Abstract:
The current risk prediction models for mortality following coronary artery bypass graft (CABG) surgery have been developed on patient and disease characteristics alone. Improvements to these models potentially may be made through the analysis of biomarkers of unmeasured risk. We hypothesize that preoperative biomarkers reflecting myocardial damage, inflammation, and metabolic dysfunction are associated with an increased risk of mortality following CABG surgery and the use of biomarkers associated with these injuries will improve the Northern New England (NNE) CABG mortality risk prediction model. We prospectively followed 1731 isolated CABG patients with preoperative blood collection at eight medical centers in Northern New England for a nested case-control study from 2003–2007. Preoperative blood samples were drawn at the center and then stored at a central facility. Frozen serum was analyzed at a central laboratory on an Elecsys 2010, at the same time for Cardiac Troponin T, N-Terminal pro-Brain Natriuretic Peptide, high sensitivity C-Reactive Protein, and blood glucose. We compared the strength of the prediction model for mortality using multivariable logistic regression, goodness of fit and tested the equality of the receiving operating characteristic curve (ROC) area. There were 33 cases (dead at discharge) and 66 randomly matched controls (alive at discharge). The ROC for the preoperative mortality model was improved from .83 (95% confidence interval: .74–.92) to .87 (95% confidence interval: .80–.94) with biomarkers (p-value for equality of ROC areas .09). The addition of biomarkers to the NNE preoperative risk prediction model did not significantly improve the prediction of mortality over patient and disease characteristics alone. The added measurement of multiple biomarkers outside of preoperative risk factors may be an unnecessary use of health care resources with little added benefit for predicting in-hospital mortality.
Keywords: cardiopulmonary bypass, cardiac surgery, mortality, risk factors, biomarkers
Several research groups have formulated risk prediction models of mortality following coronary artery bypass graft (CABG) surgery (1–8). The addition of additional patient and disease characteristics do not improve the prediction of in-hospital mortality over the characteristics currently defined (9,10). In the past 10 years, biomarkers measuring more subtle and prevalent tissue-level injuries have become available. Thus, if there is an improvement to be made in predicting in-hospital mortality, this may be identified through the collection and analysis of multiple biomarkers of currently unmeasured risk including myocardial damage, inflammation, and metabolic dysfunction (11).
Our research group previously reported a patient’s white blood cell count (WBC), a marker of inflammation, was a significant independent predictor of mortality over other preoperative variables currently used in our risk prediction model (12). Other studies have shown preoperative troponin T and high sensitivity-C-reactive protein (hs-CRP) independently predict mortality (13–16). These findings suggest biomarkers may represent unmeasured risk not apparent from current preoperative patient characteristics. For this reason, it is thought that more sensitive markers for myocardial damage [cardiac troponin T (cTnT), N-terminal Pro-Brain Natriuretic Peptide (Nt-ProBNP)], inflammation (hs-CRP), and metabolic dysfunction (glucose and hemoglobin A1c – HbA1c) measured preoperatively might improve our ability to predict a patient’s risk of mortality prior to undergoing CABG surgery. Since preoperative biomarkers reflecting myocardial damage, inflammation, and metabolic dysfunction are associated with an increased risk of mortality following CABG surgery (13,14,17–19), we hypothesized the use of multiple biomarkers associated with these injuries will improve our current risk prediction model used by the American College Of Cardiology/American Heart Association CABG guidelines (20,21).
MATERIALS AND METHODS
This study built on the experience of the Northern New England Cardiovascular Disease Study Group (NNECDSG), a regional collaborative consortium founded in 1987. The goals were to capture 100% of the coronary revascularizations and/or valve procedures in northern New England including eight medical centers in Vermont, New Hampshire, and Maine. Members include clinicians, hospital administrators, and health care research personnel who seek to improve continually the quality, safety, effectiveness, and cost of medical interventions in cardiovascular disease. The NNECDSG has Institutional Review Board approval for data collection and analysis at all participating centers. Patients provided written informed consent for the collection of non-clinical blood specimens for the purposes of this research.
Northern New England Biomarker Study
The NNECDSG has extensive experience in risk prediction in CABG surgery (7,12,22,23). We periodically examine new patient and disease characteristics as well as biomarkers to update our multiple logistic regression risk prediction models on a regular basis to better serve our patients’ needs (7,23,24). Blood was preoperatively collected prior to incision at each participating site in a 10-mL serum tube from 2003–2007. Blood was allowed to clot at room temperature for 20 minutes to separate out the red blood cells, the tubes were centrifuged at 3500 rpm for 20 minutes, and the sera stored at the respective medical centers below −80°C until transportation on dry ice to the Laboratory for Clinical and Biochemical Research in Colchester, Vermont where they were stored at −80°C until measurement. We enrolled 1731 isolated CABG patients into the biomarker cohort. We conducted a nested case-control study with 1:2 matching from the biomarker cohort with cases (dead at discharge) and randomly matched controls (alive at discharge). Frozen serum was analyzed at a central laboratory, at the same time for biomarker measurement (cTnT, Nt-proBNP, hs-CRP, and blood glucose). All markers were measured by the Roche Diagnostics Elecsys 2010 system (F. Hoffmann-La Roche Ltd., Basel, Switzerland). Biomarker results were linked to the NNECDSG cohort to conduct the preoperative risk prediction modeling. Biomarkers were evaluated as continuous variables, log-transformation as required, by quartiles, and by 1-standard deviation increases with the following 1-standard deviation cut-points: cTnT: 1.42 ng/mL; Nt-ProBNP: 6218 pg/mL; hs-CRP: 39.4 mg/L; and blood glucose: 150.7 mg/dL.
