Abstract:
During cardiopulmonary bypass blood gases can be analyzed with laboratory equipment or with an in-line monitor giving instant results. The manufacturer of the CDI 500 in-line blood gas monitor recommends gas calibration before use. In acute cases there may not be time to perform a gas calibration. We hypothesized that after calibration against laboratory results, the CDI values of pH, pO2, and pCO2 will keep the same level of accuracy, whether the CDI has been gas calibrated or not. We performed a prospective randomized observational study using a study group without gas calibration (29 patients) and a control group with gas calibration (29 patients). Blood sampling was done at the beginning of bypass, and 30 minutes later. After each blood sample the CDI was in-vivo calibrated to the values simultaneously obtained from the ABL. Before in-vivo calibration values from the CDI without gas calibration were significantly different from the ABL-values in accuracy as well as precision, whereas the results from the gas calibrated CDI were largely consistent with the ABL. Before in-vivo calibration, the CDI without gas calibration was completely unreliable. After in-vivo calibration there was no statistical difference between the values of the CDI with and without calibration. We recommend gas calibration of the CDI before use in the period before in-vivo calibration.
Keywords: cardiopulmonary bypass, extracorporeal circulation, In-line monitoring, safety in perfusion, blood gas
Cardiopulmonary bypass (CPB) can replace the function of the heart and lungs during a period in which the patient’s own heart and lungs cannot assure the circulation and gas exchange of the body. In these cases, an oxygenator replaces the function of the lungs on the physiological level, and the regulation of the gas exchange is in the hands of the perfusionist. Maintenance of a normal physiologic acid-base status is essential for the patient, and this is achieved by running an appropriate level of gas flow and oxygen/air mixture through the oxygenator.
The laboratory standard for analysis of blood gases and electrolytes in the field of cardiopulmonary bypass is an elaborate laboratory machine, for example the ABL series from Radiometer (Radiometer Medical, Brønshøj, Denmark), of which a recent model is the ABL 800 flex. However, the advantages of continuous blood gas monitoring are evident. Stammers outlined the characteristics of an ideal point-of-care monitoring system for CPB, stressing the importance of device reliability, rapid response time, easiness of handling, low drift, and biocompatibility (1).
The CDI 500 (CDI) in-line blood gas monitor (Terumo Cardiovascular Systems Europe, Borken, Germany) provides instant in-line monitoring of blood gas values in one single surveillance device. Trowbridge and co-workers found in a two-part study that using the CDI resulted in more accurate blood gas management during bypass as well as improvement of a number of postoperative outcome variables (2,3). LeRoy and co-workers recommend that the use of continuous in-line blood gas monitoring should become standard of care in cardiopulmonary bypass (4). An editorial by David Rubsamen in the Journal of Cardiothoracic Anesthesia from 1990 (5), and the article from 1992 by LeRoy and co-workers both draw attention to the fact that the utility of in-line blood gas monitoring can reach as far as the courtroom, providing proof of good medical care during cardiopulmonary bypass in case of a lawsuit (4).
The CDI is a microprocessor-based monitor consisting of two different measurement components: a monitor connected with cables to cable heads, disposable shunt sensors for the fluorescent measurements, and a disposable hematocrit and saturation cuvette. The disposable unit is incorporated directly into the extracorporeal circuit. Based on the measured parameters, the CDI can, furthermore, calculate and display a number of additional values. The minimum blood flow for accurate performance of the arterial shunt sensor is recommended by the manufacturer at 35 mL/min. Blood gases (partial pressure of oxygen (pO2), partial pressure of carbon dioxide (pCO2), and pH) are measured using optical fluorescent technique. The CDI has two different types of settings for start-up. Either a set of fixed values called “factory default values”, which are average calibration values determined from factory experience, or “last calibration values” which are the values obtained from the last calibration performed. All measurements are performed at the blood’s actual temperature, and corrected by the microprocessor to 37°C values.
The CDI has been tested by the manufacturer for accuracy and precision on a number of samples by comparing the results with conventional analyzers. Values of interest for the present study, obtained by the manufacturer of the CDI system, and listed in the CDI 500 User’s Manual, are for pH a mean difference of .006, ± .015, for pCO2 a mean difference of −.11 ± .23 kPa, and for pO2 a mean difference of .23 ± 1.12 kPa (6).
