Abstract:
25–35% of all seriously injured multiple trauma patients are coagulopathic upon arrival to the emergency department, and therefore early diagnosis and intervention on this subset of patients is important. In addition to standard plasma based tests of coagulation, the thromboelastogram (TEG®) has resurfaced as an ideal test in the trauma population to help guide the clinician in the administration of blood components in a goal directed fashion. We describe how thromboelastographic analysis is used to assist in the management of trauma patients with coagulopathies presenting to the emergency department, in surgery, and in the postoperative period. Indications for the utilization of the TEG® and platelet mapping as point of care testing that can guide blood component therapy in a goal directed fashion in the trauma population are presented with emphasis on the more common reasons such as massive transfusion protocol, the management of traumatic brain injury with bleeding, the diagnosis and management of trauma in patients on platelet antagonists, the utilization of recombinant FVIIa, and the management of coagulopathy in terminal trauma patients in preparation for organ donation. The TEG® allows for judicious and protocol assisted utilization of blood components in a setting that has recently gained acceptance. In our program, the inclusion of the perfusionist with expertise in performing and interpreting TEG® analysis allows the multidisciplinary trauma team to more effectively manage blood products and resuscitation in this population.
Keywords: perfusionist, trauma, thromboelastography, massive transfusion, blood component therapy, organ donation
The thromboelastograph (TEG®, Haemonetics Niles, IL) analyzer is a viscoelastic point of care device that defines abnormalities in the mechanisms of thrombus initiation, amplification, propagation, and termination. The TEG® parameters and their normal ranges are noted in Table 1 (1). In conjunction with plasma based testing such as the international normalized ratio (INR), prothrombin time, and the partial thromboplastin time, blood products can be given in a “goal directed” fashion in the trauma patient (2–4). With the addition of the platelet-mapping assay, the technology can also assess platelet function and dysfunction in trauma (5). However, the instrument’s use in trauma has not been appreciated until recently. Several studies have demonstrated that TEG® analysis can detect early changes in the coagulation of trauma patients and therefore may help transfusion management (2–5). The challenge of the clinician is to balance the risks of transfusion with the risk of a thrombotic event associated with trauma (1).
Table 1.
TEG® parameters and correlations to the phases of clot formation.
| Parameter | Normal Values | Indication |
|---|---|---|
| R | 4–8 minutes | Reflects initiation and the enzymatic phase of coagulation from the time of clot formation to the detection of initial fibrin strand formation |
| K | 0–4 minutes | Measure of time from beginning of clot formation until the curve reaches arbitrary amplitude of 20 mm. Reflects amplification and kinetics of clot formation and fibrin cross linking. |
| α | 47–74 degrees | Slope of the curve drawn as a tangent from the beginning of clot formation until 20 mm elevation. Evaluation of propagation of the clot and fibrin cross-linking. Correlates with K. |
| MA | 54–72 mm | Strength of clot due to platelet-fibrin bonding, dependent on number and function of platelets. |
| LY 30 | 0–8% | Reflects the rate of fibrinolysis by measuring the rate of amplitude reduction 30 minutes after establishment of the MA. Reflects termination. |
| INR | .8–1.2 | International normalized ratio. |
| PT | 8.7–10.8 | Prothrombin time. Measures functionality of extrinsic and common pathways of the coagulation cascade. |
| aPTT | 24.8–35.7 | Activated partial thromboplastin time. Measures functionality of intrinsic and common coagulation pathways |
PT, prothrombin time; aPTT, activated partial thromboplastin time.
Perfusionists have traditionally performed TEG® monitoring during cardiac surgery and are a logical resource to perform and interpret the test during trauma (6). The division of labor in the emergency department and the operating room mandates that the emergency physicians, surgeons, and anesthesiologists multitask as they care for the patient in the immediate resuscitation phase in the emergency department and operating room. Attention to these duties can be facilitated by consultation with the perfusionist during the crucial resuscitation phase.
