Abstract:
Microemboli are implicated in neurological injury; therefore, the extracorporeal circuit (ECC) should not generate microbubbles or transmit introduced air. The venous reservoir is the first component in the ECC designed to remove introduced air. The purpose of this study was to investigate the relative safety of two kinds of adult venous reservoirs—the closed soft-shell venous reservoir (SSVR [Medtronic CBMVR 1600]) and the open hard-shell venous reservoir (HSVR [Affinity NT CVR])—in terms of microbubble generation and introduced air transmission. A recirculating in-vitro circuit was used to compare the two reservoirs with the SSVR further assessed in a fully closed or partially open state. Microbubbles were counted using a Hatteland CMD10 Doppler in the outflow of the reservoirs before (microbubble generation) and after infusing 20 mL/min of air into the venous line (microbubble transmission) while altering pump flow rates (3 L/min; 5 L/min) and reservoir prime (200 mL; 700 mL). Negligible bubble generation was noted in the SSVRs at both flow rates and either reservoir volume. However, microbubble generation was significant in the HSVR at the higher flow rate of 5 L/min and lower reservoir volume of 200 mL. When infusing air, a flow of 3 L/min was associated with insignificant to small increases in microbubble transmission for all reservoirs. Conversely, infusing air while flowing at 5 L/min was associated with significantly more microbubble transmission for all reservoirs at both low and high reservoir volumes. The SSVR is as safe as the HSVR in microbubble handling as the generation and transmission of microbubbles by the SSVR is not more than the HSVR over a range of prime volumes and flow rates. As both reservoirs transmitted microbubbles at higher pump flow rates regardless of reservoir volumes, it is important to eliminate venous air entrainment during cardiopulmonary bypass.
Keywords: hard-shell venous reservoir, soft-shell venous reservoir, microbubbles
Cardiopulmonary bypass (CPB) has been integral to the success of cardiac surgery by supporting the patient’s circulation during the procedure. While mortality rates have been declining, neurological injury remains an increasingly important complication especially as cardiac patients are becoming older and sicker (1). Etiologies of post cardiac surgery neurological injuries include hypoperfusion, systemic inflammatory response, and emboli (2). Microemboli are implicated as a major mechanism of neurological injury – particularly postoperative cognitive decline (3–5). Most microemboli occurring during cardiac surgery are gaseous
(6) with many originating from the extracorporeal circuit (ECC) as microbubbles (7,8). Sources of microbubbles derived from the ECC are varied and include entrained venous air (8), vent return, (9) and injections by the perfusionist (10). Ideally, the ECC should remove all introduced air. However, despite the air removal capabilities of the venous reservoir, oxygenator, and arterial filter, microbubbles continue to pass via the arterial line into the patient’s brain (8,10). Therefore, further improvements in the air handling capabilities of ECC components are warranted.
The venous reservoir is the first component in the ECC designed to remove introduced air. Two kinds of venous reservoirs are currently used: a more popular rigid, hard-shell venous reservoir (HSVR) and a soft-shell collapsible venous reservoir bag (SSVR) (11). Although studies have investigated the air handling capabilities of various HSVR and SSVR models (8,12–14), no known study has been published comparing an SSVR with an HSVR. To determine if an SSVR was as safe as an HSVR in terms of relative microbubble generation and transmission of introduced air, the Medtronic collapsible venous reservoir bag and Medtronic Affinity hardshell venous reservoir were compared in-vitro over a range of volumes and flow rates.
