Abstract:
A recently completed study quantified the percent of fentanyl or morphine sulfate lost to uncoated polyvinylchloride (PVC) tubing or to one of two hollow fiber oxygenators within the extracorporeal life support (ECLS) circuit. The results demonstrated the majority of drug loss was due to adsorption by the PVC tubing. The purpose of this study was to determine if a tubing coating process affects fentanyl or morphine Sulfate adsorption. The goal was to quantify fentanyl or morphine sulfate lost due to adhesion within surface modified tubing. The following surface modifications were studied: 1) Maquet Safeline® (synthetic immobilized albumin); 2) Maquet Softline® (a heparin free biopassive polymer); 3) Maquet Bioline® (recombinant human albumin + heparin) (Maquet Cardiopulmonary AG, Hirrlingen, Germany); 4) Terumo X Coating™ (poly2methoxylacrylate)) (Terumo Cardiovascular Systems Corporation, Ann Arbor, MI); 5) Medtronic Carmeda® (covalently bonded heparin); and 6) Medtronic Trillium® (covalently bonded heparin) (Medtronic, Minneapolis, MN). A total of 36 individual circuits were built from the above six available modified surface coatings, for a total of six individual circuits of each coating type. Blood samples were drawn at 5 minutes, 120 minutes, and 360 minutes followed by High-Performance Liquid Chromatography to determine available circulating levels of either fentanyl or morphine sulfate. Fentanyl concentrations decreased to an average final available concentration of 35% (±5%) within the 18 circuits. Morphine sulfate however, decreased to a final available concentration of 57% (±1%) in all Maquet tubing and the Medtronic Trillium tubing, while it decreased to a final concentration of 35% (±1%) in the Medtronic Carmeda coated tubing and in the Terumo X Coating tubing. Biocompatible ECLS circuit surface coatings affected drug-adsorption and availability. Further evaluation is necessary to understand the adsorptive loss of other drugs administered to our patients while on modified surface coated ECLS circuits.
Keywords: extracorporeal life support, drug adsorption, morphine, fentanyl, modified surface coating
Drug adsorption within the ECLS circuit by various components at various rates is an ongoing problem that complicates the entire course of patient care (1). Several studies have demonstrated that drug adsorption rates by uncoated polyvinyl chloride (PVC) tubing affects circulating levels of the common sedatives fentanyl and morphine sulfate (2–5). In a previous study, two different hollow fiber oxygenators (Quadrox D (Maquet Cardiopulmonary AG, Hirrlingen, Germany) and the Baby-RX™ (Terumo Cardiovascular Systems Corp., Ann Arbor, MI)) and uncoated PVC tubing were evaluated to determine if the surface of the hollow fiber oxygenators would adsorb more drug than the PVC tubing (4). After reviewing this data, it was determined the next logical step would be to experiment with a variety of PVC tubing with modified surface coatings to establish potential drug loss to this ECLS component.
The purpose of this study was to evaluate the drug adsorptive effect of modified surface coatings within the ECLS circuit. We set out to determine the relationship between sedation dose and drug levels at various times, by testing the hypothesis that the drug will be adsorbed less by the coated tubing than previously reported in uncoated PVC tubing.
MATERIALS AND METHODS
A total of 36 individual circuits were built from six available modified surface coatings, for a total of six individual circuits of each coating type. Three circuits of each modified surface coating received morphine sulfate, while the remaining three circuits of each modified surface received fentanyl. The modified circuit surface coatings used were: six Medtronic Carmeda® and six Medtronic Trillium®, both covalently bonded heparin coating (Medtronic, Minneapolis, MN); six Terumo X Coating ™ (poly2methoxylacrylate) (Terumo Cardiovascular Systems Corporation, Ann Arbor, MI); and six each Maquet Safeline® (synthetic immobilized albumin), Softline® (a heparin free biopassive polymer), and Bioline® (recombinant human albu min + heparin) (Maquet Cardiopulmonary AG, Hirrlingen, Germany). All tubing except the Super Tygon (Saint-Gobain Performance Plastics Corp., Aurora, OH) tubing had a durometer of 68.