The choices of fluids and the ratio of blood products given during trauma resuscitation have changed recently and require monitoring of physiologic parameters that include the TEG® (4). These changes have challenged the established recommendation from the American College of Surgeons Advanced Trauma Life Support (ATLS) guidelines that two liters of crystalloid be given prior to the administration of blood products in the hypotensive trauma patient (7,8). These guidelines for initial resuscitation are being replaced with a strategy of “hemostatic,” also referred to as “damage control,” resuscitation. This protocol recommends an approximate 1:1:1 red blood cell to plasma to platelet ratio with minimal administration of crystalloids in unstable trauma patients requiring transfusion. This damage control resuscitation strategy has been tested in the battlefields of Iraq and Afghanistan for resuscitation of soldiers in hemorrhagic shock due to penetrating injuries as well as in the civilian population of massively transfused trauma patients (3,8). This aggressive method of resuscitation of the trauma patient requires the early detection of coagulopathies so that blood component therapy (BCT) may be undertaken judiciously (2,4,8–10). Despite the recent enthusiasm for such pre-emptive coagulation factor replacement in the multiple trauma patients who require BCT, the lack of real time assessment and therefore the early treatment of traumatic coagulopathy has hindered the widespread adoption of the TEG® as a means for guiding BCT in the setting of trauma. This absence of a clear methodology for the performance and reporting of the TEG® at the bedside in a fashion that allows for real time monitoring of the need for blood components has been cited in the literature as an impediment to its adoption in the trauma setting (4). In an attempt to address these problems of real time performance and interpretation, some trauma centers have installed a large computer screen in the trauma operating room in an attempt to provide real time TEG® guided goal directed blood component therapy and also have used the rapid TEG® or rTEG® in an attempt to provide information more quickly (4). Also, there is concern for the administration of large volumes of fresh frozen plasma and platelets without laboratory confirmation of their necessity (4). The early presence of a perfusionist at the bedside both in the emergency department (ED) and the operating room (OR) and post operative period in trauma cases can help guide BCT and address this problem.
We describe our experience at a Level II trauma center in a community teaching hospital where perfusionists and physicians collaborate in the management of blood components in a “goal- directed” fashion driven by clinical and laboratory parameters, including TEG® monitoring. The following examples illustrate the usefulness of TEG® analysis and the role of the perfusionist in performing the analysis, interpreting the results, and collaborating with physicians and basic scientists regarding transfusion management. Institutional Review Board approval was obtained for presentation of the cases.
Implementation of TEG®-Directed Massive Transfusion Protocol (MTP)
The MTP consists of a transfusion package of one unit of packed red blood cells (PRBC), fresh frozen plasma (FFP), and platelets approaching a ratio of (1:1:1). This transfusion package reflects the experience of Holcomb and others in wartime and civilian situations (3,7–10). TEG® monitoring is initiated as soon as a blood sample can be obtained once a seriously injured patient arrives in the trauma center. After initiation of the protocol, a blood bank technician is dispatched to the resuscitation site emergency department, surgery, or intensive care unit to facilitate the delivery and to maintain the inventory of blood components. Two units of thawed FFP are available at all times near the ED for every Level I (highest) trauma activation. This practice has evolved to a transfusion protocol nearer to a 1:1:1 PRBC to FFP to platelet ratio for all trauma patients who require BCT. Once the trauma patient is determined to be a candidate for the MTP by meeting established criteria, the patient is type and crossed and immediately transfused 6 units of O negative or O positive PRBC until type specific and cross matched blood is available, as well as 6 units of FFP and one single donor (SD) apheresis platelets. Calcium is given with the first round of blood products. During this period, a complete plasma based coagulation diagnostic profile is ordered. Further BCT is guided by clinical indicators as well as the TEG® and other laboratory parameters. See Table 2.
Table 2.
