Abstract:
This prospective randomized study compares the inflammatory response and fibrinolytic activation of fully coated/uncoated and open/closed extracorporeal circuits (ECC) in high risk patients. Over a 2-month period, 48 patients with EuroSCOREs 6 or greater undergoing coronary revascularization were pro spectively randomized to one of the four perfusion protocols: Group 1: Closed and totally hyaluronan based heparin free coated (Vision HFO-GBS-HF™, Gish Biomedical, Rancho Santa Margarita, CA) ECC with a soft-shell coated venous reservoir (SVR11S2-HFC™, Gish Biomedical) and a hard-shell cardiotomy (CAPVRF44, Gish Biomedical) (n = 12); Group 2: Closed and totally uncoated identical ECC with soft-shell uncoated venous reservoir and a hard-shell cardiotomy (n = 12); Group 3: Open, totally hyaluronan based heparin free coated ECC (n = 12); and Group 4: Control-open, uncoated ECC (n = 12). Blood samples were collected at T1: Baseline; T2: 15 minutes after cardiopulmonary bypass (CPB) initiation; T3: before cessation of CPB; T4: 15 minutes after protamine reversal, and T5: in the intensive care unit. Serum IL-6 levels were significantly lower at T2 in all study groups, at T3 for coated groups, and T4 for closed+coated group (p < .05 versus control). Creatine kinase M-band (MB) levels in coronary sinus blood demonstrated well preserved myocardium after CPB in both coated groups versus Control (p < .05). Neutrophil CD11b/CD18 levels were significantly lower for all study groups versus control at T2, for both coated groups at T3 and only for closed+coated group at T4 (p < .05). Postoperative hemorrhage (mL) was 510 ± 40 in closed+coated and 536 ± 40 in open+coated groups (control: 784 ± 48, p ≤ .05). No significant differences in thrombin-antithrombin complex and free plasma hemoglobin were observed. Desorbed protein amount on ECC (mg/dL) was 1.7 ± .01 in closed+coated, 2.01 ± .01 in open+coated, and 3.3 ± .015 in control groups (p ≤ .05). Use of a closed and completely heparin free coated ECC may reduce neutrophil degradation, cytokine release characterized by improved clinical outcomes including reduced blood loss, reduced requirement for inotropes, and reduced atrial fibrillation.
Keywords: cardiopulmonary bypass, inflammatory mediators, hemodynamics
Despite tremendous progress since its introduction into clinical practice, cardiopulmonary bypass (CPB) is still far from perfect and hence continuous efforts to study and improve hemocompatibility of both conduct and components of CPB are crucial (1).
Recently introduced, closed and miniaturized CPB circuits appear to be a fundamental innovation, because they lack an open reservoir and cardiotomy suction. The use of closed miniaturized circuits has been shown to improve clinical outcomes, compared with conventional open circuits (2,3). To assess the effects of closed miniaturized circuits, we should clearly distinguish between those due to the “closed” and “miniaturized” aspects, because the miniaturization of CPB circuits brings various advantages owing to the avoidance of excessive hemodilution and the excessive reduction of blood-circuit contact area.
Eliminating blood-air interface has been addressed by several closed operations (no hard shell reservoir). Yet, other such circuits have reduced circuit length and prime volume to limit transit time of blood through foreign surfaces but, retained an open configuration that exposed the blood to an air interface in the hard shell reservoir. This negated some of the advantage of coating the circuit and reducing the length. Other issues of limited safe operation such as trapped air removal from a purely closed circuit have reduced field acceptance of closed circuits (4).
Implementation of heparin coated CPB circuits in cardiac operations has proved to attenuate the activation of biologic cascades and the presence of thromboresistant heparinized surfaces has further allowed better perioperative outcomes (5). However, clinical benefits have not been demonstrated in most studies on surface heparinized circuits when used in low-risk patients (6). The emerging problem of heparin induced thrombocytopenia and thrombocytosis has led to use of heparinless strategies for patients with antibodies and use of alternative “heparinless” surface coatings and direct thrombin inhibitors for anticoagulation.
Hyaluronan is a polysaccharide of the glycosaminoglycans class. It is a unique biopolymer, which is found in all tissues and body fluids in every mammalian species as well as in microorganisms. It is very hydrophilic; its viscous solutions have most unusual rheological properties and are exceedingly lubricious (7).