MATERIALS AND METHODS
Test Circuit
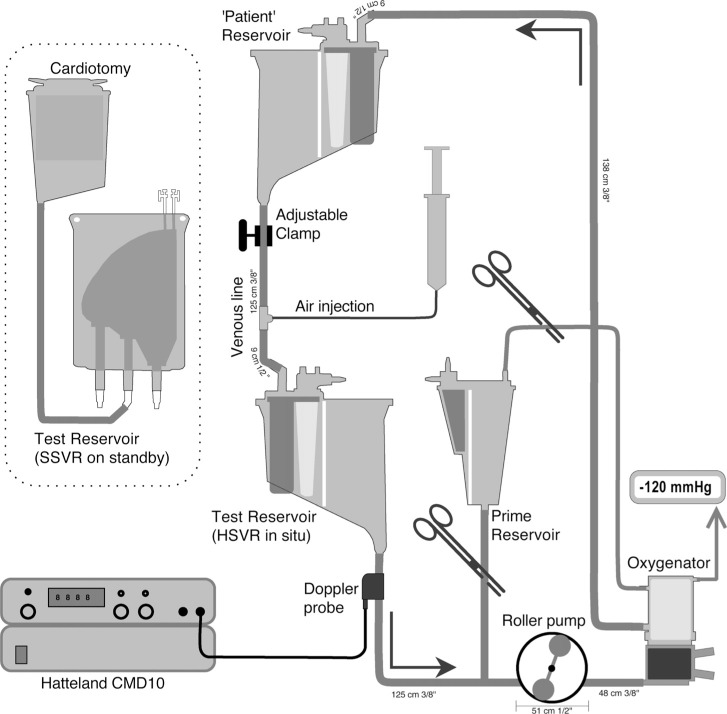
A recirculating in-vitro circuit was constructed (see Figure 1 for circuit details). The patient was simulated by a HSVR (Trillium Affinity NT 541T Integrated CVR, Medtronic, Minneapolis, MN). This “patient” reservoir was filled to the 1000 mL level marker and kept at a constant height (100 cm between the top of its fluid level to the outlet of the test reservoir) to aid in deairing the recirculating fluid and provide a constant siphonage drainage pressure.
Figure 1.
In-vitro circuit. See text for details. Note: not to scale.
During recirculation, the “patient” reservoir volume was maintained by adjusting a variable clamp positioned on the “venous line” connecting the “patient” reservoir to the test reservoir. Fluid entering the test reservoir was pumped into a hollow fiber membrane oxygenator (Trillium Affinity NT 541T, Medtronic, Minneapolis, MN) by a correctly occluded and calibrated roller pump (Sarns 8000, Terumo, Australia). This oxygenator was used to remove bubbles that had been introduced into the circuit; a process that was facilitated by connecting the oxygenator’s gas inlet port to suction (−120 mmHg) while sealing its other gas exhaust ports (thereby increasing the pressure gradient for microbubble elimination). From the oxygenator the fluid was returned back into the “patient” reservoir to be further debubbled. All circuit components were new.
Rapid and precise changes in test venous reservoir volumes were made by adding or draining prime via a volume calibrated cardiotomy reservoir (CB1351, Medtronic, Minneapolis, MN) positioned between the test reservoir and the roller pump. When setting a baseline, the test reservoir was bypassed. With a confirmed 700 mL in this “priming” reservoir and 1000 mL in the “patient” reservoir, the test reservoir was installed. The test reservoir was then primed by draining 700 mL or 200 mL as needed from the “priming” reservoir. No volume adjustment was made to account for hold-up volumes (dynamic prime volume) at the two flows used (3 L/min or 5 L/min). The prime consisted of 2500 mL of 4% albumin (Albumex 4, CSL Bioplasma, Australia) kept at room temperature (19–21°C)
Test Venous Reservoirs
Two reservoir models – both manufactured by Medtronic (Minneapolis, MN) –were assessed for their air handling performance: the soft-shell collapsible venous reservoir bag (model CBMVR 1600) and hard-shell open venous reservoir (Trillium Affinity NT 541T Integrated CVR). The SSVR is housed in a cage that can be opened or closed to respectively increase or decrease reservoir volumes. The SSVR was tested in a fully closed (minimal volume [SSVR-closed]) or in the one step out of latch position from its holder back plate (SSVR-open). The operation of the SSVR at both the fully closed and one latch open position was examined to understand any changes in air handling abilities between these two commonly used modes. To emulate its clinical application, the SSVR’s cardiotomy inlet was open to an attached cardiotomy positioned with its outlet level to the top of the SSVR.
Three copies of each reservoir model were used. In addition to the three HSVRs, as the three SSVRs were further investigated as fully closed or one latch open, there were three reservoir types (HSVR, SSVR-closed, SSVR-open). Thus, for the purposes of the experiment, a total of nine reservoirs were tested.
The SSVR has a 1600 mL volume capacity and incorporates an internal screen filter of 105 μm to assist in trapping air and other debris that may enter this reservoir from its venous and cardiotomy inlets. No minimum operating volume is noted in “instructions for use.” The HSVR is a top entry model consisting of a combined venous and cardiotomy reservoir. Venous blood passes a 200 μm inlet screen, before passing via a final reservoir screen filter of 150 μm. It has a recommended minimal operating volume of 200 mL.
