly different from the ‘no anxiety disorder’ group.
§Significantly different from the ‘no CAD’ group.
BMI, body mass index; BP, blood pressure; CAD, coronary artery disease.
Cox regression models were used to assess the main effect of depressive and anxiety disorders on time to first event. For each of the main outcome variables (all-cause mortality or MACE), three models were computed: model 1 included the presence of depressive disorders and the presence of anxiety disorders as separate independent variables, and was conducted using the whole sample of patients (ie, 2390 patients with and without a history of CAD). Model 2 also included depressive and anxiety disorders as the independent variables, but was conducted among patients only with CAD, while model 3 tested the effects of depressive and anxiety disorders among patients with no CAD. The first occurrence of the outcome variable was taken as the event for analysis. Owing to the known association of the following variables with CAD,19 all regression models were adjusted for baseline age, sex, smoking status, history of CAD (in model 1 only), diabetes, hypertension, dyslipidaemia, SPECT evidence of exercise-induced myocardial ischaemia and BMI. All covariates were determined a priori as recommended.20
Results
Sociodemographic and clinical characteristics
The sample was composed of 789 (33%) female and the mean (SD) age at baseline was 57 (SD=9) years. Participants were followed for a mean (SD) period of 8.8 (1.3) years (range from 1.0 to 10.1). In total, 721 (30%) patients had at least one MACE during the follow-up period, and 165 (7%) cases of mortality were observed (see figure 1). A total of 48% of all deaths were due to cancer and 32% were due to cardiac causes (table 1).
Figure 1.
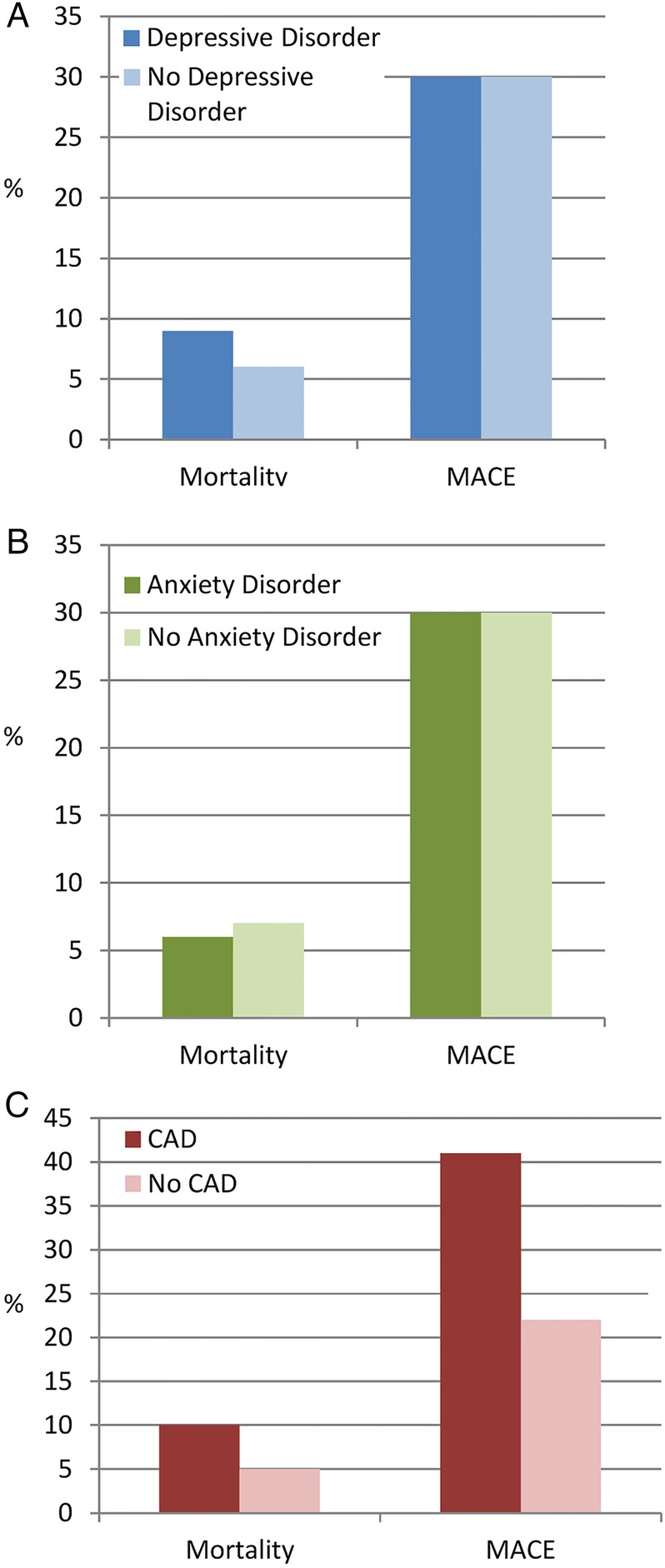
Raw proportions of all-cause mortality and major adverse cardiovascular events (MACE), according to depressive disorders, anxiety disorders and cardiovascular event (CAD) status.
