Abstract:
The delivery of gaseous microemboli (GME) by the cardiopulmonary bypass circuit should be minimized whenever possible. Innovations in components, such as the integration of arterial line filter (ALF) and ALFs with reduced priming volumes, have provided clinicians with circuit design options. However, before adopting these components clinically, their GME handling ability should be assessed. This study aims to compare the GME handling ability of different oxygenator/ALF combinations with our currently utilized combination. Five commercially available oxygenator/ALF combinations were evaluated in vitro: Terumo Capiox SX25RX and Dideco D734 (SX/ D734),Terumo Capiox RX25R and AF125 (RX/AF125),Terumo FX25R (FX), Sorin Synthesis with 102 μm reservoir filter (SYN102), and Sorin Synthesis with 40 μm reservoir filter (SYN40). GME handling was studied by introducing air into the venous return at 100 mL/min for 60 seconds under two flow/ pressure combinations : 3.5 L/min, 150 mmHg and 5 L/min, 200 mmHg. Emboli were measured at three positions in the circuit using the Emboli Detection and Classification (EDAC®) Quantifier and analyzed with the General Linear Model. All circuits significantly reduced GME. The SX/D734 and SYN40 circuits were most efficient in GME removal whilst the SYN102 handled embolic load (count and volume) least efficiently (p < .001). A greater number of emboli <70 μm were observed for the SYN102, FX and RX/AF125 circuits (p < .001). An increase in embolic load occurred with higher flow/pressure in all circuits (p < .001). The venous reservoir significantly influences embolic load delivered to the oxygenator (p < .001). The majority of introduced venous air was removed; however, significant variation existed in the ability of the different circuits to handle GME. Venous reservoir design influenced the overall GME handling ability. GME removal was less efficient at higher flow and pressure, and for smaller sized emboli. The clinical significance of reducing GME requires further investigation.
Keywords: cardiopulmonary bypass, perfusion, gaseous emboli, cardiac surgery
The susceptibility of the brain to microembolic damage has been the focus of many studies over the last four decades (1). In cardiac surgery, microemboli have been demonstrated to cause neurological deficits in patients undergoing cardiopulmonary bypass (CPB) (2). Pugsley et al. (3) showed that neuropsychologic deficits after cardiac surgery were associated with the number of microemboli delivered to the patients during CPB, highlighting the relationship between microembolic load and neuropsychological performance.
The embolic load delivered to the patient has been reported to be influenced by a multitude of different physical, technical, and human factors. A number of authors have shown that perfusion techniques (e.g., pH or temperature management) and perfusionist interventions (e.g., drug administration) contribute to the generation of gaseous microemboli (GME) in the CPB circuit (4,5). The behavior of GME within the CPB circuit is influenced by numerous physical factors including the complex interaction between flow, gaseous partial pressure, temperature, volume, solubility, fluid viscosity, buoyancy, perfusate, and circuit design (6). The design of the CPB circuits’ components have been found to influence GME handling and removal, with GME elimination rates varying among CPB circuits and components from different manufacturers (6–10). Circuit design is one of the most interesting and challenging areas for the perfusionist as the selection of components are usually under the auspice of the perfusion team. Mitchell et al. (7) reported that the most efficient removal of GME is able to be realized at those locations in the circuit where there is low blood velocity and high buoyancy exists, such as the venous reservoir; however, fluid dynamics, transit time, and the average height of the blood column in the reservoir will influence the final GME reduction.
Recent reports (8,10–12) have used the Emboli Detection and Classification (EDAC®) Quantifier (Luna Innovations Inc., Blacksburg, VA) monitoring system to evaluate the embolic load and handling capability in CPB circuits and individual circuit components. The EDAC® Quantifier, which uses fixed beam ultrasonic imaging, is purported to improve GME measurement compared with pulsed Doppler systems and is reported to record high frequency embolic events over a size range of 10 μm to 12,700 μm in diameter (13,14). Comparisons of CPB circuits and arterial line filters (ALFs) have highlighted the need for clinical teams to be aware of the different emboli handling capability of components of CPB circuits (10,11).
Development and innovations by manufacturers in CPB circuit components have provided options for clinicians in the components incorporated into the design of CPB circuits (e.g., oxygenators with integrated ALFs and components with reduced priming volumes); however, a comparison of the GME handling ability of different circuit designs should be assessed before making the decision to introduce new components or circuits into clinical practice. The primary aim of this study is to compare the GME handling ability of four different oxygenator/filter combinations and our currently used oxygenator and ALF. Our secondary aim was to determine the contribution of circuit components to the embolic load.
METHODS
CPB Circuits
The devices chosen for evaluation represent four different circuit combinations which allowed evaluation of oxygenators with integrated and non-integrated ALFs and combinations which would result in a reduction in total clinical prime volume. Four commercially available hard-shell venous reservoir (HVR), membrane oxygenator/ALF combinations were compared with our current clinical circuit, Capiox SX25RX (Terumo Cardiovascular Systems, Ann Arbor, MI) and D734 ALF (Dideco, Mirandola, Italy) (SX/D734), which afforded us a 47 μm polyester screen filter HVR and 40 μm arterial filter. We compared the Capiox RX25R (Terumo) with 47 μm polyester screen filter HVR in combination with a 37 μm AF125 arterial filter (Terumo) (RX/AF125), the Capiox FX25R (Terumo) with 47 μm polyester screen filter HVR and integrated arterial filter with pore size of 32 μm (FX), and the Synthesis with integrated arterial filter with pore size of 40 μm (Sorin Group, Milan, Italy). Two versions of the Synthesis were investigated, our initial experiments were performed with the original HVR configuration (outer filter consisted of 3 rows of 102 μm (SYN102)), whilst our final experiments were performed using the modified HVR (outer filter consisting of upper 2 rows of 102 μm and bottom row of 40 μm (SYN40)).