Air Infusion
To emulate a continuous air entrainment via a venous cannula purse string scenario, a continuous infusion of air was introduced into the “venous line.” The venous air injection site was a Luer lock connector with injection plug inserted in the “venous line” 22 cm distal to the outflow of the “patient reservoir” – after the venous occluder. A 38 mm, 25-gauge hypodermic needle pierced the plug with the needle tip positioned in the midstream of the connector. A syringe pump (Graseby 3500, Smiths Medical Australasia Pty. Ltd., Queensland, Australia) was used to pump air in a 50 mL syringe through a 150 cm extension line into the injection site. Air was injected at 20 mL/min (0.33 mL/sec) for 65 seconds; after the first 5 seconds to purge the needle and reach a steady state, 60 seconds of recording was made to determine microbubble transmission.
Microbubble Detection
A Hatteland CMD 10 pulsed Doppler micobubble detector (Hatteland Instrumentation, Royken, Norway) was used to detect and count bubbles in the outflow line of the test venous reservoirs. A transluminal 13 mm (ID) microbubble detection probe was attached to the 3/8’’ tubing approximately 8 cm distal to the outflow of the test venous reservoir. The detection probe was attached to the Hatteland CMD 10; itself connected to a COMAC data acquisition unit interface that was supported by BUBMON version 2.6 software (Hatteland Instrumentation, Royken, Norway) running on a laptop computer. The Hatteland Doppler was configured for continuous detection at high resolution with attenuation set at 0 dB, and depth at 2.2 cm.
Calibration of this device was performed as per the CMD10 user manual using glass microspheres of known size in a closed loop circuit. Confirmation was made of the ability of the Hatteland to detect microbubbles in an approximately 20–100 μm size range. For the purposes of this study, only total microbubble counts were used. Ultrasonic gel was used to couple the probe to the tubing to ensure no air existed between the probe and the tubing. Also a Velcro strap was applied around the probe and a spacer to ensure a snug and consistent fit between the probe and tube, which was reapplied for every test run. To maximize the reproducibility of all readings, identical instrument settings were used and only one probe was applied; thereby, avoiding issues of subtle differences in probe sensitivities.
Test Procedure
At the beginning of each trial the microbubble detection probe was temporarily positioned on the “venous line” tubing immediately distal to the “patient” reservoir while air was infused at 20 mL/min over several minutes. This confirmed that the circuit was effective at removing the air that was infused into the “venous line” by observing that the recirculation of microbubbles was negligible (<5 counts/min).
The nine test reservoirs (3 HSVR, 3 SSVR-closed, 3 SSVR-open) were randomly subjected to two pump flows (3 L/min; 5 L/min) and two static reservoir volumes (200 mL; 700 mL). Both flow rates were seen as being representative of adult pump flows; the levels ranged from the minimum static recommended HSVR volume to a commonly occurring level. The sequencing of the reservoirs was also randomized. Thus, 36 test runs were performed in one trial; a total of three trials were run for a total of 108 tests.
Each of the test runs at the specified pump flow (3 L/min or 5 L/min) or added prime volume (200 mL or 700 mL) consisted of a first part whereby the microbubble generation of any test reservoir was examined by a baseline measurement of 60 seconds with no air injection, and a second part whereby the test reservoir’s introduced microbubble transmission performance was determined with air injected as described above. The SSVR was deaired before each trial by aspirating air via its highest port.
Statistical Analysis
Microbubble counts in tables are presented as medians and interquartile range. To compare the differences between the three venous reservoirs’ microbubble generation and transmission counts, non parametric comparisons (using Kruskal-Wallis test for more than two groups) were computed. The paired non parametric test (Wilcoxon Signed Rank test for paired groups) was used to compare the venous reservoir’s baseline versus air infusion microbubble transmission, and to compare the test venous reservoir’s difference in microbubble generation and transmission counts at varying flow rates and volumes. A null hypothesis of no difference between the median microbubble generation or transmission between three reservoir types was rejected if p < .05. Statistical analyses were performed using StatView (StatView; Abacus Concepts, Berkeley, CA).
