Abstract:
The recent global threat of a severe pandemic influenza outbreak has suggested that extracorporeal life support will begin to play an evolving role in the care of critically ill influenza stricken patients. The highly communicable attributes of influenza could result in widespread infection and an associated increased need for advanced life support. Supply and demand equilibrium may be abruptly disrupted, and ethical decisions regarding the allocation of life saving resources will inevitably need to be made. Protocol oriented planning, research analysis, and advanced technologies are critical factors in averting catastrophe. This review article details the epidemiology, diagnostic techniques, and interventions for the influenza A virus, including H1N1.
Keywords: extracorporeal membrane oxygenation, extracorporeal life support, pandemic, H1N1, influenza
The potential for a catastrophic global influenza pandemic has brought about much apprehension regarding large-scale management and advance life support treatments. Recent consortium data shows that the pandemic H1N1 viral influenza is the most dominant strain that is currently circulating throughout the modern world, with 399,218 laboratory confirmed cases as of January 23, 2010 (1). The H1N1 virus was first detected in people of the United States in April of 2009 (2); and on June 11, 2009, the World Health Organization (WHO) declared that a global pandemic of H1N1 was underway (3). Current indicators predict that in the event of a pandemic surge; between 12–30% of the global population will develop clinically diagnosed pandemic influenza as compared to 5–15% of the population typically infected with the seasonal strain of influenza (4,5). In many cases, patients presenting with clinically diagnosed H1N1 are likely to require hospitalization and advanced critical care such as ventilator support and extracorporeal life support (ECLS). Predictions estimate that 4% of all H1N1 patients will require hospitalization, and one out of every five hospitalized will present in a manner commanding critical care (4,5).
According to the Centers for Disease Control (CDC), there have been inaccurate estimations regarding H1N1 related deaths. Misleading estimations can be attributed to incomplete testing, erroneous test results, and inaccurate diagnosis. Additionally, the CDC does not know exactly how many people are infected with seasonal influenza each year. Statistical methods are commonly used to estimate the annual number of seasonal influenza related deaths (2). However, unlike seasonal influenza A which usually infects immune compromised individuals, H1N1 seemingly affects young and otherwise healthy individuals with no obvious immune suppression (6,7).
Microbiology of Influenza
Influenza A is the most prevalent of the five genera in the family Orthomyxoviridae and includes many different subtypes (1). The most common subtypes include the pandemic H1N1, avian H5N1, and seasonal H3N2 strains (8,9). Influenza type A viruses are initially characterized by their eight negative-sense single-strand ribonucleic acid (RNA) segmented genomes (10). They are further classified by the sub-typing of their surface glycoproteins that include the hemagglutinin (HA) and neuraminidase (NA) glycoproteins (11). Standard nomenclature relating to influenza virus identification considers the species from which it was isolated, the location of isolation, isolate number, isolate year, and specifically for the type A influenza, it’s HA and NA subtype (12). Currently, there are multiple genetically unique subtypes that have been isolated from circulating influenza A viruses; there are 16 HA subtypes and 9 NA subtypes (8). Though there are many different subtypes, only three HA (H1, H2, and H3) and two NA (N1, N2) have been documented as causative of human epidemics with respect to widespread, sustained human-to-human transmission (11).
The influenza A genome consists of an eight-segment genome, which predisposes the virus to an increase in frequency of antigenic shifts amongst different subtypes of influenza A (12). Antigenic shifts are major changes due to the recombination of genes that are typically possessive of pandemic capabilities (13). As a result of a shift, the virus may develop completely new antigenic properties of which the human population is highly vulnerable and immunologically naïve (13). Pandemic H1N1, also called swine origin influenza virus, is a reassortant virus made up of two swine strains, one human strain, and one avian strain (14,15). The name swine flu came about because the initial antigenic shift took place in the swine population. Different influenza A strains infected a pig, and it acted as a genetic mixing vessel for the reassortant of the various strains which yielded the pandemic H1N1 virus. The reassortant pathogen may be remarkably virulent, spreading easily from person to person and eventually progressing to a global pandemic, as seen with the H1N1 strain (12). Antigenic drift, which is less serious than antigenic shift, is caused by minor mutations that take place within a particular strain. Due to antigenic drift, new vaccines are needed annually to provide active immunization against mutated influenza A strain (13,16). The current H1N1 virus is very similar to previous strains that have been documented throughout history, but over time there have been enough antigenic shifts to render previously used vaccinations useless. Researchers are still uncertain as to the exact time in which the genetic re-assortment that created the 2009 H1N1 influenza virus occurred (17).
Influenza A virions are approximately 100 nm in diameter and 300 nm in length. Each virion is enclosed in a host cell-derived lipid membrane that is made up of HA and NA glycoprotein projections that appear in a 4:1 ratio as well as matrix (M2) ion channels (11,18). Within the envelope, M1 matrix is responsible for enclosing the virion core that houses the nuclear export protein (NEP) and the ribonucleoprotein (RNP). The NEP and RNP both consist of viral RNA segments coated in nucleoprotein and RNA polymerase molecules that allow the virus to replicate once viral entry of the host cell takes place (12).
Influenza Pathophysiology
Influenza virions recognize a potential host cell by detecting the presence of N-acetylneuraminic acid conjugate sites on the host cell surface (12). N-acetylneuraminic acid, also known as sialic acid, is a nine-carbon monosaccharide that is found on nearly all animal cells, and it is the site at which the HA viral glycoprotein binds to the host cell (12). Carbon-2 of the terminal end of a sialic acid molecule on a potential host cell preferentially binds to the carbon-3 or carbon-6 of a galactose molecule, forming either an alpha-2,3 or alpha-2,6-linkage which are both specifically recognized by the HA membrane protein found on Influenza A virions (12).
In ducks and other avian species, the alpha-2,3 linkage is more common than the alpha-2,6 linkage and is primarily found in the epithelial tissues of the gut (19,20). Conversely, in humans the alpha-2,6 configuration is found more often in the upper respiratory tract, and the alpha-2,3 linkage tends to be found deep within the lungs and alveoli (21,22). Avian influenza (H5N1) has a lower efficacy in humans due to its association with and, higher affinity for, the alpha-2,3 linkage-binding site (21,23). For the virus to reach the human analog alpha-2,3 linkage, it would have to be transmitted deep within the lungs to the alveoli, an area difficult for airborne viral particles to access. The small percentages of avian influenza infections currently on record, ubiquitously present with severe pneumonia, resulting in a 60% mortality rate (24).
One reason that humans are so susceptible to the H1N1 (swine flu), as opposed to the H5N1, is that the H1N1 strain of influenza A commonly binds to the alpha-2,6 receptors which are located in the upper respiratory tract of humans, making it a much more readily accessible target (8). Once an influenza virion recognizes a potential host cell and attachment takes place, viral entry begins by a process known as receptor-mediated endocytosis (25). The viral envelope fuses with the host cell membrane, and M2 proteins, which are specific to the influenza virus (12), form ion channels between the virus and host cell. The M2 ion channels allow for the passage of RNPs from the virion into the cellular matrix of the host cell (26), making it possible for viral uncoating to take place (27,28). Once the RNPs are released into the host cell’s cytoplasm, they are incorporated into the nucleus with the help of viral protein nuclear localization signals (29). The viral RNA then commandeers the host cell and replicates, creating more virions that bud off from the host cell to infect additional cells (13). Autopsy of pandemic H1N1 infected lung tissue revealed severe intra-alveolar edema, alveolar hemorrhage, erythrophagocytosis, and prolific hyaline membrane formation (9,30).
Traditional methods for classifying a suspected influenza infection included isolating the virus from field samples using embryonated chicken eggs or by performing an antigen-capture enzyme-linked immunosorbent assay (ELISA) test. An enzyme immune assay (EIA) may also be used for bedside diagnosis of flu like symptoms; but it is not sensitive enough to decipher between different strains of influenza A, and therefore it is not used when H1N1 is suspected (31). Additionally, the use of fluorescent antibody technology has also been used, but along with the ELISA and the EIA, it cannot differentiate between the various strains of influenza A virus (32).
To tell the difference between seasonal influenza A and the pandemic H1N1 influenza, a real-time reverse-transcriptase polymerase chain reaction (RT-PCR) assay is used (33). Typically the RT-PCR assay has been effective at classifying the specific strain of influenza A with a 95% confidence interval, which contributes to better diagnosis and improved treatment options (15). The RT-PCR test usually has about a 48-hour turn-around time when testing equipment can be used locally (31). Another less widely used method of further classification includes the use of an electrospray ionization mass spectrometry (15).
Pharmacological Interventions
The class of anti-influenza A drugs known as amantadines were developed to specifically target the M2 ion channel protein of the viral membrane (28). The goal of this drug class was to block the function of the M2 ion channels, which would slow the transmission of viral RNPs into the host cell, as well as depress viral un-coating (27). The 2009 H1N1 influenza A strain possesses the genetic marker S31N which is indicative of amantadine resistance (34).