Southworth and co-workers evaluated the performance of the CDI in respect to pH, pCO2, and pO2 in a multi-center trial and found that the values obtained with the CDI 500 system meet the Clinical Laboratory Improvement Amendments accuracy standards for laboratory analyzers (7). The manufacturer of the CDI recommends an in vitro gas calibration of the CDI before each case. The gas calibration is performed using the CDI gas calibrator, by means of two different mixtures of oxygen and carbon dioxide gases in bottles specially made by the CDI manufacturer. These gases calibrate the pH, pO2, and pCO2 measurement parts of the shunt sensor. The gas calibration takes 10 minutes, during which the CDI cannot be used for blood gas monitoring. In acute cases when it is necessary to go on bypass urgently, there may not be time to gas calibrate the CDI. In these cases bypass is initiated without previous gas calibration, and then, once on bypass, the procedure of storage and in-vivo calibration against the ABL results is performed. In our institution the standard procedure is that after the start of bypass when a steady state of perfusion has been reached, blood is drawn in vivo for a blood gas analysis on the Radiometer ABL 800 flex, and simultaneously the values displayed on the CDI are stored to be used for the subsequent in vivo calibration against the results from the ABL.
To our knowledge there is no study where the reliability of the non-gas calibrated CDI has been evaluated. We therefore set out to investigate this, and formed the following hypothesis. After in-vivo calibration, the level of accuracy and precision is the same for a gas calibrated and a non gas calibrated CDI, when compared to a standard laboratory analyzer. The aim of this study was to compare the performance of the gas calibrated CDI and the CDI without gas calibration, after in-vivo calibration against ABL results.
MATERIALS AND METHODS
Patients
Fifty-eight normothermic (core temperature 34–37°C) adult valve replacement and/or coronary artery bypass grafting procedures with CPB were randomized at the patient’s arrival in the operating theatre to either without gas calibration (Study Group), or with gas calibration (Control Group).
Ethics
The use of the CDI is part of the standard procedure of the department. Blood gas analysis on the ABL was immediately available during all procedures in the study. The local Ethical Committee was consulted and concluded that patient consent was not necessary for this study.
Equipment and Utensils
Custom pack tubing sets with incorporated hard-shell venous reservoir and oxygenator from two different manufacturers were used: 1) Maquet Cardio-pulmonary with a Quadrox Softline coated oxygenator (Maquet Cardiopulmonary, Hirrlingen, Germany), and 2) Sorin with a PrimO2x PC coated oxygenator (Sorin Group, Mirandola, Italy).
Priming consisted of 1700 mL of Ringer’s Lactate (Fresenius Kabi, Uppsala, Sweden) and 5000 International Units of Bovine Heparin (Leo Pharma A/S, Ballerup, Denmark). Gas anesthesia and CO2 flushing were not used in the study.
The same CDI monitor (Terumo Cardiovascular Systems Europe, Frankfurt, Germany) was used for every case in the study, and had been set to start up with the factory default values to have the same starting point for the different values, at the beginning of each case. The management of the CDI was carried out according to the manufacturer’s instructions, except that gas calibration before use was omitted in the Study Group cases. For all cases, ABL analysis was immediately available to the perfusionist.
Gas calibration was done with a CDI gas calibrator, and all CDI shunt sensors were from one single batch. Anesthesia, surgery, and CPB were performed according to the standards of the department and will not be described in detail because they have no influence on the parameters examined in this study.
Pre-CPB Handling
For the patients allocated to the Study Group, the CDI 500 was not gas calibrated prior to bypass. For the patients allocated to the Control Group, the CDI was gas calibrated prior to bypass according to the manufacturer’s instructions. The heart-lung machine was primed and tested according to the protocol in the department, while the patient was being anesthetized. CO2-flushing of the bypass-system before priming was not used.
The shunt sensor was unpacked immediately prior to use, and placed either directly in the primed bypass line from arterial filter to venous reservoir, before the sample manifold (Study Group) or in the gas calibrator for gas calibration, and after completion of gas calibration, inserted into the primed bypass line from arterial filter to venous reservoir, before the sample manifold (Control Group). The CPB system was then recirculated with the prime solution without gas flow for a variable period depending on the actual operation, from 15 minutes until more than one hour, until the start of bypass.
Value Storage on CDI and Blood Samples for ABL Analysis:
Measurement series 1: after 5–15 minutes of steady state bypass.
Measurement series 2: 30 minutes after series 1.
Procedure
The values on the CDI (CDI estimates) were stored using the “store/recall” function on the CDI, and immediately thereafter blood was drawn via the sample line for ABL blood gas analysis. One mL of arterial blood was drawn directly into a 2 mL syringe without purging. Air bubbles were removed from the syringe immediately. Analysis of the arterial sample by the ABL, situated in a room adjacent to the theatre, was performed inside of 10 minutes after the blood was drawn. Intermediate ABL-analysis could be performed anytime, so that gas and blood flow adjustments could be made if necessary. When the perfusionist had received the results of the ABL analysis, the stored values on the CDI were recalled and the calibration was done by replacing the stored values with the ABL analysis results (ABL calibration).