Patient, procedural, and outcome data were collected from patients undergoing isolated CABG surgery; those undergoing CABG incidental to heart valve repair or replacement, resection of a ventricular aneurysm, or other surgical procedure were not included. Variables included were: patient sex and age; presence of comorbid illness (diabetes mellitus, chronic obstructive pulmonary disease, vascular disease, preoperative renal failure, and serum creatinine ≥2 mg/dL); prior CABG or valve surgery; cardiac catheterization results including: ejection fraction (EF), number of diseased coronary arteries, and the percentage stenosis of the left main coronary artery; history of myocardial infarction (≤7 days); and WBC count. The severity of coronary artery disease was expressed as the number of diseased vessels (25). The ejection fraction results were categorized as follows: EF < 40%, EF 40–49%, EF 50–59%, and EF ≥60% (26). Acuity of surgery was assessed by the cardiothoracic surgeons using definitions previously described (22). A technical paper describing our method of predicting risk has been previously published (7).
Primary Endpoint
The primary endpoint of the study was all-cause in-hospital mortality from the index admission. Hospital mortality was confirmed using hospital discharge data.
Statistical Analysis
Standard statistical methods were used for the calculation of the odds ratio, the chi-squared test for a specified number of degrees of freedom (27,28). Logistic regression analysis was used to calculate the predicted mortality rate and was performed using the Stata 11.0 statistical program (Stata Corp., College Station, TX). First order interaction terms were created, and models with and without the interaction terms were compared using the likelihood ratio chi-squared test; all interaction terms were assessed and ruled out (29). Model calibration was assessed using plots of observed versus expected values by quintile of risk (based on using regression coefficients) and the Lemeshow and Hosmer (30). The discriminating ability of the regression model was assessed by the c-statistic, which is the area under the receiver operating characteristic curve (ROC) (31). The bootstrapping method was used to calculate 95% confidence intervals around the ROC-curve (32). Log-likelihood tests were performed to assess whether or not the inclusion of biomarkers significantly improved the prediction of in-hospital mortality. We tested the equality of the area under the Northern New England (NNE) model ROC against the area under the ROC for the NNE biomarker model (12) to determine if the biomarker model added improvement to the model without biomarkers (33).
We repeated our analysis by calculating the risk score from the NNE risk model based on previous registry subjects and used the predicted score as the one covariate in the logistic model with and without biomarkers to limit over-fitting of the logistic model. We then conducted a propensity matched analysis identifying the nearest neighbor matches using the NNE risk score without biomarkers for the propensity score by matching 24 cases with 24 controls and compared the ROC for the propensity matched NNE model with and without biomarkers according to the methods described above.
A χ-pie chart was developed for the NNE risk model with the addition of biomarkers according to methods previously described (34). To determine the χ2 value of each contributing risk factor, the model was calculated eliminating one risk factor at a time with the four biomarkers being removed as a group. The reduced model χ2 was then recorded for each factor. Each risk factor’s χ2 was subtracted from the full model’s χ2 to determine the percent contribution. A pie chart was then plotted to show the relative contribution of each risk factor for the prediction of in-hospital mortality (Figure 2).
Figure 2.
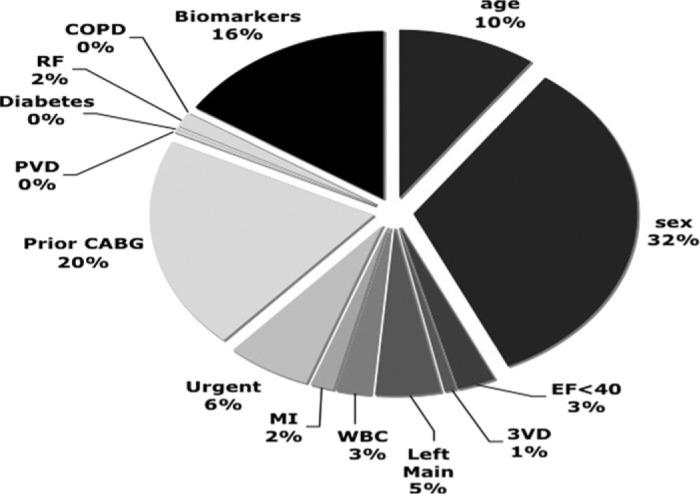
Relative contribution of risk factors predicting in-hospital mortality. The relative contributions of predictors for in-hospital mortality are plotted based on the NNE preoperative risk model with the addition of biomarkers cTnT, Nt-ProBNP, hs-CRP, and blood glucose. The size represents the percent of contribution to the prediction model: all biomarkers, age, sex, EF <40%, three vessel disease (3VD), left main disease >50%, WBC count >12,000, MI within 7 days of surgery, urgent priority of surgery, prior CABG surgery, peripheral vascular disease (PVD), any diabetes, preoperative renal failure and serum creatinine ≥2 mg/dL (RF), and chronic obstructive pulmonary disease (COPD).
