Abstract:
The purpose of this study was to investigate the clinical outcome, inflammatory response and myocardial function in high-risk patients undergoing three different leukocyte depletion strategies. Over a four-month period, forty patients (EuroSCORE 6+) undergoing coronary revascularization were prospectively randomized to one of the four perfusion protocols: Group 1 (N = 10): Conventional circuits (ECC) + two leukocyte filters (LG6B, Pall, USA) with the method of two-phase (continuous + strategic) leukofiltration; Group 2 (N = 10): ECC + single leukocyte filter with the method of continuous leukofiltration; Group 3 (N = 10): ECC + single leukocyte filter with the method of strategic leukofiltration; Group 4 (N = 10) Control: ECC without leukocyte filtration. Blood samples were collected at T1: Baseline, T2: On CPB, T3: X-Clamp, T4: Off CPB, T5: ICU24 and T6: ICU48. Perioperative follow-up was thoroughly monitored. Leukocyte counts in double filter and strategic filtration groups demonstrated significant differences at T4 (p < .05 vs. control). TNF-alpha levels were significantly lower in Group 1 at T4 and procalcitonin levels at T5 and T6 (p < .05 vs. control). CKMB levels demonstrated well preserved myocardium in double filter group (p < .05 vs. control). Brain natriuretic peptide levels in double filter group were significantly lower at T5 and T6 with respect to Group 2 (p < .05) and control (p < .001). Matrixmetallopeptidase 9 and D-Dimer levels in double filter group were significantly lower at T5 and T6 (p < .05 vs. control). Two-phase leukofiltration is associated with some compound benefit over continuous deployment in high-risk patients. A larger more powerful study than this pilot one is warranted for further evaluation.
Keywords: cardiopulmonary bypass, leukapheresis, reperfusion injury, leukocyte filtration
Despite many theoretical opportunities and possibilities, the goal of complete attenuation of inflammation and ischemia-reperfusion injury after cardiopulmonary bypass (CPB) remains elusive. Attempts have been made to attenuate the activation of these cascades to reduce the negative effects of CPB. Past efforts to reduce the inflammatory response have consisted of the use of pharmacologic regimens, bioactive-coated circuits, elimination of the air-blood interface, circuit miniaturization, leukofiltration, ultrafiltration, and retrograde autologous priming (1–3).
Leukocyte filtration was introduced for the purpose of enhancing extracorporeal filtration beyond conventional gaseous microemboli, aggregate, and particulate to selectively filter activated leukocytes, but methodologic discrepancies remained within the literature. Two perfusion protocols have evolved for the use of leukofiltration: “continuous,” throughout the extracorporeal circulation; “strategic,” during the rewarming phase. The strategic method involves bypassing the leukocyte filter up to the final 10–30 minutes before removal of the aortic cross-clamp and for the duration of CPB beyond this point (4,5). Clinical support for this technique arises theoretically in an attempt to maximize filtration efficiency at the peak point of leukocyte activation and for avoidance of filter pressurization, which has been reported in the literature (6). The continuous leukofiltration concept is based on the fact that cellular activation has been active at the outset of surgical intervention with median sternotomy, aortic manipulation, and initiation of CPB and that protection from neutrophil-mediated injury is needed from the onset of CPB.
In previous animal studies, we showed that cerebrovascular injury appears early after the onset of CPB. These studies further suggest that that the loss of endotheliumdependent regulatory factors in the cerebral microcirculation after CPB may enhance vasoconstriction, leading to impaired cerebrovascular function; this may be an important mechanism underlying the development of neurologic injury after CPB (7). We also showed the efficacy of both strategic and continuous leukocyte filtration in high-risk patients (8–10).
The purpose of this study was to investigate the efficiency of three leukocyte depletion strategies. In this study, comparison was made between groups of patients in which continuous filter deployment, strategic filtration, and deployment of two filters: one used in the early phase and one deployed after release of the aortic cross-clamp and no leukocyte depleting filter. The effect of these strategies on inflammatory markers, myocardial function, and any correlation with clinical outcome was also a focus of this study.