Statistical Analysis
Data were reported as the mean ± standard deviation (SD). The relative error of the CDI estimates with and without gas calibration, for values of pO2, pCO2, and pH, was defined as the difference between the CDI estimates and the ABL measurements divided by the ABL measurements and expressed as percentage. Bland Altman plots were prepared for pO2, pCO2, and pH respectively. The relative errors of each CDI estimate were plotted against the reference ABL measurements (8), enabling assessment of the overall accuracy and precision of CDI estimates, with and without gas calibration, compared to the ABL method. The mean and SD of the relative errors of the CDI estimates were interpreted as measures of accuracy and precision of the CDI method, respectively. Significance was set at p < .05.
RESULTS
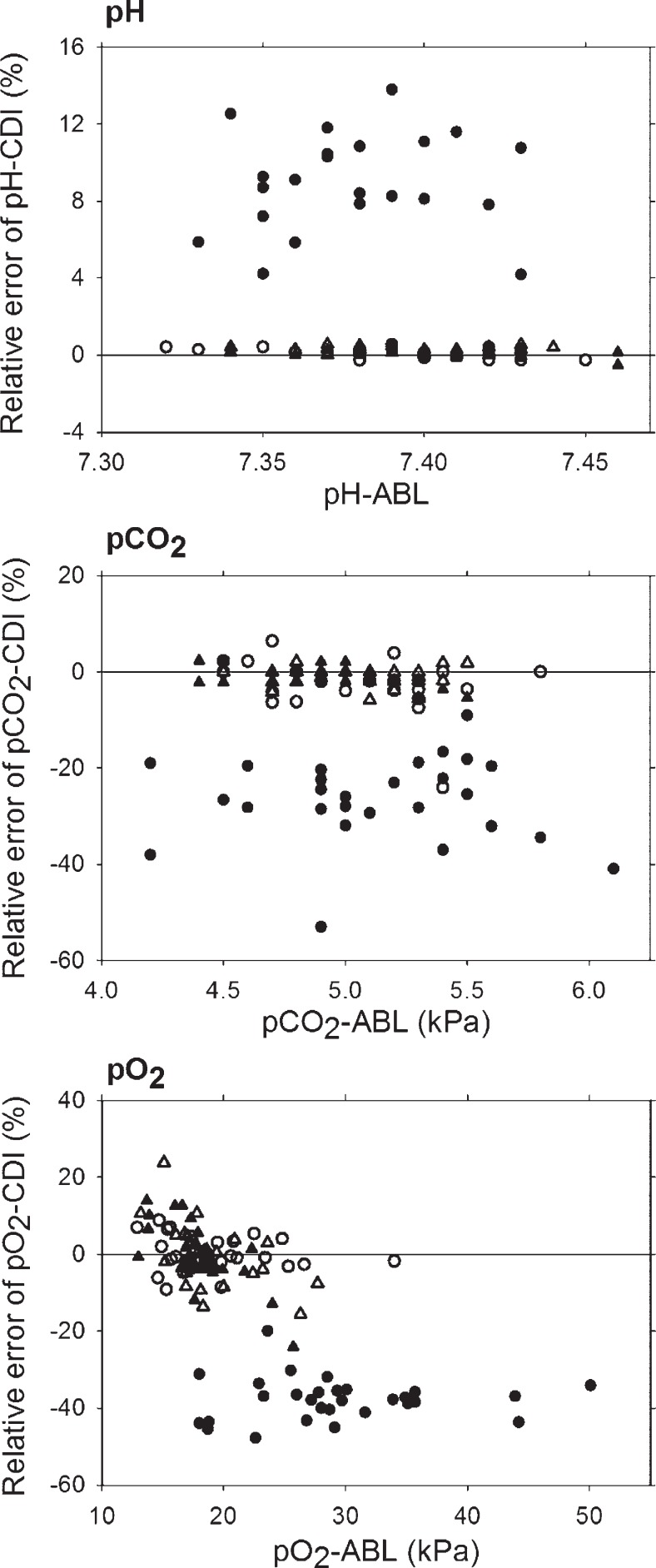
Twenty-nine estimates of pH, pCO2, and pO2 respectively were obtained under each state of the CDI, without gas calibration and before ABL calibration, without gas calibration after ABL calibration, with gas calibration before ABL calibration, and with gas calibration after ABL calibration. However, without gas calibration and before ABL calibration the CDI failed to report pH in seven cases. The accuracy and precision of the CDI method was determined for pH, pCO2, and pO2 within the following ranges: pH: 7.31 ± 7.46, pCO2: 4.2 ± 6.1 kPa, pO2: 12.4 ± 50.1 kPa. Bland and Altman analyses were performed on the data from all patients. Plots are shown in three panels of Figure 1 for pH (top), pCO2 (middle), and pO2 (bottom). Accuracy and precision of the CDI method with and without gas calibration and before and after ABL calibration for pH, pCO2, and pO2 are shown in Table 1. The CDI method without gas calibration and before ABL calibration significantly overestimated pH by 9.0 ± 2.6%, whereas pCO2 was significantly underestimated by −26.5 ± 8.8%, and pO2 by −37.8 ± 5.6%. The mean relative errors of the CDI method without gas calibration after ABL calibration, with gas calibration before ABL calibration, and with gas calibration after ABL calibration were not significantly different from zero for pH, pCO2, and pO2. The variation of the relative errors of CDI estimates without gas calibration before ABL calibration were significantly larger than the remaining measurement series for pH (p = .001) and pCO2 (p = .007), whereas it was within the same range for pO2. A tendency for precision to be slightly better, with smaller SD values, for the gas calibrated CDI estimates than for the CDI estimates without gas calibration, even after ABL calibration, was observed for pO2, pCO2, and pH. For the pO2 estimates, independent of calibration method, we observed a negative correlation between the relative errors of the CDI estimates and the ABL measurements (Figure 1 bottom).
Figure 1.
Bland-Altman plots for the agreement between the CDI and ABL methods for pH (top), pCO2 (center), and pO2 (bottom); with gas calibration, before in vivo calibration (○), with gas calibration, after in vivo calibration (△), without gas calibration, before in vivo calibration (●), without gas calibration, after in vivo calibration (▴). The relative errors of each CDI estimate are plotted against the reference ABL measurements The solid line in each plot represents zero relative error of CDI estimates compared to ABL measurements.