All-cause mortality
Main effects of depressive and anxious disorders: whole sample
When adjusting for covariates, depressive disorders, but not anxiety disorders, were associated with all-cause mortality, such that patients who suffered from a depressive disorder at baseline were nearly three times more likely to die compared with patients with no depressive disorder at baseline (table 2).
Table 2.
Main effects of depressive and anxiety disorders on all-cause mortality and MACE
| Variables main effect | All-cause mortality* |
MACE* |
||
|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | |
| Depressive disorders | ||||
| All | 2.84 | 1.25 to 6.49 | 1.03 | 0.68 to 1.58 |
| Non-CAD | 4.39 | 1.12 to 17.21 | 1.44 | 0.73 to 2.82 |
| CAD | 2.32 | 0.78 to 6.88 | 0.88 | 0.49 to 1.59 |
| Anxiety disorders | ||||
| All | 0.86 | 0.81 to 2.36 | 1.44 | 0.91 to 2.27 |
| Non-CAD | 0.61 | 0.13 to 3.04 | 1.25 | 0.62 to 2.51 |
| CAD | 0.95 | 0.25 to 3.69 | 1.62 | 0.88 to 3.00 |
*Adjusted for age, sex, smoking, history of CAD, diabetes, hypertension, dyslipidaemia, exercise-induced myocardial ischaemia and BMI.
BMI, body mass index; CAD, coronary artery disease; MACE, major adverse cardiovascular events; RR, relative risk.
Main effects of depressive and anxiety disorders: stratified by CAD status
Our results revealed an association between depressive disorders and all-cause mortality in the non-CAD group only. Specifically, depressed patients without CAD were more than four times more at risk of dying than non-depressed patients without CAD. However, patients with CAD who also had a depressive disorder were not at increased risk of mortality when compared with their non-depressed counterparts (figure 2). Finally, there was no association between anxiety disorders and all-cause mortality among either the CAD or the non-CAD group.
Figure 2.