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agreed to the manuscript as written.
RESULTS
We enrolled 1731 isolated CABG surgery patients. There were 33 deaths at discharge (cases). Cases were randomly matched 1:2, with 66 patients alive at discharge (controls). The baseline risk factors included in the modeling are reported in Table 1 with percents and univariate logistic regression analysis. The multivariate prediction model statistics and model parameters are reported in Table 2.
Table 1.
Preoperative risk factors for in-hospital mortality.
| Risk Factor | Patients (%) |
In-Hospital Mortality | |||
|---|---|---|---|---|---|
| Death (%) |
Odds Ratio |
95% CI |
p Value | ||
| Preoperative Risk Factors | |||||
| Age category | |||||
| <60 | 25.3 | 24.0 | 1.00 | Ref. | |
| 60–69 | 31.3 | 25.8 | 1.10 | .33–3.73 | .877 |
| 70–75 | 19.2 | 36.8 | 1.85 | .50–6.83 | .358 |
| 76–79 | 11.1 | 45.5 | 2.64 | .59–11.83 | .205 |
| ≥80 | 13.1 | 53.9 | 3.69 | .89–15.37 | .072 |
| Gender | |||||
| Male | 72.7 | 23.6 | 1.00 | Ref. | |
| Female | 27.3 | 59.3 | 4.71 | 1.84–12.06 | .001 |
| Ejection fraction | |||||
| ≥40% | 91.9 | 33.0 | 1.00 | Ref. | |
| <40% | 8.1 | 37.5 | 1.22 | .27–5.45 | .795 |
| Diseased vessels | |||||
| <3 | 32.3 | 34.4 | 1.00 | Ref. | |
| ≥3 | 67.7 | 32.8 | .93 | .38–2.27 | .879 |
| Left main | |||||
| <50% | 65.7 | 29.2 | 1.00 | Ref. | |
| 50–89% | 29.3 | 37.9 | 1.48 | .59–3.72 | .405 |
| ≥90% | 5.1 | 60.0 | 3.63 | .56–23.50 | .176 |
| White blood cell count | |||||
| ≤12,000 | 95.0 | 31.9 | 1.00 | Ref. | |
| >12,000 | 5.1 | 60.0 | 3.20 | .51–20.17 | .216 |
| Timing of MI | |||||
| >7 days | 81.8 | 32.1 | 1.00 | Ref. | |
| ≤7 days | 18.2 | 38.9 | 1.35 | .47–3.87 | .581 |
| Priority | |||||
| Elective | 26.3 | 19.2 | 1.00 | Ref. | |
| Urgent | 72.7 | 37.5 | 2.52 | .85–7.46 | .095 |
| Emergent | 1.0 | 100.0 | NA | ||
| Prior CABG | |||||
| No | 95.0 | 30.9 | 1.00 | Ref. | |
| Yes | 5.1 | 80.0 | 8.97 | .96–83.76 | .054 |
| Peripheral vascular disease | |||||
| No | 68.7 | 27.9 | 1.00 | Ref. | |
| Yes | 31.3 | 45.2 | 2.12 | .88–5.14 | .095 |
| Diabetes | |||||
| No | 64.7 | 35.9 | 1.00 | Ref. | |
| Yes | 35.4 | 28.6 | .71 | .29–1.74 | .458 |
| Renal failure | |||||
| No | 97.0 | 32.3 | 1.00 | Ref. | |
| Yes | 3.0 | 66.7 | 4.19 | .37–48.03 | .249 |
| Serum creatinine > 2.0 | |||||
| No | 92.9 | 30.4 | 1.00 | Ref. | |
| Yes | 7.1 | 71.4 | 5.71 | 1.05–31.25 | .044 |
| COPD | |||||
| No | 79.8 | 30.4 | 1.00 | Ref. | |
| Yes | 20.2 | 45.0 | 1.88 | .69–5.11 | .219 |
| Preoperative Biomarkers | |||||
| cTnT | |||||
| <1SD | 93.9 | 33.3 | 1.00 | Ref. | |
| ≥1SD (1.42 ng/mL) | 6.1 | 33.3 | 1.00 | .17–5.76 | 1.000 |
| Nt-ProBNP | |||||
| <1SD | 89.9 | 29.2 | 1.00 | Ref. | |
| ≥1SD (6218 pg/mL) | 10.1 | 70.0 | 5.65 | 1.36–23.57 | .017 |
| hs-CRP | |||||
| <1SD | 91.9 | 34.1 | 1.00 | Ref. | |
| ≥1SD (39.4 mg/L) | 8.1 | 25.0 | .65 | .12–3.39 | .604 |
| Blood glucose | |||||
| <1SD | 82.8 | 37.8 | 1.00 | Ref. | |
| ≥1SD (150.7 mg/dL) | 17.2 | 11.8 | .22 | .05–1.02 | .054 |
Table 2. Prediction of the risk of in-hospital mortality after CABG surgery.