Massive transfusion protocol.
| Clinical Triggers (2 or more) | Heart rate > 120 bpm | |||||
| Systolic blood pressure < 90 mmHg | ||||||
| Base deficit > −6 | ||||||
| Positive focused abdominal sonography in trauma | ||||||
| Positive computerized tomography | ||||||
| Difficult to control hemorrhage | ||||||
| Transpelvic/multi-cavity gunshot wound | ||||||
| Physician discretion | ||||||
| OR charge nurse to notify perfusionist on call for TEG® and platelet-mapping | ||||||
| Call blood bank to activate MTP | ||||||
| Draw baseline labs (as needed) | Type and cross STAT | |||||
| Complete blood count | ||||||
| PT/PTT | ||||||
| Fibrinogen | ||||||
| Lactic acid | ||||||
| Draw and hold extra green- and blue-top tubes for perfusionist | ||||||
| MTP Packs | Administer products by infuser/warmer except as noted | |||||
| Pool Number | RBC | Plasma | Platelets (not warmed) | Cryo (not warmed) | CaCl | |
| 1 | 6 | 6 | 1 | 0 | 1g | |
| 2 | 6 | 6 | 1 | 0 | 1g | |
| Further transfusion guided by TEG® | ||||||
| Consider Factor VIIa early if pH > 7.20 (Dose 90 mcg/kg IV) | ||||||
| Order labs | I-stat 8+ | |||||
| Complete blood count | ||||||
| PT/PTT | ||||||
| Fibrinogen | ||||||
| FDP/D-dimer and platelet function assay 100 as indicated | ||||||
| Magnesium | ||||||
| (at 30 minutes then q 60 minutes while MTP is active) | ||||||
| Repeat TEG® and platelet mapping per perfusionist/physician recommendation | ||||||
PT, prothrombin time; PTT, partial thromboplastin time; FDP/D, Fibrinogen Degradation Production.
Integral to the evolution of this transfusion protocol is the staff cardiac surgery perfusionist’s availability. The MTP was revised to include the early paging of the perfusionist on call. This approach has increased the perfusionist’s role in trauma to that of a central consultant for all trauma cases requiring massive transfusion.
Diagnosis and Treatment of Occult Coagulopathy Caused by Platelet Antagonist and Primary Platelet Dysfunction of Trauma
Platelet dysfunction can occur in the trauma setting (11), and in particular, in that population taking anti platelet agents. Often, the patients are unaware or unable to give the history of taking these agents and platelet mapping can determine the need for platelets despite a normal platelet count. A 68-year-old male was involved in a motor vehicle crash where he was a restrained driver struck on the driver’s side. He was seemingly uninjured from the crash and walked home. However, he fainted at home and the paramedics found the patient hypotensive and in shock. The patient’s hemoglobin and platelet count were normal but his blood pressure was 90/60. A nondisplaced pubic ramus pelvic fracture was found with a disproportionately large pelvic hematoma on a computerized tomography of the abdomen and pelvis. This subtle fracture with a large clot led the emergency physician to order a TEG® with platelet mapping. The TEG® revealed no abnormality. However, there was significant inhibition of platelets to arachidonic acid (96.6%) and adenosine diphosphate aggregation (95.7%). Despite the normal platelet count, the patient was transfused two SD apheresis platelets and taken to interventional radiology where the bleeding pelvic vessels were coiled to secure cessation of bleeding. Once recovered from shock the patient was able to recall that he had been taking aspirin that could explain the arachidonic acid inhibition, but not the adenosine diphosphate inhibition that could be explained by the traumatic shock of hemorrhage (1,5,11). Although there are published guidelines for thresholds for platelet counts during trauma and surgery (9), there are few studies concerning the effect of trauma on platelet function or an established protocol for evaluating patients for platelet dysfunction during the coagulation screening of the trauma patient (5,11). With the increasing use of platelet antagonists in the aging trauma population, occult platelet dysfunction in trauma patients is likely to be more frequently diagnosed and require treatment.
Persistent Bleeding Due to Mechanical Bleeding Requiring Reoperation—The Diagnostic Value of Normal TEG®
An elderly patient on warfarin because of atrial fibrillation had persistent bleeding following soft tissue injury due to a ground level fall that caused a compartment syndrome of the thigh. After correction of the INR with FFP, there was continued bleeding. FFP was used because at this time Prothrombin Complex Concentrate was not available. Three subsequent TEG® tracings were normal, suggesting a mechanical cause for the bleeding (12) (Figure 1). Cessation of the bleeding occurred after reoperation that revealed continued arterial and venous bleeding. This case confirms that a normal TEG® tracing has a high predictive value of mechanical bleeding (12).
Figure 1.