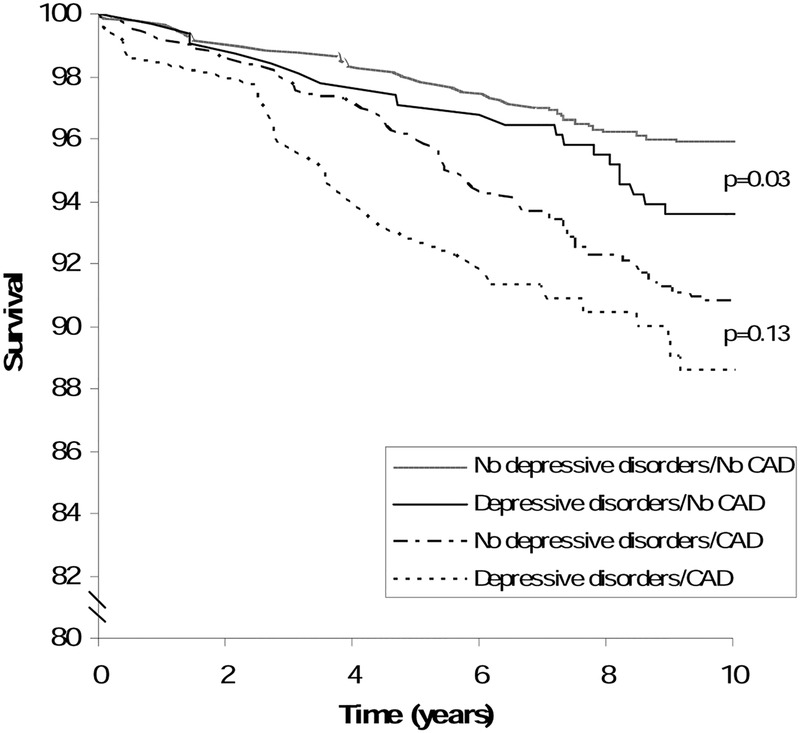
Survival curves in depressed and non-depressed patients with and without cardiovascular event (CAD).
Sensitivity analyses: the association between depressive and anxiety disorders, and specific causes of death
In order to better understand how depressive and anxiety disorders influence the risk of mortality, we conducted sensitivity analyses assessing the association between depressive and anxiety disorders, and cancer and CVD deaths separately. Results of this analysis yielded no significant association between depressive (adjusted RR=2.30; 95% CI 0.58 to 10.00) or anxiety disorders (adjusted RR=0.26; 95% CI 0.03 to 2.03) on CVD mortality. On the other hand, results suggested a trend for an association between depressive disorders and cancer deaths (adjusted RR=3.06; 95% CI 0.90 to 10.39), but no association between anxiety disorders and cancer deaths (adjusted RR=1.06; 95% CI 0.25 to 4.52).
Major adverse cardiovascular events
Main effects of depressive and anxiety disorders: whole sample
Results suggested no association between depressive disorders and the risk of MACE in the whole sample. On the other hand, there was a non-statistically significant trend for an increased risk of MACE among patients with anxiety disorders (table 2).
Main effects of depressive and anxiety disorders: stratified by history of CAD
Our results revealed non-statistically significant trends for associations between depressive disorders and MACE in the non-CAD group, and between anxiety disorders and MACE in both the CAD and the non-CAD groups (table 2).
Discussion
Results of the present study partially confirmed our hypotheses by indicating that patients with a depressive, but not anxiety disorder, were at an increased risk of all-cause mortality. When our sample was stratified according to CAD status, we also observed that depressive disorders remained associated with mortality, but in patients without CAD only. Of note, these relationships were independent of traditional CAD risk factors, as well as anxiety disorders. These results are consistent with others who have found symptoms of depression (rather than disorders), but not anxiety, to be associated with an increased risk of all-cause mortality among patients with heart failure and among patients referred for an exercise stress test.21–23
The implications of our results are that depression and anxiety may act differently on health-related factors associated with mortality. Although both disorders have been associated with some similar behavioural and physiological mechanisms that may increase the risk of mortality (eg, increased cigarette smoking24 25 and alcohol consumption,26 27 impaired hypothalamic–pituitary–adrenal (HPA) axis function28 29), these disorders have also been found to differ according to some other important health factors. For example, a meta-analysis on the effects of anxiety and depression on patient adherence to medical treatment30 found the associations between anxiety and non-adherence with medical regimens to have small and non-significant effect sizes. In contrast, the relationships between depression and non-adherence were significant and considerable. Compared with depressed patients, individuals with anxiety disorders might be more hypervigilant and proactive regarding their health, possibly because they are known to be more sensitive to even small bodily changes.11 31 This hypervigilence might bring patients to consult their physicians more often and to better follow their recommendations. These protective behaviours may counterbalance some adverse effects of other known mechanisms associated with anxiety, such as sympathetic hyperactivity and platelet aggregation.32
Consistent with results for the entire sample, there was no effect of anxiety on the risk of mortality in patients with or without CAD. However, depressive disorders were predictive of mortality, but only among patients with no CAD. The explanations for this unexpected finding are unclear. It is possible that CAD itself confers more important physiological consequences than depressive disorders, and would therefore explain the risk of mortality better than depression among patients who already have confirmed CAD. It is also possible that this result may be driven by cause of death in patients with and without CAD. Indeed, patients with CAD may have been more likely than the non-CAD to die from cardiovascular than from other causes such as cancer, and cardiovascular deaths were not associated with depression in sensitivity analyses. In order to test this hypothesis, we conducted an exploratory analysis, and found that patients with CAD died more often from cardiovascular diseases then from other causes, when compared with patients without CAD (F=13.87, p<0.001). This could explain the absence of significant association between depressive disorders and mortality among patients with a history of CAD.