MATERIALS AND METHODS
Patients
This study was approved by the Medical Ethics Committee of the Institution. Informed consent was obtained from each patient included in the study.
During the period from March until June 2008, 40 patients (high risk: Euroscore 6+) undergoing coronary artery bypass grafting (CABG) were prospectively randomized to one of the four perfusion protocols with the investigators blinded to the allocation.
The study groups were constructed as follows: group 1 (n = 10), compactflo EVO circuits (Dideco, Mirandola, Italy) + two leukocyte filters (LG6B; Pall Biomedical Pro ducts, East Hills, NY) with the method of two-phase leukofiltration; group 2 (n = 10), Compactflo EVO circuits + single leukocyte filter with the method of continuous leukofiltration; group 3 (n = 10), compactflo EVO circuits + single leukocyte filter with the method of strategic leukofiltration; group 4 (n = 10; control), compactflo EVO circuits without leukocyte filtration.
Operative Technique
Anesthesia was induced by fentanyl (35 μg/kg), and muscle relaxation was established with pancuronium (.1 mg/kg). The patients were intubated endotracheally and ventilated with 100% oxygen. A Swan-Ganz catheter was placed using internal jugular vein. All patients were administered 3 mg/kg heparin (Liquemine; Roche, Istanbul, Turkey). After cross-clamping of aorta, the heart was arrested using 10–15 mL/kg crystalloid potassium cardioplegia and continued with cold blood cardioplegia at 20-minute intervals. Warm blood cardioplegia was administered before releasing the aortic cross-clamp. Rewarming was initiated during the last distal coronary anastomosis. When a nasopharyngeal temperature of 36.5°C was reached, CPB was discontinued, and heparin was reversed with a 3.1-mg/kg dose of protamine sulphate (Protamine; Roche) after decannulation.
Cardiotomy and suction lines were not connected to the circuit. Shed blood was not re-transfused and processed in cell-saver (Dideco-Shiley Therapeutic Autotransfusion System). Prime volume was identical for all patient groups: 60 mL of mannitol 20% + 1000 mL of hydroxyethyl starch (Voluven 130.4; Fresenius, Istanbul, Turkey) and 300 mL of crystalloid (Plasmalyte A; Eczacibasi, Istanbul,Turkey) with a total of 1360 mL.
Oxygen transfer rate and pressure drop of circuits were recorded at different flows and FiO2.
Methods of Leukocyte Filtration
The pump was set up identically for both patient groups. Using sterile technique, an 18-in section of the arterial line between the outlet of the oxygenator and arterial line filter was removed and replaced with LG6B filter (first leukofilter). A one-way purge line was attached to the luer port of the filter and connected to a three-way stopcock placed on the cardiotomy reservoir. The line pressure before and after the filters was monitored by a special monitor on the heart lung machine at the purge ports for the purpose of maintaining the pressure <350 mmHg for avoidance of pressurization induced hemolytic cell damage or thrombocytopenia as a result of an adverse event. Pressure drop was documented every 15 minutes by adjustment of pump flow ∼2.2 L/m2/min (Figure 1). A security bypass loop to avoid any potential pressurization associated with the leukofilters was always ready to use.
Figure 1.
Pump design for leukocyte filtration with two filters during pump run.
The circuit was CO2 flushed for 5 minutes to ensure that the filters had been thoroughly treated. All lines including filters were primed and debubbled. The circuit was allowed to recirculate while the patient was prepared for surgery. The circuit was brought to the table.
In group 1, continuous leukofiltration started as soon as the CPB was on using the first leukofilter. Approximately 30 minutes before cross-clamp release, the first filter was clamped, and the second leukofilter was deployed strategically. In group 2, continuous leukofiltration started as soon as the CPB was on until the end of the procedure. In group 3, the single filter was deployed strategically ∼30 minutes before cross-clamp release.
Blood Samples and Assays
Complete blood count [hemoglobin, hematocrit, erythrocyte, leukocytes (white blood cell) and platelet count] was evaluated. Standard blood and urine biochemistry and total protein, albumin, and globulin fractions were documented.