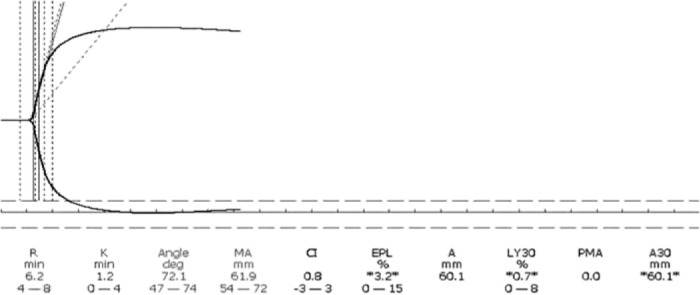
Eighty-eight-year-old after first surgery for compartment syndrome has normal INR and normal TEG® tracing. A re-operation was prompted by normal TEG® tracing, indicating a mechanical bleed, which was found and repaired at re-operation.
TEG® Guided Factor rVIIa Administration
The patient had severe closed head injury with large scalp laceration, bilateral hemothoraces, pulmonary contusions with adult respiratory distress syndrome, grade II splenic injury, grade I liver laceration, left adrenal hematoma, large retroperitoneal hematoma, gluteal and pudendal artery hemorrhage, pelvic fracture, bladder rupture, and left femur fracture following a motor vehicle versus pedestrian crash. Surgical procedures included scalp closure, liver laceration repair, splenectomy, bladder repair, and intraoperative ligation of the left superior gluteal and pudendal arteries with subsequent open reduction and internal fixation of the left femur fracture. During the initial damage control surgery, there was continued bleeding in spite of aggressive damage control resuscitation with institution of the MTP. The TEG® revealed a diffuse coagulopathy with an reaction time (R) of 8.3 minutes, clot formation time (K) of 7.2 minutes, alpha angle of 37.6 degrees, and an maximum amplitude (MA) of 30.4 mm. Because of the patient’s failure to respond to MTP and damage control surgery the surgeon ordered a single dose of 90 μg/kg rFVIIa. Within a few minutes of infusion there was cessation of bleeding. Repeat TEG® analyses revealed an improvement in the coagulopathy with a normal R, K, alpha angle and a reduced but improved MA of 38.3 mm. Following the MTP protocol, 29 units of PRBC, three apheresis platelets, six units of FFP, and six units of cryoprecipitate were administered in the ED and the OR within 4 hours, a clear indication for MTP (8). Because of the failure to respond to the MTP and the persistence of shock due to bleeding with a globally abnormal TEG® , rFVIIa was administered. This patient’s case exemplifies the utility of the TEG® in guiding BCT resuscitation during surgery and the concomitant use of rFVIIa therapy for a patient with a very high Injury Severity Score of 57 that correlates with mortality at 85% (the highest ISS of 75 correlates with 100% mortality) (13). This patient’s clinical condition met criteria for administration of rFVIIa that is not effective if administered too late to patients in profound shock (14).
TEG® Directed Hemostatic Resuscitation for Penetrating Injuries or Traumatic Brain Injury Resulting in Survival for Organ Donation
A 17-year-old male presented to the ED in asystole after a stab wound of the innominate artery. Immediate damage control resuscitation resulted in return of spontaneous circulation in the ED after eight units of PRBC and two units of FFP. The patient was transferred to the OR for surgical repair of the artery. The initial TEG® tracing in the OR was abnormal for all parameters and was corrected with the perfusionist’s assistance using a TEG® guided algorithm during the surgery. The patient received a total of 25 units of PRBC, 19 units of FFP, two SD apheresis platelets, and six units of cryoprecipitate (Figures 2 and 3). Normalization of hemostasis and stabilization of vital signs preceded brain death due to prolonged cerebral hypoxia prior to resuscitation. However, immediate damage control resuscitation, quick repair of the vessel, and post-surgical stabilization with a simple algorithm based TEG® guided blood component protocol allowed survival and time for discussion of organ donation with the family, resulting in a donation of seven organs (Table 3).
Figure 2.