Finally, it is possible that the failure to find an association between depression and mortality in patients with CAD is due to differences in the populations and recruitment centres. For example, our sample was recruited from the MHI, which is a highly specialised tertiary care cardiology centre. For example, this cardiology hospital includes a Department of Psychosomatic Medicine, whose mission is to ‘provide medical and psychiatric expertise to help doctors diagnose and treat mental symptoms that may appear during a hospital stay or as a result of surgery or that may be detected during a consultation in an outpatient clinic’. The MHI's treatment and referral practices, for both cardiovascular disease and depressive disorders, might therefore differ from those of other studies who have recruited from general hospital settings.33 34 This may have improved patient's psychological and cardiovascular prognoses and study outcomes. This difference could also explain the lower mortality rate observed in our study, versus rates observed in previous studies.22 33 34
In contrast to patients with CAD, depressive disorders in patients with no CAD were predictive of worse mortality, which may be explained by many of the well-documented mechanisms in the previous literature. Depression's association with decreased motivation and compensatory behaviours11 has been linked to higher rates of poor health behaviours (eg, non-adherence to medical regimens,30 increased cigarette smoking35 36 and alcohol consumption,37 poor diet and physical inactivity38), which have all been associated with higher rates of chronic disease mortality.39–42 Depression has also been associated with dysregulated physiological processes (eg, impaired HPA axis function,29 43 endothelial dysfunction,44 reduced heart rate variability45 and increased inflammatory processes such as increased levels of interleukin-646 47), which are also associated with many fatal chronic diseases.48 49
Our results further suggested non-statistically significant trends for associations between depressive disorders and MACE in the non-CAD group, and between anxiety disorders and MACE in both the CAD and the non-CAD groups. It is possible that with a larger sample size and/or more events, these associations may become statistically significant. As suggested above, the non-statistically significant finding for an association between anxiety disorders and risk for MACE may be explained through hypervigilence to changes in bodily symptoms and subsequent proactive behaviours (eg, more frequent medical visits,50 better adherence to medical regimens51). These behaviours may result in earlier detection and treatment of symptoms, which may alter the adverse physiological effect of anxiety and lower the strength of the association between anxiety disorders and MACE. It is noteworthy that these results for anxiety disorders are partly consistent with results reported in the meta-analysis from Roest et al.9 In this meta-analysis, a non-significant trend is reported for the association between anxiety and non-fatal MI. The HR and 95% CI reported in Roest study (HR=1.43, 95% CI 0.85 to 2.40) is also very similar to what we observed in the present study (HR=1.44, 95% CI 0.91 to 2.27).