| Current NNE Model | NNE Multi-marker Model | |||||
|---|---|---|---|---|---|---|
| Risk Factor | Adjusted Odds Ratio | Coefficient | p Value | Adjusted Odds Ratio | Coefficient | p Value |
| Preoperative Risk Factors | ||||||
| Age (years) | ||||||
| 60–69 | 1.09 | .0870 | .920 | .99 | −.0124 | .990 |
| 70–75 | 2.50 | .9158 | .358 | 2.13 | .7549 | .485 |
| 76–79 | 3.50 | 1.2539 | .222 | 4.18 | 1.4300 | .210 |
| ≥80 | 4.74 | 1.5561 | .108 | 7.81 | 2.0559 | .086 |
| Female | 6.36 | 1.8494 | .003 | 9.80 | 2.2828 | .002 |
| Ejection fraction < 40% | 3.08 | 1.1252 | .250 | 1.21 | .1906 | .902 |
| 3 vessel disease | .94 | −.0668 | .918 | 1.49 | .3982 | .573 |
| Left main 50–89% | 1.09 | .0880 | .891 | 1.18 | .1682 | .819 |
| Left main ≥ 90% | 2.54 | .9309 | .448 | 2.90 | 1.0631 | .422 |
| White blood cell count > 12,000 | 4.25 | 1.4458 | .203 | 2.02 | .7019 | .572 |
| MI within 7 days | .56 | −.5742 | .484 | .70 | −.3606 | .726 |
| Urgent priority | 2.73 | 1.0036 | .183 | 1.50 | .4054 | .603 |
| Emergent priority | NA | |||||
| Prior CABG | 37.35 | 3.6203 | .007 | 23.73 | 3.1669 | .020 |
| Peripheral vascular disease | 1.12 | .1110 | .865 | 1.09 | .0857 | .901 |
| Diabetes | .62 | −.4716 | .451 | 1.57 | .4530 | .556 |
| Renal failure | .95 | −.0510 | .981 | .19 | −1.6781 | .563 |
| Serum creatinine > 2.0 | 6.13 | 1.8131 | .283 | 1.54 | .4297 | .812 |
| COPD | .84 | −.1741 | .835 | .79 | −.2371 | .798 |
| Preoperative Biomarkers | ||||||
| By ≥ 1 standard deviation | ||||||
| cTnT | .32 | −1.1445 | .517 | |||
| Nt-ProBNP | 112.02 | 4.7187 | .059 | |||
| hs-CRP | .06 | −2.7981 | .118 | |||
| Blood glucose | .12 | −2.1586 | .053 | |||
| Model statistics | ||||||
| Intercept | −2.9755 | −2.9009 | ||||
| Model ROC, 95%CI | .8274 | (.74–.92) | .8714 | (.80–.94) | .091 | |
p-value for multivariate logistic regression model.
COPD, chronic obstructive pulmonary disease; NA, not applicable.
Biomarkers with 1 standard deviation increase: cTnT, Nt-ProBNP, hs-CRP.
The NNE model without biomarkers significantly predicted the occurrence of in-hospital mortality (model χ2 = 32.58, p-value .019). The NNE model discriminated well between patients alive and dead at discharge following CABG surgery [ROC: .83; 95% confidence interval (CI): .74–.92, Figure 1]. The model was well calibrated among deciles of observed and expected risk (Hosmer-Lemeshow χ2 = 14.02, p-value .08). Observed and expected deciles of risk were correlated (R = .83, p-value .003).
Figure 1.
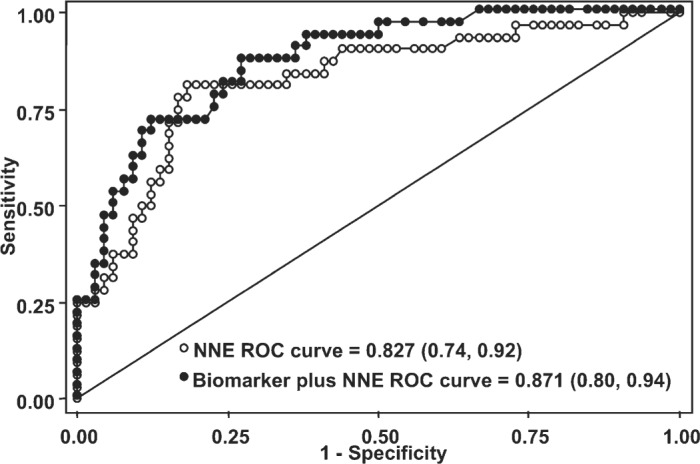
ROC curves for NNE preoperative mortality risk model with and without biomarkers. Receiver operating characteristic (ROC) curve for NNE model without biomarkers (white dots) and NNE model with biomarkers (black dots) using a 1-standard deviation cut-off for biomarkers Nt-ProBNP, cTnT, hs-CRP, and blood glucose. The c-statistic for each model with 95% confidence intervals is listed (p-value = .091, see Table 2).