The membrane oxygenator and arterial filter combinations were studied in an in vitro circuit which replicated our clinical circuit (Figure 1). The tubing used in the circuit was SMART 3/8*3/32 (Sorin Group, Arvada, CO), with the exception of the venous return line (SMART 1/2*3/32, (Sorin)) and the pump boot (PHYSIO 3/8*3/32, (Sorin Group, Milan, Italy)). A second membrane oxygenator (Capiox SX25RX oxygenator) was used as a microemboli removal device (MRD) by applying vacuum to the inlet and outlet gas ports, a technique adapted from Dickinson et al. (10) and originally reported by Rudolph et al. (15). The venous reservoir of this device was used as a surrogate patient, positioned at a height corresponding to the operating table.
Figure 1.
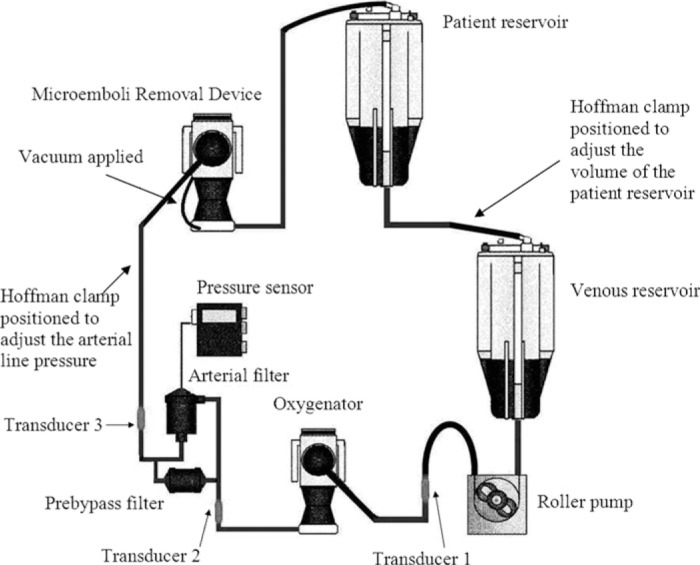
The in vitro experimental circuit depicting the non-integrated arterial filter and oxygenator components. Emboli were measured post venous reservoir and roller pump (Transducer 1), post oxygenator (Transducer 2), and post arterial filter (Transducer 3). In circuits with integrated arterial filters, the pre-bypass filter was retained, and emboli were measured post venous reservoir and roller pump (Transducer 1), and post-oxygenator and filter at Transducer 2 and also at Transducer 3 at an equivalent distance from the arterial outlet of the oxygenator as for those with non-integrated arterial line filters.
The systems were flushed with CO2 for 3 minutes and primed according to the manufacturer’s instructions using 3000 mL of Plasmalyte solution (Baxter, Australia). The occlusion of the arterial roller pump was set as per institutional protocol (by filling the arterial line to a height of 60 cm and adjusting the occlusion so that fluid level falls at a constant rate of 1 cm/30 seconds), the prime recirculated through a Pre-bypass Plus® filter (Pall Corporation, NY) for 2 minutes, and 1000 mL priming solution was removed. Heparin 10,000 IU (Roche, Basel, Switzerland) was added into the circuit and six units of donor blood were added to achieve a hematocrit of 22–25%. The donor blood was obtained from the Australian Red Cross Blood Service (Agreement Number 10–05SA-04). This study was approved by the Flinders Clinical Research Ethics Committee (Research Application 280/09).
Air Delivery Technique
The site for venous air entry was created by connecting an injection port onto the first luer connector on the oxygenator venous inlet. Air was delivered at a flow rate of 100 mL/min for 1 minute using a roller pump. A length of 1/4″ tubing connected to a 19 G needle was used to deliver the air, with GME measured for 3 minutes from the introduction of air into the circuit.
Emboli Detection
The EDAC® device was used to detect emboli in each experiment using three 5-mHz sonar-based transducers, located at fixed positions in the CPB circuit using three 3/8″ EDAC® connectors. The first transducer was positioned after the arterial roller pump and at a fixed distance (15 cm) from the inlet of the oxygenator, the second detector was positioned at a fixed distance (20 cm) from the outlet of the oxygenator, and a third was positioned a fixed distance (40 cm) after the ALF. For circuits with an integrated ALF, the position of the third transducer was the same fixed distance from the arterial outlet of the oxygenator as for those with non-integrated ALFs. Prior to measurement, recirculation through the circuit was maintained until the establishment of a minimal baseline embolic count rate. The EDAC® records the number of microemboli and displays output in 10 μm range increments. The emboli band recorded below 10 μm was not reported as per the manufacturer’s instructions. No microemboli >120 μm were detected.
Experimental Conditions
Three circuits of each of the five combinations were examined; for each circuit, air emboli challenges were measured five times at a flow rate of 3.5 L/min and a pressure of 150 mmHg, and five times at a flow rate of 5.0 L/min and a pressure of 200 mmHg. Experimental conditions were achieved by using a roller pump (Cobe Cardiovascular, Arvada, CO) and a gate clamp positioned on the arterial line. Solution temperature was maintained at 34 ± .5°C using a Sub Zero heater-cooler unit (Cincinnati, Ann Arbor, MI). Room air gas flow through the test oxygenator was maintained at 1.5 L/min. In all test circuits, the rate of drainage from the MRD was controlled by a gate clamp to maintain the volume of 1000 mL in the MRD reservoir and 1000 mL in the test reservoir. Oxygenator sampling manifolds remained open whilst oxygenator and ALF purge lines were closed to simulate our standard clinical protocols.