RESULTS
Part A) Baseline – Microbubble Generation
-
Microbubble generation for each venous reservoir type
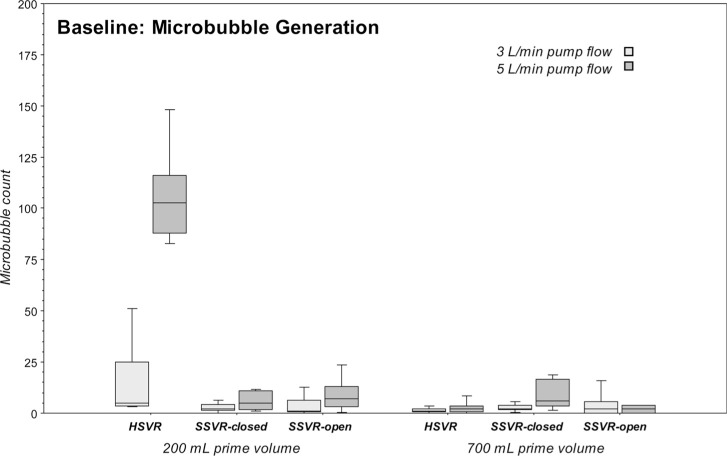
The microbubble generation differences of each venous reservoir type at pump flows 3 L/min versus 5 L/min at 200 mL or 700 mL prime were computed. No statistically significant changes in microbubble counts were seen with the higher flow rate of 5 L/min versus 3 L/min with the notable exceptions of a significant increase in the microbubbles generated by the HSVR at 200 mL (p = .017) and a much more moderate increase in microbubbles generated by the SSVR-closed at the 700 mL level (p = .049). See Table 1 and Figure 2.
-
Microbubble generation – comparison of all three venous reservoir types
The microbubble generation differences amongst the three venous reservoirs at both pump flows and both primes were computed. Bubble generation was relatively low for all venous reservoirs at all flow rates and reservoir volumes with the exception of the significant increase in the microbubbles generated by the HSVR at 5 L/min and 200 mL level (p = .0003). A more moderate though statistically significant higher microbubble generation was associated with the SSVR-closed at 5 L/min and 700 mL level (p = .023). See Table 1 and Figure 2.
Table 1.
Baseline microbubble generation: Median microbubbles (counts/min) in the outflow of the three venous reservoir types at pump flows of 3 L/min or 5 L/min, and reservoir volumes of 200 mL or 700 mL.
| 200 mL |
700 mL |
|||||||
|---|---|---|---|---|---|---|---|---|
| Reservoir type | 3 L/min | IQR | 5 L/min | IQR | 3 L/min | IQR | 5 L/min | IQR |
| HSVR | 5 | 22 | 103 | 28 | 1 | 2 | 2 | 3 |
| SSVR-closed | 2 | 3 | 5 | 9 | 2 | 2 | 6 | 13 |
| SSVR-open | 1 | 6 | 7 | 10 | 2 | 6 | 2 | 4 |
IQR, interquartile range.
Figure 2.
Boxplots of microbubble generation (counts/min) in the outflow of the three venous reservoir types at pump flows of 3 L/min or 5 L/min and reservoir volumes of 200 mL or 700 mL.
Part B) Air Infusion – Microbubble Transmission
-
Microbubble transmission for each venous reservoir type
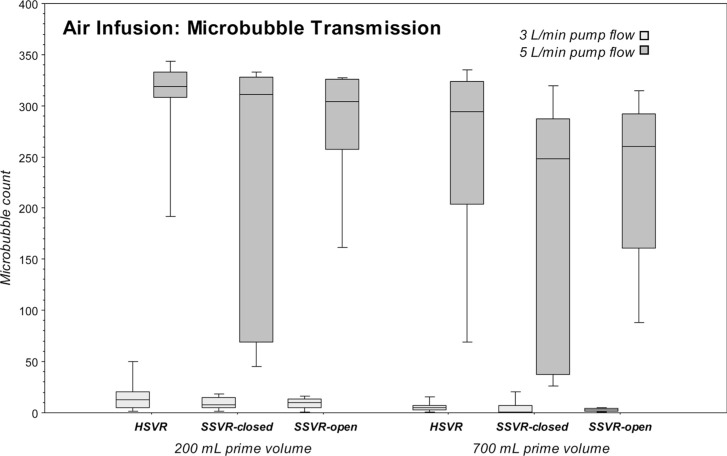
While air was being introduced into the venous line, all the reservoir types transmitted significantly more microbubbles with the higher flows of 5 L/min versus 3 L/min at both 200 mL and 700 mL levels (all reservoirs at 700 mL level: p = .0077; SSVR-closed and SSVR-open at 200 mL: p = .0077; HSVR at 200 mL: p = .017). See Table 2 and Figure 3.
-
Microbubble transmission – comparison of all three venous reservoir types
There were no significant differences in transmitted introduced air between the three venous reservoirs at equivalent flow rates and volumes. See Table 2 and Figure 3.