An alternative group of drugs that the virus appears to be sensitive to, targets the NA glycoproteins of the virion as opposed to M2 proteins. The NA proteins assist the virus by breaking down mucins in the respiratory tract, which makes the host cell epithelium more penetrable. Neuraminidase inhibitors help to block the ability of the NA proteins to break down mucins in the respiratory epithelium, diminishing the infectivity of the virus (12). A couple mildly effective prescription anti-viral medications that seem to lessen the effects of the H1N1 virus include the anti-virals oseltamivir (Tamiflu®) and zanamivir (Relenza®) (31,35). Studies show that it is of paramount importance that early initiation of appropriate antiviral treatments such as oseltamivir or zanamivir be used, and empirical antiviral treatment is advised for all hospitalized patients with suspected H1N1 infections (36–38).
All current treatments emphasize prevention and focus on immunization and public reduction of exposure by implementation of infection control (39). The primary component in currently licensed influenza virus vaccines is HA surface glycoproteins from the influenza A virus (34). By injecting these surface antigens into the body, active immunity creates antibodies which will subsequently defend against future viral infections of the particular strain presented in the vaccine. Aside from vaccines according to the CDC, hand washing should help to prevent the spread of influenza. The use of ethanol hand sanitizers that are at least 60% ethanol by volume, have also shown to slow the spread of the virus.
Reports have shown that in immune compromised hosts, pandemic H1N1 can become drug resistant. Oseltamivir-resistant sub-strains of H1N1 in patients with suboptimal immune systems need to be carefully considered. Continued administration of the medication may lead to the emergence of additional resistant virions, compromising the potential patency of viable treatment options (40). Pandemic H1N1 oseltamivir resistant influenza can emerge as a result of genetic reassortment with seasonal H1N1, due to the fact that seasonal H1N1 is regularly resistant to the drug (8). The WHO and the Global Influenza Surveillance Network have reported 31 cases as of March 2010 of H1N1 viruses that show the H275Y mutation, conferring resistance to the anti-viral oseltamivir.
Symptomatic Presentation, Risk Factors, and Comorbidity Management
Initial infection of pandemic H1N1 is associated with symptoms of headache, nasal congestion, fatigue, pyrexia, vomiting, and diarrhea, all of which usually begin within a week of initial exposure (17). Once infected, the individual remains communicable for approximately 8 days following the onset of symptoms (41). The severity of symptomatic presentation ranges from mild to severe depending on the hosts comorbidities, immune system, and age. H1N1 is particularly dangerous for people who have one or more previously underlying medical conditions such as pregnancy, diabetes, heart disease, obesity, asthma, and kidney disease. According to the CDC, approximately 70% of H1N1 patients hospitalized have had one or more preexisting medical conditions, placing them in the high-risk category with potential serious complications such as septic shock. In addition, the very young and elderly are considered high-risk individuals (42).
The most devastating manifestation of pandemic H1N1 infection includes rapidly progressive acute respiratory distress syndrome (ARDS), acute lung injury (ALI), and the subsequent multiple system organ failure due to severe hypoxemia (43). ARDS is typically caused by pneumonia, aspiration of gastric contents, sepsis, multiple blood product transfusion, as well as some other less common factors (44). Prolonged hospital stays associated with ARDS and H1N1 commonly increase exposure to the nosocomial infections Streptococcus pneumoniae and Staphylococcus aureus, which further complicate treatment and recovery (33). According to the Journal of Heart and Lung transplantation (31) and the CDC, to minimize viral infectivity, patients should be placed in isolation and all health care interactions should be governed by droplet precaution protocol, as well as the use of personal protective equipment (PPE), including respiratory protection such as the N95 1860/1860s or 1870 (3M, St. Paul, MN). The CDC recommends this equipment as a means of protection against particulate aerosols that might contain viral material. The N95 mask is not oil resistant and it filters 95 percent of particulate matter that is as small as .3 microns in size mass median aerodynamic diameter (42,45). When using this equipment, it is imperative that it is properly fit tested and users are trained to attain optimal protection. In addition to respirators, infection control should be maintained at a high standard to prevent against widespread nosocomial transmission of infection, as was seen in the spring 2003 severe acute respiratory syndrome (SARS) outbreak in Canada (46). In an attempt to attenuate viral transmission, several sources (31,47,) suggest that health care workers who may come in contact with potentially infected patients should get vaccinated for the pandemic H1N1 influenza virus; as well as the seasonal virus as soon as vaccinations become available.
In severe cases of ARDS, lung function often stops altogether for days or weeks and may take up to a month to possibly regain adequate function. The pathophysiology of ARDS leads to severe inflammation of the lungs and pulmonary edema, which drastically impairs gas exchange. In addition, pandemic H1N1 can result in pulmonary hypertension, alveolar atelectasis, decreased surfactant production, and increased respiratory straining. All of these factors result in hypercapnea, hypoxia, and acidosis, which eventually lead to total lung dysfunction and multi-system organ failure. By utilizing loop diuretic therapy with a continuous infusion of furosemide or bumetanide, hypoxia due to pulmonary edema can be attenuated (43). Furthermore, studies show that by placing the patient in the prone position improved oxygenation and reduced pulmonary edema can be achieved (48,49). In addition, some studies (50) have shown that the use of inhaled nitrous oxide has improved alveolar ventilation and vasoregulation. Inhaled prostacyclin has also been shown to be effective in attenuating the effects of pulmonary edema associated with ARDS and ALI (51). Other beneficial treatments for lung injury include maintaining a positive end expiratory pressure (PEEP) of at least 10 cm H2O while the patient is on ventilator support (52). PEEP maintains alveolar flexibility and inflation, which both contribute to better gas exchange and reduced fluid recruitment (53). Additionally, studies have shown that PEEP prevents alveolar over distention and permanent injury caused by the repetitive opening and closing of the alveoli (54).
To allow time for the restoration of lung function, a patient with pulmonary dysfunction that cannot be managed using a ventilator, can be supported by extracorporeal membrane oxygenation (ECMO). ECMO allows the patient to maintain adequate body perfusion while the lung tissue heals, and gas exchange abilities are sufficiently restored.
Extracorporeal Life Support
ECMO was first widely accepted and used in the mid 1970s and was based on the work and ideas of Dr. John Gibbon who invented the first heart-lung machine using primitive oxygenation methods. It was not until the mid to late 70s that ECMO was successfully and repeatedly used for life support (55). It has been widely used for the treatment of over 40,000 critically ill patients of all ages and has traditionally been used to treat patients with compromised respiratory function; a large percentage of these patients are neonates and infants (56). Studies have shown that neonates tend to have a lower mortality rate than adults (57), and this may be attributed to a reduction in hemodynamic and circuit stress due to lower flow rates, respectively (58). Survival rates near 72% in newborns and 74% in children have been achieved using ECMO (59). A recent retrospective multi-center database study showed that for adults presenting with respiratory failure who were supported with ECMO, there was a 50% survival rate (56,60).
The role of ECMO is to return the patients physiological parameters to within normal limits by taking over for the injured lungs. When on ECMO, the patient’s tissue oxygen delivery and carbon dioxide removal are managed via the extracorporeal circuit (58). ECMO is used for patients that need respiratory or circulatory support due to underlying health conditions that compromise their native systems. For this reason ECMO has become an effective and an ever growing means of life support for patients with H1N1, allowing patients to bridge the gap from respiratory distress to total body recovery.
When ARDS develops as a result of a severe influenza infection, and prior treatments prove ineffective, ECMO is often used in a timely fashion to attenuate the possible occurrence of multiple system organ failure (25,61). The majority of patients treated with ECMO are patients with advanced and life threatening ARDS (52). To assess the degree of respiratory failure and determine whether ECMO support is indicated, the Murray scoring system is used. A Murray score is based on the patients PaO2/FiO2 ratio, PEEP, dynamic lung compliance, and the number of quadrants infiltrated on a chest radiograph (62). A Murray score of ≥3 or a PaO2/FiO2 ratio of <50 is usually an indication for ECMO utilization (52). According to the Extracorporeal Life Support Organization (ELSO), a PaO2 of less than 80 mmHg on an FiO2 of 1.0, and/or hypotensive shock while being optimally treated with two vasoactive drugs are indications for ECMO (56).
ECMO should be implemented within 6 or 7 days of initiating mechanical ventilation, and before the irreversible fibro proliferative phase of infection begins (47,60). The timing of ECMO implementation is considered with respect to symptom onset to minimize reperfusion injury. A recent study suggests that ECMO should be implemented before severe tissue hypoxia occurs, so that the damage associated with uncontrolled reactive oxygen species can be minimized, allowing endogenous antioxidants to maintain functionality (63). According to ELSO, when ECMO is used within 6 days of intubation, survival rates can be as high as 72%, as opposed to 30% after 7 days (47).
Due to the risks associated with ECMO, such as intracranial hemorrhaging, it is only used in the treatment of critically ill patients and performed in experienced centers with adequate equipment. The limiting factors with respect to ECMO utilization are equipment availability and healthcare staff with expertise in ECMO management.
ECMO Equipment and Techniques
ECMO circuits are comprised of a pump, tubing, oxygenator, heat exchanger, and often times other ancillary equipment such as a hemoconcentrator and safety monitoring devices. According to ELSO, servo-regulated occlusive roller head pumps are the most common type of ECMO pump. However, the centrifugal pump has proven to be very effective and safe due to its afterload dependent traits, and it has recently become more popular for ECMO use (64,65). Precautions need to be taken with respect to the potential for retrograde flow if the pump stops during ECMO bypass while using centrifugal technology (47). Anticoagulation with heparin must also be practiced to prevent blood clot formation at the artificial interface created during extracorporeal circulation (57).