Statistical Analysis
The variability of the emboli volume and count data necessitated either logarithmic or square root transformation to approximate normality. Data were analyzed with the General Linear Model (SPSS version 18.0, SPSS Inc., Chicago, IL). We describe comparisons of GME volume and GME count in size ranges. A comparison of each overall circuit was made by analyzing Transducer 3 values using analysis of variance according to counts in terms of GME size. Comparisons of emboli count were made at all three transducer sites, and emboli volume at Transducer 1 and 3. Analysis of emboli count and volume included consideration of the controlled manipulations of flow rate and pressure to compare the overall circuit, and the HVR. Analysis of variance post-hoc tests used Fisher’s Least Significant Difference correction method with p < .05 considered statistically significant. Estimate of effect sizes (ηp2) were defined as small (.20), medium (.50), and large (.80) as described by Cohen (16).
RESULTS
Raw data of emboli counts and volume are displayed in Table 1 and Table 2 respectively.
Table 1.
Total emboli count versus circuit type, flow rate and pressure, transducer, and size range.
| Size Range (μm) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transducer | Circuit | Pressure/Flow | N | 10–20 | 20–30 | 30–40 | 40–50 | 50–60 | 60–70 | 70–80 | 80–90 | 90–100 | 100–110 | 110–120 |
| 1 | SX/D734 | A | 15 | 2257 (734) | 1009 (297) | 394 (107) | 111 (36) | 46 (18) | 20 (7.6) | 11 (5.7) | 6.3 (3.3) | 3.5 (2.5) | 1.4 (.9) | 1.5 (1.6) |
| B | 15 | 3999 (847) | 1848 (316) | 877 (170) | 293 (70) | 114 (37) | 46 (16) | 21 (10) | 9.4 (5.5) | 6.9 (3.6) | 3.4 (3.0) | 1.5 (1.3) | ||
| RX/AF125 | A | 15 | 2247 (428) | 1099 (174) | 456 (80) | 152 (25) | 61 (17) | 26 (8.1) | 14 (7.3) | 7.5 (5.1) | 4.0 (2.3) | 1.5 (1.2) | 1.2 (1.5) | |
| B | 15 | 3507 (762) | 1695 (301) | 844 (132) | 312 (52) | 147 (32) | 66 (21) | 35 (15) | 19 (11) | 13 (7.7) | 6.1 (5.0) | 4.7 (4.4) | ||
| FX | A | 15 | 1643 (425) | 785 (232) | 289 (90) | 86 (32) | 31 (11) | 11 (4.1) | 4.7 (3.4) | 2.5 (2.1) | .6 (.7) | 0 | .1 (.4) | |
| B | 15 | 2572 (1001) | 1214 (582) | 507 (257) | 153 (86) | 56 (31) | 20 (9.5) | 10 (6.9) | 4.0 (2.3) | 1.2 (.9) | .93 (1.4) | .6 (1.1) | ||
| SYN102 | A | 15 | 1378 (632) | 694 (308) | 425 (189) | 217 (95) | 125 (59) | 73 (39) | 47 (25) | 26 (13) | 18 (9) | 11 (6.7) | 8.2 (4.7) | |
| B | 15 | 1484 (543) | 748 (263) | 463 (179) | 238 (96) | 148 (70) | 85 (47) | 49 (25) | 33 (20) | 25 (15) | 14 (8.9) | 10 (7.2) | ||
| A | 15 | 198 (57) | 60 (22) | 15 (6.7) | 3.2 (1.9) | 1.5 (1.1) | .5 (.6) | .4 (.7) | 0 | 0 | 0 | 0 | ||
| B | 15 | 599 (221) | 284 (118) | 105 (50) | 30 (14) | 11 (5.5) | 3.9 (2.3) | 2.1 (1.5) | 1.5 (1.8) | .5 (.8) | .2 (.6) | 0 | ||
| 2 | SX/D734 | A | 15 | 63 (44) | 10 (7.7) | 2.1 (3) | .3 (.6) | .1 (.4) | .1 (.3) | 0 | 0 | 0 | 0 | 0 |
| B | 15 | 188 (164) | 41 (41) | 6.0 (6.4) | 1.0 (1.5) | .1 (.4) | .07 (.3) | 0 | .1 (.3) | 0 | 0 | 0 | ||
| RX/AF125 | A | 15 | 152 (83) | 31 (16) | 5.3 (3.1) | 1.3 (1.4) | .6 (.6) | .1 (.4) | 0 | 0 | 0 | 0 | 0 | |
| B | 15 | 457 (166) | 110 (45) | 22 (6.8) | 3.9 (1.5) | 1.7 (1.3) | .5 (.7) | .1 (.3) | 0 | 0 | 0 | 0 | ||
| FX | A | 15 | 96 (47) | 15 (12) | 3.7 (3.2) | 1.1 (1.8) | .3 (.5) | .1 (.5) | .1 (.3) | 0 | 0 | 0 | 0 | |
| B | 15 | 261 (105) | 57 (33) | 14 (9.9) | 3.1 (2.3) | 1.5 (2.3) | .4 (1.1) | .3 (.6) | .1 (.3) | .1 (.3) | 0 | 0 | ||
| SYN102 | A | 15 | 297 (183) | 151 (83) | 69 (35) | 18 (6.5) | 7.1 (4.1) | 3.1 (1.7) | 1.2 (1.3) | .5 (.8) | .1 (.3) | .1 (.4) | .1 (.3) | |
| B | 15 | 378 (143) | 232 (93) | 122 (41) | 41 (12) | 17 (6.3) | 5.1 (3.4) | 2.1 (2.0) | 1.1 (1.1) | .4 (.6) | .3 (.6) | .4 (.6) | ||
| SYN40 | A | 15 | 0 | .1 (.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| B | 15 | 5.9 (13) | 2.3 (6.7) | 1.7 (4.5) | .4 (1.3) | .1 (.3) | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | SX/D734 | A | 15 | .8 (1.3) | .2 (.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 15 | 3.0 (3.1) | .6 (.9) | .2 (.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| RX/AF125 | A | 15 | 5.9 (4.9) | 5.9 (4.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| B | 15 | 28 (19) | 5.5 (3.9) | 1.3 (1.2) | .1 (.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| FX | A | 15 | 10 (7.2) | 1.6 (2.6) | .5 (.6) | .1 (.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| B | 15 | 46 (31) | 9.7 (6.1) | 2.4 (1.9) | .5 (.6) | .1 (.3) | 0 | .1 (.3) | 0 | 0 | 0 | 0 | ||
| SYN102 | A | 15 | 221 (132) | 98 (53) | 40 (25) | 10 (6.8) | 4.3 (3.2) | 1.9 (3.3) | 1.0 (1.6) | .5 (.5) | .2 (.6) | .1 (.4) | 0 | |
| B | 15 | 288 (114) | 156 (61) | 77 (30) | 31 (12) | 9.6 (3.6) | 4.1 (2.7) | 1.1 (1.1) | .3 (.5) | .2 (.4) | 0 | .1 (.3) | ||
| SYN40 | A | 15 | .7 (1.1) | 0 | .7 (.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| B | 15 | 3.3 (8.4) | 1.9 (5.3) | .5 (1.6) | .2 (.8) | .1 (.5) | 0 | 0 | 0 | 0 | 0 | 0 | ||
Raw data presented as mean (±standard deviation) with the emboli size range in μm.