-
Microbubble transmission versus baseline for each venous reservoir type
All three venous reservoirs were effective at not transmitting significantly more gaseous microbubble during air infusion than baseline at lower flow rate of 3 L/min with the exceptions of HSVR at 700 mL (p = .049) and the SSVR-closed at 200 mL (p = .0077); though differences in median microbubble counts were small. However, dramatic and significant transmissions were seen in all venous reservoirs when flows were increased to 5 L/min irrespective of reservoir volume (p = .0077 except for HSVR at 200 mL where p = .0117). See Tables 1–2 and Figures 2–3.
Table 2.
Microbubble transmission: Median microbubbles (counts/min) in the outflow of the three venous reservoir types during venous line air infusion at 20 mL/min at pump flows of 3 L/min or 5 L/min, and reservoir volumes of 200 mL or 700 mL.
| 200 mL |
700 mL |
|||||||
|---|---|---|---|---|---|---|---|---|
| Reservoir type | 3 L/min | IQR | 5 L/min | IQR | 3 L/min | IQR | 5 L/min | IQR |
| HSVR | 13 | 16 | 319* | 25 | 5 | 4 | 294 | 120 |
| SSVR-closed | 8 | 10 | 311 | 258 | 1 | 7 | 248 | 250 |
| SSVR-open | 10 | 9 | 304 | 68 | 3 | 2 | 260 | 131 |
IQR, interquartile range.
2 data points missing
Figure 3.
Boxplots of microbubble transmission (counts/min) in the outflow of the three venous reservoir types during venous line air infusion at 20 mL/min at pump flows of 3 L/min or 5 L/min and reservoir volumes of 200 mL or 700 mL.
DISCUSSION
The results of this study show that the generation and transmission of microbubbles by the Medtronic collapsible venous reservoir bag is not more than the Medtronic Affinity hardshell venous reservoir over a range of prime volumes and flow rates suggesting that the SSVR is as safe as the HSVR.
The venous reservoir is the first and most important component within the ECC to remove both macrobubbles and microbubbles. Whether from the vents and suckers, purge lines, or venous line itself, a potentially large volume of air unavoidably enters the venous reservoir. Although the oxygenator and arterial filter also aid in air removal, gaseous microbubbles are still able to traverse these components (7,15). Thus it is incumbent upon venous reservoir design to remove as much of the introduced air as possible without itself being a source of microbubbles.
The venous reservoir – being a site in the ECC that has a low blood velocity and thus high gas buoyancy – passively removes introduced air (16). However, it can also be a source of air by inducing blood cavitation and turbulence, or when accidentally 31 emptied (17). Different models of venous reservoirs have been shown to vary considerably in their air handling capabilities and their propensity to generate microbubbles (8,13,14,18).
HSVRs are associated with increased cerebral microemboli counts when running at higher pump flows or lower volumes (12,19,20). Higher flow rates and low reservoir volumes not only reduce the transit time for the passive removal of air, but also exacerbate any turbulent waterfall effect. Here, blood tumbles down from the cardiotomy and splashes into the venous reservoir causing turbulence and mixing of air and blood with subsequent microbubble generation. This phenomenon varies with various HSVR designs emphasizing the importance of adhering to the manufacturer’s recommendation of minimal volumes for operation. Nielsen et al. investigated the relationship between reservoir levels and microbubble generation in three brands of HSVR, including the same HSVR as this study, within an in-vitro circuit (8). Although an inverse relationship was seen between lowering reservoir levels and increasing bubble counts, no dramatic rise in count was seen. However, their lower level was 250 mL; i.e., 50 mL above the manufacturer’s recommended minimum. Our study’s static reservoir volume of 200 mL would dynamically lower to 100–150 mL level under higher flows. Though this is lower than recommended, it does emphasize the ability of the SSVR (a device that has not had its safe lower operating level identified) not to generate microbubbles at equivalent operating volumes. A further study to identify the critical flow-level relationship for microbubble generation in both reservoirs by introducing a greater range of pump flows and reservoir volumes would be informative.
The relationship between increased cerebral emboli or arterial line emboli with introduced venous air (whether from the patient or via sampling ports) is well documented in HSVRs (8,21–23). We were able to show that both the HSVR and SSVR were relatively effective at removing introduced air at the lower flow rate of 3 L/min at low and higher reservoir volumes. However, even at the higher reservoir volumes, both reservoir designs transmitted significant quantities of microbubbles at 5 L/min flow. Thus it is essential to ensure that the venous line is never entraining air – particularly at higher flows – as these reservoirs will transmit some of this air.