The recent development of the poly-4-methyl-1-pentene (PMP) diffusion membrane allows for the use of hollow-fiber technology with a true (nonmicroporous) membrane. This has enabled the use of this low resistance device with all its inherent advantages, without plasma leakage necessitating circuit change out (66). Quadrox D® (Maquet, Rastatt, Germany) PMP oxygenators (Figure 1) are used in about 75% of adult ECMO cases as opposed to the hollow fiber oxygenators commonly used during cardiopulmonary bypass. The new Quadrox-iD has been developed for use on pediatric patients. It has a decreased surface area and prime volume all while maintaining the same benefits of the PMP technology. Studies have shown that the long-term use of microporous hollow fiber oxygenators leads to plasma leakage and eventual impairment of gas exchange (65). Additionally it is imperative that the membrane oxygenator be mounted below the level of the patient to avoid entraining air in the micropores (47).
Figure 1.
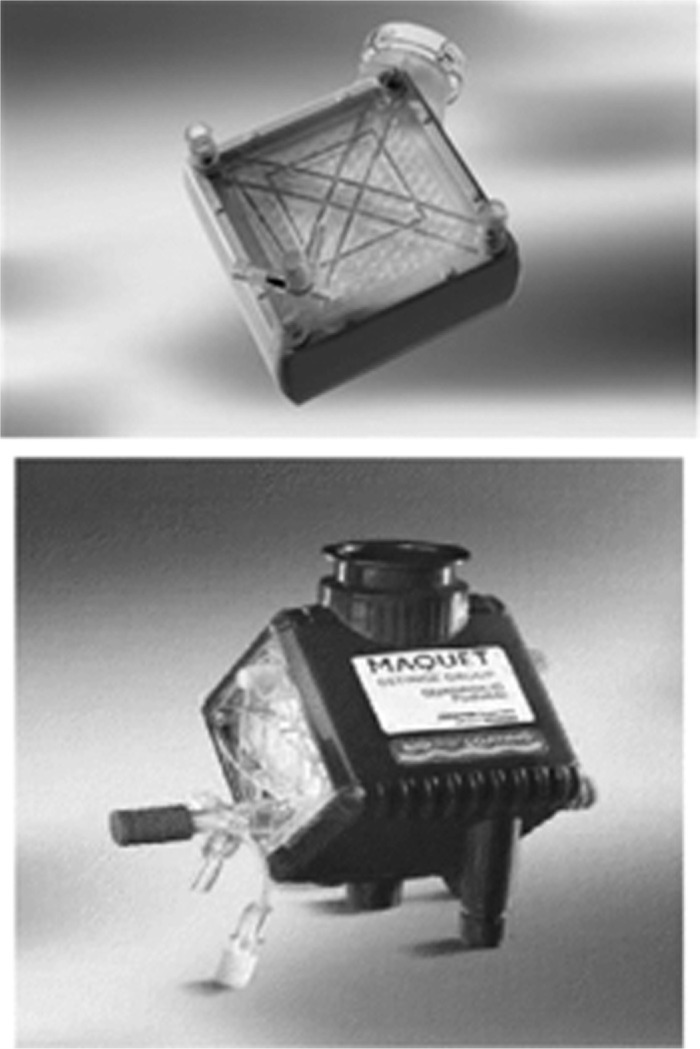
Maquet Quadrox D (top) and Quadrox iD (bottom) PMP oxygenators (Maquet, Rastatt, Germany). Quadrox D has a 250 mL prime volume with a flow range of .5–7 L/min. Quadrox iD has a prime volume of 81 mL and a flow range of .2–2.8 L/min.
Approximately 67% of ECMO centers primarily use heparin bonded circuits such as the Carmeda® (Medtronic, Inc., Minneapolis, MN) coating (65). Alternate surface coatings include the SmartX® (Sorin, Arvada, CO) and Physio® (Sorin) both of which use a polycaprolactone polysiloxane and polycaprolactone polydimethylsiloxane surface coating, respectively. Terumo has developed the X-coating® (Terumo, Ann Arbor, MI) which utilizes a poly-2-methoxyethylacrylate surface coating. Medtronic’s Trillium® coating is an improvement to their previous Carmeda® coating that utilizes polyethylene oxide and heparin, which resists flaking to a greater degree than the covalently bonded coating Carmeda®. For a patient with heparin-induced thrombocytopenia, the SmartX®, Physio®, and X-coating® are indicated since they are heparin free.
Most ECMO circuits incorporate a heat exchanger, cooler-heater, and safety devices such as a flow meter and bubble detector (58). According to a recent survey by Sutton et al. (67) of 49 registered ECMO centers, 67% used point-of-care blood gas devices, 71% used a bubble detector, 95% used an in-line arterial/venous oxygen saturation monitor, and 70% used cerebral saturation monitoring devices (Table 1). Additionally, 37% of the centers used the Thromboelastograph® (TEG®, Haemonetics Corp., Braintree, MA), and 61% used a plasma-free hemoglobin monitoring device to provide better quality ECMO care.
Table 1. Safety devices used during ECMO based on a 2007 survey of registered ECMO centers.
| Frequency of Monitoring and Safety Devices Utilization | ||
|---|---|---|
| CDI ™* in-line blood gas monitoring | 23 (55%) | 19 (45%) |
| Point-of-care blood gases | 29 (67%) | 14 (33%) |
| Oxygen analyzer | 20 (50%) | 20 (50%) |
| In-line venous/arterial oxygen saturation | 39 (95%) | 2 (5%) |
| Venous line pressure | 37 (90%) | 4 (10%) |
| Arterial line pressure | 33 (83%) | 7 (17%) |
| Blood flowmeter | 31 (76%) | 10 (24%) |
| Pre and post oxygenator pressure | 40 (93%) | 3 (7%) |
| Bubble detector | 30 (71%) | 12 (29%) |
| Expired CO2 | 18 (46%) | 21 (54%) |
| Battery back-up | 41 (98%) | 1 (2%) |
| Data management system (computerized charting) | 18 (45%) | 22 (55%) |
| Cerebral oximetry | 28 (70%) | 12 (30%) |
CDI™, Terumo Cardiovascular, Ann Arbor, MI.
Table from Sutton et al. (67), A 2007 survey of extracorporeal life support members: Personnel and equipment. J Extra Corpor Technol. 2009;41:172–9.
ECMO that is used for isolated respiratory support typically utilizes venovenous cannulation (Figure 2). The majority of patients with severe ARDS are managed with venovenous ECMO (43). In adults presenting with ARDS, blood flows of at least 60 mL/kg/min are advised to maintain adequate whole body perfusion (43).
Figure 2.
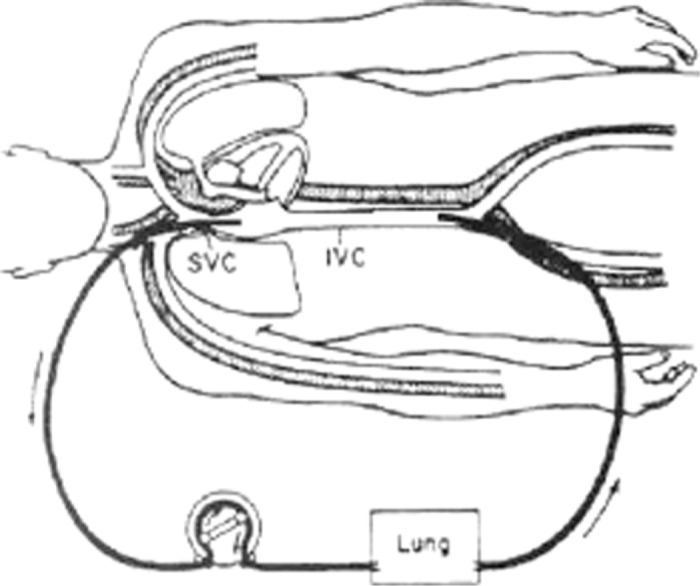
Simple veno-venous ECMO circuit with cannulation of the right atrium and femoral vein.
Venovenous ECMO is less challenging than venoarterial and many centers have proposed the use of simplified circuits to make this more easily managed by the critical care staff (Figure 3). Venovenous cannulation exclusively supports the respiratory function of the patient (57). It is the preferred method of cannulation for H1N1 patients not experiencing cardiac failure, because of the lower rate of associated complications (64,68). Blood is generally drained from the body via the femoral vein, passed through the membrane oxygenator of the ECMO circuit, and returned to the body via the contralateral femoral vein. Additionally, venovenous ECMO may use cannulation via the right jugular vein or directly into the right atrium. An alternative to using two cannulae for venous-venous ECMO is the single, bicaval, dual lumen cannula, called the Avalon Elite® (Avalon Laboratories, Rancho Dominguez, CA). It is placed percutaneously in the right internal jugular vein and simultaneously drains blood from the vena cavae and returns blood to the right atrium (Figure 4). Typically, in adults, a 27 Fr to 31 Fr Avalon cannula is inserted using the Seldinger technique and in some cases transesophageal echocardiography is used to ensure proper placement of the dual lumen cannula (43). Studies show that venovenous ECMO may be associated with fewer complications and mortality as compared to venoarterial ECMO (69,70).
Figure 3.