Serum interleukin 6 (IL-6), human tumor necrosis factor α (TNF-α), and procalcitonin levels were measured by ELISA (Biosource International, Camarillo, CA). Creatine kinase MB (CKMB) levels were measured in the samples obtained from the retrograde cardioplegia catheter (coronary sinus blood) before the start and after cessation of CPB.
Further inflammatory response was evaluated by the documentation of serum levels of brain natriuretic peptide (BNP), matrix metalloproteinase 9 (MMP 9), S-100β, and D-dimer (Triage Stroke Panel; Biosite, Belfast, UK).
Blood samples were obtained through the radial artery catheter in potassium-EDTA tubes at the following intervals: T1 (baseline), after induction of anesthesia (before administration of heparin); T2 (on CPB), 5 minutes after initiation of CPB; T3 (X-clamp), 5 minutes after cross-clamping of aorta; T4 (off CPB), 5 minutes after cessation of CPB; T5 (ICU24), first postoperative day at 8:00 am; T6 (ICU48), second postoperative day at 8:00 am.
Additional samples were obtained through the lines before and after the leukocyte filters in potassium-EDTA tubes every 15 minutes for documentation of percentage reduction rate of neutrophils and platelets.
Thromboelastography
Platelet function was evaluated by thromboelastography (TEG; ROTEG; Pentapharm, Munich, Germany) during the operation. Coagulation time (CT), clot formation time (CFT), α-angle, mean clot firmness (MCF), and A5 were measured in samples T1–T4.
Perioperative Follow-Up
For each patient, the following factors were evaluated before discharge and documented: hemodynamic parameters, perfusion and cross-clamp duration, intubation period, postoperative hemorrhage, the use of blood and plasma, incidence of arrhythmia [atrial fibrillation (AF)], use of inotropic support, complications, the duration of intensive care unit and hospital stay, perioperative mortality, NY Heart Association Classification, and Doppler echocardiography. Comparison among groups was performed retrospectively.
Statistical Analysis
Data are expressed as the mean ± SE. The Mann-Whitney U test was used to compare demographic and non-parametric data. Two-way analysis of variance with factor group and repeated factor time was used to analyze differences over time in each group and for differences between groups. A post hoc test (Bonferroni correction) was applied whenever a significant difference was detected. p < .05 was considered significant. Data were analyzed using SPSS program (Version 10.0; Microsoft, Istanbul, Turkey).
RESULTS
Demographic data are presented in Table 1.
Table 1.
Demographic data.
| Double Filter | Single Continuous | Single Strategic | Control | p | |
|---|---|---|---|---|---|
| Age (years) | 68.3 ± 2.2 | 64.5 ± 2.4 | 67.1 ± 2.65 | 70 ± 2.7 | NS |
| Male sex | 6 | 4 | 5 | 4 | NS |
| BSA (m2) | 1.63 ± 0.05 | 1.59 ± 0.05 | 1.61 ± 0.05 | 1.68 ± 0.06 | NS |
| NYHA class | 3.2 ± 0.15 | 3.0 ± 0.15 | 3.25 ± 0.15 | 3.1 ± 0.16 | NS |
| Ejection fraction | 0.35 ± 0.08 | 0.30 ± 0.1 | 0.36 ± 0.06 | 0.33 ± 0.09 | NS |
| LVEDP (mmHg) | 18.1 ± 1.6 | 17.4 ± 1.5 | 19.5 ± 1.9 | 19.2 ± 1.6 | NS |
NS, not significant.