The NNE biomarker model significantly predicted the occurrence of in-hospital mortality (model χ2 = 42.16, p-value .006). The NNE biomarker model improved discrimination between patients alive and dead at discharge following CABG surgery (ROC: .87; 95%CI: .80–.94, Figure 1) and was well calibrated among deciles of observed and expected risk (Hosmer-Lemeshow χ2 = 5.27, p-value .73). Observed and expected deciles of risk were highly correlated (R = .95, p-value < .001).
The ROC for the preoperative mortality model was improved from .83 (95%CI: .74–.92) to .87 (95%CI: .80–.94) with multiple biomarkers including Nt-ProBNP, cTnT, hs-CRP, and blood glucose; the ROC comparison statistic was not statistically significant with χ2 = 2.85, p-value for equality of ROC areas was .09.
We calculated the relative contribution of each risk factor’s predictive ability in the preoperative risk prediction model for in-hospital mortality (Figure 2). All four biomarkers (Nt-ProBNP, cTnT, hs-CRP, and blood glucose), age, sex, and prior CABG surgery were the largest contributors in the model accounting for 16%, 10%, 32%, and 20%, respectively. Although we did not find a statistically significant improvement in the model with the addition of preoperative biomarkers, the multi-marker measurement provided 16% of the model’s predictive ability of mortality over currently measured risk factors.
In a sub-analysis we repeated our analysis by generating the predicted score from the NNE risk model and used it as the one covariate in the logistic model with and without biomarkers to limit over-fitting the model. The ROC for the NNE model without biomarkers was .83 (95% CI: .74, .92) and the NNE model ROC with biomarkers was .85 (95% CI: .77, .93); the ROC comparison p-value was .117. We then conducted a propensity-matched analy sis identifying the nearest neighbor matches using the NNE risk model without biomarkers for the propensity score. We were able to match 24 cases with 24 controls. The ROC for the propensity matched NNE model without biomarkers was .71 (95% CI: .57, .86) and the ROC for the propensity matched NNE model with biomarkers was .78 (95% CI: .65, .91) with p-value .216.
DISCUSSION
In this regional observational study, we developed a prospective regional blood cohort to develop a new multivariate prediction model with relevant biomarkers of currently unmeasured risk and calculated each patient’s predicted risk to attempt better discrimination. We discovered the addition of multiple biomarkers (Nt-ProBNP, cTnT, hs-CRP, and blood glucose) did not significantly improve our prediction of in-hospital mortality improving our ROC curve from .83–.87 (p = .09). However the addition of Nt-ProBNP and blood glucose performed the best in the multi-marker approach to risk prediction with near statistical significance and may provide added predictive risk for preoperative risk assessment.
Multi-marker risk prediction has not been evaluated for preoperative risk prediction in cardiac surgery, while some have shown mixed results for the ability of a similar set of multi-marker risk prediction for cardiovascular events and death in population studies (35,36). However, various studies have evaluated individual markers used in our multi-marker model to determine risk of mortality. cTnT was suggested to be useful for risk-stratifying patients and inpatient decision making and prognosis (37–40). Two studies, by Carrier and colleagues and Lyon and colleagues, identified pre- and post-operative cTnT levels ≥.2 ng/mL as independent predictors of mortality and morbidity following CABG (13,14), suggesting cTnT to be useful for risk-stratifying patients and playing a role in patient decision making and prognosis (37–40). In our multi-marker model, the use of cTnT was not a significant predictor of mortality, therefore suggesting that the use of cTnT values does not add increased risk assessment over currently known patient characteristics. One such characteristic relevant to cTnT was the risk factor of an myocardial infarction (MI) within 7 days of surgery. It is likely that cTnT levels do not provide added prediction over the clinical risk factor of a known MI within 7 days of surgery; this is plausible since our definition of MI includes a diagnosis from ST-elevation, CK-MB, cTnT, or cTnI biomarker levels. We repeated our modeling and excluded MI from both the NNE risk score and the biomarker model and demonstrated that without the MI risk factor, the NNE risk score ROC decreased to .81 and the biomarker ROC remained the same at .87 with the p-value for testing the equality of the ROC areas at .045, thus showing a statistically significant improvement in prediction with the biomarkers. However, this was not consistent with the primary hypotheses of testing the ability of biomarkers on top of clinically relevant risk factors.
Brain natriuretic peptide (BNP) and Nt-proBNP were shown to predict mortality preoperatively in surgical patients (41,42), thereby suggesting Nt-proBNP as a prognostic marker for risk stratification by a patient’s preoperative BNP levels. Although we did not show statistical improvement in our preoperative prediction model, the strongest biomarker in the model was a Nt-ProBNP level above 6218 (ng/L) with a strong independent association with mortality with odds ratio: 5.65 (95% CI: 1.36–23.57, p = .017); our results confirming previous findings of the independent effect of Nt-ProBNP and modest improvements to prediction in cardiovascular disease progression and non-cardiac surgery (43–45). It is a likely explanation that the strength of the Nt-ProBNP marker in improving the risk score was a result of not capturing ventricular wall stress among the other risk factors in addition to the NNE risk score not using congestive heart failure in the current model.