The patient population referred for coronary artery bypass grafting has become more challenging. The results of this displacement could be altered to the benefit of the patients undergoing open heart surgery by continual improvement of the operative techniques as well as the technology of CPB systems.
Risk recognition and risk stratification have received wide acceptance in cardiac surgery over the past decade. Researchers focus on factors associated with patient risk, develop and test strategies designed to improve the margin of safety, and lead to risk neutralization (8).
Coating of extracorporeal circuits may be a solution to prevent adverse effects induced by contact of blood elements and proteins with foreign surfaces and the use of a closed system minimizes the gas to blood interface, which may play a significant role in activation. This prospective randomized study compares the inflammatory response and fibrinolytic activation of fully coated/uncoated and open/closed extracorporeal circuits in high risk patients.
PATIENTS AND METHODS
Patients
This study was approved by the Medical Ethics Committee of the Institution (Issue no: 16/2009) and supported by University of Kirikkale Research Fund (Issue no: 09/27). Informed consent was obtained from each patient included in the study.
Over a 2-month period, 48 EuroSCORE 6+ patients undergoing coronary revascularization were evaluated according to the Euroscore before the operation and then prospectively randomized (closed envelope allocation) to one of the four perfusion protocols with the investigators blinded to the allocation: Group 1: Closed and totally hyaluronan based heparin free coated (Vision HFO-GBS-HF™, Gish, CA) extracorporeal circuits with a soft-shell coated venous reservoir (SVR11S2-HFC™) and a hard-shell cardiotomy (CAPVRF44) (n = 12) (Figure 1); Group 2: Closed and totally uncoated identical ECC with soft-shell uncoated venous reservoir and a hard-shell cardiotomy (n = 12); Group 3: open, totally hyaluronan based heparin free coated ECC (n = 12); Group 4: Control-open, uncoated ECC (n = 12).
Figure 1.

Closed and totally coated extracorporeal circuit with a soft-shell coated venous reservoir and a hard-shell cardiotomy.
CPB was instituted on roller pump (System 1, TCM Heat exchanger, Terumo Cardiovascular, Ann Arbor, MI) for all groups. Table 1 demonstrates the features of the components of ECC.
Table 1. Specifications of extracorporeal circuits.
| Model No. | Priming Volume | Surface Area | Blood Flow Rate | |
|---|---|---|---|---|
| Vision Oxygenator | HFO | 280 mL | 2.45 m2 | 1–8 L/min |
| Model No. | Operating Capacity | Min. Operating Capacity | Filter Type | |
| CAPVRF44 hard-shell venous reservoir | CAPVRF44 | 4400 mL | 200 mL | 160 μ screen/20 μ depth |
| SVR11S2 soft-shell venous reservoir | SVR11S | 1100 mL | 200 mL | 105 μ screen |
Anticoagulation or antiplatelet therapy was discontinued 7 days before the operation in all patients. Exclusion criteria consisted of known coagulopathy, endocarditis, and inability to obtain informed consent. The rest of the patients that did not correspond to exclusion criteria were enrolled in the study with respect to preoperative Euroscore classification.
Operative Technique
Anesthesia was induced by fentanyl (35 μg/kg) and muscle relaxation was established with pancuronium (.1 mg/kg).
The patients were intubated endotracheally and ventilated with 100% oxygen. A Swan-Ganz catheter (Bicakcilar, Turkey) was placed via internal jugular vein. Patients were administered full dose - 300 IU/kg heparin (Liquemine, Roche, Turkey) with target activated clotting time (ACT) over 480 seconds. ACT was measured by Hemochron 801 (International Technodyne Corp., Edison, NJ). Cardiotomy, suction, and left ventricle decompression lines were not connected to the circuit. Shed blood was not retransfused and processed in cell-saver (DIDECO-SHILEY Therapeutic Autotransfusion System, Turkey). The ascending aorta was cannulated for arterial inflow, and the right atrium for venous return. Patients were cooled down to 30°C. Blood flow was maintained 2.0–2.4 L/min/m2 during CPB. After cross clamping of aorta, the heart was arrested with 4:1 blood cardioplegia, 10–15 mL/kg, and maintained at 20-minute intervals. Warm blood cardioplegia was administered before the aortic cross clamp was released. Bilateral internal mammary artery or radial artery grafts were used for coronary artery lesions, if not used in the first operation and saphenous vein grafts were used as alternatives in other instances. Single partial occlusion clamping technique was used for proximal anastomoses. Rewarming was initiated during last grafting. At 36.5°C, CPB was discontinued and heparin was reversed by protamine sulphate (Protamine, Roche, Istanbul, Turkey), 3.1 mg/kg. The adequacy of protamine reversal was checked by ACT and corrected, when necessary. No anti-fibrinolytics were used. Prime for CPB was different for open and closed circuits. For open circuits, 60 mL of mannitol 20% + 1000 mL of hydroxyethyl starch (Voluven 130.4, Fresenius, Turkey) and 300 mL of crystalloid (Plasmalyte A, Eczacibasi, Turkey) with a total of 1360 mL was used. For closed circuits, 60 mL of mannitol 20% + 600 mL of hydroxyethyl starch and 150 mL of crystalloid with a total of 810 mL was used.