A: Flow rate of 3.5 L/min and a pressure of 150 mmHg.
B: Flow rate of 5.0 L/min and a pressure of 200 mmHg.
N, number of experiments.
Table 2.
Total emboli volume versus circuit type, flow rate and pressure, and transducer.
| Volume (mL) |
||||||
|---|---|---|---|---|---|---|
| Circuit | Pressure/Flow | N | Transducer 1 | Transducer 2 | Transducer 3 | |
| SX/D734 | A | 15 | 4.2 (1.2) | .025 (.022) | .00078 (.002) | |
| B | 15 | 8.8 (2.2) | .084 (.082) | .0013 (.0017) | ||
| RX/AF125 | A | 15 | 4.9 (1.3) | .072 (.036) | .0015 (.0012) | |
| B | 15 | 11 (3.8) | .25 (.079) | .013 (.0075) | ||
| FX | A | 15 | 2.6 (.81) | .046 (.036) | .0040 (.0037) | |
| B | 15 | 4.5 (2.2) | .16 (.11) | .039 (.054) | ||
| SYN102 | A | 15 | 15 (8.7) | .58 (.25) | .38 (.25) | |
| B | 15 | 17 (9.9) | 1.1 (.34) | .69 (.27) | ||
| SYN40 | A | 15 | .16 (.048) | .000081 (.00016) | .00032 (.0005) | |
| B | 15 | 1.0 (.51) | .0091 (.026) | .0057 (.018) | ||
Raw data presented as mean × 10−5 (±standard deviation × 10−5).
A: Flow rate of 3.5 L/min and a pressure of 150 mmHg.
B: Flow rate of 5.0 L/min and a pressure of 200 mmHg.
N, number of experiments.
Overall Gaseous Microemboli Handling Ability (Transducer 3)
The analysis of emboli count data recorded at Transducer 3 showed that there was a significant difference in the way the different circuit combinations influenced the measured embolic load [F (4, 1540) = 1294.33, p < .001, ηp2 = .77]. The SX/D734 and SYN40 circuits were comparable and had the lowest total emboli counts. The RX/AF125 and FX circuits were comparable whilst the SYN102 had the greatest emboli counts. Examination of the distribution of total emboli count according to their size indicated that significantly less emboli were recorded above the 70–80 μm range [F (10, 1540) = 660.61, p < .001, ηp2 = .82]. The interaction between circuit, emboli size, and emboli count shown in Figure 2 suggests that a greater number of smaller emboli were observed for SYN102, FX and RX/AF125 circuits. The interaction between flow/pressure and emboli size suggested more emboli were recorded at the higher flow and pressure [F (10, 1540) = 24.81, p < .001, ηp2 = .14].
Figure 2.
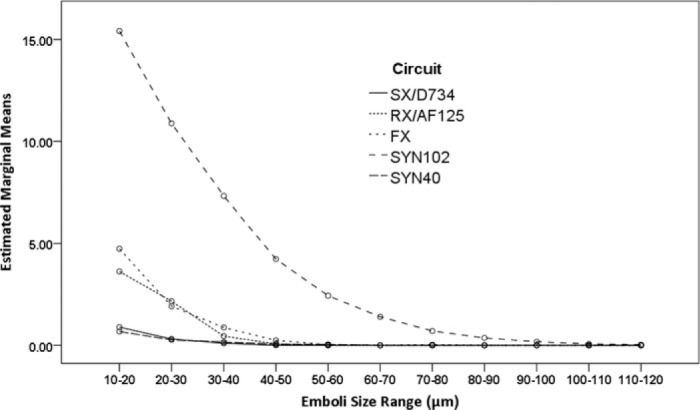
Estimated marginal means of square root transformed counts of emboli detected at Transducer 3 (after the arterial filter) at 3.5 L/min, 150 mmHg, and 5.0 L/min, 200 mmHg according to the size range of emboli. The SX/D734 and SYN40 circuits had the lowest total emboli counts, followed by the RX/AF125 and FX circuits, whilst the SYN102 had the greatest emboli counts (p < .001). Significantly less emboli were recorded above the 70–80 μm range (p < .001). (SX/D734; Capiox SX25RX oxygenator and D734 arterial filter, RX/AF125; Capiox RX25R oxygenator and AF125 arterial filter, FX; Capiox FX25R oxygenator, SYN102; Synthesis oxygenator with original venous reservoir, SYN40; Synthesis oxygenator with modified venous reservoir).