Each individual circuit was assembled with a coated 3/8″ × 3/32″ venous line 60″ (152 cm), a coated 1/4″ × 3/32″ arterial line 60″ (152 cm), and an uncoated Super Tygon S-65-HL 3/8″ × 3/32″ boot 21″ (53 cm). A 3/8″ × 1/4″ luer sampling port was inserted into the arterial line immediately post roller head while each venous line contained a 3/8″ × 3/8″ luer injection port immediately pre-roller head (Figure 1).
Figure 1.
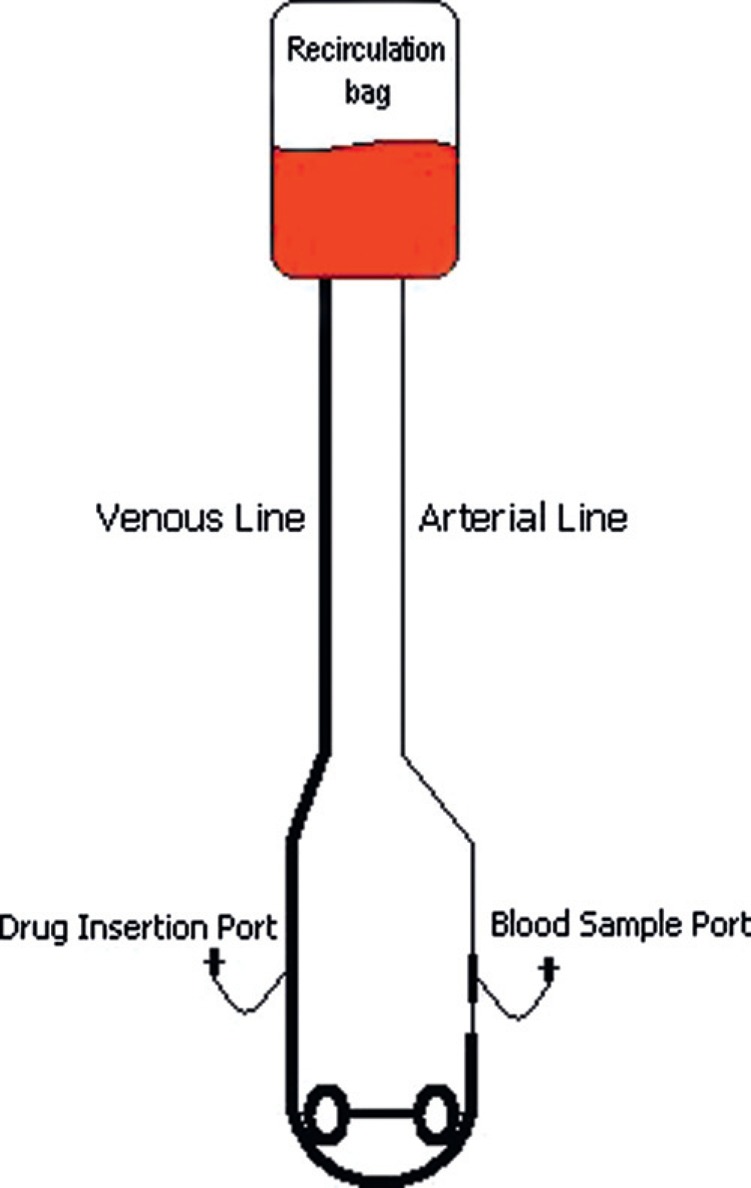
Schematic of circuit used in this experiment.