Perioperative Follow-up
For each patient, the following factors were evaluated before discharge and documented: Hemodynamic parameters, perfusion and cross clamp duration, intubation period, postoperative hemorrhage, the use of blood (as units) and fresh frozen plasma (blood product-as units) during the hospital stay, incidence of arrhythmia, use of inotropic support, complications and infection, the duration of intensive care unit stay and hospital stay, perioperative mortality, New York Heart Association Classification, and Doppler echocardiography. Comparison among groups was performed retrospectively.
Blood Samples and Assays
Complete blood count [hemoglobin, hematocrit, erythrocyte, white blood cell (WBC), and platelet counts] was recorded. Serum interleukin 6 (IL-6) levels were measured by enzyme linked immunosorbent assay (Bender Medsystems, Vienna, Austria) (coefficient of variation < 10% and sensitivity < 1.4 pg/mL). Creatine kinase MB levels were measured in the samples obtained from retrograde cardioplegia catheter (coronary sinus blood) before and at the end of CPB before protamine infusion.
Blood samples were collected in potassium-EDTA (ethylene diamine tetra acetic acid) tubes using a radial or femoral artery catheter at the following intervals:
Baseline: After induction of anesthesia (before administration of heparin) (T1)
On CPB: 15 minutes after initiation of CPB (T2)
Off CPB: Before cessation of CPB (T3) (before protamine infusion)
Protamine: 15 minutes after reversal with protamine (T4)
ICU: First postoperative day at 8:00 a.m. (T5)
Flow Cytometry
Neutrophil CD11b/CD18 expressions were determined at T1 up to T4 by flow cytometry (EPICS Elite ESP, Coulter Corporation, Hialeah, FL) as previously described (9). Cursors were set to measure the mean relative fluorescence intensity and the values were expressed as a percentage of the baseline. All steps from blood sampling to cell staining were carried out at 0°C in an ice-water bath to minimize artifactual increases in adhesion molecule expression caused by sample handling.
Thrombin Antithrombin III Complex
Thrombin generation and inactivation by antithrombin-III were measured by plasma determination of levels of thrombin antithrombin III complex (TAT-III) to quantify the degree of stimulation to the coagulation system. TAT-III measurements (Behring Diagnostics, Westwood, MA) were performed according to the instructions provided by kit manufacturers.
Free Plasma Hemoglobin
Hemolysis was evaluated by plasma determination of free plasma hemoglobin (FHb). FHb measurements (Behring Diagnostics, Westwood, MA) were done according to the instructions provided by kit manufacturers.
Spectrophotometry
At the termination of CPB, the complete circuit was rinsed with saline solution. The oxygenator was removed, treated with glutaraldehyde solution, and dismantled by a saw under sterile conditions. Hollow fibers were collected for later protein desorption studies (10). A mean number of 300 fibers (6 cm) were put into a 15-mL plastic tube with 1% sodium dodecyl sulfate (Pharmacia Biotechnology, Sweden) and 1% Triton X-100 solution (Bio-Rad, Cambridge, MA). The tube was then placed in a 38 kHz, 80 W ultrasonic washer (Kaijyo, Japan) for 1 hour and treated in phosphate buffered saline buffer solution at pH 7.4 under constant temperature of 25°C for 6 hours. The sample was passed through a filter (Millipore Corporation, Bedford, MA). The amount of desorbed protein (microalbumin) in each specimen for every patient was evaluated quantitatively with a COBAS MIRA Spectrophotometer (Roche Diagnostics Systems Inc, Branchburg, NJ) with its range adjusted to greater than .01.