When total emboli volume were analyzed at Transducer 3, post-hoc comparisons suggested that the SX/D734 and SYN40 circuits were comparable and produced significantly less total emboli volume, the RX/AF125 and FX fared equally next best followed by the SYN102 [F (4, 140) = 277.79, p < .001, ηp2 = .89] (Figure 3). The interaction between circuit and flow/pressure suggested that higher emboli volume was observed, on average, at higher flow and pressure [F (4, 140) = 7.380, p < .001, ηp2 = .17].
Figure 3.
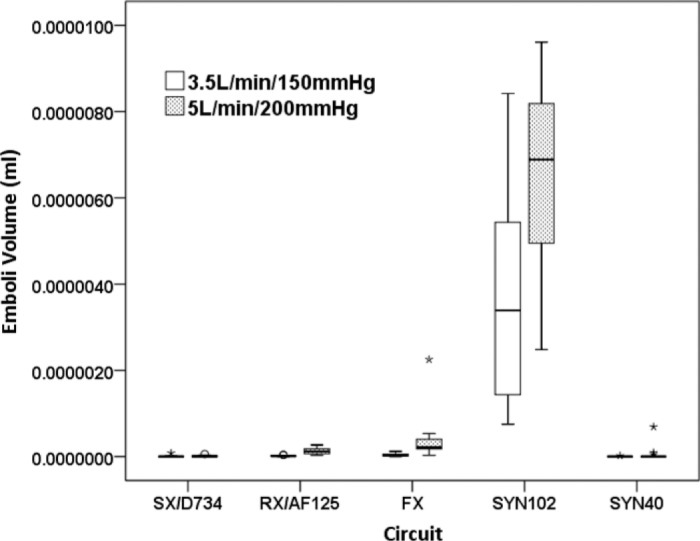
The lowest emboli volume was measured at Transducer 3 in the SX/D734 and SYN40 circuits, followed by the RX/AF125 and FX, and highest in the SYN102 (p < .001). Emboli volume was increased at higher flow and pressure (p < .001). (SX/D734; Capiox SX25RX oxygenator and D734 arterial filter, RX/AF125; Capiox RX25R oxygenator and AF125 arterial filter, FX; Capiox FX25R oxygenator, SYN102; Synthesis oxygenator with original venous reservoir, SYN40; Synthesis oxygenator with modified venous reservoir).
Component Gaseous Microemboli Handling
Post Venous Reservoir and Post Roller Pump (Transducer 1):
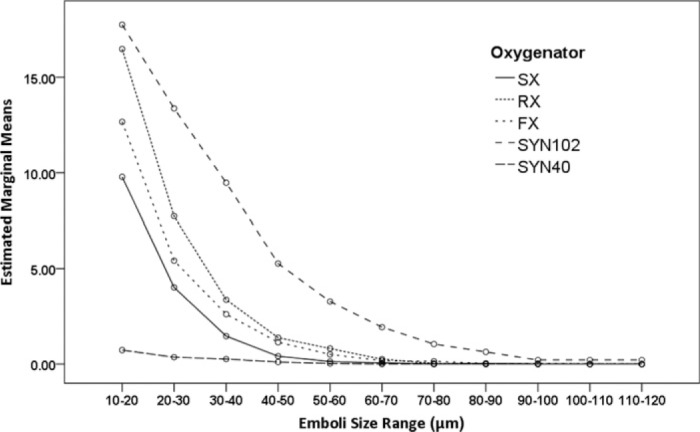
The analysis of emboli count data recorded at Transducer 1 showed there was a significant difference in the way the HVR handled the delivered embolic load [F (4, 1540) = 1190.72, p < .001, ηp2 = .76]. All HVRs performed differently from each other, SYN40 HVR had the lowest total emboli counts, followed by the FX, SX, RX, and the SYN102. Comparisons of the effect of emboli size indicated that differences were observed over the various size ranges measured [F (10, 1540) = 2804.94, p < .001, ηp2 = .95]. The interaction of HVR and emboli size, shown in Figure 4 suggests that in general a greater number of smaller emboli were detected for each HVR. The interaction of flow/pressure and emboli size suggested more emboli were recorded at the higher flow rate [F (10, 1540) = 5.63, p < .001, ηp2 = .04].
Figure 4.
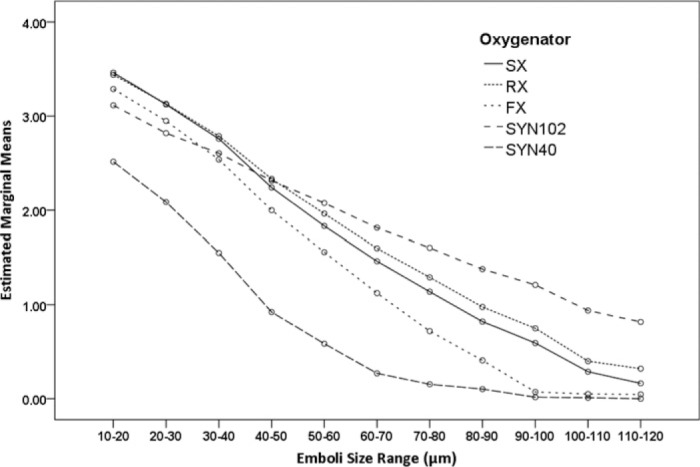
Estimated marginal means of log transformed counts of emboli detected after the venous reservoir at 3.5 L/min, 150 mmHg, and 5.0 L/min, 200 mmHg according to the size range of emboli. There was a difference in the way the venous reservoir handled the delivered embolic load (p < .001); the SYN40 reservoir had the lowest total emboli counts, followed by the FX25R, SX25, RX25R, and the SYN102. These differences were maintained over each size range. (SX; Capiox SX25RX oxygenator, RX; Capiox RX25R oxygenator, FX; Capiox FX25R oxygenator, SYN102; Synthesis oxygenator with original venous reservoir, SYN40; Synthesis oxygenator with modified venous reservoir).