The operation of the SSVR at both the fully closed and one latch open position essentially yielded clinically negligible differences in microbubble transmission. Thus no advantage could be determined in minimizing microbubble transmission by operating the SSVR in either position under the study’s parameters of flows and volumes. Clinically, opening the cage facilitates exsanguination of the patient by allowing the reservoir bag to expand more fully and increase its volume capacity.
HSVRs are predominantly used in Australia and New Zealand (11). Their appeal stems from the practical convenience of the integrated venous reservoir-cardiotomy coupled with the oxygenator-heat exchanger design; making for rapid installation and set-up. Unlike the SSVR, the HSVR passively removes macrobubbles with minimal perfusionist intervention, while the SSVR needs its highest port to be intermittently aspirated to remove any accumulating air (24). However, the HSVR’s presumed superior ability to remove infused venous air was not evident in this study, and at very low volumes it may itself be a source of microbubble generation. Future investigations to identify microbubble transmission of both reservoirs by introducing a greater range of infused venous air volumes and rates would further elucidate their air handling properties.
Another issue of the open design of the HSVR is that the venous blood is exposed to air and contacts the cardiotomy filters with exposure to antifoam-A. These filters increase the blood contact activation area of the perfusion circuit. Also, any turbulence associated waterfall effect at low volumes induces microbubble formation and increases blood shear stresses. Indeed, evidence of increased blood damage with associated bleeding and need for red cell transfusions have been demonstrated with HSVRs in clinical studies comparing HSVRs with SSVRs (25–27).
By the simplicity of their design and negligible blood-air interface, SSVRs provide a more physiological environment. Furthermore, isolating the cardiotomy blood in closed systems affords additional blood handling benefits. Cardiotomy blood has not only been activated by exposure to negative suction pressures and foreign surfaces but contains gaseous microemboli (28). Returning cardiotomy blood has been shown to be associated with increased microbubble activity in the outflow of HSVRs (9). Isolating this blood to the cardiotomy and preventing a constant trickle back to the venous reservoir – with intermittent release of this cardiotomy blood after sufficient time for debubbling – may prove to be another beneficial feature of the SSVR system warranting further investigation.
Another more physiological blood handling feature of the SSVR occurs when initiating CPB; it can be achieved more gradually with less impact on the patient’s hemodynamics by isolating the cardiotomy reservoir and operating the SSVR at its smallest volume. Once on CPB, the SSVR can be opened one step, and/or the line to the cardiotomy opened to empty the heart. Adjustment of the cardiotomy height (if open to the reservoir) further contributes to the venous return. It is this ability to manipulate reservoir volumes, adjust the pressure gradient for the venous return, control the mixing of the cardiotomy blood back with the systemic blood, and gradually go onto and come off CPB that marks the SSVR as a more patient customizable per-fusion system than the one-approach-fits-all simplicity of the HSVR.
An albumin prime was used because it is more physiological than water or electrolyte solution without the complexities of using whole blood. Also, the protein prime was deemed to be more stable, and therefore more reliable than blood. As gaseous microemboli in blood become coated in platelets (29), consumption of platelets may alter the behavior of bubbles in in-vitro circuits thereby changing the prime’s microbubble handling over time. Ideally, an animal model would be used to maintain the blood’s quality; moreover, it is a perfect eliminator of introduced microbubbles. In a study investigating microbubble removal in in-vitro ECCs, Jones et al. using both water and blood as primes, showed a decreased ability of venous reservoirs to remove microbubbles using blood (presumably due to the bubbles forming more stable platelet-lipoprotein capsules) (22). The behavior of our physiological protein prime would be expected to be within the range of that seen between blood and water, i.e., less microbubble removal might be expected if the study was repeated with blood.
The prime was kept at ambient temperature, as heating the prime was not considered to have yielded more insight. Although colder solutions theoretically can absorb more gas, a paradoxical increase in transmitted microbubbles was seen when cooling blood prime in an in-vitro ECC (30). Whether warming the prime would improve the air handling capability of the venous reservoirs is arguably a moot point as our study was designed to compare bubble generation and removal under comparable conditions; although absolute bubble numbers may vary clinically, relative differences would still be valid.