Statistical Analysis
Based on data from previous studies (11,12), a sample size of 12 patients in each subgroup would have a power of 90% and a type 1 error of .05 to detect a 25% difference in IL-6. Data are expressed as the mean ± the standard error of the mean. Mann Whitney U test was used to compare demographic and non-parametric data. Two-way analysis of variance with factor group and repeated factor time was used to analyze differences over time in each group and for differences between groups. Post-hoc test (Bonferroni correction) was applied whenever a significant difference was detected. A p value less than .05 was considered significant. Data were analyzed using SPSS program.
RESULTS
Four patients were excluded from the study. Three of them refused to participate in this study and one was reported to have history of coagulation factor insufficiency. Demographic data is presented in Table 2.
Table 2. Preoperative characteristics of patient cohorts.
| Group 1 (Closed-Coated) | Group 2 (Closed-Uncoated) | Group 3 (Open-Coated) | Control (Open-Uncoated) | p | |
|---|---|---|---|---|---|
| Age (y) | 58 ± 2.6 | 61.7 ± 2.5 | 59.3 ± 2.2 | 64.9 ± 2.38 | NS |
| Male sex | 7 | 6 | 8 | 7 | NS |
| Body surface area (m2) | 1.88 ± .05 | 1.91 ± .04 | 1.83 ± .05 | 1.79 ± .02 | NS |
| NYHA class | 3.1 ± .13 | 2.76 ± .2 | 3.2 ± .15 | 2.6 ± .11 | NS |
| Ejection fraction | .41 ± .01 | .43 ± .02 | .35 ± .08 | .43 ± .01 | NS |
| Preoperative Euroscore | 9.2 ± 1.2 | 8.91 ± 1.3 | 8.75 ± 1.7 | 9.1 ± 1.9 | NS |
| LVEDP (mmHg) | 18.2 ± 2.3 | 14.1 ± 2.4 | 19.1 ± 2.6 | 17. ± 2.9 | NS |
NYHA, New York Heart Association Classification; LVEDP, left ventricular end-diastolic pressure.
Intragroup Comparisons over Time
In all groups, WBC counts were significantly higher at T3, T4, and T5 (p < .01) with respect to T1. Platelet counts were significantly lower throughout the procedure compared with T1 in all groups (p < .01). Serum interleukin-6 levels were significantly higher at T2, T3, T4, and T5 (p < .01) with respect to T1 in all groups.
Intergroup Comparisons over Time
WBC counts demonstrated significant increases at T3 for both coated groups and T4 for closed+coated group versus control (Figure 2) (p < .05 versus control). Platelet counts were preserved significantly better at T3, T4 in closed+coated group (p < .05 versus control) (Figure 3). Serum IL-6 levels were significantly lower at T2 in all study groups, at T3 for coated groups, and T4 for closed+coated group (Figure 4) (p < .05 versus control). Creatine phosphokinase-MB levels in coronary sinus blood demonstrated well preserved myocardium after CPB in both coated groups versus control (p < .05) (Figure 5). Flow cytometric analysis is demonstrated in Figure 6. Neutrophil CD11b/CD18 levels were significantly lower for all study groups versus control at T2, for both coated groups at T3, and only for closed+coated group at T4 (p < .05).
Figure 2.
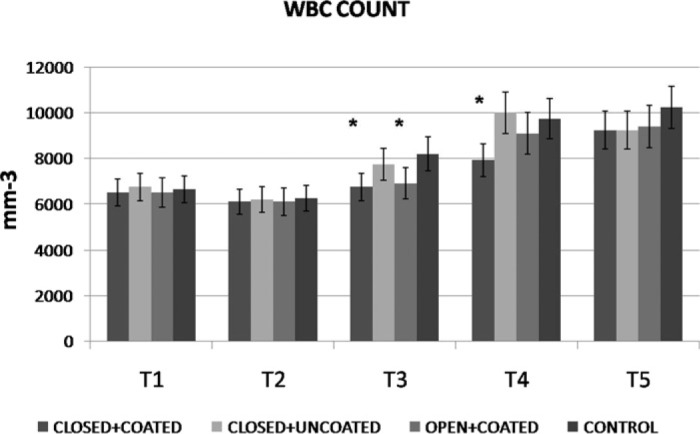
White blood cell count (mm−3) throughout the procedure for all groups. *p< .05 versus control
Figure 3.