There was an overall effect on total volume of emboli measured with different HVRs [F (4, 140) = 89.34, p < .001, ηp2 = .72]. Significantly lower emboli volume was recorded after the HVR of the SYN40; the FX produced the second least GME volume followed by the SX and RX HVRs, whereas higher emboli volumes were recorded distal to the SYN102 HVR (Figure 5). The interaction of HVR and flow/pressure suggested that higher emboli volume was observed, on average, at higher flow and pressure [F (1, 140) = 37.90, p < .001, ηp2 = .21].
Figure 5.
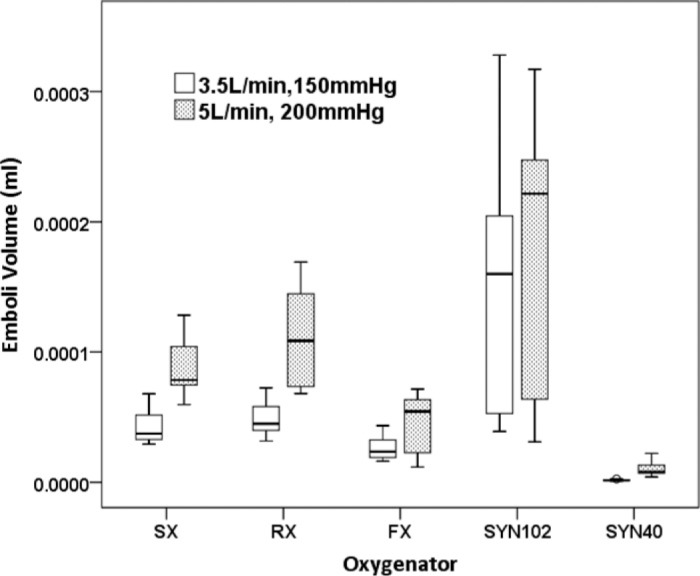
Total emboli volume was lower after the venous reservoir of the SYN40, followed by the FX, the SX, and RX, and the SYN102 oxygenators. Emboli volume was increased at higher flow and pressure (p < .001). (SX; Capiox SX25RX oxygenator, RX; Capiox RX25R oxygenator, FX; Capiox FX25R oxygenator, SYN102; Synthesis oxygenator with original venous reservoir, SYN40; Synthesis oxygenator with modified venous reservoir).
Post Oxygenator with or without Arterial Filter (Transducer 2):
The analysis of count data at Transducer 2 showed an effect for HVR/oxygenator type [F (4, 1540) = 494.96, p < .001, ηp2 = .56], with the SYN40 having the lowest total emboli counts, followed by the SX, FX, RX, and SYN102 (Figure 6).
Figure 6.
Estimated marginal means of square root transformed counts of emboli detected post oxygenator (or post oxygenator and arterial filter in the integrated circuits) at 3.5 L/min, 150 mmHg, and 5.0 L/min, 200 mmHg according to the size range of emboli. The SYN40 had the lowest total emboli counts, followed by the SX, FX, RX, and SYN102. (SX; Capiox SX25RX oxygenator, RX; Capiox RX25R oxygenator and AF125 arterial filter, FX; Capiox FX25R oxygenator, SYN102; Synthesis oxygenator with original venous reservoir, SYN40; Synthesis oxygenator with modified venous reservoir).
DISCUSSION
The decision to introduce changes to the components of the clinical CPB circuit requires rigorous evaluation to ensure quality assurance measures are met prior to any adoption of change. Often, the lack of published independent data may impair our ability to adequately assess new technology. In this report, we evaluated four alternative oxygenator/ALF combinations, which were commercially available for our use. All circuits removed the majority of introduced air; however, we were able to clearly demonstrate that there were variations in the ability of different circuits to handle the embolic load we delivered. Similar findings have been observed in previous studies of GME handling ability of different circuits (8–10).
Our overall circuit comparison compared GME recorded at Transducer 3 and has the benefit of simply demonstrating whether a difference in GME between various circuits would, if used in clinical practice, be delivered through the arterial line. Comparisons at Transducer sites 1 and 2 allowed us to examine the possible influence of different components on GME transit through the circuit. Examination of our overall circuit comparisons demonstrated that the only circuit equivalent to our current circuit (SX/D734) in terms of GME handling ability was the SYN40. The variation in GME handling ability of the circuits was influenced by components ability to handle GME. Transducer 1 recordings reflect GME observed after transiting through the HVR and provides a baseline estimate of the total load delivered to the oxygenator and ALF. We observed that there were differences both within and between manufacturers HVRs. This difference is most evident in the improvement in the modified Sorin Synthesis HVR, in which the lowest of the three screen filters has been replaced by a 40 μm filter, compared with the previous version with three rows of 102 μm. Variation in HVR GME handling has previously been reported (17,20).
At Transducer 2 we compared the ability of the oxygenator or oxygenator/integrated arterial filter components to remove GME, and we observed differences both within and between circuits of different manufacturers. Comparing the non-integrated design, we found the SX25RX to be better than the RX25R. We also observed differences in the integrated design, notably the modified SYN40 was better than the unmodified SYN102 and the FX. It appears that the number and distribution of emboli size plays a role in GME handling ability. In the SYN102 trials a greater number of smaller emboli (<70 μm) were delivered by the HVR and these were transmitted through the oxygenator and filter resulting in a greater embolic load at Transducer 2 than the SYN40. Evaluating two versions of the Synthesis oxygenator provided us with the opportunity to compare the influence of the different HVRs on identical oxygenator/ALF components. Conversely, evaluating the SX25RX and RX25 oxygenators provided us with the opportunity to compare different oxygenator and ALF combinations using identical HVRs. Although we observed a statistical difference in the embolic load after all HVRs, the distribution of emboli measured with the SX25RX and RX25 HVRs was similar and may be attributed to the variation and number of emboli encountered at this location in the circuit.