Once constructed, each circuit was primed with Normosol-R™ (Hospira, Inc., Lake Forest, IL). Each circuit’s raceway was loaded into the roller of either a Sarns 8000 (Terumo Cardiovascular Systems Corporation, Ann Arbor, MI), Cobe Century (Cobe Cardiovascular, Arvada, CO), or Stockert CAPS (Sorin Cardiopulmonary, Arvada, Colorado) heart lung machine. The occlusion of each circuit was set, then all 36 circuits were recirculated together for 2 hours through two uncoated Terumo CAPIOX® venous reservoirs and through one Baby-RX™ oxygenator (Terumo Cardiovascular Systems Corporation, Ann Arbor, MI) (6). The reservoirs were subsequently drained and filled with less than 2-hour-old adult, single donor bovine whole blood. At collection, the fresh bovine blood was anticoagulated with heparin (4 IU/mL) and required no further anti-coagulation throughout the study period. The bovine blood was recirculated continuously at room temperature, ensuring all 36 circuits contained the same physiologic blood. While recirculating, room air sweep gas was administered to the Baby-RX™ oxygenator to a final pre-separation blood gas of pH 7.432, pCO2 39.2 mmHg, pO2 119 mmHg, HCO3 26.2 Meq/L, BE 2, Hct 35%, and Hgb 11.9 g/dL. Two samples of blood, 3 mL each, were withdrawn for baseline High-Performance Liquid Chromatography (HPLC) measurement of morphine sulfate and fentanyl.
All 36 circuits were then separated from the two central reservoirs and Baby-RX™, each connected to a 250 mL Viaflex® (Baxter Healthcare Corporation, Deerfield, IL) sterile bag such that 36 individual circuits now existed. Total circuit volume, including (100 mL) volume within the Viaflex bag, equaled 295 mL. After separation, each circuit was maintained at a blood flow rate of 300 mL/min throughout the remainder of the study.
Fentanyl 100 μcg/70 mL blood (dosing for general anesthesia is 50–150 μcg/Kg, 2005 American Hospital Formulary Service (AHFS) Drug information) bolus was added to 18 of the circuits, such that three Bioline, Carmeda, Safeline, Softline, Trillium, and X Coating™ circuits received fentanyl. Morphine sulfate 100 μcg morphine/70 mL (dosing is 5–20 mg every 4 hours, 2005 AHFS Drug information) bolus was added to the remaining 18 circuits for a final concentration of 1429 ng/mL of drug per circuit, such that three Bioline, Carmeda, Safeline, Softline, Trillium, and X Coating™ circuits received morphine. Samples were drawn at 5 minutes, 120 minutes, and 360 minutes for analysis. Drug concentrations were determined via HPLC. HPLC was performed at NMS Labs (Willow Grove, PA) to measure the concentration of drug remaining in the samples at each time interval. Knowing the difference between the initial quantity and the quantity determined by HPLC revealed the amount of drug lost to the tubing.
RESULTS
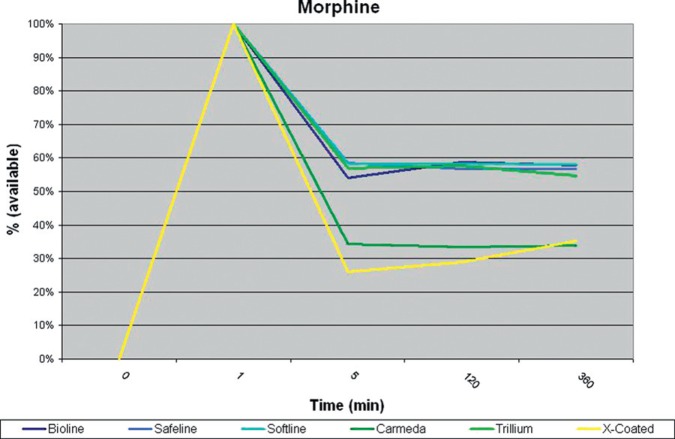
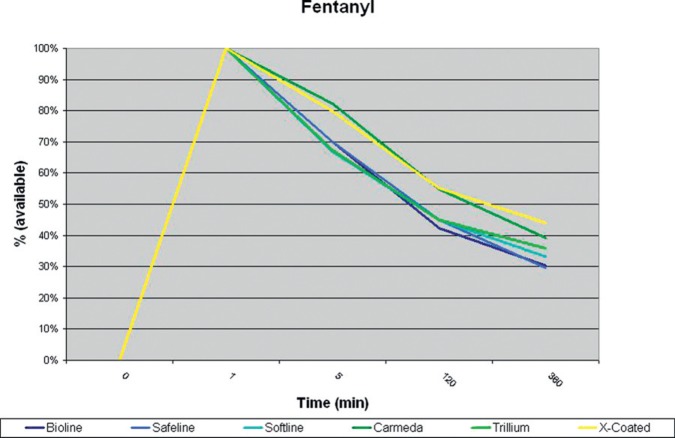