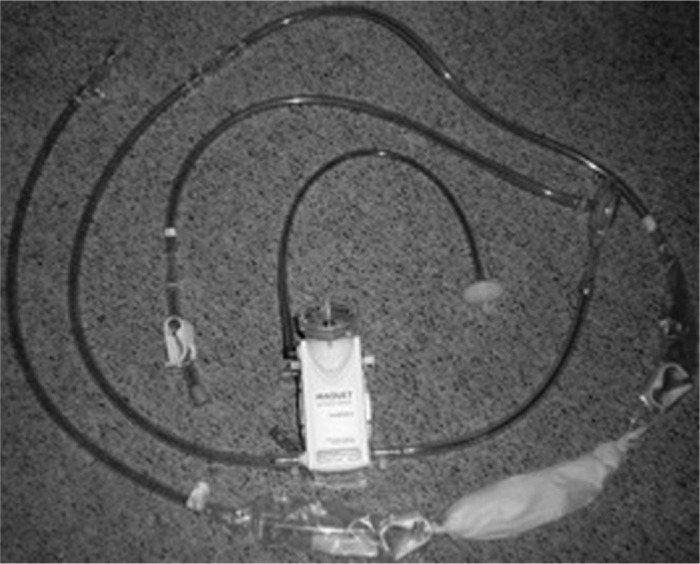
Simplified veno-venous ECMO circuit with a Quadrox D PMP oxygenator. Figure courtesy of Robert Brown.
Figure 4.
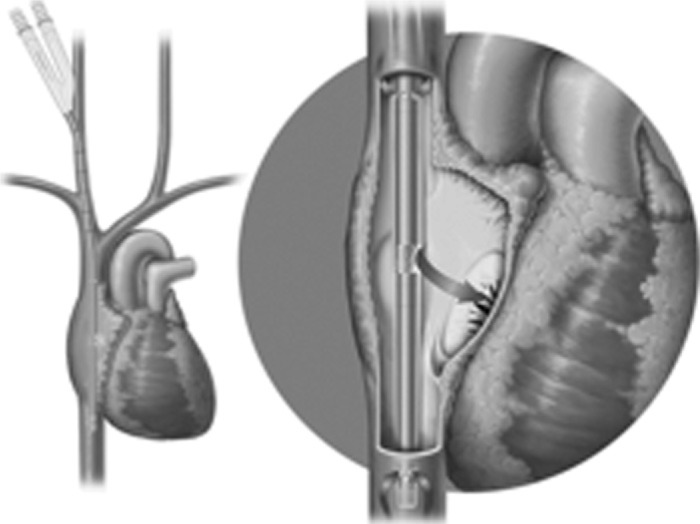
Avalon Elite® bi-caval dual lumen catheter placement. Catheter is inserted percutaneously via the internal jugular vein into the vena cavae and right atrium. Notice the cannula fenestrations in the superior and inferior vena cava where deoxygenated blood is removed from the patient. Oxygenated blood is then returned to the right atrium via the alternate lumen of the catheter.
Venoarterial ECMO is used in patients experiencing respiratory and hemodynamic shock that may be associated with pandemic H1N1 infection (43). Typically venoarterial ECMO is considered for cardiac support because it is used during heart failure following cardiac surgery (Figure 5). For this method of ECMO, blood is drained from the internal jugular vein, right atrium, proximal vena cava, or femoral vein, and returned to the circulation by way of right common carotid artery, axillary artery, femoral artery, or the aorta (proximal to the head vessels) (64). Percutaneous cannulation of the femoral artery may be performed using a 17 or 19 Fr cannula such as the Bio-Medicus® Carmeda-Coated Cannula, (Medtronic, Inc., Minneapolis, MN) and cannulation of the femoral vein may be performed using a 25 Fr multi-port cannula such as a Bio-Medicus® Carmeda Coated Cannula, (Medtronic, Inc.) (33). Distal limb perfusion is typically provided using a 8.5 Fr cannula such as the Super Arrow-Flex® Percutaneous Sheath (Arrow International, Inc., Reading, PA) (71). Venoarterial ECMO alleviates the workload of the heart and lungs, allowing time for cardiac and respiratory recovery with an emphasis on the cardiac support that it provides.
Figure 5.
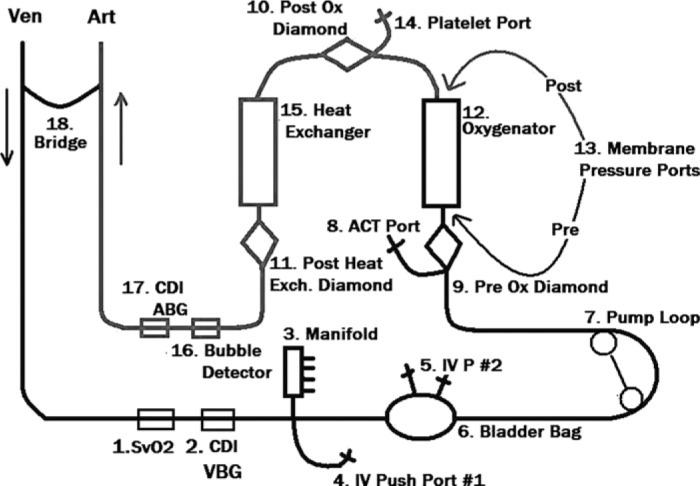
Typical veno-arterial ECMO circuit with safety devices and monitoring ports. Figure courtesy of Robert Brown.
The patient is weaned from ECMO when gas exchange can be maintained at a FiO2 of less than .30 (57). Prophylactic low molecular weight heparin is often used post ECMO to attenuate the likelihood of deep vein thrombosis and thromboemboli at the cannulation sites (47).
Khan et al. (72) cited ECMO as an accepted therapy to treat life threatening complications following lung transplantation such as primary graft dysfunction, acute rejection, or airway dehiscence. ECMO using the Levitronix CentriMag® pump (Levitronix, Waltham, MA) may be ideal for medium term support for lung transplant recipients suffering serious complications. Khan et al. cited the need for larger studies to elucidate the efficacy of the device (72). Herlihy et al. demonstrated in two case studies the use of the tandem heart as an “off label” option for ECMO in patients with cardiopulmonary failure (73). An advantage of ECMO in combination with the TandemHeart® (Cardiac Assist Inc, Pittsburgh, PA) (Figure 6) or CentriMag® pump (Figure 7) is utilization of one circuit to support both the ventricles and lungs (72,73).
Figure 6.
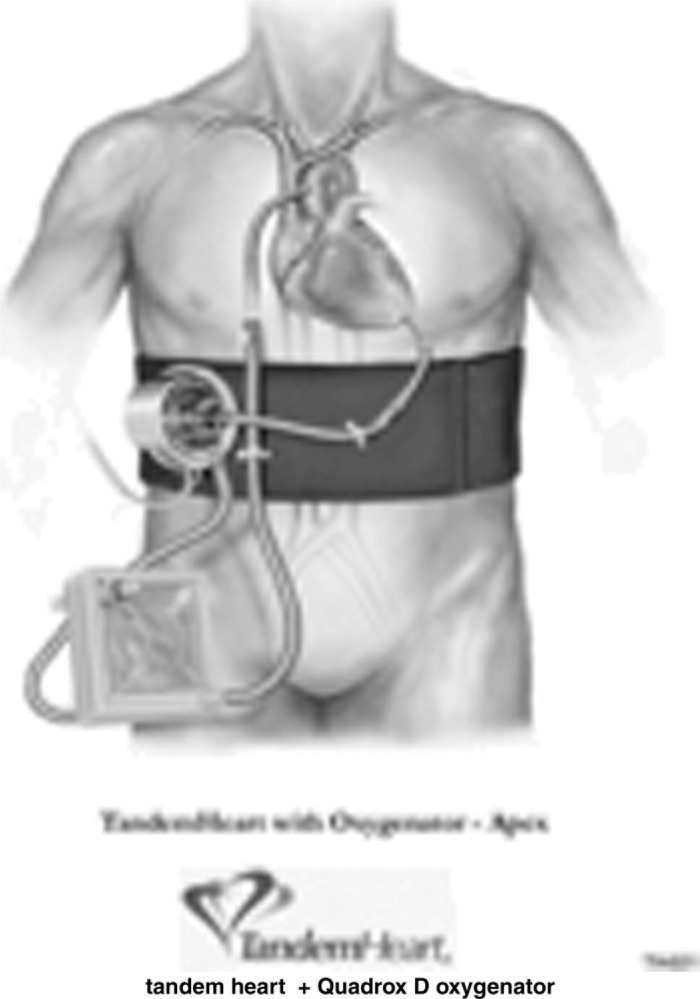
TandemHeart® ECMO circuit. The centrifugal pump is driven by a three phase, brushless, DC servomotor, has a 10 cc prime volume, and is capable of delivering flows up to 5 L/min.
Figure 7.

CentriMag® ECMO circuit. This extracorporeal blood pump is bearingless, works by magnetic levitation, and has been approved as a right ventricular assist device for up to 30 days. It has a 31 mL prime volume and is capable of delivering flows up to 10 L/min.