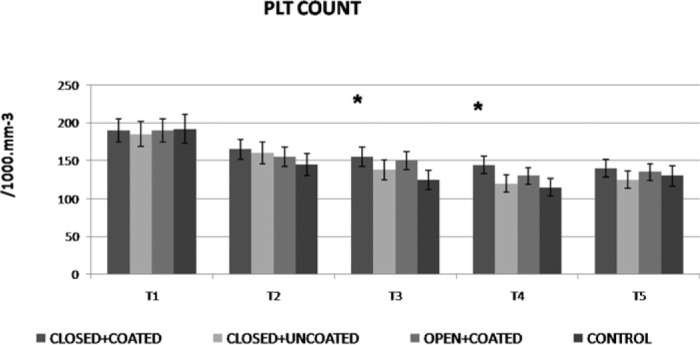
Platelet count (1000 mm−3) throughout the procedure for all groups. *p< .05 versus control
Figure 4.
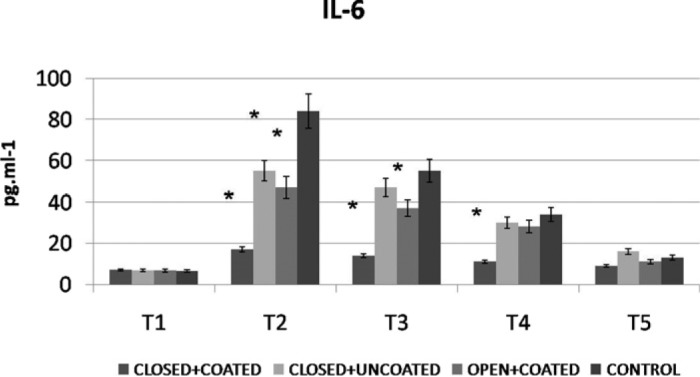
IL-6 levels (pg/mL) throughout the procedure for all groups. *p< .05 versus control
Figure 5.
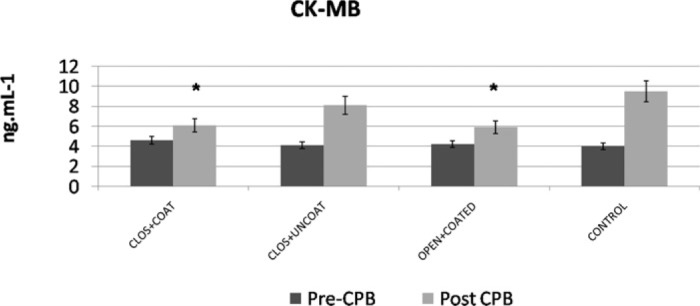
Coronary sinus creatine kinase MB (ng/mL) evaluation before (T1) and post CPB (T4) for all groups. *p< .05 versus control
Figure 6.
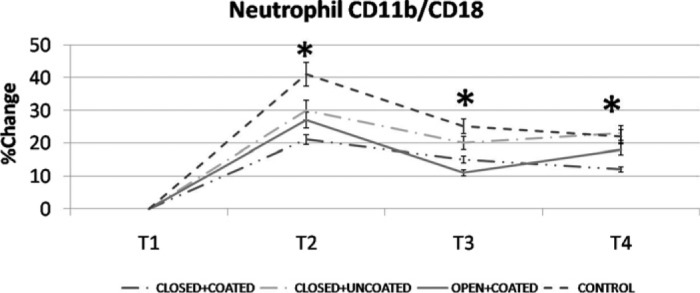
Neutrophil CD11b/CD18 upregulation (%) detected by flow cytometry for all groups. *p< .05 versus control
In all cohorts, ACT was significantly higher 5 minutes after heparin administration and remained high until the start of protamine reversal compared with the preoperative ACT in all groups. TAT-III was significantly greater 10 minutes after the start of CPB than baseline. It continued to increase during CPB and reached a maximum value of 5 minutes after protamine administration. Thereafter, TAT-III started to decrease. No significant differences in TAT-III were observed among any groups at any time point. There was no significant difference in the amount of cardiotomy suction blood in all groups.