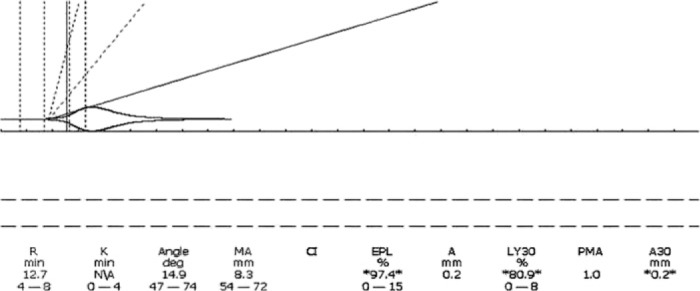
Innominate artery laceration. Asystole in emergency department; eight units of PRBC, two units of FFP in ED. Fibrinolysis was not treated with antifibrinolytic agents since the source of the coagulopathy was the bleeding vessel and control of hemorrhage imminent.
Figure 3.
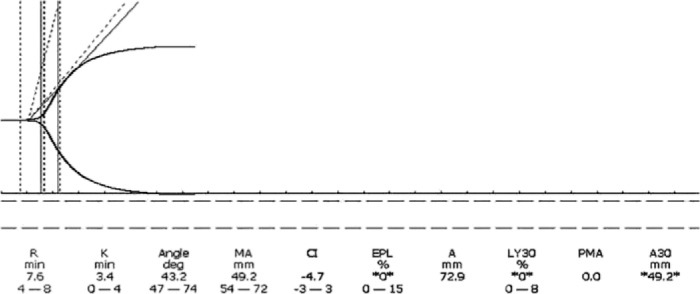
Innominate laceration after repair. Total BCT 25 units of PRBC, 19 units of FFP, 1450 cc auto transfusion, six units cryoprecipitate, and two single donor apheresis platelets. Fibrinolysis corrected without use of antifibrinolytic agent. Outcome: Seven organs donated.
Table 3.
Algorithm for thromboelastographic-guided blood component therapy.*
| TEG® Abnormality | Blood Component Therapy |
|---|---|
| Prolonged R | Fresh frozen plasma |
| Prolonged K and/or reduced α angle | Cryoprecipitate |
| Low MA | Platelets consider DDAVP |
| Elevated LY 30% | Consider antifibrinolytics |
Address other etiologies of coagulopathy: hypothermia, acidosis and continued hemorrhage, hypocalcemia, and dilution. Consider early surgery and 1:1:1 PRBC, FFP, platelets for damage control resuscitation and rFVIIa. Look for combined and occult causes of coagulopathy and primary fibrinolysis.
MA, maximum amplitude; LY, percent clot lysis at 30 minutes; DDAVP, 1-desamino-8-D-arginine vasopressin.
This example illustrates the TEG®’s utility in assisting in the resuscitation of otherwise moribund patients, whose continued resuscitation depends on family wishes for organ donation. This cases also demonstrates resolution of fibrinolysis with goal directed MTP resuscitation and surgical control of hemorrhage.
A victim of gunshot wound to the cerebellum revealed abnormal pre-operative TEG® values for the R, K, a, and LY 30% indicating a profoundly hypocoagulopathic state with fibrinolysis. The patient underwent TEG® guided resuscitation until the TEG® tracing was normalized. Unfortunately, he progressed to brain death, but eight organs were donated for transplantation after discussion with the family. This patient’s care represents the goal of our protocol, which is to save the life first through aggressive resuscitation. Since cerebellar bleeds respond to rapid evacuation, there was a good chance for survival of this patient, particularly since he was alert in the ED prior to decompensation. This case affirms that organ donation is a secondary goal after saving the patient’s life.
DISCUSSION
These examples provide a view into how a trauma team implements TEG® analysis for trauma patients. We treat these coagulopathies using an adaptation of the TEG®based algorithm developed by Shore-Lesserson et al. for directing BCT during cardiac surgery (Table 3) (6). Other algorithms have been published for titrating blood components and factor rVIIa with the guidance of the TEG® and clinical information (2,15,16). Our algorithm does not correlate specific numbers of units of blood components to specific changes of the TEG® parameters, but rather defines the threshold TEG® value that will trigger the administration of PRBC, FFP, platelets cryoprecipitate, or rFVIIa. This approach allows the clinician more flexibility in the administration of blood products and conforms to the fluid nature of trauma resuscitation where frequent re-evaluation is important for patient survival.
