
Robert Groom
Three original articles in this issue by Potger (1), Liu (2), and Burnside (3) remind us of the invisible gaseous micro-emboli that are ever present in, and commonly released from, cardiopulmonary bypass circuits. These three in vitro studies examine the vulnerability of the extracorporeal system, when challenged with entrained air. Furthermore, they provide new knowledge about how component design and operating conditions relate to embolic activity. So, should we really worry about gaseous microemboli? That question never fails to bring to my mind an image from Muth and Shank’s review article on gaseous emboli published in The New England Journal of Medicine in 2000 (4). The striking illustration provides a visual description of the plausible causes of injury from a 30–60 micron embolism trapped in a small arteriole in cerebral tissue (Figure 1). The mechanism of injury includes ischemia, mechanical disruption of the endothelium, and a host of both cellular and immune mechanisms.
Figure 1.
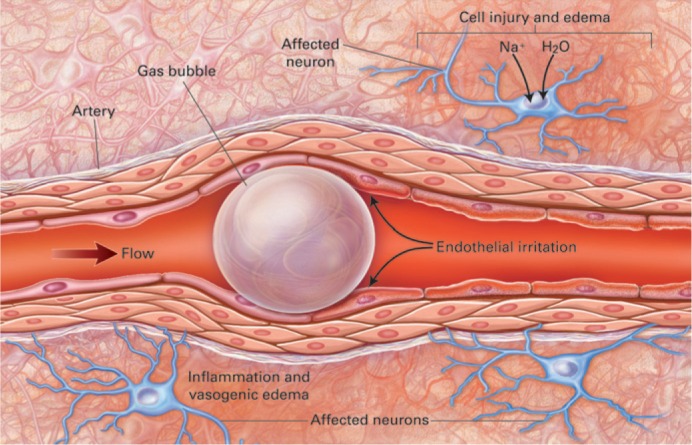
Bubble obstructing end-arterial flow in a cerebral vessel with a diameter of 30 to 60 μm, causing distal ischemia. The obstruction causes the metabolic processes of neurons to fail. Sodium and water enter the neuron, and cytotoxic edema develops. The surface of the bubble generates a foreign-body response through cellular and humoral immune mechanisms. The bubble also mechanically irritates the arterial endothelium. Both processes result in vasogenic edema and greater impairment of perfusion. The neuronal injury extends beyond the area of obstruction. (Reprinted with permission from N Engl J Med 2000;342: 476–823)
Jeffrey Riley’s choice of a classic article is a report from 1982, by Sakauye and colleagues from Shiley Laboratories, some of the earliest work published about micro emboli detection (5). This 30-year-old article reminds us that this is not a new problem.
There are other invisible hazards in the operating room. Heart surgery teams work within systems that involve a number of professionals (humans), numerous devices (made by humans), and processes that include thousands of steps, creating a frightening number of hidden latent conditions that could produce a catastrophe on any given day. Matte et al.’s (6) report of an air embolism subsequent to a pressurized venous reservoir evoked letters from several readers, which are published in this issue (see pages 168–9). The time for an international blame-free reporting system of perfusion related incidents is long overdue. Such a system would alert us to areas where our programs are vulnerable, providing us with the opportunity to “design out” some of these weaknesses and to create rapidly deployable mitigation strategies to recover from incidents when they occur. Our colleagues down under have had a Perfusion Incident Reporting System in place for Australia and New Zealand for more than a decade. A report on this system presented at the Perfusion Down Under conference this August will be published in the December issue of the Journal.
So, how might we move forward to improve our systems? I am reminded of the work of John P. Kotter and Dan S. Cohen, experts on leadership and change. In their best seller, The Heart of Change, they introduce a powerful paradigm, “see-feel-change” based on the concept of showing people potent reasons for change that will drive them to be steadfast in their pursuit of challenging the status quo and introduce change that is both large in scale and durable (7). The first step is to identify a problem or a solution to a problem and then allow others to see the problem in a way that is visual and concrete.
We are fortunate to have investigators that report their research findings and also clinicians that report adverse events. By making problems more visual and concrete, they heighten our awareness and prompt us to take action. Let us put Kotter and Cohen’s powerful formula “see-feel-change” to work. This will make the operating room as safe as possible for patients, sooner rather than later.
REFERENCES
- 1.Potger KC, McMillan D, Ambrose M.. Microbubble generation and transmission of Medtronic’s Affinity hardshell venous reservoir and collapsible venous reservoir bag: An in-vitro comparison. J Extra Corpor Technol. 2011;43:115–22. [PMC free article] [PubMed] [Google Scholar]
- 2.Liu S, Newland RF, Tully PJ, Tuble SC, Baker RA.. In vitro evaluation of gaseous microemboli handling of cardiopulmonary bypass circuits with and without integrated arterial line filters. J Extra Corpor Technol. 2011;43:107–14. [PMC free article] [PubMed] [Google Scholar]
- 3.Burnside J, Gomez D, Preston TJ, Olshove VF Jr, Phillips A.. In-vitro quantification of gaseous microemboli in two extracorporeal life support circuits. J Extra Corpor Technol. 2011;43:123–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Muth CM, Shank ES.. Gas embolism. N Engl J Med. 2000;342:476–82. [DOI] [PubMed] [Google Scholar]
- 5.Sakauye L, Servas F, O’Connor K, Cottonaro C.. An in vitro method to quantitiate gaseous microemboli production of bubble oxygenators. J Extra Corpor Technol. 1982;14:445–52. [PubMed] [Google Scholar]
- 6.Matte GS, Kussman BD, Wagner JW, et al. Massive air embolism in a Fontan patient. J Extra Corpor Technol. 2011;43:79–83. [PMC free article] [PubMed] [Google Scholar]
- 7.Kotter JP, Cohen DS.. The Heart of Change—Real Life Stories of How People Change their Organizations. Boston: Harvard Business Press; 2002. [Google Scholar]