All circuits showed a loss of drug in the first 5 minutes after administration. Carmeda® and X Coating™ tubing lost significantly (p < .05) more morphine at 5 minutes than the other modified surface coatings (Table 1). Maquet Safeline®, Bioline®, and Softline® as well as Medtronic Trillium® tubing all had similar losses with regard to morphine adsorption during this study (Figure 2, Table 2). Fentanyl was lost in all coated circuits at a constant rate (Figure 3).
Table 1.
Morphine available at 5 minutes post injection into circuit.
| Drug = Morphine | Time |
|---|---|
| Coating | 5 minutes |
| Bioline | 54% |
| Safeline | 59% |
| Softline | 58% |
| Trillium | 57% |
| X Coating | *26% |
| Carmeda | *34% |
= significant (p < .05)
Figure 2.
Morphine sulfate, percent available at each time interval.
Table 2.
Mean (±SD) in ng/mL of drug available at each time point for the three circuits of each specific coating type tested.
| Morphine (ng/mL) | ||||||
| 5 minutes | 120 minutes | 360 minutes | ||||
| Circuits | Mean | SD | Mean | SD | Mean | SD |
| Bioline | 773 | 31 | 840 | 17 | 827 | 35 |
| Safeline | 837 | 6 | 810 | 62 | 810 | 46 |
| Softline | 833 | 6 | 833 | 15 | 830 | 40 |
| Trillium | 813 | 25 | 823 | 32 | 783 | 15 |
| X Coating | 370 | 151 | 410 | 125 | 500 | 82 |
| Carmeda | 487 | 107 | 473 | 45 | 480 | 60 |
| Fentanyl (ng/mL) | ||||||
| 5 minutes | 120 minutes | 360 minutes | ||||
| Circuits | Mean | SD | Mean | SD | Mean | SD |
| Bioline | 1000 | 0 | 603 | 29 | 433 | 12 |
| Safeline | 1000 | 0 | 643 | 21 | 423 | 38 |
| Softline | 953 | 50 | 643 | 12 | 473 | 23 |
| Trillium | 963 | 40 | 643 | 12 | 513 | 118 |
| X Coating | 1133 | 58 | 783 | 12 | 623 | 12 |
| Carmeda | 1167 | 58 | 777 | 15 | 555 | 49 |
Initial value of each drug 1429 ng/mL.
Figure 3.
Fentanyl, percent available at each time interval.
DISCUSSION
Earlier experiments have shown that the majority of drugs lost in the ECLS circuit are lost to the PVC tubing (3,4). The data from this experiment determined that Maquet Safeline®, Softline®, Bioline®, and Medtronic Trillium® tubing all adsorb morphine at similar rates with that previously reported for uncoated PVC tubing (4). Medtronic Carmeda® and Terumo X Coating ™ adsorb morphine at rates considerably higher than the other types of tubing evaluated in this study. The exact reason for this increase in adsorption is unclear as all circuits contained tubing that was identical in durometer and length. Additionally, this increase in adsorption was not seen in the Medtronic Trillium® tubing. It is also important to note that while 52% (±14%) of all morphine was adsorbed within 5 minutes, the circuits then appeared to achieve at a steady state. Hypothetically, adding morphine to this “steady state” circuit may provide a higher concentration of drug to the patient. If utilizing morphine as a sedative with Carmeda® or X Coating™ tubing types, a significantly greater quantity of morphine may initially be required to achieve the same level of sedation compared with any of the other surface coatings tested in this experiment. Carmeda® tubing and X Coating™ tubing adsorbed 66% and 74% of the morphine, respectively, within 5 minutes of administration of the drug into the circuit.