The Hatteland CMD-10 has been shown to be less sensitive than more contemporary devices; probably underestimating circulating microbubble counts (31). It is also a poor estimator of bubble size and volumes and thus bubble sizing was not attempted other than the determination of the gross range of bubble sizes detected by calibration. Knowing bubble size and gas volumes is important due to the varying performances of the ECC components in removing microbubbles of differing sizes (e.g., the arterial filter and oxygenator may preferably remove larger bubbles) and different clinical sequelae (e.g., several larger bubbles may have the same embolic effect as many smaller bubbles). Nevertheless, though the difference in microbubble handling between the SSVR and HSVR in terms of cerebral embolic load during cardiac surgery was not defined, the purpose of this study was to quantitatively rank the reservoirs according to total microbubble counts generated and transmitted.
In summary, we investigated the relative air handling capabilities of two kinds of adult venous reservoirs in terms of microbubble generation and transmission – the Medtronic Affinity hardshell venous reservoir and Medtronic collapsible venous reservoir bag. These reservoirs’ relative air handling capabilities were examined under equal in-vitro conditions of baseline and controlled air introduction, while varying the reservoir prime volumes and pump flow rates. We conclude that the SSVR is as safe as the HSVR in terms of microbubble handling and should be considered as an alternative venous reservoir. The SSVR has the added potential advantage of contributing to a closed ECC thereby reducing blood activation. Importantly, as both reservoir types transmitted microbubbles at higher pump flow rates regardless of reservoir volumes, it is essential to eliminate venous air entrainment during CPB.
REFERENCES
- 1.Dinh DT, Lee GA, Billah B, Smith JA, Shardey GC, Reid C.. Trends in coronary artery bypass graft surgery in Victoria, 2001–2006: Findings from the Australasian Society of Cardiac and Thoracic Surgeons database project. Med J Aust. 2008;188:214–7. [DOI] [PubMed] [Google Scholar]
- 2.Newman MF, Matthew JP, Grocott HP, et al. Central nervous system injury associated with cardiac surgery. Lancet. 2006;368:694–703. [DOI] [PubMed] [Google Scholar]
- 3.Hammon JW, Stump DA, Kon ND, et al. Risk factors and solutions for the development of neurobehavioural changes after coronary artery bypass grafting. Ann Thorac Surg. 1997;63:1613–8. [DOI] [PubMed] [Google Scholar]
- 4.Groom RC, Quinn RD, Lennon P, et al. Detection and elimination of microemboli related to cardiopulmonary bypass. Circ Cardiovasc Qual Outcomes. 2009;2:191–8. [DOI] [PubMed] [Google Scholar]
- 5.Gerriets T, Schwarz N, Sammer G, et al. Protecting the brain from gaseous and solid micro-emboli during coronary artery bypass grafting: A randomized controlled trial. Eur Heart J. 2010;31:360–8. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Omar Y, Balacumaraswami L, Pigott DW, Matthews PM, Taggart DP.. Solid and gaseous cerebral microembolisation during off-pump, on-pump, and open cardiac surgery procedures. J Thorac Cardiovasc Surg. 2004;127:1759–65. [DOI] [PubMed] [Google Scholar]
- 7.Taylor RL, Borger MA, Weisel RD, Fedorka L, Feindel CM.. Cerebral microemboli during cardiopulmonary bypass: Increased emboli during perfusionist interventions. Ann Thorac Surg. 1999;68:89–93. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen PF, Funder JA, Jenson MO, Nygaard H.. Influence of venous reservoir level on microbubbles in cardiopulmonary bypass. Perfusion. 2008;23:347–53. [DOI] [PubMed] [Google Scholar]
- 9.Myers GJ, Voorhees C, Haynes R, Eke B.. Post-arterial filter gaseous microemboli activity of five integral cardiotomy reservoirs during venting: An in vitro study. J Extra Corpor Technol. 2009;41:20–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Borger MA, Peniston CM, Weisel RD, Vasiliou M, Green REA, Feindel CM.. Neuropsychologic impairment after coronary bypass surgery: Effect of gaseous microemboli during perfusionist interventions. J Thorac Cardiovasc Surg. 2001;121:743–9. [DOI] [PubMed] [Google Scholar]