ECMO circuitry precautionary measures should be embraced when a circuit is constructed, primed, and stored for later use. Sievert et al. (65) have reported that approximately 60% of centers that perform ECMO keep a primed circuit on hand at all times. A recent study showed that wet storage led to higher leaching of di-(2-ethylhexyl) phthalate plasticizer (DEHP) from the polyvinyl chloride tubing commonly used in ECMO circuits (74). The leaching of DEHP, which is thought to be carcinogenic, depends primarily on how long the wet circuit is stored and the priming solution used. DEHP is also considered a potent inflammatory mediator, which needs to be carefully considered when it is used in ECMO circuitry (75–77).
As of February 2010, the CDC reported that there have been at least 3376 laboratory confirmed H1N1 deaths in the United States (78). Precautionary measures such as immunization, frequent hand washing, and avoiding potentially infected individuals are the best means of reducing infection and slowing the spread of the pandemic. Although the effects of H1N1 are extremely detrimental, advances in medical technology and the use of previously applied support systems, such as ECMO, provide beneficial treatments for severely compromised patients. Hospitals throughout the world are coming up with new innovative circuits to efficiently support infected patients (79).
According to a prospective study regarding the 2009 Canadian influenza season, critical illness due to influenza A (H1N1) occurred rapidly after hospitalization, and was often associated with young adults who presented with fewer comorbidities. The Canadian flu season, which peaked during the summer months of June and July, was associated with severe hypoxia, multisystem organ failure, prolonged ventilatory support, and frequent use of rescue therapies such as vasoactive drugs and inotropes (80). Of the 168 RT-PCR confirmed cases of pandemic H1N1, the mean age was 32.3 years, with 67.3% of the female gender. Only 30.4% of the total patient population presented with comorbidities such as chronic lung disease, hypertension, and obesity. Of the 168 patients studied, 29 of them expired; the majority with ARDS and hypoxic complications as the major cause of death (80). This study showed that by utilizing critical care technologies, especially ventilators, the majority of the patients presenting with confirmed pandemic H1N1 and ARDS could be assisted to recovery and eventual hospital release.
Currently no standard protocol for care of H1N1 has been developed. In addition to managing evident symptoms, it is important to consider ECMO as a potential treatment for the critically ill. As published in a recent study found in the November 4th issue of the Journal of the American Medical Association (81), emergent H1N1 cases that were treated with ECMO proved to be largely successful. According to Andrew Davies, MBBS and colleagues of Monash University and Alfred Hospital in Melbourne, Australia, 68 patients were supported with ECMO during the Southern Hemisphere’s 2009 influenza season, and 54 patients survived as of September 7, 2009 after being weaned from ECMO (81). The study showed that the most common comorbidities for those who did not survive were obesity (50%), asthma (28%), and diabetes (15%). Hemorrhagic complications were also quite common in the patients undergoing ECMO, and these complications occurred in 54% of the patients in the study. The most common sites for bleeding were cannulation sites (22%), respiratory (10%), and gastrointestinal tract (10%). Several of the 14 fatalities were attributed to intracranial hemorrhage (81). The average duration of ECMO support for the 68 patients in the series was 10 days (82).
It would be negligent not to assess the downfalls of ECMO treatment. High medical costs of approximately $2,000 a day (83), prolonged hospital stays, and the associated risks of hemorrhage and nosocomial infection are major ECMO-associated risks. Other risk factors include circuit clotting, oxygenator failure, air bubbles, and mechanical device errors. Due to the high degree of potential complications, patients undergoing intensive ECMO therapy require 24 hour monitoring. In addition, an ECMO specialist must be present in the hospital at all times to deal with any mechanical malfunctions or device failures (64).
Some contraindications of ECMO are multiple organ failure syndrome, unresponsive septic shock, uncontrollable metabolic acidosis, central nervous system injury, and patients unlikely to survive even with ECMO support (64). According to ELSO, depending on the level of leucopenia, concomitant immune suppression is also considered a contraindication.
Potentially Available Capacity
As of May 2010, 126 ECMO programs were registered with the Extracorporeal Life Support Organization in the United States (56). It is common for an ECMO center to have the capacity to operate only two ECMO systems at once. It has been stated that even if only a small portion of people who acquire pandemic H1N1 become sick enough to need ECMO support, there still could be the overwhelming number of 10 million people that need ECMO (83). Currently, all of the ECMO centers in the world could not supply ECMO services to such a large number of patients (83). As the potential scale of the pandemic increased, there would be an associated diminishment of the essential resources available to provide ECMO support. If the demand for extracorporeal support overwhelms the traditional ECMO methodology, one innovator suggested the potential for converting dialysis equipment to perform extracorporeal oxygenation and support (84). By incorporating an oxygenator and making a few minor alterations, dialysis technology could help alleviate the deficit between supply and demand for extracorporeal equipment and the associated advanced care. Another new technology that could change the quality of ECLS care is the portable ECMO system. Portable ECMO systems have provided effective inter-hospital transport of critically ill patients, as well as improved care for emergent cases. By utilizing an experienced ECMO team, patients can be transported to specialized ECMO centers where they can receive dedicated treatment from ECLS specialists.
According to a recent publication regarding the 2009 influenza season in Victoria, Australia, small diversion influenza clinics helped to relieve larger hospitals from the overwhelming demand for clinical diagnosis and initial care for influenza-like symptoms (85). This conserved hospital resources (staff, intensive care unit (ICU) beds, and ECMO equipment) for diagnostically confirmed, critically ill patients. ELSO suggests that ECMO centers should be located in tertiary centers that are able to support a minimum of six ECMO patients a year under normal circumstances (47,53). Due to the very invasive, complex, and demanding characteristics of ECMO, centers that perform a high volume of ECMO each year have better survival rates (86). Regionalization of patient care associated with pandemic influenza allows primary centers to accumulate experience in dealing with and managing the most critically ill patients, while reserving other larger hospitals for routine patient care (56,87). By accumulating experience at a select few centers, potentially lifesaving clinical trials can be streamlined, and collaborative measures can be more readily executed (88). By utilizing ECMO centers with extensive experience in the field, patient morbidity and mortality could be greatly reduced. Incentive reimbursement programs based on the benefit of care provided, and improved patient outcomes, should motivate hospitals and health professionals to embrace proven technologies while maintaining the patient’s best interest (89).
In order for best practice policies to be effective, financial concerns regarding ECMO as an optional treatment must be considered. According to the American Medical Association, in 2007, the cost of ECMO treatment consisted of, 10.53 relative value units for cannula insertion, 27.09 relative value units for the first 24 hours of ECMO care, and 15.24 relative value units for each additional 24 hours of ECMO support (68,90). The costs of infection control and isolation practices also add to the burden on the already financially overwhelmed healthcare system (91). With the threat of a pandemic outbreak and a fiscally limited healthcare system hanging in the balance, critical decisions regarding resource planning and management command a high degree of ethical responsibility.
Influenza pandemics come in waves, and according to recent model projections, the world is overdue for another pandemic of global proportions (92,93). A global pandemic of any scale presents health care providers with the necessity to make critical decisions based on ethical standards. As hospitals become overwhelmed with patients in dire need of advanced care, medical personnel are faced with the allocation of critical resources including time, space, and medications. According to the Journal of American Medical Association, the health care systems of both Canada and Mexico struggled to meet the demands of the 2009 influenza outbreak in their respective regions (87).
Understanding the epidemiology of a pandemic threat is a crucial step in developing higher quality diagnostic techniques and therapeutic services that can be used to improve patient outcomes (94). The role of ECMO must be critically evaluated based on its resource-intensive nature and its role in patient treatment. Assessing the scope and scale of a global pandemic is the first step in determining the potential role and expanse of ECMO treatment. As a pandemic develops over time, more data are collected, and the scope and scale are reevaluated to determine an updated account for resource allocation. Sequential triage methods are used as estimates of demand-capacity change, and time progresses. Sequential triage methods illicit changes in the interventional care threshold based on resource scarcity as demand increases due to global proliferation of a pandemic agent (39). Relief-demand and urgency forecasting should be used to optimally prepare regional centers for the true potential scale of an influenza pandemic (95,96). Guidelines should be established for the criteria determining patient referral and transport to regional ECMO centers. The events surrounding Hurricane Katrina tested the competency of traditional triage methods. Advanced planning, clear guidelines, and predetermined protocols regarding resource management are imperative implementations that must be embraced in preparation for the next natural disaster, particularly an influenza pandemic (97).
Ethics Behind ECMO and Allocation Planning
A recent publication in the Journal of Critical Care Medicine suggested that it would be profoundly unethical to neglect gaining valuable scientific research during a time of global crisis (94). The article suggests that to be medically and professionally responsible, protocols should be mandatory, and organized methodical research should be conducted. The complete experience of each pandemic must be accurately captured and recorded for analytical and methodological research to provide stepwise progress toward improving patient outcomes (39,98). According to Cook et al., “Failure to improve outcomes through rigorous efficient investigations during a pandemic is as ethically irresponsible as failing to provide care itself” (94).
Modeling studies suggest that a disaster similar in proportion to the 1918 influenza pandemic would require somewhere between 200% and 400% of the current ICU beds and ventilators available in the United States (99). On a regular basis the typical ICU operates at greater than 90% occupancy, and has very little available surge capacity (100). Models for the allocation of disaster relief funds are in place for natural disasters, terrorist attacks, and other global catastrophic events (96,101,102). In 2005, Hurricane Katrina caused billions of dollars in damage, and accentuated the inadequacies of current management strategies (103).