Fentanyl was adsorbed by Bioline®, Safeline®, Softline®, and Trillium® coated tubing at a greater rate than it was by either Carmeda® or X Coating™ tubing, however, this did not reach statistical significance. Fentanyl was adsorbed by the other modified tubing surfaces at a similar or slightly slower rate than was previously reported in uncoated PVC tubing (4). Fentanyl, although adsorbed more slowly, was continually lost to the tubing and a saturation point or “steady state” did not appear to be achieved during the 6 hours of this study. Morphine, while lost at a higher rate appeared to saturate the tubing possibly making additional doses available to the patient, and a potential better choice for sedation of the ECLS patient.
This was an in-vitro experiment undertaken at room temperature and did not take into account the affect of hemofiltration (which could potentially increase circulating drug levels), drug metabolism, or drug excretion, as all would have contributed to changes in circulating drug concentrations (7). Uncoated Super Tygon tubing was used for the raceway. While an area of potential drug loss, Super Tygon is currently the standard of care in long term ECLS circuit raceways. To limit the impact this tubing had on the experiment, a single lot number was used for all circuits, and all raceways were cut to the same length. The 250 mL Viaflex bag used with each circuit was also uncoated and existed as a potential site for drug loss. To decrease the impact of this product on the overall experiment, all circuits contained a Viaflex bag from the same lot.
CONCLUSION
While these adsorption experiments are difficult to accomplish due to component cost, and HPLC testing availability for these medications, further experiments with a broader range of drugs need to be conducted.
ACKNOWLEDGMENTS
The authors would like to thank Maquet Cardiopulmonary, Medtronic, and Terumo Cardiovascular Systems Corp. for their tubing donations, which made this study possible.
REFERENCES
- 1.Mulla H, Lawson G, von Anrep C, et al. . In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion. 2000;15:21–6. [DOI] [PubMed] [Google Scholar]
- 2.Buck ML.. Pharmacokinetic changes during extracorporeal membrane oxygenation: Implications for drug therapy of neonates. Clin Pharmacokinet. 2003;42:403–17. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt-Meht V, Annich G.. Sedative clearance during extracorporeal membrane oxygenation. Perfusion. 2005;20:309–15. [DOI] [PubMed] [Google Scholar]
- 4.Preston T, Hodge A, Riley J, Leib-Sargel C, Nicol K.. In vitro drug adsorption and plasma free hemoglobin levels associated with hollow fiber oxygenators in the extracorporeal life support circuit. J Extra Corpor Technol. 2007;39:234–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan O, Klein J, Gruenwald C, Bohn D, Barker G, Koren G.. Preliminary studies of the effects of extracorporeal membrane oxygenator on the deposition of common pediatric drugs. Ther Drug Monit. 1993;15:263–6. [DOI] [PubMed] [Google Scholar]
- 6.Rawn DJ, Harris HK, Riley JB, Yoda DN, Blackwell MM.. An under-occluded roller pump is less hemolytic than a centrifugal pump. J Extra Corpor Technol. 1997;29:15–8. [PubMed] [Google Scholar]
- 7.Taenzer A, Groom R, Quinn R.. Fentanyl plasma levels after modified ultrafiltration in infant heart surgery. J Extra Corpor Technol. 2005;37:369–72. [PMC free article] [PubMed] [Google Scholar]