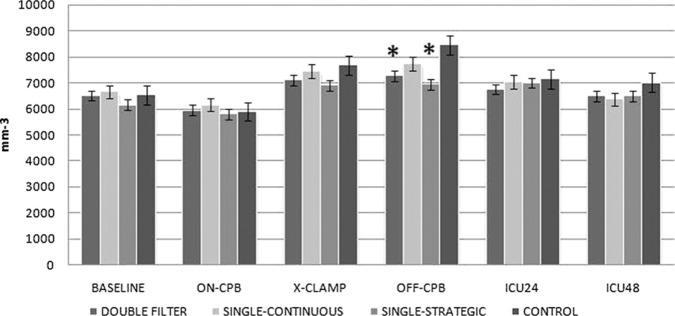
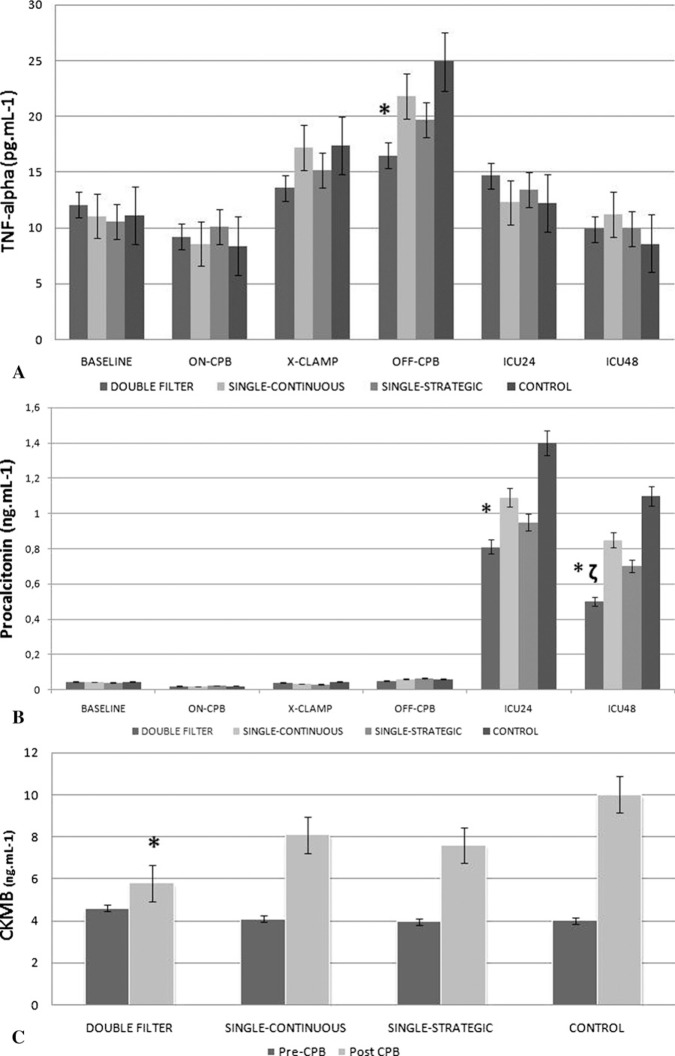
White blood cell (WBC) count in the double filter and strategic filtration groups showed significant differences at T4 (Figure 2; p < .05 vs. control). There were no significant differences in platelet count and serum IL-6 levels among groups. TNF-α levels were significantly lower in group 1 at T4 (p < .05 vs. control; Figure 3A). Procalcitonin levels were significantly lower in group 1 at T5 (p < .05 vs. control) and T6 (p < .05 vs. control and p < .05 vs. group 2; Figure 3B).
Figure 2.
White blood cell count (/mm 3) throughout the procedure.
Figure 3.
TNF-α (ng/mL) (A), procalcitonin (pg/mL) (B), and CKMB (ng/mL) (C) levels throughout the procedure.
CKMB levels in coronary sinus blood showed well-preserved myocardium in the double filter group (p < .05 vs. control; Figure 3C).