Current allocation strategies largely embrace the first come first serve approach, and focus on treatment of those likely to expire without interventional care (104). The army and civil protection agency have a four-level injury assessment system that rates patient condition based on viability and the level of care needed to ensure survivorship (105).
In an emergency situation when the supply of vital health care resources, including personnel, cannot meet demand, the only way to satisfy the equation is to decide who will get the limited resources and in what order. When it comes to widespread disasters that stress the healthcare system, such as pandemic influenza, there are several ethical schools of thought regarding resource management and allocation. The “Broad Social Value” viewpoint refers to the overall worth of an individual to society. It attempts to determine, based on summary judgments, if the past and future contributions of an individual support the common good of the community (106). Based on that judgment, the individual is provided with or denied the limited, potentially lifesaving resource.
Another school of thought regarding the ethics of resource management is “Instrumental Value,” also known as the “Multiplier Effect.” This ideology suggests that the premise for an individual receiving a limited resource is based on his or her ability to carry out a specific function that will, in turn, prevent further disintegration and assist in crisis management (107). Treatment of doctors, healthcare professionals, and people in leadership positions take precedence over other individuals. By prioritizing key “instrumental” individuals who can provide essential services to the public, there would be more of a potential supply of critical services, and ultimately more lives would be saved (106).
Other ideologies relating to the ethics of resource allocation include “Maximizing Life-Years” and the “Life Cycle” principle. The “Maximizing Life-Years” principle suggests that priority should be given to the individual who has the greatest chance of surviving for the longest period of time, assuming all other variables (age, race, gender, comorbidities) are equal (106). The “Life Cycle” principle advocates giving each individual an equal opportunity to live through the various “phases” of life, and it does not directly consider intrinsic worth or social utility (108). It supports the prioritization of younger individuals based on “intergenerational equity” and the “fair innings” theology (109). This consideration has interesting implications in that it tends to sacrifice experience for youth. From an ethics standpoint, it lends itself to little interpretation. It allows difficult, potentially biased decisions of who will get the limited care to be a little more protocol oriented.
By practicing a multi-principle approach to allocation, so as not to use any one attribute as an absolute determinant of care, one can alleviate some of the ethical weight associated with critical decision making. A multi-principle approach would clarify the difference between a potentially healthy older individual and an individual of the same age with preexisting, potentially preventable comorbidities, based on a point system. Use of a Sequential Organ Failure Assessment scoring system would help to determine who would get care based on the outcome of the scoring algorithm (106,110).
A conglomerate of the aforementioned ideologies should take into account the greatest number of possible variables. By reducing bias and considering the need for vital resources and services, pandemic protocols can be implemented prior to an actual outbreak. “The value of observational data in predicting potential resource utilization in the near term and for future pandemics cannot be overemphasized.” (81).
SUMMARY
Models have forecasted that the genetic shifting within the influenza A genera will continue, and over time it will produce highly virulent pathogenic strains of influenza for which mankind has little or no immunity (111,112). The overwhelming demand for advanced life support technologies and services such as ECMO could not possibly be met without sufficient planning, education, and preparation. Critical allocation strategies need to be studied and appropriate policies and protocols need to be developed and assessed. The best method of mitigating pandemic effects is to prepare a protocol oriented defense strategy from an ethical standpoint that clearly defines resource allocations. Strategies rooted on evidence based studies, data collection, and the scale of disaster should be continually reassessed (39,98). Extracorporeal life support will play a vital role in the preservation of life. Prospective studies that define the detailed role and implementation timing of ECLS are essential, and will aid in improving the quality of care and management. Continual exhaustive monitoring, technological improvement, advanced planning, and historical record analysis are areas of consequential and paramount importance with regards to ECLS utilization to mitigate disaster.
REFERENCES
- 1.World Health Organization (WHO). Pandemic (H1N1) 2009-update 86. Available at: http://www.who.int/csr/don/2010_02_5/en/ (Accessed February 11, 2010).
- 2.Center for Disease Control and Prevention. CDC estimates of 2009 H1N1 influenza cases, hospitalizations and deaths in the United States, April-October 17, 2009. Available at: http://www.cdc.gov/h1n1flu/estimates/April_October_17.htm (Accessed November 12, 2009).
- 3.World Health Organization (WHO). World now at the start of 2009 influenza pandemic. Available at: http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html (Accessed October 8, 2009).
- 4.Royal College of Physicians. Preparations for pandemic influenza: Guidance for hospital medical specialties on adaptations needed for a pandemic influenza outbreak. Available at: http://bookshop.rcplondon.ac.uk/contents/f2df511c-f131-4fa1-8b7b-66105a0bb8e4.pdf (Accessed March 14, 2010).
- 5.Department of Health (DH). Swine flu, guidance for planners (October 22, 2009). Available at: http://www.dh.gov.uk/en/Publications andstatistics/Publications/PublicationsPolicyAndGuidance/DH_107413 (Accessed March 14, 2010).
- 6.Ya-Shu C, van Hal SJ, Spencer PM, Gosbell IB, Collett PW.. Comparison of adult patients hospitalized with pandemic (H1N1) 2009 influenza and seasonal influenza during the “PROTECT” phase of the pandemic response. Med J Aust. 2010;192:90–3. [DOI] [PubMed] [Google Scholar]
- 7.Shinde V, Bridges CB, Uyeki TM, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med. 2009;360:2616–25. [DOI] [PubMed] [Google Scholar]
- 8.Malik-Peiris JS, Poon LLM, Guan Y.. Emergence of a novels swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol. 2009;45:169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guarner J, Falcon-Escobedo R.. Comparison of the Pathology Caused by H1N1, H5N1, and H3N2 Influenza Viruses. Department of pathology and laboratory medicine, Emory University Hospital, Atlanta, GA: Archives of Medical Research; 2010. [DOI] [PubMed] [Google Scholar]
- 10.Bouvier NM, Palese P, Shaw ML.. The biology of influenza viruses. Table 1: The genomic segments of influenza A/PuertoRico/8/1934 (H1N1) virus and their encoded proteins. Vaccine. 2008;26S:D49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palese P, Shaw ML.. Orthomyxoviridae: The viruses and their replication. In: Knipe DM, Howley PM, eds. Fields Virology. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 12.Bouvier NM, Palese P.. The biology of influenza viruses. Vaccine. 2008;26S:D49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCance KL, Huether SE.. Pathophysiology. The Biological Basis for Disease in Adults and Children, 5th Ed. St. Louis, MO: Elsevier Mosby Inc; 2006:298–305. [Google Scholar]
- 14.Patel M, Dennis A, Flutter C, Khan Z.. Pandemic (H1N1) 2009 influenza. Br J Anaesth. 2010;104:128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faix DJ, Sherman SS, Waterman SH.. Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;361:728–9. [DOI] [PubMed] [Google Scholar]
- 16.Martini FH, Nath JL.. Fundamentals of Anatomy and Physiology, 8th Ed. San Francisco, CA: Pearson Education, Inc.; 2009:796–7. [Google Scholar]
- 17.Flu.gov. Origin of 2009 H1N1 flu (swine flu): Questions and answers. Available at: http://www.flu.gov/individualfamily/about/h1n1_qa.html (Accessed November 25, 2009).
- 18.Zebedee SL, Lamb RA.. Influenza A virus M2 protein: Monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol. 1988;62:2762–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matrosovich M, Klenk HD.. Natural and synthetic sialic acid-containing inhibitors of influenza virus receptor binding. Rev Med Virol. 2003;13:85–97. [DOI] [PubMed] [Google Scholar]
- 20.Van-Riel D, Munster VJ, de-Wit E, et al. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am J Pathol. 2007;171:1215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couceiro JN, Paulson JC, Baum LG.. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 1993;29:155–65. [DOI] [PubMed] [Google Scholar]
- 22.Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD.. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc Natl Acad Sci USA. 2004;101:4620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beare AS, Webster RG.. Replication of avian influenza viruses in humans. Arch Virol. 1991;119:37–42. [DOI] [PubMed] [Google Scholar]
- 24.Gambotto A, Barratt-Boyes SM, de Jong MD, Neumann G, Kawaoka Y.. Human infection with highly pathogenic H5N1 influenza virus. Lancet. 2008;371:1464–75. [DOI] [PubMed] [Google Scholar]
- 25.Prescott LM, Harley JP, Klein DA.. Microbiology 6th Ed. New York: McGraw-Hill; 2005:390, 847–9. [Google Scholar]
- 26.Stegman T.. Membrane fusion mechanisms: The influenza hemagglutinin paradigm and its complications for intracellular fusion. Traffic. 2000;1:598–604. [DOI] [PubMed] [Google Scholar]
- 27.Pinto LH, Holsinger LJ, Lamb RA.. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–28. [DOI] [PubMed] [Google Scholar]
- 28.Wharton SA, Belshe RB, Skehel JJ, Hay AJ.. Role of virion M2 protein in influenza virus uncoating: Specific reduction in the rate of membrane fusion between virus and liposomes by amantadine. J Gen Virol. 1994;75:945–8. [DOI] [PubMed] [Google Scholar]
- 29.Cros JF, Palese P.. Trafficking of viral genomic RNA into and out of the nucleus: Influenza, Thogoto and Borna disease viruses. Virus Res. 2003;95:3–12. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Padilla R, de-la-Rosa-Zamboni D, Ponce-de-Leon S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–9. [DOI] [PubMed] [Google Scholar]
- 31.Danziger-Isakov LA, Husain S, Mooney ML, Hannan MH.. The novel 2009 H1N1 influenza virus pandemic: Unique considerations for programs in cardiothoracic transplantation. J Heart Lung Transplant. 2009;28:1341–7. [DOI] [PubMed] [Google Scholar]
- 32.Choi YK, Goyal SM, Kang SW, Farnham MW, Joo HS.. Detection and subtyping of swine influenza H1N1, H1N2 and H1N3 viruses in clinical samples using two multiplex RT-PCR assays. J Virol Methods. 2002;102:53–9. [DOI] [PubMed] [Google Scholar]
- 33.Buckley E, Sidebotham D, McGeorge A, Roberts S, Allen SJ, Beca J.. Extracorporeal membrane oxygenation for cardiorespiratory failure in four patients with pandemic H1N1 2009 influenza virus and secondary bacterial infection. Br J Anaesth. 2010;3:326–9. [DOI] [PubMed] [Google Scholar]
- 34.Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization (WHO). Pandemic (H1N1) 2009 update 69. Available at: http://www.who.int/csr/don/2009_10_09/en/ (Accessed November 1, 2009).