On the other hand, the reasons for the non-significant relationship between depressive disorders and MACE, especially among patients with CAD, are unclear, and contradict most previous studies. As hypothesised above, it is possible that the highly specialised tertiary cardiology care setting of our study explains the discrepancy between our results and those of previous studies. Nonetheless, our results are consistent with those reported by Welin et al,52 who found no increase in the risk of non-fatal MI among patients with depressive or anxious symptoms, while having reported a main effect of depressive symptoms only on all-cause mortality. Interestingly, the 10-year follow-up period in Welin's study is very close to our 9-year follow-up, and differs from the average 2-year period of most previous studies.3 It is possible that depression and/or anxiety adversely affect non-fatal prognosis early after MI, rather than having a long-term effect. In that case, the association between depression and non-fatal events would be minimised in studies including a long follow-up period. Clearly, further work is needed to disentangle these research findings.
The absence of an association between depressive disorders and the incidence of MACE, in spite of a main effect on the risk of all-cause mortality, seems to suggest that depressive disorders might represent one core mechanism underlying a large and diverse set of health risks, rather than representing a risk factor for a specific health condition such as CAD. This is further supported by our sensitivity analyses where the effect of depressive disorders did not reach significance for specific causes of death. This is also consistent with Sun et al,53 who reported an effect of depressive symptoms on all-cause mortality, but not on specific causes of death such as ischaemic heart disease, stroke or cancer.
Study limitations and strengths
This study contains methodological limitations. First, by design, this study does not infer causality, and there could be unknown factors that were not adjusted for that could have skewed our results. Second, the study population consists of patients referred for a nuclear medicine-based stress test, in which CAD was suspected or followed up. This population is therefore neither completely representative of the general population nor of patients with established CAD, which affects the generalisation of the results. Third, it is possible that the 9-year follow-up period was not long enough to capture a sufficient number of events and to further observe increased mortality due to depression. Fourth, BMI had to be imputed for about half of the sample, which could have biased our results. However, we conducted the statistical models a second time without BMI as a covariate, and we obtained the same pattern of results, suggesting that our results have not been influenced by this limitation. Fifth, we did not assess treatment for depressive and anxiety disorders nor the evolution of the disorders over the follow-up period. The attenuation of symptoms, or the complete psychological remission in some patients may have influenced the adverse effect of depressive and anxiety disorders and moderated our results. Finally, this was a primarily male (67%) sample, and may therefore not be representative of the general population. However, the proportions of men and women in this study are consistent with referral statistics to nuclear exercise stress tests.34 54
Despite the above limitations, this study has several strengths. To our knowledge, it is the first study to examine the relative and independent effect of depressive and anxiety disorders on the prospective risk of MACE and mortality among patients with and without CAD. The use of a valid structured clinical interview to assess depressive and anxiety disorders constitutes another important strength. The large sample size (n=2390), the inclusion of both men and women, the inclusion of several important covariates in the analyses, the relatively long follow-up period (8.8 years), and the use of objective administrative database data to assess outcomes are other important strengths.
Conclusion
Results of the present study suggest that patients suffering from depressive but not anxiety disorders are almost three times more likely to die over a nearly 9-year follow-up period, compared with patients without depressive disorders. When considering patients with and without CAD separately, depression appears to increase the risk of death in non-CAD only. We also found no conclusive evidence of an increased risk of MACE among patients with depressive or anxiety disorders, regardless of their CAD status. Overall, our results highlight the importance of depressive disorders as a risk factor for all-cause mortality, and therefore stress the importance of optimal screening, referral and treatment practices regarding depressive disorders among the general population, and particularly among patients who may be at risk for CAD.
Footnotes
Contributors: KLL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. RP, KLL, SLB, AA, JD, CL and LB were involved in study concept and design. KLL, CL, SLB, AA and RP were involved in acquisition of data. RP, KLL, SLB, AA, JD and CL were involved in analysis and interpretation of data. RP was involved in drafting of the manuscript. RP, KLL, SLB, AA, JD, CL and LB were involved in revision and edition of the manuscript.
Funding: The authors acknowledge grant support from the Canadian Institutes of Health Research (CIHR: MOP 79445 and 89965, KLL and SLB) and the Heart and Stroke Foundation of Canada, salary awards from the Fonds de la recherche en santé du Québec (FRSQ: KLL and SLB) and the Canadian Institutes of Health Research (CIHR: SLB), and scholarship support from the Social Sciences and Humanities Research Counsel (SSHRC: RP) and Canadian Institutes of Health Research (CIHR: RP) and the Fonds de la recherche en santé du Québec (FRSQ: RP and CL).