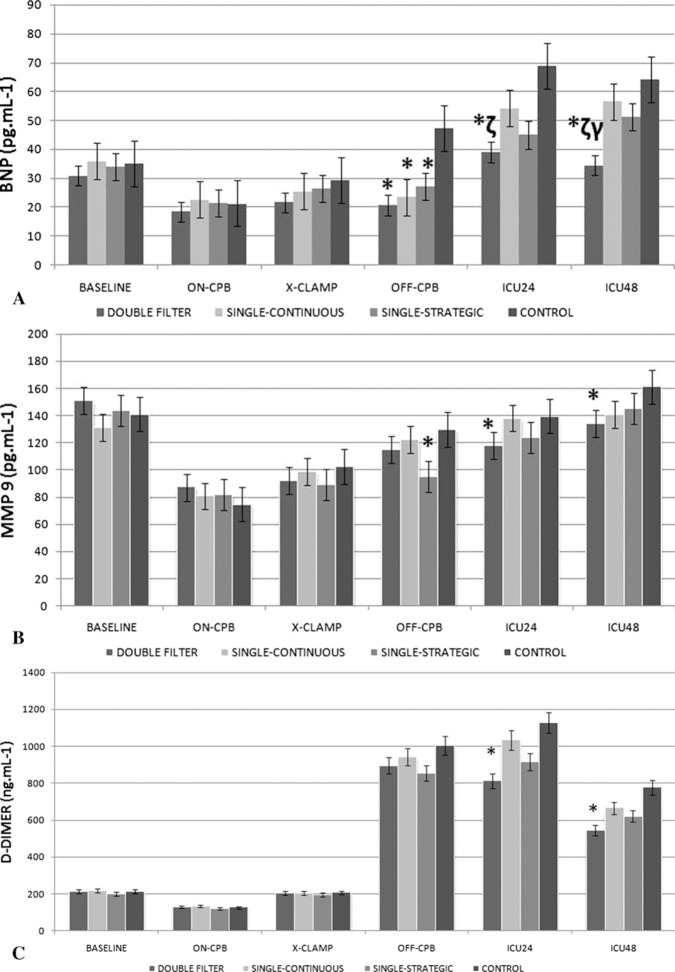
In filtrated groups, serum levels of BNP showed significant differences at T4 (p < .05 vs. control). BNP levels in the double filter group were also significantly lower at T5 and T6 with respect to group 2 (p < .05); T6 with respect to group 3 (p < .05); and T5 and T6 vs. control (p < .001; Figure 4A).
Figure 4.
BNP (pg/mL) (A), MMP-9 (pg/mL) (B), and D-dimer (ng/mL) (C) levels throughout the procedure.
MMP9 levels were significantly lower at T5 and T6 in the double filter and at T4 in the strategic leukofiltration groups (p < .05 vs. control; Figure 4B).
D-dimer levels were significantly lower at T5 and T6 in the double filter group (Figure 4C).
S-100β levels were always below detectable levels throughout CPB in all groups.
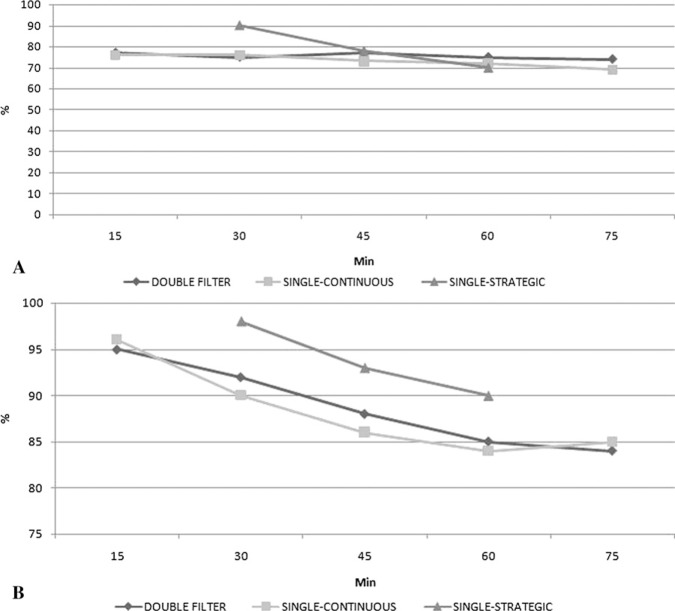
Maximal line pressures before leukofilters were 176 ± 20 mmHg for the double filter, 165 ± 20 mmHg for the single-continuous, and 147 ± 16 mmHg for the single-strategic groups. Pressure drop through LG6B filters was never over critical security level (<60 mmHg) in the filtrated groups. A similar target flow rate was maintained in each group. The adjustment of pump flow to measure pressure drop occurred in all groups. Percentage reduction of neutrophils through leukofilters is shown in Figure 5A and platelet reduction in Figure 5B.