Hs-CRP has been well studied and is correlated to the progression of atherosclerosis, plaque formation and markedly increases after plaque rupture and thrombus formation (46). Ridker and colleagues reported both men and women with hs-CRP levels >15.5 mg/L have a 3-fold increased risk of myocardial infarction (15,16). Biancari and colleagues demonstrated preoperative hs-CRP levels ≥1.0 mg/dL in CABG surgery were associated with an increased risk of mortality, cardiac death, and low-output syndrome; in multivariate analysis hs-CRP had an odds ratio of 6.97 (95% CI: 1.45–33.42), suggesting a patient with a CRP ≥1.0 (mg/dL) had a 7-fold increased risk of mortality after adjusting for patient characteristics verses a patient with a CRP <1 (mg/dL) (17). In our model, hs-CRP had no independent association with in-hospital mortality and likely did not contribute additional risk profiling over known patient and disease characteristics, such as peripheral vascular disease and white blood cell count.
Diabetes, hyperglycemia, insulin resistance, and metabolic syndrome are major risk factors for coronary artery disease by causing endothelial and cellular dysfunction resulting in an increased risk of mortality, myocardial infarction, and stroke (47–49). Recent studies have shown peri- and post-operative tight control of glucose reduces the risk of mediastinitis and mortality following CABG surgery (50–53). Studies have shown that preoperative blood glucose and HbA1c are predictive of mortality and morbidity following CABG surgery (18,19). In our multi-marker approach we were unable to investigate the role of HbA1c due to instability of HbA1c in the frozen samples stored for more than 2 weeks. Alternatively, we investigated the preoperative predictive value of blood glucose and found blood glucose levels above 150.7 mg/dL were protective against mortality, which suggests that normal blood glucose values at the time of surgery may have protected against morbidity and mortality.
We recognize potential problems and complications with a multi-center blood collection study, such as implementing a process across eight centers, patient enrollment, and specimen handling. However, we had success in implementing the operational processes and monitoring of these sites and the specimen collection. Although we collected blood specimens on a large cohort of 1731 patients, we only had 33 deaths. The limited number of deaths at discharge limited the stability of the modeling and we were at risk of over-fitting the models. To deal with this limitation, we repeated the analysis by using the predicted score from the NNE model and used that predicted score as a covariate in the logistic model with and without biomarkers. We also went back and used propensity matching, using the NNE risk score for the propensity score and identified 24 cases (dead at discharge) and 24 controls (alive at discharge) and repeated our analyses in the nearest neighbor propensity matched analysis. In both methods, we found similar results as our initial approach.
Future targeted research is needed to evaluate the clinical utility of multiple biomarkers in routine clinical practice, including evaluation in large cardiac surgery cohorts. We recommend collaborations to be developed with other larger cohorts to expand upon this work and determine the clinical benefit of measuring multiple markers for improving perioperative risk assessment. However, our findings suggest that the currently measured patient and disease risk factors are sufficient for providing accurate risk prediction without the use of multiple biomarkers until larger cohorts show clinical efficacy of multiple marker measurement for risk assessment.
In summary, the addition of biomarkers to the NNE preoperative risk prediction model did not significantly improve the prediction of mortality over patient and disease characteristics alone, although there was modest improvement in the overall model with significant improvements when corresponding risk factors (MI) are removed from the underlying model. However, the preoperative use of multiple preoperative biomarkers for risk stratification of mortality following isolated CABG surgery is not supported by our investigation. The added measurement of multiple biomarkers outside of preoperative risk factors may be an unnecessary use of health care resources with little added benefit for predicting in-hospital mortality.
ACKNOWLEDGMENT
The authors would like to acknowledge the assistance of the clinical care teams at all eight medical centers for their involvement in the NNECDSG and for the collection of blood specimens, without which we could not have completed this investigation.