Competing interests: KLL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethics approval: REB of the Montreal Heart Institute.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All patients’ reasons for hospitalisation and diagnoses, as well as cause of death and dates between 1998 and 2008 are available to KLL.
References
- 1.Guilmour H. Statistics Canada. Depression and risk of heart disease. Health Reports 2008;19:1–11. [PubMed] [Google Scholar]
- 2.Thombs BD, Bass EB, Ford DE et al. Prevalence of depression in survivors of acute myocardial infarction: review of the evidence. J Gen Intern Med 2006;21:30–8. 10.1111/j.1525-1497.2005.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meijer A, Conradi HJ, Bos EH et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry 2011;33:203–16. 10.1016/j.genhosppsych.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 4.Lichtman JH, Froelicher ES, Blumenthal JA et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350–69. 10.1161/CIR.0000000000000019 [DOI] [PubMed] [Google Scholar]
- 5.Rugulies R. Depression as a predictor for coronary heart disease: a review and meta-analysis. Am J Prev Med 2002;23:51 10.1016/S0749-3797(02)00439-7 [DOI] [PubMed] [Google Scholar]
- 6.Frasure-Smith N, Lesperance F. Depression and other psychological risks following myocardial infarction. Arch Gen Psychiatry 2003;60:627–36. 10.1001/archpsyc.60.6.627 [DOI] [PubMed] [Google Scholar]
- 7.Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation 1996;93:1976–80. 10.1161/01.CIR.93.11.1976 [DOI] [PubMed] [Google Scholar]
- 8.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psych Bull 2005;131:260–300. 10.1037/0033-2909.131.2.260 [DOI] [PubMed] [Google Scholar]
- 9.Roest AM, Martens EJ, de Jonge P et al. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol 2010;56:38–46. 10.1016/j.jacc.2010.03.034 [DOI] [PubMed] [Google Scholar]
- 10.Kessler RC, Nelson CB, McGonagle KA et al. Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US National Comorbidity Survey. Br J Psychiatry Suppl 1996;30:17–30. [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th edn Washington DC: American Psychiatric Press, 1994. [Google Scholar]
- 12.Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry 2007;29:147–55. 10.1016/j.genhosppsych.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 13.Aina Y, Susman JL. Understanding comorbidity with depression and anxiety disorders. J Am Osteopath Assoc 2006;106(5 suppl 2):S9–14. [PubMed] [Google Scholar]
- 14.Anagnostopoulos C, Harbinson M, Kelion A et al. Procedure guidelines for radionuclide myocardial perfusion imaging. Heart 2004;90(Suppl 1):i1–10. 10.1136/heart.90.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spitzer RL, Williams JB, Kroenke K et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994;272:1749–56. 10.1001/jama.1994.03520220043029 [DOI] [PubMed] [Google Scholar]
- 16.Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD: the PHQ Primary Care Study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737–44. 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- 17.Luepker RV, Evans A, McKeiguw P et al. Cardiovascular survey methods. 3rd edn Geneva: World Health Organisation, 2004. [Google Scholar]
- 18.Yuan YC. Multiple Imputation for Missing Data: Concepts and New Development (Version 9.0). SAS Institute Inc,2010:1–13.