Figure 5.
Percent reduction of neutrophils (A) and platelets (B) through leukofilters during CPB. *<.05 vs. control (group 4), ζ<.05 vs. group 2, and γ<.05 vs. group 3.
Complete blood count and urine biochemistry (urine albumin-globulin levels) were not significantly different among groups during the whole study period. TEG results never showed any abnormal level during the whole CPB period in all groups. Perioperative follow-up is summarized in Table 2.
Table 2.
Perioperative patient characteristics.
| Double Filter | Single Continuous | Single Strategic | Control | p | |
|---|---|---|---|---|---|
| Duration of CPB (minutes) | 92.5 ± 8.4 | 95.4 ± 8 | 91 ± 6.5 | 93.5 ± 8 | NS |
| t-intub (hours) | 9.4 ± 2.4 | 9.6 ± 2.5 | 9.1 ± 2 | 12.1 ± 3.5 | NS |
| Postoperative hemorrhage (mL) | 815 ± 130 | 769 ± 125 | 751 ± 150 | 791 ± 130 | NS |
| Arrhythmia (n) | AF:1* | AF:2* | AF:4 | AF:6 | <.05 |
| Blood transfusion (units) | 2.4 ± 1.3 | 2.1 ± 1.3 | 1.95 ± 1.3 | 1.9 ± 1.2 | NS |
| Blood products (units) | 2.8 ± 1.5 | 2.4 ± 1.5 | 2 ± 1.7 | 2.4 ± 1.5 | NS |
| Inotropic support (n) | 2 | 3 | 2 | 5 | NS |
| ICU stay (day) | 2.1 ± 1.1 | 2.2 ± 1.1 | 2.4 ± 1.3 | 2.6 ± 1 | NS |
| Postoperative EF (%) | 55.7 ± 6 | 59.2 ± 7 | 57 ± 8 | 53.7 ± 7 | NS |
| Hospital stay (day) | 7.4 ± 1.4 | 7.5 ± 1.4 | 7 ± 1.5 | 7.6 ± 1.4 | NS |
| Mortality rate (n) | 0 | 0 | 0 | 1 | NS |
p < .05 vs. control.
NS, not significant.
DISCUSSION
The complex pathophysiologic mechanisms of CPB-induced systemic inflammation result from interactions between activated leukocytes, platelets, endothelium, and mast cells and are mediated by a variety of adhesion molecules, cytokines, complement, clotting factors, and reactive oxygen species (11). The beneficial effects of leukocyte filtration on the outcome of cardiac surgery with CPB are probably caused by the limitation of pathogenesis mediated by over-stimulated neutrophils (12).
Postoperative AF remains the most common complication after cardiac surgery, occurring in as many as 50% of patients. Many studies suggested that a peak WBC count coincided with the time course of postoperative AF after CPB and have generated the hypothesis that inflammation played a major role in the pathogenesis (13). Leukofilters were efficient in lowering postoperative AF incidence with respect to the control group in study groups. Filtered groups had better preserved myocardium as shown by CKMB levels caused by less impact of inflammation (14).
We also documented the pressure drop and reduction rate of filters in the interest of safety. The reduction rate of neutrophils was comparable to previous laboratory evaluations. The double filtration technique was efficient in moderating the inflammatory response, but those intending to use this technique should pay attention to potential platelet interference (15). Because of the lack of any suitable screening test to identify those patients who may exhibit technical problems, particularly filter blockage, during leukofiltration, several safety strategies have been suggested. Use of a bypass loop around the filter is a good precaution to allow CPB to be maintained should a filter problem be encountered. The rate at which patients are cooled should be kept within the 10°C blood-to-patient temperature gradient. If increasing pressure drop is encountered, rewarming the blood may slow or arrest the progressive increase in pressure. The use of albumin in the priming solution may reduce problems by Vroman effect, which governs the sequential adsorption and displacement of proteins on an artificial surface over time (16). There is an increasing body of evidence that suggests that surface-modified circuits may reduce or even eliminate high pressurization problems. In addition to these precautions, the use of nitric oxide donors may also be used (17,18). In this study, we used a security bypass loop, mild-moderate hypothermia to avoid any potential pressurization associated with the leukofilters. Pressure drop and maximal pressure were always within acceptable limits; however, perhaps unsurprisingly, the continuous technique had significantly higher line pressures vs. two-phase filtration.