Table 1.
Relative error of CDI estimates with and without gas calibration.
| With Gas Calibration | Without Gas Calibration | ||
|---|---|---|---|
| Before ABL calibration | pH | .1 ± .2% | 9.0 ± 2.6%* |
| pCO2 | −2.9 ± 5.2% | −26.5 ± 8.8%* | |
| pO2 | .3 ± 4.5% | −37.8 ± 5.6%* | |
| After ABL calibration | pH | .2 ± .2% | .1 ± .2% |
| pCO2 | −1.0 ± 2.0% | −1.1 ± 2.2% | |
| pO2 | −.9 ± 7.8% | −.5 ± 8.1% |
Values are mean ± SD.
Mean values are significantly different from zero (p < .05).
DISCUSSION
During routine CPB cases, it is, in most centers, possible to have laboratory blood gas analyses performed whenever necessary, but in emergency surgery, often performed off normal working hours with little or no extra personnel available for errand running, reliable in-line monitoring is a valuable tool for the perfusionist in the acid-base management of the perfusion. The accuracy and precision of the CDI have been tested by the manufacturer with excellent results. However, these results may have been obtained in an experimental, in vitro setting, and thus may not wholly reflect the clinical setting of a CPB procedure. In their work, Southworth and co-workers found that values obtained by the CDI were comparable in accuracy to those obtained by traditional laboratory analyzers (7).
We investigated the performance of the CDI in-line blood gas monitor with and without the recommended gas calibration prior to use, with regard to the three parameters pH, pCO2, and pO2, compared to our standard, the ABL 800 flex, and found that without gas calibration and before in-vivo calibration, the CDI estimates were inaccurate and imprecise for pH and pCO2 when compared to the calibrated measurement series. In 13 cases the displayed pH value was more than 8.00, and in seven cases the display showed only -.-, presumably because the values were above the measurement range of the CDI. Thus, the relative error of the CDI estimated pH could be even more pronounced than the 9 ± 2.6% obtained with the values from the display of the CDI. For pO2 the CDI estimates were inaccurate, however with a level of precision comparable to the calibrated measurement series, corresponding to the introduction of a bias (Figure 1, bottom). Estimates of pO2 could be corrected or used for detecting relative changes. In an ideal CPB situation, steady state bypass is reached within a few minutes of bypass initiation, at which time the perfusionist can carry out an in-vivo calibration of the CDI. However, the period after the start of bypass is often characterized by an unsteady blood flow return, resulting in unsteady blood and gas flows for a period, which is not always foreseeable. If the CDI has not been gas calibrated, its values are inaccurate and imprecise for pH and pCO2, and thus unreliable in this situation, and the result may very well be a less than optimal blood gas management of this critical period of CPB.