- 36.Tsui-Mai K, Chih-Hsien W, Yee-Chun C, et al. The first case of severe novel H1N1 influenza successfully rescued by extracorporeal membrane oxygenation in Taiwan. J Formos Med Assoc. 2009;108:894–8. [DOI] [PubMed] [Google Scholar]
- 37.CDC: WHO Guidelines for Pharmacological Management of Pandemic Influenza A(H1N1) 2009 and other Influenza Viruses. (2010). Available at: http://www.who.int/csr/resources/publications/swineflu/h1n1_guidelines_pharmaceutical_mngt.pdf (Accessed December 2010). [PubMed]
- 38.Kidd IM, Down J, Nastouli E, et al. H1N1 pneumonitis treated with intravenous zanamivir. Lancet. 2009;374:1035. [DOI] [PubMed] [Google Scholar]
- 39.Baker AJ.. What is the role of extracorporeal membrane oxygenation for hypoxic failure during an influenza pandemic? Can J Anaesth. 2010;57:201–5. [DOI] [PubMed] [Google Scholar]
- 40.Center for Disease Control and Prevention. Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients—Seattle, Washington, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:893–6. [PubMed] [Google Scholar]
- 41.Daewood F, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. [DOI] [PubMed] [Google Scholar]
- 42.Center for Disease Control and Prevention. 2009 H1N1 flu. Available at: http://www.cdc.gov/h1n1flu/ (Accessed October 24, 2009).
- 43.Napolitano LM, Park PK, Raghavendran K, Bartlett RH.. Nonventilatory strategies for patients with life-threatening 2009 H1N1 influenza and severe respiratory failure. Crit Care Med. 2010;38:S1–17. [DOI] [PubMed] [Google Scholar]
- 44.Ware LB, Matthay MA.. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. [DOI] [PubMed] [Google Scholar]
- 45.3M Manufacturer. Available at: http://multimedia.3m.com/mws/mediawebserver?66666UuZjcFSLXTtMXfX4XTEEVuQEcuZgVs6EVs6E666666- (Accessed on November 10, 2010).
- 46.Ofner-Agostini M, Wallington T, Henry B, et al. Investigation of the second wave (phase 2) of severe acute respiratory syndrome (SARS) in Toronto, Canada. What happened? Can Commun Dis Rep. 2008;2:1–11. [PubMed] [Google Scholar]
- 47.Extracorporeal Life Support Organization (ELSO) Ann Arbor, MI. H1N1 Specific Supplements to the ELSO General Guidelines. (2009). Available at: http://www.elso.med.umich.edu/WordForms/ELSO%20H1N1%20Specific%20Guidelines.pdf (Accessed November 12, 2010).
- 48.Piehl MA, Brown RS.. Use of extreme position changes in acute respiratory failure. Crit Care Med. 1976;4:13–4. [DOI] [PubMed] [Google Scholar]
- 49.Gattinoni L, Tognoni G, Pesenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568–73. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman GM, Ross RA, Day SE, et al. Inhaled nitric oxide reduces the utilization of extracorporeal membrane oxygenation in persistent pulmonary hypertension of the newborn. Crit Care Med. 1997;25:352–9. [DOI] [PubMed] [Google Scholar]
- 51.Zwissler B, Gregor K, Habler O, et al. Inhaled prostacyclin (PGI2) versus inhaled nitric oxide in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:1671–7. [DOI] [PubMed] [Google Scholar]
- 52.Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J.. Emerging technology review. Extracorporeal Membrane Oxygenation for Treating Severe Cardiac and Respiratory Disease in Adults: Part 1—Overview of Extracorporeal Membrane Oxygenation. J Cardiothorac Vasc Anesth. 2009:23:886–92. [DOI] [PubMed] [Google Scholar]
- 53.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–36. [DOI] [PubMed] [Google Scholar]
- 54.Adams AB, Simonson DA, Dries DJ.. Ventilator-induced lung injury. Respir Care Clin N Am. 2003;9:343–62. [DOI] [PubMed] [Google Scholar]
- 55.Joubert-Huebner E.. History of ECMO/ECLS [Powerpoint slides]. Available at: http://www.ecc-book.com/History_of_ECMO_Lecture_1.pdf (Accessed May 2, 2010).
- 56.ECMO Registry of the Extracorporeal Life Support Organization (ELSO). Ann Arbor, Michigan. Available at: http://www.elso.med.umich.edu/H1N1Info.html (Accessed May 27, 2010).
- 57.Zwischenberger BA, Clemson LA, Lynch JE, Zwischenberger JB.. ECMO to artificial lungs: Advances in long-term pulmonary support. In: Mongero LB, Beck JR, eds. On Bypass: Advanced Perfusion Techniques. Totowa, NJ: Human Press; 2008:251–77. [Google Scholar]
- 58.Meurs KV.. ECMO Specialist Training Manual, 2nd Ed. Ann Arbor, MI: Extracorporeal Life Support Organization; 1999:19 67, 101, 123–69. [Google Scholar]
- 59.University of Michigan Department of Surgery; ECMO. Available at: http://surgery.med.umich.edu/pediatric/research/section/ecmo.shtml (Accessed May 22, 2010). [Google Scholar]
- 60.Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL.. Extracorporeal membrane oxygenation in adults with severe respiratory failure: A multi-center database. Intensive Care Med. 2009;35:2105–14. [DOI] [PubMed] [Google Scholar]
- 61.Mongero LB, Beck JR, Oz M, Argenziano, M.. On Bypass: Advanced Perfusion Techniques. Totowa, NJ: Human Press; 2008:251–77. [Google Scholar]
- 62.Peek GJ, Clemens F, Elbourne D, et al. CESAR: Conventional ventilator support vs. extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Services Registry. BMC Health Services Research. 2006;6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grist G, Whittaker C, Merrigan K, Fenton J, Pallotto E, Lofland G.. Defining the late implementation of extracorporeal membrane oxygenation (ECMO) by identifying increased mortality risk using specific physiologic cut-points in neonatal and pediatric respiratory patients. J Extra Corpor Technol. 2009;41:213–9. [PMC free article] [PubMed] [Google Scholar]
- 64.Meurs KV, Lally KP, Peek G, Zwischenberger JB.. ECMO Extracorporeal Cardiopulmonary Support in Critical Care, 3rd Ed. Ann Arbor, MI: Extracorporeal Life Support Organization; 2005:88, 108, 133,–51, 364,–78, 412–5. [Google Scholar]
- 65.Sievert AN, Shackelford AG, McCall MM.. Trends and emerging technologies in extracorporeal life support: Results of the 2006 ECLS survey. J Extra Corpor Technol. 2009;41:73–8. [PMC free article] [PubMed] [Google Scholar]
- 66.Horton S, Thuys C, Bennett M, Augustin S, Rosenberg M, Brizard C.. Experience with the Jostra Rotaflow and QuadroxD oxygenator for ECMO. Perfusion. 2004;19:17–23. [DOI] [PubMed] [Google Scholar]
- 67.Sutton RG, Salatich A, Jegier B, Chabot DA.. 2007 survey of extracorporeal life support members: Personnel and equipment. J Extra Corpor Technol. 2009;41:172–9. [PMC free article] [PubMed] [Google Scholar]
- 68.Schuerer DJE, Kolovos NS, Boyd KV, Coopersmith CM.. Extracorporeal membrane oxygenation: Current clinical practice, coding, and reimbursement. American College of Chest Physicians. Chest. 2008;134:179–84. [DOI] [PubMed] [Google Scholar]
- 69.Cengiz P, Seidel K, Rycus PT, Brogan TV, Roberts JS.. Central nervous system complications during pediatric extracorporeal life support: Incidence and risk factors. Crit Care Med. 2005;33:2817–24. [DOI] [PubMed] [Google Scholar]
- 70.Varnholt V, Lasch P, Sartoris J, et al. Prognosis and outcome of neonates treated either with veno-arterial (VA) or veno-venousus (VV) ECMO. Int J Artif Organs. 1995;18:569–73. [PubMed] [Google Scholar]
- 71.Huang SC, Yu HY, KoW J, Chen YS.. Pressure criterion for placement of distal perfusion catheter to prevent limb ischemia during adult extracorporeal life support. J Thorac Cardiovasc Surg. 2004;128:776–7. [DOI] [PubMed] [Google Scholar]
- 72.Khan NU, Al-Aloul M, Shah R, Yonan N.. Early experience with the Levitronix Centrimag® device for extra-corporeal membrane oxygenation following lung transplantation. Eur J Cardiothorac Surg. 2008;34:1262–4. [DOI] [PubMed] [Google Scholar]
- 73.Herlihy JP, Loyalka P, Jayaraman G, Kar B, Gregoric ID.. Extracorporeal membrane oxygenation using the Tandem Heart System’s catheters. Tex Heart Inst J. 2009;36:337–41. [PMC free article] [PubMed] [Google Scholar]
- 74.Horne DC, Torrance I, Modine T, Gourlay T.. The effect of priming solutions and storage time on plasticizer migration in different PVC tubing types—implications for wet storage of ECMO systems. J Extra Corpor Technol. 2009;41:199–205. [PMC free article] [PubMed] [Google Scholar]
- 75.Kavlock R, Boeckelheide K, Chapin R, et al. NTP Centre for the evaluation of risks to human reproduction: Phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol. 2002;16:529–653. [DOI] [PubMed] [Google Scholar]
- 76.Gourlay T, Stefanou DC, Taylor KM.. The effect of methanol washing of plasticized polyvinyl chloride on biomaterial-contact-mediated CD11b (mac-1) expression in a rat recirculation model. Artif Organs. 2002;26:5–9. [DOI] [PubMed] [Google Scholar]
- 77.Asberg AE, Videm V.. Activation of neutrophil granulocytes in an in vitro model of a cardiopulmonary bypass. Artif Organs. 2005;29:927–36. [DOI] [PubMed] [Google Scholar]
- 78.Pandemic flu information (PFI) (2009). Available at: http://homepage.mac.com/monotreme1/Outbreak%20folder/H1N1Outbreaks.html (Accessed March 24, 2009).