The rate of formation of FHb at the end of CPB after protamine infusion (T4) was not significantly different in four patient groups. FHb levels were 7.1 ± 2.3 μmol.L-1 (closed+coated), 6.95 ± 2.5 μmol.L-1 (closed+uncoated), 7.76 ± 3.2 μmol.L-1 (open-coated), and 7.88 ± 3.5 μmol.L-1 for control groups. Desorbed protein amount (microalbumin) on fibers were significantly lower for both coated groups (Figure 7) (p < .05). Perioperative follow-up is summarized in Table 3.
Figure 7.
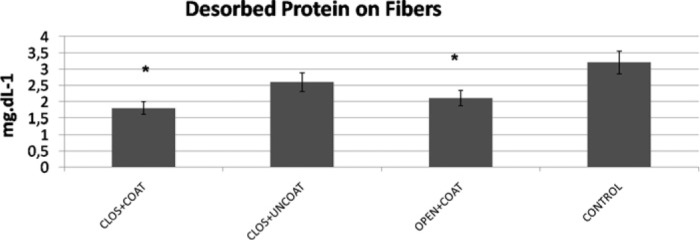
Desorbed protein amount (microalbumin mg/dL) on oxygenator fibers. *p< .05 versus control
Table 3. Perioperative follow-up of patient cohorts.
| Group 1 (Closed+Coated) | Group 2 (Closed+Uncoated) | Group 3 (Open+Coated) | Control (Open+Uncoated) | p | |
|---|---|---|---|---|---|
| Duration of CPB (min) | 121 ± 7 | 129 ± 7 | 112 ± 7 | 134 ± 8 | NS |
| t-intub (h) | 9.4 ± 2* | 13 ± 2 | 10.2 ± 1* | 15.9 ± 2 | < .05 |
| Postoperative hemorrhage (mL) | 510 ± 40* | 710 ± 42 | 536 ± 40* | 784 ± 48 | < .05 |
| Arrhythmia (%) | 15* | 35 | 25 | 45 | < .05 |
| Blood transfusion (unit) | 2.2 ± .5 | 2.0 ± .5 | 2.1 ± .5 | 2.6 ± .5 | NS |
| Inotropic support (%) | 40%* | 50% | 35%* | 60% | < .05 |
| ICU stay (day) | 3.2 ± .1* | 4.2 ± .1 | 2.9 ± .1* | 5.8 ± .1 | < .05 |
| Postoperative EF (%) | 46 ± 8 | 42.1 ± 8 | 40 ± 8 | 37 ± 7 | NS |
| Hospital stay (day) | 8.7 ± 2 | 9.4 ± 2 | 9.1 ± 2 | 11.4 ± 2 | NS |
| Mortality rate (%) | 4% | 6% | 6% | 6% | NS |
p < .05 vs. Group 4 (control).
t-intub, respiratory support time; EF, ejection fraction.
DISCUSSION
Given the deleterious impact of the inflammatory response to CPB, the most vulnerable patients are of high risk requiring complex procedures. A strategy that uses multiple agents to limit activation would appear to be logical. This kind of broad approach may, however, impact the coagulation cascade and immune system, and the value of this anti-inflammatory strategy must be balanced against the individual patient’s risk (13).
Coated circuits, closed systems, and centrifugal pumps have previously been demonstrated individually to reduce inflammatory and hemostatic activation, although contradictory results exist (14,15). The intention of recent introduction of mini-CPB is to reduce priming volume and to minimize contact of blood with polymers and air in a closed system. We demonstrated contribution of minimized and condensed circuits in several studies before (16,17). Considering the few studies on direct evaluation of closed circuits on inflammatory outcome and lower cost with regards to minimized configuration, we planned to assess the effects of solely closed and/or coated circuits. We studied “closed” and “coated” aspects separately on conventional roller pump, because the minimized concept circuits have additional safety features and centrifugal pump that may increase the cost.
Therefore we studied a dual approach combining closed circuit for prevention of blood-air interface/less prime volume and surface coating. Also, risk stratification has been considered to enhance the data on clinical outcomes.