The common uses of the TEG® in trauma are in guiding MTP, diagnosing platelet dysfunction in trauma patients with and without previous exposure to platelet antagonists, the diagnosis of mechanical bleeds with normal TEG®s, and assisting in guiding the administration of rFVIIa. For example, the presence of unanticipated pelvic bleeding in the elderly patient with a subtle pelvic fracture alerted the emergency physician to a diagnosis of platelet dysfunction caused by undetected previous platelet antagonists. Where unexplained bleeding continues, a normal TEG® may direct the surgeon toward exploratory surgery, such as in case of the patient with atrial fibrillation on warfarin whose coagulopathy was corrected properly and who had three normal TEG®s before the surgeons took the patient back to surgery to find a mechanical cause of the bleeding.
The advantage of the TEG® in guiding the use of rFVIIa has been noted in the literature as in the case of the patient who suffered multiple life threatening injuries unresponsive to standard MTP damage control resuscitation who benefited from the addition of the TEG® to guide the intraoperative resuscitation of the patient (14). The above mentioned examples demonstrate that the TEG® with platelet mapping can be helpful in the early recognition and treatment of trauma induced coagulopathy in a “goal directed” fashion confirming the experience of others in the trauma community who have found a coagulopathy in 25–35% of patients arriving with multiple trauma to the emergency department (4,8,17,18).
TEG® analysis also allows the conservation and judicious use of blood products earlier in cases that are clinically deemed futile, as with patients who donated organs who require blood products while waiting for organ donation (19).
Routine use of TEG® analysis has not yet been adopted in most trauma centers. To date, 10 studies involving more than 700 patients have evaluated the viscoelastic point of care hemostatic assays such as the TEG® in trauma (18). TEG® analysis can predict BCT and mortality in multiple trauma patients as well as anticipate hyper and hypocoagulable states (5,20).
A limitation to the widespread adoption of this technique in small or large trauma centers has been attributed to a lack of standardization, quality control, and consistency of TEG® analysis by non-laboratory personnel who perform and interpret the TEG® (14). The applicability of the TEG® is made difficult given the need for prompt correction of coagulopathy. The early involvement of the perfusionist in BCT management is our attempt to address this concern for real time analysis of hemostasis in trauma that has been attempted by others with prehospital collection of blood samples in trauma patients and the use of the computerized kaolin and rapid TEG® tracings in the OR (4,5). Another limitation that has not been cited in the literature is the failure of the trauma community to assign a member of the trauma team the responsibility of performing the TEG® analysis. The unique nature of our experience stems from the division of labor at our Level II trauma center. Because of the limited number of personnel involved in the care of the trauma patient, we have been able to quickly enlist the cooperation of the pathologists, anesthesiologists, emergency physicians, perfusionists, and surgeons. The responsibility for performing the TEG® analysis, quality assurance monitoring, and maintenance has been assumed by the perfusionist under the authority of the trauma surgeons.
The perfusionist can be of great assistance in the proper use of the TEG® in the assessment of coagulopathies in trauma patients, whether they are in hemorrhagic shock requiring MTP or have continuous bleeding in general, orthopedic, or neurosurgical surgery. As a result of the more readily available perfusionists capable of performing TEG® analysis in settings other than the MTP, the indications for the use of the TEG® in trauma have expanded at our institution (Table 4).
Table 4.
Suggested indications for TEG® analysis in trauma population.
| 1. Massive transfusion |
| 2. Severe traumatic brain injury with bleeding |
| 3. Cerebral hemorrhage while on anticoagulants or platelet inhibitors |
| 4. Unexplained continued bleeding |
| 5. Suspected platelet dysfunction |
| 6. Recombinant Factor VIIa use |
| 7. Potential organ donor with coagulopathy |
| 8. Early cessation of resuscitation in severe trauma with poor prognosis |
| 9. Identification of hypercoagulable patient |
ACKNOWLEDGMENTS
This work was supported by a research grant from Memorial Hospital. Haemonetics Corporation donated equipment used in this study.