The accuracy and precision of the calibrated CDI estimates, gas or ABL calibration, for pH, pCO2, and pO2 were not significantly different from the ABL values, and thus the calibrated CDI can be a valuable tool for the perfusionist in maintaining a stable and physiologically correct acid-base balance during CPB.
Comparison with Other Studies
To our knowledge, CDI estimates with and without calibration have not been previously compared. Our results for the gas calibrated CDI correspond with the results found by Southworth et al. (7) for pH and pCO2. In their multicenter clinical evaluation study they found that the CDI overestimated the pO2 values when compared to their standard, and the authors speculated that the discrepancy could be caused by the methodology of the analyzers. We found no such overestimation in our study.
Study Limitations
The response time from when the blood comes into contact with the CDI sensor and until the results are displayed on the screen is less than 1 minute, and the display of the CDI is updated every 6 seconds. This means, however, that the values stored on the CDI when blood is drawn for ABL analysis may not correspond exactly with the actual values of the drawn blood. To reduce this possible difference the blood for ABL analysis was drawn at steady state bypass, meaning that blood and gas flows and blood gas values on the display were stable. This may not altogether have eliminated the risk of measurement/registration errors, but has reduced the error size considerably.
This study included CPB cases conducted in normothermia, i.e., at 37°C, or slight cooling to 34°C. The solubility of gases in liquids changes with temperature. When the temperature of the liquid is lowered, the solubility of the gas in the liquid increases, and the partial pressure of the gas in the liquid will decrease. The CDI measures blood gas parameters at the actual temperature and converts them to 37°C values to be displayed on the screen. The ABL 800 flex warms the blood to 37°C before analysis. Both instruments thus give the blood gas results at 37°C values. It has not been possible to obtain the formula, which is used by the CDI to convert the values from the actual temperature to 37°C values, and therefore we cannot exclude the possibility of an error source in this context.
CONCLUSION
The overall accuracy and precision of the CDI estimates of pH, pCO2, and pO2 were not significantly different when calibrated, gas or ABL calibration. However, if not calibrated CDI estimates were inaccurate and imprecise for pH and pCO2 when compared to the calibrated measurement series. For pO2, CDI estimates were inaccurate, however with a level of precision comparable to the calibrated measurement series, corresponding to the introduction of a bias.
Since CPB gas and blood flow conditions are often very unstable for an unknown length of time after the start of bypass, during which period it is not possible to calibrate the CDI with a reliable result, we recommend gas calibration of the CDI 500 prior to use. Our recommendation for the use of the CDI in emergency cases where there is no time to gas-calibrate the CDI before the start of bypass, is to not trust the values on the CDI display until after an invivo calibration has been performed against standard laboratory results.
REFERENCES
- 1.Stammers A.. Monitoring controversies during cardiopulmonary bypass: How far have we come? Perfusion. 1998;13:35–43. [DOI] [PubMed] [Google Scholar]
- 2.Trowbridge C, Vasques M, Stammers AH, et al. The effects of continuous blood gas monitoring during cardiopulmonary bypass: A prospective, randomized study — part I. J Extra Corpor Technol. 2000;32:120–8. [PubMed] [Google Scholar]
- 3.Ferries LH.. The effects of continuous blood gas monitoring during cardiopulmonary bypass: A prospective, randomized study — part II. J Extra Corpor Technol. 2000;32:129–37. [PubMed] [Google Scholar]
- 4.Ferries LH.. Standards of care in perfusion: Should not continuous in-line blood gas monitoring be one? J Extra Corpor Technol. 1992;24:45–8. [PubMed] [Google Scholar]
- 5.Rubsamen D.. Continuous blood gas monitoring during cardiopulmonary bypass — how soon will it be the standard of care? Journal of Cardiothoracic Anesthesia. 1990;4:1–4. [DOI] [PubMed] [Google Scholar]
- 6.Terumo. CDI™ Blood Parameter Monitoring System 500 Operators Manual. Frankfurt, Germany: Terumo CVS. Terumo Cardiovascular Systems Europe; 2000. [Google Scholar]
- 7.Southworth R, Sutton R, Mize S, et al. Clinical evaluation of a new in-line continuous blood gas monitor. J Extra Corpor Technol. 1998;30:166–70. [PubMed] [Google Scholar]
- 8.Krouwer JS.. Why Bland-Altman plots should use X, not (Y+X)/2 when X is a reference method. Stat Med. 2008;27:778–80. [DOI] [PubMed] [Google Scholar]