Most modern extracorporeal circuits incorporate a venous reservoir. This might be integrated either as an open hard shell reservoir with an integral cardiotomy filter or as a closed soft shell reservoir without a filter but with a separate cardiotomy reservoir. Aspects of practicability may favor the use of an open venous reservoir system as opposed to the closed venous reservoir system. However, all venous blood continuously has to pass the filter of the open reservoir during bypass. Moreover, because it is an open reservoir, blood also contacts air. In contrast to the hard shell venous reservoirs included in the open circuit, which require high blood levels to avoid the threat of embolism, the soft bag reservoirs, included in the closed circuit reduce the blood gas interface, and by collapsing, reduce the risk of massive emboli. Therefore, the features of an open venous reservoir imply that one may expect to find more material-dependent blood activation with the use of this reservoir than with a closed venous reservoir (18,19).
In our study, the preventive effects on systemic inflammatory response dominated WBC counts, IL-6, and integrin expression in both coated groups. Coated circuits preserved platelets during CPB and provided better perioperative period via decreasing mechanical ventilation time, postoperative hemorrhage, inotropic support need, and ICU stay. Surface coating also reduced protein adsorption on fibers. Closed system contributed more by less arrhythmia incidence and additional anti-inflammatory action.
The additional effects on the incidence of atrial fibrillation are not easy to explain. However, there is evidence that surface modification may act via modulation of systemic inflammatory response, which may be one of the precursors of atrial fibrillation in the postoperative period (11,12). For the same reasons, one might expect that shear forces on blood of patients are higher in the open than in the closed venous reservoir and may induce more hemolysis.
In the present study, the levels of TAT-III complex insidiously increased during CPB, indicating that thrombin was generated during bypass in all groups. FHb levels were not different among groups indicating similar status for hemolysis.
Because the degree of both material-dependent and independent blood activation is related to whole body inflammatory reaction and thus hemostasis, one would expect to find differences in the amount of bleeding and the need for donor blood postoperatively with the open and closed circuits (20). The use of the closed circuit provided a statistical significant reduction of the priming volume, which amounted to 810 (550 mL lower versus open circuit). The coated groups’ impact on blood conservation was clearly delineated by the significantly lower postoperative hemorrhage. The effects of coating on postoperative bleeding are not unexpected because of platelet preservation. We have documented less postoperative bleeding in both coated groups but not any difference in use of blood products. This may be linked with the limited patient population.
We have not reported any complication or technical discomfort regarding the use of closed-CPB during the cases. The mean number of distal anastomoses was similar between groups.
This study had several limitations. First, the sample size was not sufficiently large to allow comparisons among groups in terms of clinical results such as required transfusions or morbidity. Second, platelet viability and function were not measured. Third, we did not measure the amount or coagulofibrinolytic marker content of the cardiotomy suction blood, and were therefore unable to determine their relationships with the magnitude of elevations in the markers in the open group but we excluded this amount by using cell saver.
The future of cardiac surgery will be marked by an increasingly complex, high-risk group of patients and a greater need for multiple modalities for reducing bleeding. Anti-inflammatory approaches that attenuate the activation of the hemostatic system and inflammation need to be used to decrease coagulopathies and the need for allogeneic blood administration. Obviously, very many different factors may contribute to post–CPB morbidity and mortality in each patient, not all of which are related to biocompatibility. The present knowledge about pathogenesis is probably fragmentary rendering it difficult to find efficient measures to reduce risk. Inflammatory response to CPB is multifactorial and combined therapies may be more efficient than a single intervention to improve outcome.