- 19.Rosengren A, Hawken S, Ounpuu S et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:953–62. 10.1016/S0140-6736(04)17019-0 [DOI] [PubMed] [Google Scholar]
- 20.Altman DG, Schulz KF, Moher D, CONSORT GROUP (Consolidated Standards of Reporting Trials). The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001;134:663–94. 10.7326/0003-4819-134-8-200104170-00012 [DOI] [PubMed] [Google Scholar]
- 21.Jiang W, Kuchibhatla M, Cuffe MS et al. Prognostic value of anxiety and depression in patients with chronic heart failure. Circulation 2004;110:3452–6. 10.1161/01.CIR.0000148138.25157.F9 [DOI] [PubMed] [Google Scholar]
- 22.Herrmann C, Brand-Driehorst S, Buss U et al. Effects of anxiety and depression on 5-year mortality in 5057 patients referred for exercise testing. J Psychosom Res 2000;48:455–62. 10.1016/S0022-3999(99)00086-0 [DOI] [PubMed] [Google Scholar]
- 23.Friedmann E, Thomas SA, Liu F et al. Relationship of depression, anxiety, and social isolation to chronic heart failure outpatient mortality. Am Heart J 2006;152:940.e1–8. 10.1016/j.ahj.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 24.Almeida OP, Pfaff JJ. Depression and smoking amongst older general practice patients. J Affect Disord 2005;86:317–21. 10.1016/j.jad.2005.02.014 [DOI] [PubMed] [Google Scholar]
- 25.Lawrence D, Considine J, Mitrou F et al. Anxiety disorders and cigarette smoking: results from the Australian Survey of Mental Health and Wellbeing. Aust N Z J Psychiatry 2010;44:520–7. 10.3109/00048670903571580 [DOI] [PubMed] [Google Scholar]
- 26.Saban A, Flisher AJ. The association between psychopathology and substance use in young people: a review of the literature. J Psychoactive Drugs 2010;42:37–47. 10.1080/02791072.2010.10399784 [DOI] [PubMed] [Google Scholar]
- 27.Lukassen J, Beaudet MP. Alcohol dependence and depression among heavy drinkers in Canada. Soc Sci Med 2005;61:1658–67. 10.1016/j.socscimed.2005.03.019 [DOI] [PubMed] [Google Scholar]
- 28.Cameron OG, Abelson JL, Young EA. Anxious and depressive disorders and their comorbidity: effect on central nervous system noradrenergic function. Biol Psychiatry 2004;56:875–83. 10.1016/j.biopsych.2004.08.007 [DOI] [PubMed] [Google Scholar]
- 29.Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am 1998;21(2):293–307. 10.1016/S0193-953X(05)70006-X [DOI] [PubMed] [Google Scholar]
- 30.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101–7. 10.1001/archinte.160.14.2101 [DOI] [PubMed] [Google Scholar]
- 31.Hoehn-Saric R, McLeod DR, Funderburk F et al. Somatic symptoms and physiologic responses in generalized anxiety disorder and panic disorder: an ambulatory monitor study. Arch Gen Psychiatry 2004;61:913–21. 10.1001/archpsyc.61.9.913 [DOI] [PubMed] [Google Scholar]
- 32.Zafar MU, Paz-Yepes M, Shimbo D et al. Anxiety is a better predictor of platelet reactivity in coronary artery disease patients than depression. Eur Heart J 2010;31:1573–82. 10.1093/eurheartj/ehp602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irvine J, Basinski A, Baker B et al. Depression and risk of sudden cardiac death after acute myocardial infarction: testing for the confounding effects of fatigue. Psychosom Med 1999;61:729–37. 10.1097/00006842-199911000-00001 [DOI] [PubMed] [Google Scholar]
- 34.Carney RM, Blumenthal JA, Catellier D et al. Depression as a risk factor for mortality after acute myocardial infarction. Am J Cardiol 2003;92:1277–81. 10.1016/j.amjcard.2003.08.007 [DOI] [PubMed] [Google Scholar]
- 35.Ellis EM, Orom H, Giovino GA et al. Relations between negative affect and health behaviors by race/ethnicity: differential effects for symptoms of depression and anxiety. Health Psychol 2015;34:966–9. 10.1037/hea0000197 [DOI] [PubMed] [Google Scholar]
- 36.Escobedo LG, Kirch DG, Anda RF. Depression and smoking initiation among us Latinos. Addiction 1996;91:113–19. 10.1111/j.1360-0443.1996.tb03166.x [DOI] [PubMed] [Google Scholar]