The incorporation of an ALF into the oxygenator potentially affords a number of benefits including ease of setup and reduced number of connectors, decreased priming volume, and the reduced requirement for CO2 priming (18). Cardiopulmonary bypass circuit design influences GME handling and this and previous studies demonstrate that the ability to handle GME in new circuit technologies must be investigated (10). We found that only the integrated ALF design of the SYN40 was equivalent to the SX/D734 combination we currently use.
One clinically related factor in the circuits that we chose for this evaluation was that each resulted in a reduction in our overall prime volume. Specifically, the combined priming volume of the SX/D734 combination was 590 mL, compared with 375 mL for the RX/AF125 combination, 260 mL for the FX, and 430 mL for the SYN102 and SYN40. From this investigation the only reduced priming volume circuit alternative to have equivalent GME handling ability in comparison to our current circuit was the SYN40, however we did not investigate the independent influence of priming volume on GME handling ability. The issues concerning the relative benefits of reduced priming volume versus the influence of GME handling ability in relation to clinical outcome are outside of the scope of this evaluation.
It is evident in the results of this study that an increase in both flow rate and pressure had an influence on embolic load, with higher counts and volumes observed for all circuits. According to the ideal gas law, an increase in pressure should result in a decrease in gas volume, highlighting the influence of flow on embolic load. The influence of flow and pressure were not studied independently, as we aimed to simulate two conditions of flow and pressure that may be encountered clinically. This is consistent with the finding of DeSomer et al. (12).
Dickinson et al. (10) reported a comparison of oxygenator GME handling ability using the EDAC®, at which time they described the lack of a standard in GME measurement and reporting. To date, although the EDAC® has been consistently reported for GME measurement, a standard of reporting has not yet emerged. Both in vitro and in vivo settings have been reported in the evaluation of CPB circuit GME handling (6,10,17–19). Some issues with comparative in vivo evaluations include the variation in embolic load observed in the clinical setting, evident in recent studies using EDAC® (18,19), and the lack of generalizability of the findings due to the variation in individual practices. Major contributing factors leading to generation of GME may include entrained venous air and the use of the aortic or ventricular vent suction (17,19). The comparative GME handling of integrated and non-integrated ALF designs has been reported in an in vivo study in a pediatric population (18). The authors were unable to demonstrate any significant difference between integrated and non-integrated components. This potentially was due to the large variation in embolic load reported resulting in the sample size being inadequate to detect statistically significant differences.
The objective of comparative GME handling evaluations from a quality assurance perspective is ultimately to determine whether alternate devices will differ from that of the device(s) currently used clinically. The in vitro setting allows a standardized embolic load to be delivered under controlled conditions. Although the clinical relevance of the embolic load we used may be questioned, the methodology allows reproducibility, reduced variation in measurement, and therefore, increased likelihood that the objectives of the study will be achieved. The most important advantage of assessment of GME handling in vitro is that it allows alternative devices to be evaluated without subjecting patients to a potential increase in GME.
The practical implications of this study relate to arterial GME delivery, and the influence of the manufacturer’s component modifications in the reduction of GME. The influence of the manufacturer’s modification to the venous reservoir in the Synthesis oxygenator highlights the efficiency in removing emboli prior to delivery to the roller pump. These results support a paradigm shift in the contemporary emphasis on the oxygenator and arterial filter as GME removal components, as previous literature by Myers et al. (17), DeSomer (20), and Groom et al. (21) describe improved GME removal using cardiotomy and venous filtration attributed to reduced fractionation of GME. There is no consensus as to the most appropriate metric of emboli (count, size, or volume) in the investigation of the association with clinically significant deteriorations in cognitive functioning after cardiac surgery. Similarly whilst there are only limited studies evaluating microbubble and cognitive function, dismissing small emboli as being clinically not relevant may be a serious misjudgment. The complex interactions of microbubbles and the microvasculature has been demonstrated, with microbubbles causing significant damage to the glycocalyx and an obstruction to blood flow, resulting in tissue damage, and activation of the complement and inflammatory response (22). We encourage further investigation as to the merits of measuring GME, clarification of how best to analyze the resultant data sets, and the impact of GME on the microcirculation, neuropsychological sequelae, and mood states following cardiac surgery.
Limitations
In vitro design affords control over the experimental environment but removes the real life elements of the clinical situation. We recorded GME activity with three EDAC® transducers and our aim was to evaluate GME at three points in the circuit after the HVR; however we were unable to confirm the embolic load delivered to the circuit. We adopted a standardized method of air introduction to minimize variation, which demonstrated in preliminary experiments an acceptable variation in GME counted. We found that to minimize variation, the air was infused via the luer on the venous inlet to the HVR, hence making a confirmation measurement impossible. These comparative findings are limited to one type of embolic delivery method to the venous line of the circuits. DeSomer et al.’s (12) most recent results suggest that the EDAC® may in fact not completely nor accurately report the embolic activity that may occur in the CPB situation. They found the activity reported by the EDAC® to be 18% of total count compared with industrial reference techniques at 3 L/min and 3% at 6 L/min. Although the quantitative values reported with the EDAC® should be interpreted with caution as they may be under estimated, these limitations should not compromise the value of comparative quality assurance studies performed with the EDAC® and suggest future continued development is required. In addition a potential source of bias for our findings arises from the necessity to perform the SYN40 series of experiments after the completion of the other four groups. However, all experiments were performed by the same operator under identical conditions.