REFERENCES
- 1.Califf RM, Phillips HR III, Hindman MC, et al. . Prognostic value of a coronary artery jeopardy score. J Am Coll Cardiol. 1985;5:1055–63. [DOI] [PubMed] [Google Scholar]
- 2.Clark RE, Edwards FH, Schwartz M.. Profile of preoperative characteristics of patients having CABG over the past decade. Ann Thorac Surg. 1994;58:1863–5. [DOI] [PubMed] [Google Scholar]
- 3.Doliszny KM, Luepker RV, Burke GL, Pryor DB, Blackburn H.. Estimated contribution of coronary artery bypass graft surgery to the decline in coronary heart disease mortality: The Minnesota Heart Survey. J Am Coll Cardiol. 1994;24:95–103. [DOI] [PubMed] [Google Scholar]
- 4.Grover FL, Johnson RR, Marshall G, Hammermeister KE.. Factors predictive of operative mortality among coronary artery bypass subsets. Ann Thorac Surg. 1993;56:1296–306, discussion 1306–7. [DOI] [PubMed] [Google Scholar]
- 5.Hannan EL, Kilburn H Jr, O’Donnell JF, Lukacik G, Shields EP.. Adult open heart surgery in New York State. An analysis of risk factors and hospital mortality rates. JAMA. 1990;264:2768–74. [PubMed] [Google Scholar]
- 6.Higgins TL, Estafanous FG, Loop FD, Beck GJ, Blum JM, Paranandi L.. Stratification of morbidity and mortality outcome by preoperative risk factors in coronary artery bypass patients. A clinical severity score. JAMA. 1992;267:2344–8. [PubMed] [Google Scholar]
- 7.O’Connor GT, Plume SK, Olmstead EM, et al. . Multivariate prediction of in-hospital mortality associated with coronary artery bypass graft surgery. Northern New England Cardiovascular Disease Study Group. Circulation. 1992;85:2110–8. [DOI] [PubMed] [Google Scholar]
- 8.Parsonnet V, Dean D, Bernstein AD.. A method of uniform stratification of risk for evaluating the results of surgery in acquired adult heart disease. Circulation. 1989;79:I3–12. [PubMed] [Google Scholar]
- 9.Jones RH, Hannan EL, Hammermeister KE, et al. . Identification of preoperative variables needed for risk adjustment of short-term mortality after coronary artery bypass graft surgery. The Working Group Panel on the Cooperative CABG Database Project. J Am Coll Cardiol. 1996;28:1478–87. [DOI] [PubMed] [Google Scholar]
- 10.Peterson ED, DeLong ER, Muhlbaier LH, et al. . Challenges in comparing risk-adjusted bypass surgery mortality results: Results from the Cooperative Cardiovascular Project. J Am Coll Cardiol. 2000;36:2174–84. [DOI] [PubMed] [Google Scholar]
- 11.Braunwald E.. Application of current guidelines to the management of unstable angina and non-ST-elevation myocardial infarction. Circ J Am Heart Assoc. 2003;108:28–37. [DOI] [PubMed] [Google Scholar]
- 12.Dacey LJ, DeSimone J, Braxton JH, et al. . Preoperative white blood cell count and mortality and morbidity after coronary artery bypass grafting. Ann Thorac Surg. 2003;76:760–4. [DOI] [PubMed] [Google Scholar]
- 13.Lyon WJ, Baker RA, Andrew MJ, Tirimacco R, White GH, Knight JL.. Relationship between elevated preoperative troponin T and adverse outcomes following cardiac surgery. ANZ J Surg. 2003;73:40–4. [DOI] [PubMed] [Google Scholar]
- 14.Carrier M, Pelletier LC, Martineau R, Pellerin M, Solymoss BC.. In elective coronary artery bypass grafting, preoperative troponin T level predicts the risk of myocardial infarction. J Thorac Cardiovasc Surg. 1998;115:1328–34. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH.. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH.. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circ J Am Heart Assoc. 1998;98:731–3. [DOI] [PubMed] [Google Scholar]
- 17.Biancari F, Lahtinen J, Lepojarvi S, et al. . Preoperative C-reactive protein and outcome after coronary artery bypass surgery. Ann Thorac Surg. 2003;76:2007–12. [DOI] [PubMed] [Google Scholar]
- 18.Medhi M, Marshall MC Jr, Burke HB, et al. . HbA1c predicts length of stay in patients admitted for coronary artery bypass surgery. Heart Dis. 2001;3:77–9. [DOI] [PubMed] [Google Scholar]
- 19.Zindrou D, Taylor KM, Bagger JP.. Admission plasma glucose: An independent risk factor in nondiabetic women after coronary artery bypass grafting. Diabetes Care. 2001;24:1634–9. [DOI] [PubMed] [Google Scholar]
- 20.Eagle KA, Guyton RA, Davidoff R, et al. . ACC/AHA guidelines for coronary artery bypass graft surgery: Executive summary and recommendations: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1991 guidelines for coronary artery bypass graft surgery). Circ J Am Heart Assoc. 1999;100:1464–80. [DOI] [PubMed] [Google Scholar]
- 21.Eagle KA, Guyton RA, Davidoff R, et al. . ACC/AHA guidelines for coronary artery bypass graft surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1991 Guidelines for Coronary Artery Bypass Graft Surgery). American College of Cardiology/American Heart Association. J Am Coll Cardiol. 1999;34:1262–347. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor GT, Plume SK, Olmstead EM, et al. . A regional prospective study of in-hospital mortality associated with coronary artery bypass grafting. The Northern New England Cardiovascular Disease Study Group. JAMA. 1991;266:803–9. [PubMed] [Google Scholar]
- 23.Surgenor SD, O’Connor GT, Lahey SJ, et al. . Predicting the risk of death from heart failure after coronary artery bypass graft surgery. Anesth Analg. 2001;92:596–601. [DOI] [PubMed] [Google Scholar]