- 11.Baker RA, Wilcox TW.. Australian and New Zealand perfusion survey: Equipment and monitoring. J Extra Corpor Technol. 2006;38:220–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell SJ, Willcox T, McDougal C, Gorman DF.. Emboli generation by the Medtronic maxima hard-shell adult venous reservoir in cardiopulmonary bypass circuits:A preliminary report. Perfusion. 1996;11:145–55. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell SJ, Willcox T.. Bubble generation and venous air filtration by hard-shell venous reservoirs: A comparative study. Perfusion. 1997;12:325–33. [DOI] [PubMed] [Google Scholar]
- 14.Tyndal CM, Berryessa R, Tornabene SP.. An in vitro comparison of macro air passage in the venous reservoir bag. J Extra Corpor Technol. 1986;18:101–5. [Google Scholar]
- 15.Riley JB.. Arterial line filters ranked for gaseous micro-emboli separation performance: An in vitro study. J Extra Corpor Technol. 2008;40:21–6. [PMC free article] [PubMed] [Google Scholar]
- 16.De Somer F.. Impact of oxygenator characteristics on its capability to remove gaseous microemboli. J Extra Corpor Technol. 2007;39: 271–3. [PubMed] [Google Scholar]
- 17.Kurusz M, Butler BD, Katz J, Conti VR.. Air embolism during cardiopulmonary bypass. Perfusion. 1995;10:361–91. [DOI] [PubMed] [Google Scholar]
- 18.Dickinson TA, Riley JB, Crowley JC, Zabetakis PM.. In vitro evaluation of the air separation ability of four cardiovascular manufacturer extra-corporeal circuit designs. J Extra Corpor Technol. 2006;38:206–13. [PMC free article] [PubMed] [Google Scholar]
- 19.Moehle DA.. Neuromonitoring in the cardiopulmonary bypass surgical patient: Clinical applications. J Extra Corpor Technol. 2001;33:126–34. [PubMed] [Google Scholar]
- 20.Rodriquez RA, Williams KA, Babaev A, Rubens F, Nathan HJ.. Effect of the perfusionists technique on cerebral embolization during cardiopulmonary bypass. Perfusion. 2005;20:3–10. [DOI] [PubMed] [Google Scholar]
- 21.Willcox TW, Mitchell SJ, Gorman DF.. Venous air in the bypass circuit: A source of arterial line emboli exacerbated by vacuum-assisted drainage. Ann Thorac Surg. 1999;68:1285–9. [DOI] [PubMed] [Google Scholar]
- 22.Jones TJ, Deal DD, Vernon JC, Blackburn N, Stump DA.. Does vacuum-assisted venous drainage increase gaseous microemboli during cardiopulmonary bypass? Ann Thorac Surg. 2002;74:2132–7. [DOI] [PubMed] [Google Scholar]
- 23.Rodriquez RA, Rubens F, Belway D, Nathan HJ.. Residual air in the venous cannula increases cerebral embolization at the onset of cardiopulmonary bypass. Eur J Cardiothorac Surg. 2006;29:175–80. [DOI] [PubMed] [Google Scholar]
- 24.Hessel AE.. Cardiopulmonary bypass circuitry and cannulation techniques. In: Hessel Gravlee GP, Davis RF, Utley JR, eds. Cardiopulmonary Bypass–Principles and Practice. Baltimore: Williams & Wilkins; 1993:55–92. [Google Scholar]
- 25.Schönberger JPAM, Everts PAM, Hoffmann JJ.. Systemic blood activation with open and closed venous reservoirs. Ann Thorac Surg. 1995;59:1549–55. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney CB, Donnelly JE.. Impact of closed versus open venous reservoirs on patient outcomes in isolated coronary artery bypass graft surgery. Perfusion. 2000;15:467–72. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka H, Oshiyama T, Narisawa T, et al. Clinical study of biocompatibility between open and closed heparin-coated cardiopulmonary bypass circuits. J Artif Organs. 2003;6:245–52. [DOI] [PubMed] [Google Scholar]
- 28.Lilly KJ, O’Gara PJ, Treanor PR, et al. Heparin-bonded circuits without a cardiotomy: A description of a minimally invasive technique of cardiopulmonary bypass. Perfusion. 2002;17:95–7. [DOI] [PubMed] [Google Scholar]
- 29.Eckmann DM, Armstead SC, Mardini F.. Surfactants reduce platelet-bubble and platelet-platelet binding induced by in vitro air embolism. Anesthesiology. 2005;103:1204–10. [DOI] [PubMed] [Google Scholar]
- 30.Sleep J, Syhre I, Evan E.. Blood temperature management and gaseous microemboli creation: An in-vitro analysis. J Extra Corpor Technol. 2010;42:219–22. [PMC free article] [PubMed] [Google Scholar]
- 31.Stump DA, Vernon JC, Deal DD.. A comparison of the Hatteland CMD10 versus the embolus detection and classification system (Abstract). Outcomes 2004 Abstracts. Heart Surg Forum. 2004;7:E617–8. [Google Scholar]