Leukocyte-depleting filters have been shown to perform very well under test conditions, but there has been scant correlation between the laboratory data and real clinical benefit. The literature on this subject remains controversial, with many discrete studies with the same methodology and technique during CPB. We did not have a power calcuobjectives yielding different results in the clinical setting lation to justify the sample size. Our primary aim was to (19–21). These contradictory results suggest that perhaps show that double filters could function well and safe durthere are response mechanisms that are specific to indi-ing a long CPB duration. Therefore, we studied high-risk vidual patients that may interfere with outcomes or may cohorts as a population that adds far more confounding render these patients incompatible with the leukofiltra-variables than an uncomplicated routine, first time corotion technique. This study is the first pilot study to test nary bypass operation. High-risk patients had low ejection the safety and effectiveness of the two-phase filtration fraction rates preoperatively. The high standard of current CPB systems has made it increasingly difficult to test technical improvements in clinical studies involving relatively small patient groups. In most cases, the statistical power of such studies will not suffice to show a significant clinical benefit associated with changes in the CPB circuit. We believe performing such evaluations in high-risk patients are needed for a clear picture. However, we could not show any difference in routine laboratory examinations among groups (22,23).
It is clear from this study that two-phase deployment of leukofiltration is associated with some compound benefit over continuous deployment. The control group had no treatment, and institutional ethics board approved this protocol because leukofiltration has not been accepted as a standard anti-inflammatory strategy during CPB, and there are many different positive and negative publications. Although we were able to show statistically significant and near-statistically significant results from this small study, the high standard of current CPB systems makes it increasingly difficult to test such technical improvements in the clinical setting. Considering the primary outcome of this study, after showing the feasibility of the application, we tried to include various sensitive inflammatory parameters for an initial evaluation of the impact of this technique in a small patient population. This is a small-scale initial evaluation study with a low power. Clinical and laboratory outcomes were not distinct enough to conclude important strategies. It seems clear from the increasing body of supportive evidence that there is a role for leukocyte filtration during CPB; however, it is now necessary to look more critically at the application of this technology in clinical practice, particularly with regard to identifying the patient cohort who will benefit most from this technique. This will require a larger more powerful study than the present one, and it might merit an international multi-center trial.
REFERENCES
- 1.Asimakopoulos G, Gourlay T.. A review of anti-inflammatory strategies in cardiac surgery. Perfusion. 2003;18(Suppl 1):7–12. [DOI] [PubMed] [Google Scholar]
- 2.De Somer F.. Optimization of the perfusion circuit and its possible impact on the inflammatory response. J Extra Corpor Technol. 2007;39:285–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Rimpilainen R, Biancari F, Wistbacka J, et al. Outcome after coronary artery bypass surgery with miniaturized versus conventional cardio-pulmonary bypass. Perfusion. 2008;23:361–7. [DOI] [PubMed] [Google Scholar]
- 4.Gunaydin S, McCusker K, Vijay V.. Strategic leukofiltration in cardiac surgery. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:323–31. [DOI] [PubMed] [Google Scholar]