- 24.Peterson ED, DeLong ER, Muhlbaier LH, et al. . Challenges in comparing risk-adjusted bypass surgery mortality results: results from the Cooperative Cardiovascular Project. J Am Coll Cardiol. 2000;36:2174–84. [DOI] [PubMed] [Google Scholar]
- 25.The Principal Investigators of CASS and Their Associates: The National Heart, Lung, and Blood Institute Coronary Artery Surgery Study (CASS). Circulation 1981;63(Suppl I):I-1–I-81. [Google Scholar]
- 26.Pierpont GL, Kruse M, Ewald S, Weir EK.. Practical problems in assessing risk for coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1985;89:673–82. [PubMed] [Google Scholar]
- 27.Hennekens CH, Buring JE.. Epidemiology in Medicine. Boston, MA: Little Brown & Co; 1988. [Google Scholar]
- 28.Armitage P, Berry G.. Statistical Methods in Medical Research. London: Blackwell Scientific Publications; 1987. [Google Scholar]
- 29.Kleinbaum DG, Kupper LL, Morgenstern H.. Epidemiologic Research: Principles and Quantitative Methods. Belmont, CA: Wadsworth Inc; 1982. [Google Scholar]
- 30.Lemeshow S, Hosmer DW.. The use of goodness-of-fit statistics in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. [DOI] [PubMed] [Google Scholar]
- 31.Hanley JA, McNeil BJ.. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 32.Efron B, Tibshirani R.. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54–77. [Google Scholar]
- 33.DeLong ER, DeLong DM, Clarke-Pearson DL.. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 34.Brown JR, Cochran RP, Leavitt BJ, et al. . Multivariable prediction of renal insufficiency developing after cardiac surgery. Circulation. 2007;116(Suppl):I139–43. [DOI] [PubMed] [Google Scholar]
- 35.Melander O, Newton-Cheh C, Almgren P, et al. . Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zethelius B, Berglund L, Sundstrom J, et al. . Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–16. [DOI] [PubMed] [Google Scholar]
- 37.Hamm CW, Ravkilde J, Gerhardt W, et al. . The prognostic value of serum troponin T in unstable angina. N Engl J Med. 1992;327:146–50. [DOI] [PubMed] [Google Scholar]
- 38.Wu AH, Abbas SA, Green S, et al. . Prognostic value of cardiac troponin T in unstable angina pectoris. Am J Cardiol. 1995;76:970–2. [DOI] [PubMed] [Google Scholar]
- 39.Ohman EM, Armstrong PW, Christenson RH, et al. . Cardiac troponin T levels for risk stratification in acute myocardial ischemia. GUSTO IIA Investigators. N Engl J Med. 1996;335:1333–41. [DOI] [PubMed] [Google Scholar]
- 40.Venge P, Lagerqvist B, Diderholm E, Lindahl B, Wallentin L.. Clinical performance of three cardiac troponin assays in patients with unstable coronary artery disease (a FRISC II substudy). Am J Cardiol. 2002;89:1035–41. [DOI] [PubMed] [Google Scholar]
- 41.Bergler-Klein JK, Klaar U, Heger M, et al. . Prognostic importance of natriuretic peptides in aortic stenosis. Circ J Am Heart Assoc. 2003;108:2343. [Google Scholar]
- 42.Berendes E, Schmidt C, Van Aken H, et al. . A-type and B-type natriuretic peptides in cardiac surgical procedures. Anesth Analg. 2004;98:11–9. [DOI] [PubMed] [Google Scholar]
- 43.Di Angelantonio E, Chowdhury R, Sarwar N, et al. . B-Type natriuretic peptides and cardiovascular risk. Systematic review and meta-analysis of 40 prospective studies. Circulation 2009;120:2177–87. [DOI] [PubMed] [Google Scholar]
- 44.Karthikeyan G, Moncur RA, Levine O, et al. . Is a pre-operative brain natriuretic peptide or N-terminal pro-B-type natriuretic peptide measurement an independent predictor of adverse cardiovascular outcomes within 30 days of noncardiac surgery? A systematic review and meta-analysis of observational studies. J Am Coll Cardiol. 2009;54:1599–606. [DOI] [PubMed] [Google Scholar]
- 45.Rutten JH, Steyerberg EW, Boomsma F, et al. . N-terminal pro-brain natriuretic peptide testing in the emergency department: beneficial effects on hospitalization, costs, and outcome. Am Heart J. 2008;156:71–7. [DOI] [PubMed] [Google Scholar]
- 46.Pearson TA, Mensah GA, Alexander RW, et al. . Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circ J Am Heart Assoc. 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 47.Farrer M, Fulcher G, Albers CJ, Neil HA, Adams PC, Alberti KG.. Patients undergoing coronary artery bypass graft surgery are at high risk of impaired glucose tolerance and diabetes mellitus during the first postoperative year. Metabolism. 1995;44:1016–27. [DOI] [PubMed] [Google Scholar]
- 48.Sheetz MJ, King GL.. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288:2579–88. [DOI] [PubMed] [Google Scholar]
- 49.Beckman JA, Creager MA, Libby P.. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–81. [DOI] [PubMed] [Google Scholar]
- 50.Furnary AP, Gao G, Grunkemeier GL, et al. . Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007–21. [DOI] [PubMed] [Google Scholar]
- 51.Finney SJ, Zekveld C, Elia A, Evans TW.. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041–7. [DOI] [PubMed] [Google Scholar]
- 52.Guvener M, Pasaoglu I, Demircin M, Oc M.. Perioperative hyperglycemia is a strong correlate of postoperative infection in type II diabetic patients after coronary artery bypass grafting. Endocr J. 2002;49:531–7. [DOI] [PubMed] [Google Scholar]
- 53.van den Berghe G, Wouters P, Weekers F, et al. . Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67. [DOI] [PubMed] [Google Scholar]