- 79.World Health Organization (WHO). Preparing for the second wave: Lessons from current outbreaks. Available at: http://www.who.int/csr/disease/swineflu/notes/h1n1_second_wave_20090828/en/index.html (Accessed November 1, 2009).
- 80.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A (H1N1) in Canada. Available at: http://jama.amaassn.org/content/early/2009/10/12/jama.2009.1496.full.pdf (Accessed November 12, 2010).
- 81.The Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95. Available at: http://jama.ama-assn.org/cgi/content/full/302/17/1888 (Accessed November 12, 2010). [DOI] [PubMed] [Google Scholar]
- 82.Neale T.. Most patients with severe H1N1 treated with ECMO survive. MedPage Today; 2009. Available at: http://www.medpagetoday.com/InfectiousDisease/URItheFlu/16381 (Accessed November 18, 2009).
- 83.Elsevier Global Medical News. Flu pandemic pushing demand for ECMO. Available at: http://www.medconnect.com (Accessed October 8, 2009).
- 84.Gaspard G.. Winnipeg surgeon “MacGyvers” artificial lung. Dialysis machine transformed into heart/lung bypass, saves newborn. National Review of Medicine. 2007; Available at: http://www.nationalreviewofmedicine.com/issue/2007/03_15/4_advances_medicine1_5.html (Accessed November 12, 2010). [Google Scholar]
- 85.Lum ME, McMillan AJ, Brook CW, Lester R, Piers LS.. Impact of pandemic (H1N1) 2009 influenza on critical care capacity in Victoria. Med J Aust. 2009;191:502–6. [DOI] [PubMed] [Google Scholar]
- 86.Grasselli G, Foti G, Patroniti N, et al. A case of ARDS associated with influenza A-H1N1 infection treated with extracorporeal respiratory support. Minerva Anestesiol. 2009;75:741–5. [PubMed] [Google Scholar]
- 87.White DB, Angus DC.. Preparing for the sickest patients with 2009 influenza A (H1N1). JAMA. 2009;302:1905–6. [DOI] [PubMed] [Google Scholar]
- 88.Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfield GD.. Hospital volume and outcomes of mechanical ventilation. N Engl J Med. 2006;355:41–50. [DOI] [PubMed] [Google Scholar]
- 89.Diamond GA, Kaul S.. Evidence-based financial incentives for healthcare reform: Putting it together. Circulation: Cardiovas cular Quality and Outcomes. 2009;2:134–40. [DOI] [PubMed] [Google Scholar]
- 90.American Medical Association. 2007 National Physician Fee Schedule Relative Value Units. Chicago, IL: American Medical Association; 2007. [Google Scholar]
- 91.Denholm JT, Gordon CL, Johnson PD, et al. Hospitalized adult patients with pandemic (H1N1) 2009 influenza in Melbourne, Australia. The Doctor’s Health Fund. Med J Aust. 2010;192:84–6. [DOI] [PubMed] [Google Scholar]
- 92.Morse SS, Garwin RL, Olsiewski PJ.. Next flu pandemic: What to do until the vaccine arrives? Science. 2006;314:929. [DOI] [PubMed] [Google Scholar]
- 93.Kilbourne ED.. Influenza pandemics of the 20th century. Emerg Infect Dis. 2006;12:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cook D, Burns K, Finfer S, et al. Clinical research ethics for critically ill patients: A pandemic proposal. Crit Care Med. 2010;38:S1–5. [DOI] [PubMed] [Google Scholar]
- 95.Sheu JB.. Dynamic relief-demand management for emergency logistics operations under large-scale disasters. Transp Res, Part E Logist Trans Rev. 2010;46:1–17. [Google Scholar]
- 96.Sheu JB.. An emergency logistics distribution approach for quick response to urgent relief demand in disasters. Transp Res, Part E Logist Trans Rev. 2007;43:687–709. [Google Scholar]
- 97.Okie S.. Dr. Pou and the hurricane-implications for patient care during disasters. N Engl J Med. 2008;358:1–5. [DOI] [PubMed] [Google Scholar]
- 98.Chan V, Fraser R.. ECMO and the elephant in the corner. Perfusion. 2009;24:223–4. [DOI] [PubMed] [Google Scholar]
- 99.Toner E, Waldhorn R, Maldin B, et al. Hospital preparedness for pandemic influenza. Biosecur Bioterror. 2006;4:207–17. [DOI] [PubMed] [Google Scholar]
- 100.Rubinson L, Nuzzo JB, Talmor DS, O’Toole T, Kramer BR, Inglesby TV.. Augmentation of hospital critical care capacity after bioterrorist attacks or epidemics: Recommendations of the Working Group on Emergency Mass Critical Care. Crit Care Med. 2005;33:2393–403. [DOI] [PubMed] [Google Scholar]
- 101.Morris SS, Wodon Q.. The allocation of natural disaster relief funds: Hurricane Mitch in Honduras. World Dev. 2003;7:1279–89. [Google Scholar]
- 102.Fiedrich F, Gehbaur F, Rickers U.. Optimized resource allocation for emergency response after earthquake disasters. Saf Sci. 2000;35:41–57. [Google Scholar]
- 103.Rawls CG, Turnquist MA.. Pre-positioning of emergency supplies for disaster response. Technology and Society. 2009;9:1–9. [Google Scholar]
- 104.Pesik N, Keim NE, Iserson KV.. Terrorism and the ethics of emergency medical care. Ann Emerg Med. 2001;37:642–6. [DOI] [PubMed] [Google Scholar]
- 105.Fries G, McCalla G, Levitt A, Cordova R.. A prospective comparison of paramedic judgment and the trauma triage rule in the prehospital setting. Ann Emerg Med. 1994;24:885–9. [DOI] [PubMed] [Google Scholar]
- 106.White DB, Katz MH, Luce JM, Lo B.. Who should receive life support during a public health emergency? Using ethical principles to improve allocation decisions. Ann Intern Med. 2009;150:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Department of Health and Human Services. Draft Guidance on Allocating and Targeting Pandemic Influenza Vaccine. Washington, DC: U.S. Department of Health and Human Services; 2007. Available at: www.pandemicflu.gov/vaccine/prioritization.pdf, http://www.flu.gov/individualfamily/vaccination/allocationguidance.pdf (Accessed April 14, 2010). [Google Scholar]
- 108.Emanuel EJ, Wertheimer A.. Public health. Who should get influenza vaccine when not all can? Science. 2006;312:854–5. [DOI] [PubMed] [Google Scholar]
- 109.Williams A.. Intergenerational equity: An exploration of the ‘fair innings’ argument. Health Econ. 1997;6:117–32. [DOI] [PubMed] [Google Scholar]
- 110.Christian MD, Hawryluck L, Wax RS, et al. Development of a triage protocol for critical care during an influenza pandemic. CMAJ. 2006;175:1377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Webster RG.. Predictions for future human influenza pandemics. J Infect Dis. 1997;176:S14–9. [DOI] [PubMed] [Google Scholar]
- 112.Vincent AL, Ma W, Lager KM, Janke BH, Richt JA.. Swine influenza viruses: A North American perspective. Adv Virus Res. 2008;72:127–54. [DOI] [PubMed] [Google Scholar]