- 5.Matheis G, Scholz M, Simon A, Henrich D, Wimmer-Greinecker G, Moritz A.. Timing of leukocyte filtration during cardiopulmonary bypass. Perfusion. 2001;16(Suppl):31–7. [DOI] [PubMed] [Google Scholar]
- 6.Asimakopoulos G.. The inflammatory response to CPB: The role of leukocyte filtration. Perfusion. 2002;17(Suppl):7–10. [DOI] [PubMed] [Google Scholar]
- 7.Modine T, Azzaoui R, Ouk T, et al. Changes in cerebral vascular reactivity occur early during cardiopulmonary bypass in the rat. Ann Thorac Surg. 2006;82:672–8. [DOI] [PubMed] [Google Scholar]
- 8.Gunaydin S, McCusker K, Vijay V, et al. Comparison of polymethoxyethylacrylate-coated circuits with leukocyte filtration and reduced heparinization protocol on heparin-bonded circuits in different risk cohorts. Perfusion. 2006;21:329–42. [DOI] [PubMed] [Google Scholar]
- 9.Gunaydin S, Sari T, McCusker K, et al. Clinical evaluation of strategic leukofiltration with surface modification: Enhanced preservation or fantasy. Filtration. 2005;1:47–58. [Google Scholar]
- 10.Gunaydin S, McCusker K, Vijay V, et al. Clinical significance of strategic leukocyte filtration in different risk cohorts undergoing cardiac surgery. Filtration. 2005;1:95–106. [Google Scholar]
- 11.Day JR, Taylor KM.. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int J Surg. 2005;3:129–40. [DOI] [PubMed] [Google Scholar]
- 12.Gourlay T, Fleming J, Taylor KM.. Laboratory evaluation of the Pall LG6 leukocyte depleting arterial line filter. Perfusion. 1992;7:131–40. [DOI] [PubMed] [Google Scholar]
- 13.Fontes ML, Mathew JP, Rinder HM, Zelterman D, Smith BR, Rinder CS; Multicenter Study of Perioperative Ischemia. (McSPI) Research Group . Atrial fibrillation after cardiac surgery/cardiopulmonary bypass is associated with monocyte activation. Anesth Analg. 2005;101:17–23. [DOI] [PubMed] [Google Scholar]
- 14.Gunaydin S, Ayrancioglu K, Dikmen E, et al. Clinical effects of leukofiltration and surface modification on post-cardiopulmonary bypass atrial fibrillation in different risk cohorts. Perfusion. 2007;22:279–88. [DOI] [PubMed] [Google Scholar]
- 15.Ogiwara M, Kyo S, Ohuchi H, et al. High pressure occlusion of leukocyte depletion arterial line filter in patients with cardio-pulmonary bypass surgery. Kyobu Geka. 2001;54:759–63. [PubMed] [Google Scholar]
- 16.Noh H, Fogler EA.. Volumetric interpretation of protein adsorption: Competition from mixtures and the Vroman effect. Biomaterials. 2007;28:405–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel AN, Sutton SW, Livingston S, et al. Clinical benefits of leukocyte filtration during valve surgery. Am J Surg. 2003;186:636–9, discussion 639–40. [DOI] [PubMed] [Google Scholar]
- 18.Whitaker DC, Stygall JA, Newman SP, Harrison MJ.. The use of leucocyte-depleting and conventional arterial line filters in cardiac surgery: A systematic review of clinical studies. Perfusion. 2001;16:433–46. [DOI] [PubMed] [Google Scholar]
- 19.Leal-Noval SR, Amaya R, Herruzo A, et al. Effects of a leukocyte depleting arterial line filter on perioperative morbidity in patients undergoing cardiac surgery: A controlled randomized trial. Ann Thorac Surg. 2005;80:1394–400. [DOI] [PubMed] [Google Scholar]
- 20.Scholz M, Simon A, Matheis G, et al. Leukocyte filtration fails to limit functional neutrophil activity during cardiac surgery. Inflamm Res. 2002;51:363–8. [DOI] [PubMed] [Google Scholar]
- 21.Sahlman A, Ahonen J, Salo JA, Rämö OJ.. No impact of a leucocyte depleting arterial line filter on patient recovery after cardiopulmonary bypass. Acta Anaesthesiol Scand. 2001;45:558–63. [DOI] [PubMed] [Google Scholar]
- 22.Royston D, Kovesi T, Marczin N.. The unwanted response to cardiac surgery: Time for a reappraisal? J Thorac Cardiovasc Surg. 2003;125:32–5. [DOI] [PubMed] [Google Scholar]
- 23.Landis RC, Arrowsmith JE, Baker RA, et al. Consensus statement: Defining minimal criteria for reporting the systemic inflammatory response to cardiopulmonary bypass. Heart Surg Forum. 2008;11:E316–22. [DOI] [PubMed] [Google Scholar]