- 37.Dixit AR, Crum RM. Prospective study of depression and the risk of heavy alcohol use in women. Am J Psychiatry 2000;157:751–8. 10.1176/appi.ajp.157.5.751 [DOI] [PubMed] [Google Scholar]
- 38.Goodman E, Whitaker RC. A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics 2002;110:497–504. 10.1542/peds.110.3.497 [DOI] [PubMed] [Google Scholar]
- 39.Hershman DL, Shao T, Kushi LH et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 2011;126:529–37. 10.1007/s10549-010-1132-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farrell SW, Finley CE, McAuley PA et al. Cardiorespiratory fitness, different measures of adiposity, and total cancer mortality in women. Obesity (Silver Spring) 2011;19:2261–7. 10.1038/oby.2010.345 [DOI] [PubMed] [Google Scholar]
- 41.Paganini-Hill A. Lifestyle practices and cardiovascular disease mortality in the elderly: the leisure world cohort study. Cardiol Res Pract 2011;2011:983764 10.4061/2011/983764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neblett RC, Hutton HE, Lau B et al. Alcohol consumption among HIV-infected women: impact on time to antiretroviral therapy and survival. J Womens Health 2011;20:279–86. 10.1089/jwh.2010.2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heidt T, Sager HB, Courties G et al. Chronic variable stress activates hematopoietic stem cells. Nat Med 2014;20:754–8. 10.1038/nm.3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavoie KL, Pelletier R, Arsenault A et al. Association between clinical depression and endothelial function measured by forearm hyperemic reactivity. Psychosom Med 2010;72:20–6. 10.1097/PSY.0b013e3181c2d6b8 [DOI] [PubMed] [Google Scholar]
- 45.Gehi AM, Dennis MD, Pipkin S et al. Depression and heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry 2005;62:661–6. 10.1001/archpsyc.62.6.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bremmer MA, Beekman AT, Deeg DJ et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord 2008;106:249–55. 10.1016/j.jad.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 47.Empana JP, Sykes DH, Luc G et al. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME). Circulation 2005;111:2299–305. 10.1161/01.CIR.0000164203.54111.AE [DOI] [PubMed] [Google Scholar]
- 48.Jokinen J, Nordström P. HPA axis hyperactivity and cardiovascular mortality in mood disorder inpatients. J Affect Disord 2009;116(1–2):88–92. 10.1016/j.jad.2008.10.025 [DOI] [PubMed] [Google Scholar]
- 49.Noori N, Kovesdy CP, Dukkipati R et al. Racial and ethnic differences in mortality of hemodialysis patients: role of dietary and nutritional status and inflammation. Am J Nephrol 2011;33:157–67. 10.1159/000323972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katon WJ, VonKorff M, Lin E. Panic disorder: relationship to high medical utilization. Am J Med 1992;92:7S–11S. 10.1016/0002-9343(92)90130-4 [DOI] [PubMed] [Google Scholar]
- 51.Kim HK, Park JH, Park JH et al. Differences in adherence to antihypertensive medication regimens according to psychiatric diagnosis: results of a Korean population-based study. Psychosom Med 2010;72:80–7. 10.1097/PSY.0b013e3181c4e3e9 [DOI] [PubMed] [Google Scholar]
- 52.Welin C, Lappas G, Wilhelmsen L. Independent importance of psychosocial factors for prognosis after myocardial infarction. J Intern Med 2000;247:629–39. 10.1046/j.1365-2796.2000.00694.x [DOI] [PubMed] [Google Scholar]
- 53.Sun W, Schooling M, Chan WM et al. The association between depressive symptoms and mortality among Chinese Elderly: a Hong Kong cohort study. J Gerontol A Biol Sci Med Sci 2011;66:459–66. 10.1093/gerona/glq206 [DOI] [PubMed] [Google Scholar]
- 54.Yusuf S, Hawken S, Ounpuu S et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52. 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]