REFERENCES
- 1.Wegner J, Popovsky MA.. Clinical utility of thromboelastography: One size does not fit all. Semin Thromb Hemost. 2010;36:699–706. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez E, Pieraici FM, Moore EE, Kashuk JL.. Coagulation abnormalities in the trauma patient: The role of point of care thromboelastography. Semin Thromb Hemost. 2010;36:723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plotkin AJ, Wade CE, Jenkins DH, et al. A reduction in clot formation rate and strength assessed by thromboelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma. 2008;64:S64–8. [DOI] [PubMed] [Google Scholar]
- 4.Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: Goal directed resuscitation via POC thromboelastography. Ann Surg. 2010;251:604–14. [DOI] [PubMed] [Google Scholar]
- 5.Carroll RC, Craft RM, Langson RL, et al. Early evaluation of acute traumatic coagulopathy by thromboelastography. Transl Res. 2009;154:34–9. [DOI] [PubMed] [Google Scholar]
- 6.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin A.. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88:312–9. [DOI] [PubMed] [Google Scholar]
- 7.Kortbeck JB, Turki SA, Ali J, et al. Advanced trauma life support, 8th edition, the evidence for change. J Trauma. 2008;64:1638–50. [DOI] [PubMed] [Google Scholar]
- 8.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–58. [DOI] [PubMed] [Google Scholar]
- 9.Ketchum L, Hess JR, Hiippala S.. Indications for early FFP, cryoprecipitate and platelet transfusion in trauma. J Trauma. 2006;60:S51–8. [DOI] [PubMed] [Google Scholar]
- 10.Riskin DJ, Tsai TC, Riskin L, et al. Massive transfusion protocols:The role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg. 2009;209:198–205. [DOI] [PubMed] [Google Scholar]
- 11.Davenport RA, Brohi K.. Coagulopathy in trauma patients: Importance of thrombocyte function? Curr Opin Anaesthesiol. 2009;22:261–6. [DOI] [PubMed] [Google Scholar]
- 12.Cammerer U, Dietrich W, Rampf T, Braun SL, Richter JA.. The predictive value of modified computerized thromboelastography and platelet function analysis for postoperative blood loss in routine cardiac surgery. Anesth Analg. 2003;96:51–7. [DOI] [PubMed] [Google Scholar]
- 13.Baker SP, O’Neill BB, Haddon W, et al. The Injury Severity Score: A method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–96. [PubMed] [Google Scholar]
- 14.Bartal C, Yitzak A.. The role of thromboelastography and recombinant factor VIIa in trauma. Curr Opin Anaesthesiol. 2009;22:281–8. [DOI] [PubMed] [Google Scholar]
- 15.Nylund CM, Borgman MA, Holcomb JB, Jenkins D, Spinella PC.. Thromboelastography to direct the administration of recombinant activated factor VII in a child with traumatic injury requiring massive transfusion. Pediatr Crit Care Med. 2009;10:e22–6. [DOI] [PubMed] [Google Scholar]
- 16.Trowbridge CC, Stammers AH, Cicarelli N, et al. Dose titration of recombinant factor VIIa using thromboelastography monitoring in a child with hemophilia and high titer inhibitors to factor VII. A case report and brief review. J Extra Corpor Technol. 2006;38:254–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Brohi K.. Diagnosis and management of coagulopathy after major trauma. Br J Surg. 2009;96:963–4. [DOI] [PubMed] [Google Scholar]
- 18.Johansson PI, Stissing T, Bochsen L, Ostrowski SR.. Thromboelastography and thromboelastometry in assessing coagulopathy in trauma. Scand J Trauma Resusc Emerg Med. 2009;17:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prowner DJ.. Thromboelastography during adult donor care. Prog Transplant. 2010;20:163–7. [DOI] [PubMed] [Google Scholar]
- 20.Jeger J, Zimmermann H, Exadaktylos K.. Can rapid TEG®accelerate the search for coagulopathies in the patient with multiple injuries? J Trauma. 2009;66:1253–7. [DOI] [PubMed] [Google Scholar]