CONCLUSION
Each oxygenator/ALF combination resulted in the majority of introduced venous air being removed; however significant variation was observed in the ability of the different circuits to handle GME. The SX/D734 and SYN40 circuits performed significantly better than the RX/AF125 and FX, followed by the SYN102. The design of the venous reservoir influenced the ability of the oxygenator/arterial filter combinations. The reduced priming volume circuit alternatives had reduced GME handling ability in comparison to our current circuit, the integrated ALF design alternative was equivalent only with the SYN40 oxygenator. The clinical significance of reducing GME requires further investigation.
ACKNOWLEDGMENTS
The authors wish to acknowledge the Australian Red Cross Blood Service for their provision of red blood cells used for our experiments, and to Cellplex Pty Ltd and Terumo Corporation Australian branch for the provision of perfusion disposables, and Luna Innovations Inc. for the supply of the EDAC® Quantifier and disposables.
REFERENCES
- 1.Barak M, Katz Y.. Microbubbles: Pathophysiology and clinical implications. Chest. 2005;128:2918–32. [DOI] [PubMed] [Google Scholar]
- 2.Stump DA.. Embolic factors associated with cardiac surgery. Semin Cardiothorac Vasc Anesth. 2005;9:151–2. [DOI] [PubMed] [Google Scholar]
- 3.Pugsley W, Klinger L, Paschalis C, Treasure T, Harrison M, Newman S.. The impact of microemboli during cardiopulmonary bypass on neuropsychological functioning. Stroke. 1994;25:1393–9. [DOI] [PubMed] [Google Scholar]
- 4.Borger MA, Peniston CM, Weisel RD, Vasiliou M, Green RE, Feindel CM.. Neuropsychologic impairment after coronary artery bypass surgery. Effect of gaseous microemboli during perfusionist interventions. J Thorac Cardiovasc Surg. 2001;121:743–9. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez RA, Williams KA, Babaev A, Rubens F, Nathan HJ.. Effect of perfusionist technique on cerebral embolization during cardiopulmonary bypass. Perfusion. 2005;20:3–10. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell SJ, Willcox T, McDougal C, Gorman DF.. Emboli generation by the Medtronic Maxima hard-shell adult venous reservoir in cardiopulmonary bypass circuits: A preliminary report. Perfusion. 1996;11:145–55. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell SJ, Willcox T, Gorman DF.. Bubble generation and venous air filtration by hard-shell venous reservoirs: A comparative study. Perfusion. 1997;12:325–33. [DOI] [PubMed] [Google Scholar]
- 8.Jones TJ, Deal DD, Vernon JC, Blackburn N, Stump DA.. How effective are cardiopulmonary bypass circuits at removing gaseous micro-emboli. J Extra Corpor Technol. 2002;34:34–9. [PubMed] [Google Scholar]
- 9.Weitkemper HH, Oppermann B, Spilker A, Knobl HJ, Körfer R.. Gaseous microemboli and the influence of microporous membrane oxygenators. J Extra Corpor Technol. 2005;37:256–64. [PMC free article] [PubMed] [Google Scholar]
- 10.Dickinson TA, Riley JB, Crowley JC, Zabetakis PM.. In vitro evaluation of the air separation ability of four cardiovascular manufacturers extra-corporeal circuit designs. J Extra Corpor Technol. 2006;38:206–13. [PMC free article] [PubMed] [Google Scholar]
- 11.Riley JB.. Arterial line filters ranked for gaseous micro-emboli separation performance: An in vitro study. J Extra Corpor Technol. 2008;40:21–6. [PMC free article] [PubMed] [Google Scholar]
- 12.De Somer FMJJ, Vetrano MR, Van Beeck JPAJ, Van Nooten GJ.. Extracorporeal bubbles: A word of caution. Interact Cardiovasc Thorac Surg. 2010;10:995–1001. [DOI] [PubMed] [Google Scholar]
- 13.Lynch JE, Riley JB.. Microemboli detection on extracorporeal bypass circuits. Perfusion. 2008;23:23–32. [DOI] [PubMed] [Google Scholar]
- 14.Emboli Detection and Classification (EDAC®) Quantifier Users Guide. Blacksburg, VA: Luna Innovations; 2008. [Google Scholar]
- 15.Rudolph JL, Tilahun D, Treanor PR, et al. Use of a large bore syringe creates significantly fewer high intensity transient signals (HITS) into a cardiopulmonary bypass system than a small bore syringe. Perfusion. 2006;21:67–71. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J.. A power primer. Psychol Bull. 1992;112:155–9. [DOI] [PubMed] [Google Scholar]
- 17.Myers GJ, Voorhees C, Haynes R, Eke B.. Post-arterial filter gaseous microemboli activity of five integral cardiotomy reservoirs during venting: An in vitro study. J Extra Corpor Technol. 2009;41:20–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Preston TJ, Gomez D, Olshove VF, Phillips A, Galantowicz M.. Clinical gaseous microemboli assessment of an oxygenator with integral arterial filter in the pediatric population. J Extra Corpor Technol. 2009;41:226–30. [PMC free article] [PubMed] [Google Scholar]
- 19.Willcox TW, Mitchell SJ.. Microemboli in our bypass circuits: A contemporary audit. J Extra Corpor Technol. 2009;41:31–7. [PMC free article] [PubMed] [Google Scholar]
- 20.De Somer FMJJ.. Impact of oxygenator characteristics on its capability to remove gaseous microemboli. J Extra Corpor Technol. 2007;39:271–3. [PubMed] [Google Scholar]
- 21.Groom RC, Quinn RD, Lennon P, et al. Detection and elimination of microemboli related to cardiopulmonary bypass. Circ Cardiovasc Qual Outcomes. 2009;2:191–8. [DOI] [PubMed] [Google Scholar]
- 22.Barak M, Nakhoul F, Katz Y.. Pathophysiology and clinical implications of microbubbles during hemodialysis. Semin Dial. 2008;21:232–8. [DOI] [PubMed] [Google Scholar]