REFERENCES
- 1.Rizzi G, Scrivani A, Fini M, Giardino R.. Biomedical coatings to improve the tissue-biomaterial interface. Int J Artif Organs. 2004;27:649–57. [DOI] [PubMed] [Google Scholar]
- 2.von Segesser LK, Tozzi P, Mallbiabrrena I, Jegger D, Horisberger J, Corno A.. Miniaturization in cardiopulmonary bypass. Perfusion. 2003;18:219–24. [DOI] [PubMed] [Google Scholar]
- 3.Groom RC.. A systematic approach to the understanding and redesigning of cardiopulmonary bypass. Semin Cardiothorac Vasc Anesth. 2005;9:159–61. [DOI] [PubMed] [Google Scholar]
- 4.Norman MJ, Sistino JJ, Acsell JR.. The effectiveness of low-prime cardiopulmonary bypass circuits at removing gaseous emboli. J Extra Corpor Technol. 2004;36:336–42. [PubMed] [Google Scholar]
- 5.Ovrum E, Tangen G, Oystese R, Ringdal MAL, Istad R.. Comparison of two heparin coated extracorporeal circuits with reduced systemic anticoagulation in routine coronary artery bypass operations. J Thorac Cardiovasc Surg. 2001;121:324–30. [DOI] [PubMed] [Google Scholar]
- 6.Videm V, Mollnes TE, Fosse E, et al. Heparin coated cardiopulmonary bypass equipment: Biocompatibility markers and development of complications in a high risk population. J Thorac Cardiovasc Surg. 1999;117:794–802. [DOI] [PubMed] [Google Scholar]
- 7.Gunaydin S, McCusker K, Vijay V.. Clinical performance and biocompatibility of hyaluronan based heparin bonded extracorporeal circuits. J Extra Corpor Technol. 2005;37:290–5. [PMC free article] [PubMed] [Google Scholar]
- 8.Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: Analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816–23. [DOI] [PubMed] [Google Scholar]
- 9.Repo H, Jansson SE, Leirisalo-Repo M.. Flow cytometric determination of CD11b upregulation in vivo. J Immunol Methods. 1993;164:193–202. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka M, Motomura T, Kawada M, et al. Blood compatible aspects of poly(2-methoxyethylacrylate) relationship between protein adsorption and platelet adhesion on PMEA surface. Biomaterials. 2000;21:1471–81. [DOI] [PubMed] [Google Scholar]
- 11.Gunaydin S, Farsak B, Mccusker K, et al. Clinical and biomaterial evaluation of hyaluronan-based heparin-bonded extracorporeal circuits with reduced versus full systemic anticoagulation in reoperation for coronary revascularization. J Cardiovasc Med. (Hagerstown) 2009;10:135–42. [DOI] [PubMed] [Google Scholar]
- 12.Gunaydin S, McCusker K, Sari T, Onur MA, Zorlutuna Y.. Clinical performance and biocompatibility of hyaluronan-based heparin-bonded extracoporeal circuits in different risk cohorts. Interact Cardiovasc Thorac Surg. 2010;10:371–6. [DOI] [PubMed] [Google Scholar]
- 13.Zamora E, Delgado L, Castro MA, et al. Coronary artery bypass surgery using the mini-extracorporeal circulation system: A Spanish unit’s experience. Rev Esp Cardiol. 2008;61:376–81. [PubMed] [Google Scholar]
- 14.Moen O, Fosse E, Dregelid E, et al. Centrifugal pump and heparin coating improves cardiopulmonary bypass biocompatibility. Ann Thorac Surg. 1996;62:1134–40. [DOI] [PubMed] [Google Scholar]
- 15.Nishida H, Aomi S, Tomizawa Y, et al. Comparative study of biocompatibility between open circuit and closed circuit in cardiopulmonary bypass. Artif Organs. 1999;23:547–51. [DOI] [PubMed] [Google Scholar]
- 16.Gunaydin S, Sari T, McCusker K, Schonrock U, Zorlutuna Y.. Clinical evaluation of minimized extracorporeal circulation in high-risk coronary revascularization: Impact on air handling, inflammation, hemodilution and myocardial function. Perfusion. 2009;24:153–62. [DOI] [PubMed] [Google Scholar]
- 17.Gunaydin S, McCusker K, Vijay V.. Clinical and biomaterial evaluation of a new condensed dual-function extracorporeal circuit in reoperation for coronary artery bypass surgery. Int J Artif Organs. 2009;32:802–10. [DOI] [PubMed] [Google Scholar]
- 18.Casalino S, Stelian E, Novelli E, et al. Reduced transfusion requirements with a closed cardiopulmonary bypass system. J Cardiovasc Surg (Torino). 2008;49:363–9. [PubMed] [Google Scholar]
- 19.Fukada J, Morishita K, Ingu A, et al. Comparative study of the effect on clinical outcome of the use of an open circuit and the use of a closed circuit in cardiopulmonary bypass for a graft replacement of the descending thoracic or thoracoabdominal aorta. Surg Today. 2004;34:11–5. [DOI] [PubMed] [Google Scholar]
- 20.Nakahira A, Sasaki Y, Hirai H, et al. Closed cardiopulmonary bypass circuits suppress thrombin generation during coronary artery bypass grafting. Interact Cardiovasc Thorac Surg. 2010;10:555–60. [DOI] [PubMed] [Google Scholar]


